- 1Center for Psychiatry and Psychotherapy, Triaplus Clinic Zugersee, Zug, Switzerland
- 2Department of Consultation-Liaison-Psychiatry and Psychosomatic Medicine, University Hospital of Zurich, Zurich, Switzerland
- 3Faculty of Medicine, University of Zurich, Zurich, Switzerland
- 4Privatklinik Hohenegg, Meilen, Switzerland
Objective: Delirium is an acute, life-threatening neuropsychiatric disorder frequently occurring among hospitalized patients. Antipsychotic medications are often recommended for delirium management but are associated with cardiovascular risks. This study aimed to investigate the frequency and magnitude of QTc interval prolongation and clinically relevant side effects occurring in delirium patients managed with haloperidol and/or pipamperone.
Methods: This descriptive retrospective cohort study evaluated 102 elderly (mean age: 73.2 years) inpatients with delirium treated with either haloperidol, pipamperone, a combination of both, or neither in a naturalistic setting over the course of up to 20 days or until the end of delirium.
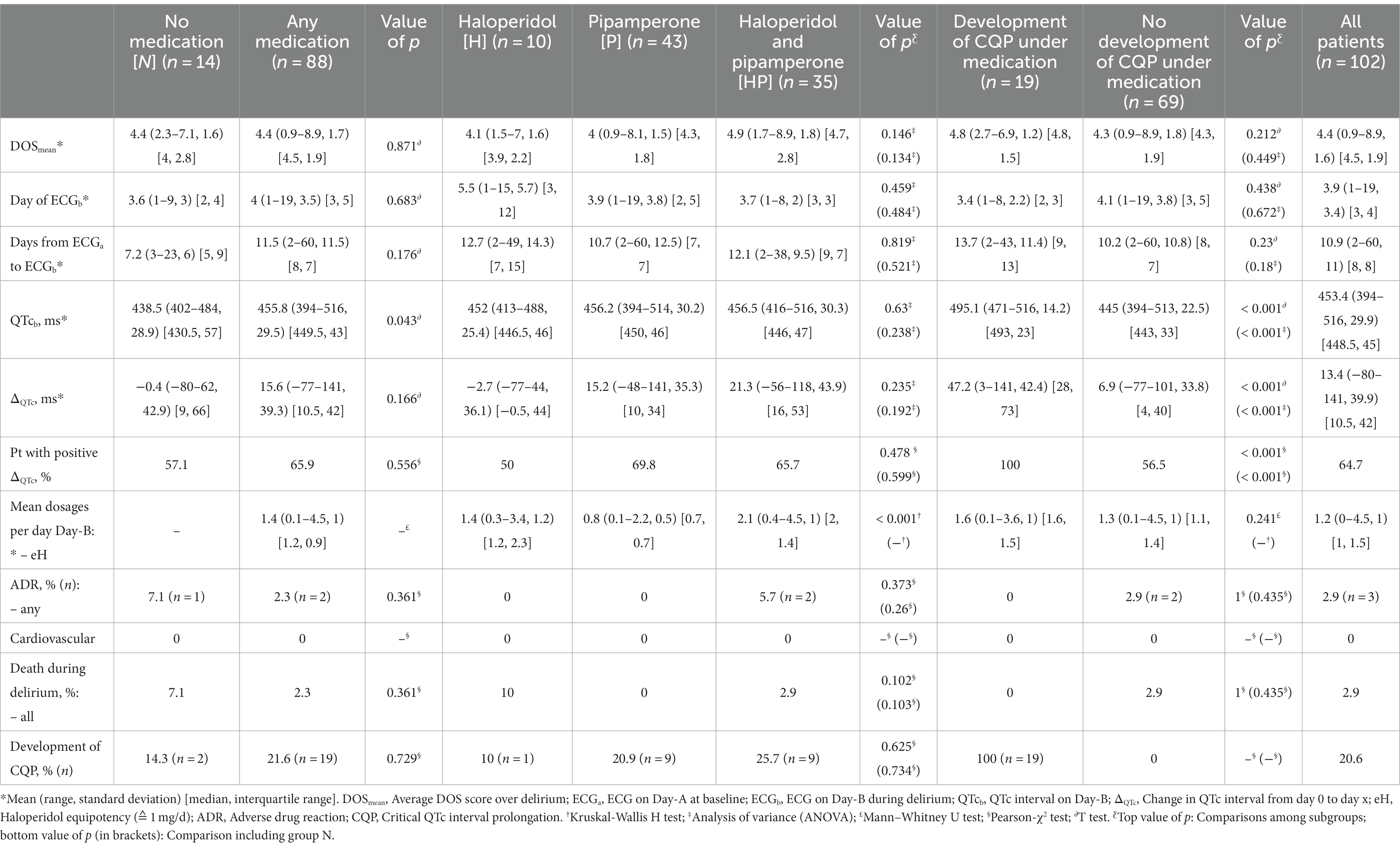
Results: A total of 86.3% of patients were treated with haloperidol and/or pipamperone at a mean daily haloperidol-equipotent dose of 1.2 ± 1 mg. Non-cardiovascular side effects were registered in 2.9% of all patients and correlated with higher scores on the Delirium Observation Screening Scale. They did not occur more frequently under antipsychotic treatment. The frequency of QTc interval prolongation was comparably common among all groups, but prolongation magnitude was higher under antipsychotic treatment. It was positively correlated with antipsychotic dosage and the total number of QTc interval-prolonging substances administered. Critical QTc interval prolongation was registered in 21.6% (n = 19) of patients in the group treated with antipsychotics compared to 14.3% (n = 2) of patients in the unmedicated group; however, the difference was not statistically significant. Polypharmacy was associated with a higher risk of critical QTc interval prolongation and increased mortality during delirium.
