- 1Department of Psychiatry, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 2School of Public Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Background: Perinatal depression, characterized by the presence of depressive symptoms during pregnancy and/or within the first 12 months postpartum, poses a significant global public health concern. It contributes to a multitude of health risks for mothers, their infants, and their families. Understanding of perinatal depression and its associated factors is crucial for effective prevention and intervention strategies. However, there is a lack of comprehensive research on this topic in Ethiopia. Therefore, this study aims to determine the prevalence and factors contributing to perinatal depression among Ethiopian women.
Methods: An institutional-based cross-sectional study was conducted, involving 552 women receiving perinatal services at Kutaber district health institution and Boru Meda General Hospital. Study participants were selected through systematic random sampling techniques. Perinatal depression was assessed using the Depression, Anxiety, and Stress Scale-21 (DASS-21). The associations between various determinants and perinatal depression were examined using binary logistic regression, and factors with a p-value of less than 0.2 were included in the multiple logistic regression analysis. A p-value less than 0.05 was considered statistically significant.
Results: The prevalence of perinatal depression was found to be 32.2%. The prevalence of perinatal depression was found to be 32.2%. Factors significantly associated with perinatal depression included being a student [adjusted odds ratio (AOR) = 4.364, 95% confidence interval (CI): 1.386, 13.744], experiencing excessive pregnancy-related concerns (AOR = 1.886, 95% CI: 1.176, 3.041), past substance use (AOR = 2.203, 95% CI: 1.149, 4.225), the presence of anxiety symptoms (AOR = 3.671, 95% CI: 2.122, 6.352), experiencing stress symptoms (AOR = 6.397, 95% CI: 3.394–12.055), and daytime sleepiness (AOR = 2.593, 95% CI: 1.558, 4.316).
Conclusion: The findings of this study indicate a relatively high prevalence and valuable factors associated with perinatal depression. It highlights the need for a comprehensive approach to perinatal mental health that takes into account not only the biological aspects of pregnancy but also the psychological, social, and lifestyle factors that can impact a person’s mental well-being during this critical period.
Introduction
Perinatal depression, a prevalent mental health issue, affects pregnant individuals and new mothers during the perinatal period, encompassing pregnancy and the postpartum period (1, 2). While extensively studied in developed countries, perinatal depression remains a significant concern in developing countries, where healthcare resources may be limited (3–17). Perinatal depression is characterized by the occurrence depressive symptoms during pregnancy and/or within 12 months following delivery (15–18). Globally, perinatal depression is a major public health concern due to its direct association with altered mother-to-child interaction, diminished outcomes in child development, and considerable personal, economic, as well as social costs (15, 19, 20). It is the most common mental health problems experienced by a woman during perinatal period (3, 4, 10–14) and is linked with increased risks of maternal & infant mortality and morbidity (5–9).
Due to physiological, psychological, hormonal, and social changes during pregnancy and the postpartum period, the likelihood of experiencing emotional disturbances such as depression may increase (21–23). Approximately 70% to 80% of all new mothers experience some negative psychological feelings or mood swings during pregnancy, delivery, and the first year after giving birth (24). Becoming a mother is a significant life transition that impacts a woman’s sense of self and identity. It involves navigating various emotional, physical, and social changes. These changes often require women to reassess their roles, goals, and aspirations both within and outside of motherhood. This process of identity development is complex and can contribute to a mother’s depression (25, 26). In the perinatal period, parents acquire new roles, responsibilities, and knowledge and respond to changes in personal identity, relationships, and family dynamics (27–31).
Depressive symptoms during the perinatal period may have devastating consequences not only for maternal health but also for mother-to-child interaction and their child’s biological, physiological, social, and cognitive development (5, 7, 27, 32, 33).
Identity development before becoming a parent plays a crucial role in one’s ability to cope with the responsibilities and challenges of parenthood. Developing a solid adult identity allows individuals to have a strong sense of self, a clear understanding of their values, and a firm grasp on their personal goals and aspirations (34). Transitioning to parenthood can be overwhelming in itself, and without a strong sense of self, individuals may find it challenging to navigate the changes and adjustments that come with this new role. They may feel a loss of personal freedom and struggle to find a balance between their own identity and the responsibilities of being a parent. When individuals enter parenthood without a well-established identity, they may face various challenges. Firstly, they may struggle with a lack of self-confidence and uncertainty about their abilities as a parent. Secondly, they may experience difficulties in setting boundaries and establishing a sense of self within the parental role. They may struggle with balancing their own needs and desires with the demands of their child, leading to feelings of frustration, resentment, or guilt (35).
Regarding perinatal depression, neurobiological birth dynamics play a pivotal role. The perinatal period is characterized by significant hormonal fluctuations, especially during pregnancy and after childbirth, which can influence maternal mental health. Additionally, the neurobiological changes associated with pregnancy and childbirth can impact the mother’s stress response and emotional regulation systems, contributing to the risk of perinatal mood disorders such as depression and anxiety (36–38).
In developing countries maternal mental health problems especially depression during perinatal period become the most challenging issue (39–41). According to WHO reports depression is the fourth leading cause of disability worldwide (42). Depression is also predicted to become the leading cause of disease burden by 2030, and it is already the leading cause of disease burden in women worldwide (42, 43). Perinatal depression rates vary globally, with studies reporting different prevalence rates. In the USA, rates ranged from 18.4% to 40.4% among women (44). In rural China, 13% exhibited symptoms of depression (14) while in Italy, 18.7% of perinatal caregiver professionals reported depression symptoms (45, 46). Low- and middle-income countries showed a wide range of 3%–50% prevalence (47). In Malaysia, depression rates during the perinatal period varied from 1.9% to 82.1% in developing countries and 5.2% to 74.0% in developed countries (48).
During the COVID-19 outbreak in Mexico, 39.2% of women developed depression symptoms (30), and in Portugal, 13%–16% experienced postnatal depression (32). Among Australian fathers, 10% experienced perinatal depression (49), and in Brazil, the prevalence was 24.3% during pregnancy and 10.8% postpartum (50). In Egypt, 40% of university students exhibited depressive symptoms (51). and in Ghana, 9.9% experienced antenatal depression (52). Various studies among pregnant women reported rates ranging from 24.94% to 58% for depressive symptoms (53–58). Several factors were associated with perinatal depression across these studies. Unemployment (45) and financial problems (13) were common determinants. Low social support, single status, lower education, unemployment, financial instability, and older age increased the risk (4, 59). Other systematic review and meta-analysis study in China shows that educational level and economic status of families were significantly correlated with perinatal depression (60). The absence of a partner (50), maternal age less than 30 years old, never being married (52), and low economic status and low economic status (50, 52) were also significant factors with perinatal depression.
Clinically, a history of depression, physical trauma (61), lack of physical activity (62), poor physical health (63), sleep disturbance (27), poor health status, and history of traumatic experiences (64), physical & sexual abuse (28), domestic violence (60), poor relationships with their parent (7), family conflict, lack of decision-making power and poor social support (14) were linked to perinatal depression. Lifetime stressful event exposure (7, 64, 65) perinatal smoking and/or the use of alcohol (50, 60, 66), and husband smoking status (57) were additional contributing factors.
Obstetric factors such as multiparity (50, 60), unintended pregnancy (52, 54, 55), and previous pregnancy loss (52, 67) having more than 4 living children (7, 57, 68), emotional detachment during childbirth (69), history of lifetime abortion (54), age at marriage (57), obstetric complications in previous and/or this pregnancy (57, 58), violence during pregnancy (70, 71) were also factors associated with perinatal depression. Generally, perinatal depression in developing countries particularly in Ethiopia poses a significant public health challenge, with potentially severe consequences for maternal and child well-being. Understanding the prevalence and associated factors in developing countries is crucial for effective prevention and intervention strategies. However, there is a lack of comprehensive research addressing the prevalence and the factors contributing to perinatal depression in Ethiopia. So, this study aimed to determine the prevalence and its associated factors of perinatal depression among women in Ethiopia.
Methods and materials
Study areas, design, and period
An Institution based cross-sectional quantitative study design was conducted to assess the prevalence and associated factors of perinatal depression among women attending perinatal services in Kutaber district public health facilities and Boru Meda general hospital from January to August 2022.
