- 1Department of Neurology, Sichuan Taikang Hospital, Chengdu, China
- 2Department of Respiratory, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, China
- 3Department of Neurology, Qionglai People’s Hospital, Chengdu, China
Background: Observational studies have suggested that COVID-19 increases the prevalence of psychiatric disorders, but the results of such studies are inconsistent. This study aims to investigate the association between COVID-19 and the risk of psychiatric disorders using Mendelian randomization (MR) analysis.
Methods: We used summary statistics from COVID-19 Host Genetics Initiative genome-wide association study (GWAS) of COVID-19 involving 2,586,691 participants from European ancestry. Genetic variations of five psychiatric disorders including autism spectrum disorder (ASD) (N = 46,351), bipolar disorder (BID) (N = 51,710), major depressive disorder (MDD) (N = 480,359), anxiety disorder (N = 83,566), and schizophrenia (SCZ) (N = 77,096) were extracted from several GWAS of European ancestry. The inverse-variance weighted (IVW) method as the main MR analysis conducted. We further performed sensitivity analyzes and heterogeneity analyzes as validation of primary MR results.
Results: The IVW analysis found that COVID-19 hospitalization phenotype was the risk factor for BID (OR = 1.320, 95% CI = 1.106–1.576, p = 0.002) and SCZ (OR = 1.096, 95% CI = 1.031–1.164, p = 0.002). Moreover, we detected a significant positive genetic correlation between COVID-19 severity and two psychiatric traits, BID (OR = 1.139, 95% CI = 1.033–1.256, p = 0.008) and SCZ (OR = 1.043, 95% CI = 1.005–1.082, p = 0.024). There was no evidence supporting the causal relationship between COVID-19 susceptibility and psychiatric disorders.
Conclusion: Our results found that the COVID-19 hospitalization phenotype and COVID-19 severity phenotype might be the potential risks of BID and SCZ in European populations. Therefore, patients infected with SARS-CoV-2 should have enhanced monitoring of their mental status.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which usually has respiratory symptoms as the main clinical manifestation and can also lead to multisystem involvement (1–3). Since the COVID-19 outbreak in December 2019, the World Health Organization has classified the COVID-19 outbreak as an international public health emergency (4, 5). Some clinical investigations suggest that the COVID-19 epidemic is a highly stressful event, which acts as an important stressor disrupting the physiological and psychological balance of individuals. This imbalance can potentially lead to various degrees of mental health problems in both social groups and individuals (6). Among patients in COVID-19 epidemic areas, the more common mental symptoms are nervousness, anxiety, worry, fear, insomnia and other symptoms or various symptoms of physical discomfort. In severe cases, some patients even develop psychiatric disorders, such as major depressive disorder and anxiety disorder, or relapse of their original mental illness (7–9). However, the strength and significance of the observed associations of COVID-19 with psychiatric disorders remain controversial.
An observational study involving 56,679 participants conducted in China showed that the risk of depression was 3.27 [95% confidence interval (CI): 1.84–5.80], anxiety disorder was 2.48 (95% CI: 1.43–4.31), and insomnia was 3.06 (95% CI: 1.73–5.43) in patients with COVID-19 (10). A cohort study conducted in the United States comprising 153,848 people showed that the risk of psychiatric disorders in patients with COVID-19 was 1.46 (95% CI: 1.40–1.52), including 1.41 (95% CI: 1.40–1.52) for sleep disorders, 1.38 (95% CI: 1.34–1.43) for stress disorders, and 1.35 (95% CI: 1.30–1.39) for anxiety disorders (11). A cross-sectional population study in South Korea showed that participants with moderate or severe depressive symptoms and anxiety symptoms accounted for 12.6 and 6.8%, respectively (12). In addition, during the COVID-19 pandemic, the disruption of the daily lives, physical activities, social life and educational progress of children and adolescents also had an impact on their physical and mental health, with symptoms such as anxiety, depression, post-traumatic stress disorder (PTSD), sleep problems and non-suicidal self-injury (13). A cross-sectional survey conducted after 2 months of COVID-19 quarantine in Saudi Arabia revealed that among adolescents, 15.5% had no symptoms, 44.1% experienced mild symptoms, and 13.0% exhibited potential PTSD symptoms (14).
SARS-CoV-2 infection or the presence of residual virus may lead to persistent psychiatric symptoms such as brain fog, memory loss, and decreased thinking and reaction ability (15). Additionally, the immune response triggered by the virus can have a long-lasting impact on the brain and other organs, including hormone feedback systems and blood biochemical transduction signaling systems (16). These effects can potentially trigger various biological responses. For example, under chronic stress, the brain signals the adrenal gland to release cortisol for extended durations, resulting in a malfunctioning hormone system and an overactive immune system. These factors contribute to an increased susceptibility to anxiety, depression, and other mental disorders (17).
A growing number of observational studies suggest that COVID-19 may potentially trigger the onset of mental disorders, such as anxiety disorders, depression, and even schizophrenia. However, traditional observational studies are often interfered by a variety of confounding factors, such as living environment, education level, and eating habits. Mendelian randomization (MR) is a causal inference method that relies on genetic variation. Its fundamental principle is to utilize the impact of randomly assigned genotypes on phenotypes in nature to infer the influence of biological factors on diseases, which can largely avoid potential confounding factors and reverse causality (18). Therefore, MR analysis, as an epidemiological method, is widely used to verify the causal relationship found in observational studies (19). In short, MR studies use genetic variation as an instrumental variables (IVs) to avoid confounding factors and reverse causality (20). In this study, we conducted a two-sample MR study to assess the causal relationship between COVID-19 and five important psychiatric disorders included autism spectrum disorder (ASD), major depressive disorder (MDD), bipolar disorder (BID), schizophrenia (SCZ), and anxiety disorder as outcomes.
2. Methods
2.1. Study design
We conducted a two-sample MR analysis to investigate the causal relation of COVID-19 traits on the risk of psychiatric traits (21). It is well known that MR studies are carried out under the assumption that instrumental variable (IVs) associated with exposure is independent of known or unknown confounders and that IVs affects outcomes only through exposure and not through other pathways (19). In this MR study, three COVID-19 traits (COVID-19 susceptibility, COVID-19 hospitalization, COVID-19 severity) as exposure, five psychiatric traits including autism spectrum disorder (ASD), major depressive disorder (MDD), bipolar disorder (BID), schizophrenia (SCZ), and anxiety disorder as outcomes.