Conclusion: Delirium treatment with haloperidol and/or pipamperone was not associated with a higher risk of QTc-interval prolongation in this naturalistic patient sample but was greater in magnitude and correlated with equipotent dosage and the number of QT interval-prolonging substances used. Polypharmacy was associated with higher mortality and increased risk of critical QTc prolongation.
1. Introduction
Delirium is an acute, potentially life-threatening neuropsychiatric disorder (1) that frequently occurs in hospitalized patients and is characterized by fluctuating vigilance, disorganized thinking, affective lability, vegetative disinhibition, and psychotic symptoms. It is categorized into three subtypes: hypoactive, dominated by apathy and a decrease in executive functions; hyperactive delirium with predominant restlessness and often aggressive behavior; and the mixed form of delirium in which characteristics of both conditions are present, changing within the course of hours. The complex pathophysiology of delirium involves various neurotransmitter systems (e.g., acetylcholine, glutamate, and gamma-aminobutyric acid) and is not fully understood (2). Estimated prevalence rates of delirium among medical inpatients vary between 11 and 42% (1), and it tends to be underdiagnosed, especially its hypoactive subtype (3). In intensive care unit (ICU) settings, the prevalence can easily exceed 80% (4). Correct diagnosis and consecutive management of delirium are crucial since its persistence is associated with prolonged hospitalizations, elevated risk for various complications, residual neurocognitive deficits, and increased mortality (5).
Treatment of delirium is a challenging issue and can be partially contradictory in its dilemma of providing optimal relief for suffering patients while also following the “Nihil nocere” principle of primarily not causing harm through therapy (6). Treatment guidelines for non-withdrawal delirium primarily recommend the elimination of underlying causes (e.g., anemia, electrolyte imbalance, infections, drug effects, and pain) and non-psychopharmacological strategies such as orientation aids, mobilization, optimization of circadian activation, involvement of family, or reduction of environmental stimuli (7). In addition, antipsychotics are frequently prescribed, especially if severe agitation and distress occur; however, their use correlates with increased mortality (8) and provides conflicting results on the duration and outcome of delirium (9, 10). Haloperidol is usually considered the established standard (7), although, in practice, other antipsychotics are frequently used as well (e.g., risperidone, pipamperone, and quetiapine) and have been found to be effective (11, 12). While some authors point out the delirogenic potential of benzodiazepines (13), a recent meta-analysis demonstrated better outcomes from the addition of lorazepam to haloperidol (1).
A common side effect of any antipsychotic pharmacotherapy is the prolongation of QTc interval. It is caused by the blocking of specific cardiac cellular hERG-potassium channels (14) and can lead to lethal ventricular arrhythmia (torsades de pointes), although the relation between the risk for torsades de pointes and the magnitude of QTc interval prolongation is not strictly linear (15), with the latter nonetheless being an important surrogate marker (16). Not all antipsychotics have the same pro-arrhythmic potential (14), and our knowledge about the relative cardiac risks under different combinations of antipsychotic drugs is still insufficient. In this study, we retrospectively evaluated QTc intervals and other possible therapy side effects among 102 cases of inpatients with delirium receiving either no pharmacotherapy, haloperidol, pipamperone, or both.
