
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 11 January 2024
Sec. Neurostimulation
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1308437
This article is part of the Research Topic Translational Approaches in Neurostimulation Research: Challenges and Opportunities for Neuropsychiatry View all 6 articles
 Xin Wei1†
Xin Wei1† Zhan-Ming Shi2†
Zhan-Ming Shi2† Xian-Jun Lan1
Xian-Jun Lan1 Zhen-Juan Qin1
Zhen-Juan Qin1 Yu Mo1
Yu Mo1 Hua-Wang Wu3
Hua-Wang Wu3 Xing-Bing Huang3
Xing-Bing Huang3 Qing-Bin Zeng4
Qing-Bin Zeng4 Li-Xia Luo5
Li-Xia Luo5 Xin-Hu Yang3*
Xin-Hu Yang3* Wei Zheng3*
Wei Zheng3*Background: In randomized clinical trials (RCTs) investigating the application of transcranial alternating current stimulation (tACS) in schizophrenia, inconsistent results have been reported. The purpose of this exploratory systematic review of RCTs was to evaluate tACS as an adjunct treatment for patients with schizophrenia based on its therapeutic effects, tolerability, and safety.
Methods: Our analysis included RCTs that evaluated adjunctive tACS’ effectiveness, tolerability, and safety in schizophrenia patients. Three independent authors extracted data and synthesized it using RevMan 5.3 software.
Results: Three RCTs involving 76 patients with schizophrenia were encompassed in the analysis, with 40 participants receiving active tACS and 36 receiving sham tACS. Our study revealed a significant superiority of active tACS over sham tACS in improving total psychopathology (standardized mean difference [SMD] = −0.61, 95% confidence interval [CI]: −1.12, −0.10; I2 = 16%, p = 0.02) and negative psychopathology (SMD = −0.65, 95% CI: −1.11, −0.18; I2 = 0%, p = 0.007) in schizophrenia. The two groups, however, showed no significant differences in positive psychopathology, general psychopathology, or auditory hallucinations (all p > 0.05). Two RCTs examined the neurocognitive effects of tACS, yielding varied findings. Both groups demonstrated similar rates of discontinuation due to any reason and adverse events (all p > 0.05).
Conclusion: Adjunctive tACS is promising as a viable approach for mitigating total and negative psychopathology in individuals diagnosed with schizophrenia. However, to gain a more comprehensive understanding of tACS’s therapeutic effects in schizophrenia, it is imperative to conduct extensive, meticulously planned, and well-documented RCTs.
Schizophrenia is a debilitating condition characterized by impaired cognitive, emotional, and thinking functions. It is frequently a chronic and persistent illness (1, 2). Besides constituting a substantial disability, schizophrenia burdens families and society significantly while profoundly impacting the quality of life for affected individuals (3). The inadequacy of current treatments for schizophrenia could lead to the emergence of aggressive and violent behaviors among patients, exacerbating societal issues and intensifying the associated stigma (4–6). Enhancements in schizophrenia treatment could have broader implications for public health.
Currently, therapeutic strategies for schizophrenia encompass pharmacological, psychological, and physical interventions. However, pharmacological treatments encounter challenges, including inadequate efficacy in certain patients, resulting in treatment-resistant forms of schizophrenia (7). Cognitive-behavioral therapy (CBT), a prominent psychological intervention, has been extensively utilized for schizophrenia treatment (8). However, its implementation requires the patients to be stable, entails extended intervention durations, is accompanied by high costs, and demands substantial patient cooperation (9). There has been a growing utilization of non-pharmacological interventions to augment the effectiveness of antipsychotic treatments within clinical settings. These interventions encompass adjunctive non-invasive brain stimulation (NIBS) techniques, including electroconvulsive therapy (ECT) (10, 11), transcranial magnetic stimulation (TMS) (12), magnetic seizure therapy (MST) (13), transcranial direct current stimulation (tDCS), (14), and transcranial alternating current stimulation (tACS) (15).
Over the past two decades, tACS, an electrical brain stimulation method, has gained widespread acceptance within the medical field (16). Its applications include the treatment of mental disorders (17), attention enhancement (18), cognitive ability improvement (19), and sleep pattern regulation (20). Following extensive research and development, tACS technology will become a vital tool for tailoring medical treatments in these areas (21). Contrary to traditional techniques such as tDCS and TMS, tACS completely avoids sensory stimulation, it employs sinusoidal and biphasic alternating currents to stimulate cortical neurons, thereby regulating intrinsic brain oscillations and governing the synchronization and desynchronization of neural activity within the cerebral cortex. Consequently, this modulation of cortical excitability and brain function occurs (22). tACS potentially induces synaptic plasticity changes and regulates neurotransmitter levels (23, 24), enhancing long-term cognitive function and alleviating psychiatric symptoms. Consequently, this technology demonstrates significant potential for further development within schizophrenia.
