- 1Unit of Behavioral Neurology and Center for Cognitive Disorders and Dementias (CDCD), IRCCS Mondino Foundation, Pavia, Italy
- 2Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
- 3Department of Mathematics, University of Pavia, Pavia, Italy
- 4Pavia Unit, National Institute for Nuclear Physics (INFN), Pavia, Italy
- 5Laboratory of Neuropsychology, IRCCS Mondino Foundation, Pavia, Italy
- 6Neuroradiology Department, Advanced Imaging and Radiomics Center, IRCCS Mondino Foundation, Pavia, Italy
Background: Neuropsychiatric symptoms (NPSs) are a distressful aspect of dementia and the knowledge of structural correlates of NPSs is limited. We aimed to identify associations of fronto-limbic circuit with specific NPSs in patients with various types of cognitive impairment.
Methods: Of 84 participants, 27 were diagnosed with mild cognitive impairment (MCI), 41 with Alzheimer’s disease (AD) dementia and 16 with non-AD dementia. In all patients we assessed regional brain morphometry using a region of interest (ROI)-based analysis. The mean cortical thickness (CT) of 20 cortical regions and the volume (V) of 4 subcortical areas of the fronto-limbic system were extracted. NPSs were rated with the Neuropsychiatric Inventory (NPI). We used multiple linear regression models adjusted for age and disease duration to identify significant associations between scores of NPI sub-domains and MRI measures of brain morphometry.
Results: All significant associations found were negative, except those between irritability and the fronto-opercular regions in MCI patients (corresponding to a 40-50% increase in CT) and between delusions and hippocampus and anterior cingulate gyrus (with a 40-60% increase). Apathy showed predominant involvement of the inferior frontal regions in AD group (a 30% decrease in CT) and of the cingulate cortex in non-AD group (a 50-60% decrease in CT). Anxiety correlated in MCI patients with the cingulate gyrus and caudate, with a CT and V decrease of about 40%, while hallucinations were associated with left enthorinal gyrus and right amygdala and temporal pole. Agitation showed associations in the AD group with the frontal regions and the temporal pole, corresponding to a 30-40% decrease in CT. Euphoria, disinhibition and eating abnormalities were associated in the MCI group with the entorhinal, para-hippocampal and fusiform gyri, the temporal pole and the amygdala (with a 40-70% decrease in CT and V). Finally, aberrant motor behavior reported a significant association with frontal and cingulate regions with a 50% decrease in CT.
Conclusion: Our findings indicate that specific NPSs are associated with the structural involvement of the fronto-limbic circuit across different types of neurocognitive disorders. Factors, such as age and disease duration, can partly account for the variability of the associations observed.
1 Introduction
Dementia is a clinical syndrome characterized by a decline in cognition that interferes with activities of daily living. According to the World Health Organization, around 55 million of people are presently living with dementia worldwide, with a trend to triple by 2050 (1). In addition to cognitive impairment, neuropsychiatric symptoms (NPSs) are a core clinical feature of dementia. These symptoms, often referred to as behavioral and psychological symptoms of dementia (BPSD), broadly include depression, apathy, agitation, psychosis, sleep disturbances and eating abnormalities. NPSs affect almost all demented patients at least once during the disease course, even in early stages, and are associated with accelerated progression to severe dementia, increased risk of institutionalization and earlier death (2, 3). Noteworthy, NPSs appear within the diagnostic criteria of specific types of dementia, such as Dementia with Lewy bodies (DLB) (i.e. visual hallucinations and rapid eye movement (REM) sleep behavior disorders) (4) and the behavioral variant of Frontotemporal Dementia (FTD) (i.e. disinhibition, apathy, aberrant motor behavior, and eating disorders) (5). They are the most distressful aspect of dementia and often lead to lower quality of life for both patients and caregivers (6), although in clinical settings they represent potentially reversible conditions (7). Therefore, early recognition of NPSs in subjects with cognitive impairment is relevant for diagnostic implications, as well as for therapeutic management and disease outcome.
Neuroimaging techniques have been used in cognitive impaired patients to provide clues on the pathophysiology of the most disabling NPSs. Evidence from morphological, perfusion and metabolic studies suggests that alterations in specific cortical regions, predominantly in the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC), are associated with most of NPSs in patients with AD (8). Similarly, the anterior cingulate and subcortical regions are specifically related to apathy in AD, the anterior cingulate and frontal regions to depression, and the amygdala to anxiety (3). Studies in subjects with MCI are scarce, but reported a link between apathy and hypoperfusion of the temporal and frontal lobes (9), as well as atrophy of the inferior temporal gyrus and anterior cingulate (10). Interestingly, it has been shown that prefrontal subregions and amygdala play a key role in the emotion regulation (11) and are involved in psychiatric diseases such as major depressive disorder (12).
This early evidence focused on single neuropsychiatric symptoms or single diagnostic groups seems to suggest that the limbic lobe and its frontal interconnections are variously involved in the onset of neuropsychiatric symptoms in individuals with cognitive disorders. In this work, we studied the associations between the occurrence of neuropsychiatric symptoms and morphostructural parameters of cortical (cortical thickness) and subcortical (volume) regions in three diagnostic groups that differ in severity and etiology of cognitive decline. The aim is to exploratively investigate the involvement of the fronto-limbic circuit in the full range of neuropsychiatric disorders, taking into account the severity and the type of cognitive decline. The results of this work will provide the basis for future correlation studies focused on specific components of this circuit.
2 Materials and methods
This study was approved by the IRCCS Mondino Foundation Ethics Committee (n. 20210032261) and carried out in accordance with the ethical standards of the Helsinki Declaration. All subjects provided written informed consent for image acquisition and anonymized use of their data.
2.1 Participants
We enrolled 84 cognitively impaired patients from the Behavioral Neurology Unit of the IRCSS Mondino Foundation (Pavia, Italy), referred to our Institute consecutively between June 2018 and February 2021. We included subjects aged 50 to 90 years referring for first evaluation and subsequently diagnosed with MCI (amnesic or non-amnesic/single or multiple domain) (13) or dementia (behavioral variant of frontotemporal dementia (bvFTD) (5), dementia with Lewy bodies (DLB) (4) or vascular dementia (VD)). Subjects with psychiatric disease, epilepsy or any uncontrolled medical condition that could contribute to cognitive impairment (e.g., nephropathy, liver disease, brain tumor, alcohol or drug abuse, normal pressure hydrocephalus) were excluded. None of the patients were receiving cholinesterase inhibitors, antidepressants or antipsychotic drugs at the time of the assessment.
2.2 Study design
This study was designed as a single-site cross-sectional case-control study in which the controls were not healthy but demented subjects without NPSs (patients with NPSs versus patients without NPSs). This design was intended to allow for understanding whether the atrophy is associated with NPSs and not just related to the disease or to physiological ageing (the latter is expected to be equally represented in both groups). For the same reason, we investigated the NPSs in different etiological groups, in order to ascertain that atrophy is associated with NPSs in a reliable manner across different etiological groups. As part of the routinely diagnostic workup, all enrolled participants underwent neurological and neuropsychological evaluation, Neuropsychiatric Inventory (NPI) (14) assessment and morphological magnetic resonance imaging (MRI); cerebrospinal fluid (CSF) was collected in seventy-nine subjects (detailed analytic procedure has been previously described in 15). Subjects with dementia had a clinical dementia rating (CDR) score ≥ 1 (16) and received an etiological diagnosis of typical AD [n=41; (17)], bvFTD [n=5; (5)], DLB [n=1; (4)] or VD [n=4; (18)]. All patients with non-vascular dementia had a score < 4 on the Modified Hachinski Ischemic Scale (19). Six demented patients, with negative AD biomarkers, could not receive a diagnosis with sufficient confidence, and were therefore classified into not-otherwise specified dementias (Dem NOS). The flowchart in Figure 1 summarizes the study design and analyses performed.
