- 1Faculty of Medicine, Institute of Legal Medicine, Saarland University, Homburg, Germany
- 2Department for Thoracic and Cardio-Vascular Surgery, Saarland University Medical Center, Homburg, Germany
- 3Department of Psychiatry, Clinic for Psychiatry, Psychotherapy and Psychosomatics, SHG-Kliniken Sonnenberg, Saarbrücken, Germany
- 4Institute of Legal Medicine, University Hospital, Goethe-University of Frankfurt, Frankfurt, Germany
As the population ages, the prevalence of heart failure and individuals wearing an implanted cardiac device is increasing. The combination of different underlying pathophysiologies and (the combination of) implanted cardiac devices can become a challenge with regard to the determination of cause and manner of death in such individuals. Additionally, heart disease is frequently associated with mental disease, ranging from anxiety and depression to suicidality and suicide (attempts). At the same time, the correct diagnosis of cause and manner of death is the basis for quality assurance, further therapeutic advances, legal safety, and suicide prevention. By that, an interdisciplinary field between legal medicine, clinicians, and law enforcement opens up. In this field, the different participants can simultaneously benefit from and need each other. For example, legal medicine experts need investigatory results and clinical expertise for the interpretation of readout data of implanted cardiac devices in order to correctly determine the cause of death. A correctly determined cause of death can assist law enforcement and help clinicians to further improve various therapeutic approaches based on correct mortality data collection. In addition, it is the basis for identification of suicides of device carriers, allowing psychological and psychiatric experts to better understand the burden of mental disease in this particular cohort. Against this interdisciplinary background, this manuscript summarizes information about psychiatric comorbidities and suicidality while being on a device. Thereby, basic information on complications and malfunctions of implanted cardiac devices, device-associated deaths with particular emphasis on device manipulation is displayed as basic information needed for correct determination of the cause of death. Also, legal and ethical issues in this field are outlined. The final result is a proposal of an interdisciplinary assessment workflow for a conjoint approach to improve the diagnosis of deaths associated with implanted cardiac devices. It will allow for a differentiation between an individual who died with or due to the device.
1 Introduction
Implanted cardiac devices (IcarDs; abbreviations summarized in Supplementary material SA) are well known in the antemortem and the postmortem routine. Many clinical specialties are exposed to IcarDs. For example, cardiologists employ them to treat cardiac arrhythmias (1), or cardiac surgeons implant total artificial hearts to bridge a patient to transplantation (2). Also, palliative care specialists encounter them during end-of-life decisions (3, 4). In the postmortem field, IcarDs can be employed to estimate time since death (5, 6), identify unidentified bodies (7, 8), or to clarify the cause of death (5, 9). Additionally, the legal medicine experts contribute to the work-up of IcarD-associated deaths [(e.g., 10)] including IcarD associated suicides [(e.g., 11)]. However, both fields are facing increasing numbers of IcarD carriers [(e.g., 12, 13)]. This could be attributed to the accelerated aging of the 21st century’s population (14). At the same time the incidence of heart failure (HF) is increasing (12, 13). This would fit the growing spectrum of IcarDs complementing the pharmaceutical HF therapy (15, 16). Thereby, for both fields the differentiation whether a person died with an IcarD, or due to an IcarD is relevant. For the clinicians this information is an important outcome measure, and by that, fundamental for further improvement of patient care in terms of safety and quality (17), like demonstrated for HF (18). For the legal medicine expert, clarification of the exact cause of death is a central task to support law enforcement [(e.g., 19, 20)]. This is also relevant in cases dealing with malpractice allegations, especially considering its increasing number in the field of internal medicine/cardiology from 2020 to 2021 in Germany (21). In this context, it has to be pointed out, that it is not the task of legal medicine to assess indication, quality of the preimplantation diagnostics, and so forth. Therefore, pertinent clinical experts must be consulted. Also, in cases with assumed deaths due to assumed device malfunction an exact diagnosis of both, the cause and manner of death, is crucial. Respective cases are non-scientifically published (22), and consecutively devices has been withdrawn by the United States Food and Drug Administration (FDA) (23). However, in the ante-and the postmortem setting, the correct differentiation between death with or due to an IcarD is necessary to correctly fill the death certificate. This again has general legal implications (24) such as the opening of a death investigation. But although important, this differentiation is far from trivial. This can be attributed to several factors. One is the complex pathophysiology of HF (summary see Supplementary material SB) and its high mortality (16). Additionally, there is a broad spectrum of IcarDs available nowadays (summary see Supplementary material SC). The author group made these observations on the occasion of two cases reported in Supplementary material SD. In both cases a left ventricular assist device (LVAD) was employed to commit suicide.
Because two cases of suicide by LVAD prompted the present narrative literature review, psychiatric co-morbidity as one cause of suicide is first discussed below. Subsequently, topics that are indispensable for the correct classification of the cause of death will be dealt with. These are, complications, mortality, and device readout as a source of information. Afterwards, further legal and ethical aspects such as the classification of the manner of death are discussed. Finally, a proposal for an assessment workflow of deaths associated with IcarDs is presented.
2 Materials and methods
The present article mostly resembles a narrative review article. A particular focus on IcarD associated suicidality is applied. Thus, the outline of the current knowledge of mental illness and suicidality in device carriers is based on a systematic literature search. Therefore, the data base PubMed® was searched. The search algorithms employed are given in the respective paragraph. The identified literature was selected by screening title and abstract. An article was selected, if it dealt with suicide in association with an IcarD. The summary results of each data base query are provided in Supplementary material SE.
3 Psychiatric comorbidity of cardiac devices and heart failure
In order to discuss suicide of IcarD carriers, first the question of why these individuals commit suicide will be addressed. Afterwards, the different devices are revisited regarding there associated mental burden. Overall, the suicidality seems attributable to the immense burden of mental co-morbidity in heart disease.
First it must be highlighted, that “cardiac patients” in general show increased depression rates. A clinical trial of coronary artery disease and arterial hypertension patients demonstrated, that at least 14% of the patients had suicidal thoughts within the past 2 weeks. This trial also points out, that the mental health state in their cohort seems to be not attributed to the heart disease only. For example, they found associations of suicidality with loneliness, retirement and stroke in their cardiac patients. They, also found females to have more frequent suicidal ideations compared to males (25). Taken together, this study underlines the relevance of the biopsychosocial model and the associated transdisciplinarity (26).
This trend towards suicidality is known since decades. For example, in a long-term study in 1986 of individuals after correction of tetralogy of Fallot, suicide was observed as a leading cause of death (27). Especially after cardiac events such as acute myocardial infarction, the suicide rate peaks (28). Subsets of cardiac patients of particular relevance for the present review are HF patients and IcarD carrier.
HF patients are exposed to psychological distress (16), considered as overall severe burden of mental disease (29, 30). This includes depression and anxiety disorders in particular (31). In general, up to 30% of individuals suffering from HF experience depression (32). Even higher rates of depression, up to 70%, have been reported in individuals with end-stage HF (29). Among these, depressed HF patients have worse clinical outcomes compared with non-depressed HF patients (32). Correspondingly, psychological comorbidity in HF has been associated with disease progression (31). Also, readmission rates after the procedure of depressed individuals undergoing LVAD implantation are reportedly higher (33). Approximately 2% of the LVAD recipients have attempted or completed suicide (11).
Carrying an IcarD in general seems to be associated with mental illness also. For example, carrying an implantable cardioverter/defibrillator (ICD) or pacemaker (PM) has been associated with anxiety and depression (34). PM patients show depression rates as high as 45% (35). ICD carrier present with depression rates of approximately 23% (34). Even adolescents carrying an ICD present with anxiety and/or depression (36).
Depression progression after ICD implantation has been observed in approximately 23.6% of the affected individuals (37). The depression rates are reported to be significantly higher in ICD carriers compared to PM carriers and a control group (34). As ICDs can be indicated in HF (16), this could be attributable to the HF patients among the ICD carriers. The patients often name a “mental vacuum” (38) as their reason for suicidality. Interestingly, not only the IcarD carriers, but also their partners exhibit psychological distress. For ICD patients it has been shown that patients’ partners also show elevated depression and anxiety rates (39).
In the given context, one must also consider that physically ill individuals are significantly more likely to choose nonviolent suicide (40). So, IcarDs could be seen as a nonviolent option to commit suicide. From a legal medicine perspective, therefore, they must be considered “potential kill switches“. This means that manipulation of its soft- or hardware could potentially cause someone to “die” (41).
3.1 Pacemaker
For PMs, 85 publications were identified [last database query: 3rd May 2023; 5:45 pm; search algorithm: (suicide) AND (pacemaker)]. Of these, 9 publications dealt with suicide in PM carriers (35, 42–49). The other articles mostly dealt with the therapeutic use of pacemakers in the treatment of individuals after they have attempted suicide by various substances such as thioridazine (50), nebivolol (51), or propafenone and captopril (52). Also, ethical aspects in end-of-life-scenarios [(e.g., 53, 54)], or therapeutic aspects in accidental digoxin overdosing (55) are discussed in the literature. However, 8 articles deal with suicide attempts by targeting the aggregate and/or the leads (35, 42, 43, 45–49). In one case report, the individual attempted suicide by jumping out of the window after PM implantation (35). In some instances, the individuals combine the PM removal attempt with intoxication [(e.g., 43, 46, 48)]. Only in one reported case no mental illness was known prior to the suicide attempt (35). In all remaining cases, mental illnesses were prior known (42–49). No literature on a possible association between cardiac resynchronization therapy (CRT) and suicide could have been identified [last database query: 4th May 2023, 03:00 pm; search algorithm: (suicide) AND (cardiac resynchronization therapy)]. The algorithm found 2 articles on sudden death in general (56) and ethical issues of ICD withdrawal (57).