The results of a randomized single-blind study suggest that gamma-tACS could be more effective than tDCS in improving working memory in people with schizophrenia (25). While in three recent double-blinded randomized controlled trials (RCTs), tACS has been examined for its feasibility, efficacy, and safety in the treatment of adult schizophrenia patients (26–28), their results have been varied. Chang et al. observed more substantial decreases in Positive and Negative Syndrome Scale (PANSS) negative subscale scores following theta-frequency tACS (θ-tACS) stimulation in the active condition (13.84%) compared to the sham condition (3.78%), accompanied by significant effect size (26). However, the two remaining RCTs examining alpha-frequency tACS (α-tACS) in patients with schizophrenia did not find significant improvements in total psychopathology (27, 28).
A systematic review aimed to detect the impact of tACS on cognition, depression, and schizophrenia (29), however, it included only limited studies (2 RCTs, n = 51) examining the impact of tACS on schizophrenia, resulting in insufficient statistical power. The objective of this exploratory systematic review, incorporating a recent RCT (28), was to acquire more substantial evidence about the effectiveness and safety of adjunctive tACS when combined with antipsychotic medications.
Three investigators (ZMS, ZJQ, and XJL) independently searched four international databases (PubMed, EMBASE, PsycINFO, and Cochrane Library) from the database’s inception to February 6, 2023. The search terms used were: (“transcranial alternating current stimulation” OR tACS) AND (schizophrenia [MeSH] OR schizophrenic disorder OR disorder, schizophrenic OR schizophrenic disorders OR schizophrenia OR dementia praecox). Furthermore, the researchers manually examined the reference lists of the included studies (26–28), systematic review (29) and scoping review (30) on tACS for patients with schizophrenia to identify any missing RCTs.
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, studies meeting the following PICOS criteria were included (31). Participants: Individuals with schizophrenia or schizoaffective disorder, regardless of diagnostic criteria. Intervention versus Comparison: Antipsychotic medications combined with active tACS versus those combined with sham tACS. Outcomes: The primary outcome assessed was the post-tACS change in total psychopathology, measured using standardized instruments such as the PANSS (32) or the Brief Psychiatric Rating Scale (BPRS) (33). Secondary outcomes included positive, negative, and general psychopathology scores on the PANSS or BPRS, the Scale for the Assessment of Negative Symptoms (SANS) or the Scale for the Assessment of Positive Symptoms (SAPS), auditory hallucinations scores measured using the Auditory Hallucination Rating Scale (AHRS) (34), cognitive function, discontinuation for any reason, and adverse events. When a study employed multiple measures to assess positive and negative psychopathology, preference was given to PANSS subscale scores to minimize heterogeneity. Study: Only published double-blinded RCTs on adjunctive tACS for patients with schizophrenia were eligible for inclusion. A randomized single-blind study focusing on γ-tACS for schizophrenia was notably excluded (25). We also excluded studies comparing active tACS with tDCS or other forms of physical therapy, review articles, and case reports/series.
Data were extracted from each included RCT by three independent researchers (ZJQ, XJL, and ZMS). Any discrepancies were resolved through collaborative discussions involving a senior author (WZ). A standardized form was used to collect information, including authorship details, publication year, study design, tACS protocol, and primary and secondary outcomes. The original study authors were contacted to obtain missing data when further information was required. Only pre-crossover data were extracted if the eligible RCT had a crossover design (28).
To evaluate the quality of the RCTs, three independent researchers (ZJQ, XJL, and ZMS) used the Jadad scale (35) and the Cochrane risk of bias tool (36). RCTs with a Jadad score of ≥3 were categorized as “high quality” (37). For all meta-analyzable outcomes, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system was employed.
We conducted statistical analyses with RevMan software (version 5.3) using random effects models (38). Risk ratio (RR) and standard mean difference (SMD) were computed for dichotomous and continuous outcomes, respectively, along with 95% confidence intervals (CIs). Heterogeneity among the studies was assessed using Cochrane’s Q and I2 test, with Q < 0.1 or I2 ≥ 50% indicating significant heterogeneity (39). For primary outcomes with I2 ≥ 50%, sensitivity and subgroup analyses were conducted to elucidate the heterogeneity. In all analyses, publication bias was assessed with funnel plots and Egger’s test (40), with 5% significance at two-tailed p-values.
Following the search strategy, 192 trials were retrieved. On screening the titles, abstracts, and full texts, three RCTs that met the inclusion criteria (26–28) were analyzed in this meta-analysis (Figure 1).
Table 1 summarizes the participant characteristics and tACS parameters of the three included RCTs. These RCTs (n = 76) that were published between 2018 and 2022 compared active tACS (n = 40) and sham tACS (n = 36) in patients with schizophrenia or schizoaffective disorder. Among them, two RCTs (66.6%) were conducted in the United States, and one (33.3%) was performed in China (Table 1). In this study, the average age of participants was 41.5 years old (range 18–70) and 59.2% of participants were men (range 50–73%). All three RCTs employed a 2 mA current intensity (6 Hz-tACS in one RCT or 10 Hz-tACS in two RCTs), with stimulation sessions scheduled either twice daily for 20 min (26, 27) or once daily for 40 min over five consecutive days (28). The follow-up period for the included three RCTs ranged from one (2 RCTs) (26, 27) to two months (1 RCT) (28).
