- 1Department of Psychiatry, Shandong Daizhuang Hospital, Jining, China
- 2Institute of Mental Health, Tianjin Anding Hospital, Tianjin, China
- 3Department of Medical Imaging, Affiliated Hospital of Jining Medical University, Jining, China
- 4Department of Psychiatry, Jining Medical University, Jining, China
Background: In recent years, studies on the clinical features and cognitive impairment of patients with different first-episode types of bipolar disorder have received increasing attention. The patients with bipolar disorder may present with different symptoms at first onset. The aim of this study is to assess the cognitive functions of a patient’s index episode of bipolar disorder, depression or mania, on risk factors of effecting on cognitive functions.
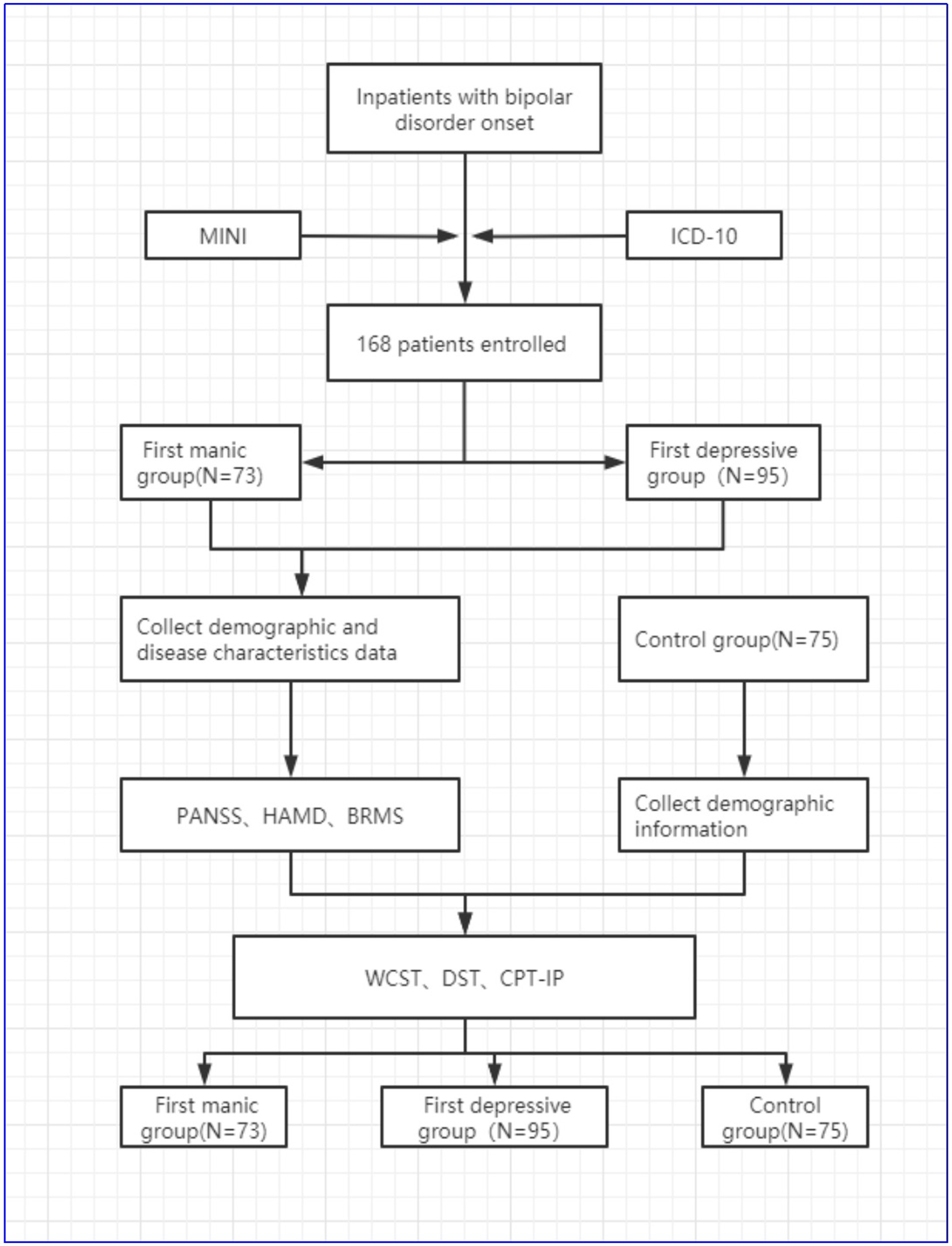
Method: One hundred sixty eight patients with bipolar disorder diagnosed for the first time were enrolled in the study. All patients were divided into two groups according to their index episode of bipolar disorder, either depression or mania. Seventy three patients of the cohort had an index episode mania and 95 patients had initial symptoms of depression. Demographic and clinical disease characteristic data of all enrolled patients were collected. Meanwhile, 75 healthy controls were included. Demographic data of controls were collected. The cognitive functions of all patients and controls were detected by continuous performance test (CPT), digital span test (DST) and Wisconsin card sorting test (WCST). The main cognitive functions data were compared among the mania group, depression group and control group. The relevant risk factors affecting cognitive function were analyzed.
Results: (1) Most patients with bipolar disorder had an index episode depression (56.55% vs. 43.45%). Compared with the depression group, the mania group had later age of onset [(24.01 ± 4.254) vs. (22.25 ± 6.472), t = 2. 122, p = 0.035]. The education level of patient groups was lower than control group (p < 0.001). (2) The healthy control group’s DST, WCST and CPT scores were better than the patient groups (All p < 0.05). The mania group’s DST (forward, reverse, sum), WCST (total responses, completed classifications, correct responses, incorrect responses, percentage of correct responses, completed the number of responses required for classification, the percentage of conceptualization level, the number of persistent responses, non-persistent errors), CPT (2 digit score, 3 digit score, 4 digit score) was better than the depression group (p < 0.05). (3) In mania group, correlation analysis showed that all CPT parameter, inverse digit span, and the sum of DST was negatively correlated with the education level (All p < 0.05). The CPT-4 digit score was negatively correlated with onset age (p < 0.05). In the WCST, the number of correct responses, the percentage of correct responses and the percentage of conceptualization level were positively correlated with the BRMS score (All p < 0.05). The number of false responses and persistent responses were negatively correlated with the BRMS score (All p < 0.05). The number of persistent errors and percentage of persistent errors was positively correlated with education years (All p < 0.05). In depression group, there was a positive correlation between inverse digit span and the education level (p < 0.05).
Conclusion: In our study, there were cognitive impairments in attention, memory, and executive function of patients with different onset syndromes of bipolar disorder. Compared with the mania group, the degree of cognitive impairments in bipolar patients with the depressive episode was more severe. The risk factors affecting cognitive impairments included the age of onset, education level, number of hospitalizations and severity of illness.
1. Introduction
Bipolar disorder (BD) is a chronic and recurrent psychiatric disease affecting more than 1% of the population worldwide (1, 2). Bipolar disorder is characterized by a high prevalence rate, high disability rate, and high comorbidity rate (3, 4), that results in significant disability, heavy economic burden on patient and their families. Bipolar disorder occurs mainly in early adulthood, and most patients’ first onset is between 20 and 30 years old (4). Bipolar disorder is characterized by pathological changes in mood as well as recurring episodes of mania, hypomania, depression and mixed symptoms. The most common symptoms are manic episodes and depressive episodes, which manifests as manic symptoms clusters and depressive symptoms clusters.
Patients with bipolar disorder (BD) differ in their relative predominance of types of episode. Predominant polarity has predictive value in predicting group differences in course of illness. Compared to patients with depressive polarity, patients with manic polarity may represent a more distinct subgroup regarding illness course, suicide attempts, and psychiatric comorbidity (5). Colom et al. (6) thought depression was the dominant polarity both for bipolar I and bipolar II patients. Prevention of mania and depression would be equally important in the case of bipolar I patients. Prevention of depression is crucial for the maintenance treatment of bipolar II patients, whilst predominant polarity is a valid prognostic parameter with therapeutic implications. Most patients with bipolar disorder were found to have depressive episodes as their first symptom, and those with depressive episodes as their first type had a higher risk of suicide. Popovic et al. (7) found that patients with manic episodes as the dominant symptom were later diagnosed with bipolar I disorder, characterized by male predominance, earlier age of onset and first hospitalization, more hospitalizations, higher rates of substance abuse, and higher rates of concomitant psychotic symptoms; while patients with depressive episodes as the first symptom were later diagnosed with bipolar II disorder, with more depressive episodes, higher rates of stressful events, and higher risks of suicide attempts.