Population, sample size and sampling procedure
All women who attend antenatal care, postnatal care & child vaccination program in the last 12 months after delivery in the selected public health facilities of the district and Boru Meda general hospital. Women who attended perinatal health services in the selected public health of Kutaber district and Boru Meda General Hospital during the study period. Inclusion criteria for the enrolling women were the age of ≥18 years, having regular ANC & PNC follow up as well as women who came for delivery during the study period if they are volunteers. Women who are unable to communicate, severely ill and previous diagnosis of psychiatric disorder were excluded from the study. To study the associated risk factors with perinatal depression Epi. Info. Version 7, for double population proportion, was used with an assumption of; a two-sided confidence level (1-alpha) (95%), power (chance of detecting) (80%), the ratio of controls to cases (1), the hypothetical proportion of controls with exposure, and hypothetical proportion of cases with exposure from previous research findings of related works (54, 57). Accordingly, the researcher gets the largest study participant in the second objectives sample size calculation which is 502 and takes it by adding 10% non-response rate. Finally, the population was also proportionally allocated. The study respondents were recruited using a systematic random sampling method. The total estimated number of women that visited the five public health centers and the one general hospital per day is 65 patients. Since the number of required test subjects were 552, a sampling interval of three were used as the constant difference between subjects. The first starting number of each study site was selected randomly using the lottery method from the registration counter. A structured interviewer-administered questionnaire was used to obtain socio-demographic and relevant associated information from the respondents.
Data collection tools and procedure
Perinatal depression was measured using the depression content of Depression Anxiety Stress Scale-21 (DASS-21). DASS-21 has been widely used in studying perinatal psychological health (72–76). Each item in DASS-21 is rated using a 4-point scale (0 for always false or not applicable to 3 for always true or totally applicable). Higher scores indicate greater distress levels. The internal consistency reliability of DASS-21 was very impressive in Ethiopia with Cronbach’s alpha 0.75, 0.72, 0.86, and 0.95 for DASS depression, anxiety, stress, and total scales, respectively (77). These coefficients demonstrated good internal consistencies. The cut-off values for DASS depression, anxiety and stress were 9, 7, and 14, respectively (74, 75). Based on the will of the women those who had scores for depression, anxiety, and stress scale higher than the cut point were linked to the psychiatric ward for further diagnostic investigation.
The Epworth Sleepiness Scale was used as a subjective measure of a woman day time sleepiness. The test had a list of eight situations in which women were rated to become sleepy on a scale of 0, no chance of dozing, to 3, high chance of dozing (78, 79). The total score was based on a scale of 0 to 24. The scale estimates whether women were experiencing excessive sleepiness that possibly requires medical attention (78–82). Socio-demographic characteristics and obstetric variables: trimester, having previous pregnancy, previous pregnancy & labor complication, previous history of stillbirth, previous history of abortion, plan current pregnancy, previous ANC follow-up, current pregnancy complication and previous psychiatric history was collected by a structured and pre-tested questionnaire.
The Baby’s father’s support, partner’s feeling on current pregnancy, community support, and substance use history was also collected by a structured questionnaire.
Operational definition
Perinatal period
The period starting from pregnancy (antenatal) until the end of the first year after a baby is born (postnatal) (6, 57, 83, 84).
Anxiety symptoms
Scoring above the cut-off scores (7) on DASS-21 scales used for assessing perinatal anxiety symptoms (11).
Stress symptoms
Scoring above the cut-off scores (14) on DASS-21 scales used for assessing perinatal stress symptoms (11).
Daytime sleepiness
Those women having ESS score of >10 (85–91).
Data quality assurance
To assure the data quality high emphasis was given in designing data collection instruments. The questionnaires were pretested Haroye health post on 10% of the sample size to check consistency and length of time each questioner took, sampling method and techniques.
Training was provided for data collectors, and supervisors and before data collection the questioners were checked its simplicity, clarity and understandability. Checking and re-checking of the data was employed to identify whether the data was completely filled or not by double data entry. Daily supervision of the data collection process was implemented. One day of training was given for data collectors and supervisors. To ensure the quality of data, a properly designed standardized data collection tool was used.
Data analysis procedure
Each completed questionnaire was coded. The data was checked and cleaned by entering into Epi data version 4.6 and was exported into Statistical Package for the Social Sciences SPSS window version 26, for analysis. Descriptive statistics were employed to estimate the prevalence of perinatal depression. Bivariate analyses (binary logistic regression) were carried out between the predictors and outcome variables.
Using significant variables (p < 0.2) from binary logistic regression models, a multivariable logistic regression model was fitted to identify the independent predictors of perinatal depression. The strength of association was measured by odds ratios with 95% confidence intervals. Statistical significance was declared at p < 0.05. To measure the fitness of the data to the model Hosmer–Lemeshow test was conducted. The small p-value <0.05 of the goodness of fit test means that the model is not good fit. And also apply to the binary logistics regression assumptions were conducted that were linearity of the independent variable, the inclusion of relevant variable, meaningful coding, independent observation, no multicollinearity and sufficiently large sample size.
Ethical consideration
The studies involving human participants were reviewed and approved by Ethical Committee of Wollo University College of Medicine and Health Science with an ethical review number (RCSPG-191/14). All procedures performed were in accordance with the ethical standards of the institutional and national research committee at which the studies were conducted and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. A written informed consent was obtained from each participant or their parents &/or legal guardians.
Results
Sociodemographic and husband-related characteristics of the study subject
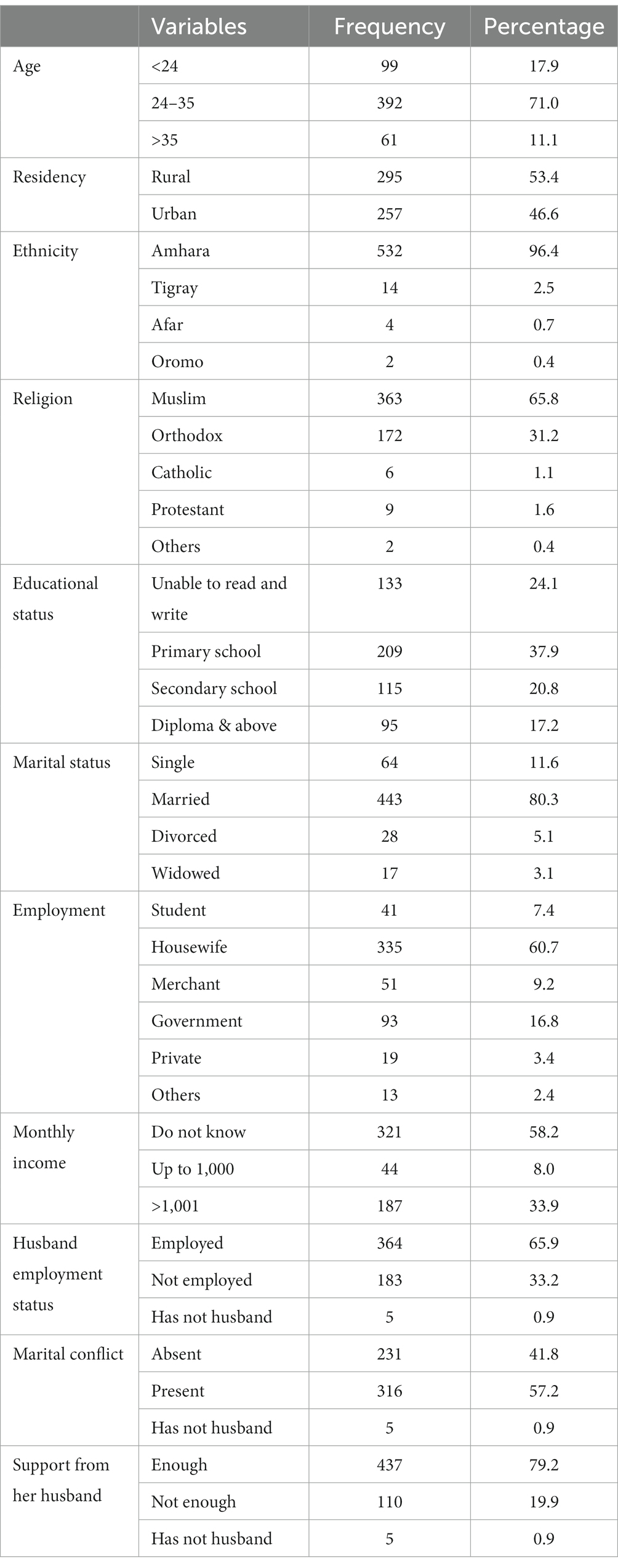
A total of 552 women were recruited for the study, and all of them completed the questionnaire, resulting in a 100% response rate. The respondents were all aged between 18 and 45 years, with a mean age of 29.6 ± 5.385, a median of 29, and a mode of 30. Geographically, 295 (53.4%) lived in rural areas. The majority of the participants, 532 (96.4%), were of Amhara ethnicity, 363 (56.8%) were Muslim, 209 (37.9%) had completed primary school, 443 (80.3%) were married, 321 (58.2%) reported not knowing their monthly income, and 245 (44.4%) had medium levels of social support. Of the total 552 respondents, 333 (60.3%) had employed husbands (Table 1).
