- 1Dermatology Hospital, Southern Medical University, Guangzhou, China
- 2Department of Dermatology, University of California, San Francisco, CA, United States
- 3Dermatology Service, San Francisco VA Medical Center,San Francisco, CA, United States
- 4Department of Dermatology, The People’s Hospital of Baoshan, Baoshan, China
- 5Department of Dermatology, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 6Department of Dermatology, The First Affiliated Hospital of Soochow University, Suzhou, China
Autism spectrum disorder (ASD) is a common neurological disorder. Although the etiologies of ASD have been widely speculated, evidence also supports the pathogenic role of cutaneous inflammation in autism. The prevalence of ASD is higher in individuals with inflammatory dermatoses than in those without inflammatory diseases. Anti-inflammation therapy alleviates symptoms of ASD. Recent studies suggest a link between epidermal dysfunction and ASD. In the murine model, mice with ASD display epidermal dysfunction, accompanied by increased expression levels of proinflammatory cytokines in both the skin and the brain. Children with ASD, which develops in their early lifetime, also exhibit altered epidermal function. Interestingly, improvement in epidermal function alleviates some symptoms of ASD. This line of evidence suggests a pathogenic role of cutaneous dysfunction in ASD. Either an improvement in epidermal function or effective treatment of inflammatory dermatoses can be an alternative approach to the management of ASD. We summarize here the current evidence of the association between the skin and ASD.
1. Introduction
Neurological disorders are common and can involve both children and adults with various prevalence, depending on geographic region, age, and gender. For instance, the prevalence of autism spectrum disorder (ASD), also termed autism, is approximately 2.21% in adults (1) and 1.4–2.8% in children aged 8 years in the United States (2, 3). In children, the prevalence of ASD increases with age, with a higher prevalence in boys (4.4% at the age of 10 years) than in girls (1.2% at the age of 10 years) (4). Similarly, a higher portion of adults with ASD are men (69.3% vs. 30.7%) (5). However, the gender differences in prevalence decline after age of 35 years (5). Moreover, a higher prevalence was observed in North America and high-income countries than in other regions and low-income countries (6). In adults, ASD is associated with intellectual, physical, and mental disabilities (5). Children with ASD display delayed mental development in multiple domains, with a negative correlation of developmental levels with disease severity (7). The annual medical costs for adult outpatients with ASD are five times of that for those without ASD ($4,375 vs. $824) (8). Average annual medical costs for children with ASD are nine times for those without ASD ($9,980 vs. $1102) (9). Thus, ASD is common and can negatively impact patients’ lives and economy.
ASD includes non-syndromic and syndromic autism. The latter is mainly caused by chromosomal abnormalities or monogenic alterations (10). Regarding the etiologies of non-syndromic ASD, it is still unclear yet. Although genetic and environmental factors can contribute to the development of ASD (11), evidence also indicates an association of ASD with immune function. For example, the prevalence of allergies and autoimmune diseases is higher in individuals with ASD than in those without ASD in both children and young adults (12–15). Epidemiological studies reveal a higher prevalence of ASD in individuals with inflammatory skin disorders, such as atopic dermatitis and psoriasis (12, 16). A recent study demonstrates epidermal dysfunction, which can provoke cutaneous and systemic inflammation, in children with ASD (17). In this perspective review, we summarize the evidence of a link between the skin and ASD and discuss the implication.
2. Link between dermatoses and ASD
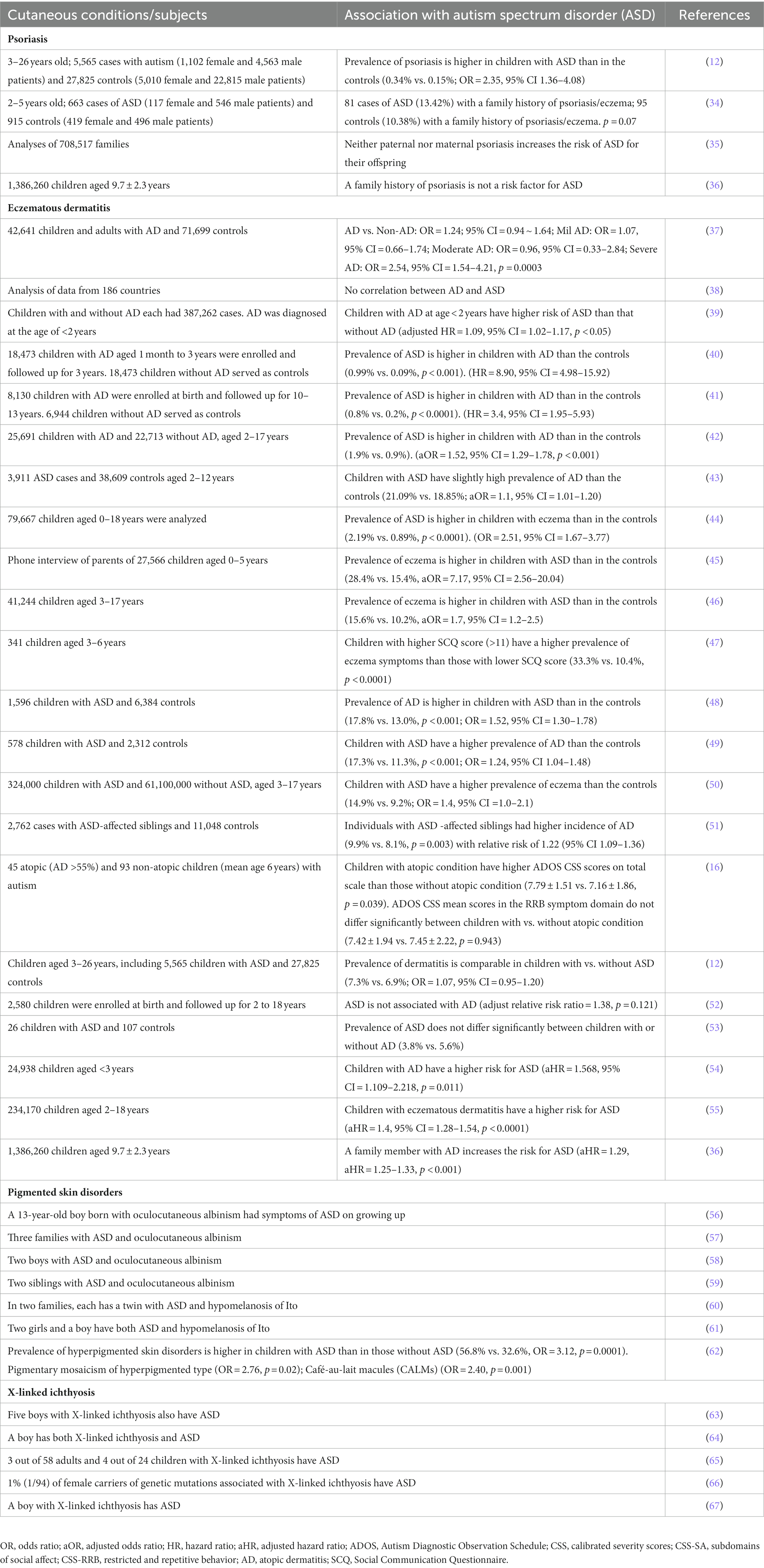
The pathogenic role of inflammation in ASD has been well demonstrated (18, 19). Individuals with ASD display the upregulation of activator protein-1-mediated neuroinflammation and insulin/insulin-like growth factor-1 signaling pathways in the brain (20) although one study showed no significant differences in expression levels of either proinflammatory or anti-inflammatory genes, including IL-1β, in both the gray and white matter of the brain in normal vs. individuals with ASD (21). Moreover, the activation state of astrocytes is increased in both the gray and white matter of prefrontal areas in individuals with ASD, indicating the presence of chronic inflammation in the brain (22). Furthermore, circulating levels of some proinflammatory cytokines, such as IL-1β, IL-6, TNF-α, and IFN-γ, are also higher in individuals with ASD than in the controls (23–25), while circulating levels of anti-inflammatory cytokines, such as IL-10 and IL-1Ra, are decreased in individuals with ASD (26). Although this line of evidence suggests a pathogenic role of inflammation in ASD, the origins of inflammation are still inconclusive. Some studies demonstrate the contribution of inflammation associated with dysbiosis and/or with the increased gut permeability to the pathogenesis of ASD (26–30). Maternal immune activation is also a risk factor for ASD in offspring (31–33). However, a great bulk of evidence indicates a link between cutaneous inflammation to ASD. Moreover, the association of other cutaneous conditions with ASD has also been reported. The evidence of the link between the skin and ASD is summarized in Table 1 (12, 16, 34–67).