Some researchers had studied the distribution of onset type in bipolar disorder patients. Rangappa et al. (8) studied 285 bipolar I patients with a course of more than 5 years and found that manic episode was the main attack type (79%). The type of the first episode determined the main episode type in the lifetime episode, and the type of the first episode affected the treatment and prognosis of the disease. Patients with the first type of manic episode seem to have a better prognosis. For the distribution of first-episode types in bipolar disorder patients, Rangappa et al. (8) found that manic episodes were the predominant episode type (79%) in 285 patients with type I bipolar disorder with a disease duration of more than 5 years, that the first-episode type determined the predominant episode type throughout the future course of the disease, and that the first-episode type influenced the treatment and prognosis of the disease, with patients whose first-episode type was a manic episode appearing to have a better prognosis. The study of Baldessarini et al. (9) on 1,081 cases of bipolar disorder showed that the first symptom types of bipolar disorder were in the order of depressive episode (59%), manic episode (13%), episode with psychotic symptoms (8.0%), anxiety (7.6%), mild manic episode (6.7%), and mixed state (5.5%). Overall, depressive episodes as the first symptom were a more consistent finding in most studies, and patients with bipolar disorder with depressive episodes as the first symptom seemed to have a worse prognosis. According to Barcelona proposal for predominant polarity (10), 20.6% of the BD patients belonged to depressive predominant polarity, 45.8% belonged to manic predominant polarity and 33.6% belonged to indeterminate polarity. Those patients with depressive predominant polarity were more often having bipolar disorder II, had later age of onset, more time in episodes stage, more residual depressive symptoms, less residual manic symptoms, and so on.
Cognitive deficits are considered to be one of the core features of bipolar disorder, and the study of cognitive function in bipolar disorder is one of the current research hotspots (11, 12). Cognitive deficits predict the overall functional outcome of patients with bipolar disorder, and persistent cognitive deficits suggest a poor prognosis for patients with bipolar disorder (13). Cognitive deficits in patients with bipolar disorder are manifested in multiple domains, including attention, executive functioning, memory, intelligence, and judgmental and analytical skills (13).
Cognitive impairment has been found to exist not only during the acute onset of bipolar disorder (14, 15) but also during the remission phase of the disease (16, 17). Bora et al. (18) showed that deficits in neurocognitive development are already present in the early stages of bipolar disorder onset and that patients with bipolar disorder in the early stages of onset have significant neuropsychological deficits in IQ, verbal and deficits in IQ, verbal and visual memory, verbal fluency, and reasoning. However, individuals in the acute and clinically stable phases of bipolar disorder have deficits in different domains of cognitive functioning (including executive function, attention, memory, verbal comprehension, etc.) (19), suggesting that some of the indicators of cognitive deficits in bipolar disorder are state markers of the patient that change as the condition changes and some are genetic markers that remain relatively stable across the stages of change. Since bipolar disorder is highly familial (17, 18), it is entirely possible that some cognitive deficits exist in first-degree relatives of patients with bipolar disorder who do not have the condition (20). Therefore, exploring the clinical characteristics of patients with bipolar disorder and clarify the features of cognitive impairments is conducive to a more appropriate assessment the patients’ condition. It is beneficial to optimize the treatment method of patients with bipolar disorder.
In conclusion, BD patients commonly have cognitive deficits, and cognitive deficits affect the overall prognosis (21). Although cognitive function in BD patients has been studied extensively (22, 23), there is a paucity of literature examining whether cognitive impairment exists between BD patients with different first episode types, so our study focused on exploring this point. Based on previous findings, we hypothesized that BD patients with different first episode types has cognitive impairment during the stable phase of the disease and that there are differences in the characteristics of cognitive impairment, so we further clarified this issue by comparing the characteristics of cognitive impairment in BD patients with depressive symptoms as the first symptom and those with manic symptoms as the first symptom, in order to provide a comprehensive understanding of cognitive function in bipolar disorder patients, and provide a reference for better clinical intervention strategies.
2. Participants and methods
2.1. Study subjects
2.1.1. Patients group
There were 168 BD inpatients recruited from Shandong Daizhuang Hospital in China between July 1, 2020 and June 30, 2021. Inclusion criteria were as follows: (1) primary diagnosis meeting the BD diagnosis based on International Classification of Diseases-10 (ICD-10) criteria, confirmed by two experienced psychiatrists; (2) Bech-Rafaelsen Mania Rating Scale (BRMS) ≤5 points for a minimum of 2 weeks; (3) Hamilton Depression Scale (HAMD) (24-item version) ≤7 points for a minimum of 2 weeks; (4) age 18–55 years old; (5) no gender limits; (6) having at least junior school education, understand the research project required in this study; (7) Han ethnicity, Chinese. Exclusion criteria were as follows: (1) diagnoses consistent with other psychiatric disorders in ICD-10; (2) patients with severe somatic diseases, including severe cardiovascular system diseases, immune system diseases, neurological diseases, etc.; (3) electroconvulsive therapy within 2 weeks; (4) alcohol, drug and various types of drug abuse; (5) female patients who are pregnant or breastfeeding; (6) disagree to participate in this study.
Manic episodes and depressive episodes are common types of bipolar disorder. All enrolled patients were divided into two groups according to the type of first episodes. One hundred sixty eight patients with bipolar disorder were divided into the first mania group (N = 73 patients) and the first depression group (N = 95 patients). These patients were treated with sodium valproate combined with olanzapine; antidepressant treatment was available as needed during depression episodes.
2.1.2. Control group
The healthy controls group were staff, interns or registrars of our hospital during the same period, with gender and age matched to the patient group. A total of 75 healthy controls were enrolled, 44 males and 31 females, aged 18–55 years, with a mean of (25.31 ± 4.239) years.
The study protocol was reviewed and approved by the Ethics Committee of Shandong Daizhuang Hospital.
2.2. Research method
2.2.1. Information collection questionnaire
A self-designed questionnaire was used to collect information on age, sex, occupation, family history, marriage, education level, and onset age in patients group. Information on age, sex, marital status, and years of education was collected from the health control group.
2.2.2. Assessments of mental condition
1. Bech-Rafaelsen Mania Rating Scale (BRMS) (24).
The BRMS mainly assesses the severity of the subject’s manic symptoms. A score of 0 represents no symptoms or a level similar to the subject’s normal level, 1 represents mild symptoms, 2 represents moderate symptoms, 3 represents significant symptoms, and 4 represents severe symptoms. The total score reflects the severity of the disease, the higher the total score, the more serious the disease, 0–4 is no obvious manic symptoms, 6–10 is mild manic symptoms, ≧22 is severe manic symptoms.
2. Hamilton Depression Scale (24-item version) (HAMD-24) (25).
HAMD is the most commonly used scale to assess depressive symptoms in patients with psychiatric disorders in clinical practice. There are three versions of this scale, including 17-item, 21-item, and 24-item versions. The 24-item version was used in this study, and the 24 items were scored using a five-point scale from 0 to 4. 0-none, 1-mild, 2-moderate, 3-severe, and 4-very severe. Total score <8: normal; total score from 8 to 20: possible depression; total score from 20 to 35: definitely depression; total score ≧35: severe depression.
3. The Positive and Negative Syndrome Scale (PANSS) (26, 27).
The PANSS is mainly used to assess the presence or absence of psychiatric symptoms and the severity of each symptom in patients with psychiatric disorders. This scale was used in this study to assess the presence or absence of psychotic symptoms of the enrolled patients. Item scoring criteria: each item of PANSS has a clear definition and a clear 7-point scale, and the 7-point scales are: 1-none; 2-very mild; 3-mild; 4-moderate; 5-moderate-severe; 6-severe; and 7-very severe.
2.3. Cognitive measurement instruments
1. Wisconsin card sorting test (WCST).
The WCST is a neuropsychological test that reflects subjects’ cognitive functions such as neuropsychological processes, generalization, working memory, cognitive transfer, information extraction, attention, categorization switching, categorization maintenance, stimulus recognition and processing, sensory input and motor output. The test consists of 4 stimulus cards and 128 response cards. The WCST is suitable for measuring the cognitive function of clinical patients.