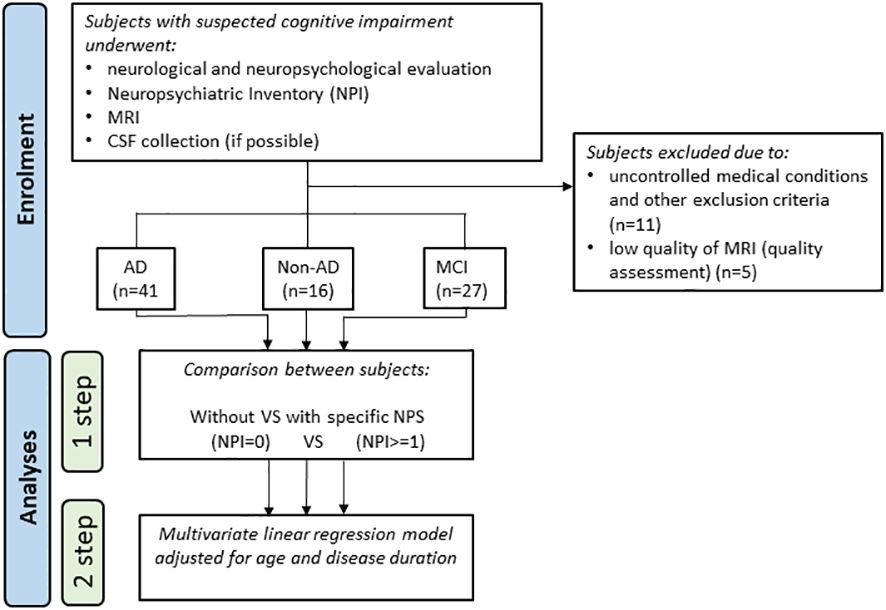
Figure 1 Flowchart of the study design and the performed analyses. CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; NPS, neuropsychiatric symptom.
2.3 Neuropsychological and behavioral assessment
The neuropsychological evaluation included tests for global cognitive efficiency (Mini-Mental State Examination, MMSE), memory (Verbal Span, Digit Span, 15 Item Memory Test, Corsi Test, Story Recall Test, Rey Complex Figure delayed recall), logical and executive functioning (Raven’s Colored Matrices, Frontal Assessment Battery), attention (Trail Making Test A/B, Attentive Matrices, Stroop Test), language (Semantic and Phonemic fluency tests) and visual-spatial perception (Rey Complex Figure copy).
NPI was used to assess behavioral changes associated with dementia. The questionnaire begins with screening questions addressed to the caregiver to investigate whether the patients had experienced any neuropsychiatric symptoms over the past month. In case of positive screening, the caregivers are asked to rate with dedicated scale the frequency (range 1-4), the severity (range 1-3), and their level of distress for the corresponding NPS (range 0-5); the total score for each NPS is the product of the ratings for frequency and severity (14).
2.4 Neuroimaging
MRI scans were acquired at the Neuroradiology Unit of IRCCS Mondino Foundation, Pavia. We analyzed eighty-four 3D T1-weighted sequences acquired with Magnetom Skyra 3T (Siemens Healthcare). A 32-channel coil was used for this study. Imaging parameters were: magnetization-prepared rapid acquisition with gradient echo (MPRAGE) with time of repetition = 2300 ms, echo time = 2.98 ms; inversion time = 900 ms; flip angle = 9°; voxel size = 1.0 x 1.0 x 1.0 mm (n = 68) or 1.2 x 1.2 x 1.2 mm, (n = 16) with no interslice gap; matrix size = 256 x 256.
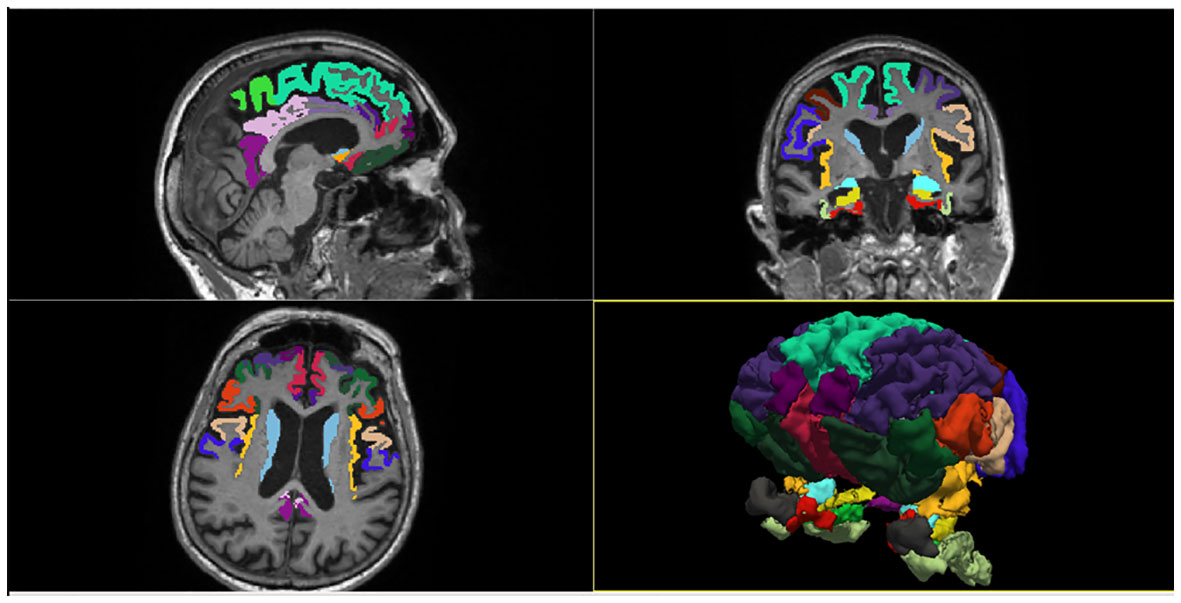
We used the commercial software FreeSurfer v.6 (https://surfer.nmr.mgh.harvard.edu) to evaluate regional brain morphometry on MRI scans. Freesurfer is a set of software tools for the study of cortical and subcortical anatomy. It provides a full processing stream for structural MRI data, including skull stripping, B1 bias field correction and gray-white matter segmentation. It also allows the reconstruction of the cortical surface (by identifying the gray-white matter boundary and pial surface) and the labeling of cortical and subcortical regions. In the present study, we extracted the cortical thickness (CT) of 20 cortical regions and the volume (V) of 4 subcortical regions included in the fronto-limbic system, which were parceled and labeled according to Desikan-Killiany anatomical atlas (Figure 2). The earliest descriptions of the anatomical composition of the fronto-limbic circuit date back to the ‘90s (20) and include as major regions: the prefrontal cortex (dorsolateral, ventromedial, and orbitofrontal), amygdala, ventral striatum (nucleus accumbens, caudate), anterior cingulate, and insula. Later, evidence in psychiatric field expanded the areas involved in the fronto-limbic circuitry to include the temporo-lateral regions and the hippocampus (21). Therefore, we placed ROIs in the above regions including also adjacent cortical areas and the posterior portion of the cingulate gyrus, in order to be as inclusive as possible. The chosen 24 regions of interest (ROI) were: Entorhinal cortex, Parahippocampal gyrus, Temporal pole, Fusiform gyrus, Superior frontal gyrus, Middle frontal gyrus (Caudal and Rostral division), Inferior frontal gyrus (Pars opercularis, Pars triangularis, Pars orbitalis), Orbitofrontal cortex (Lateral and Medial division), Frontal pole, Precentral gyrus, Paracentral lobule, Cingulate cortex (Rostral anterior division, Caudal anterior division, Posterior division, Isthmus division), Insula, Accumbens, Amygdala, Caudate, Hippocampus. We adjusted volumes for total intracranial volume (TIV) to account for individual difference in brain size. Imaging results were inspected individually by a trained radiologist (LMF) for quality assessment. An in-house Matlab v.2020b code was then used to extract and store patient-specific anatomical measures of interest for subsequent statistical analysis. CT and subcortical volumes adjusted for TIV were statistically verified to be not significantly dependent from the different T1 MPRAGE spatial resolutions through a Mann-Whitney test (Supplementary Table 1).
