- 1Alexandria Faculty of Medicine, Alexandria University, Alexandria, Egypt
- 2Faculty of Medicine, Al-Azhar University, Medical Research Group of Egypt, Cairo, Egypt
- 3Faculty of Medicine, The Pavlov First State Medical University of St. Petersburg, St. Petersburg, Russia
- 4Faculty of Medicine, Zagazig University, Medical Research Group of Egypt, Cairo, Egypt
- 5Faculty of Medicine, South Valley University, Qena, Egypt
- 6Neurology Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
- 7Nursing Department, Hamad Medical Corporation, Doha, Qatar
Background and aims: Smoking cigarettes is a major global health problem that affects appetite and weight. The aim of this systematic review was to determine how smoking affected plasma leptin and ghrelin levels.
Methods: A comprehensive search of PubMed, Scopus, Web of Science, and Ovid was conducted using a well-established methodology to gather all related publications.
Results: A total of 40 studies were included in the analysis of 11,336 patients. The overall effect showed a with a mean difference (MD) of −1.92[95%CI; −2.63: −1.20] and p = 0.00001. Subgroup analysis by study design revealed significant differences as well, but with high heterogeneity within the subgroups (I2 of 82.3%). Subgroup by sex showed that there was a significant difference in mean difference between the smoking and non-smoking groups for males (MD = −5.75[95% CI; −8.73: −2.77], p = 0.0002) but not for females (MD = −3.04[95% CI; −6.6:0.54], p = 0.10). Healthy, pregnant, diabetic and CVD subgroups found significant differences in the healthy (MD = −1.74[95% CI; −03.13: −0.35], p = 0.01) and diabetic (MD = −7.69[95% CI, −1.64: −0.73], p = 0.03). subgroups, but not in the pregnant or cardiovascular disease subgroups. On the other hand, the meta-analysis found no statistically significant difference in Ghrelin serum concentration between smokers and non-smokers (MD = 0.52[95% CI, −0.60:1.63], p = 0.36) and observed heterogeneity in the studies (I2 = 68%).
Conclusion: This study demonstrates a correlation between smoking and serum leptin/ghrelin levels, which explains smoking’s effect on body weight.
Systematic review registration: https://www.crd.york.ac.uk/ prospero/display_record.php, identifier (Record ID=326680).
Introduction
Smoking is a major global health problem that has been associated with various metabolic and neurologic effects on appetite (1, 2). Smoking has been linked to the development of central obesity, resistance to insulin, low levels of high-density lipoprotein, and type 2 diabetes (3, 4). In the short term, the nicotine found in cigarettes can increase energy expenditure and decrease appetite, which may explain why smokers generally have lower body weight than non-smokers. However, when smokers quit, they often experience weight gain. On the other hand, individuals who smoke heavily tend to have higher body weights than light smokers or non-smokers (5). Leptin is a hormone primarily secreted by adipocytes in white adipose tissue and helps regulate energy balance by decreasing hunger (6). Leptin controls hunger and aids in maintaining a healthy weight. When it functions properly, it regulates the balance between the amount of food consumed and the amount of fat stored in the body. High levels of leptin signal to the brain that the fat cells are saturated, thereby reducing hunger (7). However, the relationship between smoking and serum leptin is still debated. Some studies have found that smoking has been linked to increased leptin levels, while smoking cessation has been associated with decreased leptin levels (8–10) and some other studies showed the reverse (11, 12). Ghrelin, another hormone primarily secreted by the stomach, regulates energy balance, metabolism, and gastrointestinal function. Ghrelin stimulates hunger and promotes lipogenesis (13, 14). Studies have shown that smoking cigarettes can increase ghrelin levels in the blood, and smoking cessation causes it to decrease (15–17).
To date, no systematic review or meta-analysis has attempted to assess the effect of smoking on plasma leptin and ghrelin concentrations. The purpose of this study is to determine the effect of cigarette smoking on plasma levels of leptin and ghrelin, thus adding a cornerstone to the evidence about understanding the effect of smoking cigarettes on body weight.
Methods
This systematic review and meta-analysis were conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (18) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (19). The study protocol was registered in PROSPERO (CRD42022326680), and any deviations from the protocol were reported in the Methods section.
Eligibility criteria
Inclusion criteria
a. Original articles that report on the effect of smoking on plasma leptin and ghrelin levels.
b. Clinical studies, including randomized controlled trials and observational studies such as cohort and case-control studies.
c. Adult subjects (18 years and above) were included in the studies.
d. Studies that used nicotine/smoking as the intervention and measured serum ghrelin and leptin levels as the outcome measures.
Exclusion criteria
a. Studies with unclear data or missing information.
b. Studies that used other food supplements containing nicotine as the intervention.
c. Short-term studies (duration of less than 1 week) were excluded from the analysis.
d. Studies did not report the effect of smoking on plasma leptin and ghrelin levels.
e. Studies that did not report the data necessary for the meta-analysis, such as means and standard deviations for ghrelin and leptin levels.
Information sources and search strategy
We thoroughly searched four internet databases (PubMed, Scopus, Web of Science, and Ovid). The search strategy for each database is presented in (Supplementary material). The listed studies’ references were carefully examined for any possible eligible articles. The search was carried out by two independent reviewers (NS and AS).
Selection process
Endnote (Clarivate Analytics, United States) was used to get rid of duplicates, and then the returned references went through two steps of screening:
Step 1: title and abstract screening.
The first step involved screening the titles and abstracts of all papers retrieved by the search strategy for relevance by two independent reviewers (RD and AS). Studies that did not meet the inclusion criteria or had any exclusion criteria were discarded.
Step 2: full-text screening.
The full texts of the remaining studies were then downloaded and reviewed in detail by two independent reviewers (RD and AS). These reviewers evaluated the eligibility of the studies based on the predefined inclusion and exclusion criteria. Any disagreements on the eligibility of a particular study were resolved through discussion between the reviewers or by a third reviewer (NS).
Data extraction
After the eligibility of the studies was determined, the reviewers extracted relevant data from the included studies. This data included information such as study design, sample size, population characteristics, and the main outcome measures. Three reviewers independently performed data extraction (RD, SS, and AM), and any discrepancies were resolved through discussion or by a third reviewer (NS).
Quality assessment of the included studies
The risk of bias of the included studies in the meta-analysis was assessed using the Newcastle-Ottawa Scale (NOS) (20) by two independent authors (MH and SS). The NOS is a tool commonly used to assess the quality of observational studies. The guidelines for using the NOS were followed for each study design, including case-control, cohort, and cross-sectional studies, without any modifications to the scale. The NOS evaluates the risk of bias in three domains: selection, comparability, and exposure/outcome assessment.
For each domain, the studies were given a certain number of stars based on the level of quality. The final scores were then converted to The Agency for Healthcare Research and Quality (AHRQ) standards of good, fair, and poor quality. One or two stars in the comparability domain, three or four stars in the selection domain, and two or three stars in the exposure/outcome domain were given to studies to indicate their high quality. If a study earned two stars for selection, one or two stars for comparability, and two or three stars for exposure/outcome, it was deemed to be of fair quality. No or one star in the domain of selection, the domain of comparability, and the area of exposure/outcome indicated a low-quality study (20).
Data analysis
The meta-analysis utilized RevMan 5.4 for calculation of weighted mean differences (WMDs) and standard errors (SEs) in serum ghrelin and leptin levels. WMDs, representing the difference in mean hormone levels between smokers and non-smokers, were weighted by the inverse of variance. SEs were computed as the square root of within-study and between-study variance sums. Random-effects model, accommodating heterogeneous studies, assumed a normal distribution of true effects with a mean and variance. Mean differences (MD) as the effect size were calculated for uniformity. Heterogeneity was assessed via chi-square test and I-squared statistic, considering significance at p < 0.05 and I2 > 50%. Funnel plots and Egger’s regression/Begg and Mazumdar tests were used for publication bias analysis. Subgroup analysis explored heterogeneity sources, categorizing studies by design, gender, health status, and pregnancy. Significance was set at p < 0.05 for chi-square tests in subgroup analysis. The studies with small numbers of effects did not show different treatment effects than large ones even after performing Hartung-Knapp adjustment (21).
