- 1Department of Basic Psychology, School of Psychology, Army Medical University, Chongqing, China
- 2Chongqing City Mental Health Center, Southwest University, Chongqing, China
- 3Department of Radiology, Southwest Hospital, Army Medical University, Chongqing, China
- 4Department of Medical Equipment and Metrology, College of Biomedical Engineering, Army Medical University, Chongqing, China
- 5School of Psychology, Army Medical University, Chongqing, China
Anhedonia is a hallmark symptom of depression that often lacks adequate interventions. The translational gap remains in clinical treatments based on neural substrates of anhedonia. Our pilot study found that depressed individuals depended less on goal-directed (GD) reward learning (RL), with reduced reward prediction error (RPE) BOLD signal. Previous studies have found that anhedonia is related to abnormal activities and/or functional connectivities of the central executive network (CEN) and salience network (SN), both of which belong to the goal-directed system. In addition, it was found that real-time functional magnetic resonance imaging (rt-fMRI) neurofeedback (NF) could improve the balance between CEN and SN in healthy individuals. Therefore, we speculate that rt-fMRI NF of the CEN and SN associated with the GD system may improve depressive and/or anhedonic symptoms. Therefore, this study (1) will examine individuals with anhedonic depression using GD-RL behavioral task, combined with functional magnetic resonance imaging and computational modeling to explore the role of CEN/SN deficits in anhedonic depression; and (2) will utilize network-based rt-fMRI NF to investigate whether it is feasible to regulate the differential signals of brain CEN/SN of GD system through rt-fMRI NF to alleviate depressive and/or anhedonic symptoms. This study highlights the need to elucidate the intervention effects of rt-fMRI NF and the underlying computational network neural mechanisms.
Introduction
Anhedonia is a transdiagnostic psychiatric symptom typical of psychiatric disorders, such as depression, posttraumatic stress disorder (PTSD), and schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), and is associated with an elevated risk of suicide and chronicity of the disease (1). The biological endophenotypes of anhedonia include reduced anticipation of upcoming rewards, reduced experienced pleasure during the reward presentation, and impaired reward learning (2). Specifically, reward learning (RL) is the ability to adapt to the contingencies between action and the reward feedback, with two systems working together: The habitual system supports routine responses to deal with regular situations, while the goal-directed (GD) system is sensitive to environmental change to flexibly adapt to unfamiliar situations. The deficits in goal-directed systems play an important role across the lifespan and in various mental disorders, such as subclinical depression, trauma-related disorders, addiction, and obsessive-compulsive disorder (OCD) (3–6). Specifically, goal-directed reward learning (GD-RL) could discriminate between depressed patients and healthy controls using a machine learning algorithm (Naive Bayes) and could predict the concurrent depressive symptoms and depression traits 1 year later (7). To sum up, deficits in the goal-directed system may contribute to depression during both the symptomatic and clinical phases. However, a translational gap still remains in the clinical imaging study of the goal-directed system (8).
Neurally, the goal-directed system may involve functional interactions between the central execution network (CEN) and the salience network (SN). The CEN, including the medial frontal gyrus (MFG), superior frontal gyrus (SFG), and the anterior cingulate cortex (ACC), especially the perigenual ACC (pgACC), is essential for external, goal-directed cognitive processes (9, 10). The SN, including the anterior insula (aINS), the dorsal anterior cingulate cortex (dACC), the amygdala (AMY), the substantia nigra/ventral tegmental area (SN/VTA), ventral striatum (VS), and thalamus, is responsible for detecting salient stimuli in the environment and allocating cognitive resources to facilitate goal-directed behavior (10, 11). SN may function as a gate to detect the salience of stimuli and co-activate task-relevant networks such as CEN to facilitate goal-directed behavior (12), through the shift between default mode network (DMN) and CEN (13). Previous studies showed global circuit–phenotype associations across clinical anxiety and depression, with distinct CEN (e.g., ACC and dlPFC) and SN (e.g., aINS and AMY) circuits (14). Therefore, abnormalities in the goal-directed system, and in particular the CEN/SN decoupling, may represent distinctive neural phenotypes of depression.
On the one hand, the dysfunctional networks of CEN and SN persist during depressive episodes. We previously found that depressed patients showed impaired goal-directed behavior, accompanied impairments in CEN, such as over-activation in the left lateral PFC (LPFC), as well as SN, such as under-activation in VTA, dorsal striatum (DS), and orbital frontal cortex (OFC) (7) (Figure 1), the last of which plays a critical role in controlling the transition between goal-directed and habitual behavior (15). The neural abnormalities in VTA during habitual learning do not normalize during remission (16). Further evidence suggested that enhanced resting-state functional connectivity (RSFC) between subregions of ACC (including pgACC and sgACC) and caudate could predict future anhedonia symptoms in adolescent depression (17, 18). Specifically, neural activation to uncertain rewards in ACC during reward learning tasks could predict anhedonia symptoms 1 year later when baseline anhedonia is controlled for (19). Collectively, these studies suggest that neural impairments in the CEN and SN within the goal-directed system are not only present during the acute phase of depression but also predict the course of the disease.
