
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 25 September 2023
Sec. Mood Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1268290
 Aleksandra Gorostowicz1
Aleksandra Gorostowicz1 Sakina J. Rizvi2
Sakina J. Rizvi2 Sidney H. Kennedy2
Sidney H. Kennedy2 Adrian Andrzej Chrobak1
Adrian Andrzej Chrobak1 Dominika Dudek1
Dominika Dudek1 Katarzyna Cyranka1
Katarzyna Cyranka1 Joanna Piekarska3
Joanna Piekarska3 Eve Krawczyk4
Eve Krawczyk4 Marcin Siwek5*
Marcin Siwek5*Background: Anhedonia is the core symptom of depression. Its presence has been linked to worsened prognosis. The Dimensional Anhedonia Rating Scale (DARS) is a scale measuring desire, motivation, effort and consummatory pleasure across different domains. The aim of this paper was to confirm factor structure, assess reliability and validity of the Polish adaptation of the DARS in a clinical sample of patients with mood disorders and healthy controls (HC).
Methods: The study sample included 161 participants aged 18–65 years - 34 HC, 72 patients with bipolar disorder and 55 with major depressive disorder (in depressive episode or remission). Reliability of the Polish adaptation of the DARS was assessed using Cronbach’s α and the average inter-item correlation (AIC). Convergent and divergent validity was established by Pearson’s correlations between the DARS and the Snaith-Hamilton Pleasure Scale (SHAPS), the Quick Inventory of Depressive Symptomatology- self-report (QIDS-SR), the Hospital Anxiety and Depression Scale (HADS). The structure of the scale was examined by factor analysis.
Results: The factor structure was consistent with the original scale. Strong internal consistency for the DARS total score (Cronbach’s α = 0.95) and all subscales (0.86–0.93) was observed. The DARS demonstrated good convergent (moderate to strong correlations with measures of anhedonia and depression) and divergent validity (weak correlations with anxiety level).
Conclusion: The Polish DARS demonstrated excellent internal consistency and very good validity. The scale is a valuable contribution to the psychometrics of anhedonia measures in patients with mood disorders.
Anhedonia is defined by the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders 5th edition) diagnostic criteria as “markedly diminished interest or pleasure in all, or almost all, activities of the day, nearly every day (as indicated by either subjective account or observation)” (1). Based on the currently used diagnostic classifications for major depressive disorder (MDD) anhedonia is one of the two (1, 2) or three (3) main symptoms necessary for the diagnosis of a depressive episode. Anhedonia is also present in other psychiatric and neurological conditions: schizophrenia (4), bipolar disorder (BD) (5), substance use disorders (SUDs) (6), personality disorders (7), chronic pain (8) and Parkinson’s disease (9, 10). Clinically relevant anhedonia has been observed in about 70% of patients with MDD (11). The results of one of a recent meta-analysis indicated that patients with current MDD reported significantly higher than all other groups analyzed (i.e., patients with schizophrenia, Parkinson’s disease, chronic pain, SUD and healthy controls) (10).
The presence of anhedonia has been linked to a worsened prognosis in MDD patients – longer time to remission and recovery (12, 13), poorer treatment outcomes (14, 15) and higher suicide risk (independently of other depressive symptoms) (16, 17). Accumulating evidence suggests that commonly used first-line antidepressants (i.e., SSRIs - selective serotonin reuptake inhibitors) do not adequately treat anhedonia (18, 19) or can even induce emotional blunting and apathy (with prevalence of SSRI-induced apathy ranging between 20 and 92%) (20).
Anhedonia is a multifaceted construct reflecting deficits in reward processing (18). Results from neurobiological studies have shown that the hedonic process consists of different components, namely: interest/desire, reward anticipation, motivation/effort and consummatory pleasure (21). Thus, anhedonia is a broad term used to describe deficits across various areas: reward liking (consummatory anhedonia), wanting (motivational anhedonia) and reward learning (learning the associations between a stimuli and an outcome, based on past rewards and punishments) (22, 23). It has been observed that different parts of reward processing are characterized by both distinct and common brain circuits and neurotransmitters – for example dopamine has been typically linked to motivational deficits, while endogenous opioids may be more important for experiencing pleasure (21, 22, 24). Many areas of reward circuitry appear to play a role in the development of anhedonia, including both subcortical (nucleus accumbens, amygdala, hippocampus, insula, lateral habenula, ventral pallidum, ventral tegmental area), and cortical structures (orbitofrontal cortex, ventromedial prefrontal cortex, anterior cingulate cortex) (11, 25).
There are 4 validated measures of anhedonia that are typically used in clinical studies: the Snaith-Hamilton Pleasure Scale (SHAPS) (26), the Fawcett-Clark Pleasure Scale (FCPS) (27), the Revised Chapman Physical Anhedonia Scale (CPAS) and the Chapman Social Anhedonia Scale (CSAS) (28). These measures differ with respect to the aspects of anhedonia they measure (e.g., consummatory pleasure, motivation, effort). Some items included in the FCPS, CSAS and CPAS are culturally biased, while the SHAPS provides responders with more general categories of hedonic events/activities (potentially not detecting the most pleasant ones) (21).
In order to overcome the limitations of the above mentioned tools, a few second-generation scales measuring anhedonia have been developed (21). One such tool is the Dimensional Anhedonia Rating Scale (DARS), a 17-item scale that assesses desire, motivation, effort and consummatory pleasure across different domains (hobbies, foods/drinks, social activities and sensory experiences) (29). The DARS is unique in that individuals provide their own examples of pleasurable activities/experiences within each domain. The DARS has been validated in both clinical and non-clinical populations, and demonstrates high reliability, good convergent and divergent validity (29–31). The DARS has also demonstrated additional utility over SHAPS in predicting treatment-resistance in a population of depressed patients (29). It has been validated in German, Spanish and Chinese (30–32).