2.5 Statistical analysis
Shapiro-Wilk test was used to investigate the distribution normality of the different variables. Demographic and clinical characteristics among diagnostic groups were compared using Kruskall-Wallis test for continuous variables, and Chi-square test (χ2) for categorical variables.
The following statistical analyses were performed separately in three different diagnostic groups: MCI, AD dementia and non-AD dementia. Differences in CT of cortical ROIs and V of subcortical ROIs between patients presenting with a specific NPS (total NPI sub-domain score ≥ 1) compared to those who did not (total NPI subdomain score = 0) were examined using Mann-Whitney test. Associations between NPI sub-domains scores and CT or V were investigated within each group using multivariate linear regression models adjusted for age, disease duration and MMSE. Since all the statistical tests were conducted for exploratory purposes, no multiple testing correction for the number of comparisons has been applied. Statistical computations were performed using R v. 4.1.2 (The R Foundation for Statistical Computing). A two-sided p-value < 0.05 was considered statistically significant.
3 Results
3.1 Patients’ characteristics
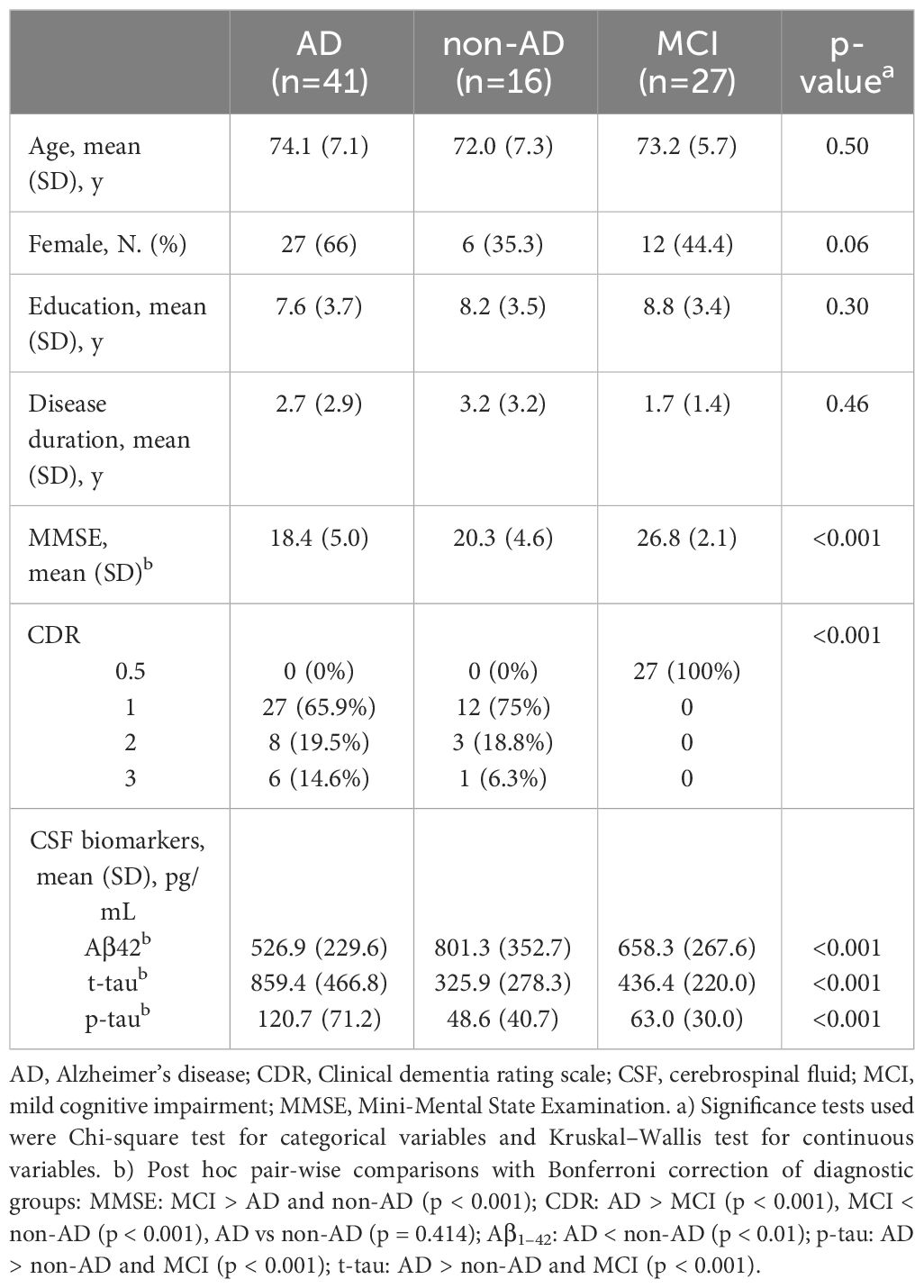
Demographic and clinical characteristics of the study population are shown in Table 1. We considered three different diagnostic groups: MCI (n=27, 13 with CSF biomarkers positive for Alzheimer pathology; age: 73.2 ± 5.7 years), AD dementia (n=41; age: 74.1 ± 7.1 years) and non-AD dementia (n=16; age: 72.0 ± 7.3 years). Non-AD dementia group included 5 patients with FTD, 1 DLB, 4 VD and 6 Dem NOS (Supplementary Table 2). Mean MMSE score was 26.8 ± 2.1 in subjects with MCI, 18.4 ± 5.0 in patients with AD dementia and 20.3 ± 4.6 in those with non-AD dementia. No significant difference was found among diagnostic groups with respect to age, gender and education. As expected, AD group showed lower Aβ42 levels, and higher p-tau and t-tau levels, compared to non-AD and MCI groups. The composite score for each item of the NPI, along with distribution of NPSs within the diagnostic groups are shown in Table 2. Depression and anxiety were the most prevalent NPSs in the MCI and AD-dementia groups (55.6% and 63.4%, respectively), while in non-AD patients apathy was the most common (68.8%). When considering all groups, more than 80% of the subjects had two or more NPSs at one time. The prevalence of apathy, night-time behavior disturbances and hallucinations significantly differed between the 3 groups of patients (p = 0.014, p = 0.035, p = 0.011 respectively). Supplementary Table 3 includes mean and standard deviation (SD) of CT (mm) and V (mm3) of the ROIs in AD, non-AD and MCI patients.