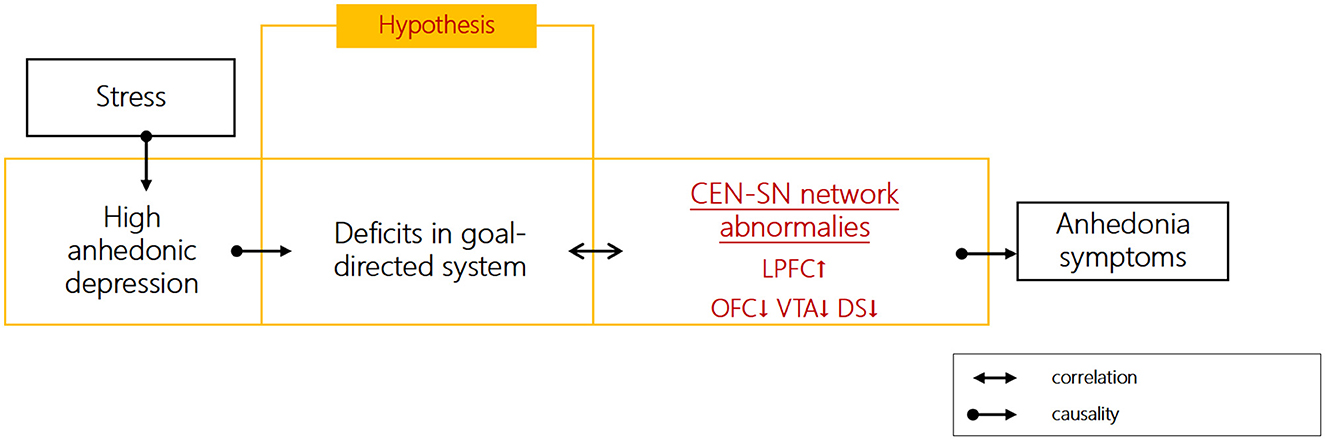
Figure 1. Hypothesized role of goal-directed function impairments in anhedonia symptoms of depression.
On the other hand, the stress-resilience model of depression provides a framework for understanding distinct subnetworks (CEN and SN) underlying anhedonic symptoms of depression. Emerging findings from functional abnormalities of higher functional connectivity between CEN (dlPFC and pgACC) and SN (striatum and VTA) during reward-related tasks were shown in depressed patients, as well as hypo-activation in CEN (pgACC and mOFC) and SN (VS/DS) (20). Anhedonia was associated with SN overactivity in the resting state, lack of SN activation in response to positive stimuli in task-related states, and SN overactivity in response to negative stimuli (21). Stress mediates the relationship between goal-directed reward learning and anhedonia (7). Stress may contribute to impaired goal-directed processes by enhancing the connectivities between CEN and SN circuits (e.g., dlPFC and amygdala/VS) (22). The resilience to stress involves the interactions among heightened CEN (PFC) and lessened SN (mesostriatal reward circuits) neural activities (23). Taken together, goal-directed behavior depends on the adaptive redistribution of neural resources between the CEN (↓) and SN (↓), and stress may lead to an imbalance between the CEN (↓) and SN (↓), as well as aberrant connectivity during reward processing, which may contribute to anhedonia symptoms.
Neurofeedback based on real-time functional magnetic resonance imaging (rt-fMRI) uses measured changes in brain activation to help participants regulate activity in selected regions or networks. During rt-fMRI neurofeedback, participants are provided with visually presented brain network activation information in real time (24). The rt-fMRI neurofeedback targeting the brain areas within SN involved in emotion processing (e.g., amygdala, insula, and striatum) decreased the anhedonic or depressive scores (25, 26). However, the effects of rt-fMRI neurofeedback (NF) based on CEN-SN on anhedonia to the best of our knowledge have not been investigated previously. Network-based NF can be used to recalibrate the balance between brain networks (27). It has been recommended that researchers use NF methods that consider neural response across regions (i.e., functional connectivity) (28). Recent studies have found that participants can flexibly regulate the balance between the CEN and SN through rt-fMRI neurofeedback which could promote resilience to stress (29). Therefore, the use of rt-fMRI neurofeedback to improve goal-directed reward learning is a potential intervention that involves the interaction between CEN and SN. Additionally, reward learning studies have typically adopted computational modeling during behavioral tasks to estimate expected value and reward prediction errors (RPEs) (20). Therefore, the current study was designed to adopt reward learning tasks (30) and computational modeling in anhedonic individuals to examine goal-directed brain network dynamics. Network-based rt-fMRI neurofeedback will be utilized to improve the balance of CEN/SN within the goal-directed brain network (Figure 1). The within-group changes of depressive symptoms after rt-fMRI NF training of goal-directed brain network will be examined to assess the effects of rt-fMRI NF intervention to improve depressive and/or anhedonic symptoms for depressed patients. The goal-directed RL, as well as CEN/SN changes (CEN-SN functional connectivity) at 3- and 6-month follow-ups, will also be examined to evaluate the long-term rt-fMRI neurofeedback training effects. This study may provide evidence to elucidate the effect of rt-fMRI NF based on the goal-directed system in alleviating depressive/anhedonic symptoms. The rt-fMRI NF training may have clinical implications with improved safety and minor side effects (31).
Methods and analysis
Aim
• To determine whether rt-fMRI neurofeedback of the goal-directed system could improve the depressive and/or anhedonic symptoms of depressed patients after training and at follow-ups;
• To examine the short-term and long-term effects of rt-rMRI neurofeedback on the goal-directed RL behavioral and/or connectivity imaging mediators.
Participants
The study was approved by the ethical review committee at the Army Medical University (AMU, PRC) and conformed to the Declaration of Helsinki. The study has been submitted to the Chinese Clinical Trials Registry (ChiCTR, https://www.chictr.org.cn/). The trial identifier will be made available upon approval of the registration. After signing the informed consent form, the clinical data and general information of all participants will be collected, including age, gender, duration of illness, years of education, and medication history. The clinical diagnosis and symptom assessment will be completed by psychiatrists according to DSM-5 combined with a Mini-international Neuropsychiatric Interview (MINI). We will use the Hamilton Depression Scale-17 (HAMD-17) and the Montgomery–Åsberg Depression Rating Scale (MADRS) to assess anhedonia symptoms. Participant selection and recruitment processes were not conducted prior to the submission of the study.
(1) High anhedonic depression group. Inclusion criteria: ① diagnosed with major depression, with at least one core symptom (loss of pleasure or interest in all or nearly all activities) (MADRS item 8 ≥ 4) or non-reactive mood (items 1 or 2 ≥5, ② at least three following symptoms during the most severe phase of this depressive episode, including (a) significant psychomotor disturbance (HAMD items 8 or 9 ll1); (b) appetite/weight loss (HAMD items 12 or 16 = 2); (c) late insomnia (waking at least 2h earlier than usual) (HAMD item 6 ≥ 1); and (d) guilt (HAMD ≥ 1) (32); ③right-handedness. Exclusion criteria: ① comorbid with other mental disorders or drug or alcohol dependence; ② has received any previous antidepressant or antipsychotic medication (including benzodiazepines and other sedatives and hypnotics); ③ with severe physical diseases such as heart, liver, or kidney impairment, endocrine disorders, or infectious diseases; ④ substance dependence or abuse; ⑤ vision impairments; ⑥ pregnant or breastfeeding women; ⑦ MRI contraindications; and ⑧ currently receiving any emotional ability training.