Currently only the SHAPS and CPAS/CSAS have been translated into Polish for use in clinical or research practice (33, 34). These studies have demonstrated excellent and acceptable reliability of the SHAPS and CPAS/CSAS, respectively. To our knowledge, the DARS was validated in Polish among anonymous participants from a community sample only in the context of a 2017 Master’s thesis that is yet to be published (35). We decided to perform a separate adaptation and validation in order to: (1) conduct a translation that includes a back-translation from Polish to English by a native speaker, which is an essential step for scale translation; (2) validate the DARS in a clinical sample with mood disorders as well as healthy controls; and (3) establish convergent validity with a gold standard scale (e.g., the SHAPS). Additional goals of this study were to confirm the reliability and factor structure of the DARS in Polish.
We enrolled patients from the Department of Adult, Child, and Adolescent Psychiatry, University Hospital in Cracow (both in- and outpatients) if they met the following inclusion criteria: age 18–65 years; met criteria for a DSM-5 diagnosis of MDD (first episode or recurrent depression, in depressive episode or remission) or bipolar disorder (BD; in depressive episode or remission); no severe or unstable medical illness; and no substance use disorder (apart from nicotine or caffeine) in the last 12 months. As psychiatric comorbidities are highly prevalent in patients with mood disorders (36, 37), we decided to enroll patients with additional diagnoses (anxiety disorders, eating disorders, personality disorders, attention deficit hyperactivity disorder [ADHD]) if their intensity was mild (i.e., not impairing a patient’s functioning distinctly and not being the primary reason for seeking medical help at the time of enrollment). Healthy controls (HC) were recruited from local volunteers. Inclusion criteria for the control group were: age 18–65; no history of psychiatric treatment; no use of psychotropic medications; no significant medical illness; no first degree family members with psychiatric diagnoses; no substance use disorder (apart from nicotine or caffeine).
The study was approved by the Bioethics Committee of the Jagiellonian University in Krakow (approval No. 1072.6120.45.2019). Patients and healthy controls were recruited between December 2019 and August 2022. All participants provided informed, written consent to take part in the study.
The original DARS scale was translated from English to Polish by two independent clinicians with proficiency in English (one being a native speaker of both English and Polish and another with a Master of English Philology). The final Polish version was back-translated into English by a native speaker of Polish and sent to the authors of the scale for feedback. All corrections were addressed, and the final version was accepted by the authors. The Polish version of the DARS is shown in Appendix 1.
The DARS is a self-administered 17-item scale divided into four categories – hobbies, foods/drinks, social activities (four items each) and sensory experiences (five items). For each question respondents are asked to give 2–3 examples of their own favorite activities/experiences and rate their interest, desire, motivation and pleasure “right now” on a 5-point Likert scale (Not at all = 0; Slightly = 1; Moderately = 2; Mostly = 3; Very much = 4). The final score is a sum of all items (minimum score is 0 and maximum is 68), with lower scores indicating more severe anhedonia (29).
The SHAPS is a self-administered tool with 14 items measuring pleasure from different experiences. Each question has four answers: strongly agree, agree, disagree and strongly disagree. The first two responses are rated 0 points and the last two 1 point. Total score ranges from 0 to 14 with higher scores indicating higher level of anhedonia (26).
The QIDS-SR (Quick Inventory of Depressive Symptomatology- self-report) is a 16-item scale measuring severity of depressive symptoms (during “the last 7 days”) and based on the DSM criteria for MDD. The total score ranges from 0 to 27 and higher scores indicate more severe depressive symptoms (38).
The HADS (Hospital Anxiety and Depression Scale) is a self-rated, 14-item tool measuring presence of anxiety (HADS-A: Anxiety subscale – seven items) and depression (HADS-D: Depression subscale – seven items) during the last week. Each item is scored from 0 to 3. The total scores range from 0 to 21 for each subscale, with higher scores indicating higher anxiety or depression (39, 40).
For the analysis of convergent and divergent validity it is essential to include scales assessing partially similar (i.e., depression) and different constructs (anxiety). Therefore, we decided to include additional tools measuring depressive (QIDS-SR, HAD-D) and anxiety symptoms (HAD-A).
Participants enrolled in the study were assessed during one visit to the Department of Psychiatry. The medical interview was performed by a trained clinician in order to establish and verify psychiatric diagnoses (according to the DSM-5 criteria). Both patients and HC were interviewed with a structured psychiatric interview (MINI - Mini International Neuropsychiatric Interview (41)) to ensure that the inclusion criteria for each group were met. Both medical and sociodemographic data were collected. Participants were then asked to fill in the following scales using the Polish versions: DARS, SHAPS, QIDS-SR, and HADS.
Basic socio-demographic and clinical data are presented as mean and standard deviation (SD; for normally distributed quantitative data), median with interquartile range (IQR; for non-normally distributed quantitative data) or percentages for nominal data. Normality was assessed by the analysis of histograms and z-scores for skewness and kurtosis – values <1.96 indicate approximation of the normal distribution.
Comparisons of quantitative data between two groups were performed with an independent samples t-test or Mann–Whitney test. Differences across three groups were assessed with a one-way ANOVA or Kruskal-Wallis test, depending on the normality of the data. Bonferroni correction was applied to all post-hoc tests (pairwise comparisons).
Internal reliability of the DARS total scale and the four subscales was assessed in the group of depressed MDD and BD patients using Cronbach’s α (with values ≥0.7 considered acceptable) and the average inter-item correlation (AIC) (42, 43). Convergent and divergent validity were established by calculating correlations between the total DARS score and subscale scores and the SHAPS, QIDS-SR, HADS-A (anxiety subscale) and HADS-D (depression subscale). Pearson’s or Spearman’s rank correlation coefficient was selected based on the assessment of normality of distribution.
