
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 23 February 2023
Sec. Public Mental Health
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.999934
 Yuan Zhou1,2,3*
Yuan Zhou1,2,3* Yuwen He1,4
Yuwen He1,4 Yuening Jin1,2
Yuening Jin1,2 Peter Zeidman5
Peter Zeidman5 Lianlu Gao1,2
Lianlu Gao1,2 Bei Rong6
Bei Rong6 Huan Huang6
Huan Huang6 Yuan Feng3
Yuan Feng3 Jian Cui3
Jian Cui3 Shudong Zhang3
Shudong Zhang3 Yun Wang3
Yun Wang3 Gang Wang3
Gang Wang3 Yu-Tao Xiang4,7
Yu-Tao Xiang4,7 Huiling Wang6,8*
Huiling Wang6,8*Introduction: The amygdala plays an important role in stress responses and stress-related psychiatric disorders. It is possible that amygdala connectivity may be a neurobiological vulnerability marker for stress responses or stress-related psychiatric disorders and will be useful to precisely identify the vulnerable individuals before stress happens. However, little is known about the relationship between amygdala connectivity and subsequent stress responses. The current study investigated whether amygdala connectivity measured before experiencing stress is a predisposing neural feature of subsequent stress responses while individuals face an emergent and unexpected event like the COVID-19 outbreak.
Methods: Data collected before the COVID-19 pandemic from an established fMRI cohort who lived in the pandemic center in China (Hubei) during the COVID-19 outbreak were used to investigate the relationship between amygdala connectivity and stress responses during and after the pandemic in 2020. The amygdala connectivity was measured with resting-state functional connectivity (rsFC) and effective connectivity.
Results: We found the rsFC of the right amygdala with the dorsomedial prefrontal cortex (dmPFC) was negatively correlated with the stress responses at the first survey during the COVID-19 outbreak, and the rsFC between the right amygdala and bilateral superior frontal gyri (partially overlapped with the dmPFC) was correlated with SBSC at the second survey. Dynamic causal modeling suggested that the self-connection of the right amygdala was negatively correlated with stress responses during the pandemic.
Discussion: Our findings expand our understanding about the role of amygdala in stress responses and stress-related psychiatric disorders and suggest that amygdala connectivity is a predisposing neural feature of subsequent stress responses.
Psychosocial stressor increases anxiety, depression, and other negative emotions and thus influences individuals’ physical and mental health (1–3). Individual differences in reactions to psychosocial stressors have been observed in some studies (4–6), in which some individuals are more vulnerable to stressors or traumatic events than others. In this context, timely and precise identification of individuals who are vulnerable to stressors is urgent and important. It promotes a more proper allocation of public resources to aid vulnerable individuals, which in turn decreases the likelihood of these potentially vulnerable individuals to develop stress-related psychiatric disorders.
Amounting studies have demonstrated the importance of the amygdala in stress responses and stress-related psychiatric disorders [e.g., post-traumatic stress disorder (PTSD)] (7, 8). For example, previous studies have found abnormal amygdala connectivity in adults with early life stressors (9, 10), relationship between amygdala activity or connectivity with experimentally induced acute stress (11, 12), and altered spontaneous activity or functional connectivity of amygdala in patients with stress-related psychiatric disorders (13–15). These evidences suggest that the amygdala activity or connectivity may be a neurobiological vulnerability marker for stress responses or stress-related psychiatric disorders and will be useful to precisely identify the vulnerable individuals before stressors or traumatic events. However, few studies directly examine this possibility, due to two major difficulties in experimental design. First, the occurrence of natural stressors cannot be foreseen. Second, the acquisition of neuroimaging data before the occurrence of natural stressors is difficult. Two experimental studies have attempted to investigate whether task-induced amygdala activity reflects a vulnerability to trauma exposure (1, 2). These studies reveal that the increased amygdala’s reactivity to stress-related stimuli predicts the subsequent stress symptoms. However, these studies cannot capture the role of amygdala connectivity in predicting real chronic stressors in natural settings.
In December 2019, a mass outbreak of a novel coronavirus infection, named as the novel coronavirus disease (COVID-19), occurred in Wuhan, Hubei province, China. Then, the COVID-19 pandemic affects people around the world. Besides the physical influence due to the infection, the COVID-19 also influences individuals’ mental health (16, 17), not only among the infected patients but also among the general public (18, 19). Thus, the COVID-19 pandemic is taken as an uncertain and unpredictable psychosocial stressor (20). Individual differences in reactions to this stressor have also been observed (21, 22). The current study grasps the unique chance of COVID-19 outbreak and leverages data from an established healthy cohort in Hubei province to investigate whether amygdala connectivity measured before the COVID-19 pandemic is related to subsequent stress responses in the individuals in the geographic pandemic center (i.e., Hubei, China).
Resting-state functional magnetic resonance imaging (fMRI) is a powerful tool to uncover the neural basis of individual differences in human cognitive abilities and behavioral tendencies (23–27). Two studies from one research group have found that the resting-state functional connectivity (rsFC) can predict the feelings of stress or anxiety related to the COVID-19 pandemic (28, 29); however, neither of the studies focused on amygdala connectivity, instead they focused on functional connectome of the whole brain regions. Therefore, it is still unclear whether amygdala connectivity before experiencing stress is a predisposing neural feature of subsequent stress responses while facing an emergent and unexpected event, like the COVID-19 outbreak.
It is noteworthy that these studies applied rsFC to investigate the potential linkage between amygdala connectivity and subsequent stress responses (28, 29). This method, rsFC, which examines correlations between fMRI time series across the brain, does not reveal the causal influence of one neural system on another and thus only describes a non-directed functional interaction (30). Animal studies have demonstrated that the directed interaction between amygdala and other brain regions, such as dorsomedial prefrontal cortex (dmPFC), is highly correlated with the increased anxiety-like behavior in stressed mice (31). Therefore, it is more interesting to investigate whether the causal influences related to the amygdala predict subsequent stress responses in humans.
To capture how the directional interaction between amygdala and brain regions predicts subsequent stress responses in humans, we incorporated the dynamic causal modeling (DCM), a widely adopted framework for effective connectivity analysis (32). DCM can better disclose the causal and directed nature of coupling between intrinsic modes of brain activity (30). This approach has been used to predict individual differences in the cognition of healthy participants and in treatment responses of depressed patients (33, 34). Technologies called stochastic and spectral dynamic causal modeling (spDCM) are the most recent approaches to characterize effective connectivity during rest (35). Compared to its stochastic counterpart, spDCM, which operates in the frequency domain rather than the time domain, is more computationally efficient and more accurate and sensitive to group differences (36). Therefore, we used the spDCM to estimate effective connectivity and test the hypothesis that the directed connectivity of amygdala or its self-connection, as a predisposing neural feature, is related to subsequent stress responses.
In brief, this study investigates whether resting-state functional and effective connectivity of amygdala measured before the COVID-19 pandemic is related to subsequent stress responses by analyzing data from an established healthy cohort in Hubei, China. Previous studies suggest that the amygdala works together with other brain regions, especially the medial prefrontal cortex (mPFC), to tune the expression of stress-related emotions, such as fear and anxiety (31, 37). Impaired functional interaction between the amygdala and mPFC has been repeatedly reported in both psychiatric patients and animal models and recognized as one of the core neurobiological features across stress-related psychiatric disorders (14, 38–40). Thus, we speculate that the rsFC between amygdala and mPFC measured before the COVID-19 pandemic is related to subsequent stress responses. Then, we furthermore explored how the causal and directed nature of coupling between amygdala and the target region(s) (e.g., mPFC) is related to subsequent stress responses. We are also interested in self-connection within each region. Previous studies have found hyper-responsivity within the amygdala in stress-related psychiatric disorders, such as PTSD (40, 41), and found that amygdala activity is positively correlated with the severity of PTSD symptoms (42). Self-connection in the frame of DCM can be considered as parameterizing the interplay between inhibitory interneurons and pyramidal cells within a brain region, thus reflecting the gain or excitability of neuronal populations (43–45), which is an analogy of activity responsivity during rest. Moreover, we speculate that such relationship between amygdala connectivity and subsequent stress responses will disappear when the stressor weakened. Therefore, we also explored the relationship between amygdala connectivity and stress responses measured after 3 months of the COVID-19 outbreak in Hubei.