2. Materials and methods
2.1. Patients
In this retrospective, descriptive cohort study, 102 patients, who were treated at different departments of the University Hospital Zurich between March 1, 2012, and June 26, 2015, were selected from an ongoing delirium evaluation that was part of a larger research project. Inclusion criteria were adult age (18 years or older by the time of admission), a diagnosis of hyperactive, hypoactive, or mixed delirium, an existing electrocardiography (ECG) performed at baseline within 60 days before the onset of delirium (ECGa, Day-A), and a further ECG performed later during delirium (ECGb, Day-B). We included only patients who received no potentially QTc interval-prolonging substances (QIPSs) other than haloperidol and pipamperone on Day-B or who had an established therapy with any QIPS continued and unaltered between days a and b.
We used the CredibleMeds® database (17) from the Arizona Center for Education and Research on Therapeutics (AZCERT) as a reference to determine QTc interval-prolonging potential of QIPSs.
2.2. Ethics approval and consent to participate
This study is part of a larger research project, which was approved by the Ethics Committee of the canton of Zurich, Switzerland (KEK-ZH-Nr: 2012-0263), and was carried out in accordance with the Declaration of Helsinki, considering local regulations and standards.
2.3. Definition of delirium
Delirium was defined by fulfilling its diagnostic criteria according to the tenth edition of the International Classification of Mental and Behavioral Disorders, ICD-10 (alterations of consciousness and attention span, cognitive decline, psychomotor change, sleep disturbances, and affective symptoms with typical circadian fluctuation) (18) and by scoring positively on the 13-item Delirium Observation Screening Scale (DOS) (19). From suspicion of incident delirium, DOS scores were measured by specifically trained nursing staff every 8 h, and delirium assessment was performed until the end of delirium, 20 days at maximum. The first day of delirium was defined as day 1. Persistence of delirium was assumed if the mean DOS score during that day exceeded 2, at least one DOS score during the day exceeded 3, and/or at least two DOS scores exceeded 2. Remission of delirium was defined as a continuous absence of delirium over 24 h. Single missing DOS scores were reconstructed by calculating the arithmetic means of their adjacent scores. We calculated the mean DOS score on day 1 (DOS1) and over the duration of delirium (DOSmean).
2.4. Delirium management
Antipsychotics were used (haloperidol and pipamperone), sometimes combined with various benzodiazepines (midazolam, lorazepam, diazepam, alprazolam, triazolam, oxazepam, bromazepam, and clonazepam), to manage delirium. All patients received standard non-pharmacological antidelirious therapy. Patients treated with haloperidol monotherapy were summarized as group H, those with pipamperone represented group P, and those receiving a combination of both represented group HP. Group N consisted of patients receiving neither haloperidol nor pipamperone. Additional benzodiazepines were used in patients of all groups with comparable incidence (p = 0.978).
Haloperidol equipotency (1 eH 1 mg/d haloperidol 50 mg/d pipamperone) was estimated and calculated for pipamperon to compare dosages (20). Exposure to antipsychotics was formally assumed for eH ≥ 0.02.
2.5. Electrocardiography
In cases where multiple ECGs were performed within 60 days before delirium, the one conducted closest to day 1 and under the least exposure to antipsychotics and any QIPS was selected as ECGa. To determine the latter, we estimated plasma concentrations for all drugs involved based on a pharmacokinetic model with approximated half-life durations. QTc intervals on days a and b (QTca, QTcb) were measured both automatically and manually, with excellent accordance between the measurements (r > 0.999, p < 0.001), applying the widely used Bazett correction formula. The difference between QTca and QTcb was calculated for all patients (ΔQTc). In cases where more than one ECG was performed during delirium, the one conducted under the highest exposure to antipsychotic pharmacotherapy was chosen for ECGb.
Applying the limits suggested by the American Heart Association, we defined QTc intervals of >470 ms for men and > 480 ms for women (15) as critical QTc interval prolongations (CQPs).
2.6. Statistical methods
All analyses were performed using the IBM Statistical Package for Social Sciences (SPSS), version 20/25. We calculated means, standard deviations, medians, and interquartile ranges (IQRs) for descriptive statistics (QTc intervals, age of patients, day of ECG, dosages of medications, numbers of QIPS, etc.). T-tests (Mann–Whitney tests for non-parametric data) were used to compare means between the treatment subgroups. Analyses of variance (Kruskal-Wallis tests for non-parametric data) with post-hoc Bonferroni correction were performed for multiple comparisons. Pearson χ2 tests were applied to detect correlations between non-nominal variables. In samples of n < 20, we used Fisher’s exact test. The Kolmogorov–Smirnov method was applied to test for normal distribution. Subgroups of n < 10 were excluded from statistical analysis. A probability value of p < 0.05 was defined as the level of statistical significance.