2.2. Data sources and genetic instruments
We obtained summary-level data for COVID-19 susceptibility, hospitalization, severity from the latest version of the COVID-19 Host Genetics Initiative (HGI) GWAS meta-analyzes, round 6 (22). Diagnosis of COVID-19 cases relies on laboratory-confirmed infection of SARS-CoV-2, as well as electronic health record documentation or physician diagnosis of COVID-19. Additionally, self-reported COVID-19 infection from the patient is also considered. The exposure of COVID-19 susceptibility phenotype compared 112,612 European COVID-19 patients with a control population of 2,474,079 without a history of COVID-19. Patients who were diagnosed with COVID-19 and hospitalized due to COVID-19 were considered as a COVID-19 hospitalized cohort. The exposure of COVID-19 hospitalization phenotype compared 24,274 patients who were hospitalized due to COVID-19 with a control group (N = 2,061,529) consisting of individuals who were diagnosed with COVID-19 but were not hospitalized or were free of COVID-19. The COVID-19 severe cohort includes hospitalized patients who died from COVID-19, and those who developed respiratory failure and needed respiratory support (including tracheotomy, tracheal intubation, non-invasive ventilator-assisted ventilation, invasive ventilator-assisted ventilation, etc.) The exposure of COVID-19 severity phenotype compared 8,779 severe hospitalized individuals with a controls who were without severe COVID-19, or who were free of COVID-19 (N = 1,001,875).
We obtained summary-level data for five psychiatric traits from published multiplied studies with large sample sizes of European ancestry (23). Genome-wide association study data for Autism Spectrum Disorder (ASD), Major Depressive Disorder (MDD), Bipolar Disorder (BID), and Schizophrenia (SCZ) were sourced from the Psychiatric Genomics Consortium (PGC) (24–27). The PGC is an international consortium of scientists committed to meta-analysis of genome-wide genetic data, specifically focusing on psychiatric disorders. Summary-level data for anxiety disorders were obtained from a meta-analyzed, involving 83,566 participants (25,453 cases and 58,113 controls) from the UK Biobank (28, 29). The basic characteristics of GWASs, including exposures and outcomes, are listed in Table 1.
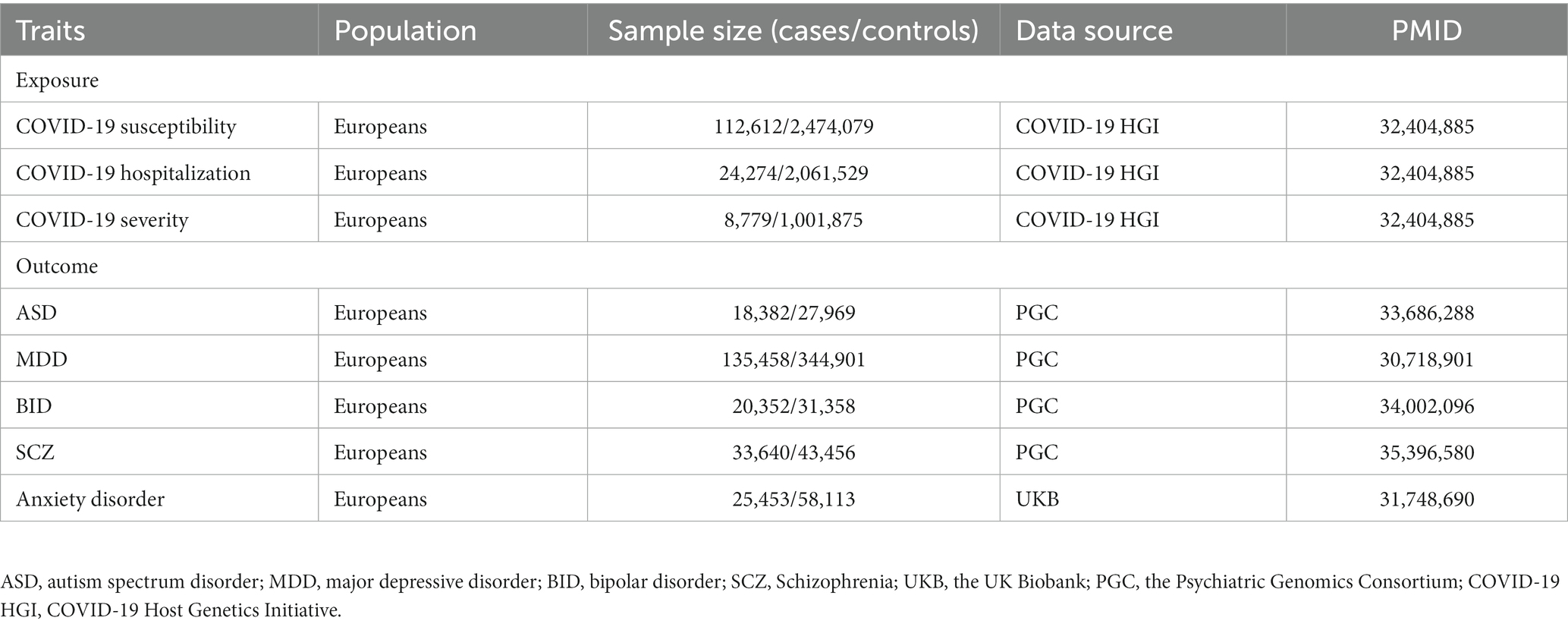
Table 1. Detailed information of the studies and datasets used for Mendelian randomization analyzes.
2.3. IVs selection
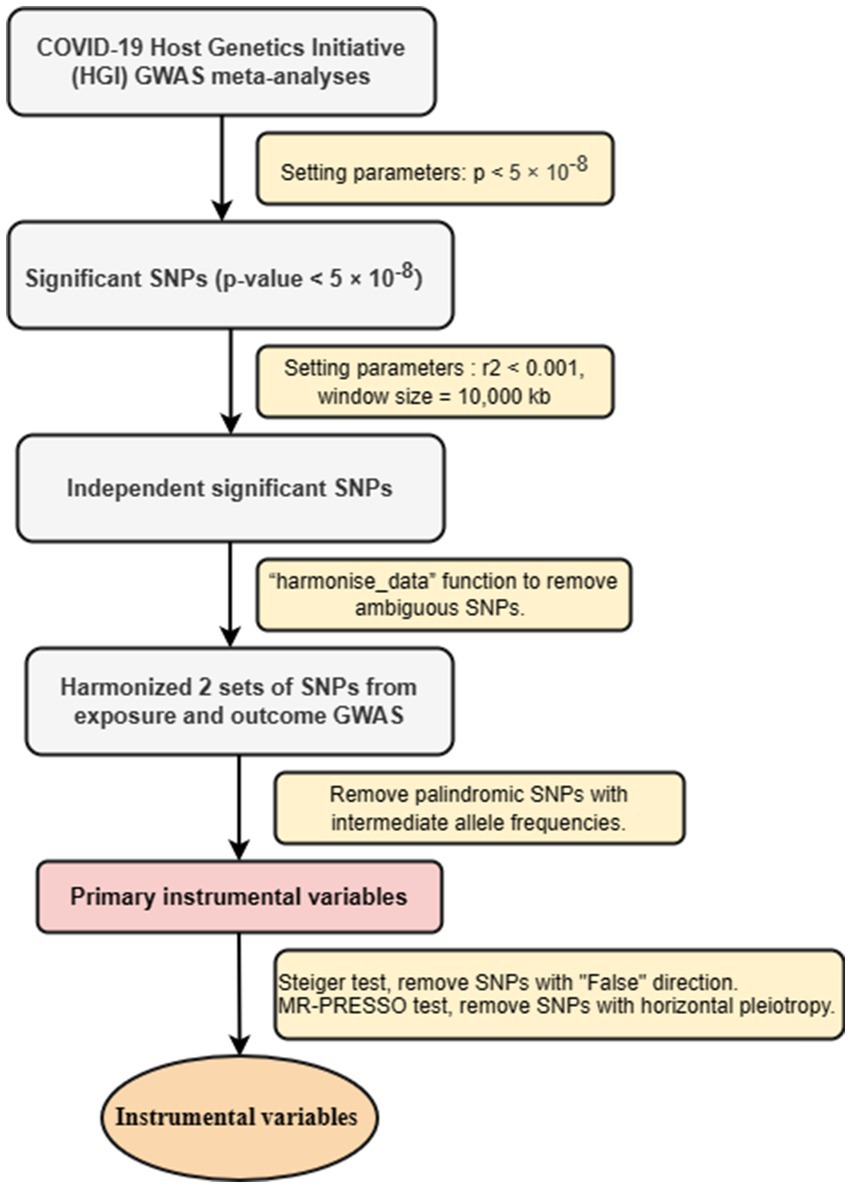
To ensure that all screened IVs meet MR analysis standards, we have adopted a series of strict control steps. First step, to ensure the relevance of IVs, we extracted genome-wide significant single nucleotide polymorphisms (SNPs) from exposed GWASs. Only SNPs that value of p < 5 × 10−8 were considered strongly associated with exposure used as IVs. In the second step, to ensure the independence of IVs, we conducted linkage disequilibrium (LD) clumping (r2 < 0.001, window size = 10,000 kb) to select independent significant SNPs. In the third step, using the screened SNPs, we extracted SNPs from the outcome (psychiatric disorders) GWAS (30). We harmonized the exposure and outcome datasets using the “harmonise_data” function to remove ambiguous SNPs and create a new data frame combining