Fifty neurologically normal participants were recruited from an established cohort that belongs to an fMRI study conducted at the Renmin Hospital, Wuhan University from June 2012 to July 2019 (46, 47). All participants had reported no history of major psychiatric or neurological illness when they were recruited. Detailed inclusion and exclusion criteria for participants were provided in the original studies (46, 47). All of the participants were invited to complete two surveys, covering from the peak of the outbreak in China in February 2020 to the remission period in June 2020 (Figure 1). Importantly, based on their self-reports, none of the participants were suspected cases or patients with the COVID-19. The first survey was conducted from 15 to 29 February 2020, when residents in Hubei experienced the most serious period of the COVID-19 pandemic. The second survey was conducted 3 months after the first survey (from 28 May to 8 June 2020), when the COVID-19 pandemic had been effectively controlled in China, as indicated by the fact that Wuhan, a Hubei city which was the most seriously affected by the COVID-19, lifted lockdown on 8 April 2020 due to the sharp reduction of daily increased diagnosed cases. Among of these participants, only the data from those participants who lived in Hubei province when they were recruited in the original project, and were living or still lived in Hubei province half a year before the COVID-19 outbreak in Hubei at the time of questionnaire data collection of this study, were used in the current study. These participants (the Hubei Cohort) were assumed to experience a high level of stressors at the first survey.
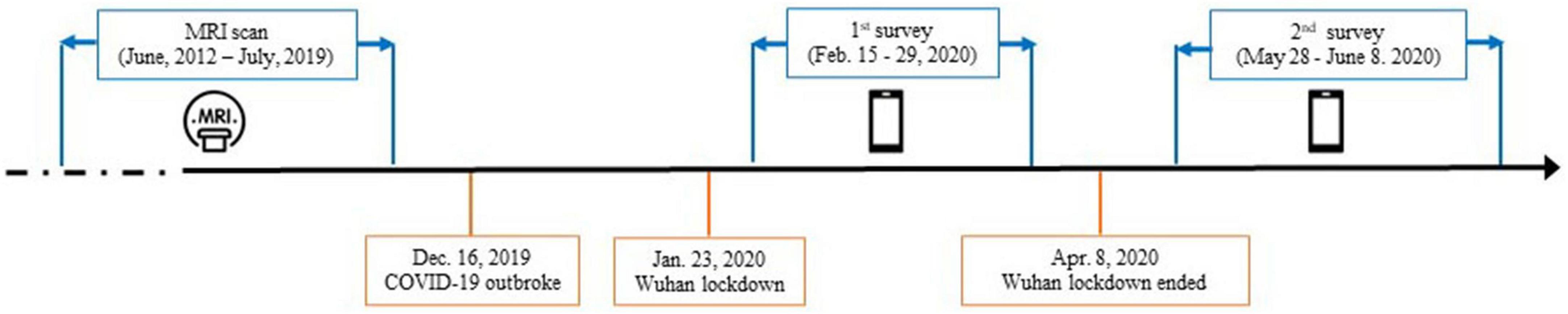
Figure 1. Timeline of data collection with the dates of major events relevant to the COVID-19 pandemic.
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University, and the Institutional Review Board of the Institute of Psychology, Chinese Academy of Sciences. All of the participants gave informed consents online.
Following self-report scale development principles (48), we developed a 14-item Stress Behavior Scale (induced by COVID-19) (SBSC) to assess the stress responses specifically related to the COVID-19 pandemic. The sampled behaviors in SBSC are different from the general stress behaviors in existing scales (e.g., Perceived Stress Scale-10, PSS-10). The detailed procedures were included in the Supplementary Material I. SBSC measures the extent to which individuals exhibit several most common COVID-19 induced stress behaviors and feelings. Sample behaviors and feelings in the SBSC include “Repeatedly takes temperature,” “Rushes to buy or hoards daily necessities and food,” “Worries that self or family members would be infected,” and so on. A full list of items is presented in Supplementary Table 1. Participants indicated that the degree to which each of the fourteen items matched their own behaviors and feelings during the COVID-19 pandemic on a six-point Likert scale ranging from 1 = does not match at all to 6 = matches to a great extent. The scale had good internal validity with Cronbach’s alpha amounting to 0.87 and 0.89, respectively for the Hubei Cohort and the non-Hubei Cohort (the validation sample described below). Explorative factor analysis on the combined sample generated one common factor with eigenvalue exceeding 1 and with factor loadings of all items on the common factor exceeding 0.40. We also administered the State-Trait Anxiety Inventory (S-TAI) (49), the PSS-10 (50), and the Patient Health Questionnaire-9 (PHQ-9) (51).
We recruited a group of healthy participants from another established non-clinical cohort conducted in Beijing (N = 58, non-Hubei Cohort) (6), administered two surveys during and after the pandemic, and conducted various analyses on the external validity of SBSC. The sample information, survey administration and analytical strategies is provided in Supplementary Material II.
The MRI data were acquired before the COVID-19 pandemic and have been used in previous studies (46, 47). MRI scanning was performed on a 3.0T General Electric Signa HDxt MR scanner in the Department of Radiology, Renmin Hospital of Wuhan University. Resting-state functional images were obtained by using an echo-planar imaging (EPI) sequence [repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 220 mm × 220 mm, matrix = 64 × 64, 32 slices, slice thickness = 4 mm, and gap = 0.6 mm) and 240 volumes were obtained. Structural images were collected using a 3D Bravo T1-weighted sequence (TE = 7.8 ms, TR = 3.0 ms, flip angle = 7°, inversion time = 1,100 ms, FOV = 256 mm × 256 mm, matrix = 256 × 256, 188 slices, and voxel size 1 mm × 1 mm × 1 mm). During the resting-state scanning, all participants were instructed to close their eyes and to focus on nothing in particular.
All imaging data preprocessing procedures were carried out with Data Processing Assistant for Resting-state fMRI version 4.3,1 which is based on Statistical Parametric Mapping 12.2 The preprocessing procedures include removing first 5 time points, slice-time correction, realignment, co-registration, segmentation for structural images, nuisance covariates regression, normalization to MNI space, and spatial smoothing. The nuisance covariates included the 5 principal components from the individual segmented white matter and the cerebrospinal fluid, 24 motion parameters (6 head motion parameters, 6 head motion parameters one time point before, and the 12 corresponding squared items), and linear and quadratic trends. Particularly, volume-based scrubbing regression by including scrubbing regressors was also included into the multiple linear regression model (52). The time points with a threshold of framewise displacement (FD) >0.5 mm as well as one back and two forward frames were identified and then modeled as a separate regressor in the regression model of the realigned resting fMRI data. After that, the preprocessed images were temporal filtering. For rsFC, a temporal filtering (0.01–0.1 Hz) was conducted. For effective connectivity, a general linear model (GLM) and an F-contrast analysis were used to identify the low frequency fluctuation in effective connectivity analysis based on previous studies (52, 53). Specifically, the voxels showing low frequency fluctuations were identified using a GLM containing a discrete cosine basis set with frequencies ranging from 0.0078 to 0.1 Hz. An F-contrast was specified across the discrete cosine transforms, producing an SPM that identified regions exhibiting BOLD fluctuations within the frequency band.