Regarding random sequence generation and allocation concealment, two RCTs (66.7%) were rated as ‘low risk’ (Figure 2). The blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting of RCTs were all rated as being ‘low risk’. The mean Jadad score was 4.3 (range = 3–5), classifying all included RCTs as high-quality studies (Jadad score ≥ 3) (Table 1). According to the GRADE approach (Supplementary Table 1), evidence quality was moderate for all primary and secondary outcomes (100%).
Adjunctive active tACS demonstrated superiority over the sham tACS group in improving total psychopathology (SMD = −0.61, 95% CI: −1.12, −0.10; I2 = 16%, p = 0.02) as measured by the PANSS, and in decreasing negative psychopathology (SMD = −0.65, 95% CI: −1.11, −0.18; I2 = 0%, p = 0.007), calculated using the PANSS-negative symptoms subscale. However, no significant differences were observed between groups in terms of changes in positive psychopathology (2 RCTs, n = 40, SMD = 0.12, 95% CI: −0.50, 0.75; I2 = 0%, p = 0.70, Figure 3) (27, 28) assessed by the PANSS-positive symptoms subscale, general psychopathology (2 RCTs, n = 40, SMD = 0.03, 95% CI: −0.75, 0.80; I2 = 31%, p = 0.95, Figure 3) (27, 28), measured with the PANSS-general psychopathology subscale, and auditory hallucinations (2 RCTs, n = 40, SMD = −0.04, 95% CI: −0.66, 0.58; I2 = 0%, p = 0.90; Table 2) (27, 28) as measured by the AHRS.
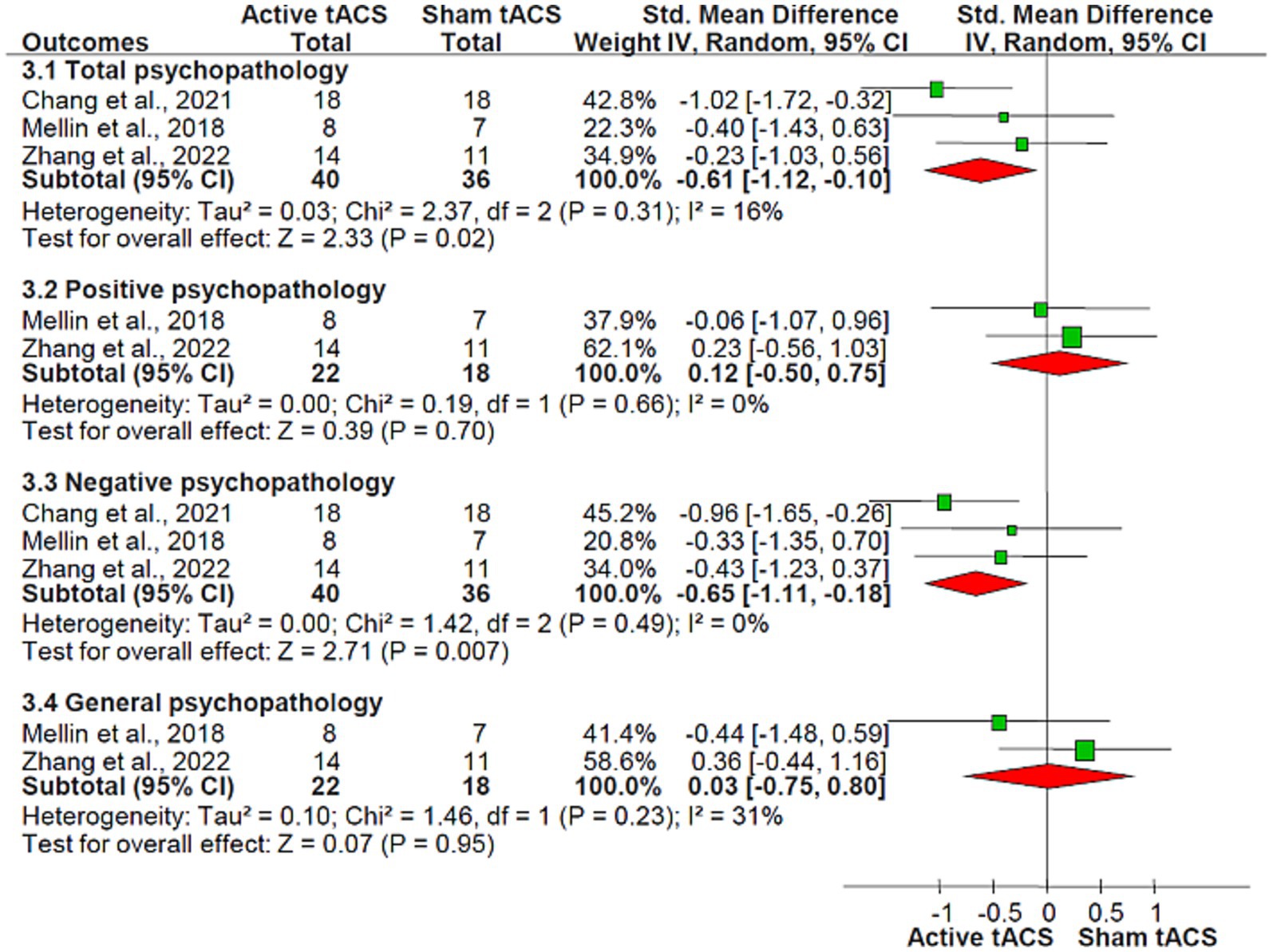
Figure 3. tACS for schizophrenia: the forest plot of overall, positive, negative, and general psychopathology scores measured by PANSS Abbreviations: CI, confidence interval; PANSS, positive and negative syndrome scale; tACS, transcranial alternating current stimulation.