3.2 Implantable cardioverter/defibrillator
A literature search regarding ICDs identified 27 publications [last database query, 4th May 2023; 11:30 am; search algorithm: (suicide) AND (implantable cardioverter defibrillator)]. One article was identified describing a case involving a suicide attempt by medication overdose due to phantom shocks (58). Two cases are reported in which the ICD aborted suicide attempts. One of these describes, that the ICD terminated a self-electrocution-induced ventricular tachycardia (59). The other article describes an individual who attempted suicide by overdosing “diazepam, coumadin, and other pills” (60). Again, the ICD aborted the suicide attempt (60). Furthermore, a report was found describing an individual who had ventricular tachycardia due to a myocardial scar. This was caused by a bullet during a suicide attempt 28 years ago (61). The ICD was used to treat the consequences of this failed suicide attempt (61). No other articles on ICD associated suicide could have been found. The vast majority of articles found by the algorithm addressed ethical and legal aspects of device deactivation in end-of-life-scenarios [(e.g., 62)].
3.3 Mechanical circulatory support
Corresponding to the cases reported in Supplementary material SD, in this section particular focus on ventricular assist devices (VADs) is applied. For mechanical circulatory support (MCS) in general 18 articles have been identified by the algorithm [last database query, 4th May 2023; 12:20 pm; search algorithm: (suicide) AND (mechanical circulatory support)]. For VAD specific, 22 articles have been found [last database query, 4th May 2023; 12:25 pm; search algorithm: (suicide) AND (ventricular assist device)]. The results of the algorithms overlapped [(e.g., 3, 63, 64)]. Most articles identified dealt with ethical and/or legal aspects in end-of-life-scenarios.
For MCS, no article was found describing a suicide or suicide attempt on a device. Instead several articles describe MCS-systems used to treat people after pharmacological suicide attempts. Thereby, cases with the use of an extracorporeal membrane oxygenation (ECMO) (63, 65, 66), so-called extracorporeal cardiopulmonary support (ECHLS) (67), or intra-aortic balloon pump (IABP) (68) are reported. However, in one author’s experience (KH), at least one suicide was completed by a patient on the so-called “awake ECMO.” This term describes cases in which the patient is awake while on ECMO (69, 70). In this case, the patient removed one of the cannulas. Thus, there is a paucity of literature on the description and/or assessment of an association between MCS systems and suicide.
Regarding VADs, there are two case reports of suicide (attempts). In one, a depressed patient committed suicide by disconnecting its LVAD’s drive line (71). The other case report describes a suicide attempt of a LVAD carrier by overdosing zolpidem (72). This individual is reported to have attempted suicide because he felt that all the trouble associated with receiving his LVAD was not worth it due to the restrictions and limitations imposed by the recent severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) pandemic (72). Also, two observational studies has been identified. One of these reports on severe psychological distress in VAD carriers (73). Suicidal ideation and anxiety were observed in 17.5% of the individuals. Depression was found in 14.9% of the patients (n = 121) (73). Another study reported that 10 of 494 LVAD patients (approximately 2%) completed or attempted suicide during an 18-month follow-up period after hospital discharge following LVAD implantation. Suicide was completed in eight cases. Either the driveline was disconnected, respectively, cut, or an intoxication was caused. Unsuccessful attempts were found in two instances (1 attempt by targeting the drive line, 1 attempt by intoxication). Nine of these individuals were men. Only 2 of these 10 had a known psychiatric comorbidity. Of these 10 individuals, three did not undergo psychiatric evaluation before LVAD implantation (11).
Additionally, an article has been found, that describes the sociocultural risks of LVAD carriers in the Middle East. This region appears to have a high incidence of HF. Here, advanced HF occurs much earlier in life. Therefore, LVADs are used as bridge-to-destination, as heart transplantation is rarely available. At the same time, this region struggles with terrorism and suicide bombers. The article describes several cases in which LVAD carriers were mistaken for suicide bombers due to the belt, batteries, and drive line. Some were even at risk of having their drive line cut after a car accident as they were mistaken as suicide bombers (74).
3.4 Summary: mental disease and suicidality in implanted cardiac devices
Reviewing the association of various IcarDs with suicide, it appears that most case reports are available for PM carriers, whereas the best evidence with observational studies is available for LVAD patients. Nevertheless, the literature seems to be incomplete so far regarding the association of other-than-VAD-MCS systems and suicide.
Cases, analogous to the two reported cases (Supplementary material SD), are reported in which suicide was committed by “pulling the plug.” Thus, at least, LVADs can be considered potential “kill switches.” This emphasizes the importance of a pre-implantation psychological/psychiatric assessment. The results of the literature search indicate, that a respective assessment is mandatory in suicide prevention. The literature findings underpin, that not only individuals prior to VAD implantation (16) but also individuals undergoing implantation of other IcarDs should undergo psychosocial assessment (43). Interestingly, the literature findings showed that LVADs and PMs are targets of suicide (attempts), whereas ICDs rarely seem to be associated with suicide. The ICD was in most cases somehow “beneficial.” Contrasting, PMs’ aggregates and/or leads were the target or trigger of the respective suicide attempt. This aligns with the understanding of ICD carriers. Generally, they rate their IcarD as beneficial (75). It could be speculated that this is due to the preventive character ICDs have. The arrhythmias they can terminate are in turn associated with psychological and physical stress (76). PM carrier targeting their aggregate or the leads, also contrast with LVAD carriers. Latter generally preferred more non-violent attempts by intoxication and/or unplugging. Following this line of thought, Rosenthal and colleagues raised the question as early as 1980 whether the PM devices and leads should or could somehow be concealed from their carrier (47). From this perspective, leadless PM technology – originally developed to prevent lead-associated complications (77) – seems useful in the prevention of suicide attempts as well.
While knowledge on the associated psychiatric co-morbidity is fundamental for understanding the why, knowledge on device associated complications, malfunction and mortality is the fundamental for the correct diagnosis of device associated suicide.
4 Complications
Foremost it must be stated that the complications associated with IcarDs are “case- and device specific.” This is due to differences in the underlying pathophysiology, the characteristics of different devices, and others. However, different clusters of complications can be distinguished such as “surgical complications” (e.g., bleeding or right heart failure) (78) and “medical complications” (e.g., ischemic neurological events) (79). For LVADs it can be additionally distinguished between LVAD specific complications, such as device malfunction, and LVAD-associated complications, like aortic regurgitation (80). Also, complications can be assigned to several groups at the same time, such as infection or pump thrombosis (78–80). The group of “unusual complications” such as late onset aortic regurgitation, uterine bleeding, or display malfunction (81) is described also. Regardless of how these complications are grouped they bear a risk for the IcarD carrier. For example, a common factor of LVAD complications in general is their high mortality and morbidity (80). Besides such general clusters, there are also typical complications for each device. So, is hemolysis quite frequently in continuous flow VADs (82). Or acute lung injury (83) is considered typical for extracorporeal life support systems (ECLS). Besides the individual and the device, also the time frame has to be taken into consideration. This is due to different complications being frequent in the early (i.e., implantation, early postoperative phase), and the late phase. In the following, this will be explained using the PM as an example. Here, the most common complications shortly after implantation are pneumothorax, hematoma, lead dislodgement, or superficial phlebitis (84). In this context, especially “re-do procedures” such as PM upgrade (e.g., adding leads) may be associated with higher complication rates compared to initial interventions (85). In the late phase, especially lead-related issues (e.g., lead fracture), endocarditis, or PM-induced HF are more relevant (86). In particular, surgeon experience and patient morbidity seem to influence the long-term complication rate (87). In addition to surgical experience, the technique applied plays a pivotal role. For example, in the past, redundant leads were often abandoned (88). Contrasting, recent studies demonstrated that lead extraction can be performed safely with the laser technique (88). Summarizing, for the PM, the early phase tends to be dominated by surgical/procedural complications (e.g., pneumothorax), whereas the long-term phase tends to be dominated by device-and lead-associated complications (e.g., HF, endocarditis). A brief summary of complications associated with the different IcarDs is given in Supplementary material SF.
5 Malfunction
Talking about technical devices, malfunction must always be considered. For the IcarDs the cause of malfunction can be in the device itself (e.g., PM battery depletion), in the connection to the heart (e.g., PM lead fracture), and in the heart (e.g., increased pacing threshold) (89). A particular issue with the device itself is its so-called “programming” (90) or software (91). Programming describes the settings that clinicians make using software to make the device work according to agreed parameters. Cases have been reported in which PM firmware updates resulted in interrogation malfunction (92). So, particular attention should be paid during interrogation after a firmware update, especially for PM-dependent individuals (92). Although such updates carry a particular risk, they are still necessary. One reason therefore is the susceptibility of such devices to cyberattacks. Here appropriate updates can improve the safety (93). But, also external factors can lead to device malfunction. For example, PM malfunction can occur due to electrocautery during surgery (94) or due to radiotherapy (95). Depending on device and patient, the impact of device malfunction differs. For example, in PM carriers, malfunctions are usually not fatal (96) and can result in symptoms such as dizziness or chest pain (97). On the other hand, there are devices such as LVADs in which malfunction is more likely to be fatal (98). It should be noted that LVAD malfunction involves much more than just the pump, but also includes failure of the controller, battery, or patient cable, among others (98). Interestingly, in LVADs, patient non-compliance does not appear to be associated with device malfunction (98).