Results
Literature search results
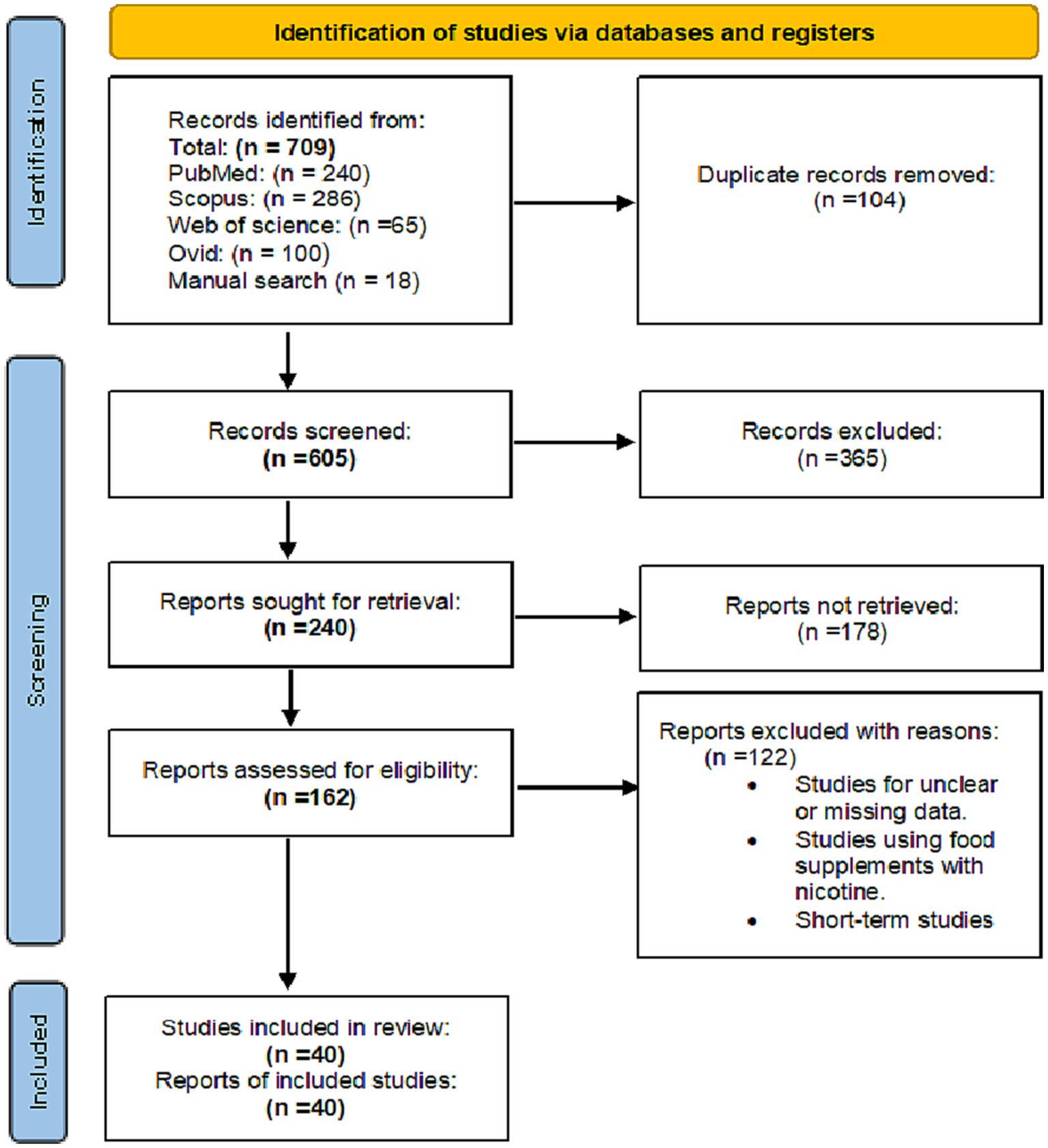
A total of 709 records were identified through searching the PubMed, Google Scholar, Scopus, Web of Science and Ovid databases. After removing duplicates, 365 records were excluded for being unrelated to the study topic. A total of 240 records were screened for relevance, and 178 were excluded after the full-text review. A total of 162 full-text articles were assessed for eligibility, and 40 studies were included in the qualitative and quantitative (meta-analysis) synthesis, with 40 studies being included in the meta-analysis (Figure 1).
Characteristics of the included studies
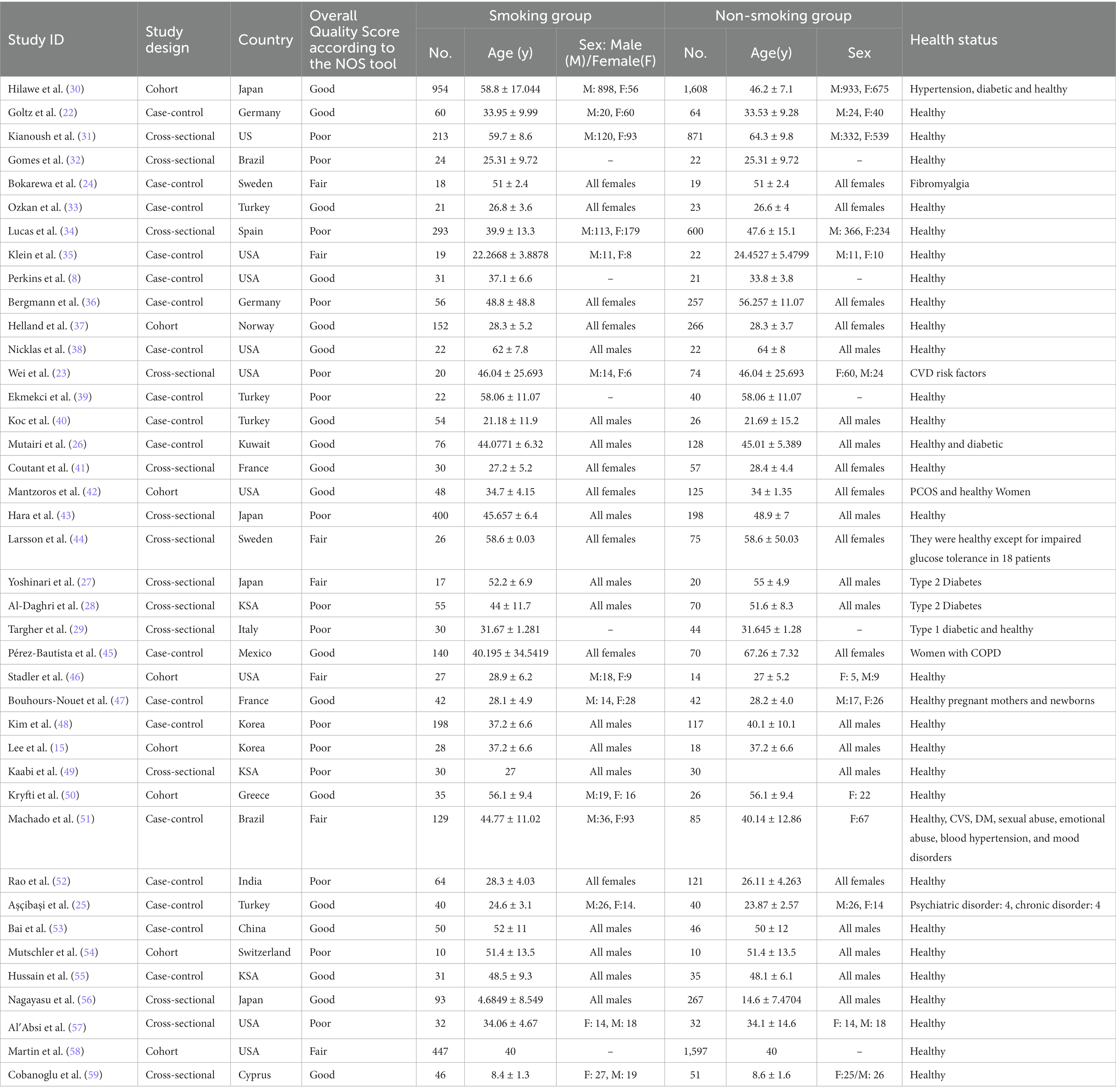
This meta-analysis included 40 studies: 18 case-control studies, 14 cross-sectional studies, and 8 cohort studies. The included studies have a pooled total of 11, 336 patients: 4,083 of whom were smokers with a mean age of 43.39 ± 0.46 years and 60% were males, while 7,253 were non-smokers with a mean age of 40 ± 0.47 and 49.7% were males. Most papers included healthy individuals except Goltz et al. (22) and Wei et al. (23) included patients with CVD risk factors, Bokarewa et al. (24) studied fibromyalgia patients, Aşçibaşi et al. (25) studied patients with psychiatric and chronic diseases and 4 papers [Mutairi et al. (26), Yoshinari et al. (27), Al-Daghri et al. (28), and Targher et al. (29)] included diabetic participants (Table 1). According to the NOS tool, eighteen of the included studies had good quality, seven had fair quality, and fifteen had poor quality. The overall bias is present in Table 1 and the details for each domain are present in (Supplementary material).
Serum leptin levels in smokers and non-smokers
Study designs-based subgroup analysis
A total of 35 studies reported serum leptin in smoking and non-smoking groups. The overall effect was significant, with a mean difference (MD) of −1.92[95%CI, −2.63:1.20] and p-value of 0.00001. Subgroup analysis by study design (cohort, case-control, and cross-sectional) revealed significant differences with a Chi2 of 11.31, df of 2, and a p value of 0.003. The heterogeneity within the subgroups was high, with an I2 of 82.3% (Figure 2).
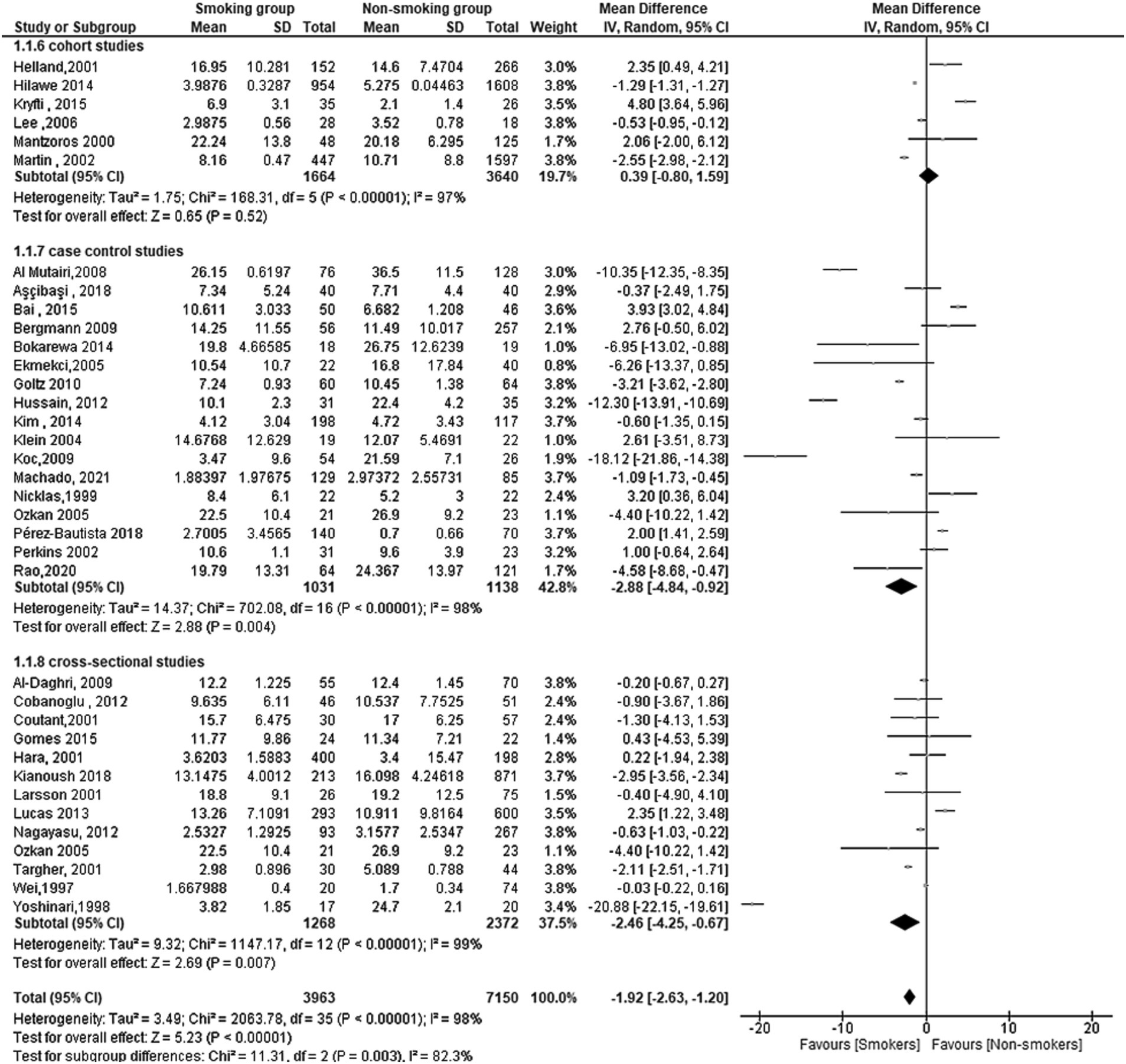
Figure 2. Forest plot diagram of serum leptin concentration between smokers and non-smokers according to the study designs.