2. Measurements of digit span test (DST) (28).
The DST mainly measures subjects’ short term memory ability and attention. The DST consists of two parts, namely, parsimonious and backward memorization. The digit span parsimony mainly reflects the memory ability and attention ability of the subjects; the digit span reversal mainly reflects the executive functions of the subjects, especially working memory and cognitive flexibility.
3. Measurements of continuous performance test-identical pairs (CPT-IP).
There are several versions of the CPT, and in this study, the CPT-IP was used to measure the sustained attention function in patients with bipolar disorder and healthy controls (see Figure 1).
2.4. Statistical analysis
All data were statistically analyzed using IBM SPSS version 20.0. For the measurement data such as age, age at first presentation, and other factors that conformed to normal distribution, they were expressed as (mean ± standard deviation), and one-way ANOVA was used for comparison between groups; those that did not conform to normal distribution were expressed as median (minimum, maximum), and non-parametric test (Kruskal–Wallis H test) was used. The chi-square test was used for gender, occupation, and other count data. The χ2 test was used to compare the differences in gender, occupation, family history, whether or not there were psychotic symptoms, whether or not there was a history of mental stimulation and marital status among the first manic group, first depressive group and normal control group, and the one-way ANOVA was used to analyze the differences in age and years of education among the first manic group, first depressive group and normal control group, and the independent sample t-test was used to compare the differences in first onset of mania and first depressive group. Non-parametric test was used to compare the differences in cognitive function scores among the first manic group, first depressive group and normal control group; Spearman’s correlation analysis was used to explore the correlation between cognitive function impairment and age at onset, years of education and number of hospitalizations in the first manic group and first depressive group. Test level α = 0.05, two-sided test.
3. Results
3.1. Demographic and clinical characteristics of bipolar disorder patients
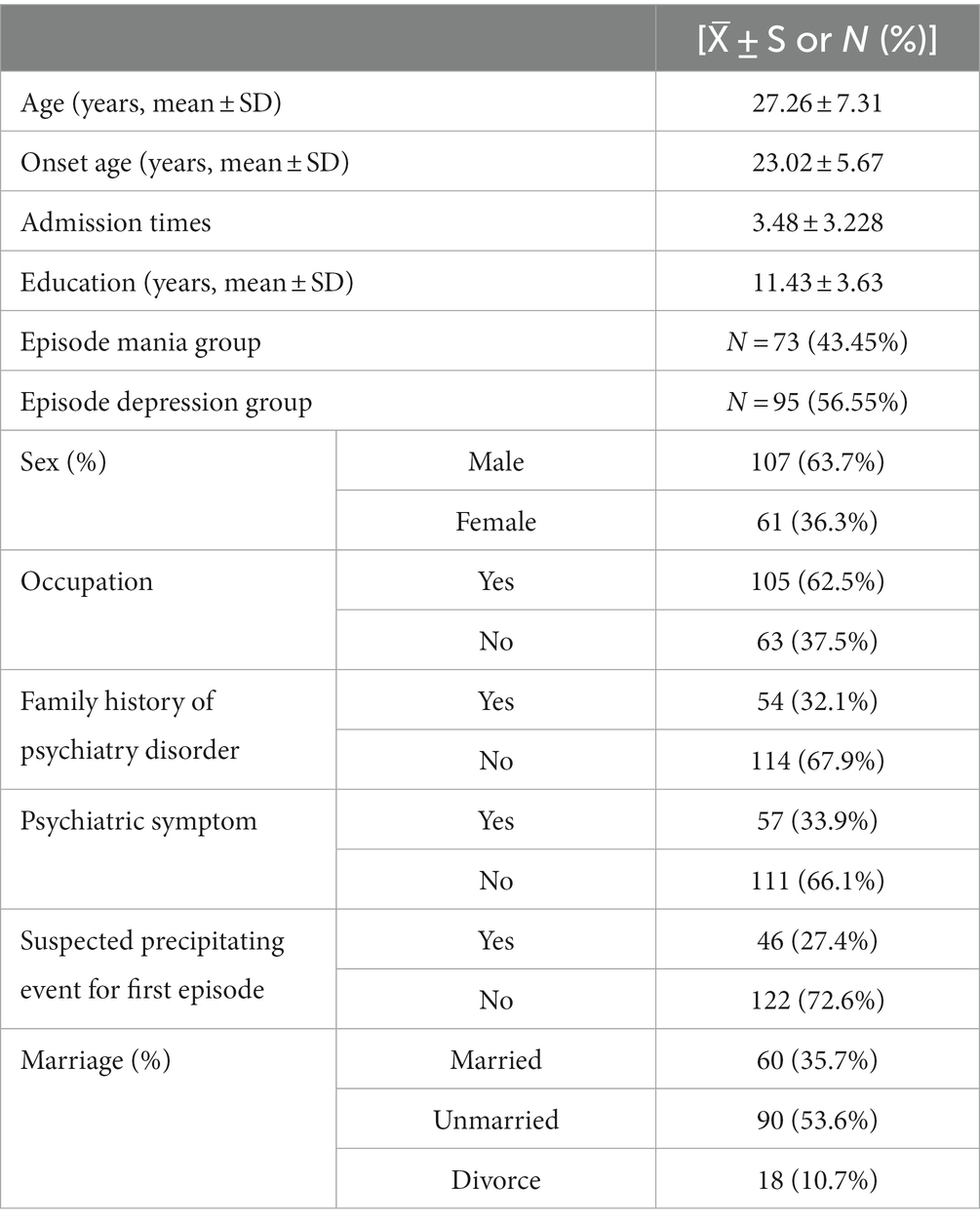
A total of 168 patients were enrolled [73 (43.45%) in the first manic group and 95 (56.55%) in the first depressive group]. The details were showed in Table 1.
3.2. Comparison of demographic and clinical characteristics among three groups
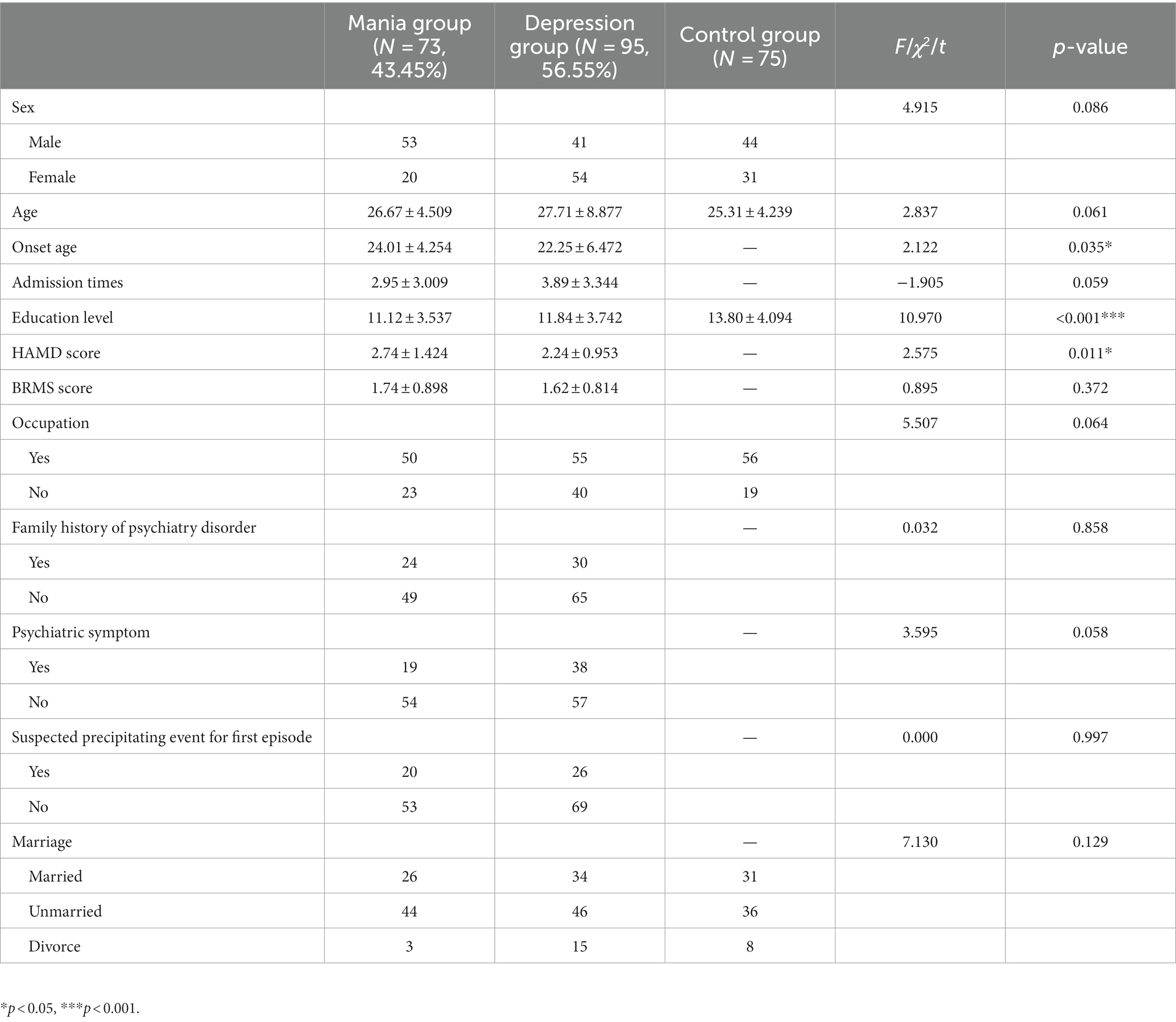
Compared with the first mania group, the first depression group had a higher percentage of patients with bipolar disorder (56.55% vs. 43.45%), the age of first onset [(24.01 ± 4.254) vs. (22.25 ± 6.472), t = 2.122, p = 0.035] was statistically significant between two patient groups, and the difference in education level among three groups was statistically significant (p < 0.001). The differences between the remaining indicators were not statistically significant (Table 2).