Two out of three RCTs (66.7%) investigated the impact of adjunctive tACS on cognitive function in schizophrenia patients, yielding mixed results (26, 27) (Supplemental Table 2). According to Chang et al.’s study (26), active tACS improves working memory significantly more than sham tACS when measured with dual n-back tasks, while Mellin et al.’s study (27) did not report such an improvement.
There were no significant differences between the groups in terms of discontinuation due to any reason (RR = 2.67, 95% CI: 0.13, 56.63; I2 = not applicable, p = 0.53; Table 2). As indicated in Supplementary Table 3, adverse events were assessed using an adverse-effects questionnaire, and the most commonly reported ones associated with tACS in the included RCTs encompassed tingling, drowsiness, scalp pain, dizziness, difficulty concentrating, itching, headaches, and a burning sensation. No relevant significant differences were detected (all p > 0.05).
As fewer than 10 RCTs were included, publication bias could not be analyzed as recommended (41).
This exploratory systematic review encompassed three double-blinded RCTs, encompassing 76 individuals diagnosed with schizophrenia. The primary findings demonstrated the superiority of active tACS over sham tACS in effectively addressing the total and negative psychopathological symptoms. Negative symptoms are inherent to schizophrenia and correlated with neurocognitive impairments (42). tACS significantly improved negative symptoms of schizophrenia, which is consistent with several other NIBS approaches, including tDCS (43) and repetitive TMS (rTMS) (44). However, it is crucial to acknowledge that the evidence quality for total and negative psychopathology presented in this exploratory systematic review is low, likely attributable to the limited sample size, which ranged from 15 to 36 participants. tACS appeared ineffective in treating positive psychopathology, general psychopathology, and auditory hallucinations inherent to schizophrenia. Only two RCTs (66.7%) investigated the cognitive effects of tACS in patients with schizophrenia, yielding inconsistent results (26, 27). This exploratory systematic review suggests that tACS is safe and well-tolerated for treating schizophrenia.
The incorporated RCTs with sample sizes ranging from 15 to 36 were published within the last five years, signifying the novelty and clinical significance of tACS in schizophrenia. The findings from this meta-analysis suggest that tACS could be a viable non-pharmacological intervention for individuals with schizophrenia. Beyond its effectiveness in improving total and negative psychopathology of schizophrenia, tACS has been explored as a treatment for major depression (MDD) (45), chronic insomnia (46), attention deficit hyperactivity disorder (ADHD) (47), and pain disorders (48). For instance, a recent meta-analysis has determined that tACS effectively alleviates depression symptoms in individuals with MDD (45) while ensuring safety. Furthermore, a systematic review of RCTs (n = 73) has demonstrated tACS as an effective and safe therapeutic approach for chronic insomnia (46). However, this systematic review found that tACS does not demonstrate efficacy in treating auditory hallucinations in individuals with schizophrenia, as assessed by the AHRS. Moreover, a recent meta-analysis encompassing eight RCTs (n = 329) suggests that a regimen of twice-daily stimulation or ten sessions of tDCS is necessary to improve auditory hallucination symptoms, as assessed by the AHRS (49). However, a comparative study revealed that there was no significant disparity in terms of safety, tolerability, and efficacy for the treatment of schizophrenia between the tACS and tDCS groups (27).