Description of respondents by obstetric & other psychosocial related factors
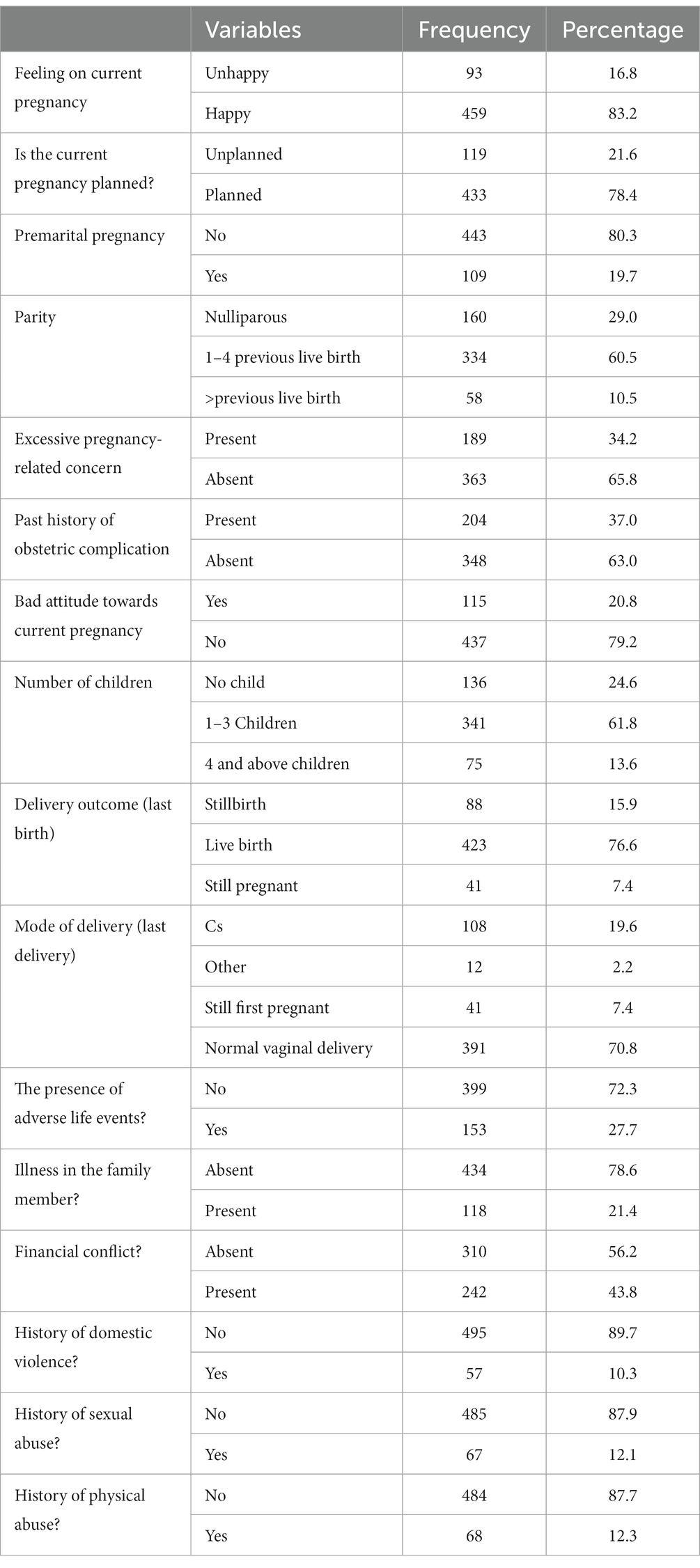
Out of the total respondents, 459 (83.2%) reported having happy feelings during pregnancy, 443 (80.3%) experienced pregnancy after marriage, 341 (61.8%) had 1–3 children, 391 (70.8%) had a history of normal vaginal delivery, and 485 (87.9%) had no history of sexual violence. Additionally, 199 (36.1%) had a history of substance use, and 153 (27.7%) were living with other chronic medical illnesses (Table 2).
Perinatal depression scores of the participants
The overall prevalence of perinatal depression was 32.2% (95% CI, 27.87–36.62). The cutoff point for depression on the DASS-21 scale was set at 9, and accordingly, 178 (32.2%) of study subjects met the criteria for depression symptoms. The depression scores on the DASS-21 scale ranged from 0 to 19, with a median score of 7 (Figure 1).
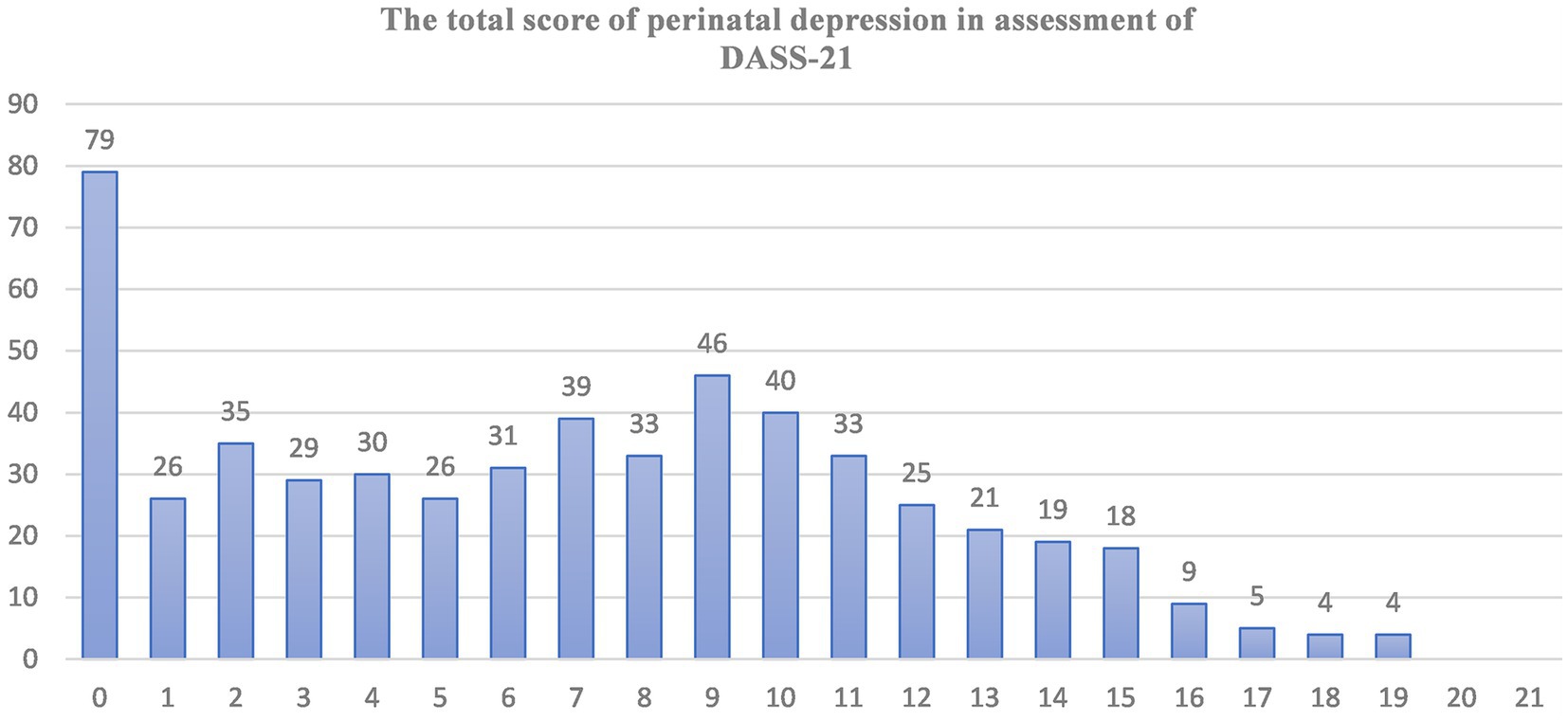
Figure 1. Graph of the total response of depression symptoms (from 0 up to 21) during perinatal period on the seven depression related items of DASS-21 scales. The X-axis indicates the sum scores (0–21) of depressions related symptoms assessment. Y-axis indicates the number of participants who score different level of depression related symptoms.