exposure and outcome data. For SNPs not found in the outcome GWAS, after reconciling the above two sets of SNPs, palindromic SNPs with intermediate allele frequencies were removed, and the remaining SNPs were retained as primary IVs. In the fourth step, we conducted Steiger test, removed SNPs with “False” direction. We perfomed MR-PRESSO test, removed SNPs with horizontal pleiotropy (29). At same time, we used the Pheno Scanner database to examine selected IVs associated with other phenotypes that might influence the results (31). The detailed information for the selected SNP is shown in the Supplementary File. IVs screening flow chart is shown in Figure 1.
2.4. Statistical analysis
To address the potential pleiotropic effects of genetic variation, this study applied three MR analyzes to assess the causal effects of COVID-19 traits on psychiatric disorders. We applied the standard inverse variance weighting (IVW) method as the primary MR methods, which combined the Wald ratio of each SNPs on the outcome and obtained a pooled causal estimate. If there is heterogeneity, we use IVW random effect method. In addition, MR-Egger and weighted median (WM) methods, as further complementary methods to MR, these methods can provide more reliable estimates in a broader range of situations (31). MR-Egger regression can provide tests for unbalanced pleiotropy and considerable heterogeneity, whereas for the same underexposed variation it requires a larger sample size (20). MR-Egger method often yields inaccurate and statistically less significant results, especially when the number of SNPs is small. In addition, the value of the MR-Egger intercept term was far from zero, indicating horizontal pleiotropy (p < 0.05) (32, 33). Therefore, in our MR study, the MR-Egger method was mainly performed to detect pleiotropy. The WM method will return an unbiased estimate if more than one-half of the IVs were valid.
Horizontal pleiotropy occurs when exposure (COVID-19) related genetic variations directly affect the results by assuming multiple pathways other than exposure (psychiatric disorders). Therefore, we further conducted Cochrane’ s Q statistic, leave-one-out (LOO) analysis and MR-Egger intercept test to detect the existence of pleiotropy and evaluate the robustness of the results (33). When the p value of the Cochrane Q test is less than 0.05, there is heterogeneity. We also evaluate the horizontal multidirectionality based on the intercept term obtained by MR-Egger regression (34). In order to determine whether the causal estimation is driven by a single SNP, we performed a LOO analysis, in which each exposure-related SNP was discarded in turn to repeat the IVW analysis.
3. Results
3.1. Association of COVID-19 susceptibility with psychiatric traits
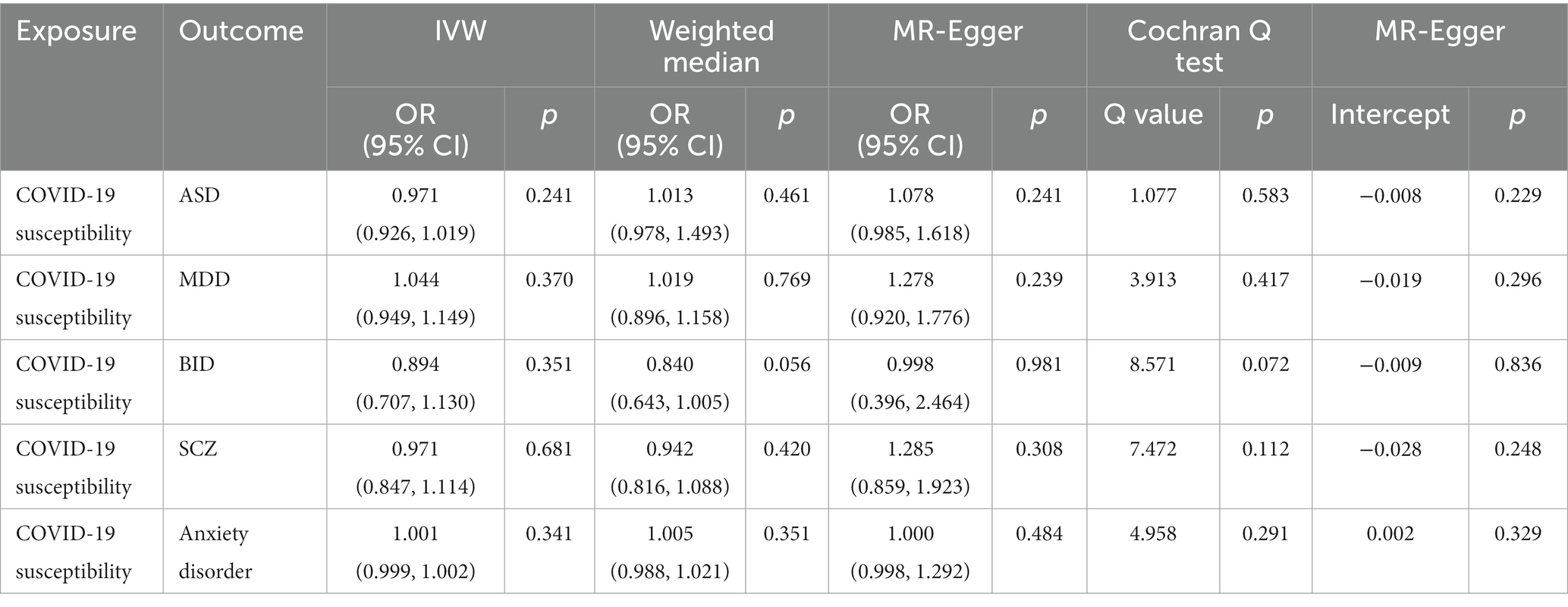
The IVW MR analysis suggested that there is no evidence supporting COVID-19 susceptibility as a risk or protective factor for five psychiatric traits, ASD (IVW: OR = 0.971, 95% CI = 0.926–1.019, p = 0.241), MDD (IVW: OR = 1.044, 95% CI = 0.949–1.149, p = 0.370), BID (IVW: OR = 0.894, 95% CI = 0.707–1.130, p = 0.351), SCZ (IVW: OR = 0.971, 95% CI = 0.847–1.114, p = 0.681), anxiety disorder (IVW: OR = 1.000, 95% CI = 0.999–1.002, p = 0.341). The MR results are shown in Table 2 and Figure 2.