3. Results
3.1. Patients characteristics
Of all patients, 72 patients (70.6%) were male, and 30 patients (29.4%) were female, with a median (IQR) age of 74.9 (11.4) years (Table 1). Most of them were treated at the departments of cardiology/cardiac surgery (44.1%), neurology/neurosurgery (22.5%), or angiology/vascular surgery (11.8%). A total of 13.7% of patients were under treatment with one or more QIPSs, mostly antidepressants (leading: citalopram, trazodone). Thirty-six patients (35.3%) were pre-diagnosed with at least one documented psychiatric disorder (most of them were organic), followed by substance use-related and mood disorders. Seventy-eight patients (76.5%) also had a cardiological diagnosis.
3.2. Specifications of delirium
The mean DOS1 of delirium was 4.8 (2.2), DOSmean was 4.4 (1.6), and the median observation duration was 5 (7) days (Tables 1, 2). Remission was observed in 73.5% of all patients within 3 (5) days after delirium onset. The results for remission rates and duration of delirium were comparable among all groups.
3.3. Pharmacological treatment of delirium: overview
Eighty-eight patients (86.3%) were under antidelirious pharmacotherapy with haloperidol, pipamperone, or both on Day-B (Table 2). Among those patients, 10 patients (11.4%) received haloperidol monotherapy, 43 patients (48.9%) received pipamperone monotherapy, and 35 patients (39.8%) received haloperidol combined with pipamperone. Pipamperone was the overall most frequently administered antipsychotic (88.6%).
Antipsychotic dosages on Day-B varied (p < 0.001) between eH = 0.1 and 4.5 (mean 1.2 ± 1) and were lowest in group P (0.8 ± 0.5) and highest in group HP (2.1 ± 1). DOS1 scores showed a tendency to correlate positively with eH on Day-B (rP = 0.188, p = 0.08).
Patients receiving antipsychotics were more likely to have been previously diagnosed with a psychiatric disorder (p = 0.031). There were no significant differences in gender, age, pre-morbidity, number of QIPS administered, days between ECGa and ECGb, or QTc interval at baseline between the treatment groups.
3.4. Adverse effects and fatal outcomes
In total, adverse drug reactions (ADRs) occurred in three patients (2.9%) on average on day 2.7 ± 0.6 of delirium (Table 2). All ADRs were non-cardiovascular (hypopnea, aspiration, and somnolence), and no case of torsades de pointes was registered. ADRs occurred in delirium with higher DOSmean (p = 0.014, rP = 0.243), and their manifestation appeared to be positively correlated with the length of QTc interval on Day-A (p = 0.026, rP = 0.221). One case of ADRs (7.1%) was registered in group N.
Among patients receiving antipsychotics, all ADRs occurred in group HP. No significant correlation to the use of any specific substance nor the dosage was found. One 87-year-old polymorbid patient (1.1% of medicated patients) died from aspiration under a cumulative dose of 1 mg haloperidol and 90 mg pipamperone on day 6 of delirium (1.1 eH).
The total number of QIPSs prescribed correlated with the risk of dying during delirium from any cause (p = 0.005, rP = 0.275).
3.5. Findings in ECG: overview
Across all patients, ECGa was performed at a median of 9.9 (6) days prior to delirium onset, and the mean QTca was 440 ± 35.4 ms (Tables 1, 2). In 18 patients (17.6%), prolonged QTc interval was found in ECGa. ECGb was performed after a median of 3.4 (4) days into delirium. The time between ECGa and ECGb (10.9 ± 11 days) did not differ significantly across treatment groups.
3.6. Findings in ECG: ΔQTc and CQP under therapy
Across all patients, QTc increased by a mean of 13.4 ± 39.9 ms from Day-A to Day-B (QTcb 453.4 ± 29.9 ms) (Table 2).
Among patients receiving any antipsychotics, 58 patients (65.9%) showed a positive ΔQTc, while only 8 patients (57.1%) showed a positive ΔQTc in group N. ΔQTc was −2.7 ± 36.1 ms in group H, 15.2 ± 35.3 ms in group P, and 21.3 ± 43.9 ms in group HP; however, these ΔQTc differences between the treatment regimens and compared to group N were not significant. In group HP, dosages above the mean correlated with higher ΔQTc (30.6 ± 46.5 ms vs. −2 ± 26 ms; p = 0.045). Overall, we found a negative correlation between ΔQTc and the incidence of ADRs (rP = −0.234, p = 0.018).