3.3. Comparison of sustained attention tests among three groups
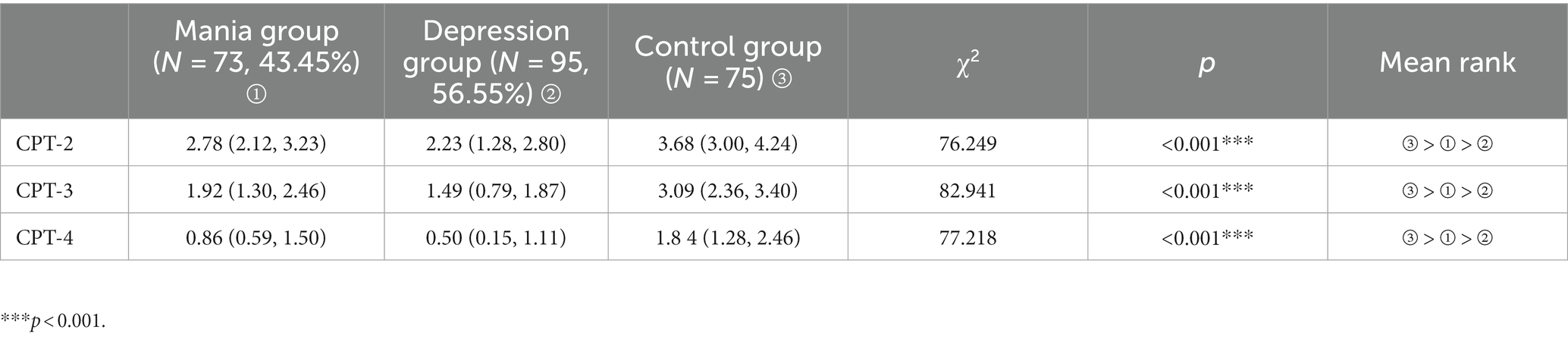
The CPT scores of the first-episode mania group, first-episode depression group, and healthy control group did not conform to normal distribution, the median (minimum, maximum) was used to compare the CPT scores among the three groups using a nonparametric test (Kruskal–Wallis H test). The differences of CPT-2 digit score, CPT-3 digit score and CPT-4 digit score were statistically significant (all p < 0.001) among three groups (Table 3).
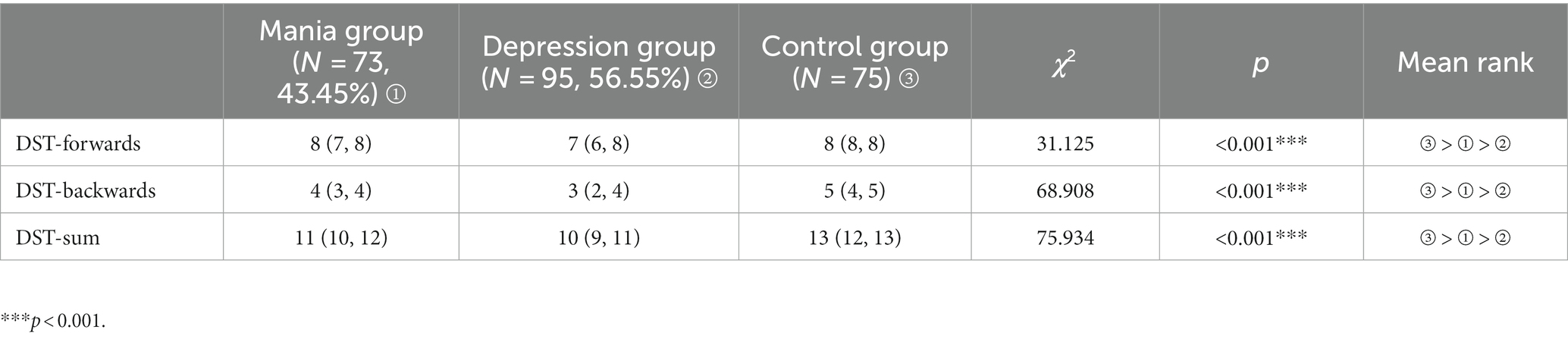
3.4. Comparison of numerical breadth measures among three groups
A non-parametric test (Kruskal–Wallis H test) was used to analysis three groups of numerical breadth measures. The differences in DST-forwards, DST-backwards, DST-sum were statistically significant among three groups (all p < 0.001). The details were showed in Table 4.
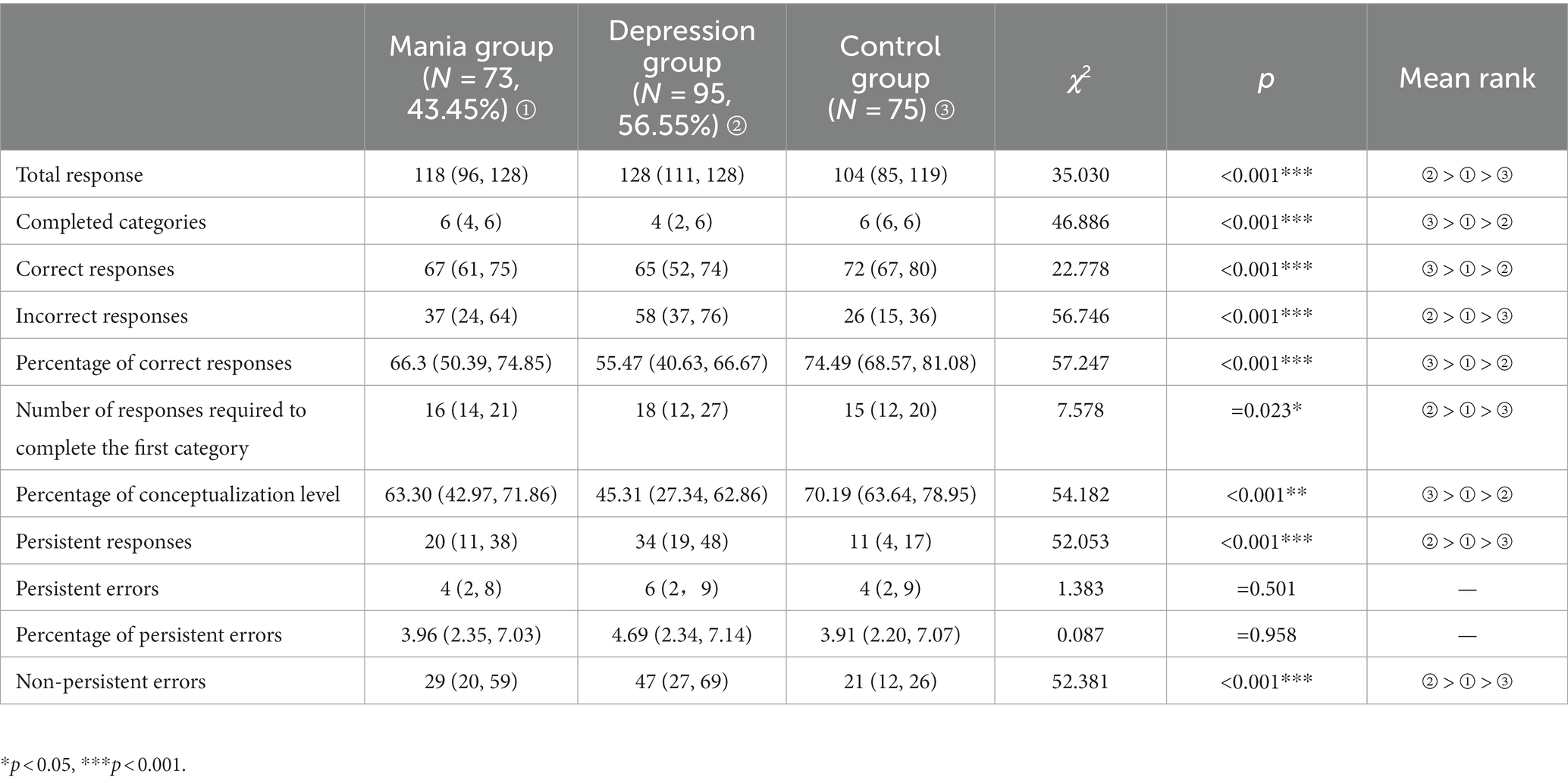
3.5. Comparison of WCST among three groups
There were statistically significant difference in total responses, number of completed categories, number of correct responses, number of incorrect responses, percentage of correct responses, number of responses required to complete the first category, percentage of conceptualization level, number of persistent responses, and non-persistent errors among three groups (all p < 0.05, or p < 0.001). Further comparison showed all above indicators of healthy control group were better than patients groups. These indicators of the first mania group were more better than the first depression group. Details were showed in Table 5.