The potential neuronal mechanism underlying the efficacy of tACS in ameliorating negative symptoms could be attributed to its ability to entrain brain network oscillations (50). These adverse symptoms are commonly linked with impairments in cognitive function and dysregulated dopaminergic transmission within the mesocorticolimbic pathway, including the ventral tegmental area (VTA), ventral striatum (VS), hippocampus (HP), and prefrontal cortex (PFC) (26). An aberrant functional coupling between the PFC, VTA, and HP is believed to play a crucial role in the manifestation of these abnormalities (51, 52). Accumulating research suggests that theta-rhythm oscillations coordinate neuronal activity within the PFC-VTA-HP axis when engaged in cognitive processes, such as working memory (53, 54). This phenomenon is illustrated by rTMS. Intermittent theta-burst stimulation (iTBS) targets the left dorsolateral prefrontal cortex (DLPFC) at a theta rhythm. iTBS has demonstrated the ability to alleviate negative symptoms while influencing neural transmission in the PFC, VS, and HP (26, 55, 56). Chang et al. found that θ-tACS could modulate frontoparietal networks (26) while treating schizophrenia. The potential neuronal mechanisms underlying the clinical effectiveness of tACS in individuals with schizophrenia could involve synchronizing intrinsic brain oscillations with the stimulation frequency and establishing long-range oscillatory connections between distant brain regions, such as the PFC-VTA-HP axis.
Another primary objective of alternative NIBS techniques, such as tDCS and TMS, is to assess their neurocognitive function. For instance, a previous meta-analysis revealed that the supplementary use of tDCS demonstrates a significant therapeutic impact on ameliorating working memory impairments in individuals with schizophrenia (14). There is a common link between major mental disorders and cognitive impairments, especially schizophrenia. However, only two RCTs (66.6%, 2/3), with inconsistent findings, have evaluated the neurocognitive function of tACS in schizophrenia. In this systematic review, the rates of discontinuation and adverse events were similar across the active and sham groups, indicating that tACS may be a safe and well-tolerated treatment strategy for patients with schizophrenia in clinical practice. The electrical stimulation techniques (tDCS and tACS) possess several advantageous features such as cost-effectiveness, portability, suitability for home use, and compatibility with training or rehabilitation interventions (57). Importantly, as compared with tDCS, tACS seemed to cause fewer adverse effects (58). Therefore, tACS has been also proven as a safe intervention for patients suffering from MDD (45), chronic insomnia (46), and healthy participants (59).
Several limitations are present in this exploratory systematic review. First, analyses were limited due to the small sample size (n = 76) and the number of RCTs included (3 RCTs), the current findings are still preliminary and exploratory. Second, unpublished RCTs with negative results can affect the interpretation of these findings since publication bias cannot be conducted in this exploratory systematic review. Third, the parameters of tACS differ across the included three RCTs in this exploratory systematic review. Consequently, there is a need for determining the optimal parameters (e.g., frequency of daily sessions and total sessions) of tACS for schizophrenia. Fourth, the included studies did not implement long-term follow-up (e.g., beyond 2 months) despite the fact that maintaining the antipsychotic effects remains a major concern for tACS. Finally, the protocol of this systematic review was not registered.
The use of adjunctive tACS shows promise as a viable approach to alleviate overall and negative psychopathology in individuals diagnosed with schizophrenia. However, it is imperative to conduct extensive, meticulously planned, and well-documented RCTs to better understand tACS’s therapeutic effects in schizophrenia. Additional research is necessary to investigate the optimal parameters for tACS, encompassing the identification of the most effective frequency for daily sessions, the total number of sessions, and the temporal distribution of treatment days.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
