
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Psychiatry , 31 October 2023
Sec. Addictive Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1307732
This article is part of the Research Topic Stress and Addictive Disorders View all 6 articles
Editorial on the Research Topic
Stress and addictive disorders
Stress events include chronic stress, acute stress, traumatic stress, natural disasters (e.g., earthquakes), sex abuse, rape, wars, and severe diseases that cause death (e.g., cancer and COVID-19) (1–4). The various types of stress events can induce impulsive and compulsive behaviors and cause vulnerability to addictive behaviors. Accordingly, many reports indicated that stress can potentially enhance the development of addictive and relapse behaviors (5, 6). In the animal model, growing body evidence showed that experiencing stress events can increase drug self-administration and drug-seeking behaviors (7–9). In these cases, chronic or acute stress alters the mesolimbic dopamine, glutamate, noradrenaline, and GABA systems; moreover, it increases the corticotropin-releasing factor of the hypothalamus-pituitary-adrenal axis and the autonomic hyperactivity (3, 10–13). Recently, the kappa receptor and endogenous ligand dynorphin were found to contribute to the occurrence of comorbidity for substance abuse with chronic stress, post-traumatic stress disorder (PTSD), and traumatic stress, indicating the opiate system is also involved in the stress-induced anxiety and addictive behaviors (14).
In particular, the reward and reinforcement pathway of the ventral tegmental area to the nucleus accumbens was sensitized by chronic stress treatments. By way of the neural sensitization in the reward system, stress contributes to the vulnerability of addictive behaviors and enhances its prevalence. Therefore, stress is highly associated with the occurrence of addictive behaviors.
How to prevent and terminate addictive behaviors remains a big issue. Until now, some pharmacotherapy and psychotherapy have been developed to ameliorate addiction, effectively (15, 16). For example, some studies have reported that psychotherapies might be effective in reducing addictive behaviors, including cognitive-behavior therapy (17), motivational enhancement therapy, behavioral enhancement therapy, psychological addiction elimination technology, mindfulness-based relapse prevention, unconditioned stimulus memory retrieval-extinction paradigm, program implantation technology under deep hypnosis, aversion therapy, individual therapy, and group therapy (15). Moreover, psychotherapy was applied to prevent and treat Internet addiction (18). Obviously, psychotherapy is an effective treatment for reduction of addictive behaviors.
Alternatively, pharmacological treatments were alternative considerations for ameliorating addictive behaviors (16, 19). For example, opiate antagonists (e.g., naloxone) (20) and agonists (e.g., methadone) (21) can reverse or lessen opiate addiction. Previous studies have reported that the approval of the USA Food and Drug Administration for medications in treatments of nicotine, alcohol, and opiate abuse by ways of affecting dopamine, GABA, serotonin, and glutamate systems in the brain (16, 19). Downregulation of the brain's dopamine levels can relieve the incentive property and reinforcement or reward of the addictive stimulus, resulting in the amelioration of drug and food addiction (22). In conclusion, psychotherapy and pharmacotherapy are essential strategies for treating drug and non-drug addictive behaviors.
In our Research Topic, some studies demonstrated that numerous stress events facilitated addictive behaviors. For example, Obuobi-Donkor et al. found that people who experienced multiple natural disasters had a high vulnerability and prevalence risk of cannabis abuse and anxiety and depression symptoms. Nin et al. showed that lower social distancing increased abused drugs during the pandemic; however, anxiety and depression were associated with higher drug use for sociodemographics (such as men, lower income, and less education). Thus, the COVID-19 pandemic-induced stress changes the use of abused drugs and the mental state. Zhang et al. revealed that perceived stress was associated with lower self-control due to a high risk of mobile phone addiction; however, the security factor moderated the relationship between perceived stress and self-control.
On the other hand, novel interventions were developed to reduce stress-induced PTSD symptoms and addictive behaviors. For example, Yu et al. used the optogenetics approach to demonstrate that the different subareas of the medial prefrontal cortex moderated alternations of the stimulus valence from reward to aversion or neutral states, and the findings can develop novel treatments for drug addiction. Lee et al. employed the PTSD animal study, which showed that the blockade of hypocretin signals in the basolateral amygdala reduced the PTSD-like behaviors induced by a novel stress protocol in mice. Their data indicated the hypocretin system plays an essential role in modulating PTSD symptoms, indicating that hypocretin can be developed as a new treatment for PTSD. Altogether, all collected research as above has shown their findings and conclusions in Table 1.
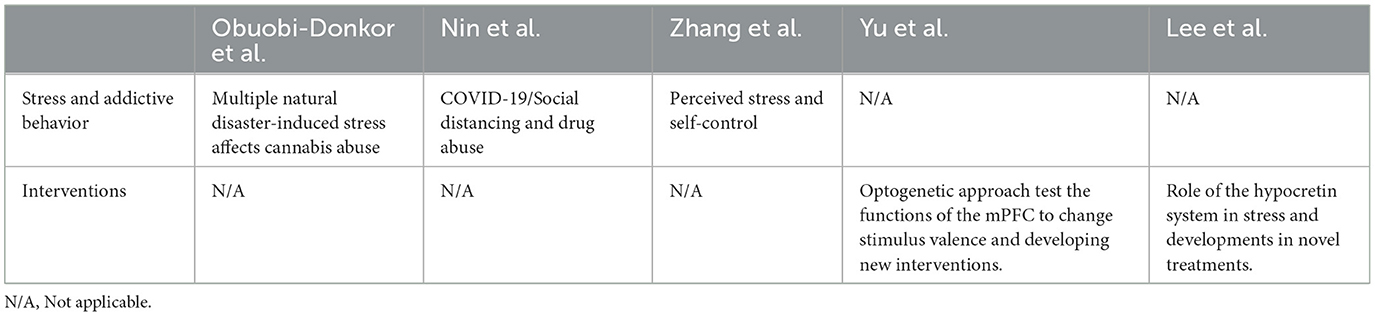
Table 1. Summary for the topic research related to the issues of stress and behavioral addiction and its interventions for addiction.