Table 2 Percentage of patients presenting with specific NPSs and mean and standard deviation of the sub-domain NPI total score in AD, non-AD and MCI groups.
3.2 Differences in fronto-limbic system morphometry between patients with and without specific NPSs
Significant differences in morphometric measures (CT and V of cortical and subcortical ROI) between patients with and without specific NPSs are shown in Table 3. For each NPS, at least one anatomic region of the 24 investigated reported a CT or V decrease, except for hallucinations for which no differences were found. Agitation in AD patients and aberrant motor behavior in non-AD patients were the NPSs associated with the higher number of atrophic ROI (11 regions for the former and 12 for the latter), without a clear hemispheric predominance but with prevalent involvement of the fronto-orbital, opercular and medial temporal regions. Delusions showed more atrophy in the fronto-orbital regions in AD and non-AD groups, depression in fronto-lateral and anterior cingulate regions in non-AD group, and apathy in fronto-orbital regions in AD group and in fronto-insular regions in non-AD group. The MCI group had fewer NPSs associated with atrophic regions compared to the AD and non-AD groups, and these differences were not only expressed in term of atrophy; apathy, night-time behavior disturbances, agitation and irritability were indeed associated to increased CT or V in specific ROIs, such as the anterior cingulate cortex, the caudate and accumbens nuclei and the rostral middle frontal gyrus.
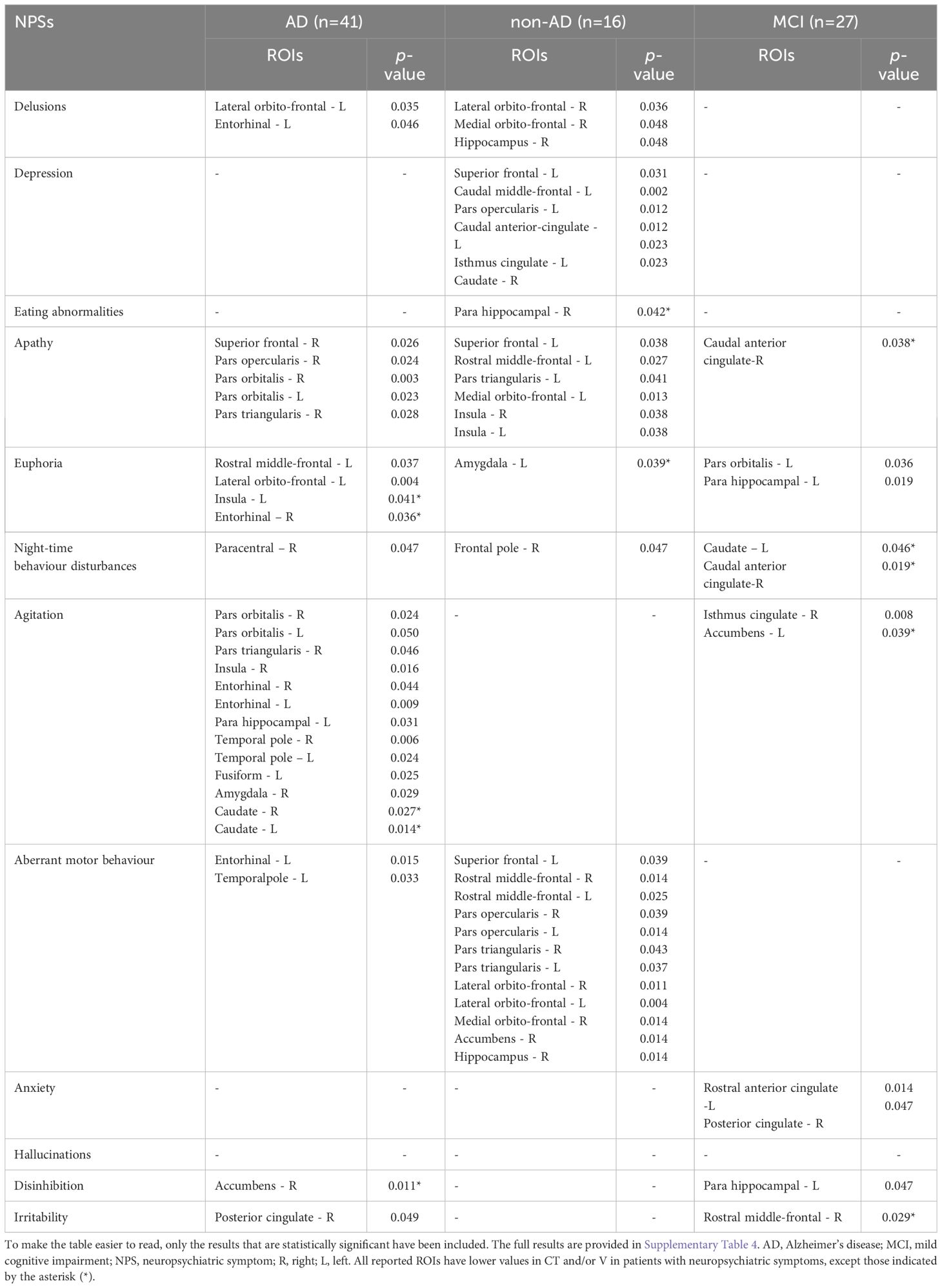
Table 3 Differences in CT of cortical ROIs and V of subcortical ROIs between patients with (total NPI sub-domain score ≥ 1) or without specific NPSs (total NPI sub-domain score = 0).
3.3 Associations between fronto-limbic circuit morphometry and NPSs
Tables 4, 5 report significant associations between total score of each NPI subdomain and CT or V of the cortical and subcortical ROIs, after correction for covariates in multiple linear regression models. All significant associations found in demented patients were generally negative (inverse), while in the MCI group positive associations were found between irritability and fronto-opercular regions (corresponding to a 40-50% increase in CT for each 100% increase in NPI score) and between delusions and hippocampus and anterior cingulate gyrus (corresponding to a 40-60% increase). Apathy showed more atrophic regions in demented patients than MCI subjects, with predominant involvement of the inferior frontal regions in AD group (corresponding to a 30% decrease in CT) and of the cingulate cortex in non-AD group (about a 50-60% decrease in CT). Anxiety correlated in MCI patients with the cingulate gyrus and caudate, with a CT and V decrease of about 40%, while hallucinations were associated with left enthorinal gyrus and right amygdala and temporal pole when the analyses were also corrected for MMSE. Agitation showed associations in the AD group with the frontal regions (superior, middle and inferior frontal gyri) and the temporal pole, corresponding to a 30-40% decrease in CT. Euphoria, disinhibition and eating abnormalities had significant associations predominantly in the MCI group. The ROIs mainly associated with these NPSs were the entorhinal, para-hippocampal and fusiform gyri, the temporal pole and the amygdala, with euphoria having a 40-70% decrease in CT and V, dishinibition a 50% decrease and eating abnormalities a 40-60% decrease. Finally, aberrant motor behavior reported a significant association with frontal and cingulate regions with a 50% decrease in CT.