For the cohort studies, the overall effect was not significant (MD = 0.39[95% CI; −0.80:1.59], p = 0.52) with high heterogeneity (Tau2 = 1.75, Chi2 = 168.31 df = 5 (p < 0.00001); I2 = 97%).
For the case-control studies, the overall effect was significant (MD = −2.88[95% CI; −4.84: −0.92], p = 0.004) with high heterogeneity (Tau2 = 14.37, Chi2 = 702.08, df = 16 (p < 0.00001); I2 = 98%).
Finally, for the cross-sectional studies, the overall effect was significant (MD = −2.46[95% CI; −4.25: −0.67], p = 0.007) with high heterogeneity (Tau2 = 9.32; Chi2 = 1147.17, df = 12 (p < 0.00001); I2 = 99%).
Risk of bias results
In summary, this meta-analysis found a significant overall difference in serum leptin levels between smoking and non-smoking groups, as the smoking group had a lower level of leptin, with subgroup analysis revealing significant differences by study design. Moreover, the heterogeneity within the subgroups was high (Figure 3).
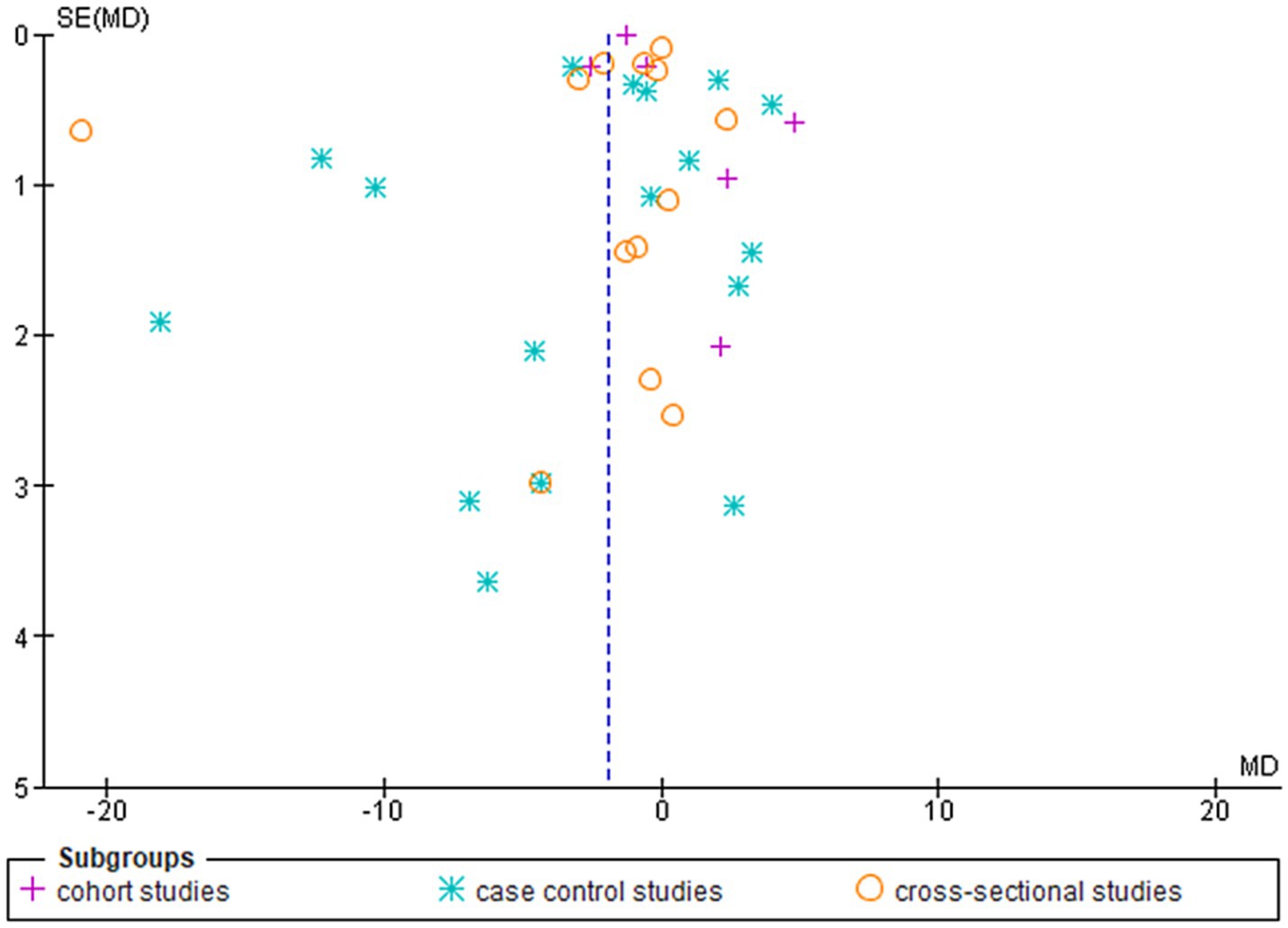
Figure 3. Shows the funnel plot investigating the publication bias among the studies in reporting serum leptin with subgrouping according to the study design.
Sex-based subgroup analysis
The results of the meta-analysis show that there is a significant difference in mean difference between smoking and non-smoking groups. So, subgroup analysis was conducted to examine the effect of gender on this relationship.
For males, the test for overall effect (MD = −5.75[95% CI; −8.73: −2.77], p = 0.0002) showed a strong association between smoking and leptin levels, with lower levels of leptin in the smoking men group. Heterogeneity was also high among the studies included in this subgroup, as indicated by Tau2 = 22.43, Chi2 = 1421.21, df = 9 (p < 0.00001) and I2 = 99%.
In contrast, the overall effect for Females was not statistically significant (MD = −3.04[95% CI; −6.6:0.54], p = 0.10). Heterogeneity was also high among the studies included in this subgroup, as indicated by Tau2 = 26.02, Chi2 = 132.57, df = 8 (p < 0.00001) and I2 = 94%. This subgroup showed also a lower level of leptin in the smoking women group despite the non-statistically significant difference (Figure 4).
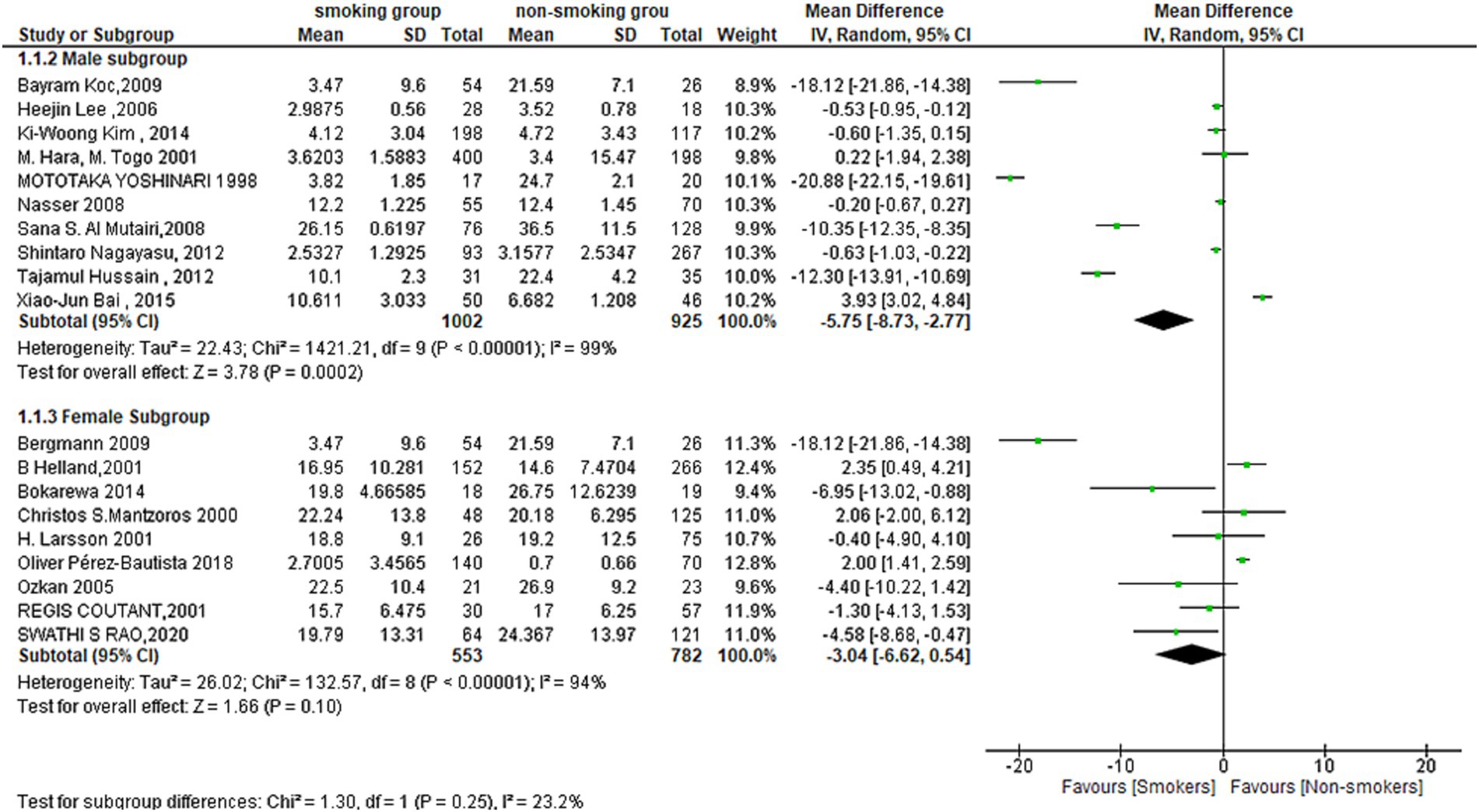
Figure 4. Forest plot diagram of serum leptin concentration between smokers and non-smokers according to the sex.