The mean QTcb in patients receiving antipsychotics was 455.8 ± 29.5 ms (ΔQTc 15.6 ± 39.3 ms) compared to 438.5 ± 28.9 ms (ΔQTc − 0.4 ± 42.9 ms) in group N (p = 0.043). QTcb of patients in groups P (456.2 ± 30.2 ms; p = 0.06) and HP (456.5 ± 30.3 ms; p = 0.064) showed a clear tendency to be longer compared to those in group N (438.5 ± 28.9 ms). The total number of QIPSs (haloperidol and pipamperone) administered tended to correlate positively with the length of QTcb (rP = 0.182, p = 0.067). Overall, each additionally administered eH predictively prolonged QTcb by approximately +5.7 ms (F[1, 100] = 4.133, p = 0.045; R2 = 3%).
The rate of CQP among all patients was 20.6%, occurring after a median time of 2 (3) days. Nineteen patients (21.6%) with antipsychotics newly developed CQP on Day-B (mean 495.1 ± 14.2 ms); however, there was no significant difference with two patients in group N (14.3%, 478.1 ± 8.5 ms). The incidence of CQP did not differ significantly between groups H (10%), P (20.9%), and HP (25.7%). In patients receiving haloperidol plus pipamperone combination therapy, dosages above the mean correlated with a higher rate of CQP (36% vs. 0%; χ2[1] = 4.8, p = 0.036). Furthermore, no clear dose dependency on the risk of developing CQP was found in any group. The manifestation of CQP did not correlate with the incidence of ADRs.
4. Discussion
4.1. Summary of main findings
Pharmacological delirium management with antipsychotics, especially pipamperone, was very common (86.3%). Generally, antipsychotic dosages administered were low (mean eH on Day-B = 1.2 ± 1), and the use of combination therapy with haloperidol and pipamperone was correlated with higher eH on Day-B (2.1 ± 1).
ADRs were rare (2.9%) and mainly non-cardiovascular and occurred in patients receiving haloperidol plus pipamperone combination therapy. The probability of ADRs correlated positively with higher DOS scores and the length of QTc interval at baseline. Polypharmacy with multiple QIPSs was correlated with higher mortality during delirium.
QTc interval prolongation on Day-B was very common in all groups (haloperidol: 50%; pipamperone: 69.8%; haloperidol plus pipamperone: 65.7%; no antipsychotics: 57.1%) and was positively correlated with the dosages administered, especially under combination therapy with haloperidol and pipamperone. Treatment with antipsychotics per se was not associated with a higher incidence of QTc interval prolongation, but if prolongation did occur, it was 17.3 ms longer than in patients without haloperidol and/or pipamperone. The length of QTc interval on Day-B was positively correlated with the total number of QIPSs at baseline. The use of pipamperone monotherapy and haloperidol plus pipamperone combination, but not haloperidol monotherapy, tended to be associated with positive ΔQTc on Day-B.
Overall, CQP was common (20.6%), and its occurrence did not significantly differ between the groups. Combination therapy with haloperidol and pipamperone dose-dependently increased the risk of CQP.
4.2. Interpretation
The finding that particularly the haloperidol plus pipamperone combination therapy was dose-dependently associated with an increased risk of CQP provides further evidence to favor monotherapy over polypharmacy whenever possible. However, in line with the existing literature (21), we found that the use of combination therapy was accompanied by higher equipotent antipsychotic dosages, which is suspected to be the main reason for the QTc interval-prolonging effect in polypharmacy with antipsychotics. The design of our study, the number of patients, and the low drug dosages used do not allow for any causal attribution, but further research on the complex interactions between antipsychotic polypharmacy, equipotent dosages, and QTc interval changes could provide clarification.
The use of pipamperone, which, in the light of its proven antidelirious property (12) and the de facto lack of anticholinergic effects, is often used to treat geriatric patients, was associated with an increase in QTc interval length. Despite the descriptive intent of this study, this finding supports the recommendation for frequent ECG control under pipamperone administration, particularly in vulnerable patient populations such as the one in this study.
The unexpected result that haloperidol monotherapy per se was not associated with the prolongation of QTc interval might be due to the low dosages used in the study. In addition to that, the mean QTc interval length at baseline in the haloperidol group was rather high (454.7 ± 38.3 ms), which might indicate a distorting effect of the QT interval correction method used. The Bazett formula is known to slightly overestimate elevated heart rates (22), by which patients with tachycardia of any etiology (anemia, infection, etc.) might have been diagnosed with baseline QTc interval prolongation, which, as a consequence, would falsely reduce ΔQTc.