The structure of the ‘Polish DARS’ was examined by factor analysis using direct oblimin rotation with delta = 0 (oblique rotation method was selected due to the suspected correlation between factors). Correlation matrix (demonstrating correlations between each pair of items) was checked for values lower than 0.3 or higher than 0.9. The determinant was also examined to avoid multicollinearity (value >0.00001 would be accepted). Sampling adequacy was assessed with a Kaiser-Meyer-Olkin (KMO) measure and Bartlett’s test (42). The number of factors for extraction was determined using Kaiser’s criteria (44) and analysis of a scree plot (Cattell’s method) (45). As each of these two methods yields different results, a confirmatory factor analysis (CFA) was performed to compare models. The following model fit indices were selected to compare models: root mean square error of approximation (RMSEA), comparative fit index (CFI), Tucker Lewis index (TLI), standardized root mean square residual (SRMR). Values for CFI and TLI higher than 0.95, for RMSEA <1.0 and for SRMR <0.08 were considered accurate (indicating good fit of the model) (46–48).
Statistical analyses were carried out using Statistical Package for the Social Sciences (SPSS) version 28.0. CFA was performed with IBM AMOS version 28. The level of significance was set at p < 0.05.
In total 161 Caucasian participants were included in the study – 72 patients with BD, 55 patients with MDD and 34 HC. A total of 70.8% of the BD group and 89.1% of the MDD group met criteria for a current depressive episode. The remaining patients were in clinical remission. Among the BD patients, 15 had type I, 50 had type II and 7 others had unspecified BD. Basic socio-demographic and clinical data are presented in Table 1. Distributions of age, gender, level of education, presence of medical disorders and psychiatric comorbidities were not statistically different across the BD and MDD groups. There was a significant difference between BD and MDD patients in median duration of illness (longer duration for BD patients – 7 vs. 4.5 years, p = 0.025).
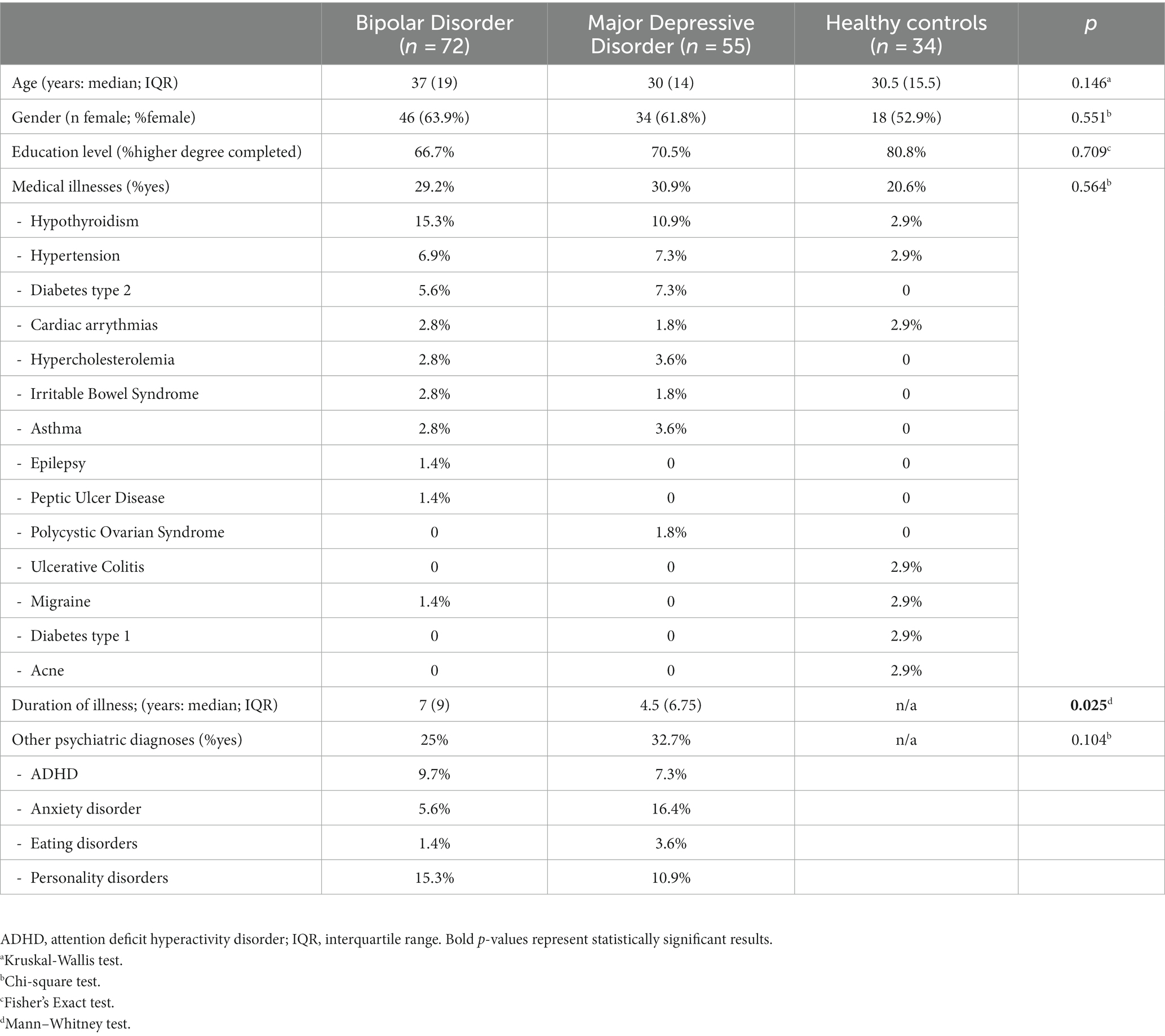
Table 1. The distribution of basic socio-demographic and clinical data across groups of BD, MDD patients and healthy controls.
The DARS scores (total and subscales) and SHAPS scores in BD and MDD patients in a current depressive episode, as well as HCs is presented in Table 2. Statistically significant differences in all measures were observed when comparing BD or MDD depressed patients with HC. No significant differences were shown when comparing anhedonia between BD and MDD depressed patients.