Figure 2. Associations of COVID-19 susceptibility with five psychiatric traits based on the IVW method. ASD, autism spectrum disorder; MDD, major depressive disorder; BID, bipolar disorder; SCZ, Schizophrenia.
The Cochrane’ s Q test suggested that there was no heterogeneity in the main MR analysis among the five psychiatric traits (all p values >0.05). Additionally, no horizontal pleiotropy was found, with an insignificant intercept from the MR-Egger test (all p values >0.05). The results of leave-one-out sensitivity analyzes suggested that the causal associations between COVID-19 susceptibility traits and psychiatric disorders were not affected by any individual SNP (Supplementary Figures S1–S5).
3.2. Association of COVID-19 hospitalization with psychiatric traits
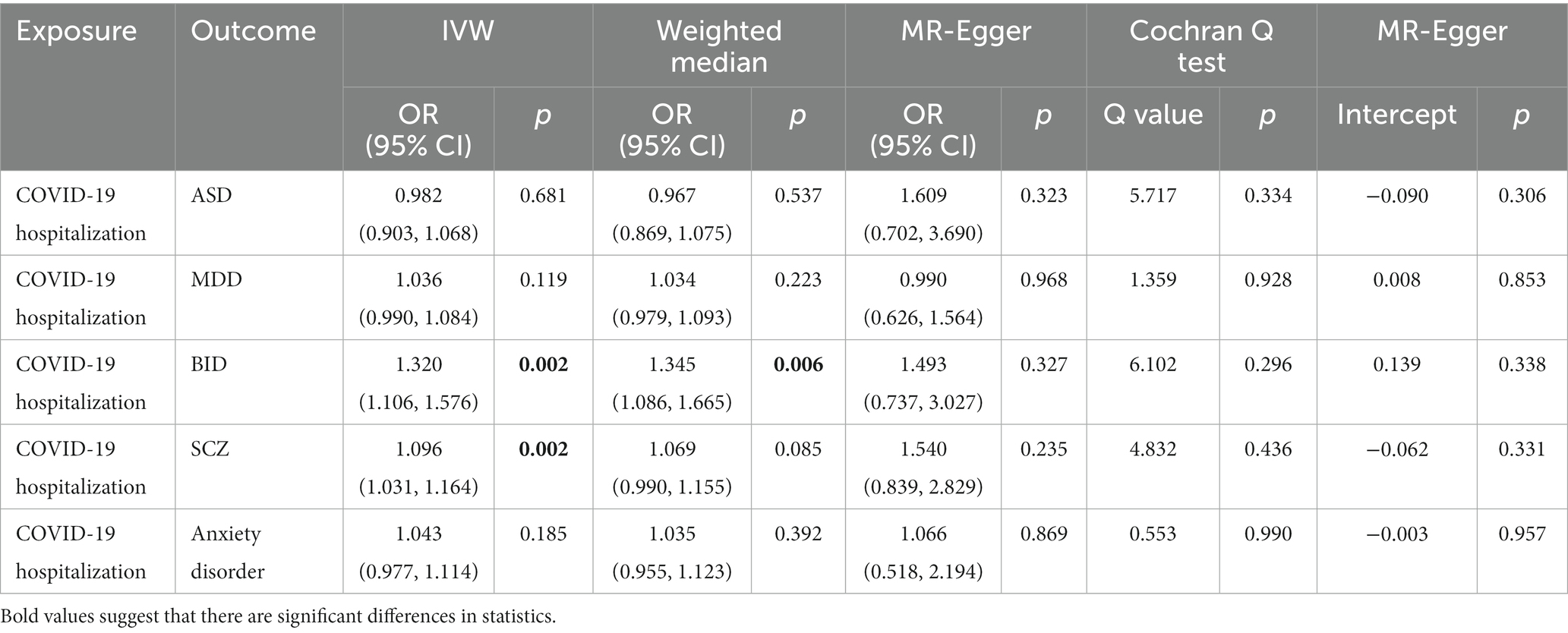
In the IVW analyzes, one unit increase in log odds of hospitalization of COVID-19 was suggestively associated with higher BID risk (IVW: OR = 1.320, 95% CI = 1.106–1.576, p = 0.002) and SCZ risk (IVW: OR = 1.096, 95% CI = 1.031–1.164, p = 0.002). The IVW MR analysis suggested that there is no evidence supporting COVID-19 hospitalization trait as a risk or protective factor for ASD (IVW: OR = 0.982, 95% CI = 0.903–1.068, p = 0.681), MDD (IVW: OR = 1.036, 95% CI = 0.990–1.084, p = 0.119), and anxiety disorder (IVW: OR = 1.043, 95% CI = 0.977–1.114, p = 0.185). The MR results are shown in Table 3 and Figure 3.