A gray matter mask was generated by including the voxels in which 90% of participants contained EPI signal and the mean gray matter values were larger than 0.2. All of the following analyses were conducted within this mask.
The left and right amygdala derived from the SPM Anatomy toolbox (54, 55) were used as two seed regions for rsFC analysis separately. We calculated the Pearson correlation between the mean time series of the seed and the time series of each voxel within the gray matter mask. After transforming the Pearson correlations into z-values, the resulting z-valued functional connectivity maps of each seed were entered to the multiple regression analyses to investigate the relationship between amygdala’s functional connectivity and the SBSC score. To remove the confounding effects, we included gender, age, and mean FD as covariates to the regression model. Statistical significance was set at a cluster-defined threshold p < 0.001 in conjunction with cluster wise FWE p < 0.025 (Bonferroni correction for two seed-based rsFC analyses) to correct for multiple comparisons.
According to the results of functional connectivity analyses, spDCM was used to reveal the relationship between directed connectivity and stress responses induced by the COVID-19 pandemic. Specifically, we took the right amygdala and the region identified by functional connectivity analysis (i.e., dmPFC) as the volume of interest (VOI). The principal eigenvariate of the voxels in each VOI was computed separately. Then spDCM analysis was conducted using DCM12.5 implemented in the SPM12 (revision 7497, see text footnote 2). For each participant, a fully connected model was built to investigate whether the stress responses was related to the top-down regulation effect from the dmPFC to the right amygdala, or the down-up regulation effect from the right amygdala to the dmPFC. We were also interested in whether the stress responses were related to the self-connections within each region. Therefore, we constructed a full model consisting of the directed connections between the right amygdala and the dmPFC as well as self-connections within each region.
The inversion of DCM at the first level was performed using spDCM, which fits the complex cross-spectral density using a power-law model of endogenous neuronal fluctuations (35, 36). Then we used Parametric Empirical Bayes (PEB) (56) to model how individual connections relate to group means and individual differences in stress responses related to COVID-19 outbreak indicated by the SBSC scores. Using the Bayesian model comparison implemented in the PEB framework, we compared reduced models that encoded different hypotheses to find the best model, who told us whether there was an effect of SBSC scores on the effective connectivity and, if so, where it was expressed (57, 58). To address this, we performed an automatic search over the reduced PEB models, in which an efficient (greedy) search of the model space was conducted by scoring the evidence for different models (with certain connections switched on or off) based on log model evidence or free energy. In the PEB framework, group-level analysis is conducted using Bayesian inference. It avoids the need to contend with the multiple-comparison problem of classical inference, because the objective is to quantify the posterior probability for effects, rather than determine whether they exceed a significance threshold (59). We computed the Bayesian posterior probability for our effects of interest using variational Bayesian methods, as implemented in the PEB framework. Then, we computed the Bayesian Model Average, which is the average of the parameters from different models weighted by the models’ posterior probabilities, to present the results. Here we focus on effects with posterior probability >0.95, which is considered “strong evidence” for an effect (60). The detailed guidance of conducting these group-level analyses could be found in the tutorials (56, 58).
We repeated the abovementioned functional connectivity analyses to explore the correlations between the rsFC of amygdala and the SBSC scores at the second survey. If the correlation found at the first survey still remained at the second survey, then effective connectivity was furthermore analyzed; otherwise, no more analyses were conducted.
In addition, we explored the relationship between changes in SBSC scores and the rsFC or effective connectivity of amygdala identified in the abovementioned analyses.
Forty-five participants who completed the first survey were recruited in the Hubei Cohort. Among of them, 30 participants also completed the second survey. Table 1 showed the demographic characteristics and measurements on COVID-19 related stress responses in the first and second survey of the Hubei Cohort, and the score differences between the two surveys with paired sample t-tests. As shown, after 3 months, when the COVID-19 pandemic had been effectively controlled in China, stress responses measured by the SBSC scores decreased in the Hubei Cohort (26.30 ± 11.43) relative to the first survey (34.73 ± 13.44) in the 30 participants who completed the second survey [t(29) = 3.24, p = 0.003].
Sample characteristics of the validation sample (i.e., non-Hubei Cohort) are provided in Supplementary Table 2. Validation analyses of SBSC convergently showed good external validity of SBSC (for details, refer to Supplementary Material III).
We found the connectivity between the right amygdala and the dmPFC (peak coordinates: [8, 48, 36], cluster size = 172 voxels, cluster-level FWE p = 0.016) was negatively correlated with the SBSC scores of the first survey, suggesting that the individuals with weaker correlation between the right amygdala and the dmPFC exhibited more stress responses (Figure 2). No significant correlations between the rsFC of the left amygdala and the SBSC scores were found.
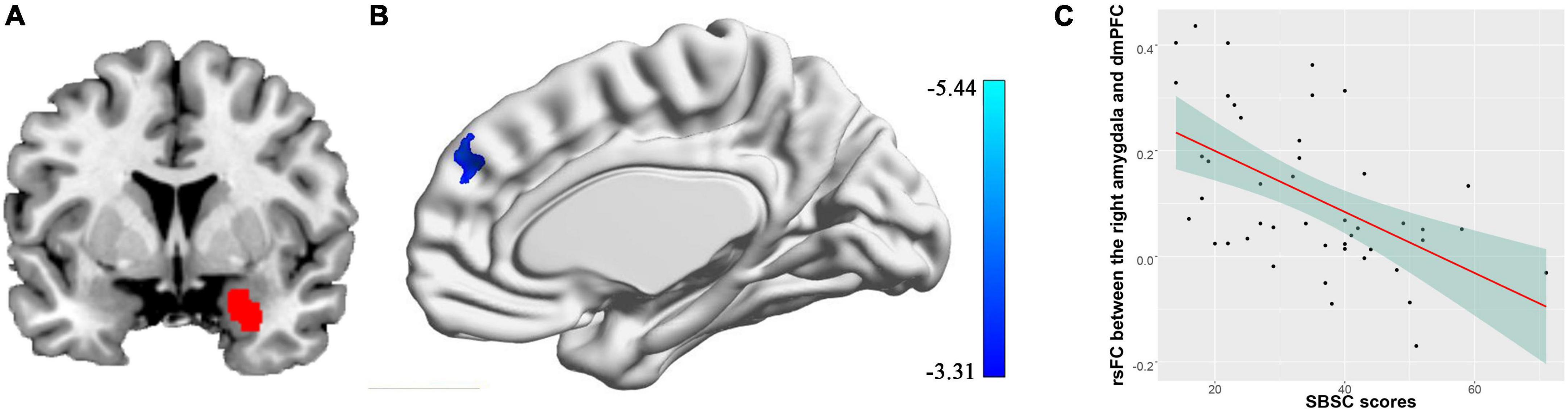
Figure 2. Correlation between rsFC of the right amygdala with the dmPFC and stress responses in a sample consisting of 45 participants who completed the first survey. (A) The right amygdala; (B) the dmPFC whose rsFC with the right amygdala correlated with the stress behaviors during the COVID-19 outbreak; (C) a scatter plot showing the relationship between the rsFC and the stress responses.