6 Mortality
Mortality varies from case to case due to different underlying diseases, the procedure chosen, the device used, and so on. Therefore, each case in legal medicine casework that addresses the question of IcarD-associated death requires a case-specific evaluation. This must take into account as much information as possible, such as situation of finding the body, signs of extraneous involvement, autopsy findings, and the results of subsequent further analyzes (e.g., histology), as appropriate. Additionally, consultation of appropriate experts such as perfusionists or cardiac surgeons, has to be considered to clarify specific issues at hand. One issue could be the device readout and its interpretation. Thereby, the choice of the expert must make sure that in the respective case there is no conflict of interest. For example, in the legal medicine setting, the cardiac surgeon who did the assessed and disputed surgery, is not appropriate. In the experience of the author group, it has proven useful to consult higher-level entities, such as the manufacturer or the coordinator of the respective VAD program. This can help to find appropriate experts without conflict of interest and also provide equipment for reading out the information.
How mortality can vary is illustrated in the following for the MCS setting. In short-term MCS treatment, 54% of all individuals died on the device (99). Contrasting, long-term MCS therapy as bridge to destination, can be associated with a two-year survival rate of up to 62% (100). Also, how the devices are installed has to be considered. For example, there are several ways to implement an ECLS. They can be installed through an open chest to allow for larger cannulas with central cannulation of the right atrium and the aorta (101). Or they can be installed via the femoral arteries and veins (102). Overall, ECLS has been associated with in-hospital mortality rates of up to 55.5% (103). Herein, associated pathological changes such as need for blood transfusion can lead to higher mortality (103). With the open chest approach being associated with higher transfusion rates (104), the implantation technique may influence the mortality rate observed.
7 Readout and associated problems
The data obtained by device readout may be useful for reconstructing events prior to death (10). However, the readout can also raise new questions, as illustrated by the case study by Junge et al. (10). They found a magnetically reversed ICD in a 66-year-old HF patient. As well, 5 ICD interventions in the last 4 days before death has been found in the memory (10). For this reason, the authors doubted the “naturalness” (10) of death in this particular case. The authors state that in a case without readout and without autopsy, the cause of death in ICD carriers “must be reported as unknown” (10). This indicates that device readout does complement the autopsy, for example, by allowing for detection of magnetically reversed devices (105). This cognition, facilitates to evaluate the role of the reversed device in the causal chain leading to death.
8 Device manipulation
It is important to note, that device manipulation can occur in various occasions, such as end-of-life decisions or intentionally for suicide. Additionally, plenty external factors can influence or manipulate the device. So, the device can become electrically damaged (106), can dislocate (107) or a cannula can be accidentally removed (106). By that, IcarDs can complicate the determination of the manner of death, especially since there can be discrepancies in the interpretation of deaths between clinicians and (forensic) pathologists (10). In a series examining IcarD data obtained before cremation, single cases with magnet reversion were identified (105). It was not possible to clarify whether this was performed by clinicians because the end of life had been reached or resembled suicide or homicide (105).
8.1 Implanted cardiac devices and magnets
Connecting to the aforementioned series, it must be highlighted, that IcarDs can not only be manipulated by magnets, but that the magnet can also damage the data stored on the device (10). At this point, it is important to note that not all magnets per se bear the potential to manipulate an ICarD. The magnet must have a particular strength. For example, it has been demonstrated that the magnetic field of a dermatoscope is not capable of influencing IcarDs (108). Furthermore, it should be noted that even stronger magnetic fields, such as those encountered during magnetic resonance imaging (MRI), do not necessarily lead to severe or even fatal changes in IcarD function (109, 110). Since safety regarding the magnetic field in MRI depends on an interdisciplinary approach and appropriate precautions (111), the unconscious magnetic manipulation of an IcarD can lead to potentially severe adverse events for its carrier even in a clinical setting (112). Hereby, it must be noted, that some everyday technologies, such as certain phones, can cause magnetic fields strong enough to turn IcarDs into magnetic mode, as indicated in a 2021 FDA notification (113). At least in the recent years, a rapidly growing number of smartphones has been reported (114), along with an increasing number of users with more than one device (115). Also, elderly use such handheld devices (116) and smartphones are even being investigated in Alzheimer’s Disease therapy (117). Therefore, accidental magnetic manipulation of IcarDs must also be considered. This points out the need for a thorough postmortem examination along with the analysis of perimortem circumstances. Such “unintentional” magnetism is also relevant in a clinical setting. The HeartMate 3 LVAD exhibits a fully magnetically levitated rotor. Its magnetic field can interfere with the function of various ICD models (118). Therefore, a safety margin of at least 10 centimeters should be maintained during the implantation of the LAVD and/or ICD (118).
Although devices can be magnetically manipulated, a literature search failed to find a report of suicide by magnetic device manipulation (Data base: PubMed®; search terms: (suicide) AND (magnet) AND (pacemaker); last query 10th January 2022). This is somehow surprising considering the prior outlined suicidality of “cardiac patients,” and that patients are informed regarding the interference of their device and magnets (112, 119). This raises the question of whether there are no cases of completed suicide involving magnet application or whether they are simply not detected. However, there is a case report of a medical professional who used defibrillator pads to commit suicide by self-electrocution (120). This underlines that “knowledgeable” individuals can abuse their knowledge to commit suicide.
8.2 Effects of device manipulation
The effect of turning off or manipulating a device is not uniform again. Instead, the effects are highly dependent on the device type and manufacturer, the underlying disease, and the current disease state, concomitant diseases and therapies, etc. This is exemplified in the following for the PM (Figure 1). First, the area of underlying disease and thus PM-dependence is addressed. PM-dependence can be defined as a high risk of “serious injury or death from sudden pacemaker failure” (121). For example, if the PM was indicated due to atrial fibrillation with bradyarrhythmic episodes, that individual may have an apparently high probability of surviving without the device during non bradyarrhythmic phases. In contrast, if this individual is PM-dependent in permanent complete atrioventricular-block, sudden device failure can be fatal. This has already been highlighted in a case report of an individual with sick sinus syndrome who died due to PM malfunction (9). Overall the prevalence of PM-dependence is comparably low. A clinical cohort study reports approximately 2% of the analyzed individuals to be PM-dependent (121). These clinical data fits with observations in the postmortem setting. A study analyzing PMs and ICDs explanted before cremation showed that approximately one third of the explanted devices had low or no power (105). Nevertheless, knowledge of the indication for device implantation and the current disease state is necessary to assess the role of an IcarD in the causal chain leading to death. Next, the aspect of the different devices is displayed for magnetic device manipulation. Plenty manufacturers offer a variety of different PM devices (119). It has been reported that, except for Sorin PM, removal of a magnet results in reversal to the usual preprogrammed setting in most other PM devices (119). In general, most devices are turned into a so-called asynchronous mode by the magnetic effect. What exactly happens in this mode depends on the manufacturer and the device (119). At the end, one can even generalize, that finding a cardiac device at autopsy does not mean that the deceased required the device at the time of death.