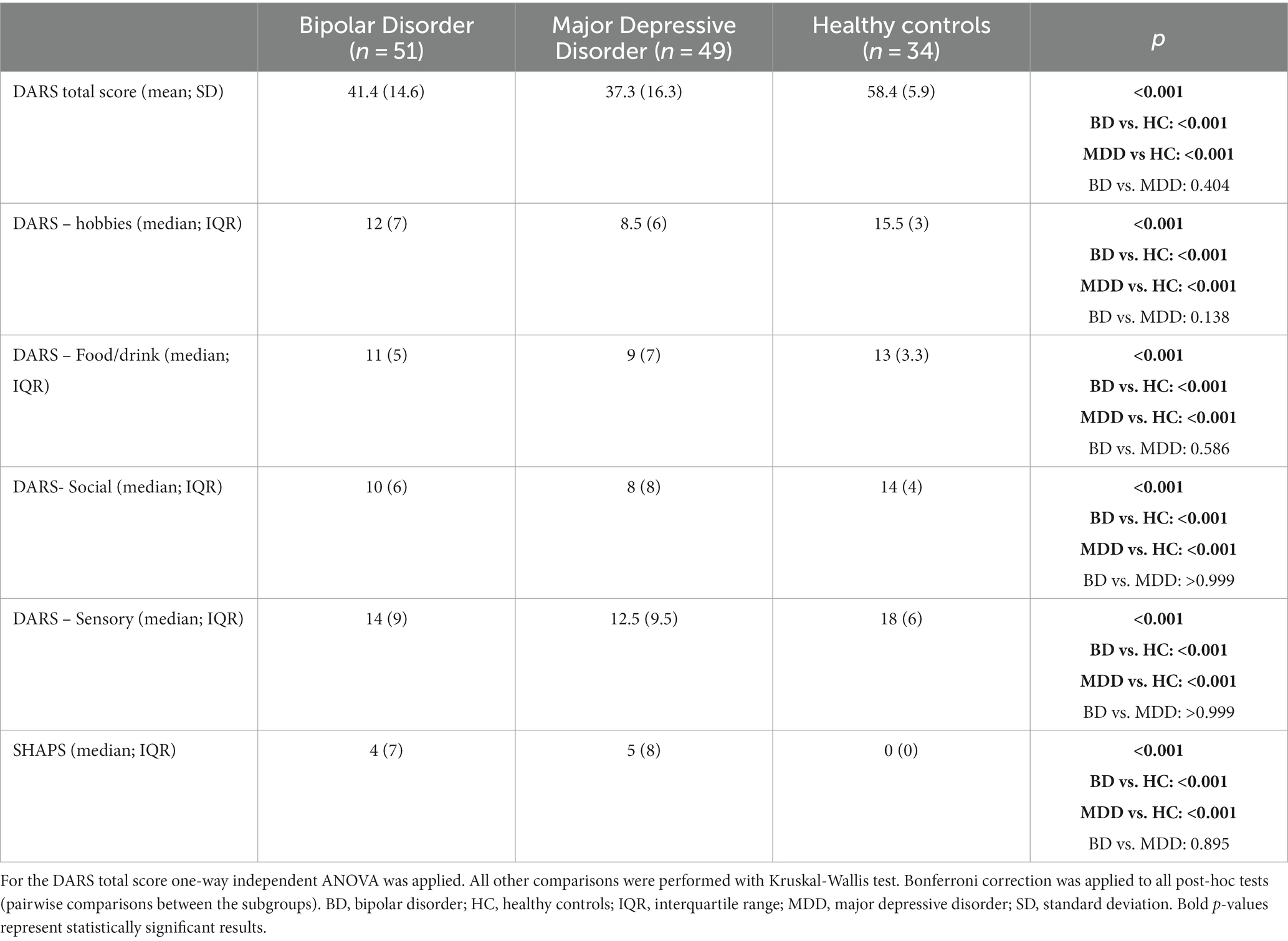
Table 2. Comparison of the distribution of anhedonia rating scales (DARS – total and subscales; SHAPS) in BD, MDD (both in a current depressive episode) and HC groups.
When the DARS total score was compared within subpopulations of patients in a current depressive episode with those in remission, statistically significant differences in mean DARS score were observed between BD depressed vs. BD remitted patients [41.4 vs. 54.2; t(67.9) = 5.18, p < 0.001] and between MDD depressed vs. MDD remitted patients (37.3 vs. 52.5; Mann–Whitney U = 61, p = 0.023; Figure 1). No statistically significant differences were shown when comparing DARS total score between remitted BD and MDD patients (U = 57, p = 0.76).