Distribution of perinatal depression among sociodemographic factors of the respondent
Among the respondents who developed perinatal depression, 125 (70.2%) were in the age group of 24–35 years, 92 (51.7%) lived in rural areas, 132 (74.2%) were married, 120 (67.4%) were employed, 94 (52.8%) did not have a known monthly income, and 85 (47.8%) reported having a moderate level of social support. Among the respondents who developed depression, 136 (76.4%) reported happy feelings during pregnancy, 133 (74.7%) reported that pregnancy was planned and wanted, 134 (80.3%) reported pregnancy occurring after marriage, 97 (54.4%) had a history of past obstetric pregnancy complications, 106 (59.6%) had 1–3 children, and 90 (50.6%) experienced adverse life events. Socially, 100 (56.2%) were exposed to financial conflicts. Clinically, among the women who developed depressive symptoms, 95 (53.4%) and 86 (48.3%) reported substance use in their lifetimes and in the past 3 months, respectively.
Factors associated with perinatal depression
Bivariate analyses were conducted between perinatal depression and independent variables (sociodemographic, obstetric and clinical factors). Finally, all individual factors with a p-value <0.20 in bivariate logistic regression analysis were entered into multivariate logistic regression for further analysis. According to the multivariate analysis, being a student (AOR = 4.364, 95% CI: 1.386, 13.744), excessive pregnancy-related concern (AOR = 1.886, 95% CI: 1.176, 3.041), ever substance use (AOR = 2.203, 95% CI: 1.149, 4.225), the presence of anxiety symptoms (AOR = 3.671, 95% CI: 2.122, 6.352), stress symptoms (AOR = 6.397, 95% CI: 3.394–12.055), and daytime sleepiness (AOR = 2.593, 95% CI: 1.558, 4.316) were significantly associated with perinatal depression (Table 3).
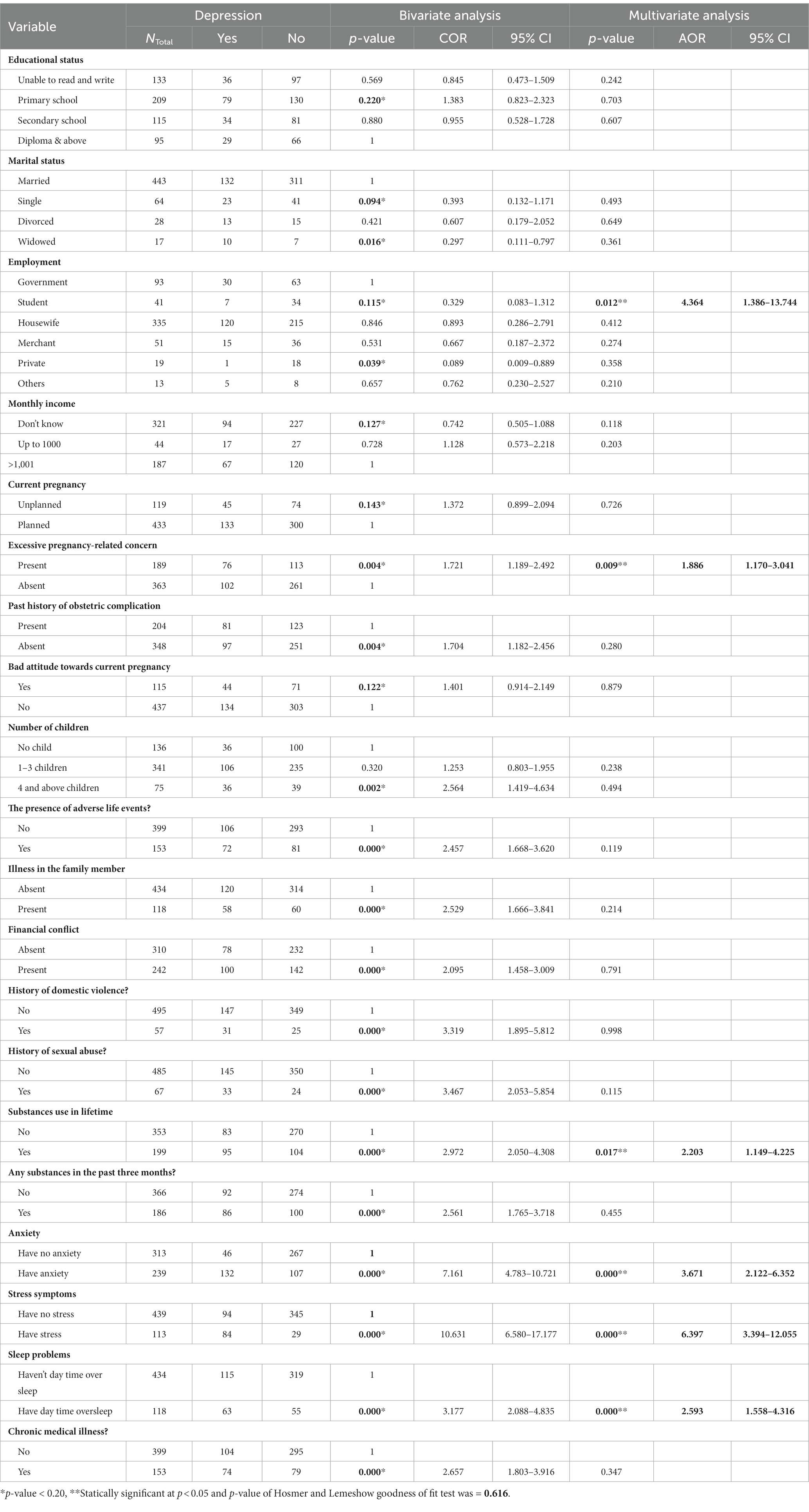
Table 3. Factors associated with perinatal depression (n = 552) (bivariate & multivariate logistic regressions).
Discussion
The prevalence of perinatal depression in this study was 32.2%, which was consistent with findings from studies conducted in Nepal 36% (92), Brazil 25.9% (67), Latin America 28.2% (93), Vietnam 29.9% (94), South Africa 39% (71) Egypt 40% (51) and Ethiopia 35.9% (54). However, it was lower than the prevalence reported in studies conducted in the United States 40.4% (44), UK 47.3% (61), Mexico 39.2% (30), Rwanda 37.6% (7) and Ethiopia 58% (56). On the other hand, it was higher than the prevalence in studies conducted in Colombia 22.36% (9), Canada 17.9% (63), Norwegian 2% (28), China 13% (14), Malaysia 12.5% (95) Australia 19.8% (41), Asia 20% & 21.8% (96), Ghana 24.3% (50) and other parts of Ethiopia (24.9, 26.7, 25.8%) (53, 57, 58). Generally, the discrepancy of the prevalence of depression during the perinatal period might be a result of a difference in assessment tools, geographical areas, sample size, health status & cultures of the study subject and the study setting. Even though there is discrepancy, the result is supported by many other studies carried out elsewhere perinatal depression as a significant public health concern among women in the perinatal period (7, 28, 30, 44, 51, 61, 67, 70, 95, 97–99).
Being a student during the perinatal period was found to be a significant risk factor for perinatal depression (AOR = 4.364, 95% CI: 1.386, 13.744). This finding suggests that the unique challenges and stressors faced by student mothers may contribute to their vulnerability to depression. Early parenting presents numerous challenges, and these challenges are closely linked to the need for a mature and well-developed adult identity. Becoming a parent requires individuals to navigate significant changes in their roles, responsibilities, and priorities. They must also grapple with their own emotional and psychological development, as they transition from being a child or young adult to assuming the role of a caregiver (34).
These stressors might include academic demands, financial pressures, and social support deficits. Academically, student mothers may experience additional stress due to the demands of attending classes, completing assignments, and studying while also being pregnant or caring for a newborn (34). Financial pressures like balancing the costs of education, such as tuition fees, textbooks, and other expenses, with the financial responsibilities of pregnancy or caring for a child can be challenging for student mothers (100). Socially, student mothers may have limited social support networks due to being away from their families or lacking access to resources that could provide assistance during pregnancy and early motherhood (100–102) This lack of support can contribute to feelings of isolation and increased stress levels. It’s important to note that these factors are not the sole causes of perinatal depression in student mothers, but they may contribute to their increased vulnerability. The combination of hormonal changes, sleep deprivation, and the demands of parenting can be particularly challenging for individuals who have not yet developed a strong sense of identity. They may struggle with feelings of isolation, inadequacy, and a sense of losing their own identity within the role of motherhood. These factors can contribute to the development or exacerbation of perinatal depression (35).
It’s crucial for healthcare professionals, educational institutions, and support networks to be aware of these challenges and provide appropriate support and resources to help student mothers during this critical period (35).