2.1. Psoriasis
Psoriasis, a chronic inflammatory disorder, is linked to a number of extracutaneous comorbidities, such as cardiovascular diseases, diabetes, and obesity (68, 69). Studies also show a link between psoriasis and ASD. For example, the prevalence of psoriasis in children with ASD is twice as that in children without ASD (0.34% vs. 0.15%, OR = 2.35, 95% CI = 1.36–4.08) (12). Interestingly, a family history of psoriasis is also a risk factor for ASD for offspring (aHR = 1.55, 95% CI = 1.07–2.23, p = 0.019) (36). Although one study did not show the link between ADS and maternal immune conditions, such as psoriasis, in children (70), Croen et al. reported that 13.4% of individuals with ASD have a maternal history of psoriasis (34). The same group conducted another study in 407 cases of ASD and 2095 controls. The results showed that the mothers of 2.7% of children with ASD had psoriasis at the time of pregnancy, while the mothers of 1% of children with ASD had psoriasis in the controls (aOR = 2.7, 95% CI = 1.3–5.8, p = 0.003) (71). This phenomenon is likely due to the inflammation in the brain of offspring, induced by IL-17A, a key cytokine in psoriasis, from the mother because injection of IL-17A into fetal lateral ventricle can induce ASD-like phenotype in mice (72). The link between psoriasis and ASD is further supported by several observations in mice and humans. First, circulating levels of IL-17 are elevated in subjects with either psoriasis or ASD (including murine models) (73–75). Similarly, increased levels of IL-17A have also been demonstrated in the brain of mice with ASD (76). Second, serum IL-17A levels are positively correlated with the severity of ASD in humans (74). The inhibition of IL-17A signaling improves ASD symptoms in a mouse model of ASD (77). Similarly, the treatment of psoriasis with Secukinumab, an IL-17A inhibitor, also alleviates psychological symptoms of ASD in humans (78). Collectively, this line of evidence indicates a link between ASD and psoriasis.
2.2. Eczematous dermatitis
Eczematous dermatitis, another common inflammatory skin condition, exhibits elevated circulating levels of cytokines, including IL-17 (79, 80), which has been linked to the development of ASD (72, 81). Several epidemiological studies demonstrate an association of eczematous dermatitis with ASD. For example, up to 25% of children with ASD had atopic dermatitis (82, 83). Similarly, a study in a large cohort showed that the prevalence of eczema is higher in children with ASD than in the controls (28.4% vs. 15.4%, aOR = 7.17, 95% CI = 2.56–20.04) (45). Similarly, more than 52% of individuals with ASD have allergic manifestations (including atopic dermatitis) (vs. 10% in subjects without ASD, p < 0.001) (84). Interestingly, children with ASD-affected siblings have a higher risk for atopic dermatitis with a relative risk ratio of 1.22 (95% CI = 1.09–1.36) (incidence 9.9% vs. 8.1%, p = 0.003) (51). On the other hand, the prevalence of ASD is higher in children with atopic dermatitis than in the controls in Chinese (0.99% vs. 0.09%, p < 0.001; HR = 8.90, 95% CI = 4.98–15.92) (40). Another study conducted in the US showed that the prevalence of ASD in children with atopic dermatitis was twice of that in children without atopic dermatitis (1.9% vs. 0.9%, aOR = 1.52, 95% CI = 1.29–1.78, p < 0.001) (42). Atopic dermatitis does not only increase the risk for ASD but also increases the severity of ASD. The Autism Diagnostic Observation Schedule Calibrated Severity scores are higher in children with both ASD and atopic dermatitis than in those with ASD alone (p < 0.05) (16). However, some studies did not show differences in the prevalence of ASD between children with vs. without atopic dermatitis (12, 52, 53). The discrepant results in the association of ASD with atopic dermatitis could be due to the severity of atopic dermatitis in the individuals because only children with severe atopic dermatitis are associated with the risk for ASD (OR = 2.54, 95% CI = 1.54–42.1, p = 0.003) (37). But odds ratios of ASD in individuals with mild and moderate atopic dermatitis are 1.07 (95% CI = 0.66–1.74) and 0.96 (95% CI = 0.33–2.84), respectively (37). Additionally, mothers who have atopic eczema at the time of pregnancy increase the risk of ASD for offspring (aOR = 1.8, 95% CI = 1.0–3.4, p = 0.04) (66). Taken together, this line of evidence demonstrates a link between ASD and eczematous dermatitis.
2.3. Other skin disorders
In addition to inflammatory dermatoses, other cutaneous conditions are also associated with ASD. There are several reports of an association between hypomelanotic disorders and ASD (56–61). For example, linear and whorled hypermelanosis complicated with ASD has also been reported (85, 86). Studies showed that up to 60% of individuals with hypomelanotic disorders have ASD [reviewed in (87)]. It has been postulated that vitamin D deficiency contributes to the pathogenesis of ASD in hypomelanotic disorders. This theory could hold true to some extent. Epidemiological studies showed that lower levels of maternal vitamin D increase the risk of ASD for offspring (88) and that children with ASD have lower levels of serum vitamin D (89). Moreover, because of the sunburn concern, individuals with hypomelanotic disorders may try to avoid sun exposure, resulting in a reduction in endogenous production of active vitamin D in the body. Vitamin deficiency has been linked to inflammatory disorders (90). This assumption is also supported by several observations: (a) serum vitamin D levels are negatively correlated with levels of inflammatory markers (C-reactive protein, calprotectin, and fibrinogen) and disease severity in individuals with either Crohn’s disease or ulcerative colitis (91); (b) supplement of vitamin D lowers levels of proinflammatory cytokines in vitro and in vivo (92, 93). However, hyperpigmented skin disorder can also be complicated with ASD. Pinheiro et al. reported that a 5-year-old boy with hyperpigmentation along the lines of Blaschko was complicated with ASD (94). Thus, further studies are needed to elucidate the underlying mechanisms by which pigmented skin disorders are linked to ASD. Other cutaneous conditions, such as alopecia areata and X-linked ichthyosis, can also be complicated with ASD. For instance, the prevalence of alopecia areata in individuals with ASD is higher than in that without alopecia areata (13.61% vs. 10.43%, HR = 1.238, 95% CI = 1.100–1.395) (95). There are also several case reports of ASD complicated with X-linked ichthyosis or microphthalmia with linear skin defects syndrome (63–67, 96). Nevertheless, these multiple lines of evidence indicate a link between skin disorders and ASD.