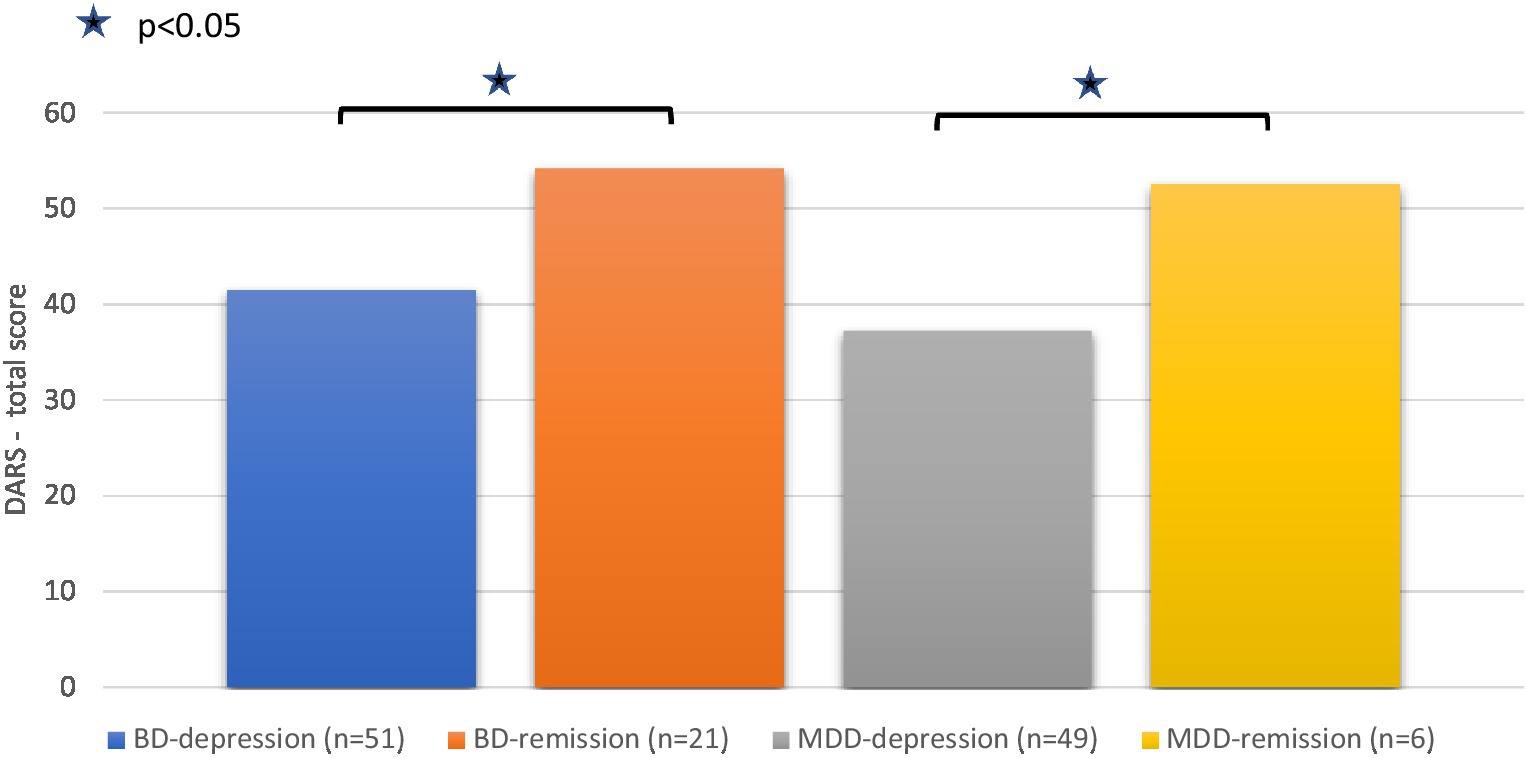
Figure 1. The DARS total score in the subgroups of BD and MDD patients (depressed and remitted). For comparing subgroups in BD and MDD patients The Independent samples t-test and Mann–Whitney test were used, respectively.
A median DARS total score was compared between patients with psychiatric comorbidities and without. In the BD depressed group, a statistically significant lower DARS score (indicating higher anhedonia) was observed among those with comorbidities (31 vs. 49.5, Mann–Whitney U = 359.5, p = 0.023). This difference was not observed in MDD depressed patients (43 vs. 32, Mann–Whitney U = 133.5, p = 0.248).
Reliability analysis was performed in the depressed BD and MDD groups. Internal consistency as measured by Cronbach’s α was high for the DARS total score (0.95) and all subscales: hobbies (0.92), foods/drinks (0.86), social activities (0.92) and sensory experiences (0.93). Analysis for Cronbach’s α after deletion of an item showed that no items would significantly increase α if deleted. The AIC for total score was 0.55 and for the subscales: 0.74 (hobbies), 0.61 (foods/drinks), 0.75 (social activities) and 0.73 (sensory experiences).
Validity analyses were performed in the group of depressed BD and MDD patients – results are presented in Table 3. The DARS total score was strongly correlated with SHAPS (rS = −0.72, p < 0.001) and HADS-D (rS = −0.72, p < 0.001) scores, moderately correlated with QIDS (rS = −0.55, p < 0.001) and weakly correlated with HADS-A (rS = −0.29, p = 0.005; negative correlation coefficients due to the fact that lower scores on the DARS represent higher level of anhedonia). All the DARS subscales were moderately correlated with SHAPS, QIDS, HADS-D, and weakly correlated with HADS-A score.
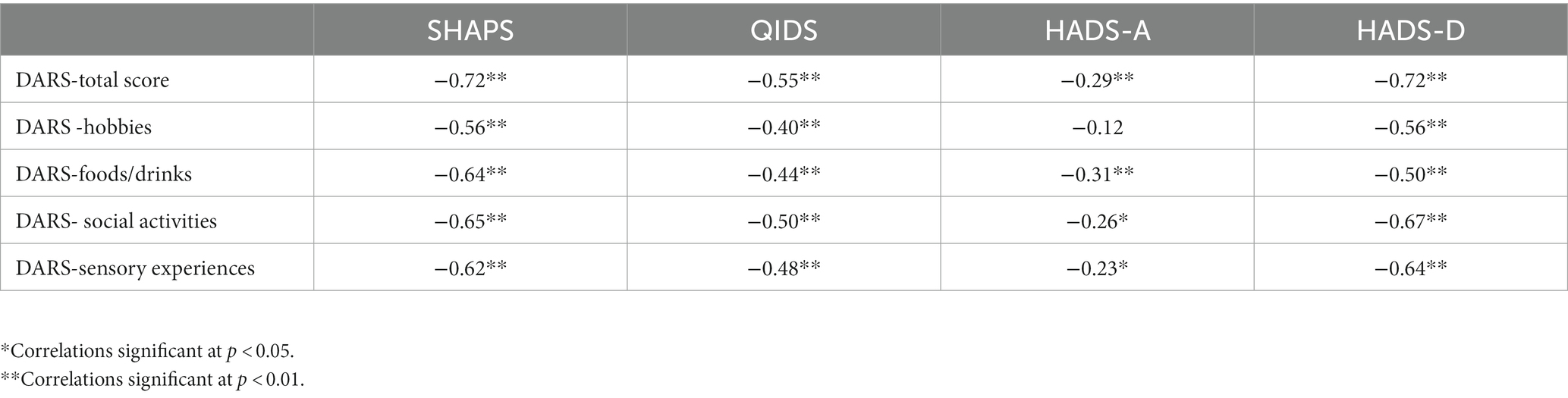
Table 3. Convergent and divergent validity of the DARS total score and subscales in depressed BD and MDD patients.