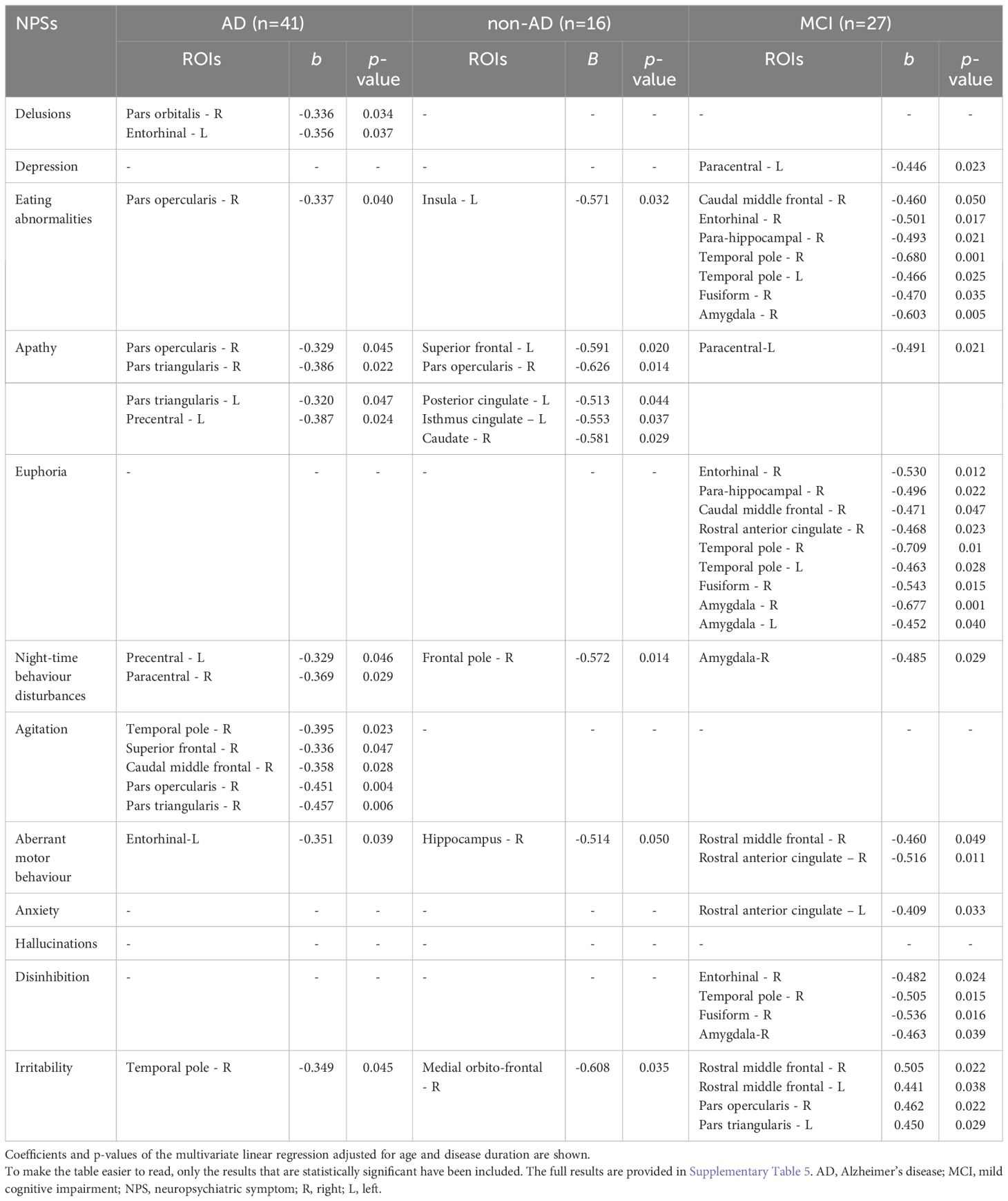
Table 4 Associations between NPI sub-domains scores (numerical variables) and CT of cortical ROIs and V of subcortical ROIs.
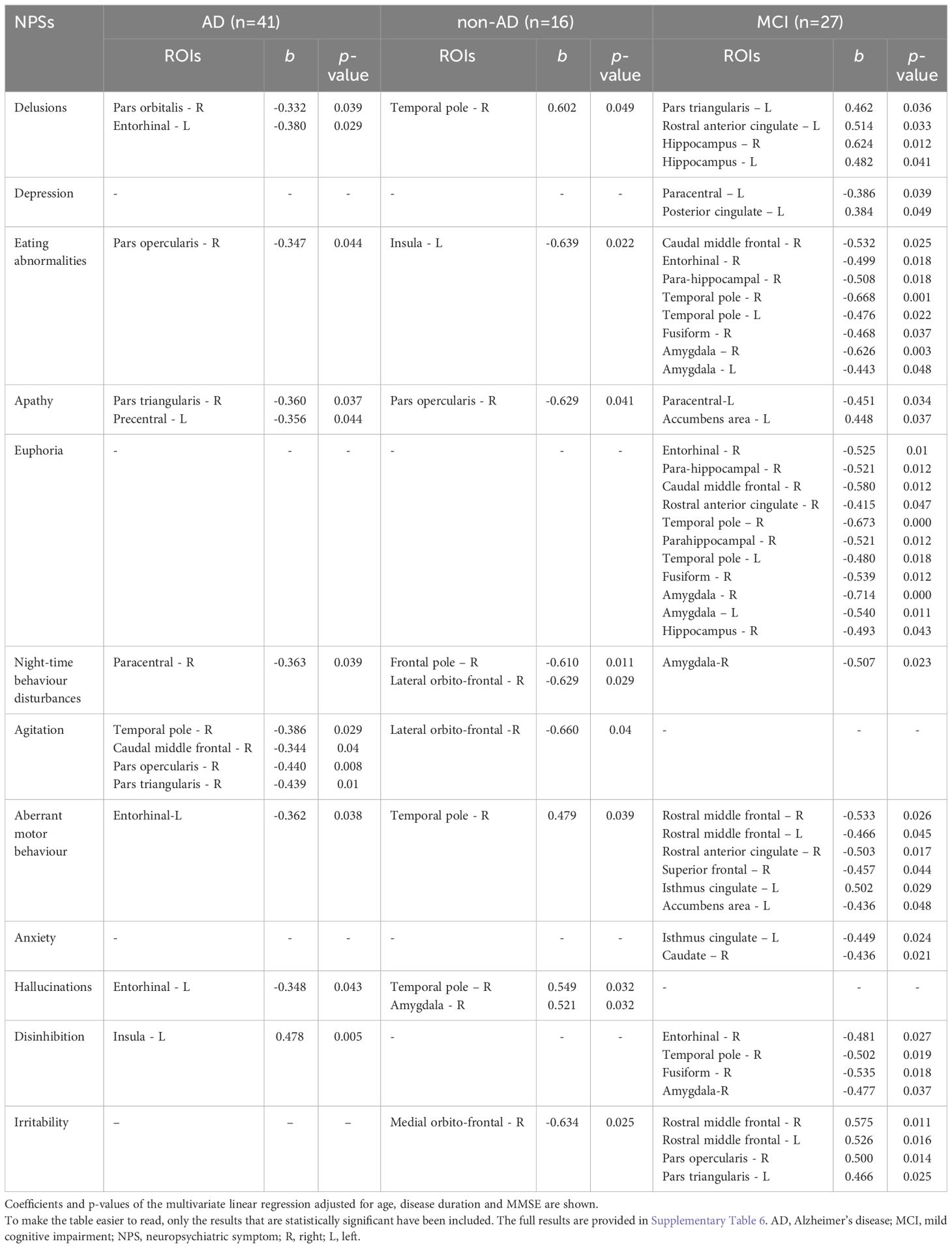
Table 5 Associations between NPI sub-domains scores (numerical variables) and CT of cortical ROIs and V of subcortical ROIs.