In addition to skin disorders, stimulation of the skin with deep pressure alleviates physiological responses, including decreased heart rate and skin conductance, in children with ASD (97, 98). However, the deep pressure-induced changes in physiological function are likely attributable to the altered neural function via efferent pathways and sudomotor impulses, resulting from the discomfort caused by deep pressure (97, 99). Moreover, the contact of the skin of the subject can also influence the symptoms of ASD, depending on the sensory responses. For example, hypo-responsiveness, but not hyper-responsiveness, to tactile stimuli is associated with aberrant social and communication (100). Hence, the skin is also associated with ASD in individuals without dermatoses.
3. Association of epidermal dysfunction and ASD
As aforementioned, ASD is associated with a number of skin disorders. Recent studies also demonstrated alterations in epidermal function in ASD. In the murine model of ASD, mice with ASD exhibit dry skin and a remarkable increase in transepidermal water loss rates, an indicator of epidermal permeability barrier function, compared to normal mice (76). Similarly, adult humans with ASD also display elevated transepidermal water loss rates (76). Recently, Wang et al. compared epidermal biophysical properties in 48 control children and 56 children with ASD (17). All participants were without a current or previous history of inflammatory skin disorders. Children with ASD exhibited higher transepidermal water loss rates, lower stratum corneum hydration levels, and elevated skin surface pH (all p < 0.0001 vs. controls), indicating epidermal dysfunction in ASD. Most importantly, the ASD symptom such as the Social Responsiveness Scale was significantly improved following the correction of epidermal functional abnormalities with topical emollient (CURECODE®) twice daily for 2 months (146.40 ± 4.83 vs. 136.8 ± 5.70, t = 2.988, p < 0.01). The autism treatment evaluation checklist and repetitive behaviors scale-revised were also markedly reduced (p = 0.0777 and p = 0.0621, respectively) (101). These results suggest that ASD is also linked to epidermal dysfunction.
4. Perspectives
ASD is a common neurological disorder with a worldwide prevalence of as high as 4.36% (102). The etiologies of ASD have been widely speculated. Evidence suggests that ASD can be attributed to genetic, epigenetic, and environmental factors in addition to microbiome (10, 103–105). Indeed, a family history of ASD increases the odds of ASD in their children by 7.4–16.2 times (106). Somatic variants account for 3–5% of ASD (107, 108). If one has ASD, the other one is more likely to have it in monozygotic twins (109). Epigenetic modifications, such as DNA methylation and histone modification, also contribute to the development of ASD (105). Moreover, several studies demonstrate the role of gut microbiota in the pathogenesis of ASD (110, 111). Children with ASD have a high incidence of gastrointestinal disorders, such as constipation and diarrhea (112). Children with constipation have a higher risk of ASD (adjusted hazard ratio = 1.431, 95% CI = 1.083–1.891) (54). Both gut microbiota and their metabolic products are altered in humans and animals with ASD (113–115). In comparison to the non-ASD controls, children with ASD have higher fecal levels of Caloramator, Sarcina, and Clostridium genera and lower levels of Eubacteriaceae (116). The pathogenic role of gut microbiota in ASD is further supported by the evidence that oral probiotics during pregnancy prevent both the development of ASD symptoms and the increases in IL-6 and IL-17a levels in the brain of offspring induced by maternal immune activation (117). Similarly, orally given human commensal bacteroides fragilis alleviates ASD symptoms, improves gut permeability, and alters microbial composition in a mouse model of ASD induced by maternal immune activation (118). Apparently, the pathogenic role of microbiota in ASD is mediated by inflammation. The structural components of the bacteria can induce the production of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α (110, 119). Individuals with ASD exhibit high intestinal permeability, resulting in an increased antigenic load from the gastrointestinal tract (113). Similarly, the brain–blood barrier is also compromised in individuals with ASD (120). Cytokines, such as IL-1β, IL-6, IFN-γ, and TNF-α, released from the intestine can easily penetrate into the circulation and cross the blood–brain barrier into the brain, consequently leading to the development of ASD (110). In support of the inflammation theory, individuals with ASD display higher levels of proinflammatory cytokines in both circulation and the brain (74, 76, 117, 121), while circulating levels of anti-inflammatory cytokines, such as IL-10 and IL-1Ra, are lower in individuals with ASD vs. normal controls (122). Accordingly, an anti-inflammation regimen improves symptoms of ASD (123). Thus, inflammation plays a central role in the development of ASD.
Consistent with the notion of the central role of inflammation in ASD, inflammatory dermatoses, such as psoriasis and eczematous dermatitis, predispose to the development of ASD. First, the development of ASD occurs approximately 6 months following the first appearance of allergic disorders in children (55). Second, in the murine model of ASD, increased cytokine levels in the brain are later than those in the skin (76). Third, the treatment of psoriasis with Secukinumab improves the symptoms of ASD (78). Coupling with the evidence of a positive correlation of the severity of ASD symptoms with the severity of atopic dermatitis, cytokines of cutaneous origin can also contribute to the development of ASD. However, a maximum of 28% of individuals with ASD have atopic dermatitis (45). Not all individuals with ASD have gastrointestinal disorders. Then, the question is where the inflammation is in individuals without either gastrointestinal disorders or inflammatory skin diseases. A bulk of evidence suggests a possible contribution of epidermal dysfunction to inflammation in both circulation and the brain. First, children with ASD, but without allergic disorders nor inflammatory dermatoses, display alterations in epidermal biophysical properties, including elevations in transepidermal water loss rates and skin surface pH and reduction in stratum corneum hydration levels (17). Second, elevation in transepidermal water loss rates increases levels of proinflammatory cytokines in both circulation and the skin (124). Third, reduction in stratum corneum hydration levels increases cutaneous inflammation (125–127), while circulating levels of proinflammatory cytokines are negatively correlated with stratum corneum hydration levels in humans without apparent inflammatory cutaneous inflammation (128). Because of the huge surface area of the skin (2 m2 for adult males and 1.7 m2 for adult females) (129), even mild cutaneous inflammation induced by epidermal dysfunction can increase proinflammatory cytokines in circulation. Elevated circulating levels of proinflammatory cytokines downregulate expression levels of a tight junction protein (occludin 1), as well as vascular cell adhesion molecule 1 in the endothelial cells of the brain, leading to an increase in blood–brain permeability, subsequently resulting in inflammation in the brain (130). Finally, improvement in epidermal function lowers circulating levels of proinflammatory cytokines in both humans and mice (124, 131) and alleviates some symptoms of ASD in children without any inflammatory dermatoses in addition to mitigation of other psychological symptoms such as cognitive impairment in adults (101, 132). Hence, this pile of evidence indicates that the development of ASD can be attributable, at least in part, to epidermal dysfunction. Because of the regulatory role of epidermal function in cutaneous inflammation, improvement in epidermal function can benefit ASD.