Figure 3 shows effective connectivity between the right amygdala and the dmPFC across all participants in the Hubei Cohort and its correlation with stress responses at the first survey, which exceeded 95% posterior probability based on comparing the approximate log evidence for models with and without each connectivity parameter. In Figure 3A, connectivity parameters are rate constants in Hertz for between-region connections, but to ensure negativity, self-connections are unitless log-scaling parameters that multiply a default value of −0.5 Hz. In terms of DCM for fMRI, a negative value indicates an inhibitory connection, showing that the brain activity of one brain region can decrease the rate of change of activity in another brain region; a positive value indicates an excitatory connection, indicating that the brain activity of one brain region can increase the rate of change of activity in another brain region (32, 56). Thus, we found that there was an inhibitory connectivity from the right amygdala to the dmPFC and an excitatory connectivity from the dmPFC to the right amygdala across participants (Figure 3A). Also, the level of self-inhibition was greater than the expected one under the priors for both regions, as shown by the positive parameter estimates. More importantly, we found a negative effect of the SBSC scores on the inhibitory self-connection of the right amygdala (posterior probability >95%), showing that individuals with weaker self-inhibition of the right amygdala had more stress responses (Figures 3B, C). The parameter in Figure 3B is the effect of the stress responses on the self-connection.
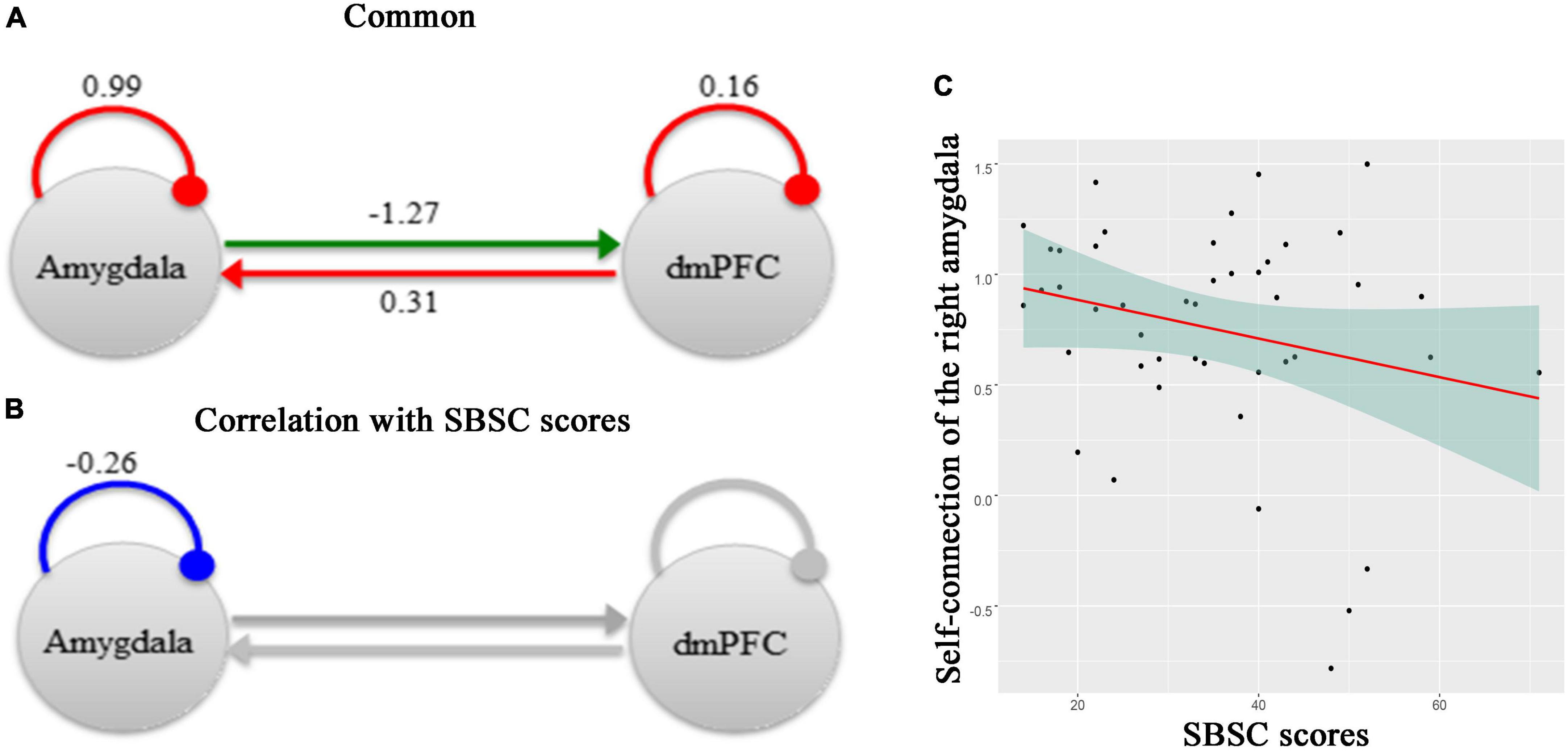
Figure 3. A schematic summarizing effective connectivity between the right amygdala and the dmPFC across participants in the Hubei Cohort and its correlation with stress responses at the first survey. (A) The green arrow represents the positive extrinsic effective connectivity and the red arrow represents the negative extrinsic effective connectivity. The parameters are the strength of connectivity. The red arcs represent self-connections. For the self-connections, the parameters are log scaling parameters, which can be converted to units of Hz by: y =–0.5 * exp(x). Where x is the log scaling parameter, −0.5 Hz is the prior and y is the self-connection strength in units of Hz. (B) The parameter is the effect of the stress responses on the self-connection. All of the other connections in this network, which had no correlations with the SBSC scores, were shown in gray. (C) A scatter plot showing the relationship between the self-connection in the right amygdala and the stress responses.
Using the data of these 30 participants in the Hubei Cohort, we could still find a negative correlation between the connectivity of the right amygdala with the dmPFC and the SBSC scores of the first survey (uncorrected voxel-wise p = 0.001, cluster-wise FWE p < 0.008, Figure 4A), which validated our main finding in a smaller sample size. No significant correlations were found between the amygdala’s rsFC and the SBSC scores of the second survey in the Hubei Cohort in the dmPFC. Instead, it was found that the rsFC of the right amygdala with the bilateral superior frontal gyri (SFG) was negatively correlated with SBSC scores at the second survey (uncorrected voxel-wise p = 0.001, cluster-wise FWE p < 0.025, Figure 4B). Because this finding is contradicting with our hypothesis that such relationship between amygdala connectivity and subsequent stress responses will disappear when the stressor weakened. We had a closer look at the findings. We found that the right SFG identified at the second survey had a small intersection with the dmPFC previously identified at the first survey (Supplementary Figure 1A). And we found a cluster located in the right SFG whose connectivity with the right amygdala was negatively correlated with the SBSC scores at the first survey at a lenient threshold (uncorrected voxel-wise p = 0.001, cluster size >50; Supplementary Figure 1B). This cluster was partly overlapped with the right SFG identified at the second survey.