The number of QIPSs was positively associated with ΔQTc in the naturalistic setting of this study, indicating the importance of carefully considering all, not only psychotropic, patient’s medications when conceptualizing antidelirious pharmacotherapy and assessing for potential risks.
We further found that the risk of ADRs was particularly present in delirium with higher DOS scores, suggesting the need for close clinical monitoring and increased caution in the administration of pharmacotherapy to this vulnerable group. We interpreted the number of QIPSs as an indirect approximative indicator for global illness severity, which could explain its positive correlation to increased mortality during delirium.
The high rate of ADRs in the group of patients not receiving any antipsychotics was surprising at first. However, many of these patients were treated with benzodiazepines. Hence, we hypothesize the latter to be the reason for therapy-related side effects, as their associated potential risk is well documented (e.g., fall hazard, anticholinergic potential, paradoxical effects, and respiratory depression) (23).
Besides being a possible manifestation of hypoactive delirium per se (9), the occurrence of somnolence and sedation under combination therapy with haloperidol and pipamperone was not unexpected, as these symptoms are common under antipsychotic medication. Pipamperone is more strongly associated with somnolence and sedation than haloperidol (24) and clinicians should carefully consider these effects in their assessment.
In line with this hypothesis, we found a concomitant association between QTc interval length at baseline and ADR risk, which we believe is explained by a tendency of physicians to be less likely to prescribe antipsychotics for patients with long QTc intervals and rather use substances with less pro-arrhythmic potential (e.g., benzodiazepines).
Similarly to ADRs, the finding that QTc prolongation occurred frequently in patients in group N was unexpected. A possible reason could be the administration of benzodiazepines, as it was previously shown that benzodiazepines are not fully electrophysiologically inert to the heart. Diazepam, for example, can prolong the QTc interval (25), and midazolam might have some inhibitory hERG-channel affinity (26). In this context, one possible explanation could be that the application of benzodiazepines to patients in group N, especially intravenous midazolam in high dosages, might have induced QTc prolonging effects. Another reason could be distortion effects due to the use of the Bazett correction formula. Future research with a focus on prospectively evaluating QTc interval under benzodiazepine therapy and with rigorous control of potentially confounding factors could further clarify this question.
4.3. Limitations
Although the total number of patients included in this study was reasonably large, the size of the subgroups became small, limiting their statistical potential. Additionally, in this descriptive presentation of a naturalistic patient sample, the median age was rather high, and men were overrepresented, limiting generalizability.
Due to the retrospective study design, a variety of known risk factors for QTc interval prolongation (e.g., kidney failure, obesity, hepatic dysfunction, and malnutrition) could not be adequately considered in our calculations, and observed associations are not to be conflated with causal relationships. Also, the potential influence of autonomic dysregulation due to delirium itself or its effects on the QTc interval (27) has not been specifically evaluated.
Although the prescription rate of benzodiazepines did not significantly differ between the four groups, the dosages given did, relativizing comparability with regard to therapy adverse effects of pharmacotherapy.
Another limitation is that baseline and control ECGs were not performed at standardized time points in all patients. Therefore, the results and interpersonal comparisons need to be interpreted cautiously due to regular QTc interval dynamics over time.
Concerning the method for QT interval correction, the application of the Bazett formula might potentially overcorrect and lead to false-positive findings of (critical) QTc interval prolongation. This effect is known to be particularly relevant in patients with higher heart rates (22), which is frequently prevalent in stressful situations such as delirium. Also, the findings of this study mainly rely on the application of the QTc interval length limits suggested by the American Heart Association (see 2.5. Electrocardiography) and it is to be mentioned, that some authors recommend lower limits (28).