5. Conclusion
ASD is common in both adults and children. Although therapeutic regimens, such as medication and behavior management, are available, there are still challenges in clinical practice. Medicines, such as risperidone and aripiprazole, can cause some side effects, including somnolence, extra-pyramidal symptoms, hyperprolactinemia, body weight gain, headache, and urinary incontinence. (133, 134). Moreover, either medication or behavior management can only improve symptoms without solving the fundamental problem, inflammation, a key contributor to the pathogenesis of ASD. However, recent studies showed that an improvement in epidermal function with topical emollients can lower cytokine levels in both the skin and circulation of mice and humans (124, 131). Similarly, topical emollients can both improve and prevent the development of inflammatory skin disorders, including atopic dermatitis and psoriasis (135–139), which both are associated with ASD, as mentioned above. Taken together with the evidence that topical emollients improve symptoms of ASD and mitigate the progression of cognitive impairment, which both are linked to inflammation, either improvement in epidermal function with emollients, especially in individuals with epidermal dysfunction or effective treatments of inflammatory dermatoses can be an alternative approach in the management of ASD. However, this speculation needs to be validated in appropriate clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
M-QM: Conceptualization, Writing – original draft, Writing – review & editing. SY: Data curation, Formal analysis, Investigation, Writing – original draft. TM: Writing – review & editing. GZ: Conceptualization, Data curation, Formal analysis, Writing – review & editing. TZ: Writing – review & editing, Conceptualization, Data curation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was partially supported with the resources from the Research Service, Department of Veterans Affairs Medical Center San Francisco, CA, United States.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dietz, PM, Rose, CE, McArthur, D, and Maenner, M. National and state estimates of adults with autism Spectrum disorder. J Autism Dev Disord. (2020) 50:4258–66. doi: 10.1007/s10803-020-04494-4
2. Christensen, DL, Braun, KVN, Baio, J, Bilder, D, Charles, J, Constantino, JN, et al. Prevalence and characteristics of autism Spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. (2018) 65:1–23. doi: 10.15585/mmwr.ss6513a1
3. Autism Prevalence Higher, According to Data from 11 ADDM Communities. Available at: https://www.cdc.gov/media/releases/2023/p0323-autism.html (Accessed May 2, 2023).
4. Rydzewska, E, Hughes-McCormack, LA, Gillberg, C, Henderson, A, MacIntyre, C, Rintoul, J, et al. Age at identification, prevalence and general health of children with autism: observational study of a whole country population. BMJ Open. (2019) 9:e025904. doi: 10.1136/bmjopen-2018-025904
5. Rydzewska, E, Hughes-McCormack, LA, Gillberg, C, Henderson, A, MacIntyre, C, Rintoul, J, et al. Prevalence of long-term health conditions in adults with autism: observational study of a whole country population. BMJ Open. (2018) 8:e023945. doi: 10.1136/bmjopen-2018-023945
6. Talantseva, OI, Romanova, RS, Shurdova, EM, Dolgorukova, TA, Sologub, PS, Titova, OS, et al. The global prevalence of autism spectrum disorder: a three-level meta-analysis. Front Psych. (2023) 14:1071181. doi: 10.3389/fpsyt.2023.1071181
7. Li, HH, Wang, CX, Feng, JY, Wang, B, Li, CL, and Jia, FY. A developmental profile of children with autism Spectrum disorder in China using the Griffiths mental development scales. Front Psychol. (2020) 11:570923. doi: 10.3389/fpsyg.2020.570923
8. Vohra, R, Madhavan, S, and Sambamoorthi, U. Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders. Autism. (2017) 21:995–1009. doi: 10.1177/1362361316665222
9. Mandell, DS, Cao, J, Ittenbach, R, and Pinto-Martin, J. Medicaid expenditures for children with autistic spectrum disorders: 1994 to 1999. J Autism Dev Disord. (2006) 36:475–85. doi: 10.1007/s10803-006-0088-z
10. Sauer, AK, Stanton, JE, Hans, S, and Grabrucker, AM. Autism Spectrum disorders: etiology and pathology In: AM Grabrucker, editor. Autism Spectrum disorders [internet]. Brisbane: Exon Publications (2021) Available at: https://www.ncbi.nlm.nih.gov/books/NBK573613/
11. Pendergrass, S, Girirajan, S, and Selleck, S. Uncovering the etiology of autism spectrum disorders: genomics, bioinformatics, environment, data collection and exploration, and future possibilities. Pac Symp Biocomput. (2014) 2014:422–6.
12. Zerbo, O, Leong, A, Barcellos, L, Bernal, P, Fireman, B, and Croen, LA. Immune mediated conditions in autism spectrum disorders. Brain Behav Immun. (2015) 46:232–6. doi: 10.1016/j.bbi.2015.02.001
13. Lyall, K, Van de Water, J, Ashwood, P, and Hertz-Picciotto, I. Asthma and allergies in children with autism Spectrum disorders: results from the CHARGE study. Autism Res. (2015) 8:567–74. doi: 10.1002/aur.1471
14. Tan, Y, Thomas, S, and Lee, BK. Parent-reported prevalence of food allergies in children with autism spectrum disorder: national health interview survey, 2011-2015. Autism Res. (2019) 12:802–5. doi: 10.1002/aur.2106
15. Xu, G, Snetselaar, LG, Jing, J, Liu, B, Strathearn, L, and Bao, W. Association of Food Allergy and Other Allergic Conditions with Autism Spectrum Disorder in children. JAMA Netw Open. (2018) 1:e180279. doi: 10.1001/jamanetworkopen.2018.0279
16. Jameson, C, Boulton, KA, Silove, N, and Guastella, AJ. Eczema and related atopic diseases are associated with increased symptom severity in children with autism spectrum disorder. Transl Psychiatry. (2022) 12:415. doi: 10.1038/s41398-022-02185-5
17. Wang, B, Li, YD, Wang, ZY, Zhao, JQ, Zhang, GQ, and Man, MQ. Alterations in epidermal biophysical properties in autistic children. Skin Pharmacol Physiol. (2023) 36:160–4. doi: 10.1159/000530140
18. Eissa, N, Sadeq, A, Sasse, A, and Sadek, B. Role of neuroinflammation in autism spectrum disorder and the emergence of brain histaminergic system. Lessons Also for BPSD? Front Pharmacol. (2020) 11:886. doi: 10.3389/fphar.2020.00886
19. Masi, A, Glozier, N, Dale, R, and Guastella, AJ. The immune system, cytokines, and biomarkers in autism Spectrum disorder. Neurosci Bull. (2017) 33:194–204. doi: 10.1007/s12264-017-0103-8
20. Cristiano, C, Lama, A, Lembo, F, Mollica, MP, Calignano, A, and Mattace, RG. Interplay between peripheral and central inflammation in autism Spectrum disorders: possible nutritional and therapeutic strategies. Front Physiol. (2018) 9:184. doi: 10.3389/fphys.2018.00184