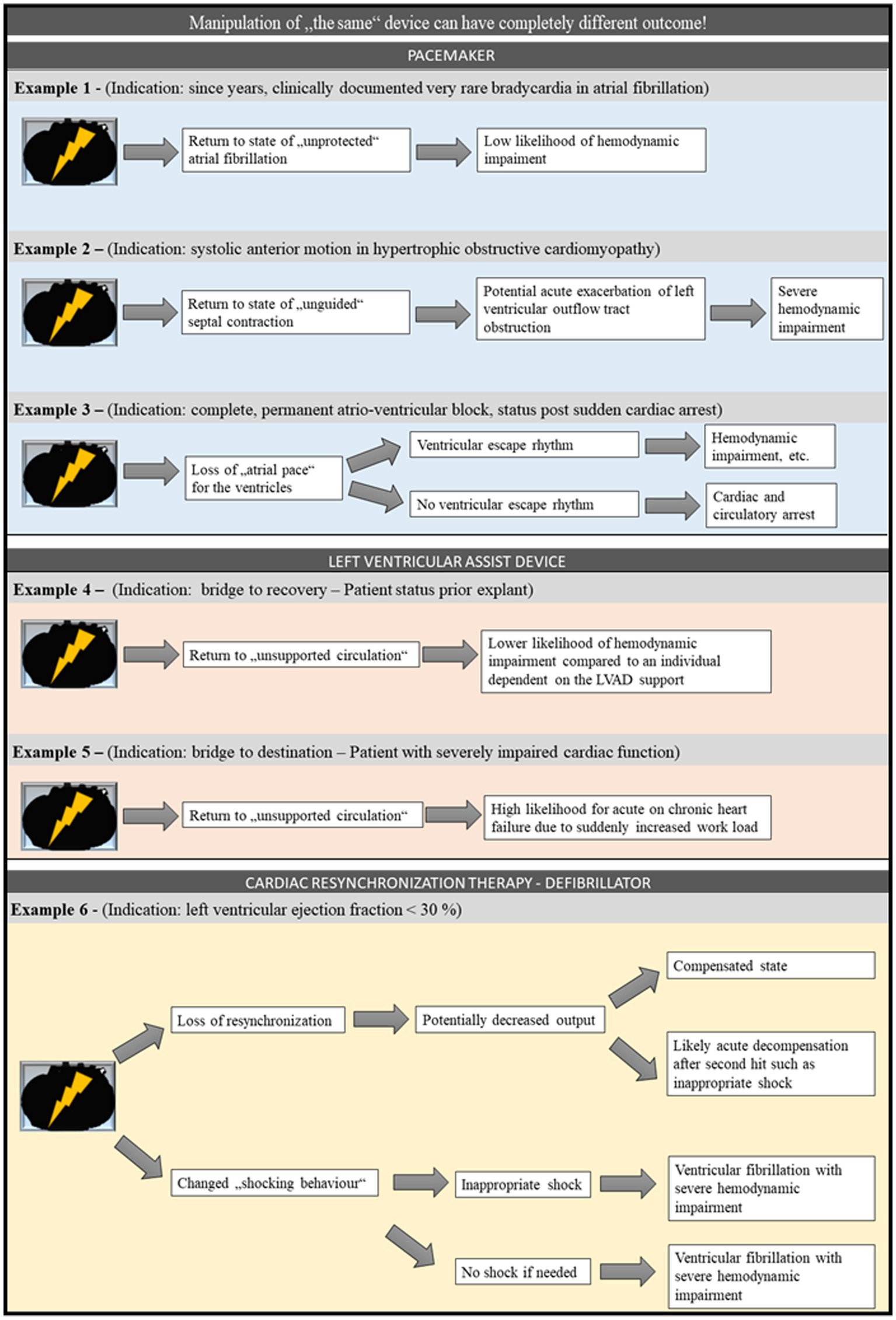
Figure 1. Example for potential consequences of loss of function of different cardiac devices. For PM, LVAD, and CRT-D, different potential scenarios starting with a loss of function of the respective device are displayed. It is highlighted that although the starting point is the same in different scenarios, each scenario reaches a different endpoint and in some cases may even reach different endpoints within a given scenario. CRT-D, cardiac resynchronization – defibrillator; LVAD, left ventricular assist device; PM, pacemaker.
9 Discussion
As indicated several times, the reconstruction of the causal chain leading to fatality can be complex and challenging. Based on the literature outlined so far, this task can best be accomplished conjoint by antemortem and postmortem experts. Such a bilateral approach has already been proclaimed for the assessment of sudden deaths (56, 122). Practical examples exist, for example, in Switzerland, where in 2015 the Swiss Society of Legal Medicine established a multidisciplinary working group together with clinicians and geneticists to jointly address sudden cardiac death (122). In the given context, beside clinical expertise, especially known psychological and psychiatric findings in the different cases has to be considered. In case these findings are ambiguous or even negative in terms of suicidality, psychological and psychiatric experts could be retrospectively involved to search for the underlying cause or overseen discrete hints. Such a retrospective psychiatric assessment of such cases could be helpful to improve suicide prevention in cardiac patients. However, such beneficial effects require the exact diagnosis of the cause of death. The current literature indicates, that in order to do so, a wide-ranging diagnostic approach is necessary. This can involve clinicians, law enforcement, legal medicine experts, and sometimes even the IcarDs manufacturer’s experts (123, 124). Prior case reports using such a broad and interdisciplinary assessment nicely outline that the presence of IcarDs never excludes a non-IcarD-associated cardiac cause of death (123). However, this shows that classifying death with and death due to an IcarD is just the first diagnostic step. In each scenario a further diagnostic segregation should be attempted, like outlined in the following.
9.1 Spectrum of death due to an implanted cardiac device
One scenario could be referred to as “accidental death due to IcarD.” This scenario would summarize all accidental malfunctions of a device leading to death, such as fateful malfunction of a LVAD. Another possible scenario would be “death due to IcarD associated with malpractice,” encompassing cases of individuals who died due to malpractice. It should be noted that in this scenario, the legal medicine expert is mainly employed to preserve evidence, and the final assessment is the responsibility of clinical experts. One might also encounter the “death due to IcarD associated with complications” scenario. This would subsume the deaths associated with a fateful fatal outcome due to complications, regardless of any short-or long-term complications about which the patients were previously informed. The two last scenarios could be termed “death due to IcarD employed for suicide” and “death due to IcarD employed for homicide.” These scenarios can only be resolved through a collaborative effort between law enforcement (e.g., presence or absence of a suicide note, presence or absence of signs of a fight), legal medicine (e.g., evidence of violation, evidence of self-harming behavior), and clinical experts (e.g., readout and interpretation of all encountered IcarDs, psychiatric medical history). Correct attribution can only be achieved by integrating all available information and may also require further subsequent analyzes, like toxicological analyzes to check for (self-)intoxication.
9.2 Spectrum of death with an implanted cardiac device
In this spectrum, mainly the area of “other than IcarD causes of death” is present, as pointed out by previous publications (123, 124). However, here the complex interplay of pathophysiology of the circulatory system, IcarD function, and the comorbidities must be considered. For example, autoptic findings must be questioned always to be able to distinguish between fatal mesenteric ischemia due to atherosclerosis and mesenteric ischemia due to cardiac low-output syndrome in the course of fatal device malfunction.
9.3 Intersection of death with and death due to an implanted cardiac device
Here, mainly one scenario has to be pointed out. This is the “clinical IcarD manipulation.” Its ambiguous character arises from the perennial ethical and legal debate about “turning off” IcarDs in end-of-life decisions. Accordingly, one could argue that the individual somehow died “accelerated” because the IcarD was turned off. But, one could also argue that the person died from an underlying cardiac disease even if the IcarD is still running. Thus, depending on one’s view and local legal and ethical circumstances, one could consider this scenario as either “death with IcarD” or “death due to IcarD.” In making this decision, it is important to keep in mind even clinical device manipulation (e.g., turning off an ICD to avoid inappropriate shocks in an individual’s final hours) does not have a uniform effect.
9.4 Legal and ethical aspects
Not only the determination of the cause of death, but also the correct classification of such is important. From a clinical perspective, the correct classification of the cause of death is considered to be helpful in determining therapeutic or preventive strategies (125). From a legal perspective, especially the manner of death has various consequences. These can vary between different countries and jurisdictions; among others, in Germany, the allowances for burial of the body (126) and potential exercise of legal jurisdiction. Ultimately, the correct determination of the cause of death and the manner of death (i.e., natural, violent or non-natural, or unclear) can have a far-reaching impact on health policy events, actions of investigating authorities, and the bereaved.
Legally, a primary criterion for a non-natural death is an “external” impact or influence (127) that establishes a chain of causation. In a medical setting, this definition is more complex and has been further defined and summarized in the International Classification of Diseases.
From a legal perspective, the intentional disconnection of a medical device shows actus reus and mens rea resulting in a criminal offense. In addition, it is an external intervention causing death and should thus technically be considered an unnatural death. However, if the patient did not communicate his/her intend, actus reus and mens rea cannot be established with certainty. An accidental interference thus raises the question what time frame constitutes a ‘sudden’ consequence of such manipulation and what time frame would be sufficient to establish a chain of causation. However, it must be noted that the determination of the cause and manner of death remains a scientific approach and not just a legal determination.
A particular type of IcarD manipulation is manipulation by a physician, such as deactivation during an end-of-life decision (128). This kind of manipulation is done, for example, to avoid inappropriate ICD shocks in an individual’s final hours. The deactivation of such devices is an ethical challenge (129) and should be assessed on an individual basis (130). An important factor in this situation is the patient’s right for self-determination (131, 132). In addition, there is a debate about the determination of “clinical deactivation” in end-of-life decisions. Some publications indicate that clinical deactivation of an IcarD could even be considered physician-assisted death (3, 4). The legality of physician-assisted death is a complex topic of ongoing discussion (133, 134) and highly dependent on the respective jurisdiction.
10 Summary and conclusion
All in all, IcarDs can be considered potential “kill switches.” This can be of relevance for the casework in legal medicine due to the severe mental burden of IcarD carriers. To allow for both, suicide prevention and support of the investigating authorities, the exact cause of death has to be determined. Thereby, a conjoint approach of medical and forensic experts is necessary. In the medical field the antemortem and the postmortem field should be involved. Clinical experts, such as cardiologists or psychiatrics, can complement the autopsy and investigatory findings with their expertise and the retrospective analyzes of medical history and readout data. Regarding the readout, it should be attempted to collect data from all IcarDs. By that, manipulation or malfunction with potential relevance to the chain of causation of death can be detected. Therefore, these information can contribute to the clarification of cause and manner of death. The readout data may also establish a timeline of events and help in the overall evaluation of the case. A proposal of an assessment workflow is displayed in Figure 2. In such a conjoint approach the selection of the experts involved always has to consider potential conflicts of interest. Apart from this, a fundamental knowledge of the legal medicine expert regarding IcarDs is essential for an appropriate autopsy procedure and, by that, for the subsequent investigations (if necessary) by additional experts (Figure 2).