Excessive worry or concern related to pregnancy was identified as another contributing factor (AOR = 1.886, 95% CI: 1.176, 3.041). This result indicates that heightened anxiety or preoccupation with pregnancy-related issues can increase the risk of perinatal depression. This might be due to the emotional toll of worrying about the health and well-being of both the mother and the baby. It’s important to consider the possible mechanisms behind this relationship. Pregnancy can be a time of immense change and uncertainty, and concerns about the baby’s health, the mother’s well-being, and the impending responsibilities of parenthood may contribute to increased levels of concern/worry. This, in turn, can impact a woman’s mental health during pregnancy and the postpartum period. Healthcare providers should be attuned to the psychological well-being of expectant mothers and offer appropriate support and resources to help them navigate their anxieties. Additionally, interventions focused on stress reduction, coping strategies, and emotional support may be beneficial in reducing the risk of perinatal depression among individuals experiencing heightened pregnancy-related worries.
The study found that a history of substance use is associated with an increased risk of perinatal depression (AOR = 2.203, 95% CI: 1.149, 4.225). This result was supported by other studies conducted in China (40), in South Africa (19) and any other countries (50, 60, 66). Substance use can have adverse effects on mental health and can exacerbate depressive symptoms. It may also impair decision-making and coping mechanisms, making it harder for individuals to manage their emotional well-being during perinatal period.
Similar to other studies in China (99, 103), presence of anxiety symptoms was strongly associated with perinatal depression (AOR = 3.671, 95% CI: 2.122, 6.352). Anxiety and depression often cooccur, and this finding underscores the importance of assessing and addressing both conditions during the perinatal period. Understanding the relationship between anxiety and perinatal depression is crucial for healthcare professionals and researchers to effectively screen, diagnose, and provide appropriate interventions. It is well-known that anxiety and depression often cooccur, and this comorbidity can worsen the overall mental health of pregnant and postpartum women.
High levels of anxiety can directly contribute to the development or exacerbation of depressive symptoms. Anxiety increases stress levels, which can disrupt a woman’s ability to cope with the challenges of pregnancy and early motherhood. Moreover, anxiety can interfere with sleep, appetite, and overall well-being, further exacerbating the risk of perinatal depression (99). Addressing both anxiety and depression during the perinatal period is crucial for optimal mental health outcomes. Early identification and intervention can help prevent the escalation of symptoms and improve overall maternal well-being. By recognizing and addressing the interconnected nature of these mental health conditions, healthcare providers can provide holistic care to pregnant and postpartum women, promoting better outcomes for both the mother and the child.
Consistent with the results of previous studies (104), stress symptoms were found to have a substantial impact on perinatal depression (AOR = 6.397, 95% CI: 3.394–12.055). High levels of stress, whether related to personal life, relationships, or external factors, can overwhelm pregnant or postpartum individuals, making them more susceptible to depression. Stress, whether stemming from personal life, relationships, or external factors, can be particularly challenging during the perinatal period (105). Pregnancy and early parenthood can bring about various stressors, such as financial concerns, changes in identity, lack of support, and sleep deprivation. Additionally, hormonal changes and physical discomforts associated with pregnancy can further exacerbate stress levels.
When stress becomes overwhelming and persistent, it can disrupt an individual’s emotional well-being and increase the likelihood of developing depression. The study highlights the importance of identifying and addressing stress symptoms during the perinatal period to mitigate the risk of depression. Interventions aimed at reducing stress and promoting emotional well-being during pregnancy and postpartum can be beneficial in preventing or minimizing the severity of perinatal depression (35). These interventions may include stress management techniques, social support networks, cognitive-behavioral therapy, and mindfulness-based approaches. Engaging in regular physical activity, practicing relaxation techniques, and seeking professional help when needed are also essential strategies for managing stress during this critical period.
Daytime sleepiness emerged as a significant factor associated with perinatal depression (AOR = 2.593, 95% CI: 1.558, 4.316). A similar finding was reported from the studies conducted in Sweden (106), and in USA (107). Disrupted sleep patterns are a common experience for many individuals during pregnancy and early parenthood. These disruptions can be caused by a variety of factors, including physical discomfort, hormonal changes, anxiety, and the demands of caring for a new infant. Such disruptions can lead to significant daytime sleepiness, which may in turn contribute to mood disturbances and increase the risk of perinatal depression (106). Addressing sleep issues as part of perinatal care can involve a range of strategies, including providing education about sleep hygiene, offering guidance on managing discomfort and anxiety, and in some cases, considering the appropriate use of medication under the supervision of a healthcare professional. By understanding and addressing sleep issues as part of perinatal mental health care, healthcare providers can potentially reduce the risk of perinatal depression and improve the overall well-being of individuals during this critical life stage.
Limitation of the study
Recall and response biases might have occurred when completing the questionnaire. In addition, some of the independent variables like physical & sexual abuse, and the presence of suicidal wish was assessed by close-ended questions which may lead some patients to respond in an indecorous manner. Because of using a cross-sectional study design, we were not demonstrating any cause and effect association between the possible determinate factors and the outcome of interest.
Conclusion
Globally perinatal depression is a public health concern. It contributes to the high burden of health risks faced by mothers, their child and their family. This study underscores the complex interplay of various factors that contribute to perinatal depression. It highlights the need for a comprehensive approach to perinatal mental health that takes into account not only the biological aspects of pregnancy but also the psychological, social, and lifestyle factors that can impact a person’s mental well-being during this critical period. Early identification, intervention, and support for individuals at risk for perinatal depression are crucial to improving maternal and child health outcomes. Additionally, public health campaigns and policies should focus on raising awareness about perinatal depression and promoting early detection and intervention to improve. Finally, further research and tailored interventions are warranted to address these associated factors effectively.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Wollo University College of Medicine and Health Science with an ethical review number (RCSPG-191/14). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NC: Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – review & editing, Investigation, Software. HY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – review & editing. EA: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Kutaber District Administration Office. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors would like to acknowledge Wollo University, Kutaber District administration office for their cooperation to provide the necessary data about the study area. Finally, would like to say thank the study participants, data collectors, and supervisors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Luciano, M, Di Vincenzo, M, Brandi, C, Tretola, L, Toricco, R, Perris, F, et al. Does antenatal depression predict post-partum depression and obstetric complications? Results from a longitudinal, long-term, real-world study. Front Psychiatry. (2022) 13:1082762. doi: 10.3389/fpsyt.2022.1082762
2. Bm, XL, Wang, S, and Wang, G. Prevalence and risk factors of postpartum depression in women: a systematic review and meta-analysis. J Clin Nurs. (2021) 30:2665–77. doi: 10.1111/jocn.16121
3. Fernández-Ordoñez, E, González-Cano-Caballero, M, Guerra-Marmolejo, C, Fernández-Fernández, E, and García-Gámez, M. Perinatal grief and post-traumatic stress disorder in pregnancy after perinatal loss: a longitudinal study protocol. Int J Environ Res Public Health. (2021) 18:2874. doi: 10.3390/ijerph18062874
4. Sorsa, MA, Kylmä, J, and Bondas, TE. Contemplating help-seeking in perinatal psychological distress—a meta-ethnography. Int J Environ Res Public Health. (2021) 18:5226. doi: 10.3390/ijerph18105226
5. Hall, SL, Hynan, MT, Phillips, R, Lassen, S, Craig, JW, Goyer, E, et al. The neonatal intensive parenting unit: an introduction. J Perinatol. (2017) 37:1259–64. doi: 10.1038/jp.2017.108
6. Byrnes, L. Perinatal mood and anxiety disorders. J Nurse Pract. (2018) 14:507–13. doi: 10.1016/j.nurpra.2018.03.010
7. Umuziga, MP, Adejumo, O, and Hynie, M. A cross-sectional study of the prevalence and factors associated with symptoms of perinatal depression and anxiety in Rwanda. BMC Pregnancy Childbirth. (2020) 20:68. doi: 10.1186/s12884-020-2747-z
8. Ganjekar, S, Thekkethayyil, AV, and Chandra, PS. Perinatal mental health around the world: priorities for research and service development in India. BJPsych Int. (2019) 17:2–5. doi: 10.1192/bji.2019.26
9. Gaviria, SL, Duque, M, Vergel, J, and Restrepo, D. Perinatal depressive symptoms: prevalence and associated psychosocial factors. Rev Colomb Psiquiatr. (2019) 48:166–73. doi: 10.1016/j.rcpeng.2017.09.011
10. Iyengar, U, Jaiprakash, B, Haitsuka, H, and Kim, S. One year into the pandemic: a systematic review of perinatal mental health outcomes during. Front Psychiatry. (2021) 12:674194. doi: 10.3389/fpsyt.2021.674194
11. Jonsdottir, SS. Effects of perinatal distress, satisfaction in partner relationship and social support on pregnancy and outcome of childbirth. (2019). (Doctoral dissertation, Linnaeus University Press).