21. Zhang, P, Omanska, A, Ander, BP, Gandal, MJ, Stamova, B, and Schumann, CM. Neuron-specific transcriptomic signatures indicate neuroinflammation and altered neuronal activity in ASD temporal cortex. Proc Natl Acad Sci U S A. (2023) 120:e2206758120. doi: 10.1073/pnas.2206758120
22. Sciara, AN, Beasley, B, Crawford, JD, Anderson, EP, Carrasco, T, Zheng, S, et al. Neuroinflammatory gene expression alterations in anterior cingulate cortical white and gray matter of males with autism Spectrum disorder. Autism Res. (2020) 13:870–84. doi: 10.1002/aur.2284
23. Vakilzadeh, G, Falcone, C, Dufour, B, Hong, T, Noctor, SC, and Martínez-Cerdeño, V. Decreased number and increased activation state of astrocytes in gray and white matter of the prefrontal cortex in autism. Cereb Cortex. (2022) 32:4902–12. doi: 10.1093/cercor/bhab523
24. Saghazadeh, A, Ataeinia, B, Keynejad, K, Abdolalizadeh, A, Hirbod-Mobarakeh, A, and Rezaei, N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: effects of age, gender, and latitude. J Psychiatr Res. (2019) 115:90–102. doi: 10.1016/j.jpsychires.2019.05.019
25. El-Ansary, A, and Al-Ayadhi, L. Neuroinflammation in autism spectrum disorders. J Neuroinflammation. (2012) 9:265. doi: 10.1186/1742-2094-9-265
26. Cao, X, Liu, K, Liu, J, Liu, YW, Xu, L, Wang, H, et al. Dysbiotic gut microbiota and dysregulation of cytokine profile in children and teens with autism Spectrum disorder. Front Neurosci. (2021) 15:635925. doi: 10.3389/fnins.2021.635925
27. Chen, Y, Fang, H, Li, C, Wu, G, Xu, T, Yang, X, et al. Gut Bacteria shared by children and their mothers associate with developmental level and social deficits in autism Spectrum disorder. mSphere. (2020) 5:e01044–20. doi: 10.1128/mSphere.01044-20
28. Zou, R, Xu, F, Wang, Y, Duan, M, Guo, M, Zhang, Q, et al. Changes in the gut microbiota of children with autism Spectrum disorder. Autism Res. (2020) 13:1614–25. doi: 10.1002/aur.2358
29. Ding, X, Xu, Y, Zhang, X, Zhang, L, Duan, G, Song, C, et al. Gut microbiota changes in patients with autism spectrum disorders. J Psychiatr Res. (2020) 129:149–59. doi: 10.1016/j.jpsychires.2020.06.032
30. Teskey, G, Anagnostou, E, Mankad, D, Smile, S, Roberts, W, Brian, J, et al. Intestinal permeability correlates with behavioural severity in very young children with ASD: a preliminary study. J Neuroimmunol. (2021) 357:577607. doi: 10.1016/j.jneuroim.2021.577607
31. Parker-Athill, EC, and Tan, J. Maternal immune activation and autism spectrum disorder: interleukin-6 signaling as a key mechanistic pathway. Neurosignals. (2010) 18:113–28. doi: 10.1159/000319828
32. Yasumatsu, K, Nagao, JI, Arita-Morioka, KI, Narita, Y, Tasaki, S, Toyoda, K, et al. Bacterial-induced maternal interleukin-17A pathway promotes autistic-like behaviors in mouse offspring. Exp Anim. (2020) 69:250–60. doi: 10.1538/expanim.19-0156
33. Dutra, ML, Dias, P, Freiberger, V, Ventura, L, Comim, CM, Martins, DF, et al. Maternal immune activation induces autism-like behavior and reduces brain-derived neurotrophic factor levels in the hippocampus and offspring cortex of C57BL/6 mice. Neurosci Lett. (2023) 793:136974. doi: 10.1016/j.neulet.2022.136974
34. Croen, LA, Qian, Y, Ashwood, P, Daniels, JL, Fallin, D, Schendel, D, et al. Family history of immune conditions and autism spectrum and developmental disorders: findings from the study to explore early development. Autism Res. (2019) 12:123–35. doi: 10.1002/aur.1979
35. Lee, H, Hsu, JW, Tsai, SJ, Huang, KL, Bai, YM, Su, TP, et al. Risk of attention deficit hyperactivity and autism spectrum disorders among the children of parents with autoimmune diseases: a nationwide birth cohort study. Eur Child Adolesc Psychiatry. (2023) 32:283–91. doi: 10.1007/s00787-021-01860-0
36. Li, DJ, Tsai, CS, Hsiao, RC, Chen, YL, and Yen, CF. Associations between allergic and autoimmune diseases with autism Spectrum disorder and attention-deficit/hyperactivity disorder within families: a population-based cohort study. Int J Environ Res Public Health. (2022) 19:4503. doi: 10.3390/ijerph19084503
37. Ahn, HJ, Shin, MK, Seo, JK, Jeong, SJ, Cho, AR, Choi, SH, et al. Cross-sectional study of psychiatric comorbidities in patients with atopic dermatitis and nonatopic eczema, urticaria, and psoriasis. Neuropsychiatr Dis Treat. (2019) 15:1469–78. doi: 10.2147/NDT.S191509
38. Tonacci, A, Pioggia, G, and Gangemi, S. Autism spectrum disorders and atopic dermatitis: a new perspective from country-based prevalence data. Clin Mol Allergy. (2021) 19:27. doi: 10.1186/s12948-021-00166-5
39. Liao, TC, Lien, YT, Wang, S, Huang, SL, and Chen, CY. Comorbidity of atopic disorders with autism Spectrum disorder and attention deficit/hyperactivity disorder. J Pediatr. (2016) 171:248–55. doi: 10.1016/j.jpeds.2015.12.063
40. Lee, CY, Chen, MH, Jeng, MJ, Hsu, JW, Tsai, SJ, Bai, YM, et al. Longitudinal association between early atopic dermatitis and subsequent attention-deficit or autistic disorder: a population-based case-control study. Medicine (Baltimore). (2016) 95:e5005. doi: 10.1097/MD.0000000000005005
41. Chen, MH, Su, TP, Chen, YS, Hsu, JW, Huang, KL, Chang, WH, et al. Is atopy in early childhood a risk factor for ADHD and ASD? A longitudinal study. J Psychosom Res. (2014) 77:316–21. doi: 10.1016/j.jpsychores.2014.06.006
42. Hou, A, and Silverberg, JI. Predictors and age-dependent pattern of psychologic problems in childhood atopic dermatitis. Pediatr Dermatol. (2021) 38:606–12. doi: 10.1111/pde.14588
43. Alexeeff, SE, Yau, V, Qian, Y, Davignon, M, Lynch, F, Crawford, P, et al. Medical conditions in the first years of life associated with future diagnosis of ASD in children. J Autism Dev Disord. (2017) 47:2067–79. doi: 10.1007/s10803-017-3130-4
44. Yaghmaie, P, Koudelka, CW, and Simpson, EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. (2013) 131:428–33. doi: 10.1016/j.jaci.2012.10.041
45. Garg, N, and Silverberg, JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. (2014) 112:525–32. doi: 10.1016/j.anai.2014.03.006
46. Schieve, LA, Gonzalez, V, Boulet, SL, Visser, SN, Rice, CE, Van Naarden, BK, et al. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006-2010. Res Dev Disabil. (2012) 33:467–76. doi: 10.1016/j.ridd.2011.10.008
47. Hori, D, Tsujiguchi, H, Kambayashi, Y, Hamagishi, T, Kitaoka, M, Mitoma, J, et al. The Association of Autism Spectrum Disorders and Symptoms of asthma, allergic Rhinoconjunctivitis and eczema among Japanese children aged 3-6 years. Health. (2017) 9:1235–50. doi: 10.4236/health.2017.98089
48. Chen, MH, Su, T, Chen, Y, Hsu, J, Huang, K, Chang, W, et al. Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: a nationwide population-based study. Res Autism Spectr Disord. (2013) 7:205–12. doi: 10.1016/j.rasd.2012.08.008