Figure 2. Assessment workflow for deaths in association with implanted cardiac devices. The selection of the different shapes was done aligned to what is known from entity-relationship models (i.e., the rectangles display an entity, ellipses represent attributes, and the diamond shape indicates a relationship). (α) The preparation technique should be adapted depending on the investigative questions and the encountered devices. So, for example if there is an CRT-D system and there is the question for lead-associated complications, the preparation should be done with special care for the venous system to be able to distinguish between antemortem findings and preparation-associated artifacts and to avoid dislocation of potentially adherent thrombi or vegetations. (β) Especially if there is a known complex pathophysiology underlying the IcarDs, one should consider a clinical consultation for conjoint interpretation of both autoptic findings together with the readout data and the known clinical history. The conjoint approach facilitates the integration of as many as possible information to a cause and by that manner of death. At this point it must be pointed out that the scenario “clinical manipulation” is somehow ambiguous. Depending on the underlying disease, the encountered IcarD or combination of IcarDs, turning off an IcarD can at least accelerate the occurrence of death (compare ethical and legal aspects of clinical IcarD manipulation in end-of-life decision). CRT-D, Cardiac Resynchronization Therapy – Defibrillator; IcarD, implanted cardiac device.
Author contributions
JF: Conceptualization, Investigation, Visualization, Writing – original draft. SP: Investigation, Visualization, Writing – review & editing. KA: Validation, Writing – review & editing. KH: Validation, Writing – review & editing. SH: Investigation, Validation, Writing – review & editing. KB: Investigation, Writing – review & editing. MR: Investigation, Writing – review & editing. MK: Investigation, Writing – review & editing. CL: Writing – review & editing. PS: Writing – review & editing. MV: Writing – review & editing. FR: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. During preparation of the present article JF was funded by the German Cardiology Society (“Deutsche Gesellschaft für Kardiologie”; Project number: DGK04/2022).
Acknowledgments
We thank the bereaved for the allows to scientifically work up the case reports presented in the supplement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1278078/full#supplementary-material
References
1. Glikson, M, Nielsen, JC, Kronborg, MB, Michowitz, Y, Auricchio, A, Barbash, IM, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: developed by the task force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European heart rhythm association (EHRA). Eur Heart J. (2021) 42:3427–520. doi: 10.1093/eurheartj/ehab364
2. Villa, CR, Moore, RA, Morales, DL, and Lorts, A. The total artificial heart in pediatrics: outcomes in an evolving field. Ann Cardiothorac Surg. (2020) 9:104–9. doi: 10.21037/acs.2020.02.15
3. Rady, MY, and Verheijde, JL. Ethical challenges with deactivation of durable mechanical circulatory support at the end of life: left ventricular assist devices and total artificial hearts. J Intensive Care Med. (2014) 29:3–12. doi: 10.1177/0885066611432415
4. Rady, MY, and Verheijde, JL. When is deactivating an implanted cardiac device physician-assisted death? Appraisal of the lethal pathophysiology and mode of death. J Palliat Med. (2011) 14:1086–8. doi: 10.1089/jpm.2011.0161
5. Lacour, P, Buschmann, C, Storm, C, Nee, J, Parwani, AS, Huemer, M, et al. Cardiac implantable electronic device interrogation at forensic autopsy: an underestimated resource? Circulation. (2018) 137:2730–40. doi: 10.1161/CIRCULATIONAHA.117.032367
6. Ondruschka, B, Babian, C, Neef, M, Zwirner, J, and Schwarz, M. Entomological and cardiologic evidence of time since death in short postmortem intervals. J Forensic Sci. (2019) 64:1563–7. doi: 10.1111/1556-4029.14010
7. Chatzaraki, V, Ampanozi, G, Thali, MJ, and Schweitzer, W. Cardiac conduction devices in the radiologic comparative identification of decedents. Forensic Sci Med Pathol. (2020) 16:157–65. doi: 10.1007/s12024-019-00181-8
8. Makinae, H, Numata, N, Kitaoka, H, Daimon, M, Yamamoto, T, and Amano, A. Use of pacemaker programmers for disaster victim identification. Forensic Sci Med Pathol. (2013) 9:551–3. doi: 10.1007/s12024-013-9432-8
9. Junge, M, Weckmüller, J, Nägele, H, Püschel, K, and Rödiger, W. “Natürlicher Tod” bei deaktiviertem Implantierbaren-Cardioverter-Defibrillator (ICD)?. (2001) Available at: https://www.researchgate.net/publication/237585401_Naturlicher_Tod_bei_deaktiviertem_Implantierbaren-Cardioverter-Defibrillator_ICD
10. Junge, M, Weckmüller, J, Nägele, H, and Püschel, K. ‘Natural death’ of a patient with a deactivated implantable-cardioverter-defibrillator (ICD)? Forensic Sci Int. (2002) 125:172–7. doi: 10.1016/S0379-0738(01)00633-8
11. Charton, M, Flécher, E, Leclercq, C, Delmas, C, Dambrin, C, Goeminne, C, et al. Suicide attempts among LVAD recipients. Circulation. (2020) 141:934–6. doi: 10.1161/CIRCULATIONAHA.119.041910
12. Savarese, G, and Lund, LH. Global public health burden of heart failure. Card Fail Rev. (2017) 3:7–11. doi: 10.15420/cfr.2016:25:2
13. Savarese, G, Becher, PM, Lund, LH, Seferovic, P, Rosano, GMC, and Coats, AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118:3272–87. doi: 10.1093/cvr/cvac013
14. Bradshaw, PJ, Stobie, P, Knuiman, MW, Briffa, TG, and Hobbs, MST. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart. (2014) 1:e000177. doi: 10.1136/openhrt-2014-000177
15. Ponikowski, P, Voors, AA, Anker, SD, Bueno, H, Cleland, JGF, Coats, AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
16. McDonagh, TA, Metra, M, Adamo, M, Gardner, RS, Baumbach, A, Böhm, M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42:3599–726. doi: 10.1002/ejhf.2333
17. Baker, R, Sullivan, E, Camosso-Stefinovic, J, Rashid, A, Farooqi, A, Blackledge, H, et al. Making use of mortality data to improve quality and safety in general practice: a review of current approaches. Qual Saf Health Care. (2007) 16:84–9. doi: 10.1136/qshc.2006.019885
18. Fonarow, GC, Yancy, CW, Hernandez, AF, Peterson, ED, Spertus, JA, and Heidenreich, PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. (2011) 161:1024–30.e3. doi: 10.1016/j.ahj.2011.01.027
19. Maeda, H, Zhu, B-L, Ishikawa, T, Quan, L, and Michiue, T. Significance of postmortem biochemistry in determining the cause of death. Legal Med. (2009) 11:S46–9. doi: 10.1016/j.legalmed.2009.01.048
20. Fujimiya, T. Legal medicine and the death inquiry system in Japan: a comparative study. Legal Med. (2009) 11:S6–8. doi: 10.1016/j.legalmed.2009.02.022
21. Medizinischer Dienst Bund. Pressekonferenz Medizinischer Dienst stellt Jahresstatistik 2021 zur Behandlungsfehlerbegutachtung vor. Essen: Medizinischer Dienst Bund (2022).
22. Parker Waichman LLP-National Personal Injury Law Firm. Heart ware ventricular assist device recall. New York: Parker Waichman LLP-National Personal Injury Law Firm (2021).
23. Center for Devices and Radiological Health. Recalls related to the HVAD system, U.S. Food and Drug Administration. Available at:. (https://www.fda.gov/medical-devices/cardiovascular-devices/recalls-related-hvad-system).
24. Dash, SK, Behera, BK, and Patro, S. Accuracy in certification of cause of death in a tertiary care hospital – a retrospective analysis. J Forensic Legal Med. (2014) 24:33–6. doi: 10.1016/j.jflm.2014.03.006
25. Lehmann, M, Kohlmann, S, Gierk, B, Murray, AM, and Löwe, B. Suicidal ideation in patients with coronary heart disease and hypertension: baseline results from the DEPSCREEN-INFO clinical trial. Clin Psychol Psychother. (2018) 25:754–64. doi: 10.1002/cpp.2305
26. Riga, S, Riga, D, Geacăr, S, and Ardelean, A. Transdisciplinarity in bio-medicine, neuroscience and psychiatry: the bio-psycho-social model. Proc Rom Acad, series B; Frequency. (2014) 16:201–8.