3.6. Correlation analysis of cognitive function scores and clinical characteristics in patients with bipolar disorder in the first onset manic group
Spearman’s correlation analysis showed the CPT-2 digit, CPT-3 digit, CPT-4 digit, DST-backwards, and DST-sum were negatively correlated with years of education, and CPT-4 digit score was negatively correlated with onset age (all p < 0.05). In WCST, the number of responses required to complete the first category was positively correlated with the number of admissions (all p < 0.05). The number of correct responses, the percentage of correct responses, and the percentage of conceptualization level were positively correlated with the BRMS score (all p < 0.05). The number of incorrect responses, the number of persistent responses, the number of persistent errors and the percentage of persistent errors were negatively correlated with the BRMS score (all p < 0.05). All details were showed in Table 6.
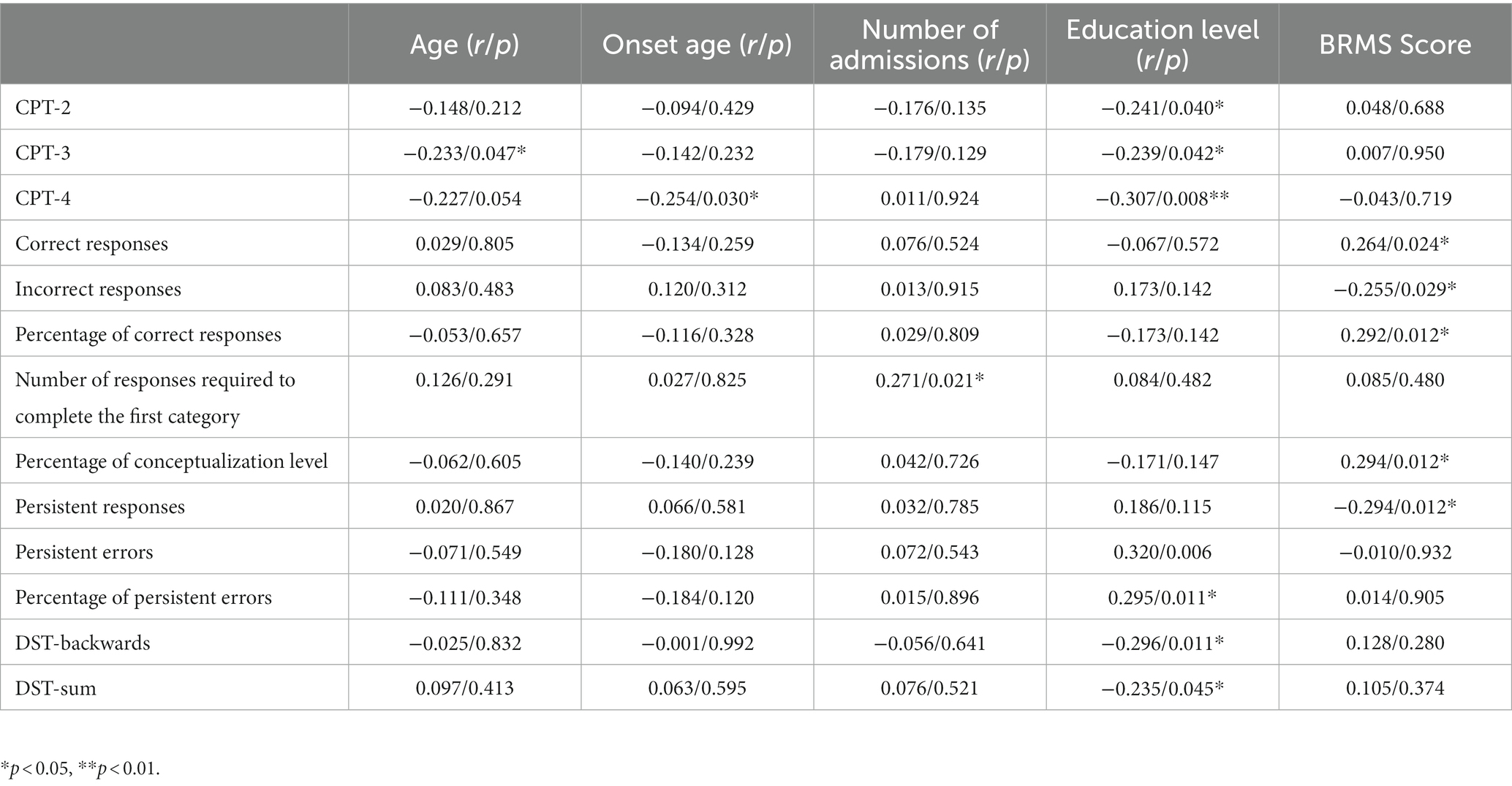
Table 6. Correlation between cognitive function scores and clinical characteristics of BD patients in the first onset manic group.
3.7. Correlation between cognitive function scores and clinical characteristics of bipolar disorder patients in the first onset depression group
Spearman correlation analysis showed DST-backwards was positively correlated with the level of education (p < 0.05). See Table 7 for details.