Figure 5 shows the funnel plot investigating the publication bias among the studies in reporting serum leptin with subgrouping according to sex.
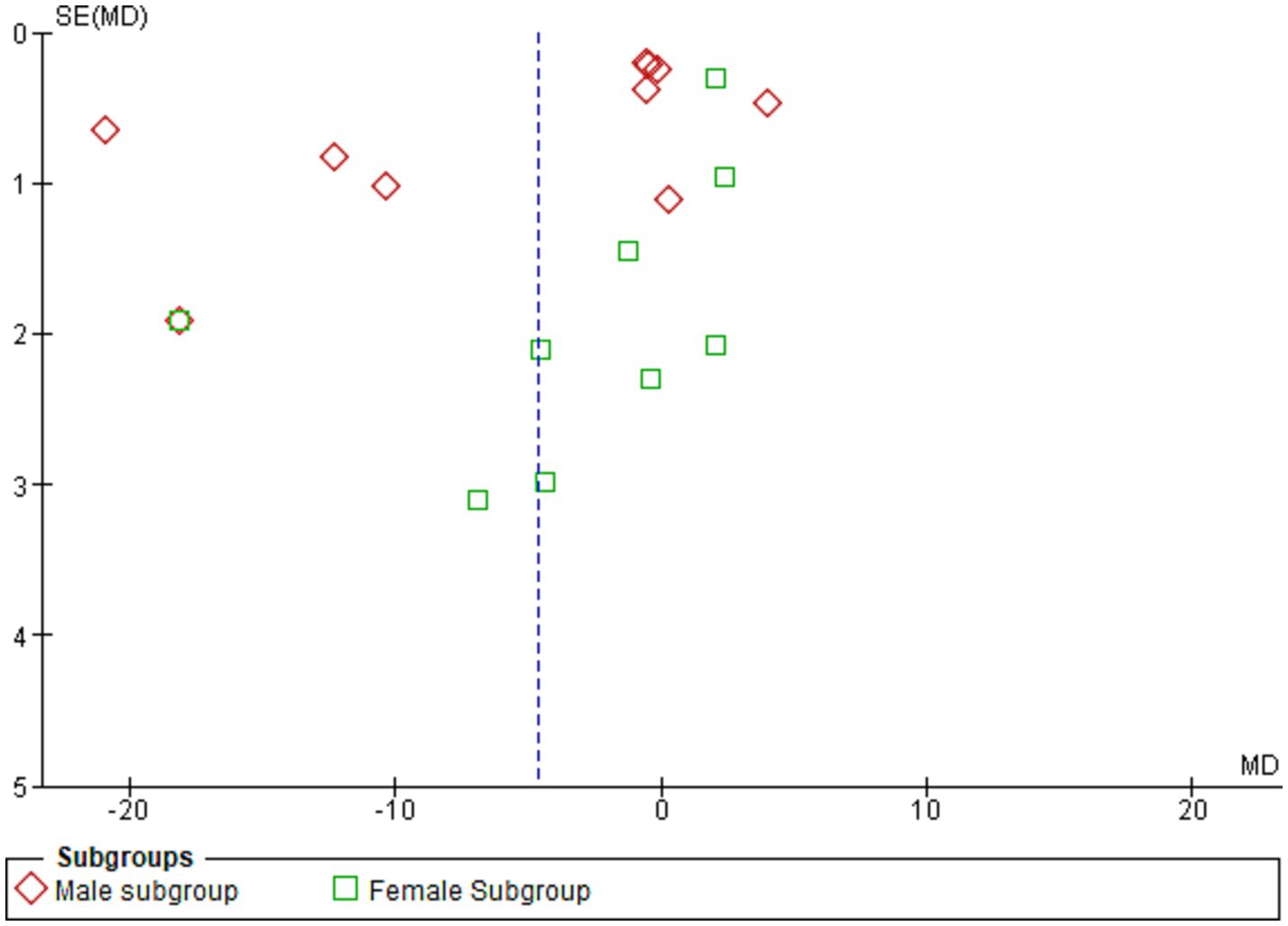
Figure 5. Shows the funnel plot investigating the publication bias among the studies in reporting serum leptin with subgrouping according to sex.
Other subgroups
The results of subgroup analysis using (healthy, pregnant, diabetic and CVD subgroups) showed that there is a statistically significant difference in leptin serum concentration between smokers and non-smokers in the healthy subgroup (MD = −1.74[95% CI; −03.13: −0.35], p = 0.01) and diabetic subgroup (MD = −7.69[95% CI; −1.64: −0.73], p = 0.03). However, there was no statistically significant difference in the pregnant subgroup (MD = −0.69[95% CI; −2.88:4.25], p = 0.71) or the subgroup with cardiovascular disease (MD = −2.09[95% CI; −7.83: −3.65], p = 0.48). The meta-analysis also found high levels of heterogeneity in the healthy subgroup (I2 = 97%), diabetic subgroup (I2 = 100%), and subgroup with cardiovascular disease (I2 = 66%) (Figure 6).
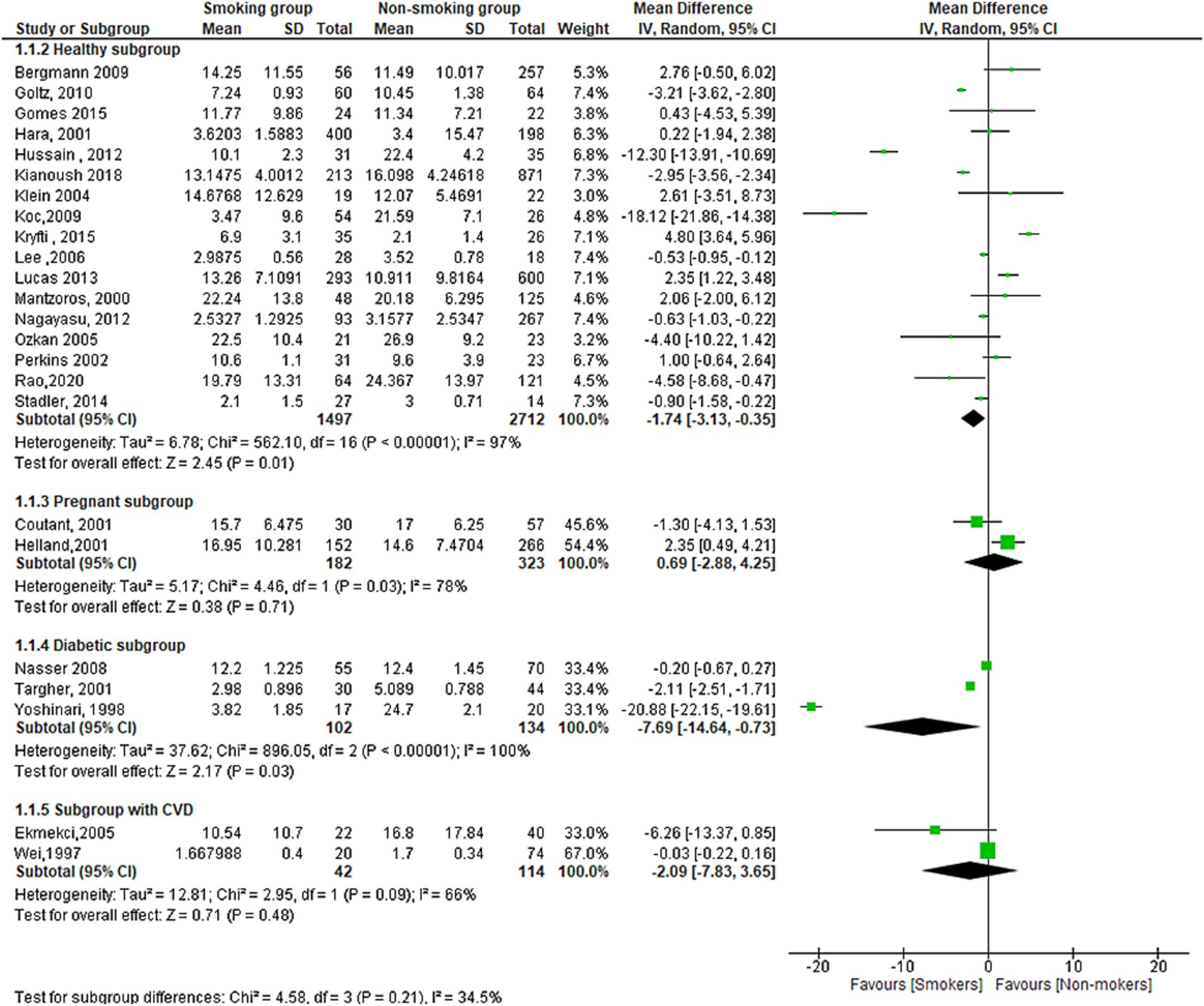
Figure 6. Forest plot diagram of serum leptin concentration between smokers and non-smokers according to the healthy status, pregnancy, diabetic, and CVD groups.
Figure 7 shows the funnel plot investigating the publication bias among the studies in reporting serum leptin with subgrouping according to the health condition.