All participants were included in the factor analysis [MDD, BD patients (depressed and remitted) and HCs]. To examine the internal structure of the Polish version of the DARS, factor analysis was performed with direct oblimin rotation. Analysis of the correlation matrix and the determinant indicated lack of significant multicollinearity. KMO measure was 0.936 and Bartlett’s test was statistically significant (χ2 = 2,358,5; df = 136; p < 0.001) which indicated sampling adequacy. Kaiser’s criteria demonstrated a three-factor structure of the scale (with the fourth factor’s eigenvalue being >0.9), but the scree plot would allow retention of four factors. Therefore, CFA was performed for the three- and four-factor model. The model fit indices for the three-factor model were: RMSA = 0.098, CFI = 0.926, TLI = 0.913, SRMR = 0.0519 and for the four-factor model: RMSA = 0.072, CFI = 0.961, TLI = 0.953, SRMR = 0.0428. The model with four factors demonstrated a better fit and was analogous to the original construct of the scale – the factors corresponded to the DARS subscales (with 79.5% of variance explained) (29). Factor loadings for each item are presented in Table 4 – all values are greater than 0.4 which is substantial (42).
We collected the most common examples of activities/experiences provided by the participants for each of the subscale (Table 5).
This study demonstrated that the psychometrics of the Polish DARS were strong, and the factor structure was consistent with the original scale (29). The reliability of the Polish DARS is in line with published data on the German (31), Spanish (30), and English (29) versions of the DARS (Cronbach’s α = 0.86, 0.92, and 0.96, respectively). The AIC for the total score and subscales was also similar to the English version of the DARS (29).
The Polish DARS also demonstrated very good convergent validity – the scores of the total scale and the subscales correlated moderately to strongly with other included measures of anhedonia (SHAPS) and depression (QIDS total score, and HADS-D total score). As anhedonia is a core symptom of depression, a moderate correlation between scales measuring these two features is not surprising. However, higher than expected correlations were observed between the DARS (total and subscales) and HADS-D, with coefficients in the range from 0.50 to 0.72. This can be potentially explained by the fact that HADS-D incudes only 7 questions about depressive symptoms and nearly half of them refer to anhedonia (“I still enjoy the things I used to enjoy:”; “I look forward with enjoyment to things:”; “I can enjoy a good book or radio or TV program:”). The Polish DARS has also shown good divergent validity as it was only weakly correlated with the level of anxiety (measured by HADS-A). The validity results with the SHAPS are similar to those in other language versions of the DARS – very strong correlation with the SHAPS was observed in the original scale (rS = −0.79) and strong in the Spanish (r = −0.51) and German versions (rS = −0.50).
The Polish version of the DARS showed an ability to detect anhedonia as a “state”; the DARS total scores were statistically significantly lower in depressed MDD and BD patients when compared to those in remission (indicating higher anhedonia in the depressive phase of illness). The scale also demonstrated potential clinical utility in discriminating patients with mood disorders from controls; statistically significant differences in the distribution of the DARS (total scale and subscales) between BD/MDD depressed patients and HC were observed. The DARS total score differed significantly between BD depressed patients with and without psychiatric comorbidities - patients with comorbidities were observed to present higher anhedonia (lower DARS score).
The examples of pleasurable activities/sensations were similar to the ones listed in the original scale (29) with the exception of foods – in the Polish version participants named a few dishes typical of Polish cuisine: dumplings, stuffed cabbage, “Polish sour soup.” The examples provided support the ability of participants to produce examples that matched what each DARS domain measured.
We are aware of several limitations in our work: (1) only patients with mood disorders and healthy controls were included, which limits the generalizability of the findings to a wider psychiatric population. However, anhedonia is a core symptom of depression, so the aim of our study was to validate the DARS firstly in the group of BD and MDD patients. (2) The study only included self-rated scales. To minimize the impact of this limitation and not to rely solely on one tool, we used a variety of scales to assess convergent and divergent validity. (3) We included patients with psychiatric comorbidities. However, comorbidity is very common in the population of patients with mood disorders (36, 37). Thus, including a population with a diagnosis not limited to only MDD or BD makes our clinical sample more naturalistic. (4) Patients with mood disorders were on different pharmacotherapy regimens, which could have impacted their DARS scores. (5) The divergent validity was tested using only the anxiety subscale of the HADS tool. (6) The sample size is relatively small. However, the subject to item ratio obtained was around 9.5:1 which is close to the optimal recommended value of 10:1 (49).
Anhedonia is a common symptom of mood disorders with an important impact on prognosis. Therefore, it is clinically relevant to adequately measure anhedonia, taking into account its different dimensions and complex nature. The Polish version of the DARS demonstrated excellent internal consistency and very good validity, comparable to the original scale. Taking into consideration the limited number of tools to measure anhedonia available in Polish, the DARS is a valuable contribution to the psychometrics of anhedonia measures in patients with mood disorders.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Bioethics Committee of the Jagiellonian University in Krakow. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review and editing. SR: Methodology, Supervision, Writing – review and editing. SK: Methodology, Supervision, Writing – review and editing. AC: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – review and editing. DD: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review and editing. KC: Conceptualization, Investigation, Methodology, Writing – review and editing. JP: Conceptualization, Investigation, Methodology, Writing – review and editing. EK: Conceptualization, Investigation, Methodology, Writing – review and editing. MS: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Additional funding for the publication fee from Jagiellonian University in Krakow.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1268290/full#supplementary-material
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn. Washington DC: American Psychiatric Publishing, (2013).
2. World Health Organization. International statistical classification of diseases and related health problems. 11th edn. (2019).