(2) Healthy control group. Inclusion criteria: ① without a history of mental illness and family history of mental illness; ② age 18–55; ③ HAMD-17 < 7; and ④ right-handedness. Exclusion criteria: ① diagnosed with depression or other axis I diseases; ② has received any previous antidepressant or antipsychotic treatment (including benzodiazepines and other sedative and hypnotic drugs); ③ with severe physical diseases such as heart, liver, renal impairment, endocrine diseases, and infectious diseases; ④ substance dependence or abuse; ⑤ vision impairment; ⑥ women pregnant or breastfeeding; ⑦ MRI contraindications; and ⑧ currently receiving any emotional ability training.
Procedure
This study is a double-blind randomized yoke-controlled rt-fMRI NF experiment, with follow-up assessments at 3 and 6 months after rt-fMRI NF (Figure 2). Participants will be randomly assigned to the experimental group (NF/YC) and given random numbers by a third party, who encodes the intervention with matching random numbers. The experimenter who will rate the outcome and/or analyze the data will be blind to group assignment. The CRED-NF checklist indicating which analyses will be performed and listed as supplementary material (available on the Open Science Framework preregistration, https://osf.io/ec396; CRED-NF checklist, https://osf.io/8wcqt).

Figure 2. Consolidated Standards of Reporting Trials (CONSORT) flow diagram summarizing the study design.
Pre- and post-test
Before and after the neurofeedback intervention, the primary and secondary outcome measures of the depressive and anhedonic symptoms will be assessed in all subjects. The behavioral tasks will be repeated post-training to evaluate the NF training effect on goal-directed behavior. The follow-up assessments include the primary outcome of depression and secondary outcome of anhedonia, the goal-directed RL (model-based weighting parameter ω), and the CEN-SN functional connectivity (Pearson's correlation) based on prediction errors (PEs) (Figure 2).
Primary and secondary outcome measures
Primary outcome measures: HAMD-17 (33). The severity of depressive symptoms will be assessed using the total score of HAMD-17. The higher the score, the more severe the depressive symptoms.
Secondary outcome measures: Snaith–Hamilton Pleasure Scale (SHAPS) (34). The scale includes four aspects of interest/entertainment, social interaction, sensory experience, and food/drink, and has a total score of 14 to 56. It has good validity and reliability to evaluate the hedonic experience of adult outpatients with MDD (35).
The changes in depression and anhedonia symptoms will be assessed using HAMD-17 and SHAPS before and after the NF intervention, respectively. Follow-up assessments of depression (HAMD) and anhedonia (SHAPS) symptoms will be conducted at 3 and 6 months after the intervention.
Behavioral task
The reward learning task (two-stage Markov decision task) (30) will be adopted. All participants will practice an 8-min Markov decision task outside of the scanner room before formal NF training, for which instructions will be provided to the participants. After practice, the participants will rest for 5 min before completing the formal NF training. The practice and formal tasks will be presented using MATLAB 2019a (https://www.mathworks.com) and Psychtoolbox 3.0 (http://psychtoolbox.org).
The task will be divided into two stages. In stage 1, participants choose one of two stimuli (red) (stage 1, left panel), which leads to the second stimulus (green) in 70% of the cases and to the third stimulus (blue) (stage 2) in 30% of the cases. The other stimulus (stage 1, right panel) leads to the second stimulus (green) in 30% of the cases and to the third stimulus (blue) in 70% of the cases. The six stimuli at the two stages will be selected from the alphabets that are unfamiliar to the participants to encourage progressive learning. The possibility that the stimulus of stage 1 changes to the second stage through the key press of participants is called the transition probability (common transition 70% vs. rare transition 30%). The selection of experimental stimuli in stage 2 may result in either a reward or no reward. To motivate participants to continue learning, the reward will follow a slowly drifting Gaussian random walk with probability changes ranging from 0.25 to 0.75 (Figure 3).

Figure 3. Schematic diagram of the Markov reward decision task adapted from Daw et al. (30).
Participants will be encouraged to make options to receive as many rewards as possible and will be informed prior to practice and formal experiments that all rewards will be paid off at the end of the experiment. The task flow is as follows (Figure 4).
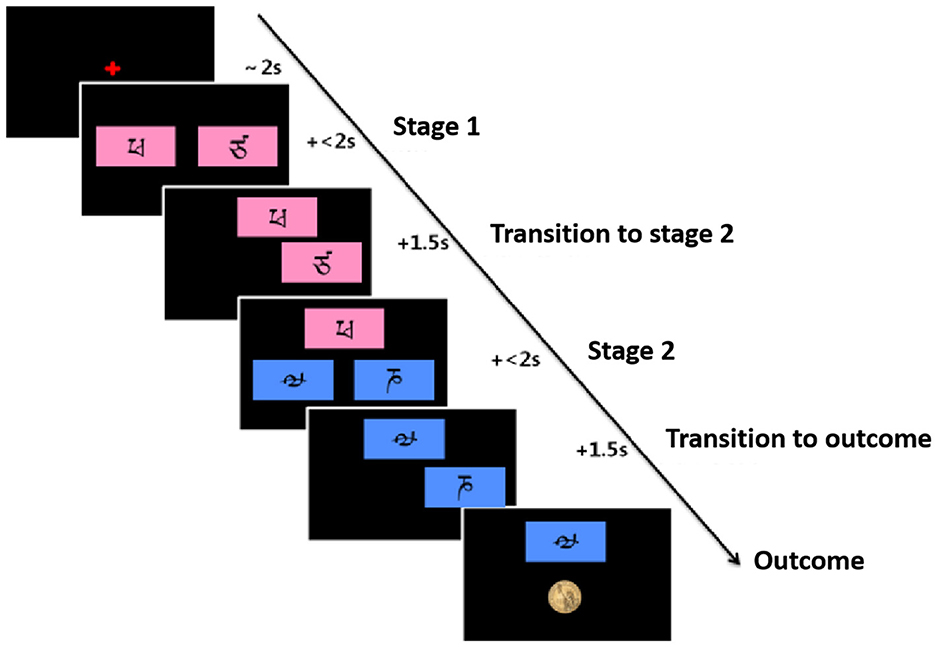
Figure 4. Flowchart of the Markov reward decision task, adapted from Daw et al. (30).