12. Antoniou, E, Stamoulou, P, Tzanoulinou, MD, and Orovou, E. Perinatal mental health; the role and the effect of the partner: a systematic review. Healthcare. (2021) 9:1572. doi: 10.3390/healthcare9111572
13. Łojko, D, Wszołek, K, Suwalska, J, Napierała, M, Bogda, P, and Suchowiak, S. Perinatal mental health during COVID-19 pandemic: an integrative review and implications for clinical practice. J Clin Med. (2021) 10:2406. doi: 10.3390/jcm10112406
14. Jiang, Q, Guo, Y, Zhang, E, Cohen, N, Ohtori, M, Sun, A, et al. Perinatal mental health problems in rural China: the role of social factors. Front Psychiatry. (2021) 12:636875. doi: 10.3389/fpsyt.2021.636875
15. Bao, C, Jin, D, Sun, S, Xu, L, Wang, C, and Tang, W. Trajectories and depressive symptoms during the perinatal period: a longitudinal population-based study in China. Front Psychiatry. (2022) 13:762719. doi: 10.3389/fpsyt.2022.762719
16. Tefera, TB, Erena, AN, Kuti, KA, and Hussen, MA. Perinatal depression and associated factors among reproductive aged group women at Goba and Robe Town of Bale Zone, Oromia Region, South East Ethiopia. Matern Health Neonatol Perinatol. (2015) 1:1–9. doi: 10.1186/s40748-015-0013-6
17. Nakku, JEM, Okello, ES, Kizza, D, Honikman, S, Ssebunnya, J, Ndyanabangi, S, et al. Perinatal mental health care in a rural African district, Uganda: a qualitative study of barriers, facilitators and needs. BMC Health Serv Res. (2016) 16:1–12. doi: 10.1186/s12913-016-1547-7
18. Lequertier, B, Mclean, MA, Kildea, S, King, S, Keedle, H, Gao, Y, et al. Perinatal depression in Australian women during the COVID-19 pandemic: the birth in the time of COVID-19 (BITTOC) study. Int J Environ Res Public Health. (2022) 19:5062. doi: 10.3390/ijerph19095062
19. Garman, EC, Schneider, M, and Lund, C. Perinatal depressive symptoms among low-income South African women at risk of depression: trajectories and predictors. BMC Pregnancy Childbirth. (2019) 19:202. doi: 10.1186/s12884-019-2355-y
20. Phoosuwan, N. Perinatal depressive symptoms among women in north-eastern Thailand: risk factors, support and prevention. Doctoral thesis. (2020). (Doctoral dissertation, Acta Universitatis Upsaliensis).
21. Din, Z, Ambreen, S, Iqbal, Z, Iqbal, M, and Ahmad, S. Determinants of antenatal psychological distress in Pakistani women. Arch Neuropsychiatr. (2016) 53:152–7. doi: 10.5152/npa.2015.10235
22. Buist, A. Perinatal mental health identifying problems and managing medications. Aust Fam Physician. (2014) 43:182–5. doi: 10.3316/informit.205997313865266
23. Ashford, MT, Olander, EK, and Ayers, S. Computer- or web-based interventions for perinatal mental health: a systematic review. J Affect Disord. (2016) 197:134–46. doi: 10.1016/j.jad.2016.02.057
24. Green, SM, Inness, B, Furtado, M, Mccabe, RE, and Frey, BN. Evaluation of an augmented cognitive behavioural group therapy for perinatal generalized anxiety disorder (GAD) during the COVID-19 pandemic. J Clin Med. (2022) 11:209. doi: 10.3390/jcm11010209
25. Bjelica, A, Cetkovic, N, Trninic-Pjevic, A, and Mladenovic-Segedi, L. The phenomenon of pregnancy—a psychological view. Ginekol Pol. (2018) 89:102–6. doi: 10.5603/GP.a2018.0017
26. Meaney, MJ. Perinatal maternal depressive symptoms as an issue for population health. Am J Psychiatry. (2018) 175:1084–93. doi: 10.1176/appi.ajp.2018.17091031
27. Cheng, C, Chou, Y, Chang, C, and Liou, S. Trends of perinatal stress, anxiety, and depression and their prediction on postpartum depression. Int J Environ Res Public Health. (2021) 18:1–12. doi: 10.3390/ijerph18179307
28. Eid, K, Torkildsen, ØF, Aarseth, J, Flemmen, HØ, Holmøy, T, Lorentzen, ÅR, et al. Perinatal depression and anxiety in women with multiple sclerosis. Neurology. (2021) 96:e2789–800. doi: 10.1212/WNL.0000000000012062
29. Osman, KM, Lara-cinisomo, S, and Anna-hernandez, KLD. Associations between religiosity and perinatal anxiety symptoms among women of Mexican descent. J Affect Disord. (2022) 294:77–84. doi: 10.1016/j.jad.2021.06.066
30. Vianey, B, Estrada-gutierrez, G, Maribel, S, Perichart-perera, O, Rodr, C, Gonz, C, et al. Prevalence of depression, anxiety, and perceived stress in postpartum Mexican women during the COVID-19 lockdown. Int J Environ Res Public Health. (2021) 18:4627. doi: 10.3390/ijerph18094627
31. Noonan, M, Jomeen, J, and Doody, O. A review of the involvement of partners and family members in psychosocial interventions for supporting women at risk of or experiencing perinatal depression and anxiety. Int J Environ Res Public Health. (2021) 18:5396. doi: 10.3390/ijerph18105396
32. Costa, J, Santos, O, Virgolino, A, Em, M, Stefanovska-petkovska, M, Silva, H, et al. MAternal mental health in the WORKplace (MAMH @ WORK): a protocol for promoting perinatal maternal mental health and wellbeing. Int J Environ Res Public Health. (2021) 18:2558. doi: 10.3390/ijerph18052558
33. Nielsen-Scott, M, Fellmeth, G, Opondo, C, and Alderdice, F. Prevalence of perinatal anxiety in low- and middle-income countries: a systematic review and meta-analysis. J Affect Disord. (2022) 306:71–9. doi: 10.1016/j.jad.2022.03.032
34. Goossens, G, Kadji, C, and Delvenne, V. Teenage pregnancy: a psychopathological risk for mothers and babies? Psychiatr Danub. (2015) 27:499–503.
35. Kristensen, IH, Juul, S, and Kronborg, H. What are the effects of supporting early parenting by newborn behavioral observations (NBO)? A cluster randomised trial. BMC Psychol. (2020) 8:1–9. doi: 10.1186/s40359-020-00467-5
36. Polese, D, Riccio, ML, Fagioli, M, Mazzetta, A, Fagioli, F, Parisi, P, et al. The newborn’s reaction to light as the determinant of the brain’s activation at human birth. Front Integr Neurosci. (2022) 16:933426. doi: 10.3389/fnint.2022.933426
37. Poletti, M, Preti, A, and Raballo, A. Focusing on modifiable early protective factors to prevent negative neurodevelopmental and psychiatric outcomes in at-risk infants. Front Psychiatry. (2023) 14:1302474. doi: 10.3389/fpsyt.2023.1302474
38. Highet, N, Stevenson, AL, Purtell, C, and Coo, S. Qualitative insights into women’s personal experiences of perinatal depression and anxiety. Women and Birth. (2014) 27:179–84. doi: 10.1016/j.wombi.2014.05.003
39. Elrassas, H, Taha, GR, El, A, Muhammed, D, Abd, S, Kareem, E, et al. Prevalence and related factors of perinatal depression in Egyptian mothers. Middle East Curr Psychiatry. (2022) 29:35. doi: 10.1186/s43045-022-00203-2
40. Hong, L, Le, T, Lu, Y, Shi, X, Xiang, L, Liu, M, et al. Distinct trajectories of perinatal depression in Chinese women: application of latent growth mixture modelling. BMC Pregnancy Childbirth. (2022) 22:1–11. doi: 10.1186/s12884-021-04316-0
41. Fisher, J, De, MC, Patel, V, Rahman, A, Tran, T, and Holmes, W. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. (2012) 90:139–149H. doi: 10.2471/BLT.11.091850