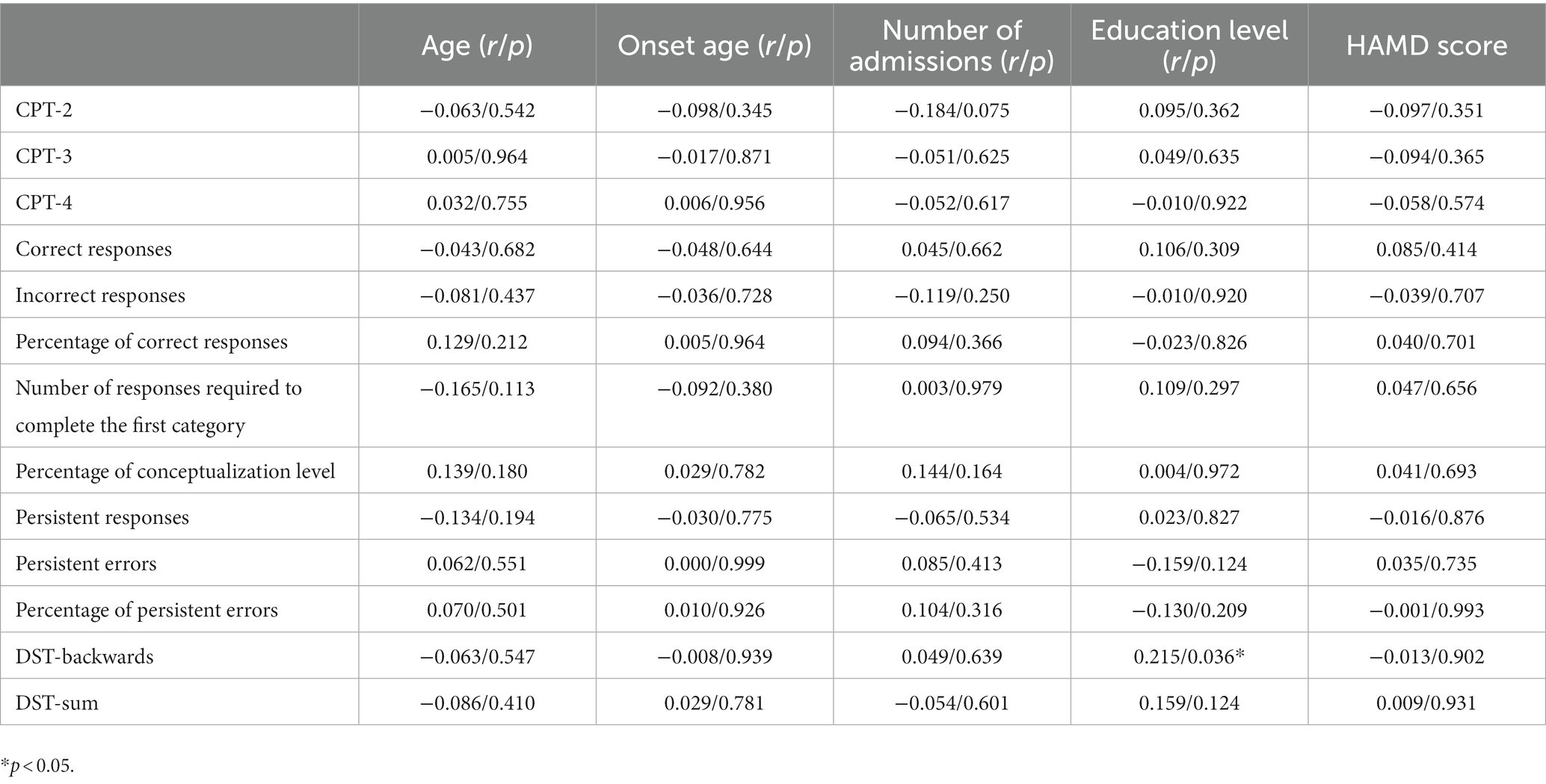
Table 7. Correlation between cognitive function scores and clinical characteristics of BD patients in the first onset depression group.
4. Discussion
4.1. The basic clinical characteristics
Our study showed that the first symptoms of BD patients are mainly depressive episodes (56.55% vs.43.45%). Our previous study (29) supported this point. Compared with the first mania group, the first depression groups had more earlier onset age. There was a significant difference in education level among three groups. The educational level of both patient groups was lower than controls. Some researchers (30) explored the educational status of BD patients, and the study included 10,065 BD patients and found that only 38.96% of BD patients had a better educational level, and the educational level was worse in the presence of co-morbid substance abuse, anxiety disorders, and personality disorders. This research suggested that the disease possibly had a negative impact on patients’ educational level.
4.2. Attention function
Attention is the ability of selecting and integrating information, including think, remember, perceive, plan, execute, and so on. Sustained attention refers to the ability of an individual to focus on a task for an uninterrupted period of time, and sustained attention is a fundamental component of attention function, and the basis for higher-level attention and cognitive function. In our study, the attention function of BD patients with different first-onset types was measured by CPT. The data showed that the CPT-2 digit score, CPT-3 digit score, and CPT-4 digit score were higher in the healthy control group than the first-onset manic group, and higher in the first-onset manic group than the first-onset depressive group, suggesting that patients with different first-onset types of bipolar disorder have impaired attention function, and the degree of attention impairment in the first-episode depression group was greater than that in the first-episode mania group. All of these studies suggest that attentional abilities are significantly impaired in patients with bipolar disorder. Young et al. (31) explored the brain imaging mechanisms underlying impaired attention in bipolar disorder, and this study suggested that impaired attention in patients with bipolar disorder may be related to damage to the parietal cortex of the patient’s brain or inadequate expression of dopamine transporter proteins.
Studies on the variability of attentional deficits among patients with different first-onset types of bipolar disorder have not been reported. Previous studies (32–34) had examined the variability in the degree of attentional impairment between bipolar I disorder and bipolar II disorder. Kung et al. (32) found that sustained attention deficits were the common cognitive impairment in bipolar disorder patients, and almost all of information processing occurred during sustained attention. Fifty-one patients with bipolar disorder (22 with bipolar I disorder and 29 with bipolar II disorder) and 20 healthy controls were included in the study. The 17-item Hamilton Depression Inventory and Young’s Mania Inventory were used to assess the severity of the condition, and the continuous attention test was used to assess the subjects’ attentional function. It was found that after controlling the influences of severity, age, and education level, bipolar I disorder patients had longer delayed response times, poorer discrimination, and more misclassification errors than bipolar II disorder patients and healthy controls. It is suggested that bipolar I disorder patients have worse impairment in attention dysfunction.
4.3. Memory function
In our study, memory function impairment was found in both the first-onset mania group and the first-onset depression group. Memory function impairment was more severe in the first-episode depression group than the first-episode mania group, and the differences among three groups were significant for the three indicators of digit breadth compliance, digit breadth reversal, and digit breadth sum (all p < 0.001). There were not articles published on the memory function impairment in patients with different first-onset types of bipolar disorder. Otherwise, there were studies (35) perform executive functions and episodic memory in bipolar disorder. Cotrena et al. (35) found bipolar disorder type I was associated with more severe and widespread impairments than bipolar II disorder, which showed smaller impairments on all functions except inhibition, where impairments were larger. This study showed that patients with bipolar I disorder had more severe and extensive cognitive impairment compared to patients with bipolar II disorder; patients with bipolar II disorder had less impairment in cognitive functions except for inhibitory functions.
McKinney’s research (36) showed that patients with bipolar II depressive episode had impaired memory function in the early stages of the disease, and patients with psychotic symptoms had more worse memory function than healthy controls. Yatham et al. (37) showed that bipolar disorder patients had significant memory and attention impairment during depressive episodes, mostly in the form of memory loss and concentration difficulties. All studies suggested that bipolar disorder patients had cognitive functions impaired, including memory function, and there was different among episode types.
4.4. Executive function
Executive functions included generally planning, organizing, managing, working memory, response inhibition, emotional self-regulation, task initiation, and so on. On account of the executive function impairment in BD patients (38), so patients had a reduced ability to analyze and judge certain problems. The WCST was used to measure the executive function impairment in BD patients with different first-episode types. Studies of Eric et al. (39) and Kozicky et al. (40) showed cognitive impairment remains for bipolar disorder in stable or remission.
Schulze et al. (41) found BD patients and their first-degree relatives had abnormal executive function, mainly in response delay time. Therefore, Schulze (41) thought executive dysfunction in BD patients was related to genetic factors, and abnormal executive function may belong to the genetic endophenotypic markers of BD patients.
Impaired executive function in BD patients may be associated with multiple factors such as genetics and neural circuitry. Kulkarni et al. (42) thought abnormalities of frontotemporal lobes and subcortical loops were associated with executive dysfunction in BD patients. Newton thought (43) that abnormal levels of serum lipid peroxidation and brain-derived neurotrophic factor expressed were associated with impaired executive function in BD patients. Ni et al. (44) found MsrA haplotype may be associated with abnormal executive function. All studies showed that impaired executive function in BD patients was associated with certain genetic inheritance.
4.5. Factors affecting cognitive function
Our study showed three CPT digits, digit breadth backward and digit breadth sum were negatively correlated with education level (all p < 0.05), and the CPT-4 digit score was negatively correlated with onset age (all p < 0.05) in the first episode mania group. In WCST, the number of correct responses, the percentage of conceptualization level were positively correlated with BRMS scores (all p < 0.05). These findings suggested that the factors affecting the attention and memory functions of mania group patients included education level and onset age. Factors affecting the executive functions of mania group patients were mainly length of stay and education level.