Real-time fMRI neurofeedback of individual goal-directed system in anhedonic depression
The anhedonic depressed participants (n = 40) will be randomized into the neurofeedback (NF) group (n = 20) and the yoke control (YC) group (n = 20). We used blocked randomization to form the allocation list (1:1 ratio) for the two comparison groups (NF and YC), by using a computer random number generator to select random permuted blocks (size = 10). The NF group will receive rt-fMRI neurofeedback intervention, with ~60 min per session, once per week for a total of four sessions. Participants in the YC group who are paired with one specific participant from the NF group will receive feedback signals from that participant during the same run. Thus, for the YC group, the sham rt-fMRI signals will not be corresponding to the participant's brain activity. The healthy control (HC) group (n = 20) will receive no intervention.
Sample size
The sample size is determined based on a heuristic justification (36) according to median recruits (n = 18) of fMRI neurofeedback (37). Moreover, given that this is a feasibility study with constraints on limited resources, a power calculation is not necessary according to the reporting and experimental design consensus in the NF field (38).
Real-time magnetic resonance imaging
MR images will be collected using 3.0T Siemens (Trio, Erlangen, Germany) with a 32-channel head coil. T1-weighted structural images will be collected from the whole brain with the magnetization-prepared rapid gradient echo (MPRAGE) sequence for anatomical localization of functional images. FOV = 256 × 256 mm, 176 sagittal slices, layer thickness = 1.0 mm, resolution = 1.0 mm2, TR/TE = 1900/2.52 ms, and flip angle = 9°. Echo-planar imaging (EPI) T2*-weighted functional images will be collected across the whole brain using a gradient echo planar imaging (EPI) sequence, FOV = 192 × 192 mm, matrix size = 64 × 64, and spatial resolution = 3 × 3 × 3 mm3, flip angle = 90°, and TR/TE = 2000/30 ms. To reduce the head motion, a strip of medical tape will be attached horizontally over the forehead of the participant and be spread to both sides of the head coil so that the participant could feel the head motion subjectively to prevent it from exceeding the range of head motion (39).
Real-time functional imaging will be achieved by using a customized function during MR image reconstruction, with TurboExport (Brain Innovation, Maastricht, The Netherlands) to convert input pixel data for each volume into images. Each image generated will be preprocessed in real time using the Turbo-BrainVoyager (Brain Innovation, Maastricht, The Netherlands). Preprocessing includes motion correction (by realigning each image to the first image of the session) and spatial smoothing (Gaussian kernel of half-height and 5 mm in full width). The computer presenting the task will communicate with Turbo-BrainVoyager via Transmission Control Protocol (TCP) to acquire preprocessed real-time data to be coregistered to the localizer anatomical image (first session) and generate feedback displays to the participants.
Network-based rt-fMRI NF intervention
The network-based rt-fMRI NF intervention of each session will be divided into 6 runs (5 NF runs and 1 transfer run), at 10 min/run. Subjects will be asked to raise or lower the height of a progress bar on the screen through cognitive effort. The height of the bar will depend on the signal intensity of regions of interest (ROIs) in the CEN and SN measured on each trial. Participants will be asked to regulate their brain signals through internal thought without specific instructions, with the feedback signaling the balance between the average signal intensities of the CEN and SN. They will know neither the source nor the calculation of the feedback signal. Before each session, participants will be explicitly instructed to try to avoid movement, including facial expressions, limb movements, and irregular breathing patterns.
During the scan, anatomical images will be recorded first (~5 min) and preprocessed immediately after image reconstruction based on a separate computer using BrainVoyager. After that, the results will be transmitted to Turbo-BrainVoyager to obtain real-time functional data aligned to the target ROIs of neurofeedback. Each run will start with a rest phase, during which the baseline and change in the feedback signal will be calculated. The feedback will be based on the differential signal between the mean values of all the voxels in the individualized ROIs in the CEN and SN. The baseline for this difference signal will be defined as the median difference between the initial resting signal of the SN and the CEN ROIs, and the lower and upper limits will be set to be two standard deviations from this baseline. The upper and lower limits will be updated to the average of the five lowest/highest median differences of this run before each block (29). The regulatory success targets the larger differential signal between CEN and SN. The transfer run will test the generalization of NF training without feedback signals during which the participants will be required to regulate their brain signals in the same way as the NF training run (40).
After each run, the participants will appraise their control over the progressive bar height (0–10 points) and their cognitive difficulties (0–10 points) with handles. Between runs, the participants will be allowed to rest for 5 min without significant head movement. After the NF intervention outside the scanner, the participants will write down the strategies they used to regulate their brain signals (e.g., through internal thought) and evaluate the effectiveness of strategies (0–10 points). To evaluate the demand characteristics, they will also indicate which group (NF or YC) they believe they belong to.
Statistical analysis
Behavioral data
Statistical analysis
IBM SPSS Statistics 22 (https://www.ibm.com/spss) will be used. The 2 (groups: high anhedonia group and healthy control group) × 2 (transition conditions: common transition and rare transition) × 2 (reward feedback: reward and non-reward) factor mixed design will be conducted. The general linear mixed model (GLMM) will be used to investigate the main effects and interactions of the group, transition condition, and reward feedback on choice behavior. The interactions of transition conditions and reward feedback will represent the effect of the goal-directed system on the choice behavior (goal-directed RL), and the main effect of reward feedback will represent the effect of the habitual system on the choice behavior (habitual RL). The three-way interaction of the group, transition condition, and reward feedback will be examined to test for group differences in goal-directed RL. The two-way interaction of group and reward feedback will be examined to test for group differences in habitual RL.