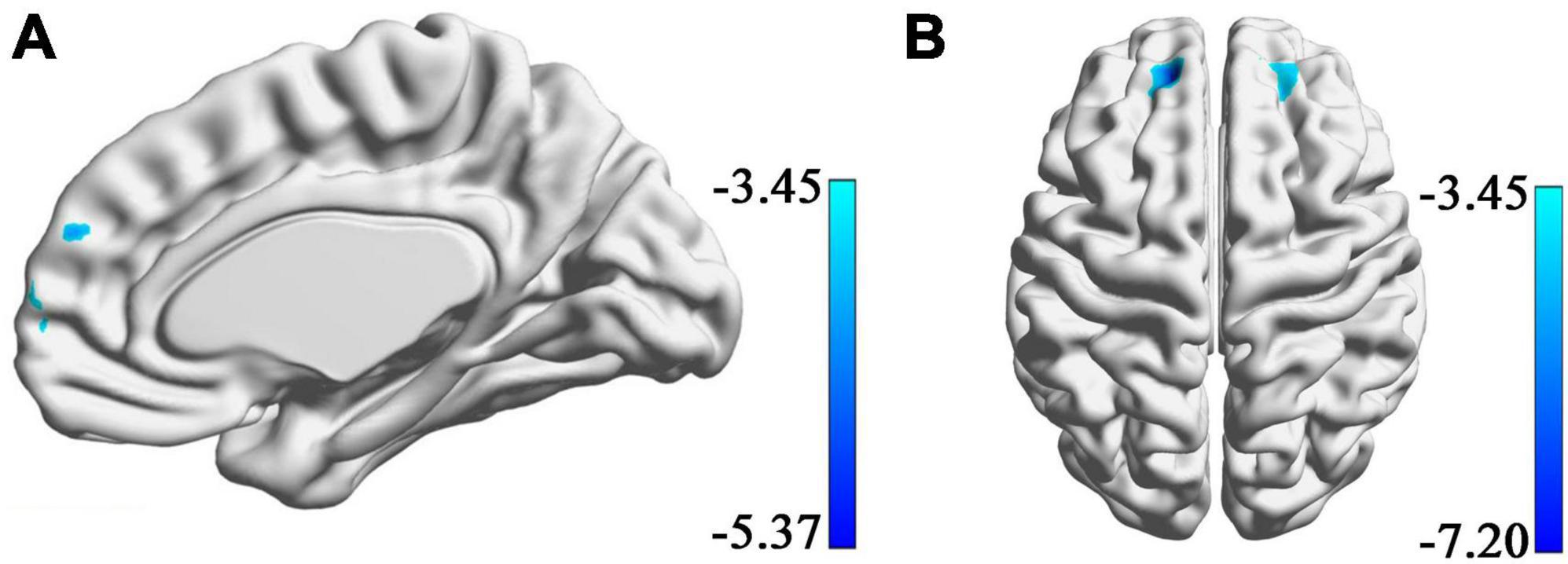
Figure 4. Regions showing correlations between rsFC with amygdala and the stress responses in a sample consisting of 30 participants who completed both of the first and the second surveys. (A) A cluster in the dmPFC whose rsFC with the right amygdala correlated with the score of SBSC at the first survey. (B) Bilateral superior frontal gyri whose rsFC with the right amygdala correlated with the score of SBSC at the second survey.
In addition, we found that there was no significant correlation between changes in SBSC scores and amygdala-dmPFC connectivity or self-connection of the amygdala.
This study demonstrated that the amygdala connectivity during rest obtained before the COVID-19 pandemic is related to stress responses during the COVID-19 outbreak in Hubei. Specifically, in an existing cohort of non-clinical population, we found that the functional connectivity between the right amygdala and the dmPFC was negatively correlated with the scores of stress responses at the early stage of the pandemic. Guided by this finding, we further found that the self-connection of the right amygdala was correlated with the scores of stress responses. This suggested that individuals with a weaker self-inhibition (i.e., disinhibition or hyper-activity) of the right amygdala before the pandemic are at a greater risk to exhibit more stress responses during the COVID-19 outbreak.
Previous studies have reported hyperactivity within the amygdala and impaired functional connectivity between the amygdala and mPFC in stress-related psychiatric disorders (14, 31, 37–40) and found that early stress can alter the amygdala’s connectivity (9, 10). Two studies found that amygdala reactivity to tasks is related to vulnerability to trauma-related psychopathology (1, 2). The current study expanded our understanding on the relationship between amygdala and stress in two important ways. First, taking advantage of an existing cohort built before the COVID-19 pandemic, we have the chance to find the evidence that the spontaneous brain activity of amygdala before stress can predict the subsequent stress responses while facing a public health emergency, suggesting that amygdala connectivity is a neural vulnerability factor to a stressful event exposure. Secondly, using effective connectivity, we provide the first evidence for the link between the self-connection in the amygdala and subsequent stress responses.
We found the negative correlation between the amygdala-dmPFC rsFC and the stress responses during the COVID-19 outbreak, suggesting that an individual with weaker amygdala-dmPFC rsFC before stress is more likely to have stress responses during the COVID-19 outbreak. The dmPFC and amygdala are extensively interconnected in the brain and amygdala-dmPFC functional coupling has a major role in fear conditioning and extinction, emotion regulation, and normal and pathological anxiety (14). The dmPFC is important in the conscious appraisal or expression of negative emotion (61), especially in threat appraisal (62). And the dmPFC has been assumed to actively regulate the amygdala through conscious evaluation and appraisal (63). According to a cognitive control model of emotion regulation (64), the neural representation of emotion regulation can be summarized as interactions between prefrontal and cortical system, including the dmPFC, and subcortical systems, especially the amygdala. Along this line, the negative correlation between the amygdala-dmPFC rsFC and the stress responses during the COVID-19 outbreak in our finding suggests that individuals with tight interaction between the dmPFC and the amygdala may have better ability of emotional regulation and thus less stress responses, indicated by the lower SBSC score. This speculation is supported by previous observations in patients with PTSD, who have impaired emotion regulation (65, 66). For example, amygdala-dmPFC connectivity to threat was decreased in patients with PTSD compared to the healthy controls (66) and functional coupling between the amygdala and the dmPFC to unpleasant stimuli was decreased in patients with high level of PTSD compared to those with low level of PTSD symptoms (67). Different from these two studies, we found the negative correlation between the amygdala-dmPFC connectivity and stress responses during rest and extended into a non-clinical population. However, it should be noted that we did not find any significant correlation between the amygdala-mPFC rsFC and trait or state anxiety in our study. Some studies showed that amygdala-mPFC rsFC is positively correlated with trait anxiety (68) or pre-scan anxiety valuation (69). Although the stress responses measured by the SBSC were significantly correlated with trait or state anxiety in this study, the sizes of correlation between SBSC and S-TAI were at small to moderate level, indicating that the stress responses that SBSC captures are somewhat different from state or trait anxiety. Therefore, it is possible that rsFC of amygdala is specifically related to the SBSC in this study.
Using DCM, we found a common model with an inhibitory connectivity from the right amygdala to the dmPFC and an excitatory connectivity from the dmPFC to the right amygdala. Although functional coupling between amygdala and dmPFC has been repeatedly reported, the directionality of the functional interactions between the two regions has only been examined in few studies. During processing of negative emotion, researchers found that the connectivity from the right amygdala to the dmPFC was significant in healthy controls using a method called Granger causality modeling (70, 71). A recent study, which also used the spDCM as we did, found inhibitory connectivity from the right amygdala to the dmPFC and excitatory connectivity from the dmPFC to the right amygdala, as well as the self-connections in both of the two regions, in healthy volunteers during rest in a network including six other regions besides the amygdala and dmPFC (72). Our finding is consistent with these previous studies.