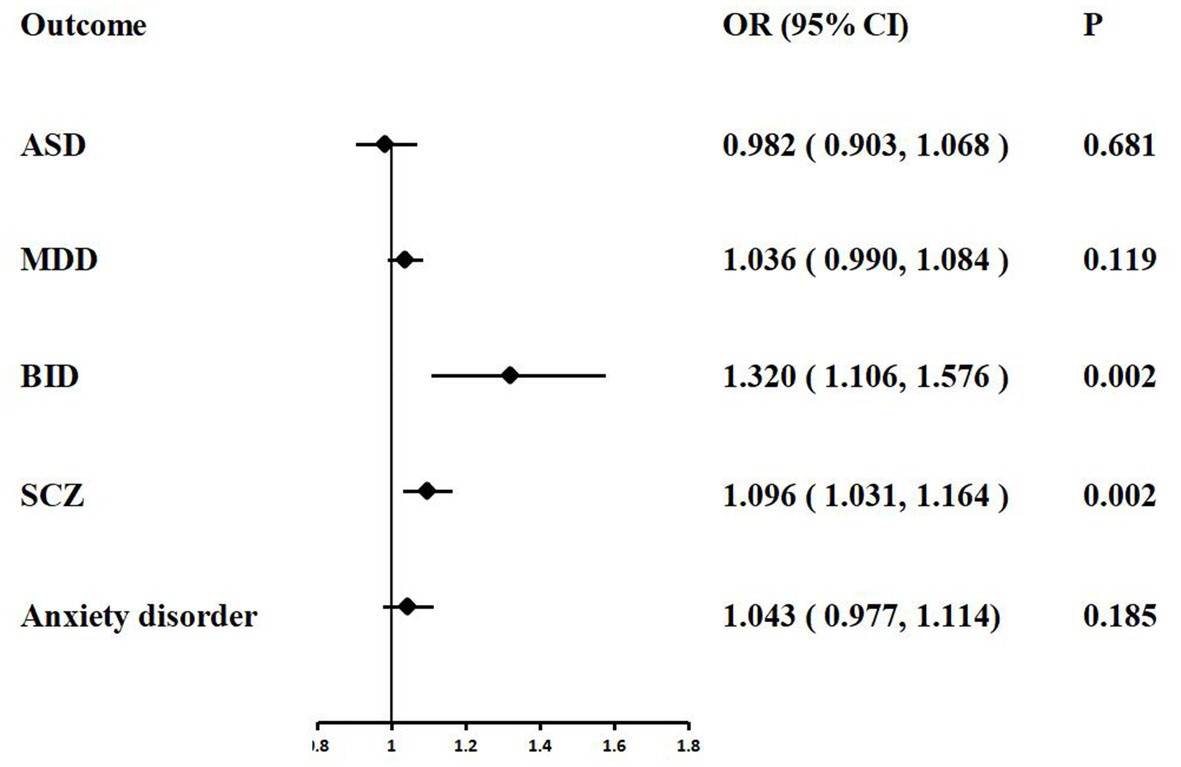
Figure 3. Associations of COVID-19 hospitalization with five psychiatric traits based on the IVW method. ASD, autism spectrum disorder; MDD, major depressive disorder; BID, bipolar disorder; SCZ, Schizophrenia.
Weak evidence of directional pleiotropy was found in the MR Egger intercept tests (all p values >0.05). The Cochrane’ s Q test suggested that there was no heterogeneity in the main MR analysis among the five psychiatric traits (all p values >0.05). The results of leave-one-out sensitivity analyzes suggested that the causal associations between COVID-19 susceptibility traits and psychiatric disorders were not affected by any individual SNP (Supplementary Figures S5–S10).
3.3. Association of COVID-19 severity with psychiatric traits
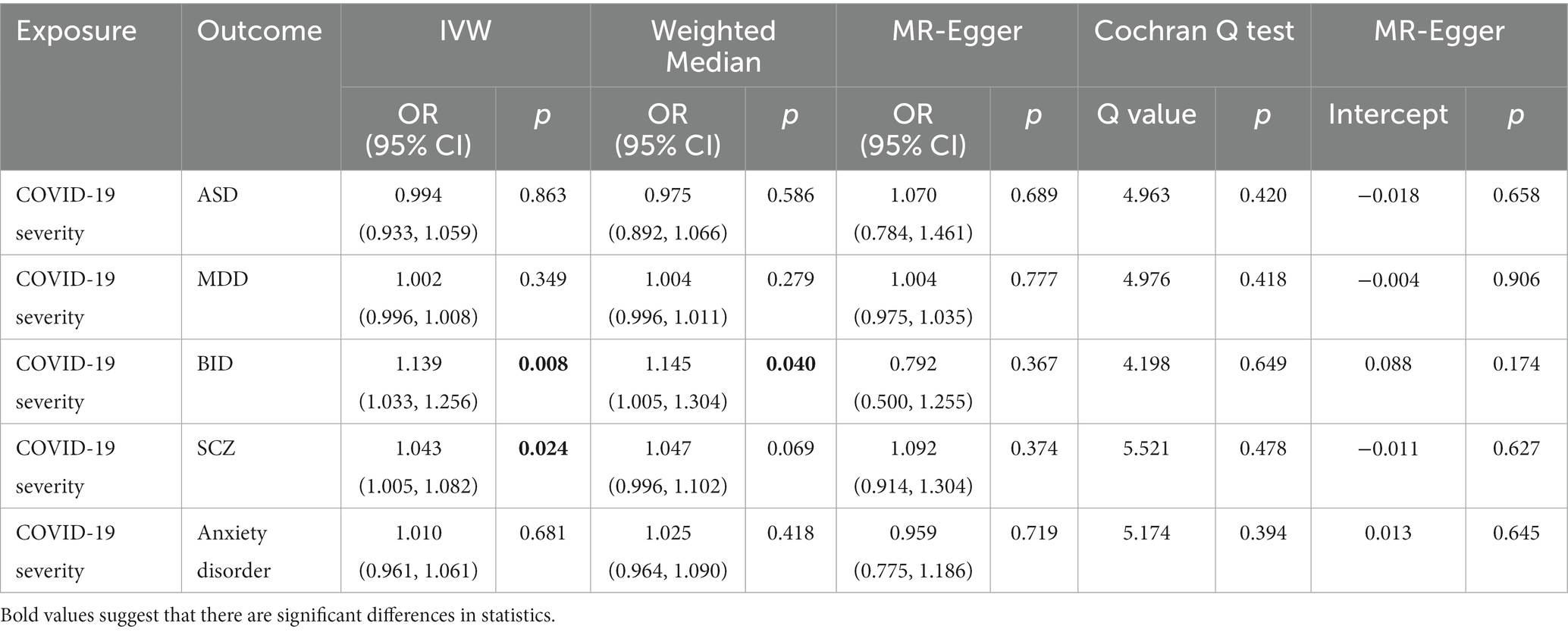
In the MR analysis, we detected a significant positive genetic correlation between COVID-19 severity and two psychiatric traits, BID (IVW: OR = 1.139, 95% CI = 1.033–1.256, p = 0.008) and SCZ (IVW: OR = 1.043, 95% CI = 1.005–1.082, p = 0.024). The IVW MR analysis suggested that there is no evidence supporting COVID-19 susceptibility as a risk or protective factor for ASD (IVW: OR = 0.994, 95% CI = 0.933–1.059, p = 0.863), MDD (IVW: OR = 1.002, 95% CI = 0.996–1.008, p = 0.349), and anxiety disorder (IVW: OR = 1.010, 95% CI = 0.961–1.061, p = 0.681). The MR results are shown in Table 4 and Figure 4.