Computational modeling
MATLAB 2019a (https://www.mathworks.com/) and the Reinforcement Learning algorithm will be used to model the behavioral data and estimate the learning rate α, inverse temperature parameter β, repetition parameter p, and model-based learning weight ω of individual subjects.
① value estimates. The state-action values for the first and second stages of each trial are determined by the weighted sum of the model-based action value QMB and the model-free action value QMF. The weighted sum is expressed as follows.
It is assumed that individual behavioral choice in the first stage is the weighted sum of goal-directed and habitual learning, with the weight of ω (0~1). If ω is close to 0, the choice mainly depends on habitual learning; if ω is close to 1, the choice mainly depends on goal-directed learning.
(a) Model-based state-action value:
(b) Model-free state-action value:
For the first and second stages, learning rates α1 and α2, respectively, represent the extent to which the reward results are used for learning.
(c) Prediction error:
The action probabilities of the first and second stages of each trial are regarded as a softmax function of the inverse temperature parameter β, the repetition parameter rep, and the indicator function p as follows.
The inverse temperature parameters β1 and β2 represent the randomness of the first- and second-stage choices. The repetition parameter p and the indicator function represent the reproducibility of the choice, that is, the tendency to choose the same choice as in the previous trial, regardless of the actual value of the choice. Additionally, p > 0 represents choices the same as the previous trial, and p < 0 represents choices different from the previous trial.
The parameters of each subject will be estimated using the R software and its Stan toolkit with the Markov Chain Monte Carlo (MCMC) estimation which has been previously described (4). The model evidence of the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) will be calculated separately to estimate the model fit of reinforcement learning to the behavior.
Imaging data
Preprocessing analysis
DPABI V7.0 software (http://rfmri.org/DPABIV7) will be used for data pre-processing, in which the DICOM data will be converted into NIFTI format. In order to remove the effects of subject maladaptation during the scan as well as the effects of inhomogeneous magnetic fields on the results, data from the first 10 time points will be removed. Then, time correction, head motion correction, image standardization, image smoothing, detrending, and filtering (0.01~0.08 Hz) will be conducted, as well as the removal of head motion parameters.
Task-state PE signal analysis
BOLD signal analysis of ROIs during the task will be performed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The PE and relative partial differential calculations in the action selection phase and the reward outcome phase will be included as regressors in the general linear model, with ω (see Equation 1) as a covariate (30). The standard hemodynamic function will be constructed for ROIs using MarsBaR (https://sourceforge.net/projects/marsbar/) with FDR correction for control of false discovery rate in voxelwise tests (41). The ROIs will be made with WFU_PickAtlas software (https://www.nitrc.org/projects/wfu_pickatlas) according to the activation map during MDT for PEs.
The ROIs within CEN/SN will be mapped to CEN and SN which are functionally defined according to 14 intrinsic connectivity networks (ICNs) that are comprised of 90 distinct ROIs (42, 43). The DMN, SN, and CEN will be constructed from network templates with the Group ICA of fMRI Toolbox (GIFT, http://mialab.mrn.org/software/gift/) for use in the constrained ICA. The differential functional image (CEN - SN strength) during the first session will be used to create individualized network masks as neurofeedback target regions during the subsequent sessions. The nuisance regressors including six motion parameters and temporal derivatives as well as their quadratic terms and 25 physiological noise components will be taken into consideration. The first two runs of the first session will be discarded without additional analysis. The time courses (extracted using Turbo-BrainVoyager online) of ROIs will be high-pass filtered (0.01 Hz/100 s) and normalized (z-transformed).
Self-regulation performance
For each subject in the patient group in each session, self-regulation performance will be assessed with the CEN-SN activation difference (computed through online time courses of ROIs extracted using Turbo-BrainVoyager) during the training. Self-regulation performance will be defined as the mean % signal change of ROIs (CEN and SN) over all the NF runs. The self-regulation performance will then be submitted to an ANOVA with the factors group (NF vs. YC) and session (four sessions) for the group and/or time effects of rt-fMRI NF training.
Additionally, to investigate the pre- and post-training difference in CEN-SN functional connectivity for each patient group, we will apply the paired t-tests or Wilcoxon signed-rank tests (depending on parametric or non-parametric distributions) between the pre- and post-training tests, while undergoing MDT behavioral task during neuroimaging.
Intervention effect analysis
Primary and secondary outcomes
We will examine the difference in depressive symptoms between the NF and YC groups at the post-test after the training. Bayesian ANCOVA with the group as a fixed factor and the baseline HAMD scores as covariates will be used, using the default JASP (https://jasp-stats.org/) multivariate Cauchy priors (44), and the statistical approach has been described previously (25). The advantage of ANCOVA over repeated-measures (RM) models has been mentioned elsewhere (45). The additional covariates include age, gender, and duration of illness. The dependent variable will be post-test HAMD scores. Similar analyses will be performed on the anhedonic symptoms (SHAPS), with the same covariates and dependent variable. Additionally, similar analyses will also be performed on the primary and secondary outcomes at 3- and 6-month follow-ups.
GD-RL behavioral and connectivity imaging indicators
The behavioral (model-based weighting parameter ω) and connectivity imaging indicators (CEN-SN activation difference) will be used as potential mediating factors to determine whether behavioral and imaging indicators could explain the changes in depressive and/or anhedonic symptoms. Within-group explorative analysis will be conducted using a stepwise regression using a bootstrapping test when multiple mediators are concerned (46) with PROCESS macro (http://www.afhayes.com/) of SPSS 22.