49. Lin, TY, Lin, PY, Su, TP, Chen, YS, Hsu, JW, Huang, KL, et al. Autistic spectrum disorder, attention deficit hyperactivity disorder, and allergy: is there a link? A nationwide study. Res Autism Spectr Disord. (2014) 8:1333–8. doi: 10.1016/j.rasd.2014.07.009
50. Gurney, JG, McPheeters, ML, and Davis, MM. Parental report of health conditions and health care use among children with and without autism: National Survey of Children's health. Arch Pediatr Adolesc Med. (2006) 160:825–30. doi: 10.1001/archpedi.160.8.825
51. Dai, YX, Tai, YH, Chang, YT, Chen, TJ, and Chen, MH. Increased risk of atopic diseases in the siblings of patients with autism Spectrum disorder: a Nationwide population-based cohort study. J Autism Dev Disord. (2019) 49:4626–33. doi: 10.1007/s10803-019-04184-w
52. Qu, X, Lee, LC, Ladd-Acosta, C, Hong, X, Ji, Y, Kalb, LG, et al. Association between atopic diseases and neurodevelopmental disabilities in a longitudinal birth cohort. Autism Res. (2022) 15:740–50. doi: 10.1002/aur.2680
53. Jyonouchi, H, Geng, L, Cushing-Ruby, A, and Quraishi, H. Impact of innate immunity in a subset of children with autism spectrum disorders: a case control study. J Neuroinflammation. (2008) 5:52. doi: 10.1186/1742-2094-5-52
54. Lee, YF, Wu, MC, Ma, KS, Huang, JY, and Wei, JC. Association of early childhood constipation with the risk of autism spectrum disorder in Taiwan: real-world evidence from a nationwide population-based cohort study. Front Psych. (2023) 14:1116239. doi: 10.3389/fpsyt.2023.1116239
55. Nemet, S, Asher, I, Yoles, I, Baevsky, T, and Sthoeger, Z. Early childhood allergy linked with development of attention deficit hyperactivity disorder and autism spectrum disorder. Pediatr Allergy Immunol. (2022) 33:10.1111/pai.13819. doi: 10.1111/pai.13819
56. Bakare, MO, and Ikegwuonu, NN. Childhood autism in a 13 year old boy with oculocutaneous albinism: a case report. J Med Case Rep. (2008) 2:56. doi: 10.1186/1752-1947-2-56
57. Delong, R. GABA(a) receptor alpha5 subunit as a candidate gene for autism and bipolar disorder: a proposed endophenotype with parent-of-origin and gain-of-function features, with or without oculocutaneous albinism. Autism. (2007) 11:135–47. doi: 10.1177/1362361307075705
58. Rogawski, MA, Funderburk, SJ, and Cederbaum, SD. Oculocutaneous albinism and mental disorder. A report of two autistic boys. Hum Hered. (1978) 28:81–5. doi: 10.1159/000152946
59. Ünsel, BG. Case report: diagnosis and treatment of attention deficit hyperactivity disorder and autism spectrum disorder in patients diagnosed with oculocutaneous albinism. Neurocase. (2020) 26:360–3. doi: 10.1080/13554794.2020.1853174
60. Zappella, M. Autism and hypomelanosis of Ito in twins. Dev Med Child Neurol. (1993) 35:826–32. doi: 10.1111/j.1469-8749.1993.tb11734.x
61. Akefeldt, A, and Gillberg, C. Hypomelanosis of Ito in three cases with autism and autistic-like conditions. Dev Med Child Neurol. (1991) 33:737–43. doi: 10.1111/j.1469-8749.1991.tb14953.x
62. Varala, S, George, R, Mathew, L, Russell, P, Koshy, B, Oommen, SP, et al. The diagnostic value of congenital and nevoid cutaneous lesions associated with autism Spectrum disorders in Indian children- a case-control study. Indian Dermatol Online J. (2021) 12:84–9. doi: 10.4103/idoj.IDOJ_275_20
63. Kent, L, Emerton, J, Bhadravathi, V, Weisblatt, E, Pasco, G, Willatt, LR, et al. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. (2008) 45:519–24. doi: 10.1136/jmg.2008.057729
64. Baek, WS, and Aypar, U. Neurological manifestations of X-linked ichthyosis: case report and review of the literature. Case Rep Genet. (2017) 2017:9086408. doi: 10.1155/2017/9086408
65. Chatterjee, S, Humby, T, and Davies, W. Behavioural and psychiatric phenotypes in men and boys with X-linked ichthyosis: evidence from a worldwide online survey. PLoS One. (2016) 11:e0164417. doi: 10.1371/journal.pone.0164417
66. Cavenagh, A, Chatterjee, S, and Davies, W. Behavioural and psychiatric phenotypes in female carriers of genetic mutations associated with X-linked ichthyosis. PLoS One. (2019) 14:e0212330. doi: 10.1371/journal.pone.0212330
67. Malik, A, Amer, AB, Salama, M, Haddad, B, Alrifai, MT, Balwi, MA, et al. X-linked ichthyosis associated with psychosis and behavioral abnormalities: a case report. J Med Case Rep. (2017) 11:267. doi: 10.1186/s13256-017-1420-2
68. Shapiro, J, Cohen, AD, David, M, Hodak, E, Chodik, G, Viner, A, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. (2007) 56:629–34. doi: 10.1016/j.jaad.2006.09.017
69. Singh, SK, and Tripathi, R. Evaluation of the Association of Metabolic Syndrome with psoriasis and its severity: a cross-sectional study. Indian J Dermatol. (2020) 65:243–4. doi: 10.4103/ijd.IJD_541_17
70. Lyall, K, Ashwood, P, Van de Water, J, and Hertz-Picciotto, I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord. (2014) 44:1546–55. doi: 10.1007/s10803-013-2017-2
71. Croen, LA, Grether, JK, Yoshida, CK, Odouli, R, and Van de Water, J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. (2005) 159:151–7. doi: 10.1001/archpedi.159.2.151
72. Fujitani, M, Miyajima, H, Otani, Y, and Liu, X. Maternal and adult interleukin-17A exposure and autism Spectrum disorder. Front Psych. (2022) 13:836181. doi: 10.3389/fpsyt.2022.836181
73. Michalak-Stoma, A, Bartosińska, J, Kowal, M, Raczkiewicz, D, Krasowska, D, and Chodorowska, G. IL-17A in the psoriatic Patients' serum and plaque scales as potential marker of the diseases severity and obesity. Mediat Inflamm. (2020) 2020:1–9. doi: 10.1155/2020/7420823
74. Al-Ayadhi, LY, and Mostafa, GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. (2012) 9:158. doi: 10.1186/1742-2094-9-158
75. Thawley, AJ, Veneziani, LP, Rabelo-da-Ponte, FD, Riederer, I, Mendes-da-Cruz, DA, and Bambini-Junior, V. Aberrant IL-17 levels in rodent models of autism Spectrum disorder: a systematic review. Front Immunol. (2022) 13:874064. doi: 10.3389/fimmu.2022.874064
76. Shin, KO, Crumrine, DA, Kim, S, Lee, Y, Kim, B, Abuabara, K, et al. Phenotypic overlap between atopic dermatitis and autism. BMC Neurosci. (2021) 22:43. doi: 10.1186/s12868-021-00645-0
77. Nadeem, A, Ahmad, SF, Al-Harbi, NO, Attia, SM, Bakheet, SA, Ibrahim, KE, et al. Nrf2 activator, sulforaphane ameliorates autism-like symptoms through suppression of Th17 related signaling and rectification of oxidant-antioxidant imbalance in periphery and brain of BTBR T+tf/J mice. Behav Brain Res. (2019) 364:213–24. doi: 10.1016/j.bbr.2019.02.031
78. Bernardini, N, Skroza, N, Marraffa, F, Prevete, E, Mambrin, A, Proietti, I, et al. A case of twins affected by psoriasis, psoriatic arthritis and autism: five years of efficacious and safe treatment with Secukinumab. Dermatol Ther. (2022) 35:e15533. doi: 10.1111/dth.15533