Based on the results that emerged in Table 3, cortical and subcortical ROIs were grouped into two main subcircuits included within the fronto-limbic circuit: circuit 1 (frontal and cingulate regions) including Superior frontal gyrus, Middle frontal gyrus (Caudal and Rostral division), Inferior frontal gyrus (Pars opercularis, Pars triangularis, Pars orbitalis), Orbitofrontal cortex (Lateral and Medial division), Cingulate cortex (Rostral anterior division, Caudal anterior division, Isthmus division), Insula, Caudate; and circuit 2 (temporal regions) including Entorhinal cortex, Parahippocampal gyrus, Temporal pole, Fusiform gyrus, Amygdala, Hippocampus. Significant associations between total score of each NPI subdomain and V of these two circuits are reported in Table 6. In MCI group, euphoria was associated with a 50% decrease in circuit 2 on both hemispheres, while anxiety was associated with a 40% decrease in circuit 1 on the right hemisphere. Conversely, irritability was associated with a 50% increase in circuit 1 on both hemispheres. These results and their relative significance depend on the number and type of regions included by the two circuits and should therefore be considered as a supplement to the previous analyses.
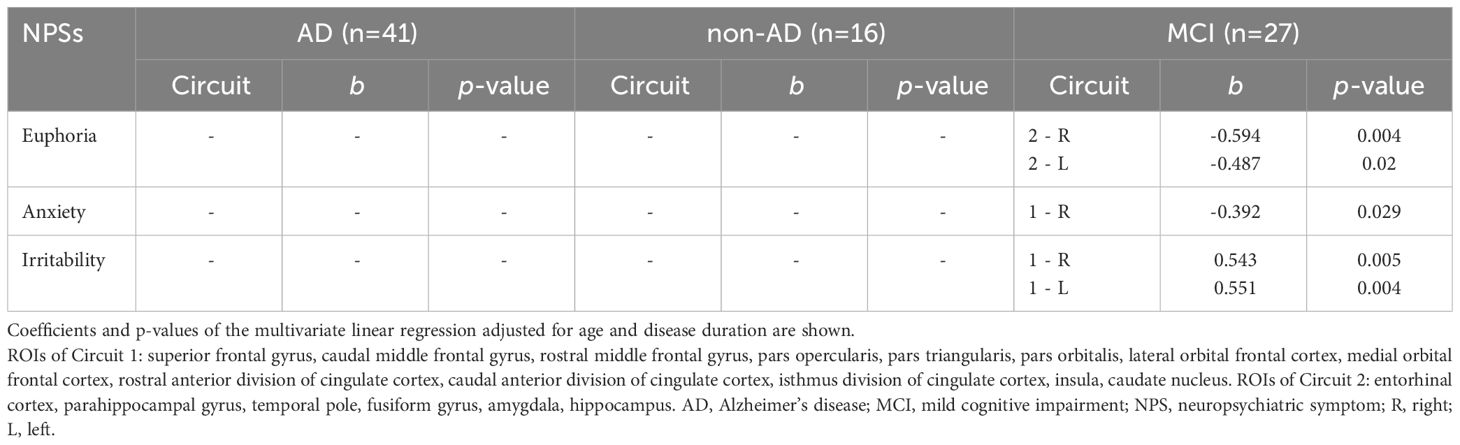
Table 6 Associations between NPI sub-domains scores (numerical variables) and volume of brain circuits composed by selected cortical and subcortical ROIs.
4 Discussion
In this study, we investigated potential associations between NPSs, assessed with the NPI questionnaire, and cortical thickness (CT) and volume (V) of 24 cerebral regions of the fronto-limbic circuit, in a cohort of patients with MCI, AD dementia and non-AD dementia. We found that different alterations in the fronto-cingulate and temporal regions were associated with presence and severity of NPSs across the different types of neurocognitive disorders. These results highlight the key role of the fronto-limbic circuit in the pathophysiology of the most frequent neuropsychiatric manifestations in cognitive disorders.
Previous neuroimaging studies reported that apathy, depression and delusions were the most prevalent NPSs associated with brain changes in AD (8). Apathy has been related to alterations of the frontal-subcortical networks, with a more severe and frequent involvement of the anterior cingulate and orbitofrontal cortices, as well as the putamen and caudate nucleus (22, 23). Our results confirmed these associations, supporting the relevance of the fronto-basal regions in modulating the behavioral initiation and reward mechanisms. Depression is known to be linked to lesions of cortical-limbic pathways (24), mainly in the dorsolateral prefrontal, cingulate and inferior temporal cortices, hypothalamus, hippocampus and insula (3). In our study, we found similar results in non-AD patients when we compared patients with and without depression (although significance did not survive correction for multiple comparisons). Conversely, we observed a negative association with the thickness of the left paracentral lobule in MCI patients and none in the AD group. Because depression has been associated in MCI subjects with atrophy of regions commonly affected by AD (frontal, parietal and temporal) (25), it is possible that this overlap of the cortical regions involved in depression and in AD attenuates the significance when comparing depressed and non-depressed patients within the AD group. Depression and apathy seem to involve more the frontal and opercular regions and the anterior portion of the cingulate (cingulate-opercular area): indeed, the fronto-opercular region and insula play an important role in the emotional control of the subject. In carrying out this function, the raw and instinctual sensory and emotional afferents coming from the hippocampus-amygdala region are filtered by the frontal and insular cortices, which play a role of inhibitory barrier and of modulation (11, 26, 27).
Concerning delusions in AD patients, we reinforced the evidence supporting the involvement of the right frontal, especially the orbitofrontal, and temporal structures (i.e. hippocampus, entorhinal cortex, amygdala) (fronto-temporal area) (21). These regions are key components of the dopaminergic, mesolimbic and mesocortical pathways, known to be involved in the control of addictive compulsive and obsessive behaviors. It has also been proposed that lesions of the right frontal and prefrontal regions may induce release and hyperactivity of the corresponding preserved contralateral frontal regions, leading to generate a creative narrator from monitoring self, memory and reality, and thus to excessive and false explanations and delusions (28).