Discussion
The deficits in RL represent a sub-phenotype of anhedonia, with partially separable neural substrates (47). The decision-theoretic view of depression favors the compromise of the GD system, rather than the habitual system in depression (48), the former of which is present in both behavioral and neural manifestations during RL for clinical and subclinical depression (5, 7, 49). Acute stress affects the allocation of neural resources within large-scale brain networks, especially the balance between the CEN/SN in the GD system due to cognitive demands (50). Additionally, the acute stress (during the last month) is supposed to trigger shifts in goal-directed vs. habitual behavior pattern, which is concomitant with specific network configurations, especially frontostriatal circuits (CEN: LPFCal SN: VTA/putamen/caudate↓, lateral/medial OFC↓) (7). Stronger PEs may contribute to highly variable momentary mood fluctuations due to stronger reward feedback dynamics, whereas the influence of PEs on RL is compromised in depressed individuals (51). In summary, these studies highlight a promising target for RL-based NF interventions for anhedonic depression to produce flexible mood changes. Moreover, the focus on the GD-RL and imaging substrates in the current protocol may provide more insights with regard to the factors driving NF outcomes (38).
The novelty of the current study is that we focus on neither single ROI nor functional connectivity limited to a few ROIs, but rather the balance between two functionally intertwined brain networks (CEN/SN), and this approach has been utilized in self-regulation and sustained attention (27, 29). However, the impact of network-based NF on reward learning is still unknown. Thus, we propose to simultaneously decrease CEN and increase SN activity (CEN: LPFC↓; SN: VTA/putamen/caudate↑, lateral/medial OFC↑), which could potentially improve mood and its fluctuations. Individualized NF training based on a goal-directed subnetwork is potentially advantageous for improving treatment response to anhedonia symptoms for depressed patients.
Additionally, the current study takes into account the potential confounding factors of motivation and demand characteristics. The mechanism underlying NF may come from NF-specific or non-specific contextual factors as well as general non-specific effects such as practice, placebo, and natural change (cognitive development or decline)(38). Therefore, a double-blind yoke-controlled design is adopted in this study to control for individual differences in motivation and effort. As a limitation, insufficient power may result from the limited sample size. To control for inter-subject variance, yoke-controlled NF will be provided for the participants to modulate the fMRI signals, in which sham feedback has been shown to regulate neural activity in cognitive control compared to passively viewing conditions (52).
The caveat of this study is the use of CEN and SN based on intermittent, rather than continuous imaging feedback signals, due to the signal calculation speed of network parameters and the high cognitive load of momentary feedback (53). Accumulating evidence indicates that intermittent feedback is more efficient than continuous feedback to reinforce the participants' internal thoughts (54, 55). However, other studies showed that continuous feedback improved regulation ability (56). Additionally, monetary reward elicits a greater extent of self-regulation than non-reward feedback (57). Therefore, further study would be able to determine the influence of intermittent vs. continuous feedback and trial-by-trial virtual monetary reward on GD-RL, though the monetary reward in this study is not contingent on participants' decision as in the Markov decision (MD) task. Feedback design for NF training should be carefully considered in future research.
Finally, accumulating evidence converges on the feasibility and effectiveness of frontostriatal connectivity as NF targets (58). Within SN, the lateral and medial part of OFC as well as ACC densely project to the dorsal (e.g., caudate) and ventral striatum, which are involved in goal-directed control of instrumental actions (59). Moreover, the clinical efficacy of NF targeting both emotion areas and higher visual areas may indicate the non-specific training effects of NF, e.g., the brain control and reinforcement components (25). Therefore, confounds from the non-specific training effects should be taken into consideration during the explanation of the results. Additionally, further empirical studies and systematic reviews may shed light on the most efficient NF targets and provide more evidence on NF targeting specific ROIs of CEN/SN and connectivity-based and network-based NF within the GD-RL systems.
Ethics statement
The study was reviewed and approved by the ethical review committee at the Army Medical University (AMU, PRC). Written informed consent to participate will be obtained from participants.
Author contributions
XW: Conceptualization, Data curation, Funding acquisition, Methodology, Writing—original draft. XZ: Data curation, Methodology, Resources, Writing—review & editing. JL: Data curation, Methodology, Resources, Writing—review & editing. YG: Formal analysis, Software, Visualization, Writing—review & editing. ZF: Supervision, Writing—review & editing, Resources.
Funding
This research was financially supported by the Talented Youth Foundation of AMU (PRC) (no. 410301053421) and the Natural Science Foundation of China (NSFC no. 81971278).
Acknowledgments
We greatly appreciate Drs. Nathaniel D. Daw, Quentin J. M. Huys, and Catherine A. Hartley for their generous guidance on the statistical and computational modeling of behavioral data for two-stage MDT. We also appreciate the technical guidance and support from Dr. Chen Liu for neuroimaging and NF.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Luca B, Flavia G, David L, Giulia F, Adele N, Andrea F, et al. Can anhedonia be considered a suicide risk factor? A review of the literature. Medicina. (2019) 55:8. doi: 10.3390/medicina55080458
2. Lucido MJ, Bekhbat M, Goldsmith DR, Treadway MT, Haroon E, Felger JC, et al. Aiding and abetting anhedonia: impact of inflammation on the brain and pharmacological implications. Pharmacol Rev. (2021) 73:1084–117. doi: 10.1124/pharmrev.120.000043
3. Banca P, Voon V, Vestergaard MD, Philipiak G, Almeida I, Pocinho F, et al. Imbalance in habitual versus goal directed neural systems during symptom provocation in obsessive-compulsive disorder. Brain. (2015) 138:798–811. doi: 10.1093/brain/awu379
4. Decker JH, Otto AR, Daw ND, Hartley CA. From creatures of habit to goal-directed learners: tracking the developmental emergence of model-based reinforcement learning. Psychol Sci. (2016) 27:848–58. doi: 10.1177/0956797616639301
5. Heo S, Sung Y, Lee SW. Effects of subclinical depression on prefrontal–striatal model-based and model-free learning. PLoS Comput Biol. (2021) 17:e1009003. doi: 10.1371/journal.pcbi.1009003
6. Sebold M, Deserno L, Nebe S, Schad DJ, Garbusow M, Hagele C, et al. Model-based and model-free decisions in alcohol dependence. Neuropsychobiology. (2014) 70:122–31. doi: 10.1159/000362840
7. Wang X. The Reward Deficits Model and Computational Neural Mechanism of Anhedonia in Major Depression [Doctor thesis]. Chongqing: Army Medical University. (2018).
8. Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatr. (2021) 110:110289. doi: 10.1016/j.pnpbp.2021.110289
9. Duan LY, Horst NK, Cranmore SAW, Horiguchi N, Cardinal RN, Roberts AC, et al. Controlling one's world: identification of sub-regions of primate PFC underlying goal-directed behavior. Neuron. (2021) 109:2485–98. doi: 10.1016/j.neuron.2021.06.003
10. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
11. Seeley WW. The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci. (2019) 39:9878–82. doi: 10.1523/JNEUROSCI.1138-17.2019
12. Shaw SB, McKinnon MC, Heisz J, Becker S. Dynamic task-linked switching between brain networks – A tri-network perspective. Brain Cogn. (2021) 151:105725. doi: 10.1016/j.bandc.2021.105725
13. Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. (2014) 99:180–90. doi: 10.1016/j.neuroimage.2014.05.052
14. Goldstein-Piekarski AN, Ball TM, Samara Z, Staveland BR, Keller AS, Fleming SL, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. (2022) 91:561–71. doi: 10.1016/j.biopsych.2021.06.024
15. Fettes P, Schulze L, Downar J. Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: promising therapeutic targets in psychiatric illness. Front Syst Neurosci. (2017) 11:25. doi: 10.3389/fnsys.2017.00025
16. Geugies H, Mocking R, Figueroa CA, Groot P, Marsman JC, Servaas MN, et al. Impaired reward-related learning signals in remitted unmedicated patients with recurrent depression. Brain. (2019) 142:2510–22. doi: 10.1093/brain/awz167
17. Rzepa E, McCabe C. Decreased anticipated pleasure correlates with increased salience network resting state functional connectivity in adolescents with depressive symptomatology. J Psychiatr Res. (2016) 82:40–7. doi: 10.1016/j.jpsychires.2016.07.013
18. Rzepa E, McCabe C. Anhedonia and depression severity dissociated by dmPFC resting-state functional connectivity in adolescents. J Psychopharmacol. (2018) 32:1067–74. doi: 10.1177/0269881118799935
19. Liu Q, Ely BA, Schwartz JJ, Alonso CM, Stern ER, Gabbay V, et al. Reward function as an outcome predictor in youth with mood and anxiety symptoms. J Affect Disord. (2021) 278:433–42. doi: 10.1016/j.jad.2020.09.074
20. Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsychopharmacology. (2022) 47:225–46. doi: 10.1038/s41386-021-01101-7
21. Pisoni A, Davis SW, Smoski M. Neural signatures of saliency-mapping in anhedonia: a narrative review. Psychiatry Res. (2021) 304:114123. doi: 10.1016/j.psychres.2021.114123
22. Maier SU, Makwana AB, Hare TA. Acute stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain's decision circuits. Neuron. (2015) 87:621–31. doi: 10.1016/j.neuron.2015.07.005
23. Tabibnia G. An affective neuroscience model of boosting resilience in adults. Neurosci Biobehav Rev. (2020) 115:321–50. doi: 10.1016/j.neubiorev.2020.05.005
24. Tursic A, Eck J, Lührs M, Linden DEJ, Goebel R. A systematic review of fMRI neurofeedback reporting and effects in clinical populations. Neuroimage Clin. (2020) 28:102496. doi: 10.1016/j.nicl.2020.102496
25. Mehler DMA, Sokunbi MO, Habes I, Barawi K, Subramanian L, Range M, et al. Targeting the affective brain-a randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology. (2018) 43:2578–85. doi: 10.1038/s41386-018-0126-5
26. Young KD, Siegle GJ, Zotev V, Phillips R, Misaki M, Yuan H, et al. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am J Psychiatry. (2017) 174:748–55. doi: 10.1176/appi.ajp.2017.16060637
27. Pamplona GSP, Heldner J, Langner R, Koush Y, Michels L, Ionta S, et al. Network-based fMRI-neurofeedback training of sustained attention. Neuroimage. (2020) 221:117194. doi: 10.1016/j.neuroimage.2020.117194
28. Fede SJ, Dean SF, Manuweera T, Momenan R. A guide to literature informed decisions in the design of real time fMRI neurofeedback studies: a systematic review. Front Hum Neurosci. (2020) 14:60. doi: 10.3389/fnhum.2020.00060
29. Krause F, Kogias N, Krentz M, Lührs M, Goebel R, Hermans EJ, et al. Self-regulation of stress-related large-scale brain network balance using real-time fMRI neurofeedback. Neuroimage. (2021) 243:118527. doi: 10.1016/j.neuroimage.2021.118527
30. Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans' choices and striatal prediction errors. Neuron. (2011) 69:1204–15. doi: 10.1016/j.neuron.2011.02.027
31. Trambaiolli LR, Kohl SH, Linden DEJ, Mehler DMA. Neurofeedback training in major depressive disorder: a systematic review of clinical efficacy, study quality and reporting practices. Neurosci Biobehav Rev. (2021) 125:33–56. doi: 10.1016/j.neubiorev.2021.02.015
32. Peters EM, Zhang Y, Lodhi R, Li H, Balbuena L. Melancholic features in bipolar depression and response to lamotrigine: a pooled analysis of five randomized placebo-controlled trials. J Clin Psychopharmacol. (2021) 41:315–9. doi: 10.1097/JCP.0000000000001393
33. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. (1960) 23:56. doi: 10.1136/jnnp.23.1.56
34. Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, et al. A scale for the assessment of hedonic tone the snaith–hamilton pleasure scale. Br J Psychiatr. (1995) 167:99–103. doi: 10.1192/bjp.167.1.99
35. Nakonezny PA, Morris DW, Greer TL, Byerly MJ, Carmody TJ, Grannemann BD, et al. Evaluation of anhedonia with the Snaith-Hamilton Pleasure Scale (SHAPS) in adult outpatients with major depressive disorder. J Psychiatr Res. (2015) 65:124–30. doi: 10.1016/j.jpsychires.2015.03.010