4.4. Conclusion
QTc-interval prolongation under haloperidol and/or pipamperone treatment of delirium in this naturalistic patient sample was not more frequent but greater in magnitude compared to patients not receiving antipsychotics. It was correlated with the dosage and the total number of administered QIPSs. Combination therapy was associated with higher mortality during delirium and a dose-dependent increase in the risk for CQP. ADRs were predicted by DOS scores and QTc-interval length at baseline. In summary, these results support the use of carefully dosed haloperidol and/or pipamperone pharmacotherapy in elderly inpatients suffering from non-withdrawal-associated delirium. Although QTc-interval prolongation is very common, treatment with antipsychotics is relatively safe regarding ADRs and critical QTc-interval alterations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the canton of Zurich, Switzerland (KEK-ZH-Nr: 2012-0263). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PB: Formal analysis, Investigation, Writing – original draft. SB: Conceptualization, Supervision, Writing – review & editing. JJ: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank all study individuals and family members for their time and participation in our study. Additionally, we would like to thank all the nurses and doctors involved, Silvana Knoepfel and Peter Hayoz for their help in data collection, as well as Richard Klaghofer and Hanspeter Mörgeli for their support throughout this study. This manuscript was edited for English Language by Charlesworth Author Services (www.cwauthors.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ECGa (ECGb), Electrocardiography performed on Day-A (B); DOS1, Delirium Observation Scale score assessed on day 1; DOSmean, Mean DOS score over the duration of delirium; QIPS, QTc interval-prolonging substances; eH, Haloperidol equipotency (1 eH ≙ 1 mg/d haloperidol); CQP, Critical QTc interval prolongation; ADR, Adverse drug reactions; QTca (QTcb), Length of QTc interval on Day-A (B); ΔQTc, Difference between QTca and QTcb; Group H, Patients treated with haloperidol; Group P, Patients treated with pipamperone; Group HP, Patients treated with haloperidol and pipamperone; Group N, Patients treated with neither haloperidol nor pipamperone.
References
1. Wu, Y-C, Tseng, P-T, Tu, Y-K, Hsu, C-Y, Liang, C-S, Yeh, T-C, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium. JAMA Psychiatry. (2019) 76:526–35. doi: 10.1001/jamapsychiatry.2018.4365
2. Santos, E, Cardoso, D, Neves, H, Cunha, M, Rodrigues, M, and Apóstolo, J. Effectiveness of haloperidol prophylaxis in critically ill patients with a high risk of delirium: a systematic review. JBI Database System Rev Implement Rep. (2017) 15:1440–72. doi: 10.11124/JBISRIR-2017-003391
3. Evensen, S, Saltvedt, I, Hylen, RA, Myrstad, M, Myrstad, C, Mellingsæter, M, et al. Delirium and cognitive impairment among older patients in Norwegian emergency departments. Tidsskr Nor Legeforen. (2018) 139:517–20. doi: 10.4045/tidsskr.18.0578
4. Lange, S, Mędrzycka-Dąbrowska, W, Friganović, A, Religa, D, and Krupa, S. Patients’ and relatives’ experiences of delirium in the intensive care unit – a qualitative study. Int J Environ Res Public Health. (2022) 19:11601. doi: 10.3390/ijerph191811601
5. Huang, D-D, and Fischer, P. Management of Delirium in the intensive care unit. Surg Clin North Am. (2022) 102:139–48. doi: 10.1016/j.suc.2021.09.006
7. National Institute for Health and Care Excellence (2018). Delirium: Prevention, diagnosis and Management in Hospital and Long-Term Care. Available at: https://www.nice.org.uk/guidance/cg103 (Accessed March 31, 2023).
8. Maust, D, Kim, H, Seyfried, L, Chiang, C, Kavanagh, J, Schneider, LS, et al. Antipsychotics, other Psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiat. (2015) 72:438–45. doi: 10.1001/jamapsychiatry.2014.3018
9. Boettger, S, and Jenewein, J. Placebo might be superior to antipsychotics in the Management of Delirium in the palliative care setting. Evid Based Med. (2017) 22:152–3. doi: 10.1136/ebmed-2017-110723
10. Inouye, S, Marcantonio, E, and Metzger, E. Doing damage in delirium: the hazards of antipsychotic treatment in elderly persons. Lancet Psychiat. (2014) 1:312–5. doi: 10.1016/S2215-0366(14)70263-9