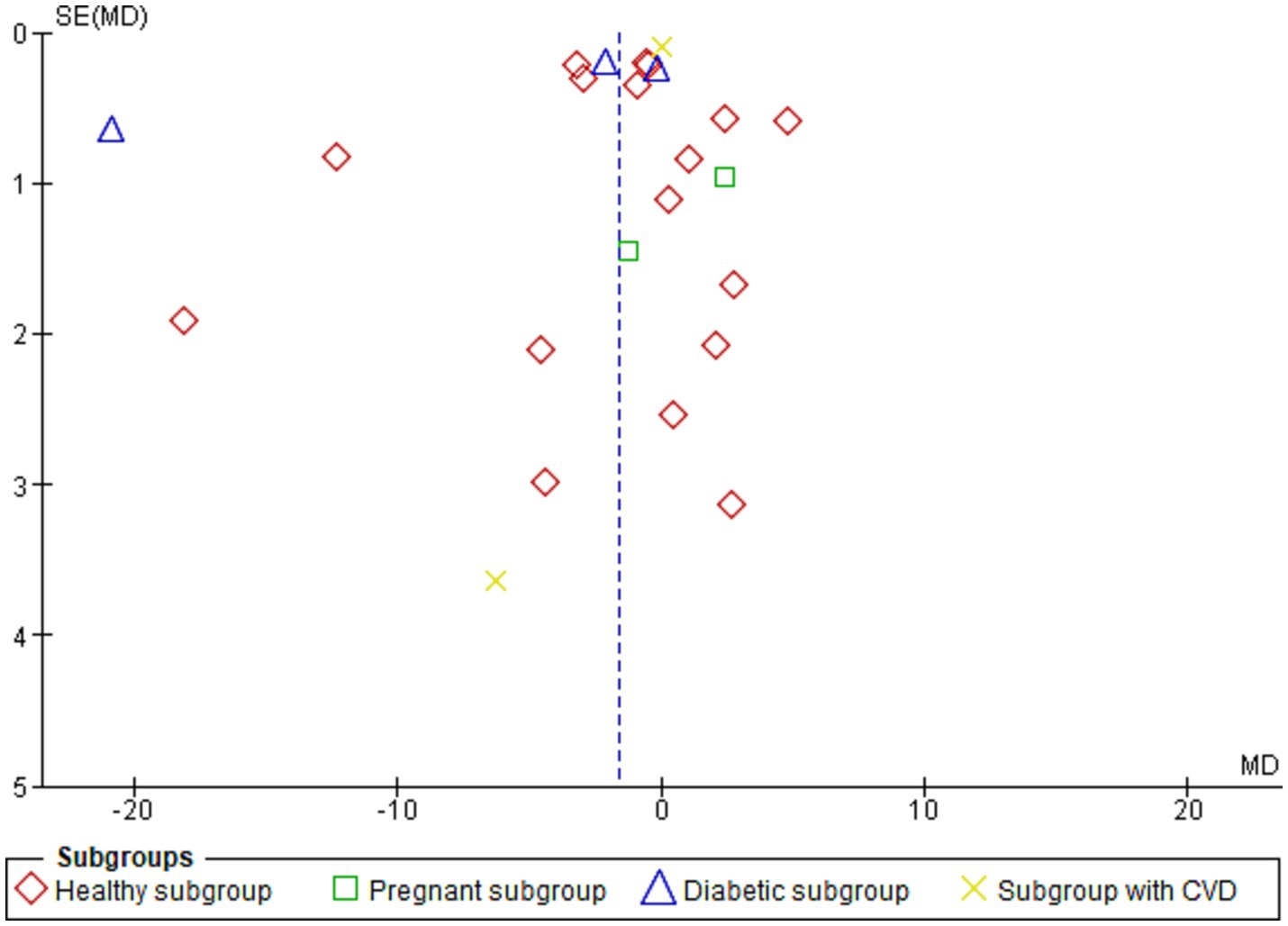
Figure 7. Shows the funnel plot investigating the publication bias among the studies in reporting serum leptin with subgrouping according to the health condition.
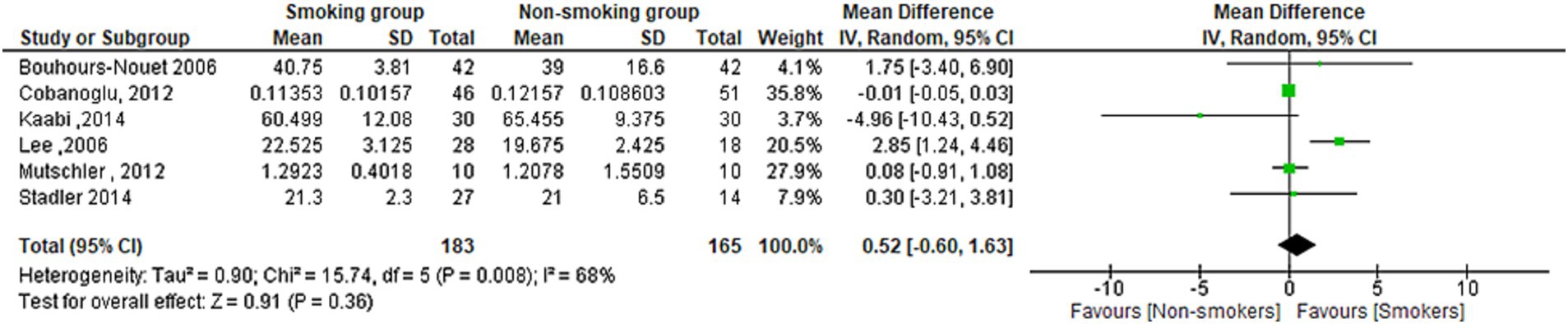
Serum ghrelin levels in smokers and non-smokers
The meta-analysis found no statistically significant difference in Ghrelin serum concentration between smokers and non-smokers (MD = 0.52[95% CI; −0.60:1.63], p = 0.36). Heterogeneity was observed in the studies (I2 = 68%) (Figure 8).
Discussion
Significance of the study
To our knowledge, this is the first comprehensive systematic review and meta-analysis investigating and summarizing the impact of smoking on leptin and ghrelin levels. This study aimed to address the current discrepancy in the literature regarding the specific impact of smoking on serum levels of leptin and ghrelin, hormones known to play a crucial role in regulating body weight with the ultimate goal of furthering our understanding of the underlying mechanisms that contribute to obesity (60).
Summary of findings
The meta-analysis included 40 studies with a pooled total of 11,363 patients. The results showed a significant overall difference in serum leptin levels between smoking and non-smoking groups, as the serum leptin was lower in the smoking group. However, when the analysis was stratified by study design, the results varied. The case-control and cross-sectional studies showed a significant difference but not the cohort studies. Both subgroups indicated a lower leptin level in the smoking group. The subgroup difference was significant, which suggests that the difference in serum leptin levels between smoking and non-smoking groups may be influenced by the study design used. The subgrouping according to the sex revealed a significant difference in the male subgroup showing lower leptin in the smoking men compared to the non-smoking men. There was also a lower level of leptin in the smoking women group despite the non-statistically significant difference. The subgrouping according to the health condition of the population investigated revealed a significantly lower leptin level in the healthy and diabetic populations but not for pregnant and individuals with CVDs. On the other hand, no significant difference was found in the term of serum ghrelin but only six studies reported it.
Explanation of the findings
Obesity, the relapsing, clinical syndrome, is one of the biggest worldwide health issues facing the world today (61–63). Obesity is characterized by a disrupted energy balance brought on by a high food intake in comparison to poor energy expenditure (64). Obesity is caused by a complex interplay of genetic, environmental, behavioural, and psychological variables; therefore, it is a multi-factorial condition (65). Weight is controlled by the hypothalamus’ weight-regulating centre through both long- and short-acting peripheral signals (66–69). Long-term peripheral impulses provide details regarding adipose tissue distribution. Leptin, a satiety hormone released by adipocytes, and insulin, a hormone secreted by the pancreas, are examples of these adiposity signals (70, 71). In response to eating and fasting, enteroendocrine cells emit a variety of peptide hormones, which are largely responsible for the regulation of energy balance through short-term peripheral signals (67). Gut hormones may be broadly categorized as hunger, orexigenic hormones, satiety, and anorexigenic hormones. Ghrelin is the primary known hormone secreted from the stomach in response to fasting, triggering the commencement of eating and hence controlling meal frequency (72). Obesity may result from disturbed control of gut hormone release, which may impact energy homeostasis (73). Studies found that obese individuals had a higher level of leptin (74–77) and lower ghrelin levels (77–79). Ardeshiripur et al. found that Regarding energy homeostasis and appetite control, these two ghrelin variants have different impacts. Food intake has been demonstrated to be inhibited by desacylghrelin, whereas acylghrelin is known to promote appetite and food intake. Moreover, acylghrelin has been connected to higher levels of fat and body weight, whereas desacylghrelin has been linked to lower levels of these parameters (80).
The relationship between obesity and smoking is still conflicting. Many studies showed that smokers often had lower body mass index (BMIs) than non-smokers (81–83). It was explained by the effects of nicotine on the brain, which suppresses appetite and shortens mealtimes, which leads to decreased food intake and weight loss (84, 85). According to our meta-analysis, we have established the cornerstone of the literature on smoking’s effects on leptin and ghrelin hormones and their effects on body weight.
The impact of smoking on appetite regulation is multifaceted, and it can have a variety of short- and long-term consequences on cravings and eating behaviors. While smoking is frequently associated with appetite suppression, the long-term effects can be quite different, leading to increased cravings and emotional eating. Smoking can function as an appetite reducer in the short term. Nicotine, the addictive ingredient in cigarettes, can temporarily suppress hunger and enhance metabolic rate (86, 87). This can lead to reduced food intake and possible weight loss. Many smokers claim that smoking helps them control their appetites or keep their weight in check. However, smoking’s long-term consequences on appetite regulation can be more complicated. Nicotine stimulates the brain’s reward system, resulting in the release of dopamine. a neurotransmitter associated with pleasure and reward. Through this mechanism, smoking can create conditioned responses, where the act of smoking becomes associated with pleasurable feelings (88).