We found the self-connection in the right amygdala was related to the stress responses during the COVID-19 outbreak. In the DCM framework, self-connections are, a priori, constrained to be inhibitory (35). This reflects the fact that inhibitory interneurons are restricted to intrinsic anatomical connectivity within the cortex. This means that an increase in self-inhibition corresponds to a reduction in the excitability of neuronal populations to their afferents (and recurrent self-connections) and on the contrary, a decrease in self-connection (i.e., disinhibition) corresponds to an increase in the excitability. Computational accounts under predictive coding interpret these changes in excitability as a failure to attenuate or modulate the precision of prediction errors; namely, the postsynaptic sensitivity of neuronal populations thought to encode prediction errors (e.g., superficial pyramidal cells) (73, 74). In these accounts, the ensuing psychopathology is often related to an imbalance between sensory and prior precision at lower and higher levels in the cortical hierarchy, respectively. The association with reduced self-inhibition (i.e., disinhibition) and the expression of stress-induced behaviors we observed is particularly interesting in light of predictive coding formulations of stress and anxiety (75, 76). The predictive processing formulations of aberrant interceptive inference (77) – in anxiety and stress – often focus on a failure to attenuate the precision of (interoceptive – and related) prediction errors. This results in a hypersensitivity to interoceptive autonomic afferents. Therefore, our findings suggest that individuals with weaker self-inhibition (i.e., disinhibition or hyperexcitability) of the right amygdala would express more stress behaviors, implying the importance of disinhibition/hyperexcitability of the right amygdala in the expression of stress responses. Previous studies have already shown that changes in the local regulation of amygdala excitability underlie behavioral disturbances in stress-related psychiatric disorders or stress responses (e.g., relapse to drug use) (78, 79) and chronic stress causes amygdala output neurons to become hyperexcitable (78, 80–82). However, in these previous studies, the hyperexcitability in the amygdala was observed while facing stressors or after experiencing stress. Our current study extends our knowledge on the role of hyperexcitability (i.e., disinhibition) of the amygdala in stress by finding that individuals with hyperexcitability in the amygdala before facing stressors will show more stress responses. It is possible that the hyperexcitability in the amygdala makes the individuals more vulnerable to uncertainty and unpredictability of environment and thus more likely to take actions while facing stressors.
We did not find the relationship between the amygdala-dmPFC connectivity with the stress responses when the stressor was not so strong, as shown after the stressor weakened, i.e., after 3 months of the COVID-19 outbreak when Wuhan has lifted lockdown. Instead rsFC between the right amygdala and bilateral SFG was found to correlate with SBSC at the second survey. However, it should be noted that there were intersections between the rsFC of the right amygdala correlated with the SBSC scores at the first survey and that at the second survey, which separately located in the right SFG and the dmPFC (Supplementary Figure 1). It indicates that there existed partially consistent rsFC pattern of amygdala correlated with SBSC scores across time. Even though this is a bit contradicting with our hypothesis, it is understandable because the SBSC in Hubei Cohort was significantly reduced at the second survey compared with the first survey, but it was still higher than that in non-Hubei Cohort at the second survey. Thus, the individuals in Hubei Cohort at the second survey still had relatively high stress responses, which might be the reason for relatively stable behavioral correlates of amygdala rsFC across time.
And this relatively stable brain-behavioral relationship may also account for the finding that there was no significant correlation between changes in SBSC scores and amygdala-dmPFC connectivity or self-connection of the amygdala. On the other hand, this negative finding might stem from the fact that stress-related vulnerability and resilience are two correlated but different psychological components that would engage different brain regions (6, 83). In our current study, we aimed to make use of neuroimaging data recorded before the stressors to predict stress response during the earlier stage of the COVID-19 pandemic (i.e., vulnerability). There might be brain connections that could predict the resilience ability to psychosocial stressors, which could be explored in our future studies.
Several limitations should be mentioned. First, the sample size in this study is small because the volunteers were recruited from an established cohort. For the same reason, we cannot find an appropriate control group to conduct formal statistics to test whether the brain-behavior relationship established in the individuals with more stress responses is statistically stronger than those with less stress responses. An ideal control group could be the individuals who took part in the scanning before the pandemic in the same site as the Hubei Cohort but they or their families were not living in Hubei province before the COVID-19 outbreak in Hubei and thus they were presumed free of the pandemic influence (there were only five cases in the current study). Future studies may use established or new cohorts with large sample size and diversified sample pool to validate the current findings. Second, we do not have baseline measures of stress responses or level. Thus, it is not known whether these participants were influenced by other stressors otherwise the COVID-19 pandemic. Third, this study suggests the potential role of amygdala connectivity in predicting stress responses in a non-clinical population. Whether the current findings can be generalized to patients with stress-related psychiatric disorders or other vulnerable populations to stress needs to be explored. Fourth, we validated the construct validity of SBSC with exploratory factor analysis using a combined sample consisting of Hubei and non-Hubei Cohort. We validated the external validity of SBSC with (1) score comparison between Hubei and non-Hubei Cohort, and (2) correlation with S-TAI in the combined sample. A limitation is that we did not additionally recruit another external sample to perform validation with different assessments of reliability and validity. Furthermore, the neurobiological mechanism behind the link between amygdala connectivity and subsequent stress response needs to be explored in future studies by using animal models. Finally, no MRI scanning was conducted during the pandemic, which prevents us from examining the changes in the brain induced by the pandemic stressors.
In conclusion, our findings support the role of functional coupling between the amygdala and dmPFC in stress responses and provide new evidence that individuals with hyperexcitability (i.e., disinhibition) in the amygdala will be more likely to exhibit stress behaviors while facing stressors. These findings expand our understanding about the role of amygdala in stress responses and stress-related psychiatric disorders and suggest that amygdala connectivity is a predisposing neural feature of subsequent stress responses.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Renmin Hospital of Wuhan University and Institutional Review Board of the Institute of Psychology, Chinese Academy of Sciences. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YZ: conceptualization, methodology, writing—review and editing, supervision, project administration, and funding acquisition. YH: conceptualization, formal analysis, and writing—original draft. YJ: formal analysis and writing—original draft. PZ and LG: writing—review and editing. BR, HH, and YF: investigation. JC and SZ: formal analysis. YW: investigation. GW: conceptualization and project administration. Y-TX: conceptualization. HW: conceptualization, supervision, project administration, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Nos. 81771473 and 81371476).
The authors gratefully acknowledge Karl Friston in the Wellcome Centre for Human Neuroimaging, University College London for his insightful comments on self-connections. The authors also acknowledge Peifu Li, Haixia Mao, Jun Chen, and the staff in the Department of Psychiatry and Department of Radiology, Renmin Hospital of Wuhan University and Chunlin Yang and the staff in the Department of Radiology, Beijing Anding Hospital for their extensive time and effort in data acquisition.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.999934/full#supplementary-material
1. McLaughlin K, Busso D, Duys A, Green J, Alves S, Way M, et al. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety. (2014) 31:834–42. doi: 10.1002/da.22284
2. Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, et al. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc Natl Acad Sci U.S.A. (2009) 106:14120–5. doi: 10.1073/pnas.0903183106
3. Kukihara H, Yamawaki N, Uchiyama K, Arai S, Horikawa E. Trauma, depression, and resilience of earthquake/tsunami/nuclear disaster survivors of Hirono, Fukushima, Japan. Psychiatry Clin Neurosci. (2014) 68:524–33. doi: 10.1111/pcn.12159
4. Bolsinger J, Seifritz E, Kleim B, Manoliu A. Neuroimaging correlates of resilience to traumatic events-a comprehensive review. Front Psychiatry. (2018) 9:693. doi: 10.3389/fpsyt.2018.00693
5. Breslau N, Davis G, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. (1991) 48:216–22. doi: 10.1001/archpsyc.1991.01810270028003
6. Zhang S, Cui J, Zhang Z, Wang Y, Liu R, Chen X, et al. Functional connectivity of amygdala subregions predicts vulnerability to depression following the COVID-19 pandemic. J Affect Disord. (2022) 297:421–9. doi: 10.1016/j.jad.2021.09.107
7. Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. (2003) 14:303–16. doi: 10.1515/REVNEURO.2003.14.4.303
8. Sergerie K, Chochol C, Armony J. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. (2008) 32:811–30. doi: 10.1016/j.neubiorev.2007.12.002
9. McLaughlin K, Weissman D, Bitran D. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol. (2019) 1:277–312. doi: 10.1146/annurev-devpsych-121318-084950
10. Herzberg M, Gunnar M. Early life stress and brain function: activity and connectivity associated with processing emotion and reward. Neuroimage. (2020) 209:116493. doi: 10.1016/j.neuroimage.2019.116493
11. Chang J, Yu R. Alternations in functional connectivity of amygdalar subregions under acute social stress. Neurobiol Stress. (2018) 9:264–70. doi: 10.1016/j.ynstr.2018.06.001
12. Orem T, Wheelock M, Goodman A, Harnett N, Wood K, Gossett E, et al. Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to psychosocial stress. Behav Neurosci. (2019) 133:203–11. doi: 10.1037/bne0000305
13. Disner S, Marquardt C, Mueller B, Burton P, Sponheim S. Spontaneous neural activity differences in posttraumatic stress disorder: a quantitative resting-statemeta-analysis and fMRI validation. Human Brain Mapp. (2018) 39:837–50. doi: 10.1002/hbm.23886
14. Kim M, Loucks R, Palmer A, Brown A, Solomon K, Marchante A, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. (2011) 223:403–10. doi: 10.1016/j.bbr.2011.04.025
15. Zhang X, Zhang J, Wang L, Li R, Zhang W. Altered resting-state functional connectivity of the amygdala in Chinese earthquake survivors. Prog Neuro Psychopharmacol Biol Psychiatry. (2016) 65:208–14. doi: 10.1016/j.pnpbp.2015.10.003
16. Holmes E, O’Connor R, Perry V, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. (2020) 7:547–60. doi: 10.1016/S2215-0366(20)30168-1
17. Xiang Y, Yang Y, Li W, Zhang L, Zhang Q, Cheung T, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. (2020) 7:228–9. doi: 10.1016/S2215-0366(20)30046-8
18. Vindegaard N, Eriksen Benros M. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. (2020) 89:531–42. doi: 10.1016/j.bbi.2020.05.048
19. Fegert J, Vitiello B, Plener P, Clemens V. Challenges and burden of the Coronavirus 2019 (COVID-19) pandemic for child and adolescent mental health: a narrative review to highlight clinical and research needs in the acute phase and the long return to normality. Child Adolesc Psychiatry Ment Health. (2020) 14:20. doi: 10.1186/s13034-020-00329-3
20. Gruber J, Prinstein M, Clark L, Rottenberg J, Abramowitz J, Albano A, et al. Mental health and clinical psychological science in the time of COVID-19: challenges, opportunities, and a call to action. Am Psychol. (2021) 76:409–26. doi: 10.31234/osf.io/desg9
21. Zacher H, Rudolph C. Individual differences and changes in subjective wellbeing during the early stages of the COVID-19 pandemic. Am Psychol. (2021) 76:50–62. doi: 10.1037/amp0000702
22. Di Crosta A, Palumbo R, Marchetti D, Ceccato I, La Malva P, Maiella R, et al. Individual differences, economic stability, and fear of contagion as risk factors for PTSD symptoms in the COVID-19 emergency. Front Psychol. (2020) 11:567367. doi: 10.3389/fpsyg.2020.567367
23. Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G. A default mode of brain function. Proc Natl Acad Sci U.S.A. (2001) 98:676–82. doi: 10.1073/pnas.98.2.676
24. Raichle M. Two views of brain function. Trends Cogn Sci. (2010) 14:180–90. doi: 10.1016/j.tics.2010.01.008
25. Finn E, Shen X, Scheinost D, Rosenberg M, Huang J, Chun M, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. (2015) 18:1664–71. doi: 10.1038/nn.4135
26. Harmelech T, Malach R. Neurocognitive biases and the patterns of spontaneous correlations in the human cortex. Trends Cogn Sci. (2013) 17:606–15. doi: 10.1016/j.tics.2013.09.014
27. Tavor I, Parker Jones O, Mars R, Smith S, Behrens T, Jbabdi S. Task-free MRI predicts individual differences in brain activity during task performance. Science. (2016) 352:216–20. doi: 10.1126/science.aad8127
28. He L, Wei D, Yang F, Zhang J, Cheng W, Feng J, et al. Functional connectome prediction of anxiety related to the COVID-19 pandemic. Am J Psychiatry. (2021) 178:530–40. doi: 10.1176/appi.ajp.2020.20070979
29. Liu P, Yang W, Zhuang K, Wei D, Yu R, Huang X, et al. The functional connectome predicts feeling of stress on regular days and during the COVID-19 pandemic. Neurobiol Stress. (2021) 14:100285. doi: 10.1016/j.ynstr.2020.100285
30. Friston K. Functional and effective connectivity: a review. Brain Connect. (2011) 1:13–36. doi: 10.1089/brain.2011.0008
31. Liu W, Zhang W, Zheng Z, Zou J, Liu X, Huang S, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. (2020) 11:2221. doi: 10.1038/s41467-020-15920-7
32. Friston K, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. (2003) 19:1273–302. doi: 10.1016/S1053-8119(03)00202-7
33. Tsvetanov K, Henson R, Tyler L, Razi A, Geerligs L, Ham T, et al. Extrinsic and intrinsic brain network connectivity maintains cognition across the lifespan despite accelerated decay of regional brain activation. J Neurosci. (2016) 36:3115–26. doi: 10.1523/JNEUROSCI.2733-15.2016
34. Vai B, Bulgarelli C, Godlewska B, Cowen P, Benedetti F, Harmer C. Fronto-limbic effective connectivity as possible predictor of antidepressant response to SSRI administration. Eur Neuropsychopharmacol. (2016) 26:2000–10. doi: 10.1016/j.euroneuro.2016.09.640
35. Friston K, Kahan J, Biswal B, Razi A. A DCM for resting state fMRI. Neuroimage. (2014) 94:396–407. doi: 10.1016/j.neuroimage.2013.12.009
36. Razi A, Kahan J, Rees G, Friston K. Construct validation of a DCM for resting state fMRI. Neuroimage. (2015) 106:1–14. doi: 10.1016/j.neuroimage.2014.11.027
37. Quirk G, Likhtik E, Pelletier J, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. (2003) 23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003
38. Hultman R, Mague S, Li Q, Katz B, Michel N, Lin L, et al. Dysregulation of prefrontal cortex-mediated slow-evolving limbic dynamics drives stress-induced emotional pathology. Neuron. (2016) 91:439–52. doi: 10.1016/j.neuron.2016.05.038
39. Robinson O, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C. The dorsal medial prefrontal (anterior cingulate) cortex-amygdala aversive amplification circuit in unmedicated generalised and social anxiety disorders: an observational study. Lancet Psychiatry. (2014) 1:294–302. doi: 10.1016/S2215-0366(14)70305-0
40. Shin L, Wright C, Cannistraro P, Wedig M, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. (2005) 62:273–81. doi: 10.1001/archpsyc.62.3.273
41. Rauch S, Whalen P, Shin L, McInerney S, Macklin M, Lasko N, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. (2000) 47:769–76. doi: 10.1016/S0006-3223(00)00828-3
42. Armony J, Corbo V, Clement M, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. (2005) 162:1961–3. doi: 10.1176/appi.ajp.162.10.1961
43. Ranlund S, Adams R, Diez A, Constante M, Dutt A, Hall M, et al. Impaired prefrontal synaptic gain in people with psychosis and their relatives during the mismatch negativity. Hum Brain Mapp. (2016) 37:351–65. doi: 10.1002/hbm.23035
44. Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. (2013) 36:181–204. doi: 10.1017/S0140525X12000477
45. Friston K, Stephan K, Montague R, Dolan R. Computational psychiatry: the brain as a phantastic organ. Lancet Psychiatry. (2014) 1:148–58. doi: 10.1016/S2215-0366(14)70275-5
46. Huang H, Shu C, Chen J, Zou J, Chen C, Wu S, et al. Altered corticostriatal pathway in first-episode paranoid schizophrenia: resting-state functional and causal connectivity analyses. Psychiatry Res Neuroimaging. (2018) 272:38–45. doi: 10.1016/j.pscychresns.2017.08.003
47. Li A, Zalesky A, Yue W, Howes O, Yan H, Liu Y, et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat Med. (2020) 26:558–65. doi: 10.1038/s41591-020-0793-8
48. Tay L, Jebb A. Scale Development. The SAGE Encyclopedia of Industrial and Organizational Psychology. Thousand Oaks, CA: Sage (2017).