XW: Methodology, Writing – original draft. Z-MS: Data curation, Writing – original draft. X-JL: Data curation, Writing – original draft. Z-JQ: Formal analysis, Writing – review & editing. YM: Data curation, Writing – original draft. H-WW: Investigation, Software, Writing – review & editing. X-BH: Conceptualization, Investigation, Writing – review & editing. Q-BZ: Conceptualization, Data curation, Writing – review & editing. L-XL: Data curation, Formal analysis, Writing – review & editing. X-HY: Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. WZ: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82101609), China International Medical Exchange Foundation (Z-2018-35-2002), the Science and Technology Program of Guangzhou (2023A03J0839, 2023A03J0436), Science and Technology Planning Project of Liwan District of Guangzhou (202201012), The Natural Science Foundation Program of Guangdong (2023A1515011383), National Clinical Key specialty construction project [(2023) 33], Guangzhou Municipal Key Discipline in Medicine (2021–2023), Guangxi Zhuang Autonomous Region Health Commission Self-Funded Research Project (Z20211399), Guangzhou High-level Clinical Key Specialty, Department of Emergency Medicine of National clinical key specialty, and Guangzhou Research-oriented Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1308437/full#supplementary-material
1. Tandon, R . The nosology of schizophrenia: toward DSM-5 and ICD-11. Psychiatr Clin North Am. (2012) 35:557–69. doi: 10.1016/j.psc.2012.06.001
2. Uzman Özbek, S , and Alptekin, K . Thought disorder as a neglected dimension in schizophrenia. Alpha Psych. (2022) 23:5–11. doi: 10.5152/alphapsychiatry.2021.21371
3. Koç, M , Tel, H , and Karakülah, K . Determining care burden and psychiatric symptom level in caregiver of schizophrenia patient. Perspect Psychiatr Care. (2021) 57:642–7. doi: 10.1111/ppc.12588
4. Imhoff, R . Zeroing in on the effect of the schizophrenia label on stigmatizing attitudes: a large-scale study. Schizophr Bull. (2016) 42:456–63. doi: 10.1093/schbul/sbv137
5. Singh, OP . Suicide attempts in achizophrenia and their relationship to depression, insight and stigma. Alpha Psych. (2022) 23:26. doi: 10.1530/alphapsychiatry.2022.2201011
6. Storvestre, GB , Valnes, LM , Jensen, A , Nerland, S , Tesli, N , Hymer, KE, et al. A preliminary study of cortical morphology in schizophrenia patients with a history of violence. Psychiatry Res Neuroimaging. (2019) 288:29–36. doi: 10.1016/j.pscychresns.2019.04.013
7. García-Carmona, JA , Simal-Aguado, J , Campos-Navarro, MP , Valdivia-Muñoz, F , and Galindo-Tovar, A . Evaluation of long-acting injectable antipsychotics with the corresponding oral formulation in a cohort of patients with schizophrenia: a real-world study in Spain. Int Clin Psychopharmacol. (2021) 36:18–24. doi: 10.1097/YIC.0000000000000339
8. Guaiana, G , Abbatecola, M , Aali, G , Tarantino, F , Ebuenyi, ID , Lucarini, V, et al. Cognitive behavioural therapy (group) for schizophrenia. Cochrane Database Syst Rev. (2022) 7:Cd009608. doi: 10.1002/14651858.CD009608.pub2
9. Shields, GE , Buck, D , Elvidge, J , Hayhurst, KP , and Davies, LM . Cost-effectiveness evaluations of psychological therapies for schizophrenia and bipolar disorder: a systematic review. Int J Technol Assess Health Care. (2019) 35:317–26. doi: 10.1017/S0266462319000448
10. İlhan Atagün, M , and Atay Canbek, Ö . A systematic review of the literature regarding the relationship between oxidative stress and electroconvulsive therapy. Alpha Psych. (2022) 23:47–56. doi: 10.5152/alphapsychiatry.2021.21584
11. Ong, Y , and Chan, LG . A systematic review on cognitive effects of electroconvulsive therapy in Asian patients. Clin Psychopharmacol Neurosci. (2022) 20:1–16. doi: 10.9758/cpn.2022.20.1.1
12. di Hou, M , Santoro, V , Biondi, A , Shergill, SS , and Premoli, I . A systematic review of TMS and neurophysiological biometrics in patients with schizophrenia. J Psychiatry Neurosci. (2021) 46:E675–e701. doi: 10.1503/jpn.210006
13. Zhang, XY , Chen, HD , Liang, WN , Yang, XH , Cai, DB , Huang, X, et al. Adjunctive magnetic seizure therapy for schizophrenia: a systematic review. Front Psych. (2021) 12:813590. doi: 10.3389/fpsyt.2021.813590
14. Sun, CH , Jiang, WL , Cai, DB , Wang, ZM , Sim, K , Ungvari, GS, et al. Adjunctive multi-session transcranial direct current stimulation for neurocognitive dysfunction in schizophrenia: a meta-analysis. Asian J Psychiatr. (2021) 66:102887. doi: 10.1016/j.ajp.2021.102887
15. Haller, N , Hasan, A , Padberg, F , Brunelin, J , Da Costa Lane Valiengo, L , and Palm, U . Gamma transcranial alternating current stimulation in patients with negative symptoms in schizophrenia: a case series. Neurophysiol Clin. (2020) 50:301–4. doi: 10.1016/j.neucli.2020.06.004