The results of our study revealed that smoking lowers leptin levels, which leads to a decreased appetite, decreasing food consumption frequency and amount, and thus causing weight loss (22, 35, 71). Our finding is agreeing with the previous studies that found an inverse relationship between smoking and body BMI (84, 85). However, it is evidenced that smoking is strongly associated with increased waist circumference and abdominal obesity despite its negative effect on the body mass index (81, 82, 89–91). Leptin is known to be positively correlated with waist circumference (92, 93), presenting a complex issue that requires more research in a variety of health populations to identify the underlying factors.
The subgroup analysis by sex revealed a significant difference for males but not for females. This suggests that the effect of smoking on leptin levels may be different between men and women. This difference in the effect of smoking on leptin levels between males and females may be due to the different hormonal milieu in men and women, as well as differences in smoking behaviour and patterns (94–97). The underlying heterogeneity can be explained by the differences among the included studies in terms of the study design, differences in sample size and population characteristics. For example, the effect of smoking on hormone levels may vary among different ethnic groups or between men and women (98, 99), as discussed previously. Additionally, the degree of obesity or the presence of other health conditions may also play a role in the relationship between smoking and hormone levels.
The relationship between smoking and ghrelin levels is also mixed. Some studies have found no difference in plasma ghrelin levels between cigarette smokers and non-smokers (54, 100), while others have found that smokers had considerably greater plasma concentrations of acetylated ghrelin than non-smokers (101, 102).
The meta-analysis did not find a significant difference in ghrelin levels between smoking and non-smoking groups. The lack of significance in the meta-analysis may be due to the limited number of studies that have investigated the effect of smoking on ghrelin levels.
Strengths
This study is a comprehensive systematic review and meta-analysis investigating the impact of smoking on leptin and ghrelin levels. The study utilized a thorough literature search and inclusion criteria to select 40 studies with a pooled total of 11,336 patients for analysis. The meta-analysis found a significant overall difference in serum leptin levels between smoking and non-smoking groups, with lower levels of leptin in the smoking group. Subgroup analysis by study design, sex, and health status also revealed significant differences. Additionally, the study is the first to investigate and summarize the impact of smoking on both leptin and ghrelin levels, providing a comprehensive overview of the topic and furthering the understanding of the underlying mechanisms that contribute to obesity.
Limitations
This study has several limitations. Firstly, the study is based on observational studies which may be subject to bias and confounding. Secondly, the studies included in the meta-analysis were from different geographical locations, which may have led to variations in study populations and measurement methods. Thirdly, the meta-analysis is based on pooled data from multiple studies, which may have led to high heterogeneity within the subgroups and may have affected the overall results. Fourthly, the study did not take into account other factors that may affect leptin and ghrelin levels, such as diet, physical activity, and medication use, which may have led to an underestimation of the effect of smoking on these hormones. Finally, the study did not consider the duration of smoking and whether it plays a role in the relationship between smoking and hormone levels.
Conclusion
The study included 40 studies with a pooled total of 11,336 patients, and the results showed a significant overall difference in serum leptin levels between smoking and non-smoking groups, with the smoking group having lower levels of leptin. The study also found no statistically significant difference in Ghrelin serum concentration between smokers and non-smokers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
NS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ASh: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. RD: Data curation, Writing – review & editing. ASa: Data curation, Writing – review & editing. OA: Writing – original draft, Writing – review & editing. SS: Data curation, Writing – review & editing. MH: Writing – review & editing. AR: Writing – review & editing. MM: Writing – review & editing. AN: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Open Access funding provided by the Qatar National Library.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1296764/full#supplementary-material
References
1. Shimokata, H, Muller, DC, and Andres, R. Studies in the distribution of body fat: III. Effects of cigarette smoking. JAMA. (1989) 261:1169. doi: 10.1001/jama.1989.03420080089037
2. Peterson, DI, Lonergan, LH, and Hardinge, MG. Smoking and taste perception. Crit Rev Food Sci Nutr. (2013) 16:219–22. doi: 10.1080/00039896196810665047
3. Liu, T, Chen, WQ, David, SP, Tyndale, RF, Wang, H, Chen, YM, et al. Interaction between heavy smoking and CYP2A6 genotypes on type 2 diabetes and its possible pathways. Eur J Endocrinol. (2011) 165:961–7. doi: 10.1530/EJE-11-0596
4. Clair, C, Chiolero, A, Faeh, D, Cornuz, J, Marques-Vidal, P, Paccaud, F, et al. Dose-dependent positive association between cigarette smoking, abdominal obesity and body fat: cross-sectional data from a population-based survey. BMC Public Health. (2011) 11:23. doi: 10.1186/1471-2458-11-23
5. Chiolero, A, Faeh, D, Paccaud, F, and Cornuz, J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. (2008) 87:801–9. doi: 10.1093/ajcn/87.4.801
6. Hariri, M, Ghiasvand, R, Shiranian, A, Askari, G, Iraj, B, and Salehi-Abargouei, A. Does omega-3 fatty acids supplementation affect circulating leptin levels? A systematic review and meta-analysis on randomized controlled clinical trials. Clin Endocrinol. (2015) 82:221–8. doi: 10.1111/cen.12508
7. Kelesidis, T, Kelesidis, I, Chou, S, and Mantzoros, CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. (2010) 152:93. doi: 10.7326/0003-4819-152-2-201001190-00008
8. Perkins, KA, and Fonte, C. Effects of smoking status and smoking cessation on leptin levels. Nicotine Tob Res. (2002) 4:459–66. doi: 10.1080/1462220021000018434
9. Eliasson, B, and Smith, U. Leptin levels in smokers and long-term users of nicotine gum. Eur J Clin Invest. (1999) 29:145–52. doi: 10.1046/j.1365-2362.1999.00420.x
10. Miell, JP, Englaro, P, and Blum, WF. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm Metab Res. (1996) 28:704–7. doi: 10.1055/s-2007-979882
11. Gonseth, S, Locatelli, I, Bize, R, Nusslé, S, Clair, C, Pralong, F, et al. Leptin and smoking cessation: secondary analyses of a randomized controlled trial assessing physical activity as an aid for smoking cessation. BMC Int Health Hum Rights. (2014) 14:1–8. doi: 10.1186/1471-2458-14-911
12. Suhaimi, MZ, Sanip, Z, Mohamed, HJJ, and Yusoff, HM. Leptin and calorie intake among different nicotine dependent groups. Ann Saudi Med. (2016) 36:404–8. doi: 10.5144/0256-4947.2016.404
13. Kojima, M, Hosoda, H, Date, Y, Nakazato, M, Matsuo, H, and Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. (1999) 402:656–60. doi: 10.1038/45230
14. Nakazato, M, Murakami, N, Date, Y, Kojima, M, Matsuo, H, Kangawa, K, et al. A role for ghrelin in the central regulation of feeding. Nature. (2001) 409:194–8. doi: 10.1038/35051587
15. Lee, H, Joe, KH, Kim, W, Park, J, Lee, DH, Sung, KW, et al. Increased leptin and decreased ghrelin level after smoking cessation. Neurosci Lett. (2006) 409:47–51. doi: 10.1016/j.neulet.2006.09.013
16. Bouros, D, Tzouvelekis, A, Anevlavis, S, Doris, M, Tryfon, S, Froudarakis, M, et al. Smoking acutely increases plasma ghrelin concentrations. Clin Chem. (2006) 52:777–8. doi: 10.1373/clinchem.2005.065243
17. Reseland, JE, Mundal, HH, Hollung, K, Haugen, F, Zahid, N, Anderssen, SA, et al. Cigarette smoking may reduce plasma leptin concentration via catecholamines. Prostaglandins Leukot Essent Fatty Acids. (2005) 73:43–9. doi: 10.1016/j.plefa.2005.04.006
18. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated updated August 2023) Cochrane, (2023)
19. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372, 36:71. doi: 10.1136/bmj.n71
20. Wells, G, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al., The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (2012)
21. Tzeng, IS. A practical approach in refining binary outcome for treatment effect of COVID-19 according to geographical diversity. Trop Med Infect Dis. (2023) 8:83. doi: 10.3390/tropicalmed8020083
22. von der Goltz, C, Koopmann, A, Dinter, C, Richter, A, Rockenbach, C, Grosshans, M, et al. Orexin and leptin are associated with nicotine craving: a link between smoking, appetite and reward. Psychoneuroendocrinology. (2010) 35:570–7. doi: 10.1016/j.psyneuen.2009.09.005
23. Wei, M, Stern, MP, and Haffner, SM. Serum leptin levels in Mexican Americans and non-hispanic whites: association with body mass index and cigarette smoking. Ann Epidemiol. (1997) 7:81–6. doi: 10.1016/S1047-2797(96)00114-7
24. Bokarewa, MI, Erlandsson, MC, Bjersing, J, Dehlin, M, and Mannerkorpi, K. Smoking is associated with reduced leptin and neuropeptide Y levels and higher pain experience in patients with fibromyalgia. Mediators Inflamm. (2014) 2014:1–8. doi: 10.1155/2014/627041
25. Aşçibaşi, K, Deveci, A, Cengiz Özyurt, B, Oran Pirinçcioğlu, A, and Taneli, F. Relationships between nicotine craving, orexin-leptin levels and temperament character traits among non-treatment seeking health professionals. Psychiatr Clin Psychopharmacol. (2018) 28:386–93. doi: 10.1080/24750573.2018.1458433
26. al Mutairi, SS, Mojiminiyi, OA, Shihab-Eldeen, AA, al Sharafi, A, and Abdella, N. Effect of smoking habit on circulating adipokines in diabetic and non-diabetic subjects. Ann Nutr Metab. (2008) 52:329–34. doi: 10.1159/000151487
27. Yoshinari, M, Wakisaka, M, and Fujishima, M. Serum leptin levels in smokers with type 2 diabetes. Diabetes Care. (1998) 21:516–7. doi: 10.2337/diacare.21.4.516