3. World Health Organization (WHO). The ICD-10 classification of mental and behavioural disorders. Geneva: World Health Organization (1993).
4. Horan, WP, Kring, AM, and Blanchard, JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. (2006) 32:259–73. doi: 10.1093/schbul/sbj009
5. Dimick, MK, Hird, MA, Fiksenbaum, LM, Mitchell, RHB, and Goldstein, BI. Severe anhedonia among adolescents with bipolar disorder is common and associated with increased psychiatric symptom burden. J Psychiatr Res. (2021) 134:200–7. doi: 10.1016/j.jpsychires.2020.12.031
6. Garfield, JBB, Lubman, DI, and Yücel, M. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Aust N Z J Psychiatry. (2014) 48:36–51. doi: 10.1177/0004867413508455
7. Marissen, MAE, Arnold, N, and Franken, IHA. Anhedonia in borderline personality disorder and its relation to symptoms of impulsivity. Psychopathology. (2012) 45:179–84. doi: 10.1159/000330893
8. Garland, EL, Trostheim, M, Eikemo, M, Ernst, G, and Leknes, S. Anhedonia in chronic pain and prescription opioid misuse. Psychol Med. (2020) 50:1977–88. doi: 10.1017/S0033291719002010
9. Loas, G, Krystkowiak, P, and Godefroy, O. Anhedonia in Parkinson’s disease: an overview. J Neuropsychiatr Clin Neurosci. (2012) 24:444–51. doi: 10.1176/appi.neuropsych.11110332
10. Trøstheim, M, Eikemo, M, Meir, R, Hansen, I, Paul, E, Kroll, SL, et al. Assessment of anhedonia in adults with and without mental illness: a systematic review and Meta-analysis. JAMA Netw Open. (2020) 3:e2013233. doi: 10.1001/jamanetworkopen.2020.13233
11. Su, YA, and Si, T. Progress and challenges in research of the mechanisms of anhedonia in major depressive disorder. Gen Psychiatry. (2022) 35:e100724. doi: 10.1136/gpsych-2021-100724
12. McMakin, DL, Olino, TM, Porta, G, Dietz, LJ, Emslie, G, Clarke, G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatmentresistant depression. J Am Acad Child Adolesc Psychiatry. (2012) 51:404–11. doi: 10.1016/j.jaac.2012.01.011
13. Khazanov, GK, Xu, C, Dunn, BD, Cohen, ZD, DeRubeis, RJ, and Hollon, SD. Distress and anhedonia as predictors of depression treatment outcome: a secondary analysis of a randomized clinical trial. Behav Res Ther. (2020) 125:103507. doi: 10.1016/j.brat.2019.103507
14. Spijker, J, Bijl, RV, De Graaf, R, and Nolen, WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands mental health survey and incidence study (NEMESIS). Acta Psychiatr Scand. (2001) 103:122–30. doi: 10.1034/j.1600-0447.2001.103002122.x
15. Uher, R, Perlis, RH, Henigsberg, N, Zobel, A, Rietschel, M, Mors, O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. (2012) 42:967–80. doi: 10.1017/S0033291711001905
16. Ducasse, D, Loas, G, Dassa, D, Gramaglia, C, Zeppegno, P, Guillaume, S, et al. Anhedonia is associated with suicidal ideation independently of depression: a meta-analysis. Depress Anxiety. (2018) 35:382–92. doi: 10.1002/da.22709
17. Ballard, ED, Wills, K, Lally, N, Richards, EM, Luckenbaugh, DA, Walls, T, et al. Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord. (2017) 218:195–200. doi: 10.1016/j.jad.2017.04.057
18. Treadway, MT, and Zald, DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. (2011) 35:537–55. doi: 10.1016/j.neubiorev.2010.06.006
19. Lally, N, Nugent, AC, Luckenbaugh, DA, Niciu, MJ, Roiser, JP, and Zarate, CA. Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. (2015) 29:596–607. doi: 10.1177/0269881114568041
20. Masdrakis, VG, Markianos, M, and Baldwin, DS. Apathy associated with antidepressant drugs - a systematic review. Acta Neuropsychiatry. (2023) 35:189–204. doi: 10.1017/neu.2023.6
21. Rizvi, SJ, Pizzagalli, DA, Sproule, BA, and Kennedy, SH. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. (2016) 65:21–35. doi: 10.1016/j.neubiorev.2016.03.004
22. Borsini, A, Wallis, ASJ, Zunszain, P, Pariante, CM, and Kempton, MJ. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci. (2020) 20:816–41. doi: 10.3758/s13415-020-00804-6
23. Thomsen, KR, Whybrow, PC, and Kringelbach, ML. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front Behav Neurosci. (2015) 9:49. doi: 10.3389/fnbeh.2015.00049
24. Liu, X, Li, L, Li, M, Ren, Z, and Ma, P. Characterizing the subtype of anhedonia in major depressive disorder: a symptom-specific multimodal MRI study. Psychiatry Res Neuroimaging. (2021) 308:111239. doi: 10.1016/j.pscychresns.2020.111239
25. Wang, S, Leri, F, and Rizvi, SJ. Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog Neuro-Psychopharmacol Biol Psychiatry. (2021) 110:110289. doi: 10.1016/j.pnpbp.2021.110289
26. Snaith, RP, Hamilton, M, Morley, S, Humayan, A, Hargreaves, D, and Trigwell, P. A scale for the assessment of hedonic tone. The Snaith-Hamilton pleasure scale. Br J Psychiatry. (1995) 167:99–103. doi: 10.1192/bjp.167.1.99
27. Fawcett, J, Clark, DC, Scheftner, WA, and Gibbons, RD. Assessing anhedonia in psychiatric patients: the pleasure scale. Arch Gen Psychiatry. (1983) 40:79–84. doi: 10.1001/archpsyc.1983.01790010081010
28. Chapman, LJ, Chapman, JP, and Raulin, ML. Scales for physical and social anhedonia. J Abnorm Psychol. (1976) 85:374–82. doi: 10.1037/0021-843X.85.4.374