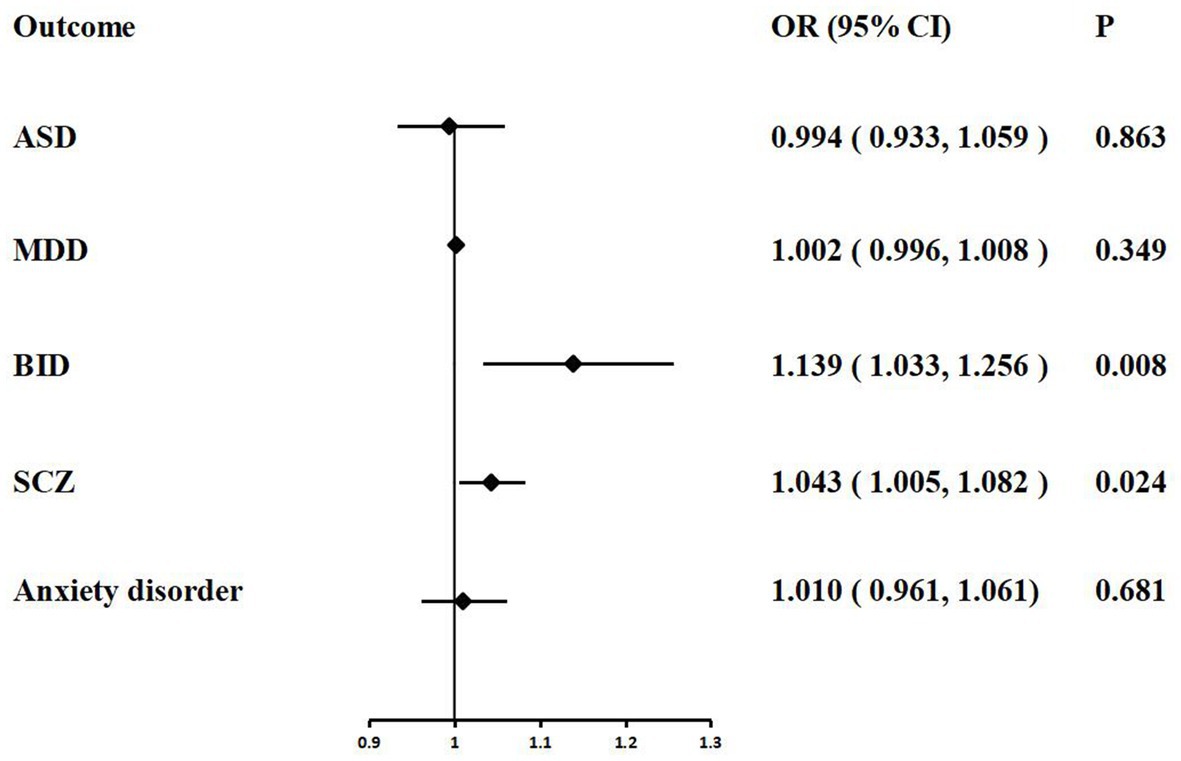
Figure 4. Associations of COVID-19 severity with five psychiatric traits based on the IVW method. ASD, autism spectrum disorder; MDD, major depressive disorder; BID, bipolar disorder; SCZ, Schizophrenia.
We performed extensive sensitivity analyzes to validate the association between COVID-19 severity and the risk of psychiatric disorders. The Cochran’ s Q test did not detect the heterogeneity of effects across the IVs (all p values >0.05, Table 4). No apparent horizontal pleiotropy was observed as the intercept of MR-Egger was not significantly deviated from zero (Table 4). The leave-one-out results suggest that the causal effect was not driven by a single instrumental variable (Supplementary Figures S11–S15).
4. Discussion
In this study, we investigated the association of three COVID-19 traits (COVID-19 susceptibility, COVID-19 hospitalization, and COVID-19 severity) on five psychiatric traits (ASD, MDD, BID, SCZ, and anxiety disorder) using MR analysis for the first time. A risk effect was found in COVID-19 hospitalization and COVID-19 severity. Specifically, hospitalization of COVID-19 increased the risk of BID and SCZ. Moreover, COVID-19 severity also increased the risk of BID and SCZ. There is no evidence supporting COVID-19 susceptibility as a risk or protective factor for five psychiatric traits.
Our findings on the increased genetic susceptibility risk for BID and SCZ due to COVID-19 hospitalization and COVID-19 severity. Our study does not support the genetic susceptibility of COVID-19 and anxiety disorder which aligns with recent epidemiological observations in the United States and the United Kingdom (9, 35). The incidence of mental health symptoms and psychiatric disorders is higher in COVID-19 patients. One reason may be that the SARS-CoV-2 increases the risk of psychiatric disorders in patients by increasing the levels of inflammatory factors, such as interleukin-6 (IL-6) and interleukin-8 (IL-8), in the blood and cerebrospinal fluid (36). In a cohort study, the risk of new psychiatric disorders was 2.87 times (95% CI: 2.45–3.35) higher in patients with severe COVID-19 than in those with mild infections (37). This may be because critically ill patients are prone to cerebral hypoxia, which increases the occurrence of psychiatric symptoms through mechanisms such as neuronal dysfunction, brain edema, and increased blood–brain barrier permeability (38). In addition to SARS-CoV-2 infection, the main epidemic factors affecting public mental health also include isolation and unemployment. A survey from Hong Kong showed that the unemployment rate in Hong Kong increased from 3.7 to 4.2% during the COVID-19 pandemic (39). Unemployment usually has a negative psychological impact on individuals, making them prone to anxiety and depression. A prospective longitudinal study in the United Kingdom found that public anxiety and depression increased in the early stages of isolation and improved as the isolation measures were gradually relaxed (40). A retrospective study from China found an increased risk of first-onset schizophrenia in older adults at the beginning of the COVID-19 outbreak compared with a similar period from 2017 to 2019 (41).
Neuropsychiatric symptoms are a significant aspect of the long-term effects of COVID-19. These symptoms commonly include cognitive impairment, sleep disturbances, depression, anxiety, post-traumatic stress symptoms, and substance use disorders (42). A meta-analysis of mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome, which included 1,458 articles, showed a significant increase in the prevalence of neuropsychiatric disorders including sleep disorders, anxiety, and depression (42). However, the mechanisms underlying COVID-19 neuropsychiatric symptoms are still poorly understood. In the acute phase of COVID-19, research data indicate that the pathophysiological basis of neuropsychiatric injury mainly includes hypoxemia, hyperinflammatory state, and hypercoagulable state (43). The increase of pro-inflammatory cytokines, especially IL-6, is a characteristic of moderate to severe COVID-19, which can cause endothelial dysfunction, increase vascular permeability, and aggravate blood–brain barrier (BBB) dysfunction (44). The neuropathological data of the COVID-19 death patients suggested endothelial injury, microbleeds, microvascular basal layer destruction, and fibrinogen extravasation into the brain parenchyma (43). The above pathological changes suggested that the BBB was ruptured, which may be mediated by the new coronary-related inflammatory state. At the same time, strong inflammation in turn leads to a hypercoagulable state, and further causes microthrombus formation and microvascular endothelial damage (45). Cumulative static brain injury, hypoxia, inflammation, BBB dysfunction, and autoimmune are the mechanisms of psychiatric disorders in the late stage of COVID-19 (46). The persistence of autoimmunity and the presence of the virus can lead to chronic inflammation in patients with COVID-19. This chronic inflammation, especially characterized by imbalances in IL-6 cytokine levels, has been found to be associated with anxiety, depression, and traumatic stress (46). It is well known that the SARS-CoV-2 virus enters the brain by mediating the angiotensin-converting enzyme-2 (ACE-2) receptor and has a great impact on the central nervous system (47). Various inflammatory mediators, such as cytokines, chemokines, and various metabolites, are poorly regulated during infection, as well as in several psychiatric disorders, leading to brain tissue hypoxia and cytokine storm syndrome. Persistence of SARS-CoV-2 infection may also lead to exacerbation of pre-existing neuropsychiatric symptoms in patients (47). Yet the SARS-CoV-2 infection during the COVID-19 epidemic was not the only factor contributing to the occurrence of psychiatric disorders. A systematic review of neuropsychological and psychiatric sequalae of COVID-19 suggested that factors that emerging risk factors for psychiatric symptoms include female sex, perceived stigma related to COVID-19, infection of a family member, social isolation, and prior psychiatry history (48).