28. Al-Daghri, NM, Al-Attas, OS, Hussain, T, Sabico, S, and Bamakhramah, A. Altered levels of adipocytokines in type 2 diabetic cigarette smokers. Diabetes Res Clin Pract. (2009) 83:e37–9. doi: 10.1016/j.diabres.2008.11.013
29. Targher, G, Zenari, L, Faccini, G, Falezza, G, Muggeo, M, and Zoppini, G. Serum leptin concentrations in young smokers with type 1 diabetes. Diabetes Care. (2001) 24:793–4. doi: 10.2337/diacare.24.4.793
30. Hilawe, EH, Yatsuya, H, Li, Y, Uemura, M, Wang, C, Chiang, C, et al. Smoking and diabetes: is the association mediated by adiponectin, leptin, or C-reactive protein? J Epidemiol. (2015) 25:99–109. doi: 10.2188/jea.JE20140055
31. Kianoush, S, DeFilippis, AP, Rodriguez, CJ, al Rifai, M, Benjamin, EJ, Hall, ME, et al. Race/ethnicity-specific associations between smoking, serum leptin, and abdominal fat: the multi-ethnic study of atherosclerosis. Ethn Dis. (2018) 28:531–8. doi: 10.18865/ed.28.4.531
32. Gomes, A S, Toffolo, MCF, Keulen,, Castro e Silva, FM, Ferreira, AP, Luquetti, SCPD, et al., Influence of the leptin and cortisol levels on craving and smoking cessation. Psychiatr Res Neuroimaging (2015); 229:126–132, doi: 10.1016/j.psychres.2015.07.060
33. Ozkan, B, Ermis, B, Tastekin, A, Doneray, H, Yildirim, A, and Ors, R. Effect of smoking on neonatal and maternal serum and breast milk leptin levels. Endocr Res. (2005) 31:177–83. doi: 10.1080/07435800500371748
34. Lucas, A, Granada, ML, Olaizola, I, Castell, C, Julián, MT, Pellitero, S, et al. Leptin and thyrotropin relationship is modulated by smoking status in euthyroid subjects. Thyroid. (2013) 23:964–70. doi: 10.1089/thy.2012.0506
35. Klein, LC, Corwin, EJ, and Ceballos, RM. Leptin, hunger, and body weight: influence of gender, tobacco smoking, and smoking abstinence. Addict Behav. (2004) 29:921–7. doi: 10.1016/j.addbeh.2004.02.023
36. Bergmann, S, and Siekmeier, R. Influence of smoking and body weight on adipokines in middle aged women. Eur J Med Res. (2009) 14:21–6. doi: 10.1186/2047-783X-14-S4-21
37. Helland, IB, Reseland, JE, Saugstad, OD, and Drevon, CA. Smoking related to plasma leptin concentration in pregnant women and their newborn infants. Acta Paediatr. (2001) 90:282–7. doi: 10.1111/j.1651-2227.2001.tb00305.x
38. Nicklas, BJ, Tomoyasu, N, Muir, J, and Goldberq, AP. Effects of cigarette smoking and its cessation on body weight and plasma leptin levels. Metabolism. (1999) 48:804–8. doi: 10.1016/S0026-0495(99)90183-X
39. Ekmekci, H, Ekmekci, OB, Sonmez, H, Ozturk, Z, Domanic, N, and Kokoglu, E. Evaluation of fibronectin, vitronectin, and leptin levels in coronary artery disease: impacts on thrombosis and thrombolysis. Clin Appl Thromb Hemost. (2005) 11:63–70. doi: 10.1177/107602960501100107
40. Koc, B, Bulucu, F, Karadurmus, N, and Şahin, M. Lower leptin levels in young non-obese male smokers than non-smokers. Ups J Med Sci. (2009) 114:165–9. doi: 10.1080/03009730902761631
41. Coutant, R, de Casson, FB, Douay, O, Mathieu, E, Rouleau, S, Beringue, F, et al. Relationships between placental GH concentration and maternal smoking, newborn gender, and maternal leptin: possible implications for birth weight. J Clin Endocrinol Metab. (2001) 86:4854–9. doi: 10.1210/jcem.86.10.7971
42. Mantzoros, CS, Cramer, DW, Liberman, RF, and Barbieri, RL. Predictive value of serum and follicular fluid leptin concentrations during assisted reproductive cycles in normal women and in women with the polycystic ovarian syndrome. Human Reproduc. (2000) 15:539–44. doi: 10.1093/humrep/15.3.539
43. Togo, M, Hashimoto, Y, Futamura, A, Tsukamoto, K, Satoh, H, Hara, M, et al. Relationship between the serum level of leptin and life-style habits in Japanese men. Horm Res. (2000) 54:169–73. doi: 10.1159/000053254
44. Larsson, H, and Ahrén, B. Smoking habits and circulating leptin in postmenopausal non-obese women. Diabetes Obes Metab. (1999) 1:57–9. doi: 10.1046/j.1463-1326.1999.00001.x
45. Pérez-Bautista, O, Montaño, M, Pérez-Padilla, R, Zúñiga-Ramos, J, Camacho-Priego, M, Barrientos-Gutiérrez, T, et al. Women with COPD by biomass show different serum profile of adipokines, incretins, and peptide hormones than smokers. Respir Res. (2018) 19:239. doi: 10.1186/s12931-018-0943-4
46. Stadler, M, Tomann, L, Storka, A, Wolzt, M, Peric, S, Bieglmayer, C, et al. Effects of smoking cessation on β-cell function, insulin sensitivity, body weight, and appetite. Eur J Endocrinol. (2014) 170:219–27. doi: 10.1530/EJE-13-0590
47. Bouhours-Nouet, N, de Casson, FB, Rouleau, S, Douay, O, Mathieu, E, Bouderlique, C, et al. Maternal and cord blood ghrelin in the pregnancies of smoking mothers: possible markers of nutrient availability for the fetus. Horm Res Paediatr. (2006) 66:6–12. doi: 10.1159/000092807
48. Kim, KW, Won, YL, Ko, KS, and Roh, JW. Smoking habits and neuropeptides: adiponectin, brain-derived neurotrophic factor, and leptin levels. J Toxicol Public Health. (2014) 30:91–7. doi: 10.5487/TR.2014.30.2.091
49. Kaabi, YA, and Khalifa, MA. Acute one-cigarette smoking decreases ghrelin hormone in saliva: a pilot study. Int J Endocrinol. (2014) 2014:1–4. doi: 10.1155/2014/575671
50. Kryfti, M, Dimakou, K, Toumbis, M, Daniil, Z, Hatzoglou, C, and Gourgoulianis, KI. Effects of smoking cessation on serum leptin and adiponectin levels. Tob Induc Dis. (2015) 13:7. doi: 10.1186/s12971-015-0054-7
51. Machado, RCBR, Vargas, HO, Zazula, R, Urbano, MR, Verri, WA, Rossaneis, AC, et al. Implications for comorbidities, maternal smoking during pregnancy, and inflammation in current smokers. J Affect Disord Rep. (2021) 6:100249. doi: 10.1016/j.jadr.2021.100249
52. Rao, SS, Preethika, A, Yeldho, DM, Kumar, YS, and Shenoy, RD. Maternal occupational tobacco exposure and newborn umbilical cord serum leptin concentration. Indian Pediatr. (2020) 57:918–21. doi: 10.1007/s13312-020-1995-3
53. Bai, XJ, Fan, LH, He, Y, Ren, J, Xu, W, Liang, Q, et al. Nicotine may affect the secretion of adipokines leptin, resistin, and visfatin through activation of KATP channel. Nutrition. (2016) 32:645–8. doi: 10.1016/j.nut.2015.12.001
54. Mutschler, J, Graf, N, Spanaus, KS, Rössler, W, Hergan, K, Binkert, CA, et al. Circulating ghrelin levels are not associated with craving and withdrawal symptoms in acute nicotine withdrawal. Psychiatr Danub. (2012) 24:229–30.
55. Hussain, T, Al-Daghri, NM, Al-Attas, OS, Draz, HM, Abd Al-Rahman, SH, et al. Plasma neuropeptide Y levels relate cigarette smoking and smoking cessation to body weight regulation. Regul Pept. (2012) 176:22–7. doi: 10.1016/j.regpep.2012.02.005
56. Nagayasu, S, Suzuki, S, Yamashita, A, Taniguchi, A, Fukushima, M, Nakai, Y, et al. Smoking and adipose tissue inflammation suppress leptin expression in Japanese obese males: potential mechanism of resistance to weight loss among Japanese obese smokers. Tob Induc Dis. (2012) 10:3. doi: 10.1186/1617-9625-10-3
57. al’Absi, M, Hooker, S, Fujiwara, K, Kiefer, F, von der Goltz, C, Cragin, T, et al. Circulating leptin levels are associated with increased craving to smoke in abstinent smokers. Pharmacol Biochem Behav. (2011) 97:509–13. doi: 10.1016/j.pbb.2010.10.004
58. Martin, LJ, Cole, SA, Hixson, JE, Mahaney, MC, Czerwinski, SA, Almasy, L, et al. Genotype by smoking interaction for leptin levels in the San Antonio family heart study. Genet Epidemiol. (2002) 22:105–15. doi: 10.1002/gepi.0135
59. Cobanoglu, N, Dalkan, C, Galip, N, Tekguc, H, Uncu, M, and Bahceciler, NN. Is calprotectin a marker of tobacco smoke related inflammation?: a pilot study in children. Inhal Toxicol. (2012) 24:486–91. doi: 10.3109/08958378.2012.693137
60. Aukan, MI, Coutinho, S, Pedersen, SA, Simpson, MR, and Martins, C. Differences in gastrointestinal hormones and appetite ratings between individuals with and without obesity-a systematic review and meta-analysis. Obes Rev. (2023) 24:13531. doi: 10.1111/obr.13531
61. Caleyachetty, R, Barber, TM, Mohammed, NI, Cappuccio, FP, Hardy, R, Mathur, R, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. (2021) 9:419–26. doi: 10.1016/S2213-8587(21)00088-7
62. Bray, GA, Kim, KK, and Wilding, JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the world obesity federation. Obesity Rev. (2017) 18:715–23. doi: 10.1111/obr.12551
63. Ng, M, Fleming, T, Robinson, M, Thomson, B, Graetz, N, Margono, C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
64. Spiegelman, BM, and Flier, JS. Obesity and the regulation of energy balance. Cell. (2001) 104:531–43. doi: 10.1016/S0092-8674(01)00240-9
65. Weinsier, RL, Hunter, GR, Heini, AF, Goran, MI, and Sell, SM. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med. (1998) 105:145–50. doi: 10.1016/S0002-9343(98)00190-9
66. Neary, NM, Goldstone, AP, and Bloom, SR. Appetite regulation: from the gut to the hypothalamus. Clin Endocrinol. (2004) 60:153–60. doi: 10.1046/j.1365-2265.2003.01839.x
67. Suzuki, K, Simpson, KA, Minnion, JS, Shillito, JC, and Bloom, SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. (2010) 57:359–72. doi: 10.1507/endocrj.K10E-077
68. Owyang, C, and Heldsinger, A. Vagal control of satiety and hormonal regulation of appetite. J Neurogastroenterol Motil. (2011) 17:338–48. doi: 10.5056/jnm.2011.17.4.338
69. Timper, K, and Brüning, JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. (2017) 10:679–89. doi: 10.1242/dmm.026609
70. Niswender, KD, and Schwartz, MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. (2003) 24:1–10. doi: 10.1016/S0091-3022(02)00105-X
71. Quan, W, Kim, HK, Moon, EY, Kim, SS, Choi, CS, Komatsu, M, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. (2012) 153:1817–26. doi: 10.1210/en.2011-1882
72. Wren, AM, Seal, LJ, Cohen, MA, Brynes, AE, Frost, GS, Murphy, KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. (2001) 86:5992–2. doi: 10.1210/jcem.86.12.8111
73. Kim, MS. The neural basis of weight control and obesity. Exp Mol Med. (2022) 54:347–8. doi: 10.1038/s12276-022-00759-3
74. Obert, R, Onsidine, VC, Inha, AKS, Ark, M, Eiman, LH, Tephens, HWS, et al. Serum Immunoreactive-leptin concentrations in Normal-weight and obese humans. NEJM. (1996) 334:292–5. doi: 10.1056/NEJM199602013340503
75. al Maskari, MY, and Alnaqdy, AA. Correlation between serum leptin levels, body mass index and obesity in Omanis. Sultan Qaboos Univ Med J. (2006) 6:27.