Agitation, aberrant motor behavior, disinhibition, euphoria, and irritability, symptoms included in the “hyperactivity syndrome” (29), were associated to alterations in multiple fronto-limbic regions, including ACC, OFC, inferior frontal gyrus, entorhinal cortex, hippocampus and amygdala. Other neuroimaging findings support these results (30, 31), corroborating the importance of neurodegeneration processes affecting the anterior salience network, that may reduce capacity to process and generate appropriate behavioral responses to salient stimuli (32). Our results agree with the data from the literature; in particular, agitation and aberrant motor disorder display a heterogeneous involvement of the fronto-temporal regions, while euphoria, disinhibition and eating abnormalities were associated with the entorhinal, para-hippocampal and fusiform gyri and the amygdala. This evidence supports the idea that fronto-temporal and cingulate-opercular areas should not be thought of as discrete and functionally independent areas, but as interconnected parts of a single circuit that operate for a proper emotional and behavioral functioning. Interestingly, in MCI subjects we found a positive association between irritability and CT of frontal and prefrontal regions; although counterintuitive, other studies obtained similar findings, indicating that in early stages mechanisms other than atrophy may be involved in the development of this symptom, such as, for instance, enhanced connectivity or increased activity (33).
In the associations between NPSs and regional correlates, we found some degree of variability among the three diagnostic groups (AD, non-AD, and MCI). In particular, few differences between patients with and without NPSs were observed in the MCI group, compared with the other two groups. This could be partly explained by both the lower levels of atrophy (which to a given extent is influenced by duration and severity of disease) and the lower frequency and severity of some NPSs in this group (see frequencies and NPI scores in Table 2). Conversely, the higher frequency of NPSs and higher NPI scores in the AD group may have allowed for differences in more regions, e.g., for symptoms such as agitation, night-time behavior disturbances, hallucinations and disinhibition. Similarly, the skewed distribution of the two groups (NPS+ versus NPS-) is another factor that might have sometimes reduced the number of associated cortical regions (e.g., in the case of euphoria). In addition, for some symptoms, such as depression, regional patterns of atrophy are described that partly overlap with disease-specific ones: thus, the differences between patients with and without NPS could be mitigated. Regression analyses in the three diagnostic groups (AD, non-AD, and MCI) showed that the number of regional correlates is lower than that obtained from comparisons when corrections for disease duration and MMSE score are made. This is an expected result as we know that the neurodegenerative process is dependent on time and disease process severity, of which cognitive impairment can be considered an indirect measure.
Overall, the fronto-limbic system seems to be characterized by multiple points of vulnerability, on which the neurodegenerative processes can act, partly in a pathology-dipendent and partly in a time-dipendent way. The underlying etiology of cognitive decline and the specific distribution of its neurodegenerative process clearly influence some of the significant correlations observed in this and other studies. For instance, a higher number of temporal structures were involved in AD compared to predominant involvement of the frontal lobe in the non-AD group, which includes FTD and VD. However, the wide variability of the evidence observed in morphometry studies suggests that the occurrence of NPSs may sometimes be associated with regions not directly involved by pathology but affected by functional changes in the brain (e.g., synaptic disconnection in the white matter). Moreover, beyond the different etiological composition of the study population, also intrinsic characteristics of the subjects (genetic, epigenetic, cultural and environmental) could modulate the expression of NPSs and their neuroimaging correlates.
Limitations. The main limitation of this study is represented by the absence of a healthy control group and the heterogeneous composition of non-AD group. The choice of a cross-sectional case-control study design in which the controls were not healthy but patients without NPSs was aimed to investigate whether the atrophy is associated with NPSs and not just related to the disease or to physiological ageing (both expected to be equally represented in both cases and controls). For the same reason, we investigated the NPSs in different etiological groups, in order to ascertain whether atrophy was associated with NPSs in a reliable manner across different etiological groups. Some types of dementias are known to be characterized by specific NPSs, and these are purposely included in the diagnostic criteria (e.g. apathy in FTD, and hallucinations in LBD). The variable contribution of these nosological entities to the non-AD group may have affected the percent presentation of NPSs and the significance of the correlations within this group. Second, the cross-sectional study design may have limited the significance of some associations, as the prevalence of neuropsychiatric disorders observed at baseline may have in fact been lower than that recorded after adequate follow-up. Prospective studies could improve the strength of evidence in this regard. Finally, the statistical analyses of this study were conducted for exploratory purposes to provide a preliminary indication of which structural features or regions of interest may be associated with NPSs in neurocognitive disorders. The results of the exploratory investigation were then reinforced by regression analysis and, taken together, may guide future investigations targeting individual components of the fronto-limbic circuit and/or individual NPSs based on more appropriate designs.
Conclusion. The results of this study indicate that specific NPSs are associated with the structural involvement of areas of the fronto-limbic circuit across different types of neurocognitive disorders with different severity, as even recently reported in a neuropathological study (34). Previous studies provided information limited to single NPSs or brain regions, never providing a comprehensive view of the fronto-limbic circuit. Moreover, much of the evidence in the literature comes from studies in patients with psychiatric disorders, such as borderline personality disorder, major depression, etc., and not with cognitive disorders. Our work supports the hypothesis that this circuit exerts an important role in the pathophysiology of the most frequent neuropsychiatric manifestations through a gain/loss of function, even if the contribution of other circuits cannot be excluded. Further neuroimaging studies are warranted to explain the heterogeneity of the correlations observed between NPSs and MR findings, as well as to investigate the biological factors (e.g., diet, physical education, vascular risk factors, genetics and epigenetics) that may influence the structure and function of cortical and subcortical regions and modulate strength and direction of the correlations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving humans were approved by IRCCS Mondino Foundation Ethics Committee (n. 20210032261) . The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: MC, GP, AC. Methodology: MC, GP, MC, LF, AP. Formal analysis and investigation: MC, GP, CI, RC, FL, LM. Writing - original draft preparation: MC, GP, CI. Writing - review and editing: MC, GP, AC. Resources: AC. Supervision: AC. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Italian Ministry of Health (Ricerca Corrente,2022-2024).
Acknowledgments
We are grateful to Dr. Matteo Gastaldi for his significant contribution in CSF immunoassay.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1231361/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; BPSD, Behavioral and psychological symptoms of dementia; CSF, cerebrospinal fluid; CT, cortical thickness; FTD, frontotemporal dementia; DLB, Dementia with Lewy bodies; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; NPI, Neuropsychiatric Inventory; NPSs, neuropsychiatric symptoms; ROI, region of interest; V, volume.
References
1. World Health Organization. Dementia (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/dementia (Accessed October 19, 2022).
2. Bellelli G, Morandi A, Di Santo SG, Mazzone A, Cherubini A, Mossello E, et al. “Delirium Day”: a nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. (2016) 14:106. doi: 10.1186/s12916-016-0649-8
3. Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener. (2021) 16:38. doi: 10.1186/s13024-021-00456-1
4. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. (2017) 89:88–100. doi: 10.1212/WNL.0000000000004058
5. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
6. Allegri RF, Sarasola D, Serrano CM, Taragano FE, Arizaga RL, Butman J, et al. Neuropsychiatric symptoms as a predictor of caregiver burden in Alzheimer’s disease. Neuropsychiatr Dis Treat. (2006) 2:105–10.