29. Rizvi, SJ, Quilty, LC, Sproule, BA, Cyriac, A, Michael Bagby, R, and Kennedy, SH. Development and validation of the dimensional anhedonia rating scale (DARS) in a community sample and individuals with major depression. Psychiatry Res. (2015) 229:109–19. doi: 10.1016/j.psychres.2015.07.062
30. Arrua-Duarte, E, Migoya-Borja, M, Barrigón, ML, Barahona, I, Delgado-Gomez, D, Courtet, P, et al. Spanish adaptation of the dimensional anhedonia rating scale (DARS). J Affect Disord. (2019) 245:702–7. doi: 10.1016/j.jad.2018.11.040
31. Wellan, SA, Daniels, A, and Walter, H. State anhedonia in Young healthy adults: psychometric properties of the German dimensional anhedonia rating scale (DARS) and effects of the COVID-19 pandemic. Front Psychol. (2021) 12:682824. doi: 10.3389/fpsyg.2021.682824
32. Ching, EW, Chiang, TP, Wong, JOY, Siu, BWM, Cheng, KM, Lui, SSY, et al. Validation of the Chinese dimensional anhedonia rating scale in depressed patients in Hong Kong. Psychol Test Adapt Dev. (2021) 2:72–9. doi: 10.1027/2698-1866/a000012
33. Linke-Jankowska, M, and Jankowski, KS. Social and physical anhedonia in relation to grandiose and vulnerable narcissism. Curr Issues Pers Psychol. (2021) 9:46–52. doi: 10.5114/cipp.2021.104595
34. Siwek, M, Gorostowicz, A, Chrobak, AA, Gerlich, A, Krupa, AJ, Juryk, A, et al. TED-trazodone efficacy in depression: a naturalistic study on the efficacy of trazodone in an extended-release formulation compared to SSRIs in patients with a depressive episode-preliminary report. Brain Sci. (2023) 13:86. doi: 10.3390/brainsci13010086
35. Szczypiński, J. Psychometric properties of polish adaptations of english-language anhedonia questionnaire. (2017) doi: 10.13140/RG.2.2.10080.38402
36. Loftus, J, Scott, J, Vorspan, F, Icick, R, Henry, C, Gard, S, et al. Psychiatric comorbidities in bipolar disorders: an examination of the prevalence and chronology of onset according to sex and bipolar subtype. J Affect Disord. (2020) 267:258–63. doi: 10.1016/j.jad.2020.02.035
37. Thaipisuttikul, P, Ittasakul, P, Waleeprakhon, P, Wisajun, P, and Jullagate, S. Psychiatric comorbidities in patients with major depressive disorder. Neuropsychiatr Dis Treat. (2014) 10:2097–103. doi: 10.2147/NDT.S72026
38. Rush, AJ, Trivedi, MH, Ibrahim, HM, Carmody, TJ, Arnow, B, Klein, DN, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. (2003) 54:573–83. doi: 10.1016/S0006-3223(02)01866-8
39. Brennan, C, Worrall-Davies, A, McMillan, D, Gilbody, S, and House, A. The hospital anxiety and depression scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res. (2010) 69:371–8. doi: 10.1016/j.jpsychores.2010.04.006
40. Czerwiński, S, Mackiewicz, J, Mytlewska, W, and Atroszko, P. Factorial validity, measurement invariance and concurrent validity of hospital anxiety and depression scale in a sample of polish undergraduate students. Psychiatr i Psychol Klin. (2020) 20:13–8. doi: 10.15557/PiPK.2020.0002
41. Sheehan, DV, Lecrubier, Y, Sheehan, KH, Amorim, P, Janavs, J, Weiller, E, et al. The Mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59 Suppl 20:22–33.
42. Field, A. Discovering statistics using IBM SPSS statistics. fifth ed. London: SAGE Publications (2018).
43. Cronbach, LJ. Coefficient alpha and the internal structure of tests. Psychometrika. (1951) 16:297–334. doi: 10.1007/BF02310555
44. Kaiser, HF. An index of factorial simplicity. Psychometrika. (1974) 39:31–6. doi: 10.1007/BF02291575
45. Cattell, RB. The scree test for the number of factors. Multivar Behav Res. (1966) 1:245–76. doi: 10.1207/s15327906mbr0102_10
46. Hu, LT, and Bentler, PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. (1999) 6:1–55. doi: 10.1080/10705519909540118
47. Hopwood, CJ, and Donnellan, MB. How should the internal structure of personality inventories be evaluated? Personal Soc Psychol Rev. (2010) 14:332–46. doi: 10.1177/1088868310361240
48. Finch, WH. Using fit statistic differences to determine the optimal number of factors to retain in an exploratory factor analysis. Educ Psychol Meas. (2020) 80:217–41. doi: 10.1177/0013164419865769
Keywords: anhedonia, validation, reliability, bipolar disorder, major depressive disorder, depression, reward, mood disorders
Citation: Gorostowicz A, Rizvi SJ, Kennedy SH, Chrobak AA, Dudek D, Cyranka K, Piekarska J, Krawczyk E and Siwek M (2023) Polish adaptation of the Dimensional Anhedonia Rating Scale (DARS) - validation in the clinical sample. Front. Psychiatry. 14:1268290. doi: 10.3389/fpsyt.2023.1268290
Received: 27 July 2023; Accepted: 12 September 2023;
Published: 25 September 2023.
Edited by:
Gábor Gazdag, Jahn Ferenc Dél-Pesti Kórház és Rendelőintézet, HungaryReviewed by:
Filipa Novais, Santa Maria Hospital, PortugalCopyright © 2023 Gorostowicz, Rizvi, Kennedy, Chrobak, Dudek, Cyranka, Piekarska, Krawczyk and Siwek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcin Siwek, bWFyY2luLnNpd2VrQHVqLmVkdS5wbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.