The advantages of using MR analysis design in this study are as follows. First, we use randomly assigned genetic variants to identify the causal effects of exposure (three important COVID-19 traits) on the results (five psychiatric traits). Based on the three basic assumptions of MR, we can reduce conventional bias and avoid reverse causality. Second, the SNPs strongly associated with COVID-19 and psychiatric traits selected in this study are from GWASs with a large sample size, which increases the reliability when interpreting the causal effect of the results. Third, this study selected five psychiatric traits, including anxiety disorders, autism spectrum disorder, major depressive disorder, bipolar disorder, and schizophrenia, in order to fully illustrate the causal relationship between COVID-19 and psychiatric disorders. Fourth, the conclusion became more convincing by confirming our results with several methods (IVW, WM, and MR-egger), and sensitivity tests (Cochran’ Q test, LOO analysis, and MR-Egger intercept test).
There are several limitations in this study that need to be acknowledged. Firstly, for three COVID-19 traits GWAS summary statistics used in this MR study were not stratified by age of onset and gender. Previous observational studies have shown that elderly patients are more likely to suffer from schizophrenia in the early stage of COVID-19 outbreak. Thus, MR analyzes could not be performed to assess the causal effect of different COVID-19 populations on psychiatric traits. Secondly, although the population of this study is from European descent. However, there may be differences in the diagnosis and treatment of COVID-19 in different European country regions, and there may be significant cross-regional differences in the prevalence as well as predictors of psychiatric disorders. Therefore, the conclusions of this study need to be viewed with caution, and cannot be applied to populations in regions such as Asia and Africa. Thirdly, despite a series of rigorous measures to screen for IVs, however, we are still unable to completely rule out SNPs with pleiotropic effect, and it is difficult to discuss the extent to which the conclusions are influenced by this phenomenon. Fourthly, epigenetic issues such as DNA methylation, RNA editing and transposon inactivation are inevitable pitfalls of MR analysis.
5. Conclusion
In conclusion, this MR study provided suggestive genetic evidence for the associations of COVID-19 hospitalization traits and COVID-19 severity traits with increased risks of BID and SCZ. Therefore, patients infected with SARS-CoV-2 should have enhanced monitoring of their psychiatric status. Further studies are required to illuminate the effectiveness of timely treating COVID-19 on reducing the risk of psychiatric disorder and investigate the potential mechanisms of these association.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
HX and LZ carried out the conception and design of the research. HX participated in the acquisition of data and carried out the analysis and interpretation of data. HX and SL drafted the manuscript. SL participated in the revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1253051/full#supplementary-material
References
1. Gasmi, A, Tippairote, T, Mujawdiya, PK, Gasmi Benahmed, A, Menzel, A, Dadar, M, et al. Neurological involvements of SARS-CoV2 infection. Mol Neurobiol. (2021) 58:944–9. doi: 10.1007/s12035-020-02070-6
2. Jiang, F, Deng, L, Zhang, L, Cai, Y, Cheung, CW, and Xia, Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med. (2020) 35:1545–9. doi: 10.1007/s11606-020-05762-w
3. Yew, MMT, Lip, JQ, and Ling, APK. COVID-19 and its effects on neurological functions. Trop Biomed. (2021) 38:435–45. doi: 10.47665/tb.38.3.086
4. Chen, S, Yang, J, Yang, W, Wang, C, and Bärnighausen, T. COVID-19 control in China during mass population movements at new year. Lancet. (2020) 395:764–6. doi: 10.1016/s0140-6736(20)30421-9
5. Sohrabi, C, Alsafi, Z, O'Neill, N, Khan, M, Kerwan, A, Al-Jabir, A, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. (2020) 76:71–6. doi: 10.1016/j.ijsu.2020.02.034
6. Huang, C, Wang, Y, Li, X, Ren, L, Zhao, J, Hu, Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/s0140-6736(20)30183-5
7. Guessoum, SB, Lachal, J, Radjack, R, Carretier, E, Minassian, S, Benoit, L, et al. Adolescent psychiatric disorders during the COVID-19 pandemic and lockdown. Psychiatry Res. (2020) 291:113264. doi: 10.1016/j.psychres.2020.113264
8. Palacio-Ortiz, JD, Londoño-Herrera, JP, Nanclares-Márquez, A, Robledo-Rengifo, P, and Quintero-Cadavid, CP. Psychiatric disorders in children and adolescents during the COVID-19 pandemic. Rev Colomb Psiquiatr (Engl Ed). (2020) 49:279–88. doi: 10.1016/j.rcp.2020.05.006
9. Taquet, M, Luciano, S, Geddes, JR, and Harrison, PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. (2021) 8:130–40. doi: 10.1016/s2215-0366(20)30462-4
10. Shi, L, Lu, ZA, Que, JY, Huang, XL, Liu, L, Ran, MS, et al. Prevalence of and risk factors associated with mental health symptoms among the general population in China during the coronavirus disease 2019 pandemic. JAMA Netw Open. 3:e2014053. doi: 10.1001/jamanetworkopen.2020.14053
11. Xie, Y, Xu, E, and Al-Aly, Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ. (2022) 376:e068993. doi: 10.1136/bmj-2021-068993
12. Lee, SY, Lee, JJ, and Lee, H. "socio-economic factors associated with mental health outcomes during the Covid-19 pandemic in South Korea." [in eng]. Front. Public Health. (2022) 10:1024751. doi: 10.3389/fpubh.2022.1024751
13. Bosch, R, Pagerols, M, Prat, R, Español-Martín, G, Rivas, C, Dolz, M, et al. Changes in the mental health of children and adolescents during the COVID-19 lockdown: associated factors and life conditions. Int J Environ Res Public Health. (2022) 19:4120. doi: 10.3390/ijerph19074120
14. Sayed, MH, Hegazi, MA, El-Baz, MS, Alahmadi, TS, Zubairi, NA, Altuwiriqi, MA, et al. COVID-19 related posttraumatic stress disorder in children and adolescents in Saudi Arabia. PLoS One. (2021) 16:e0255440. doi: 10.1371/journal.pone.0255440
15. Ceban, F, Ling, S, Lui, LMW, Lee, Y, Gill, H, Teopiz, KM, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. (2022) 101:93–135. doi: 10.1016/j.bbi.2021.12.020