7. D’Antonio F, Tremolizzo L, Zuffi M, Pomati S, Farina E. Clinical perception and treatment options for behavioral and psychological symptoms of dementia (BPSD) in Italy. Front Psychiatry. (2022) 13:843088. doi: 10.3389/fpsyt.2022.843088
8. Boublay N, Schott AM, Krolak-Salmon P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: a review of 20 years of research. Eur J Neurol. (2016) 23:1500–9. doi: 10.1111/ene.13076
9. Kazui H, Takahashi R, Yamamoto Y, Yoshiyama K, Kanemoto H, Suzuki Y, et al. Neural basis of apathy in patients with amnestic mild cognitive impairment. J Alzheimers Dis. (2017) 55:1403–16. doi: 10.3233/JAD-160223
10. Guercio BJ, Donovan NJ, Ward A, Schultz A, Lorius N, Amariglio RE, et al. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J Neuropsychiatry Clin Neurosci. (2015) 27:e22–7. doi: 10.1176/appi.neuropsych.13060141
11. Kebets V, Favre P, Houenou J, Polosan M, Perroud N, Aubry JM, et al. Fronto-limbic neural variability as a transdiagnostic correlate of emotion dysregulation. Transl Psychiatry. (2021) 11:545. doi: 10.1038/s41398-021-01666-3
12. Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. (2007) 12:360–6. doi: 10.1038/sj.mp.4001919
13. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. (2011) 7:270–9. doi: 10.1016/j.jalz.2011.03.008
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. (1994) 44:2308–14. doi: 10.1212/wnl.44.12.2308
15. Cotta Ramusino M, Perini G, Vaghi G, Dal Fabbro B, Capelli M, Picascia M, et al. Correlation of frontal atrophy and CSF tau levels with neuropsychiatric symptoms in patients with cognitive impairment: A memory clinic experience. Front Aging Neurosci. (2021) 13:595758. doi: 10.3389/fnagi.2021.595758
16. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
17. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. (2011) 7:263–9. doi: 10.1016/j.jalz.2011.03.005
18. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. (1993) 43:250–60. doi: 10.1212/wnl.43.2.250
19. Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, et al. Cerebral blood flow in dementia. Arch Neurol. (1975) 32:632–7. doi: 10.1001/archneur.1975.00490510088009
20. Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cognit Sci. (1999) 3:11–21. doi: 10.1016/s1364-6613(98)01265-0
21. Ismail Z, Nguyen MQ, Fischer CE, Schweizer TA, Mulsant BH. Neuroimaging of delusions in Alzheimer’s disease. Psychiatry Res. (2012) 202:89–95. doi: 10.1016/j.pscychresns.2012.01.008
22. Theleritis C, Politis A, Siarkos K, Lyketsos CG. A review of neuroimaging findings of apathy in Alzheimer’s disease. Int Psychogeriatr. (2014) 26:195–207. doi: 10.1017/S1041610213001725
23. Lanctôt KL, Agüera-Ortiz L, Brodaty H, Francis PT, Geda YE, Ismail Z, et al. Apathy associated with neurocognitive disorders: Recent progress and future directions. Alzheimers Dement. (2017) 13:84–100. doi: 10.1016/j.jalz.2016.05.008
24. Deckersbach T, Dougherty DD, Rauch SL. Functional imaging of mood and anxiety disorders. J Neuroimaging. (2006) 16:1–10. doi: 10.1177/1051228405001474
25. Lee GJ, Lu PH, Hua X, Lee S, Wu S, Nguyen K, et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biol Psychiatry. (2012) 71:814–21. doi: 10.1016/j.biopsych.2011.12.024
26. Pavuluri M, Feel MAI. Therefore, I am: the Insula and its role in human emotion, cognition and the sensory-motor system. AIMS Neurosci. (2015) 2:18–27. doi: 10.3934/Neuroscience.2015.1.18
27. LaLumiere RT, McGaugh JL, McIntyre CK. Emotional modulation of learning and memory: pharmacological implications. Pharmacol Rev. (2017) 69:236–55. doi: 10.1124/pr.116.013474
28. Devinsky O. Delusional misidentifications and duplications: right brain lesions, left brain delusions. Neurology. (2009) 72:80–7. doi: 10.1212/01.wnl.0000338625.47892.74
29. Aalten P, Verhey FR, Boziki M, Bullock R, Byrne EJ, Camus V, et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: part I. Dement Geriatr Cognit Disord. (2007) 24:457–63. doi: 10.1159/000110738
30. Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. (2008) 131:2455–63. doi: 10.1093/brain/awn151
31. Trzepacz PT, Yu P, Bhamidipati PK, Willis B, Forrester T, Tabas L, et al. Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. (2013) 9:S95–S104.e1. doi: 10.1016/j.jalz.2012.10.005
32. Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. (2014) 35:1237–46. doi: 10.1002/hbm.22248
33. Siafarikas N, Alnæs D, Monereo-Sanchez J, Lund MJ, Selbaek G, Stylianou-Korsnes M, et al. Neuropsychiatric symptoms and brain morphology in patients with mild cognitive impairment and Alzheimer’s disease with dementia. Int Psychogeriatr. (2021) 33:1217–28. doi: 10.1017/S1041610221000934
34. Poloni TE, Medici V, Negro G, Davin A, Chikhladze M, Zaccaria D, et al. P2-433: Association between limbic lesions (TDP-43 and/or lewy type synucleinopathy) and psychotic symptoms in patients with dementia: preliminary data from the Abbiategrasso brain bank (Italy). Alzheimers Dement. (2019) 15:P778–9. doi: 10.1016/j.jalz.2019.06.2840
Keywords: neuropsychiatric symptoms, fronto-limbic circuit, cortical thickness, brain volume, cognitive impairment
Citation: Cotta Ramusino M, Imbimbo C, Capelli M, Cabini RF, Bernini S, Lombardo FP, Mazzocchi L, Farina LM, Pichiecchio A, Perini G and Costa A (2024) Role of fronto-limbic circuit in neuropsychiatric symptoms of dementia: clinical evidence from an exploratory study. Front. Psychiatry 15:1231361. doi: 10.3389/fpsyt.2024.1231361
Received: 30 May 2023; Accepted: 15 April 2024;
Published: 10 May 2024.
Edited by:
Prabesh Kanel, University of Michigan, United StatesReviewed by:
Shefali Chaudhary, Yale University, United StatesJannik Prasuhn, Johns Hopkins University, United States
Copyright © 2024 Cotta Ramusino, Imbimbo, Capelli, Cabini, Bernini, Lombardo, Mazzocchi, Farina, Pichiecchio, Perini and Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matteo Cotta Ramusino, bWF0dGVvLmNvdHRhcmFtdXNpbm8wMUB1bml2ZXJzaXRhZGlwYXZpYS5pdA==
 Matteo Cotta Ramusino
Matteo Cotta Ramusino Camillo Imbimbo
Camillo Imbimbo Marco Capelli1
Marco Capelli1 Raffaella Fiamma Cabini
Raffaella Fiamma Cabini Sara Bernini
Sara Bernini Francesca Paola Lombardo
Francesca Paola Lombardo Laura Mazzocchi
Laura Mazzocchi Lisa Maria Farina
Lisa Maria Farina Anna Pichiecchio
Anna Pichiecchio Giulia Perini
Giulia Perini Alfredo Costa
Alfredo Costa