27. Lillehei, CW, Varco, RL, Cohen, M, Warden, HE, Gott, VL, DeWall, RA, et al. The first open heart corrections of tetralogy of Fallot. A 26-31 year follow-up of 106 patients. Ann Surg. (1986) 204:490–502. doi: 10.1097/00000658-198610000-00017
28. Wu, VC-C, Chang, SH, Kuo, CF, Liu, JR, Chen, SW, Yeh, YH, et al. Suicide death rates in patients with cardiovascular diseases - a 15-year nationwide cohort study in Taiwan. J Affect Disord. (2018) 238:187–93. doi: 10.1016/j.jad.2018.05.046
29. Chernyak, Y, Teh, L, Henderson, DR, and Patel, A. Practice issues for evaluation and Management of the Suicidal Left Ventricular Assist Device Patient. Prog Transplant. (2020) 30:63–6. doi: 10.1177/1526924819893300
30. O’Connor, CM. Suicide in HF Patients. JACC: Heart Failure. (2020) 8:243–4. doi: 10.1016/j.jchf.2020.01.008
31. Celano, CM, Villegas, AC, Albanese, AM, Gaggin, HK, and Huffman, JC. Depression and anxiety in heart failure: a review. Harv Rev Psychiatry. (2018) 26:175–84. doi: 10.1097/HRP.0000000000000162
32. Sbolli, M, Fiuzat, M, Cani, D, and O’Connor, CM. Depression and heart failure: the lonely comorbidity. Eur J Heart Fail. (2020) 22:2007–17. doi: 10.1002/ejhf.1865
33. Lundgren, S, Poon, CYM, Selim, A, Lowes, BD, Zolty, R, Burdorf, A, et al. Depression and anxiety in patients undergoing left ventricular assist device implantation. Int J Artif Organs. (2017) 41:76–83. doi: 10.5301/ijao.5000650
34. Rafsanjani, MHAP, Masoudi, S, Radmanesh, M, and Bostani, Z. Comparison of depression and anxiety among pacemaker and implantable cardioverter-defibrillator recipients: a cross-sectional study. Pacing Clin Electrophysiol. (2021) 44:235–9. doi: 10.1111/pace.14152
35. Pallangyo, P, Mgopa, L, Millinga, J, Bhalia, S, Hemed, NR, Mkojera, Z, et al. Suicide attempt following pacemaker implantation in an eighty-three-year-old male: a case report. J Med Cases. (2020) 10:345–7. doi: 10.14740/jmc3383
36. Eicken, A, Kolb, C, Lange, S, Brodherr-Heberlein, S, Zrenner, B, Schreiber, C, et al. Implantable cardioverter defibrillator (ICD) in children. Int J Cardiol. (2006) 107:30–5. doi: 10.1016/j.ijcard.2005.02.048
37. Oshvandi, K, Khatiban, M, Ghanei Gheshlagh, R, and Razavi, M. The prevalence of depression in patients living with implantable cardioverter defibrillator: a systematic review and meta-analysis. Ir J Med Sci. (2020) 189:1243–52. doi: 10.1007/s11845-020-02208-4
38. Schnyder, U, Valach, L, Bichsel, K, and Michel, K. Attempted suicide. Do we understand the patients’ reasons? Gen Hosp Psychiatry. (1999) 21:62–9. doi: 10.1016/S0163-8343(98)00064-4
39. de Ornelas Maia, ACC, Soares-Filho, G, Pereira, V, Nardi, AE, and Silva, AC. Psychiatric disorders and quality of life in patients with implantable cardioverter defibrillators: a systematic review. Prim Care Companion CNS Disord. (2013) 15:PCC.12r01456. doi: 10.4088/PCC.12r01456
40. Qin, P, Hawton, K, Mortensen, PB, and Webb, R. Combined effects of physical illness and comorbid psychiatric disorder on risk of suicide in a national population study. Br J Psychiatry. (2014) 204:430–5. doi: 10.1192/bjp.bp.113.128785
41. Adee, S. The hunt for the kill switch. IEEE Spectr. (2008) 45:34–9. doi: 10.1109/MSPEC.2008.4505310
42. Masoom, H, Berbenetz, NM, Chow, J, Acosta, JG, and Amin, F. Suicide attempt by self-dissection of permanent pacemaker leads. JACC Case Rep. (2020) 2:296–9. doi: 10.1016/j.jaccas.2019.11.070
43. Che, X, Abdelwahed, YS, Wang, X, Fang, Y, and Wang, L. Pacemaker implantation in patients with major depression, should it be of concern? A case report and literature review. BMC Cardiovasc Disord. (2020) 20:279. doi: 10.1186/s12872-020-01565-3
44. Norgaard, ML, Melchior, T, Wagner, T, and Haugan, K. Suicide attempt by complete self-removal of a 12-year-old permanent pacemaker system: case report. J Cardiovasc Electrophysiol. (2014) 25:99–100. doi: 10.1111/jce.12295
45. Bordier, P, and Robert, F. Suicide by self-removal of a pacemaker. Am J Forensic Med Pathol. (2004) 25:78–9. doi: 10.1097/01.paf.0000113858.98640.d9
46. Simon, AB, Kleinman, P, and Janz, N. Suicide attempt by pacemaker system abuse: a case report with comments on the psychological adaptation of pacemaker patients. Pacing Clin Electrophysiol. (1980) 3:224–7. doi: 10.1111/j.1540-8159.1980.tb04333.x
47. Rosenthal, R, Crisafi, BR, and Coomaraswamy, RP. Manual extraction of a permanent pacemaker: an attempted suicide. Pacing Clin Electrophysiol. (1980) 3:229–31. doi: 10.1111/j.1540-8159.1980.tb04334.x
48. Hochmeister, MN, Seifert, D, Smetana, R, and Czernin, J. Suicide attempted by aiming slaughtering gun at pacemaker. Am J Forensic Med Pathol. (1989) 10:268. doi: 10.1097/00000433-198909000-00043
49. Harthorne, JW. Attempted suicide by self-removal of implanted pacemaker. Pacing Clin Electrophysiol. (1980) 3:740–1. doi: 10.1111/j.1540-8159.1980.tb05581.x
50. Schmidt, W, and Lang, K. Life-threatening dysrhythmias in severe thioridazine poisoning treated with physostigmine and transient atrial pacing. Crit Care Med. (1997) 25:1925–30. doi: 10.1097/00003246-199711000-00036
51. Heinroth, KM, Kuhn, C, Walper, R, Busch, I, Winkler, M, and Prondzinsky, R. Acute beta 1-selective beta-receptor blocker nebivolol poisoning in attempted suicide. Dtsch Med Wochenschr. (1999) 124:1230–4.
52. Avci, A, Yilmaz, A, Celik, M, Demir, K, and Keles, F. Successful treatment of suicide attempt by megadose of propafenone and captopril. Cardiovasc Toxicol. (2013) 13:230–3. doi: 10.1007/s12012-013-9201-7
53. Ballentine, JM. Pacemaker and defibrillator deactivation in competent hospice patients: an ethical consideration. Am J Hosp Palliat Care. (2005) 22:14–9. doi: 10.1177/104990910502200106
54. McGeary, A, and Eldergill, A. Medicolegal issues arising when pacemaker and implantable cardioverter defibrillator devices are deactivated in terminally ill patients. Med Sci Law. (2010) 50:40–4. doi: 10.1258/msl.2009.009006
55. Chen, J-Y, Liu, P-Y, Chen, J-H, and Lin, L-J. Safety of transvenous temporary cardiac pacing in patients with accidental digoxin overdose and symptomatic bradycardia. Cardiology. (2004) 102:152–5. doi: 10.1159/000080483
56. Cleland, JGF, Velavan, P, and Nasir, M. Fighting against sudden death: a single or multidisciplinary approach. J Interv Card Electrophysiol. (2006) 17:205–10. doi: 10.1007/s10840-006-9077-6
57. Daeschler, M, Verdino, RJ, and Kirkpatrick, JN. The ethics of unilateral implantable cardioverter defibrillators and cardiac resynchronization therapy with defibrillator deactivation: patient perspectives. Europace. (2017) 19:1343–8. doi: 10.1093/europace/euw227
58. Lundberg, AB, Bowen, KP, Baumgart, PM, and Caplan, JP. Phantom shocks and automated implantable cardioverter defibrillators. Psychosomatics. (2015) 56:94–7. doi: 10.1016/j.psym.2014.09.005
59. Ginwalla, M, Battula, S, Dunn, J, and Lewis, WR. Termination of electrocution-induced ventricular fibrillation by an implantable cardioverter defibrillator. Pacing Clin Electrophysiol. (2010) 33:510–2. doi: 10.1111/j.1540-8159.2009.02607.x
60. Fuchs, T. Suicide attempt aborted by an implantable defibrillator. Pacing Clin Electrophysiol. (2007) 30:1020. doi: 10.1111/j.1540-8159.2007.00802.x
61. Doldi, F, Reinke, F, Yilmaz, A, and Eckardt, L. Bullet-associated ventricular tachycardia: a case report. Eur Heart J Case Rep. (2021) 5:ytab101. doi: 10.1093/ehjcr/ytab101
62. Kirkpatrick, JN, Gottlieb, M, Sehgal, P, Patel, R, and Verdino, RJ. Deactivation of implantable cardioverter defibrillators in terminal illness and end of life care. Am J Cardiol. (2012) 109:91–4. doi: 10.1016/j.amjcard.2011.08.011
63. Gariboldi, V, Grisoli, D, Tarmiz, A, and Jaussaud, N. Mobile extracorporeal membrane oxygenation unit expands cardiac assist surgical programs. Ann Thorac Surg. (2010) 90:1548–52. doi: 10.1016/j.athoracsur.2010.06.091
64. Mueller, PS. Ethical and legal concerns associated with withdrawing mechanical circulatory support: a U.S. perspective. Front Cardiovasc Med. (2022) 9:897955. doi: 10.3389/fcvm.2022.897955
65. Napp, LC, Vogel-Claussen, J, Schäfer, A, Haverich, A, Bauersachs, J, Kühn, C, et al. First-in-man fully percutaneous complete bypass of heart and lung. JACC Cardiovasc Interv. (2017) 10:e231–3. doi: 10.1016/j.jcin.2017.07.047
66. Baum, C, Bohnen, S, Sill, B, Philipp, S, Damerow, H, Kluge, S, et al. Prolonged resuscitation and cardiogenic shock after intoxication with European yew (Taxus baccata): complete recovery after intermittent mechanical circulatory support. Int J Cardiol. (2015) 181:176–8. doi: 10.1016/j.ijcard.2014.11.221