16. Thirugnanasambandam, N , Kasten, FH , and Udupa, K . Editorial: novel multimodal approaches in non-invasive brain stimulation. Front Hum Neurosci. (2021) 15:784637. doi: 10.3389/fnhum.2021.784637
17. Elias, GJB , Boutet, A , Parmar, R , Wong, EHY , Germann, J , Loh, A, et al. Neuromodulatory treatments for psychiatric disease: a comprehensive survey of the clinical trial landscape. Brain Stimul. (2021) 14:1393–03. doi: 10.1016/j.brs.2021.08.021
18. Clayton, MS , and Yeung, N . Electrical stimulation of alpha oscillations stabilizes performance on visual attention tasks. J Exp Psychol Gen. (2019) 148:203–20. doi: 10.1037/xge0000502
19. Grover, S , and Fayzullina, R . A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci Transl Med. (2023) 15:eabo2044. doi: 10.1126/scitranslmed.abo2044
20. Ayanampudi, V , Kumar, V , Krishnan, A , Walker, MP , Ivry, RB , Knight, RT, et al. Personalized transcranial alternating current stimulation improves sleep quality: initial findings. Front Hum Neurosci. (2022) 16:1066453. doi: 10.3389/fnhum.2022.1066453
21. Strüber, D , and Herrmann, CS . Modulation of gamma oscillations as a possible therapeutic tool for neuropsychiatric diseases: a review and perspective. Int J Psychophysiol. (2020) 152:15–25. doi: 10.1016/j.ijpsycho.2020.03.003
22. Fröhlich, F . Endogenous and exogenous electric fields as modifiers of brain activity: rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin Neurosci. (2014) 16:93–02. doi: 10.31887/DCNS.2014.16.1/ffroehlich
23. Pariz, A , Trotter, D , Hutt, A , and Lefebvre, J . Selective control of synaptic plasticity in heterogeneous networks through transcranial alternating current stimulation (tACS). PLoS Comput Biol. (2023) 19:e1010736. doi: 10.1371/journal.pcbi.1010736
24. Wischnewski, M , Engelhardt, M , Salehinejad, MA , Schutter, D , Kuo, MF , and Nitsche, MA . NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cereb Cortex. (2019) 29:2924–31. doi: 10.1093/cercor/bhy160
25. Hoy, KE , Whitty, D , Bailey, N , and Fitzgerald, PB . Preliminary investigation of the effects of γ-tACS on working memory in schizophrenia. J Neural Transm (Vienna). (2016) 123:1205–12. doi: 10.1007/s00702-016-1554-1
26. Chang, CC , Huang, CC , Chung, YA , Im, JJ , and Lin, YY . Online left-hemispheric in-phase frontoparietal theta tACS for the treatment of negative symptoms of schizophrenia. J Pers Med. (2021) 11:1114. doi: 10.3390/jpm11111114
27. Mellin, JM , Alagapan, S , Lustenberger, C , Lugo, CE , Alexander, ML , Gilmore, JH, et al. Randomized trial of transcranial alternating current stimulation for treatment of auditory hallucinations in schizophrenia. Eur Psychiatry. (2018) 51:25–33. doi: 10.1016/j.eurpsy.2018.01.004
28. Zhang, M , Force, RB , Walker, C , and Ahn, S . Alpha transcranial alternating current stimulation reduces depressive symptoms in people with schizophrenia and auditory hallucinations: a double-blind, randomized pilot clinical trial. Schizophrenia (Heidelb). (2022) 8:114. doi: 10.1038/s41537-022-00321-0
29. Lee, A , Yau, CE , Mai, AS , Tan, WA , Ong, BSY , Yam, NE, et al. Transcranial alternating current stimulation and its effects on cognition and the treatment of psychiatric disorders: a systematic review and meta-analysis. Ther Adv Chronic Dis. (2022) 13:11403. doi: 10.1177/20406223221140390
30. Pathak, H , Sreeraj, VS , and Venkatasubramanian, G . Transcranial alternating current stimulation (tACS) and its role in schizophrenia: a scoping review. Clin Psychopharmacol Neurosci. (2023) 21:634–49. doi: 10.9758/cpn.22.1042
31. Moher, D , Liberati, A , Tetzlaff, J , and Altman, DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. (2009) 3:e123–30. doi: 10.1371/journal.pmed.1000097
32. Kay, SR , Fiszbein, A , and Opler, LA . The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
33. Faustman, WO , and Overall, JE . The brief psychiatric rating scale. Psychol Rep. (1962) 10:799–12. doi: 10.2466/pr0.1962.10.3.799
34. Hoffman, RE , Hawkins, KA , Gueorguieva, R , Boutros, NN , Rachid, F , Carroll, K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. (2003) 60:49–56. doi: 10.1001/archpsyc.60.1.49
35. Higgins, JP , Altman, DG , Gøtzsche, PC , Jüni, P , Moher, D , Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
36. Jadad, AR , Moore, RA , Carroll, D , Jenkinson, C , Reynolds, DJ , Gavaghan, DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
37. Linde, K , Clausius, N , Ramirez, G , Melchart, D , Eitel, F , Hedges, LV, et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet. (1997) 350:834–43.