76. Ostadrahimi, A, Moradi, T, Zarghami, N, and Shoja, MM. Correlates of serum leptin and insulin-like growth factor-I concentrations in normal weight and overweight/obese Iranian women. J Women’s Health. (2008) 17:1389–97. doi: 10.1089/jwh.2007.0736
77. Haqq, AM, Sadaf Farooqi, I, O’Rahilly, S, Stadler, DD, Rosenfeld, RG, Pratt, KL, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. (2003) 88:174–8. doi: 10.1210/jc.2002-021052
78. Wang, Y, Wu, Q, Zhou, Q, Chen, Y, Lei, X, Chen, Y, et al. Circulating acyl and des-acyl ghrelin levels in obese adults: a systematic review and meta-analysis. Sci Rep. (2022) 12:1–17. doi: 10.1038/s41598-022-06636-3
79. Marzullo, P, Verti, B, Savia, G, Walker, GE, Guzzaloni, G, Tagliaferri, M, et al. The relationship between active ghrelin levels and human obesity involves alterations in resting energy expenditure. J Clin Endocrinol Metab. (2004) 89:936–9. doi: 10.1210/jc.2003-031328
80. Ardeshiripur, M, Rhein, M, Frieling, H, Bleich, S, Hillemacher, T, Muschler, M, et al. Desacylghrelin but not acylghrelin is reduced during smoking cessation. J Neurl Transm Parkinson’s Dis Dement. (2018) 125:1885–9. doi: 10.1007/s00702-018-1930-0
81. Lv, J, Chen, W, Sun, D, Li, S, Millwood, IY, Smith, M, et al. Gender-specific association between tobacco smoking and central obesity among 0.5 million Chinese people: the China Kadoorie biobank study. PLoS One. (2015) 10:124586. doi: 10.1371/journal.pone.0124586
82. Winsløw, UC, Rode, L, and Nordestgaard, BG. High tobacco consumption lowers body weight: a Mendelian randomization study of the Copenhagen general population study. Int J Epidemiol. (2015) 44:540–50. doi: 10.1093/ije/dyu276
83. Sun, M, Jiang, Y, Sun, C, Li, J, Guo, X, Lv, Y, et al. The associations between smoking and obesity in Northeast China: a quantile regression analysis. Sci Rep. (2019) 9:1–6. doi: 10.1038/s41598-019-39425-6
84. Audrain-Mcgovern, J, and Benowitz, NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. (2011) 90:164–8. doi: 10.1038/clpt.2011.105
85. Miyata, G, Meguid, MM, Varma, M, Fetissov, SO, and Kim, HJ. Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav. (2001) 74:169–76. doi: 10.1016/S0031-9384(01)00540-6
86. Bloom, EL, Farris, SG, DiBello, AM, and Abrantes, AM. Smoking-related weight and appetite concerns and use of electronic cigarettes among daily cigarette smokers. Psychol Health Med. (2019) 24:221–8. doi: 10.1080/13548506.2018.1537495
87. Chao, AM, Wadden, TA, Ashare, RL, Loughead, J, and Schmidt, HD. Tobacco smoking, eating behaviors, and body weight: a review. Curr Addict Rep. (2019) 6:191–9. doi: 10.1007/s40429-019-00253-3
88. Kober, H, Kross, EF, Mischel, W, Hart, CL, and Ochsner, KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend. (2010) 106:52–5. doi: 10.1016/j.drugalcdep.2009.07.017
89. Faulkner, G, Cohn, T, Remington, G, and Irving, H. Body mass index, waist circumference and quality of life in individuals with schizophrenia. Schizophr Res. (2007) 90:174. doi: 10.1016/j.schres.2006.10.009
90. Morris, RW, Taylor, AE, Fluharty, ME, Bjørngaard, JH, Åsvold, BO, Gabrielsen, ME, et al. Heavier smoking may lead to a relative increase in waist circumference: evidence for a causal relationship from a Mendelian randomisation meta-analysis. CARTA Consortium BMJ Open. (2015) 5:e008808. doi: 10.1136/bmjopen-2015-008808
91. Slagter, SN, Vliet-Ostaptchouk, JVV, Vonk, JM, Boezen, HM, Dullaart, RPF, Kobold, ACM, et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. (2013) 11:195. doi: 10.1186/1741-7015-11-195
92. Monti, V, Carlson, JJ, Hunt, SC, and Adams, TD. A relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. J Am Diet Assoc. (2006) 106:822–8. doi: 10.1016/j.jada.2006.03.015
93. Zoccali, C, Postorino, M, Marino, C, Pizzini, P, Cutrupi, S, and Tripepi, G. Waist circumference modifies the relationship between the adipose tissue cytokines leptin and adiponectin and all-cause and cardiovascular mortality in haemodialysis patients. J Intern Med. (2011) 269:172–81. doi: 10.1111/j.1365-2796.2010.02288.x
94. Chang, E, Varghese, M, and Singer, K. Gender and sex differences in adipose tissue. Curr Diab Rep. (2018) 18:3. doi: 10.1007/s11892-018-1031-3
95. Chaney, SE, Sheriff, SW, and Merritt, L. Gender differences in smoking behavior and cessation. Clin Nurs Stud. (2015) 3:17. doi: 10.5430/cns.v3n3p17
96. Allen, AM, Scheuermann, TS, Nollen, N, Hatsukami, D, and Ahluwalia, JS. Gender differences in smoking behavior and dependence motives among daily and nondaily smokers. Nicotine Tobacco Res. (2016) 18:1408–13. doi: 10.1093/ntr/ntv138
97. Chinwong, D, Mookmanee, N, Chongpornchai, J, and Chinwong, S. A comparison of gender differences in smoking behaviors, intention to quit, and nicotine dependence among Thai university students. J Addict. (2018) 2018:1–8. doi: 10.1155/2018/8081670
98. Kim, C, Golden, SH, Mather, KJ, Laughlin, GA, Kong, S, Nan, B, et al. Racial/ethnic differences in sex hormone levels among postmenopausal women in the diabetes prevention program. J Clin Endocrinol Metab. (2012) 97:4051–60. doi: 10.1210/jc.2012-2117
99. Windham, GC, Mitchell, P, Anderson, M, and Lasley, BL. Cigarette smoking and effects on hormone function in premenopausal women. Environ Health Perspect. (2005) 113:1285–90. doi: 10.1289/ehp.7899
100. Kokkinos, A, Tentolouris, N, Kyriakaki, E, Argyrakopoulou, G, Doupis, J, Psallas, M, et al. Differentiation in the short- and long-term effects of smoking on plasma total ghrelin concentrations between male nonsmokers and habitual smokers. Metabolism. (2007) 56:523–7. doi: 10.1016/j.metabol.2006.11.012
101. Fagerberg, B, Hultén, LM, and Hulthe, J. Plasma ghrelin, body fat, insulin resistance, and smoking in clinically healthy men: the atherosclerosis and insulin resistance study. Metabolism. (2003) 52:1460–3. doi: 10.1016/S0026-0495(03)00274-9
Keywords: smoking, leptin, ghrelin, obesity, cigarette smoking
Citation: Shaheen N, Shaheen A, Diab RA, Saad AM, Abdelwahab OA, Soliman S, Hefnawy MT, Ramadan A, Meshref M and Nashwan AJ (2023) Association of serum leptin and ghrelin levels with smoking status on body weight: a systematic review and meta-analysis. Front. Psychiatry. 14:1296764. doi: 10.3389/fpsyt.2023.1296764
Edited by:
Marc Vogel, University Psychiatric Clinic Basel, SwitzerlandReviewed by:
Alexander Glahn, Hannover Medical School, GermanyI-Shiang Tzeng, National Taipei University, Taiwan
Copyright © 2023 Shaheen, Shaheen, Diab, Saad, Abdelwahab, Soliman, Hefnawy, Ramadan, Meshref and Nashwan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulqadir J. Nashwan, YW5hc2h3YW5AaGFtYWQucWE=
 Nour Shaheen
Nour Shaheen Ahmed Shaheen
Ahmed Shaheen Rehab Adel Diab2
Rehab Adel Diab2 Abdelrahman M. Saad
Abdelrahman M. Saad Omar Ahmed Abdelwahab
Omar Ahmed Abdelwahab Mahmoud Tarek Hefnawy
Mahmoud Tarek Hefnawy Mostafa Meshref
Mostafa Meshref Abdulqadir J. Nashwan
Abdulqadir J. Nashwan