79. Klonowska, J, Gleń, J, Nowicki, RJ, and Trzeciak, M. New cytokines in the pathogenesis of atopic dermatitis-new therapeutic targets. Int J Mol Sci. (2018) 19:3086. doi: 10.3390/ijms19103086
80. Ruiz de Morales, JMG, Puig, L, Daudén, E, Cañete, JD, Pablos, JL, Martín, AO, et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. (2020) 19:102429. doi: 10.1016/j.autrev.2019.102429
81. Wong, H, and Hoeffer, C. Maternal IL-17A in autism. Exp Neurol. (2018) 299:228–40. doi: 10.1016/j.expneurol.2017.04.010
82. Mostafa, GA, Al Shehab, A, and Fouad, NR. Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J Child Neurol. (2010) 25:328–35. doi: 10.1177/0883073809339393
83. Mostafa, GA, and Al-Ayadhi, LY. The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. J Neuroimmunol. (2013) 261:77–81. doi: 10.1016/j.jneuroim.2013.04.003
84. Mostafa, GA, Hamza, RT, and El-Shahawi, HH. Allergic manifestations in autistic children: relation to disease severity. J Pediatr Neurol. (2015) 6:115–23. doi: 10.1055/s-0035-1557446
85. Di Lernia, V. Linear and whorled hypermelanosis. Pediatr Dermatol. (2007) 24:205–10. doi: 10.1111/j.1525-1470.2007.00387.x
86. Nehal, KS, PeBenito, R, and Orlow, SJ. Analysis of 54 cases of hypopigmentation and hyperpigmentation along the lines of Blaschko. Arch Dermatol. (1996) 132:1167–70. doi: 10.1001/archderm.1996.03890340027005
87. Bakare, MO, Munir, KM, and Kinney, DK. Associations of hypomelanotic skin disorders with autism: Do they reflect the effects of genetic mutations and epigenetic factors on vitamin-D metabolism in individuals at risk for autism? Hypothesis (Macon). (2011) 9:e2. doi: 10.5779/hypothesis.v9i1.200
88. Grant, WB, and Soles, CM. Epidemiologic evidence supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Dermatoendocrinol. (2009) 1:223–8. doi: 10.4161/derm.1.4.9500
89. Meguid, NA, Hashish, AF, Anwar, M, and Sidhom, G. Reduced serum levels of 25-hydroxy and 1,25-dihydroxy vitamin D in Egyptian children with autism. J Altern Complement Med. (2010) 16:641–5. doi: 10.1089/acm.2009.0349
90. Li, Y, Si, H, Ma, Y, Li, S, Gao, L, Liu, K, et al. Vitamin D3 affects the gut microbiota in an LPS-stimulated systemic inflammation mouse model. Microbes Infect. (2023) 105180:105180. doi: 10.1016/j.micinf.2023.105180
91. López-Muñoz, P, Beltrán, B, Sáez-González, E, Alba, A, Nos, P, and Iborra, M. Influence of vitamin D deficiency on inflammatory markers and clinical disease activity in IBD patients. Nutrients. (2019) 11:1059. doi: 10.3390/nu11051059
92. Calton, EK, Keane, KN, Newsholme, P, and Soares, MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. (2015) 10:e0141770. doi: 10.1371/journal.pone.0141770
93. Mousa, A, Naderpoor, N, Teede, H, Scragg, R, and de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2018) 76:380–94. doi: 10.1093/nutrit/nux077
94. Pinheiro, A, Mathew, MC, Thomas, M, Jacob, M, Srivastava, VM, Cherian, R, et al. The clinical profile of children in India with pigmentary anomalies along the lines of Blaschko and central nervous system manifestations. Pediatr Dermatol. (2007) 24:11–7. doi: 10.1111/j.1525-1470.2007.00325.x
95. Lee, SH, Lee, S, Kang, H, Lee, J, and Lee, WS. Increased risk of alopecia areata in patients with autism spectrum disorders: a Korean nationwide population-based study. J Am Acad Dermatol. (2023) 89:149–51. doi: 10.1016/j.jaad.2023.01.046
96. Margari, L, Colonna, A, Craig, F, Gentile, M, Giannella, G, Lamanna, AL, et al. Microphthalmia with linear skin defects (MLS) associated with autism Spectrum disorder (ASD) in a patient with familial 12.9Mb terminal Xp deletion. BMC Pediatr. (2014) 14:220. doi: 10.1186/1471-2431-14-220
97. Afif, IY, Manik, AR, Munthe, K, Maula, MI, Ammarullah, MI, Jamari, J, et al. Physiological effect of deep pressure in reducing anxiety of children with ASD during traveling: a public transportation setting. Bioengineering (Basel). (2022) 9:157. doi: 10.3390/bioengineering9040157
98. Afif, IY, Farkhan, M, Kurdi, O, Maula, MI, Ammarullah, MI, Setiyana, B, et al. Effect of short-term deep-pressure portable seat on behavioral and biological stress in children with autism Spectrum disorders: a pilot study. Bioengineering (Basel). (2022) 9:48. doi: 10.3390/bioengineering9020048
99. Ogawa, T, Asayama, M, Ito, M, and Yoshida, K. Significance of skin pressure in body heat balance. Jpn J Physiol. (1979) 29:805–16. doi: 10.2170/jjphysiol.29.805
100. Foss-Feig, JH, Heacock, JL, and Cascio, CJ. Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Res Autism Spectr Disord. (2012) 6:337–44. doi: 10.1016/j.rasd.2011.06.007
101. Wang, B, Li, YD, Wang, ZY, Zhao, JQ, Zhang, GQ, and Man, MQ. A Topical Emollient Improves Some Symptoms of Childhood Autism: An Open Label, Randomized Pilot Trial. (Manuscript in preparation).
102. Zeidan, J, Fombonne, E, Scorah, J, Ibrahim, A, Durkin, MS, Saxena, S, et al. Global prevalence of autism: a systematic review update. Autism Res. (2022) 15:778–90. doi: 10.1002/aur.2696
103. Mehra, A, Arora, G, Sahni, G, Kaur, M, Singh, H, Singh, B, et al. Gut microbiota and autism Spectrum disorder: from pathogenesis to potential therapeutic perspectives. J Tradit Complement Med. (2022) 13:135–49. doi: 10.1016/j.jtcme.2022.03.001
104. Rylaarsdam, L, and Guemez-Gamboa, A. Genetic causes and modifiers of autism Spectrum disorder. Front Cell Neurosci. (2019) 13:385. doi: 10.3389/fncel.2019.00385
105. Yoon, SH, Choi, J, Lee, WJ, and Do, JT. Genetic and epigenetic etiology underlying autism Spectrum disorder. J Clin Med. (2020) 9:966. doi: 10.3390/jcm9040966
106. Xie, S, Karlsson, H, Dalman, C, Widman, L, Rai, D, Gardner, RM, et al. The familial risk of autism Spectrum disorder with and without intellectual disability. Autism Res. (2020) 13:2242–50. doi: 10.1002/aur.2417
107. Freed, D, and Pevsner, J. The contribution of mosaic variants to autism Spectrum disorder. PLoS Genet. (2016) 12:e1006245. doi: 10.1371/journal.pgen.1006245
108. Krupp, DR, Barnard, RA, Duffourd, Y, Evans, SA, Mulqueen, RM, Bernier, R, et al. Exonic mosaic mutations contribute risk for autism Spectrum disorder. Am J Hum Genet. (2017) 101:369–90. doi: 10.1016/j.ajhg.2017.07.016
109. Castelbaum, L, Sylvester, CM, Zhang, Y, Yu, Q, and Constantino, JN. On the nature of monozygotic twin concordance and discordance for autistic trait severity: a quantitative analysis. Behav Genet. (2020) 50:263–72. doi: 10.1007/s10519-019-09987-2
110. Li, Q, Han, Y, Dy, ABC, and Hagerman, RJ. The gut microbiota and autism Spectrum disorders. Front Cell Neurosci. (2017) 11:120. doi: 10.3389/fncel.2017.00120
111. Taniya, MA, Chung, HJ, Al Mamun, A, Alam, S, Aziz, MA, Emon, NU, et al. Role of gut microbiome in autism Spectrum disorder and its therapeutic regulation. Front Cell Infect Microbiol. (2022) 12:915701. doi: 10.3389/fcimb.2022.915701