42. Foundation WHO and CG. Social determinants of mental health. Geneva: World Health Organization (2014).
43. Cox, J. Perinatal mental health around the world: a new thematic series. BJPsych Int. (2020) 17:1–2. doi: 10.1192/bji.2019.32
44. Mckee, K, Admon, LK, Winkelman, TNA, Muzik, M, Hall, S, Dalton, VK, et al. Perinatal mood and anxiety disorders, serious mental illness, and delivery-related health outcomes, United States, 2006–2015. BMC Womens Health. (2020) 20:150. doi: 10.1186/s12905-020-00996-6
45. Alfayumi-Zeadna, S, Bina, R, Levy, D, Merzbach, R, and Zeadna, A. Elevated perinatal depression during the COVID-19 pandemic: a national study among Jewish and Arab women in Israel. J Clin Med. (2022) 11:349. doi: 10.3390/jcm11020349
46. Luciano, M, Sampogna, G, Del Vecchio, V, Giallonardo, V, Perris, F, Carfagno, M, et al. The transition from maternity blues to full-blown perinatal depression: results from a longitudinal study. Front Psychiatry. (2021) 12:703180. doi: 10.3389/fpsyt.2021.703180
47. Baron, EC, Hanlon, C, Mall, S, Honikman, S, Breuer, E, Kathree, T, et al. Maternal mental health in primary care in five low- and middle-income countries: a situational analysis. BMC Health Serv Res. (2016) 16:53. doi: 10.1186/s12913-016-1291-z
48. Norhayati, MN, Hazlina, NHN, Asrenee, AR, and Emilin, WMAW. Magnitude and risk factors for postpartum symptoms: a literature review. J Affect Disord. (2015) 175:34–52. doi: 10.1016/j.jad.2014.12.041
49. Rodrigues, AL, Ericksen, J, Watson, B, Gemmill, AW, and Milgrom, J. Interventions for perinatal depression and anxiety in fathers: a mini-review. Front Psychol. (2022) 12:744921. doi: 10.3389/fpsyg.2021.744921
50. Melo, EF, Cecatti, JG, Pacagnella, RC, Leite, DFB, Vulcani, DE, and Makuch, MY. The prevalence of perinatal depression and its associated factors in two different settings in Brazil. J Affect Disord. (2012) 136:1204–8. doi: 10.1016/j.jad.2011.11.023
51. Salem, GM, Allah, MBA, and Said, RM. Prevalence and predictors of depression, anxiety and stress among Zagazig University students. Med J Cairo Univ. (2016) 84:325–34.
52. Weobong, B, Soremekun, S, Ha, A, Amenga-etego, S, Danso, S, Owusu-agyei, S, et al. Prevalence and determinants of antenatal depression among pregnant women in a predominantly rural population in Ghana: the DON population-based study. J Affect Disord. (2014) 165:1–7. doi: 10.1016/j.jad.2014.04.009
53. Biratu, A, and Haile, D. Prevalence of antenatal depression and associated factors among pregnant women in Addis Ababa, Ethiopia: a cross-sectional study. Reprod Health. (2015) 12:99. doi: 10.1186/s12978-015-0092-x
54. Negewo, AN. Prevalence of depression among pregnant women attending ANC follow up mother’s at higher two health center, Jimma teaching health center, Shenen Gibe Hospital and JUSH, Jimma Town, Oromia Regional State, South West Ethiopia. Clin Mother Child Health. (2019) 16:315. doi: 10.4172/2090-7214.1000315
55. Bekele, D. Prevalence and associated factors of mental distress during pregnancy among antenatal care attendees at Saint Paul’s Hospital, Addis Ababa. Obstet Gynecol Int J. (2017) 7:00269. doi: 10.15406/ogij.2017.07.00269
56. Belayihun, B, and Mavhandu-Mudzuis, AH. Psychological distress in women with obstetric fistula in Ethiopia: a multi-center, facility-based, cross-sectional study. Ethiop J Health Dev. (2018) 32:211–7.
57. Gebremichael, G, Yihune, M, Ajema, D, Haftu, D, and Gedamu, G. Perinatal depression and associated factors among mothers in southern Ethiopia: evidence from Arba Minch Zuria health and demographic surveillance site. Psychiatry J. (2018) 2018:1–12. doi: 10.1155/2018/7930684
58. Mersha, AG, Abebe, SA, Sori, LM, and Abegaz, TM. Prevalence and associated factors of perinatal depression in Ethiopia: a systematic review and meta-analysis. Depress Res Treat. (2018) 2018:1813834. doi: 10.1155/2018/1813834
59. Glasheen, C, Colpe, L, Hoffman, V, and Warren, LK. Prevalence of serious psychological distress and mental health treatment in a national sample of pregnant and postpartum women. Child Health J. (2015) 19:204–16. doi: 10.1007/s10995-014-1511-2
60. Yang, K, Wu, J, and Chen, X. Risk factors of perinatal depression in women: a systematic review and meta—analysis. BMC Psychiatry. (2022) 22:63. doi: 10.1186/s12888-021-03684-3
61. Fellmeth, G, Plugge, E, Fazel, M, Nosten, S, Oo, MM, Pimanpanarak, M, et al. Perinatal depression in migrant and refugee women on the Thai–Myanmar border: does social support matter? Philos Trans R Soc B. (2021) 376:20200030. doi: 10.1098/rstb.2020.0030
62. Susukida, R, Usuda, K, Hamazaki, K, and Tsuchida, A. Association of prenatal psychological distress and postpartum depression with varying physical activity intensity: Japan Environment and Children’s Study (JECS). Sci Rep. (2020) 10:1–9. doi: 10.1038/s41598-020-63268-1
63. Gheorghe, M. Symptoms of postpartum anxiety and depression among women in Canada: findings from a national cross-sectional survey. Can J Public Health. (2021) 112:244–52. doi: 10.17269/s41997-020-00420-4
64. Vesga-López, O, Blanco, C, Keyes, K, Olfson, M, Bridget, F, and DSH, G. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. (2008) 65:805–15. doi: 10.1001/archpsyc.65.7.805
65. Yim, IS, Stapleton, LRT, Guardino, CM, Hahn-Holbrook, J, and Schetter, CD. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. (2015) 11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426
66. Soto-Balbuena, C, De, M, Rodríguez, F, Isabel, A, Gomis, E, Javier, F, et al. Incidence, prevalence and risk factors related to anxiety symptoms during pregnancy. Psicothema. (2018) 30:257–63. doi: 10.7334/psicothema2017.379
67. Nunes, C, Brito, DO, Alves, SV, and Ludermir, AB. Postpartum depression among women with unintended pregnancy. Rev Saúde Pública. (2015) 49:33. doi: 10.1590/S0034-8910.2015049005257
68. Al-deen, ALM. Assessment of psychological distress among pregnant women in Kirkuk City. Mosul J Nurs. (2014) 2:51–7. doi: 10.33899/mjn.2014.162921
69. Polachek, IS, Harari, LH, Baum, M, and Strous, RD. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. (2014) 51:128–34.
70. Nasreen, HE, Kabir, ZN, Forsell, Y, and Edhborg, M. Prevalence and associated factors of depressive and anxiety symptoms during pregnancy: a population based study in rural Bangladesh. BMC Womens Health. (2011) 11:22. doi: 10.1186/1472-6874-11-22
71. Hartley, M, Tomlinson, M, Greco, E, Comulada, WS, Stewart, J, Le Roux, I, et al. Depressed mood in pregnancy: prevalence and correlates in two Cape Town Peri-urban settlements. Reprod Health. (2011) 8. doi: 10.1186/1742-4755-8-9
72. Lydon, K, Fp, D, Owens, L, Avalos, G, Km, S, Connor, OC, et al. Psychological stress associated with diabetes during pregnancy: a pilot study. Irish Med J. (2012) 105:26–8.
73. Bin, NR, Kaur, A, Soni, T, Por, LK, and Miranda, S. Construct validity and internal consistency reliability of the Malay version of the 21-item depression anxiety stress scale (Malay-DASS-21) among male outpatient clinic attendees in Johor. Med J Malaysia. (2017) 72:264–70.
74. Tran, TD, Tran, T, and Fisher, J. Validation of the depression anxiety stress scales (DASS) 21 as a screening instrument for depression and anxiety in a rural community-based cohort of northern Vietnamese women. BMC Psychiatry. (2013) 13:24. doi: 10.1186/1471-244X-13-24
75. Basha, E, and Kaya, M. Depression, anxiety and stress scale (DASS): the study of validity and reliability. Univ J Educ Res. (2016) 4:2701–5. doi: 10.13189/ujer.2016.041202
76. Pezirkianidis, C, Karakasidou, E, Lakioti, A, and Stalikas, A. Psychometric properties of the depression, anxiety, stress scales-21 (DASS-21) in a Greek sample. Psychology. (2018) 9:2933–50. doi: 10.4236/psych.2018.915170
77. Sileshi Bekele, MDD. Depression, anxiety and stress among first year Addis Ababa university students: magnitude, and relationship with academic achievement. J Health Med Nurs. (2018) 56:53–9.