This study showed there were relatively more disease characteristics correlated with cognitive impairment in BD patients with first episode mania. Szmulewicz et al. (45) found that neurodevelopmental abnormalities affecting on cognitive functional deficits. The metabolic syndrome has a negative impact on executive dysfunction of BD patients (46). The cognitive impairment in elderly BD patients was mainly related to the lower level of education and the higher incidence of medical diseases. Cognitive dysfunction increases disability and aggression in elderly BD patients (47). The genetic factors played an important role in the development of cognitive impairment in childhood BD patients (48). These studies suggest that there were many affecting cognitive impairment factors, and that the differences in cognitive impairment between patients with different types of first episode, and between different stages of the disease need to be studied further.
5. Conclusion
BD patients had multidimensional cognitive impairment, including attention, memory and executive function. Cognitive impairment of the first-episode depression group were more worse than the first-episode mania group. The factors affecting the cognitive function of BD patients education level, onset age, severity of illness, the number of admissions.
5.1. Limitations
The study was conducted entirely in one area of China, which may affect generalizability of findings. Another shortcoming of our study only considered the effects of drug types on cognitive function. We did not consider the effects of drug dosage on cognitive function. The method to determine the first episode rely on the patients history coming from the medical record or the patients or their relatives’ statement. We consulted patients or their relatives to identify the first episode type if it was not clear. We did not analyze the rapid cyclothymia type as a separate type, which is a shortness of this study. In addition, an assessment of what might be a protective factor of psychotherapy or counseling, often used prescribed in the prevention or mitigation of symptoms at onset, this point is not involved in our study. In future study, we will further improve the research design to make up for the above deficiencies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Shandong Daizhuang Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZW: conceptualization, formal analysis, writing—original draft, and supervision. HC: formal analysis, writing—original draft, and supervision. YC: formal analysis and writing original draft. HS: data curation and writing original draft. XJ, CW, and ZY: data curation. JL: conceptualization. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Grant (No. 2021M072) from National Technology Project Program of Shandong Province. The project was also supported by Grant (No. 2021YXNS063) for Key Research Program of Jining City. The design and results of research were conducted and explained by the authors.
Acknowledgments
All authors thank the patients with bipolar disorder and their families who agreed to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Clemente, AS, Diniz, BS, Nicolato, R, Kapczinski, FP, Soares, JC, Firmo, JO, et al. Bipolar disorder prevalence: a systematic review and meta-analysis of the literature. Braz J Psychiatry. (2015) 37:155–61. doi: 10.1590/1516-4446-2012-1693
2. Merikangas, KR, Jin, R, He, JP, Kessler, RC, Lee, S, Sampson, NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. (2011) 68:241–51. doi: 10.1001/archgenpsychiatry.2011.12
3. Mur, M, Portella, MJ, Martínez-Arán, A, Pifarré, J, and Vieta, E. Persistent neuropsychological deficit in euthymic bipolar patients: executive function as a core deficit. J Clin Psychiatry. (2007) 68:1078–86. doi: 10.4088/JCP.v68n0715
4. Cichoń, L, Janas-Kozik, M, Siwiec, A, and Rybakowski, J. Clinical picture and treatment of bipolar affective disorder in children and adolescents. Psychiatr Pol. (2020) 54:35–50. doi: 10.12740/PP/OnlineFirst/92740
5. Pallaskorpi, S, Suominen, K, Rosenström, T, Mantere, O, Arvilommi, P, Valtonen, H, et al. Predominant polarity in bipolar I and II disorders: a five-year follow-up study. J Affect Disord. (2019) 246:806–13. doi: 10.1016/j.jad.2018.12.093
6. Colom, F, Vieta, E, Daban, C, Pacchiarotti, I, and Sánchez-Moreno, J. Clinical and therapeutic implications of predominant polarity in bipolar disorder. J Affect Disord. (2006) 93:13–7. doi: 10.1016/j.jad.2006.01.032
7. Popovic, D, Torrent, C, Goikolea, JM, Cruz, N, Sánchez-Moreno, J, González-Pinto, A, et al. Clinical implications of predominant polarity and the polarity index in bipolar disorder: a naturalistic study. Acta Psychiatr Scand. (2014) 129:366–74. doi: 10.1111/acps.12179
8. Rangappa, SB, Munivenkatappa, S, Narayanaswamy, JC, Jain, S, and Reddy, YCJ. Predominant mania course in Indian patients with bipolar I disorder. Asian J Psychiatr. (2016) 22:22–7. doi: 10.1016/j.ajp.2016.04.006
9. Baldessarini, RJ, Tondo, L, and Visioli, C. First-episode types in bipolar disorder: predictive associations with later illness. Acta Psychiatr Scand. (2014) 129:383–92. doi: 10.1111/acps.12204
10. Grover, S, Avasthi, A, Chakravarty, R, Dan, A, Chakraborty, K, Neogi, R, et al. Predominant polarity in bipolar disorder: findings from the bipolar disorder course and outcome study from India (BiD-CoIN study). Compr Psychiatry. (2021) 109:152249. doi: 10.1016/j.comppsych.2021.152249
11. López-Villarreal, A, Sánchez-Morla, EM, Jiménez-López, E, Martínez-Vizcaíno, V, Aparicio, AI, Mateo-Sotos, J, et al. Progression of the functional deficit in a group of patients with bipolar disorder: a cluster analysis based on longitudinal data. Eur Arch Psychiatry Clin Neurosci. (2020) 270:947–57. doi: 10.1007/s00406-019-01050-9
12. Fellendorf, FT, Kainzbauer, N, Platzer, M, Dalkner, N, Bengesser, SA, Birner, A, et al. Gender differences in the association between physical activity and cognitive function in individuals with bipolar disorder. J Affect Disord. (2017) 221:232–7. doi: 10.1016/j.jad.2017.06.048
13. Szmulewicz, AG, Valerio, MP, Lomastro, J, Smith, JM, Chiappe, V, Martino, DJ, et al. Neurocognitive functioning in first-episode bipolar disorder: relationship with functional status. J Affect Disord. (2018) 228:97–100. doi: 10.1016/j.jad.2017.12.015
14. Lin, PY, Wang, PW, Chen, CS, and Yen, CF. Neurocognitive function in clinically stable individuals with long-term bipolar I disorder: comparisons with schizophrenia patients and controls. Kaohsiung J Med Sci. (2017) 33:260–5. doi: 10.1016/j.kjms.2017.02.004
15. Jiménez, E, Solé, B, Arias, B, Mitjans, M, Varo, C, Reinares, M, et al. Characterizing decision-making and reward processing in bipolar disorder: a cluster analysis. Eur Neuropsychopharmacol. (2018) 28:863–74. doi: 10.1016/j.euroneuro.2018.04.001
16. Kircher, T, Stein, F, and Nagels, A. Differences in single positive formal thought disorder symptoms between closely matched acute patients with schizophrenia and mania. Eur Arch Psychiatry Clin Neurosci. (2022) 272:395–401. doi: 10.1007/s00406-021-01263-x
17. Chumakov, EM, Petrova, NN, Limankin, OV, and Ashenbrenner, YV. Cognitive impairment in remitted patients with bipolar disorder. Zh Nevrol Psikhiatr Im S S Korsakova. (2021) 121:12–8. doi: 10.17116/jnevro202112104112
18. Bora, E, Can, G, Ildız, A, Ulas, G, Ongun, CH, Inal, NE, et al. Neurocognitive heterogeneity in young offspring of patients with bipolar disorder: the effect of putative clinical stages. J Affect Disord. (2019) 257:130–5. doi: 10.1016/j.jad.2019.07.015
19. Coppola, F, Courtet, P, and Olié, E. Neuropsychological profile and working memory in bipolar disorder. Can J Psychiatr. (2018) 63:314–21. doi: 10.1177/0706743717744777