112. Coury, DL, Ashwood, P, Fasano, A, Fuchs, G, Geraghty, M, Kaul, A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. (2012) 130:S160–8. doi: 10.1542/peds.2012-0900N
113. de Magistris, L, Familiari, V, Pascotto, A, Sapone, A, Frolli, A, Iardino, P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. (2010) 51:418–24. doi: 10.1097/MPG.0b013e3181dcc4a5
114. Borre, YE, O'Keeffe, GW, Clarke, G, Stanton, C, Dinan, TG, and Cryan, JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. (2014) 20:509–18. doi: 10.1016/j.molmed.2014.05.002
115. Kushak, RI, Buie, TM, Murray, KF, Newburg, DS, Chen, C, Nestoridi, E, et al. Evaluation of intestinal function in children with autism and gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. (2016) 62:687–91. doi: 10.1097/MPG.0000000000001174
116. De Angelis, M, Piccolo, M, Vannini, L, Siragusa, S, De Giacomo, A, Serrazzanetti, DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. (2013) 8:e76993. doi: 10.1371/journal.pone.0076993
117. Wang, X, Yang, J, Zhang, H, Yu, J, and Yao, Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res. (2019) 12:576–88. doi: 10.1002/aur.2079
118. Hsiao, EY, McBride, SW, Hsien, S, Sharon, G, Hyde, ER, McCue, T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cells. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
119. Al Bander, Z, Nitert, MD, Mousa, A, and Naderpoor, N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. (2020) 17:7618. doi: 10.3390/ijerph17207618
120. Fiorentino, M, Sapone, A, Senger, S, Camhi, SS, Kadzielski, SM, Buie, TM, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. (2016) 7:49. doi: 10.1186/s13229-016-0110-z
121. Molloy, CA, Morrow, AL, Meinzen-Derr, J, Schleifer, K, Dienger, K, Manning-Courtney, P, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. (2006) 172:198–205. doi: 10.1016/j.jneuroim.2005.11.007
122. Saghazadeh, A, Ataeinia, B, Keynejad, K, Abdolalizadeh, A, Hirbod-Mobarakeh, A, and Rezaei, N. Anti-inflammatory cytokines in autism spectrum disorders: a systematic review and meta-analysis. Cytokine. (2019) 123:154740. doi: 10.1016/j.cyto.2019.154740
123. Arteaga-Henríquez, G, Gisbert, L, and Ramos-Quiroga, JA. Immunoregulatory and/or anti-inflammatory agents for the Management of Core and Associated Symptoms in individuals with autism Spectrum disorder: a narrative review of randomized. Placebo-Controlled Trials CNS Drugs. (2023) 37:215–29. doi: 10.1007/s40263-023-00993-x
124. Hu, L, Mauro, TM, Dang, E, Man, G, Zhang, J, Lee, D, et al. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J Invest Dermatol. (2017) 137:1277–85. doi: 10.1016/j.jid.2017.01.007
125. Ashida, Y, Ogo, M, and Denda, M. Epidermal interleukin-1 alpha generation is amplified at low humidity: implications for the pathogenesis of inflammatory dermatoses. Br J Dermatol. (2001) 144:238–43. doi: 10.1046/j.1365-2133.2001.04007.x
126. Ashida, Y, and Denda, M. Dry environment increases mast cell number and histamine content in dermis in hairless mice. Br J Dermatol. (2003) 149:240–7. doi: 10.1046/j.1365-2133.2003.05408.x
127. Denda, M, Sato, J, Tsuchiya, T, Elias, PM, and Feingold, KR. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. (1998) 111:873–8. doi: 10.1046/j.1523-1747.1998.00364.x
128. Yang, B, Lv, C, Ye, L, Wang, Z, Kim, Y, Luo, W, et al. Stratum corneum hydration inversely correlates with certain serum cytokine levels in the elderly, possibly contributing to inflammaging. Immun Ageing. (2023) 20:7. doi: 10.1186/s12979-023-00331-1
129. Tikuisis, P, Meunier, P, and Jubenville, CE. Human body surface area: measurement and prediction using three dimensional body scans. Eur J Appl Physiol. (2001) 85:264–71. doi: 10.1007/s004210100484
130. Elahy, M, Jackaman, C, Mamo, JC, Lam, V, Dhaliwal, SS, Giles, C, et al. Blood-brain barrier dysfunction developed during Normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing. (2015) 12:2. doi: 10.1186/s12979-015-0029-9
131. Ye, L, Mauro, TM, Dang, E, Wang, G, Hu, LZ, Yu, C, et al. Topical applications of an emollient reduce circulating pro-inflammatory cytokine levels in chronically aged humans: a pilot clinical study. J Eur Acad Dermatol Venereol. (2019) 33:2197–201. doi: 10.1111/jdv.15540
132. Ye, L, Wang, Z, Kim, Y, Elias, PM, Li, T, Wen, S, et al. A topical emollient mitigates the progression of cognitive impairment in the elderly: a randomized, open-label pilot trial. J Eur Acad Dermatol Venereol. (2022) 36:1382–8. doi: 10.1111/jdv.18162
133. Al-Huseini, S, Al-Barhoumi, A, Al-Balushi, M, Al-Hosni, A, Al-Mahrouqi, T, Al-Mahrizi, B, et al. Effectiveness and adverse effects of risperidone in children with autism Spectrum disorder in a naturalistic clinical setting at a University Hospital in Oman. Autism Res Treat. (2022) 2022:1–7. doi: 10.1155/2022/2313851
134. Turgay, A, Binder, C, Snyder, R, and Fisman, S. Long-term safety and efficacy of risperidone for the treatment of disruptive behavior disorders in children with subaverage IQs. Pediatrics. (2002) 110:e34. doi: 10.1542/peds.110.3.e34
135. Hon, KL, Kung, JSC, Ng, WGG, and Leung, TF. Emollient treatment of atopic dermatitis: latest evidence and clinical considerations. Drugs Context. (2018) 7:212530:1–14. doi: 10.7573/dic.212530
136. Techasatian, L, and Kiatchoosakun, P. Effects of an emollient application on newborn skin from birth for prevention of atopic dermatitis: a randomized controlled study in Thai neonates. J Eur Acad Dermatol Venereol. (2022) 36:76–83. doi: 10.1111/jdv.17675
137. Man, MQ, Ye, L, Hu, L, Jeong, S, Elias, PM, and Lv, C. Improvements in epidermal function prevent relapse of psoriasis: a self-controlled study. Clin Exp Dermatol. (2019) 44:654–7. doi: 10.1111/ced.13888
138. Seité, S, Khemis, A, Rougier, A, and Ortonne, JP. Emollient for maintenance therapy after topical corticotherapy in mild psoriasis. Exp Dermatol. (2009) 18:1076–8. doi: 10.1111/j.1600-0625.2009.00903.x
Keywords: epidermal permeability barrier, hydration, stratum corneum, autism, inflammation
Citation: Man M-Q, Yang S, Mauro TM, Zhang G and Zhu T (2023) Link between the skin and autism spectrum disorder. Front. Psychiatry. 14:1265472. doi: 10.3389/fpsyt.2023.1265472
Edited by:
Masashi Fujitani, Shimane University, JapanReviewed by:
Magdalena Budisteanu, Prof. Dr. Alexandru Obregia Psychiatry Hospital, RomaniaMuhammad Imam Ammarullah, Universitas Pasundan, Indonesia
Copyright © 2023 Man, Yang, Mauro, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mao-Qiang Man, bXFtYW5AaG90bWFpbC5jb20=; Tingting Zhu, emh1dGluZ3RpbmcxMDVAdmlwLjE2My5jb20=
†These authors have contributed equally to this work
 Mao-Qiang Man
Mao-Qiang Man Shuyun Yang4†
Shuyun Yang4† Guoqiang Zhang
Guoqiang Zhang