67. Rygnestad, T, Moen, S, Wahba, A, Lien, S, Ingul, CB, Schrader, H, et al. Severe poisoning with sotalol and verapamil. Recovery after 4 h of normothermic CPR followed by extra corporeal heart lung assist. Acta Anaesthesiol Scand. (2005) 49:1378–80. doi: 10.1111/j.1399-6576.2005.00709.x
68. Siddaiah, L, Adhyapak, S, Jaydev, S, Shetty, G, Varghese, K, Patil, C, et al. Intra-aortic balloon pump in toxic myocarditis due to aluminum phosphide poisoning. J Med Toxicol. (2009) 5:80–3. doi: 10.1007/BF03161093
69. Langer, T, Santini, A, Bottino, N, Crotti, S, Batchinsky, AI, Pesenti, A, et al. ‘Awake’ extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care. (2016) 20:150. doi: 10.1186/s13054-016-1329-y
70. Haji, JY, Mehra, S, and Doraiswamy, P. Awake ECMO and mobilizing patients on ECMO. Indian J Thorac Cardiovasc Surg. (2021) 37:309–18. doi: 10.1007/s12055-020-01075-z
71. Tigges-Limmer, K, Schönbrodt, M, Roefe, D, Arusoglu, L, Morshuis, M, and Gummert, JF. Suicide after ventricular assist device implantation. J Heart Lung Transplant. (2010) 29:692–4. doi: 10.1016/j.healun.2009.12.005
72. Jiménez-Blanco Bravo, M, Zamorano Gómez, JL, del Prado Díaz, S, and Alonso Salinas, GL. A suicide attempt on a left ventricular assist device patient during COVID-19 pandemic: can we only blame the virus? A case report. Eur Heart J-Case Rep. (2021) 5:ytab144. doi: 10.1093/ehjcr/ytab144
73. Sladen, RN, Shulman, MA, Javaid, A, Hodgson, C, Myles, PS, Mcgiffin, D, et al. Postdischarge functional capacity, health-related quality of life, depression, anxiety, and post-traumatic stress disorder in patients receiving a Long-term left ventricular assist device. J Card Fail. (2022) 28:83–92. doi: 10.1016/j.cardfail.2021.07.019
74. Skouri, H, Shurrab, M, and Haj-Yahia, S. Saved lives at risk in the Middle East. ASAIO J. (2016) 62:359–60. doi: 10.1097/MAT.0000000000000334
75. Goldstein, NE, Mehta, D, Siddiqui, S, Teitelbaum, E, Zeidman, J, Singson, M, et al. That’s like an act of suicide’ patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med. (2008) 23:7–12. doi: 10.1007/s11606-007-0239-8
76. Derrickson, AK, Baber, JR, and Agarwal, A. A case of acute stress-induced ventricular tachycardia. Psychosom Med. (2007) 69:825. doi: 10.1097/PSY.0b013e318159e7bd
77. Winter, S, Fehske, W, Steven, D, and Sultan, A. Supplement: Perspektiven der Kardiologie-Kabellose Herzschrittmacher: Erfahrungen und Ausblick. Deutsches Ärzteblatt. (2017) 114:12. doi: 10.3238/PersKardio.2017.11.10.03
78. Kilic, A, Acker, MA, and Atluri, P. Dealing with surgical left ventricular assist device complications. J Thorac Dis. (2015) 7:2158–64. doi: 10.3978/j.issn.2072-1439.2015.10.64
79. Jezovnik, MK, Gregoric, ID, and Poredos, P. Medical complications in patients with LVAD devices. Eur Soc Cardiol. (2017) 14:37.
80. Long, B, Robertson, J, Koyfman, A, and Brady, W. Left ventricular assist devices and their complications: a review for emergency clinicians. Am J Emerg Med. (2019) 37:1562–70. doi: 10.1016/j.ajem.2019.04.050
81. Shalabi, A, Kachel, E, Kassif, Y, Faqeeh, M, Sergey, P, Sternik, L, et al. Unusual complications following left ventricular assisted device implantation: case series. J Cardiothorac Surg. (2021) 16:70. doi: 10.1186/s13019-021-01445-7
82. de Jong, MM, Lorusso, R, al Awami, F, Matteuci, F, Parise, O, Lozekoot, P, et al. Vascular complications following intra-aortic balloon pump implantation: an updated review. Perfusion. (2018) 33:96–104. doi: 10.1177/0267659117727825
83. Boulate, D, Luyt, C-E, Pozzi, M, Niculescu, M, Combes, A, Leprince, P, et al. Acute lung injury after mechanical circulatory support implantation in patients on extracorporeal life support: an unrecognized problem. Eur J Cardiothorac Surg. (2013) 44:544–50. doi: 10.1093/ejcts/ezt125
84. Carrión-Camacho, MR, Marín-León, I, Molina-Doñoro, JM, and González-López, JR. Safety of permanent pacemaker implantation: a prospective study. J Clin Med Res. (2019) 8:35. doi: 10.3390/jcm8010035
85. Gribbin, GM, Mccomb, JM, and Bexton, RS. Ventricular pacemaker upgrade: experience, complications, and recommendations. Heart. (1998) 80:420–13. doi: 10.1136/hrt.80.4.420
86. Glaser, N, Persson, M, Dalén, M, and Sartipy, U. Long-term outcomes associated with permanent pacemaker implantation after surgical aortic valve replacement. JAMA Netw Open. (2021) 4:–e2116564. doi: 10.1001/jamanetworkopen.2021.16564
87. Eberhardt, F, Bode, F, Bonnemeier, H, Boguschewski, F, Schlei, M, Peters, W, et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart. (2005) 91:500–6. doi: 10.1136/hrt.2003.025411
88. Kennergren, C, Bjurman, C, Wiklund, R, and Gäbel, J. A single-Centre experience of over one thousand lead extractions. Europace. (2009) 11:612–7. doi: 10.1093/europace/eup054
89. Bayliss, CE, Beanlands, DS, and Baird, RJ. The pacemaker-twiddler’s syndrome: a new complication of implantable transvenous pacemakers. Can Med Assoc J. (1968) 99:371–3.
90. Kowlgi, GN, Tseng, AS, Tempel, ND, Henrich, MJ, Venkatachalam, KL, Scott, L, et al. A real-world experience of atrioventricular synchronous pacing with leadless ventricular pacemakers. J Cardiovasc Electrophysiol. (2022) 33:982–93. doi: 10.1111/jce.15430
91. Lee, DS, Krahn, AD, Healey, JS, Birnie, D, Crystal, E, Dorian, P, et al. Evaluation of early complications related to De novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol. (2010) 55:774–82. doi: 10.1016/j.jacc.2009.11.029
92. Lee, JZ, Henrich, MJ, Bibby, P, Mulpuru, SK, Friedman, PA, Cha, YM, et al. Pacemaker firmware update and interrogation malfunction. Heart Rhythm Case Rep. (2019) 5:213–6. doi: 10.1016/j.hrcr.2018.12.013
93. Patel, B, and Makaryus, AN. Cardiac implantable electronic devices and cybersecurity. Expert Rev Med Devices. (2021) 18:69–77. doi: 10.1080/17434440.2021.2007075
94. Mangar, D, Atlas, GM, and Kane, PB. Electrocautery-induced pacemaker malfunction during surgery. Can J Anaesth. (1991) 38:616–8. doi: 10.1007/BF03008198
95. Zecchin, M, Morea, G, Severgnini, M, Sergi, E, Baratto Roldan, A, Bianco, E, et al. Malfunction of cardiac devices after radiotherapy without direct exposure to ionizing radiation: mechanisms and experimental data. Europace. (2016) 18:288–93. doi: 10.1093/europace/euv250
96. Sprinkle, JD, Takaro, T, and Scott, SM. Phrenic nerve stimulation as a complication of the implantable cardiac pacemaker. Circulation. (1963) 28:114–6. doi: 10.1161/01.CIR.28.1.114
97. Dalex, M, Malezieux, A, Parent, T, Zekry, D, and Serratrice, C. Phrenic nerve stimulation, a rare complication of pacemaker: a case report. Medicine. (2021) 100:e25060. doi: 10.1097/MD.0000000000025060
98. Kormos, RL, McCall, M, Althouse, A, Lagazzi, L, Schaub, R, Kormos, MA, et al. Left ventricular assist device malfunctions. Circulation. (2017) 136:1714–25. doi: 10.1161/CIRCULATIONAHA.117.027360
99. Nersesian, G, Hennig, F, Müller, M, Mulzer, J, Tsyganenko, D, Starck, C, et al. Temporary mechanical circulatory support for refractory heart failure: the German heart center Berlin experience. Ann Cardiothorac Surg. (2019) 8:76–83. doi: 10.21037/acs.2018.12.01
100. Kirklin, JK, Naftel, DC, Pagani, FD, Kormos, RL, Stevenson, L, Miller, M, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg. (2012) 144:584–603. doi: 10.1016/j.jtcvs.2012.05.044
101. McRae, K, and de Perrot, M. Principles and indications of extracorporeal life support in general thoracic surgery. J Thorac Dis. (2018) 10:S931–46. doi: 10.21037/jtd.2018.03.116
102. Pavlushkov, E, Berman, M, and Valchanov, K. Cannulation techniques for extracorporeal life support. Ann Transl Med. (2017) 5:70. doi: 10.21037/atm.2016.11.47
103. Sahli, SD, Kaserer, A, Braun, J, Halbe, M, Dahlem, Y, Spahn, MA, et al. Predictors associated with mortality of extracorporeal life support therapy for acute heart failure: single-center experience with 679 patients. J Thorac Dis. (2022) 14:1960–71. doi: 10.21037/jtd-21-1770
104. Wolbrom, D, Santore, LA, Khomutova, A, Taira, K, Cahill, J, Dhundale, K, et al. 208: does an open CHEST predict hospital mortality in adult VENOARTERIAL ECMO? Crit Care Med. (2023) 51:88. doi: 10.1097/01.ccm.0000906568.94114.30
105. Junge, M, Nägele, H, Püschel, K, and Rödiger, W. Eine Analyse von postmortal explantierten Herzschrittmachern und ICDs aus dem Jahr 2000. Aust. J Card. (2002) 9:490–4.