16. Theoharides, TC, Cholevas, C, Polyzoidis, K, and Politis, A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors. (2021) 47:232–41. doi: 10.1002/biof.1726
17. Currie, MG, Zimmer, DP, and Halushka, PV. An impaired natriuretic peptide hormone system may play a role in COVID-19 severity in vulnerable populations. FASEB Bioadv. (2020) 2:596–9. doi: 10.1096/fba.2020-00042
18. Wang, Z, Chen, M, Wei, YZ, Zhuo, CG, Xu, HF, Li, WD, et al. The causal relationship between sleep traits and the risk of schizophrenia: a two-sample bidirectional Mendelian randomization study. BMC Psychiatry. 22:399. doi: 10.1186/s12888-022-03946-8
19. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
20. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-base platform supports systematic causal inference across the human phenome. elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
21. Skrivankova, VW, Richmond, RC, Woolf, BAR, Yarmolinsky, J, Davies, NM, Swanson, SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
22. COVID-19 Host Genetics Initiative. The COVID-19 host genetics initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. (2020) 28:715–8. doi: 10.1038/s41431-020-0636-6
23. Sullivan, PF, Agrawal, A, Bulik, CM, Andreassen, OA, Børglum, AD, Breen, G, et al. Psychiatric genomics: an update and an agenda. Am J Psychiatry. (2018) 175:15–27. doi: 10.1176/appi.ajp.2017.17030283
24. Peyrot, WJ, and Price, AL. Identifying loci with different allele frequencies among cases of eight psychiatric disorders using CC-GWAS. Nat Genet. (2021) 53:445–54. doi: 10.1038/s41588-021-00787-1
25. Howard, DM, Adams, MJ, Clarke, TK, Hafferty, JD, Gibson, J, Shirali, M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. (2019) 22:343–52. doi: 10.1038/s41593-018-0326-7
26. Mullins, N, Forstner, AJ, O'Connell, KS, Coombes, B, Coleman, JRI, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. (2021) 53:817–29. doi: 10.1038/s41588-021-00857-4
27. Trubetskoy, V, Pardiñas, AF, Qi, T, Panagiotaropoulou, G, Awasthi, S, Bigdeli, TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. (2022) 604:502–8. doi: 10.1038/s41586-022-04434-5
28. Rusk, N. "The Uk biobank." [in eng]. Nat Methods. (2018) 15:1001. doi: 10.1038/s41592-018-0245-2
29. Purves, KL, Coleman, JRI, Meier, SM, Rayner, C, Davis, KAS, Cheesman, R, et al. A major role for common genetic variation in anxiety disorders. Mol Psychiatry. (2020) 25:3292–303. doi: 10.1038/s41380-019-0559-1
30. Pierce, BL, Ahsan, H, and Vanderweele, TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
31. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
32. Wu, Q, Liu, S, Huang, X, Liu, J, Wang, Y, Xiang, Y, et al. Bidirectional Mendelian randomization study of psychiatric disorders and Parkinson's disease. Front Aging Neurosci. (2023) 15:1120615. doi: 10.3389/fnagi.2023.1120615
33. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
34. Kulinskaya, E, and Dollinger, MB. An accurate test for homogeneity of odds ratios based on Cochran's Q-statistic. BMC Med Res Methodol. (2015) 15:49. doi: 10.1186/s12874-015-0034-x
35. Wang, Q, Xu, R, and Volkow, ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. (2021) 20:124–30. doi: 10.1002/wps.20806
36. Manganotti, P, Bellavita, G, Tommasini, V, D’Acunto, L, Fabris, LM, Cecotti, L, et al. Cerebrospinal fluid and serum interleukins 6 and 8 during the acute and recovery phase in COVID-19 neuropathy patients. J Med Virol. (2021) 93:5432–7. doi: 10.1002/jmv.27061
37. Taquet, M, Geddes, JR, Husain, M, Luciano, S, and Harrison, PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8:416–27. doi: 10.1016/s2215-0366(21)00084-5
38. Brownlee, NNM, Wilson, FC, Curran, DB, Lyttle, N, and McCann, JP. Neurocognitive outcomes in adults following cerebral hypoxia: a systematic literature review. NeuroRehabilitation. (2020) 47:83–97. doi: 10.3233/nre-203135
39. Kaur, M, Goyal, P, and Goyal, M. Individual, interpersonal and economic challenges of underemployment in the wake of COVID-19. Work. (2020) 67:21–8. doi: 10.3233/wor-203249
40. Fancourt, D, Steptoe, A, and Bu, F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: a longitudinal observational study. Lancet Psychiatry. (2021) 8:141–9. doi: 10.1016/s2215-0366(20)30482-x
41. Hu, W, Su, L, Li, D, Zhou, Y, and Zhu, J. Risk of first-episode schizophrenia in aged adults increased during COVID-19 outbreak. Int J Ment Health Addict. (2021) 21:1455–65. doi: 10.1007/s11469-021-00671-3
42. Premraj, L, Kannapadi, NV, Briggs, J, Seal, SM, Battaglini, D, Fanning, J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. (2022) 434:120162. doi: 10.1016/j.jns.2022.120162
43. Schou, TM, Joca, S, Wegener, G, and Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19- a systematic review. Brain Behav Immun. (2021) 97:328–48. doi: 10.1016/j.bbi.2021.07.018
44. Troyer, EA, Kohn, JN, and Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. (2020) 87:34–9. doi: 10.1016/j.bbi.2020.04.027
45. Lyra e Silva, NM, Barros-Aragão, FGQ, de Felice, FG, and Ferreira, ST. Inflammation at the crossroads of COVID-19, cognitive deficits and depression. Neuropharmacology. (2022) 209:109023. doi: 10.1016/j.neuropharm.2022.109023
46. Frontera, JA, and Simon, NM. Bridging knowledge gaps in the diagnosis and Management of Neuropsychiatric Sequelae of COVID-19. JAMA Psychiatry. (2022) 79:811–7. doi: 10.1001/jamapsychiatry.2022.1616
47. Khatun, A, Tamilanban, T, and Chitra, V. Psychiatric manifestations of COVID-19: a literature review. CNS Neurol Disord Drug Targets. (2023) 22:892–905. doi: 10.2174/1871527321666220701152821
Keywords: COVID-19, SARS-CoV-2, psychiatric disorders, schizophrenia, bipolar disorder, anxiety disorders
Citation: Xue H, Zeng L and Liu S (2023) Susceptibility and severity of COVID-19 and risk of psychiatric disorders in European populations: a Mendelian randomization study. Front. Psychiatry. 14:1253051. doi: 10.3389/fpsyt.2023.1253051
Edited by:
Mohammadreza Shalbafan, Iran University of Medical Sciences, IranReviewed by:
Lambert Zixin Li, Stanford University, United StatesTamilanban T, SRM Institute of Science and Technology, India
Copyright © 2023 Xue, Zeng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Xue, eHVlaDE4OTVAMTYzLmNvbQ==
 Hua Xue
Hua Xue Li Zeng2
Li Zeng2