20. Bora, E, and Özerdem, A. Social cognition in first-degree relatives of patients with bipolar disorder: a meta-analysis. Eur Neuropsychopharmacol. (2017) 27:293–300. doi: 10.1016/j.euroneuro.2017.02.009
21. Bora, E. Neurocognitive features in clinical subgroups of bipolar disorder: a meta-analysis. J Affect Disord. (2018) 229:125–34. doi: 10.1016/j.jad.2017.12.057
22. Ribera, C, Vidal-Rubio, SLL, Romeu-Climent, JE, Vila-Francés, J, van Rheenen, TE, and Balanzá-Martínez, V. Cognitive impairment and consumption of mental healthcare resources in outpatients with bipolar disorder. J Psychiatr Res. (2021) 138:535–40. doi: 10.1016/j.jpsychires.2021.05.003
23. Simjanoski, M, McIntyre, A, Kapczinski, F, and de Azevedo Cardoso, T. Cognitive impairment in bipolar disorder in comparison to mild cognitive impairment and dementia: a systematic review. Trends Psychiatry Psychother. (2023) 44:e20210300. doi: 10.47626/2237-6089-2021-0300
24. Shansis, F, Grevet, E, Mattevi, B, Berlim, M, Maldonado, G, Santin, A, et al. Development and application of the mania rating guide (MRG). Braz J Psychiatry. (2003) 25:91–5. doi: 10.1590/S1516-44462003000200008
25. Carneiro, AM, Fernandes, F, and Moreno, RA. Hamilton depression rating scale and Montgomery–Asberg depression rating scale in depressed and bipolar I patients: psychometric properties in a Brazilian sample. Health Qual Life Outcomes. (2015) 13:42. doi: 10.1186/s12955-015-0235-3
26. Aggarwal, NK, Tao, H, Xu, K, Stefanovics, E, Zhening, L, and Rosenheck, RA. Comparing the PANSS in Chinese and American inpatients: cross-cultural psychiatric analyses of instrument translation and implementation. Schizophr Res. (2011) 132:146–52. doi: 10.1016/j.schres.2011.08.003
27. Shafer, A, and Dazzi, F. Meta-analysis of the positive and negative syndrome scale (PANSS) factor structure. J Psychiatr Res. (2019) 115:113–20. doi: 10.1016/j.jpsychires.2019.05.008
28. Dalkner, N, Bengesser, S, Birner, A, Rieger, A, Seebauer, J, Platzer, M, et al. Body mass index predicts decline in executive function in bipolar disorder: preliminary data of a 12-month follow-up study. Neuropsychobiology. (2021) 80:1–11. doi: 10.1159/000505784
29. Wang, Z, Cao, Y, Zhu, Y, Li, K, Jiang, X, Zhuo, C, et al. Differences in demographic and clinical characteristics of patients with depressive vs. manic first episode of bipolar disorder. Front Psychiatry. (2021) 12:616415. doi: 10.3389/fpsyt.2021.616415
30. Karanti, A, Bublik, L, Kardell, M, Annerbrink, K, Lichtenstein, P, Runeson, B, et al. Patient educational level and management of bipolar disorder. BJPsych Open. (2021) 7:e63. doi: 10.1192/bjo.2021.19
31. Young, JW, Geyer, MA, Halberstadt, AL, Enkhuizen, J, Minassian, A, Khan, A, et al. Convergent neural substrates of inattention in bipolar disorder patients and dopamine transporter-deficient mice using the 5-choice CPT. Bipolar Disord. (2020) 22:46–58. doi: 10.1111/bdi.12786
32. Kung, CH, Lee, SY, Chang, YH, Wu, JYW, Chen, SL, Chen, SH, et al. Poorer sustained attention in bipolar I than bipolar II disorder. Ann General Psychiatry. (2010) 9:8. doi: 10.1186/1744-859X-9-8
33. Najt, P, Glahn, D, Bearden, CE, Hatch, JP, Monkul, ES, Kaur, S, et al. Attention deficits in bipolar disorder: a comparison based on the continuous performance test. Neurosci Lett. (2005) 379:122–6. doi: 10.1016/j.neulet.2004.12.051
34. Trivedi, JK, Dhyani, M, Sharma, S, Sinha, PK, Pratap Singh, A, and Tandon, R. Cognitive functions in euthymic state of bipolar disorder: an Indian study. Cogn Neuropsychiatry. (2008) 13:135–47. doi: 10.1080/13546800801897346
35. Cotrena, C, Damiani Branco, L, Ponsoni, A, Samamé, C, Milman Shansis, F, and Paz Fonseca, R. Executive functions and memory in bipolar disorders I and II: new insights from meta-analytic results. Acta Psychiatr Scand. (2020) 141:110–30. doi: 10.1111/acps.13121
36. McKinney, RA, Avery, SN, Armstrong, K, Blackford, JU, Woodward, ND, and Heckers, S. Relational memory in the early stage of psychotic bipolar disorder. Psychiatry Res. (2020) 294:113508. doi: 10.1016/j.psychres.2020.113508
37. Yatham, LN, Kennedy, SH, Parikh, SV, Schaffer, A, Bond, DJ, Frey, BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. (2018) 20:97–170. doi: 10.1111/bdi.12609
38. Sheffield, JM, Karcher, NR, and Barch, DM. Cognitive deficits in psychotic disorders: a lifespan perspective. Neuropsychol Rev. (2018) 28:509–33. doi: 10.1007/s11065-018-9388-2
39. Eric, YW, Halari, R, Cheng, KM, Leung, SK, and Young, AH. Cognitive performance is impaired in euthymic Chinese patients with bipolar I disorder. J Affect Disord. (2013) 151:156–63. doi: 10.1016/j.jad.2013.05.070
40. Kozicky, JM, Torres, IJ, Silveira, LE, Bond, DJ, Lam, RW, and Yatham, LN. Cognitive change in the year after a first manic episode: association between clinical outcome and cognitive performance early in the course of bipolar I disorder. J Clin Psychiatry. (2014) 75:e587–93. doi: 10.4088/JCP.13m08928
41. Schulze, KK, Walshe, M, Stahl, D, Hall, MH, Kravariti, E, Morris, R, et al. Executive functioning in familial bipolar I disorder patients and their unaffected relatives. Bipolar Disord. (2011) 13:208–16. doi: 10.1111/j.1399-5618.2011.00901.x
42. Kulkarni, KR, Reddy, PV, Purty, A, Arumugham, SS, Muralidharan, K, Reddy, YCJ, et al. Course and naturalistic treatment seeking among persons with first episode mania in India: a retrospective chart review with up to five years follow-up. J Affect Disord. (2018) 240:183–6. doi: 10.1016/j.jad.2018.07.039
43. Newton, DF, Naiberg, MR, Andreazza, AC, Scola, G, Dickstein, DP, and Goldstein, BI. Association of lipid peroxidation and brain-derived neurotrophic factor with executive function in adolescent bipolar disorder. Psychopharmacology. (2017) 234:647–56. doi: 10.1007/s00213-016-4500-x
44. Ni, P, Ma, X, Lin, Y, Lao, G, Hao, X, Guan, L, et al. Methionine sulfoxide reductase a (MsrA) associated with bipolar I disorder and executive functions in a Han Chinese population. J Affect Disord. (2015) 184:235–8. doi: 10.1016/j.jad.2015.06.004
45. Szmulewicz, A, Valerio, M, and Martino, DJ. Cognitive decline and neuroprogression in bipolar disorder: a case for Hitchens’ razor. Bipolar Disord. (2020) 22:536. doi: 10.1111/bdi.12953
46. Dalkner, N, Bengesser, SA, Birner, A, Fellendorf, FT, Fleischmann, E, Großschädl, K, et al. Metabolic syndrome impairs executive function in bipolar disorder. Front Neurosci. (2021) 15:717824. doi: 10.3389/fnins.2021.717824
47. Belvederi Murri, M, Respino, M, Proietti, L, Bugliani, M, Pereira, B, D’Amico, E, et al. Cognitive impairment in late life bipolar disorder: risk factors and clinical outcomes. J Affect Disord. (2019) 257:166–72. doi: 10.1016/j.jad.2019.07.052
Keywords: bipolar disorder, clinical characteristics, cognitive function, first onset symptoms, risk factors
Citation: Wang Z, Cao H, Cao Y, Song H, Jiang X, Wei C, Yang Z and Li J (2023) Clinical characteristics and cognitive function in bipolar disorder patients with different onset symptom. Front. Psychiatry. 14:1253088. doi: 10.3389/fpsyt.2023.1253088
Edited by:
Arghya Pal, AII India Institute of Medical Sciences, Raebareli, IndiaReviewed by:
Massimo Tusconi, University of Cagliari, ItalyTeresa Sanchez-Gutierrez, International University of La Rioja, Spain
Copyright © 2023 Wang, Cao, Cao, Song, Jiang, Wei, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhonggang Wang, d3pnOTY5QDE2My5jb20=; Jie Li, amllbGlAdGptaGMuY29t
†These authors have contributed equally to this work and share first authorship
 Zhonggang Wang
Zhonggang Wang Haiyan Cao
Haiyan Cao Yuying Cao3
Yuying Cao3 Haining Song
Haining Song Xianfei Jiang
Xianfei Jiang Jie Li
Jie Li