106. Gaudard, P, Mourad, M, Eliet, J, Zeroual, N, Culas, G, Rouvière, P, et al. Management and outcome of patients supported with Impella 5.0 for refractory cardiogenic shock. Crit Care. (2015) 19:363. doi: 10.1186/s13054-015-1073-8
107. Moses, M, Adam, M-B, Mariko, H, and Gordon, K. Percutaneous salvage of an Impella pretzel. JACC: Case Reports. (2019) 1:254–5. doi: 10.1016/j.jaccas.2019.06.017
108. Rishpon, A, Braun, R, Weinstock, MA, Kulju, S, Grenga, A, Navarrete-Dechent, C, et al. Assessment of the safety risk of Dermatoscope magnets in patients with cardiovascular implanted electronic devices. JAMA Dermatol. (2018) 154:1204–7. doi: 10.1001/jamadermatol.2018.2531
109. Shah, AD, Morris, MA, Hirsh, DS, Warnock, M, Huang, Y, Mollerus, M, et al. Magnetic resonance imaging safety in nonconditional pacemaker and defibrillator recipients: a meta-analysis and systematic review. Heart Rhythm. (2018) 15:1001–8. doi: 10.1016/j.hrthm.2018.02.019
110. Nazarian, S, Hansford, R, Rahsepar, AA, Weltin, V, McVeigh, D, Gucuk Ipek, E, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. (2017) 377:2555–64. doi: 10.1056/NEJMoa1604267
111. Bovenschulte, H, Schlüter-Brust, K, Liebig, T, Erdmann, E, Eysel, P, and Zobel, C. MRI in patients with pacemakers: overview and procedural management. Dtsch Arztebl Int. (2012) 109:270–5. doi: 10.3238/arztebl.2012.0270
112. Schulman, PM, and Rozner, MA. Case report: use caution when applying magnets to pacemakers or defibrillators for surgery. Anesth Analg. (2013) 117:422–7. doi: 10.1213/ANE.0b013e31829003a1
113. Center for Devices and Radiological Health, “Magnets in cell phones and smart watches may affect pacemakers and other implanted medical devices,” U.S. Food and Drug Administration. Available at: https://www.fda.gov/radiation-emitting-products/cell-phones/magnets-cell-phones-and-smart-watches-may-affect-pacemakers-and-other-implanted-medical-devices (Accessed May 5, 2023).
114. Price, C, Maor, R, and Shachaf, H. Using smartphones for monitoring atmospheric tides. J Atmos Sol Terr Phys. (2018) 174:1–4. doi: 10.1016/j.jastp.2018.04.015
115. Okoshi, T., Ramos, J., Nozaki, H., Nakazawa, J., Dey, A. K., and Tokuda, H.. (2015). Attelia: reducing user’s cognitive load due to interruptive notifications on smart phones, 2015 IEEE international conference on pervasive computing and communications (PerCom), pp. 96–104.
116. Mohadisdudis, H. M., and Ali, N. M.. (2014). A study of smartphone usage and barriers among the elderly, 2014 3rd international conference on user science and engineering (i-USEr).
117. Armstrong, N, Nugent, C, Moore, G, and Finlay, D. Using smartphones to address the needs of persons with Alzheimer’s disease. Ann Telecommun. (2010) 65:485–95. doi: 10.1007/s12243-010-0165-3
118. Schukro, C, Schlöglhofer, T, Khazen, C, Röhrich, M, Laufer, G, Zimpfer, D, et al. Influence of a fully magnetically levitated left ventricular assist device on functional interrogation of implantable cardioverter defibrillators. Clin Cardiol. (2019) 42:914–8. doi: 10.1002/clc.23228
119. Jacob, S, Panaich, SS, Maheshwari, R, Haddad, JW, Padanilam, BJ, and John, SK. Clinical applications of magnets on cardiac rhythm management devices. Europace. (2011) 13:1222–30. doi: 10.1093/europace/eur137
120. Nishantha Vadysinghe, A, Thambirajah, B, and Denniss, KM. Abuse of defibrillator pads: suicide by electrocution. J Forensic Legal Med. (2021) 83:102252. doi: 10.1016/j.jflm.2021.102252
121. Lelakowski, J, Majewski, J, Bednarek, J, Małecka, B, and Zabek, A. Pacemaker dependency after pacemaker implantation. Cardiol J. (2007) 14:83–6.
122. Sessa, F, Esposito, M, Messina, G, Di Mizio, G, Di Nunno, N, and Salerno, M. Sudden death in adults: a practical flow chart for pathologist guidance. Healthcare (Basel). (2021) 9:870. doi: 10.3390/healthcare9070870
123. Schyma, C, Doberentz, E, Morshuis, M, and Madea, B. Todesfall eines Patienten mit Herzunterstützungssystem. Rechtsmedizin. (2013) 23:410–4. doi: 10.1007/s00194-013-0889-2
124. Stockhausen, S, Ortmann, J, Kernbach-Wighton, G, and Madea, B. Tod eines 79 Jahre alten Mannes mit einem linksventrikulären Herzunterstützungssystem. Rechtsmedizin. (2016) 26:129–33. doi: 10.1007/s00194-015-0061-2
125. Narang, R, Cleland, JGF, Erhardt, L, Ball, SG, Coats, AJS, Cowley, AJ, et al. Mode of death in chronic heart failure. A request and proposition for more accurate classification. Eur Heart J. (1996) 17:1390–403. doi: 10.1093/oxfordjournals.eurheartj.a015074
126. Gleich, S, Viehöver, S, Stäbler, P, Graw, M, and Kraus, S. Falsch bescheinigter natürlicher Tod nach ärztlicher Leichenschau. Rechtsmedizin. (2016) 27:2–7. doi: 10.1007/s00194-016-0132-z
127. Harris, A. ‘Natural’ and ‘unnatural’ medical deaths and coronial law: a UK and international review of the medical literature on natural and unnatural death and how it applies to medical death certification and reporting deaths to coroners: natural/unnatural death: a scientific review. Med Sci Law. (2017) 57:105–14. doi: 10.1177/0025802417708948
128. Ayach, B, Malik, A, Seifer, C, and Zieroth, S. End of life decisions in heart failure: to turn off the intracardiac device or not? Curr Opin Cardiol. (2017) 32:224–8. doi: 10.1097/HCO.0000000000000366
129. Makdisi, T, and Makdisi, G. Ethical challenges and terminal deactivation of left ventricular assist device. Ann Transl Med. (2017) 5:331. doi: 10.21037/atm.2017.04.39
130. Panke, JT, Ruiz, G, Elliott, T, Pattenden, DJ, DeRenzo, EG, Rollins, EE, et al. Discontinuation of a left ventricular assist device in the home hospice setting. J Pain Symptom Manag. (2016) 52:313–7. doi: 10.1016/j.jpainsymman.2016.02.010
131. Boozang, KM. Death wish: resuscitating self-determination for the critically ill. Ariz Law Rev. (1993) 35:23–85.
132. Kelley, K. The patient self-determination act. A matter of life and death. Physician Assist. (1995) 19:59–60.
133. Potter, J. The psychological slippery slope from physician-assisted death to active euthanasia: a paragon of fallacious reasoning. Med Health Care Philos. (2019) 22:239–44. doi: 10.1007/s11019-018-9864-8
Keywords: cause of death, left ventricular assist device, mental co-morbidity in cardiac disease, end stage heart disease, ethics, implanted cardiac devices
Citation: Federspiel JM, Potente S, Abeln KB, Hennemann K, Heinbuch S, Burkhard K, Richl M, Kettner M, Lux C, Schmidt P, Verhoff MA and Ramsthaler F (2023) Forensic, legal, and clinical aspects of deaths associated with implanted cardiac devices. Front. Psychiatry. 14:1278078. doi: 10.3389/fpsyt.2023.1278078
Edited by:
Burkhard Madea, University of Bonn, GermanyReviewed by:
Toshikazu Kondo, Wakayama Medical University, JapanElke Doberentz, Innsbruck Medical University, Austria
Johanna Preuß-Wössner, Universitätsklinikum Schleswig-Hostein, Germany
Copyright © 2023 Federspiel, Potente, Abeln, Hennemann, Heinbuch, Burkhard, Richl, Kettner, Lux, Schmidt, Verhoff and Ramsthaler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan M. Federspiel, am1mZWRlcnNwaWVsQG91dGxvb2suY29t
 Jan M. Federspiel
Jan M. Federspiel Stefan Potente1
Stefan Potente1 Kai Hennemann
Kai Hennemann Mattias Kettner
Mattias Kettner