78. Fernández-Alonso, AM, Trabalón-Pastor, M, Chedraui, P, and Pérez-López, FR. Factors related to insomnia and sleepiness in the late third trimester of pregnancy. Arch Gynecol Obs. (2012) 286:55–61. doi: 10.1007/s00404-012-2248-z
79. Bourjeily, G, El Sabbagh, R, Sawan, P, Raker, C, Wang, C, and Hott, B. Epworth sleepiness scale scores and adverse pregnancy outcomes. Sleep Breath. (2013) 17:1179–86. doi: 10.1007/s11325-013-0820-9
80. Wilkerson, AK, and Uhde, TW. Perinatal sleep problems. Obstet Gynecol Clin North Am. (2018) 45:483–94. doi: 10.1016/j.ogc.2018.04.003
81. Sharma, SK, Nehra, A, Sinha, S, Soneja, M, Sunesh, K, and Sreenivas, V. Sleep disorders in pregnancy and their association with pregnancy outcomes: a prospective observational study. Sleep Breath. (2015) 20:87–93. doi: 10.1007/s11325-015-1188-9
82. Sarberg, M, Bladh, M, Josefsson, A, and Svanborg, E. Sleepiness and sleep-disordered breathing during pregnancy. Sleep Breath. (2016) 20:1231–7. doi: 10.1007/s11325-016-1345-9
83. Leung, BMY, and Kaplan, BJ. Perinatal depression: prevalence, risks, and the nutrition link—a review of the literature. J Am Diet Assoc. (2009) 109:1566–75. doi: 10.1016/j.jada.2009.06.368
84. Molenaar, J, Hanlon, C, Alem, A, Wondimagegn, D, Medhin, G, Prince, M, et al. Perinatal mental distress in a rural Ethiopian community: a critical examination of psychiatric labels. BMC Psychiatry. (2020) 20:1–10. doi: 10.1186/s12888-020-02646-5
85. Signal, TL, Paine, S, Sweeney, B, Priston, M, Muller, D, Smith, A, et al. Prevalence of abnormal sleep duration and excessive daytime sleepiness in pregnancy and the role of socio-demographic factors: comparing pregnant women with women in the general population. Sleep Med. (2014) 15:1477–83. doi: 10.1016/j.sleep.2014.07.007
86. Hein, M, Lanquart, J, Loas, G, Hubain, P, and Linkowski, P. Prevalence and risk factors of excessive daytime sleepiness in major depression: a study with 703 individuals referred for polysomnography. J Affect Disord. (2019) 243:23–32. doi: 10.1016/j.jad.2018.09.016
87. Hunskar, GS, Bjorvatn, B, Wensaas, K, Hanevik, K, Eide, GE, Langeland, N, et al. Excessive daytime sleepiness, sleep need and insomnia 3 years after Giardia infection: a cohort study. Sleep Health. (2016) 2:154–8. doi: 10.1016/j.sleh.2016.03.005
88. Boz, S, Pol, J, Anaïs, L, Marie, M, and Gwenolé, D. Risk of excessive daytime sleepiness associated to major depression in adolescents. Psychiatr Q. (2021) 92:1473–88. doi: 10.1007/s11126-021-09922-x
89. Prasad, B, Steffen, AD, HPA, VD, Pack, FM, Strakovsky, I, Staley, B, et al. Determinants of sleepiness in obstructive sleep apnea. Sleep. (2018) 41:1–9. doi: 10.1093/sleep/zsx199
90. Kawada, T. Excessive daytime sleepiness, depression and sleep-disordered breathing in patients with cardiovascular disease. Circ J. (2018) 83:692. doi: 10.1253/circj.CJ-18-0941
91. Ohashi, M, Kohno, T, Kohsaka, S, Fukuoka, R, Hayashida, K, Yuasa, S, et al. Excessive daytime sleepiness is associated with depression scores, but not with sleep-disordered breathing in patients with cardiovascular diseases. Circ J. (2018) 82:2175–83. doi: 10.1253/circj.CJ-17-1395
92. Ho-Yen, SD, Bondevik, GT, Eberhard-Gran, M, and Bjorvatn, B. Factors associated with depressive symptoms among postnatal women in Nepal. Acta Obstet Gynecol Scand. (2007) 86:291–7. doi: 10.1080/00016340601110812
93. Fonseca-Machado, MDO, Alves, LC, Cristina, J, Jos, V, Stefanello, J, and Ana, M. Depressive disorder in pregnant Latin women: does intimate partner violence matter? J Clin Nurs. (2015) 24:1289–99. doi: 10.1111/jocn.12728
94. Fisher, J, Tran, T, Kriitmaa, K, and Tran, T. Common perinatal mental disorders in northern Vietnam: community prevalence and health care use. Bull World Health Organ. (2010) 88:737–45. doi: 10.2471/BLT.09.067066
95. Lee, KW, Ching, SM, Hoo, FK, Ramachandran, V, and Chong, SC. Prevalence and factors associated with depressive, anxiety and stress symptoms among women with gestational diabetes mellitus in tertiary care centres in Malaysia: a cross-sectional study. BMC Pregnancy Childbirth. (2019) 19:367. doi: 10.1186/s12884-019-2519-9
96. Roomruangwong, C, and Epperson, CN. Perinatal depression in Asian women: prevalence, associated factors, and cultural aspects. Asian Biomed. (2011) 5:179–93. doi: 10.5372/1905-7415.0502.024
97. George, A, Luz, RF, De Tychey, C, Thilly, N, and Spitz, E. Anxiety symptoms and coping strategies in the perinatal period. BMC Pregnancy Childbirth. (2013) 13:233. doi: 10.1186/1471-2393-13-233
98. Nagandla, K, Nalliah, S, Yin, LK, Majeed, ZA, Ismail, M, Zubaidah, S, et al. Prevalence and associated risk factors of depression, anxiety and stress in pregnancy. Int J Reprod Contracept Obstet Gynecol. (2016) 5:2380–8. doi: 10.18203/2320-1770.ijrcog20162132
99. Xian, T, Zhuo, L, Dihui, H, and Xiaoni, Z. Influencing factors for prenatal stress, anxiety and depression in early pregnancy among women in Chongqing, China. J Affect Disord. (2019) 253:292–302. doi: 10.1016/j.jad.2019.05.003
100. Dadi, AF, Wolde, HF, Baraki, AG, and Akalu, TY. Epidemiology of antenatal depression in Africa: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2020) 20:251. doi: 10.1186/s12884-020-02929-5
101. Adeponle, A, Groleau, D, Kola, L, Kirmayer, LJ, and Gureje, O. Perinatal depression in Nigeria: perspectives of women, family caregivers and health care providers. Int J Ment Health Syst. (2017) 11:27. doi: 10.1186/s13033-017-0134-6
102. Dadi, AF, Yihunie, T, Id, A, Baraki, AG, and Wolde, HF. Epidemiology of postnatal depression and its associated factors in Africa: a systematic review and meta-analysis. PLoS One. (2020) 15:e0231940. doi: 10.1371/journal.pone.0231940
103. Yu, Y, Zhu, X, Xu, H, Hu, Z, Zhou, W, and Zheng, B. Prevalence of depression symptoms and its influencing factors among pregnant women in late pregnancy in urban areas of Hengyang City, Hunan Province, China: a cross-sectional study. BMJ Open. (2020) 10:e038511. doi: 10.1136/bmjopen-2020-038511
104. Richter-Levin, G, and Xu, L. How could stress lead to major depressive disorder? IBRO Rep. (2018) 4:38–43. doi: 10.1016/j.ibror.2018.04.001
105. Gelaye, B, Rondon, M, Araya, R, and Williams, MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. (2016) 3:973–82. doi: 10.1016/S2215-0366(16)30284-X
106. Sarberg, M, Bladh, M, Svanborg, E, and Josefsson, A. Postpartum depressive symptoms and its association to daytime sleepiness and restless legs during pregnancy. BMC Pregnancy Childbirth. (2016) 16:137. doi: 10.1186/s12884-016-0917-9
Keywords: perinatal mental disorder, antenatal depression, postnatal depression, perinatal care, pregnancy, Ethiopia
Citation: Seid J, Mohammed E, Cherie N, Yasin H and Addisu E (2024) The magnitude of perinatal depression and associated factors among women in Kutaber woreda public health institution and Boru Meda general hospital, Ethiopia, 2022: a cross-sectional study. Front. Psychiatry. 14:1302168. doi: 10.3389/fpsyt.2023.1302168
Edited by:
Karen Tabb, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Marco La Verde, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyDaniela Polese, Sant’Andrea University Hospital, Italy
Copyright © 2024 Seid, Mohammed, Cherie, Yasin and Addisu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jemal Seid, amVtYWxzMjgzQGdtYWlsLmNvbQ==
 Jemal Seid
Jemal Seid Emam Mohammed2
Emam Mohammed2