11. Grover, S, Kumar, V, and Chakrabarti, S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. (2011) 71:277–81. doi: 10.1016/j.jpsychores.2011.01.019
12. Boettger, S, Knöpfel, S, Schubert, M, Garcia Nuñez, D, Plichta, MM, Klaghofer, R, et al. Pipamperone and delirium: a preliminary evaluation of its effectiveness in the management of delirium and its subtypes. Swiss Med Wkly. (2017) 147:w14471. doi: 10.4414/smw.2017.14471
13. Zaal, IJ, Devlin, JW, Hazelbag, M, Klein Klouwenberg, PMC, van der Kooi, AW, Ong, DSY, et al. Benzodiazepine-associated delirium in critical ill adults. Intensive Care Med. (2015) 41:2130–7. doi: 10.1007/s00134-015-4063-z
14. Howell, S, Yarovova, E, Khwanda, A, and Rosen, SD. Cardiovascular effects of psychotic illnesses and antipsychotic therapy. Heart. (2019) 105:1852–9. doi: 10.1136/heartjnl-2017-312107
15. Ramalho, D, and Freitas, J. Drug-induced life-threatening arrhythmias and sudden cardiac death: a clinical perspective of long QT, short QT and Brugada syndromes. Rev Port Cardiol. (2018) 37:435–46. doi: 10.1016/j.repc.2017.07.010
16. Kim, A, Lim, KS, Lee, H, Chung, H, Yoon, SH, Yu, K-S, et al. A thorough QT study to evaluate the QTc prolongation potential of two neuropsychiatric drugs, quetiapine and escitalopram, in healthy volunteers. Int Clin Psychopharmacol. (2016) 31:210–7. doi: 10.1097/YIC.0000000000000124
17. Woosley, RL, Black, K, Heise, CW, and Romero, K. Credible meds: what does it offer? Trends Cardiovasc Med. (2018) 28:94–9. doi: 10.1016/j.tcm.2017.07.010
18. World Health Organization. F0: Organische, einschliesslich symptomatischer psychischer Störungen In: H Dilling, W Mombour, and MH Schmidt, editors. ICD-10: International statistical classification of diseases and related health problems (tenth revision, second edition). Goettingen: Hogrefe (2004). 91.
19. Schuurmans, MJ, Shortridge-Baggett, LM, and Duursma, SA. The delirium observation screening scale: a screening instrument for delirium. Res Theory Nurs Pract. (2003) 17:31–50. doi: 10.1891/rtnp.17.1.31.53169
21. Barbui, C, Bighelli, I, Carrà, G, Castellazzi, M, Lucii, C, Martinotti, G, et al. Antipsychotic dose mediates the association between polypharmacy and corrected QT interval. PLoS One. (2016) 11:11. doi: 10.1371/journal.pone.0148212
22. Vandenberk, B, Vandael, E, Robyns, T, Vandenberghe, J, Garweg, C, Foulon, V, et al. Which QT correction formulae to use for QT monitoring? JAHA. (2016) 5:e003264. doi: 10.1161/JAHA.116.003264
23. Seldenrijk, A, Vis, R, Henstra, M, Ho Pian, K, van Grootheest, D, Salomons, T, et al. Systematic review of the side effects of benzodiazepines. Ned Tijdschr Geneeskd. (2017) 161:d1052
24. Eugene, AR, Eugene, B, Masiak, M, and Masiak, JS. Head-to-head comparison of sedation and somnolence among 37 antipsychotics in schizophrenia, bipolar disorder, major depression, autism Spectrum disorders, delirium, and repurposed in COVID-19, infectious diseases, and oncology from the FAERS, 2004–2020. Front Pharmacol. (2021) 12:1–7. doi: 10.3389/fphar.2021.621691
25. Keller, GA, Alvarez, PA, Ponte, ML, Belloso, WH, Bagnes, C, Sparanochia, C, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. (2016) 11:86–98. doi: 10.2174/1574886311207040262
26. Jellestad, L, Stocker, L, Jenewein, J, and Boettger, S. A case of QT-interval prolongation in the context of high-dose, intravenous midazolam in a methadone maintained patient. Int J Med Pharm Case Reports. (2015) 4:101–4. doi: 10.9734/IJMPCR/2015/18840
27. Lo, SS, Mathias, CJ, and Sutton, MS. QT interval and dispersion in primary autonomic failure. Heart. (1996) 75:498–501. doi: 10.1136/hrt.75.5.498
Keywords: delirium, QTc interval, torsades de pointes, haloperidol, pipamperone
Citation: Bohny P, Boettger S and Jenewein J (2023) Dose-dependent QTc interval prolongation under haloperidol and pipamperone in the management of delirium in a naturalistic setting. Front. Psychiatry. 14:1257755. doi: 10.3389/fpsyt.2023.1257755
Edited by:
Richard Leopold Musil, Ludwig Maximilian University of Munich, GermanyReviewed by:
Konstantinos Dimitriadis, LMU Munich University Hospital, GermanyAndy R. Eugene, Medical University of Lublin, Poland
Copyright © 2023 Bohny, Boettger and Jenewein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Bohny, cGhpbGlwcC5ib2hueUB0cmlhcGx1cy5jaA==
 Philipp Bohny
Philipp Bohny Soenke Boettger3
Soenke Boettger3 Josef Jenewein
Josef Jenewein