In summary, the topic research manipulates numerous stress events and examines how stress alters addictive behaviors. Moreover, novel interventions were developed to alleviate stress-induced PTSD symptoms and addictive behaviors. The present findings can help us understand the brain mechanisms and provide some contributions and implications in clinical aspects.
AH: Conceptualization, Writing—original draft, Writing—review & editing. C-YK: Writing—review & editing. AK: Writing—review & editing. B-CS: Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a funding grant from the National Science and Technology Council of the Republic of China (Taiwan) (NSTC 112-2410-H-431-009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. (2006) 8:445–61. doi: 10.31887/DCNS.2006.8.4/jbremner
2. Puglisi A, Imperato A, Angelucci L, Cabib S. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res. (1991) 554:217–22. doi: 10.1016/0006-8993(91)90192-X
3. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. (2008) 1141:105–30. doi: 10.1196/annals.1441.030
4. Yehuda R. Post-traumatic stress disorder. N Engl J Med. (2002) 346:108–14. doi: 10.1056/NEJMra012941
5. Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. (2003) 13:435–41. doi: 10.1016/j.euroneuro.2003.08.004
6. Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. (2016) 41:335–56. doi: 10.1038/npp.2015.142
7. Blanco-Gandia MC, Aguilar MA, Minarro J, Rodriguez-Arias M. Reinstatement of drug-seeking in mice using the conditioned place preference paradigm. J Vis Exp. (2018) 136:e56983. doi: 10.3791/56983
8. Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. (2002) 301:785–9. doi: 10.1124/jpet.301.3.785
9. Liu X, Weiss F. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology. (2003) 168:184–91. doi: 10.1007/s00213-002-1267-z
10. Blum K, Thanos PK, Oscar-Berman M, Febo M, Baron D, Badgaiyan RD, et al. Dopamine in the brain: hypothesizing surfeit or deficit links to reward and addiction. J Reward Defic Syndr. (2015) 1:95–104. doi: 10.17756/jrds.2015-016
11. Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. (2014) 231:1557–80. doi: 10.1007/s00213-013-3369-1
12. Douma EH, de Kloet ER. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci Biobehav Rev. (2020) 108:48–77. doi: 10.1016/j.neubiorev.2019.10.015
13. Self DW. Neural substrates of drug craving and relapse in drug addiction. Ann Med. (1998) 30:379–89. doi: 10.3109/07853899809029938
14. Leconte C, Mongeau R, Noble F. Traumatic stress-induced vulnerability to addiction: critical role of the dynorphin/kappa opioid receptor system. Front Pharmacol. (2022) 13:856672. doi: 10.3389/fphar.2022.856672
15. He RH, Tao R. Psychotherapy. Adv Exp Med Biol. (2017) 1010:295–320. doi: 10.1007/978-981-10-5562-1_15
16. Ross S, Peselow E. Pharmacotherapy of addictive disorders. Clin Neuropharmacol. (2009) 32:277–89. doi: 10.1097/WNF.0b013e3181a91655
17. An H, He RH, Zheng YR, Tao R. Cognitive-behavioral therapy. Adv Exp Med Biol. (2017) 1010:321–9. doi: 10.1007/978-981-10-5562-1_16
18. Xu LX, Wu LL, Geng XM, Wang ZL, Guo XY, Song KR, et al. A review of psychological interventions for internet addiction. Psychiatry Res. (2021) 302:114016. doi: 10.1016/j.psychres.2021.114016
19. Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am. (2004) 27:627–48. doi: 10.1016/j.psc.2004.06.002
20. Dunn KE, Barrett FS, Bigelow GE. Naloxone formulation for overdose reversal preference among patients receiving opioids for pain management. Addict Behav. (2018) 86:56–60. doi: 10.1016/j.addbeh.2018.03.011
21. Salsitz E, Wiegand T. Pharmacotherapy of opioid addiction: “putting a real face on a false demon”. J Med Toxicol. (2016) 12:58–63. doi: 10.1007/s13181-015-0517-5
Keywords: stress, addictive behavior, drug addiction, psychological intervention, pharmacological intervention
Citation: Huang ACW, Ko C-Y, Kozłowska A and Shyu B-C (2023) Editorial: Stress and addictive disorders. Front. Psychiatry 14:1307732. doi: 10.3389/fpsyt.2023.1307732
Received: 05 October 2023; Accepted: 13 October 2023;
Published: 31 October 2023.
Edited and reviewed by: Yasser Khazaal, Université de Lausanne, Switzerland
Copyright © 2023 Huang, Ko, Kozłowska and Shyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Chih Wei Huang, Y2h3ZWlodWFuZ0BtYWlsLmZndS5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.