38. DerSimonian, R , and Laird, N . Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
39. Higgins, JP , Thompson, SG , Deeks, JJ , and Altman, DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
40. Egger, M , Davey Smith, G , Schneider, M , and Minder, C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
41. Sterne, JA , Sutton, AJ , Ioannidis, JP , Terrin, N , Jones, DR , Lau, J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
42. Gur, RE , March, M , Calkins, ME , Weittenhiller, L , Wolf, DH , Turetsky, BI, et al. Negative symptoms in youths with psychosis spectrum features: complementary scales in relation to neurocognitive performance and function. Schizophr Res. (2015) 166:322–7. doi: 10.1016/j.schres.2015.05.037
43. Valiengo, LDCL , Goerigk, S , Gordon, PC , Padberg, F , Serpa, MH , Koebe, S, et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry. (2020) 77:121–9. doi: 10.1001/jamapsychiatry.2019.3199
44. Kumar, N , Vishnubhatla, S , Wadhawan, AN , Minhas, S , and Gupta, P . A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia. Brain Stimul. (2020) 13:840–9. doi: 10.1016/j.brs.2020.02.016
45. Zheng, W , Cai, DB , Nie, S , Chen, JH , Huang, XB , Goerigk, S, et al. Adjunctive transcranial alternating current stimulation for patients with major depressive disorder: a systematic review and meta-analysis. Front Psych. (2023a) 14:1154354. doi: 10.3389/fpsyt.2023.1154354
46. Zheng, W , Lan, XJ , Qin, ZJ , Ungvari, GS , and Xiang, YT . Transcranial alternating current stimulation for chronic insomnia: a systematic review. Asian J Psychiatr. (2023b) 82:103477. doi: 10.1016/j.ajp.2023.103477
47. Dallmer-Zerbe, I , Popp, F , Lam, AP , Philipsen, A , and Herrmann, CS . Transcranial alternating current stimulation (tACS) as a tool to modulate p300 amplitude in attention deficit hyperactivity disorder (ADHD): preliminary findings. Brain Topogr. (2020) 33:191–07. doi: 10.1007/s10548-020-00752-x
48. Wandrey, JD , Kandić, M , Haberbosch, L , and Serian, A . Transcranial alternating current stimulation to modulate oscillations in pain disorders. Schmerz. (2023) 37:281–9. doi: 10.1007/s00482-022-00684-4
49. Jiang, WL , Cai, DB , Sun, CH , Yin, F , Goerigk, S , Brunoni, AR, et al. Adjunctive tDCS for treatment-refractory auditory hallucinations in schizophrenia: a meta-analysis of randomized, double-blinded, sham-controlled studies. Asian J Psychiatr. (2022) 73:103100. doi: 10.1016/j.ajp.2022.103100
50. Elyamany, O , and Leicht, G . Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci. (2021) 271:135–56. doi: 10.1007/s00406-020-01209-9
51. Brunelin, J , Fecteau, S , and Suaud-Chagny, MF . Abnormal striatal dopamine transmission in schizophrenia. Curr Med Chem. (2013) 20:397–04. doi: 10.2174/0929867311320030011
52. Goto, Y , Otani, S , and Grace, AA . The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. (2007) 53:583–7. doi: 10.1016/j.neuropharm.2007.07.007
53. Fujisawa, S , and Buzsáki, G . A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. (2011) 72:153–65. doi: 10.1016/j.neuron.2011.08.018
54. Gao, M , Liu, CL , Yang, S , Jin, GZ , Bunney, BS , and Shi, WX . Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci. (2007) 27:5414–21. doi: 10.1523/JNEUROSCI.5347-06.2007
55. Bor, J , Brunelin, J , Rivet, A , d'Amato, T , Poulet, E , Saoud, M, et al. Effects of theta burst stimulation on glutamate levels in a patient with negative symptoms of schizophrenia. Schizophr Res. (2009) 111:196–7. doi: 10.1016/j.schres.2009.03.012
56. Brunelin, J , Szekely, D , Costes, N , Mondino, M , Bougerol, T , Saoud, M, et al. Theta burst stimulation in the negative symptoms of schizophrenia and striatal dopamine release. An iTBS-[11C]raclopride PET case study. Schizophr Res. (2011) 131:264–5. doi: 10.1016/j.schres.2011.05.019
57. Bhattacharya, A , Mrudula, K , Sreepada, SS , Sathyaprabha, TN , Pal, PK , Chen, R, et al. An overview of noninvasive brain stimulation: basic principles and clinical applications. Can J Neurol Sci Le Journal Canadien Des Sciences Neurologiques. (2022) 49:479–92. doi: 10.1017/cjn.2021.158
58. Matsumoto, H , and Ugawa, Y . Adverse events of tDCS and tACS: a review. Clin Neurophysiol Pract. (2017) 2:19–25. doi: 10.1016/j.cnp.2016.12.003
Keywords: transcranial alternating current stimulation, schizophrenia, systematic review, negative symptoms, randomized clinical trial
Citation: Wei X, Shi Z-M, Lan X-J, Qin Z-J, Mo Y, Wu H-W, Huang X-B, Zeng Q-B, Luo L-X, Yang X-H and Zheng W (2024) Transcranial alternating current stimulation for schizophrenia: a systematic review of randomized controlled studies. Front. Psychiatry. 14:1308437. doi: 10.3389/fpsyt.2023.1308437
Received: 06 October 2023; Accepted: 27 December 2023;
Published: 11 January 2024.
Edited by:
Tobias Engel, Royal College of Surgeons in Ireland, IrelandReviewed by:
Chaomeng Liu, Capital Medical University, ChinaCopyright © 2024 Wei, Shi, Lan, Qin, Mo, Wu, Huang, Zeng, Luo, Yang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Hu Yang, eW91bmd4aW5odUBmb3htYWlsLmNvbQ==; Wei Zheng, emhlbmd3ZWkwNzAyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.