49. Spielberger C. State-Trait Anxiety Inventory: Bibliography. 2nd ed. Palo Alto, CA: Consulting Psychologists Press (1989).
50. Cohen S. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S editors. The Social Psychology of Health. Thousand Oaks, CA: Sage Publications, Inc (1988). p. 31–67.
51. Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
52. Yan C, Cheung B, Kelly C, Colcombe S, Craddock R, Di Martino A, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. (2013) 76:183–201. doi: 10.1016/j.neuroimage.2013.03.004
53. Power J, Barnes K, Snyder A, Schlaggar B, Petersen S. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. (2012) 59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018
54. Eickhoff S, Stephan K, Mohlberg H, Grefkes C, Fink G, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. (2005) 25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034
55. Eickhoff S, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. (2006) 32:570–82. doi: 10.1016/j.neuroimage.2006.04.204
56. Zeidman P, Jafarian A, Corbin N, Seghier M, Razi A, Price C, et al. A guide to group effective connectivity analysis, part 1: first level analysis with DCM for fMRI. Neuroimage. (2019) 200:174–90. doi: 10.1016/j.neuroimage.2019.06.031
57. Friston K, Litvak V, Oswal A, Razi A, Stephan K, van Wijk B, et al. Bayesian model reduction and empirical Bayes for group (DCM) studies. Neuroimage. (2016) 128:413–31. doi: 10.1016/j.neuroimage.2015.11.015
58. Zeidman P, Jafarian A, Seghier M, Litvak V, Cagnan H, Price C, et al. A guide to group effective connectivity analysis, part 2: second level analysis with PEB. Neuroimage. (2019) 200:12–25. doi: 10.1016/j.neuroimage.2019.06.032
59. Friston K, Penny W. Posterior probability maps and SPMs. Neuroimage. (2003) 19:1240–9. doi: 10.1016/S1053-8119(03)00144-7
60. Kass R, Raftery A. Bayes factors. J Amer Statist Assoc. (1995) 90:773–95. doi: 10.1080/01621459.1995.10476572
61. Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. (2011) 15:85–93. doi: 10.1016/j.tics.2010.11.004
62. Kalisch R, Gerlicher A. Making a mountain out of a molehill: on the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neurosci Biobehav Rev. (2014) 42:1–8. doi: 10.1016/j.neubiorev.2014.02.002
63. Hariri A, Mattay V, Tessitore A, Fera F, Weinberger D. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. (2003) 53:494–501. doi: 10.1016/S0006-3223(02)01786-9
64. Ochsner K, Gross J. The cognitive control of emotion. Trends Cogn Sci. (2005) 9:242–9. doi: 10.1016/j.tics.2005.03.010
65. Ehring T, Quack D. Emotion regulation difficulties in trauma survivors: the role of trauma type and PTSD symptom severity. Behav Ther. (2010) 41:587–98. doi: 10.1016/j.beth.2010.04.004
66. Wolf R, Herringa R. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology. (2016) 41:822–31. doi: 10.1038/npp.2015.209
67. Sadeh N, Spielberg J, Warren S, Miller G, Heller W. Aberrant neural connectivity during emotional processing associated with posttraumatic stress. Clin Psychol Sci. (2014) 2:748–55. doi: 10.1177/2167702614530113
68. Kim M, Gee D, Loucks R, Davis F, Whalen P. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. (2011) 21:1667–73. doi: 10.1093/cercor/bhq237
69. Seeley W, Menon V, Schatzberg A, Keller J, Glover G, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
70. Potvin S, Lungu O, Tikasz A, Mendrek A. Abnormal effective fronto-limbic connectivity during emotion processing in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 72:1–8. doi: 10.1016/j.pnpbp.2016.08.004
71. Lungu O, Potvin S, Tikasz A, Mendrek A. Sex differences in effective fronto-limbic connectivity during negative emotion processing. Psychoneuroendocrinology. (2015) 62:180–8. doi: 10.1016/j.psyneuen.2015.08.012
72. Ishida T, Dierks T, Strik W, Morishima Y. Converging resting state networks unravels potential remote effects of transcranial magnetic stimulation for major depression. Front Psychiatry. (2020) 11:836. doi: 10.3389/fpsyt.2020.00836
73. Bastos A, Usrey W, Adams R, Mangun G, Fries P, Friston K. Canonical microcircuits for predictive coding. Neuron. (2012) 76:695–711. doi: 10.1016/j.neuron.2012.10.038
74. Shipp S. Neural elements for predictive coding. Front Psychol. (2016) 7:1792. doi: 10.3389/fpsyg.2016.01792
75. Cornwell B, Garrido M, Overstreet C, Pine D, Grillon C. The unpredictive brain under threat: a neurocomputational account of anxious hypervigilance. Biol Psychiatry. (2017) 82:447–54. doi: 10.1016/j.biopsych.2017.06.031
76. Peters A, McEwen B, Friston K. Uncertainty and stress: why it causes diseases and how it is mastered by the brain. Prog Neurobiol. (2017) 156:164–88. doi: 10.1016/j.pneurobio.2017.05.004
77. Seth A, Friston K. Active interoceptive inference and the emotional brain. Philos Trans R Soc Lond B Biol Sci. (1708) 2016:371. doi: 10.1098/rstb.2016.0007
78. Sharp B. Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl Psychiatry. (2017) 7:e1194. doi: 10.1038/tp.2017.161
79. Prager E, Bergstrom H, Wynn G, Braga M. The basolateral amygdala gamma-aminobutyric acidergic system in health and disease. J Neurosci Res. (2016) 94:548–67. doi: 10.1002/jnr.23690
80. Rosenkranz J, Venheim E, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. (2010) 67:1128–36. doi: 10.1016/j.biopsych.2010.02.008
81. Zhang J, Liu T, He Y, Pan H, Zhang W, Yin X, et al. Chronic Stress remodels synapses in an amygdala circuit-specific manner. Biol Psychiatry. (2019) 85:189–201. doi: 10.1016/j.biopsych.2018.06.019
82. Roozendaal B, McEwen B, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. (2009) 10:423–33. doi: 10.1038/nrn2651
Keywords: amygdala, COVID-19, dorsomedial prefrontal cortex, resting-state functional connectivity, effective connectivity, stress
Citation: Zhou Y, He Y, Jin Y, Zeidman P, Gao L, Rong B, Huang H, Feng Y, Cui J, Zhang S, Wang Y, Wang G, Xiang Y-T and Wang H (2023) Amygdala connectivity related to subsequent stress responses during the COVID-19 outbreak. Front. Psychiatry 14:999934. doi: 10.3389/fpsyt.2023.999934
Received: 21 July 2022; Accepted: 02 February 2023;
Published: 23 February 2023.
Edited by:
Giorgio Di Lorenzo, University of Rome “Tor Vergata”, ItalyReviewed by:
Sunhae Sul, Pusan National University, Republic of KoreaCopyright © 2023 Zhou, He, Jin, Zeidman, Gao, Rong, Huang, Feng, Cui, Zhang, Wang, Wang, Xiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Zhou,  emhvdXl1YW5AcHN5Y2guYWMuY24=; Huiling Wang,
emhvdXl1YW5AcHN5Y2guYWMuY24=; Huiling Wang,  aGx3YW5nQHdodS5lZHUuY24=
aGx3YW5nQHdodS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.