36. Lakens D. Sample size justification. Collabra Psychol. (2022) 8:33267. doi: 10.1525/collabra.33267
37. Thibault RT, MacPherson A, Lifshitz M, Roth RR, Raz A. Neurofeedback with fMRI: a critical systematic review. Neuroimage. (2018) 172:786–807. doi: 10.1016/j.neuroimage.2017.12.071
38. Ros T, Enriquez-Geppert S, Zotev V, Young KD, Wood G, Whitfield-Gabrieli S, et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain. (2020) 143:1674–85. doi: 10.1093/brain/awaa009
39. Krause F, Benjamins C, Eck J, Luhrs M, van Hoof R, Goebel R, et al. Active head motion reduction in magnetic resonance imaging using tactile feedback. Hum Brain Mapp. (2019) 40:4026–37. doi: 10.1002/hbm.24683
40. Weiss F, Zhang J, Aslan A, Kirsch P, Gerchen MF. Feasibility of training the dorsolateral prefrontal-striatal network by real-time fMRI neurofeedback. Sci Rep. (2022) 12:75. doi: 10.1038/s41598-022-05675-0
41. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. (2002) 15:870–8. doi: 10.1006/nimg.2001.1037
42. Park Y, Cha J, Bourakova V, Lee J. Frequency specific contribution of intrinsic connectivity networks to the integration in brain networks. Sci Rep. (2019) 9:1–14. doi: 10.1038/s41598-019-40699-z
43. Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex. (2012) 22:158–65. doi: 10.1093/cercor/bhr099
44. Quintana DS, Williams DR. Bayesian alternatives for common null-hypothesis significance tests in psychiatry: a non-technical guide using JASP. BMC Psychiatry. (2018) 18:1761. doi: 10.1186/s12888-018-1761-4
45. Wan F. Analyzing pre-post randomized studies with one post-randomization score using repeated measures and ANCOVA models. Stat Methods Med Res. (2019) 28:2952–74. doi: 10.1177/0962280218789972
46. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. (2008) 40:879–91. doi: 10.3758/BRM.40.3.879
47. Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cognit Aff Behav Neurosci. (2020) 20:816–41. doi: 10.3758/s13415-020-00804-6
48. Huys QJM, Daw ND, Dayan P. Depression: a decision-theoretic analysis. Annu Rev Neurosci. (2015) 38:1–23. doi: 10.1146/annurev-neuro-071714-033928
49. Reinen JM, Whitton AE, Pizzagalli DA, Slifstein M, Abi-Dargham A, McGrath PJ, et al. Differential reinforcement learning responses to positive and negative information in unmedicated individuals with depression. Eur Neuropsychopharmacol. (2021) 53:89–100. doi: 10.1016/j.euroneuro.2021.08.002
50. Hermans EJ, Henckens MJ, Joels M, Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. (2014) 37:304–14. doi: 10.1016/j.tins.2014.03.006
51. Kao C, Feng GW, Hur JK, Jarvis H, Rutledge RB. Computational models of subjective feelings in psychiatry. Neurosci Biobehav Rev. (2023) 145:105008. doi: 10.1016/j.neubiorev.2022.105008
52. Kober SE, Witte M, Ninaus M, Neuper C, Wood G. Learning to modulate one's own brain activity: the effect of spontaneous mental strategies. Front Hum Neurosci. (2013) 7:695. doi: 10.3389/fnhum.2013.00695
53. Scheinost D, Hsu TW, Avery EW, Hampson M, Constable RT, Chun MM, et al. Connectome-based neurofeedback: a pilot study to improve sustained attention. Neuroimage. (2020) 212:1–12. doi: 10.1016/j.neuroimage.2020.116684
54. Johnson KA, Hartwell KT, Borckardt J, Morgan PS, Govindarajan K, Brady K, et al. Intermittent “real-time” fMRI feedback is superior to continuous presentation for a motor imagery task: a pilot study. J Neuroimag. (2012) 22:58–66. doi: 10.1111/j.1552-6569.2010.00529.x
55. Marxen M, Jacob MJ, Muller DK, Posse S, Ackley E, Hellrung L, et al. Amygdala Regulation Following fMRI-Neurofeedback without Instructed Strategies. Front Hum Neurosci. (2016) 10:183. doi: 10.3389/fnhum.2016.00183
56. Sorger B, Kamp T, Weiskopf N, Peters JC, Goebel R. When the brain takes 'bold' steps: real-time fMRI neurofeedback can further enhance the ability to gradually self-regulate regional brain activation. Neuroscience. (2018) 378:71–88. doi: 10.1016/j.neuroscience.2016.09.026
57. Sepulveda P, Sitaram R, Rana M, Montalba C, Tejos C, Ruiz S, et al. How feedback, motor imagery, and reward influence brain self-regulation using real-time fMRI. Hum Brain Mapp. (2016) 37:3153–71. doi: 10.1002/hbm.23228
58. Martins D, Mehta MA, Prata D. The “highs and lows” of the human brain on dopaminergics: Evidence from neuropharmacology. Neurosci Biobehav Rev. (2017) 80:351–71. doi: 10.1016/j.neubiorev.2017.06.003
Keywords: rt-fMRI neurofeedback, CEN/SN, prediction error, anhedonia, goal-directed system
Citation: Wang X, Zhou X, Li J, Gong Y and Feng Z (2023) A feasibility study of goal-directed network-based real-time fMRI neurofeedback for anhedonic depression. Front. Psychiatry 14:1253727. doi: 10.3389/fpsyt.2023.1253727
Received: 07 July 2023; Accepted: 06 November 2023;
Published: 05 December 2023.
Edited by:
Alessandro Colasanti, Brighton and Sussex Medical School, United KingdomReviewed by:
Laurel Morris, Icahn School of Medicine at Mount Sinai, United StatesDavid MA Mehler, University Hospital RWTH Aachen, Germany
Apurva Watve, University of Zurich, Switzerland
Copyright © 2023 Wang, Zhou, Li, Gong and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxia Wang, bGVtb25vd2FuZ0BnbWFpbC5jb20=; Zhengzhi Feng, Znp6QHRtbXUuZWR1LmNu
 Xiaoxia Wang
Xiaoxia Wang Xiaoyan Zhou2
Xiaoyan Zhou2 Zhengzhi Feng
Zhengzhi Feng