
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 07 September 2023
Sec. Mood Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1250925
 Andrea Stautland1*
Andrea Stautland1* Petter Jakobsen1,2
Petter Jakobsen1,2 Ole Bernt Fasmer1,2
Ole Bernt Fasmer1,2 Berge Osnes3
Berge Osnes3 Jim Torresen4
Jim Torresen4 Tine Nordgreen5,6
Tine Nordgreen5,6 Ketil J. Oedegaard1,2
Ketil J. Oedegaard1,2Background: Bipolar disorder (BD) is a chronic recurrent mood disorder associated with autonomic nervous system (ANS) dysfunction, indexed by heart rate variability (HRV). Changes in HRV between mood states are sparsely studied longitudinally. We aimed to compare HRV of hospitalized manic individuals with their own euthymic selves in a naturalistic observational study.
Methods: 34 individuals were included, of which 16 were lost to follow-up. Ultimately 15 patients provided reliable heart rate data in both a manic and euthymic state, using photoplethysmography (PPG) sensor wristbands overnight. We calculated HRV measures Root Mean Square of Successive Differences (RMSSD), High-frequency (HF: 0.15–0.40 Hz), Low-frequency (LF: 0.40–0.15 Hz), Very low-frequency (VLF: 0.0033–0.04 Hz), Total power and Sample Entropy in 5-min night-time resting samples. We compared HRV measures by mood state within individuals using paired t-tests and linear regression to control for age and sex.
Results: HRV was lower in the manic state when compared to the euthymic state for all HRV metrics (p ≤ 0.02), with large to medium effect sizes (g = 1.24 to 0.65). HRV changes were not significantly affected by age or sex.
Conclusion: This longitudinal study provides evidence of lower HRV in manic states compared to euthymia, indicating an association between ANS dysregulation and changes in bipolar mood state. This corroborates previous cross-sectional studies, although the association may be less clear or reversed in hypomanic states. Further investigation in larger longitudinal samples is warranted.
Bipolar disorder (BD) is a chronic mood disorder characterized by recurrent fluctuations in mood state and energy levels. Although it is a major cause of disability in young adults globally, the mechanisms behind BD remain unclear, and sustaining long-term mood stability is challenging (1). Mood disorders are associated with dysfunction of the autonomic nervous system (ANS), exhibited through reduced vagally mediated heart rate variability (HRV) (2). Previous reviews have described lower HRV in bipolar disorder (BD), indicating an autonomous dysregulation (2, 3). However, findings are inconsistent across studies. The current mood state at data collection is often poorly described. Furthermore, HRV changes between BD mood states are sparsely studied, especially longitudinally (4).
Studying HRV can help us better understand how the heart and ANS respond to environmental changes and stress (5). As BD is characterized by recurring energy and mood changes, corresponding ANS activity changes are anticipated (1). While there is an indication of general ANS dysregulation in BD, the role of the ANS during high-energy manic states is under-investigated. Investigating heart rate variability (HRV) during a manic episode can provide insight into ANS dysregulation and its relation to manic symptoms. Studying HRV longitudinally may provide insight into BD state-dependent changes, as resting HRV typically in relatively stable in healthy individuals (6).
ANS dysregulation has been reported in both bipolar depressed and manic states (2, 4, 7–11). Moreover, two recent studies report an inverse relationship between the severity of previous BD episodes and HRV during euthymia (11, 12). Such previous studies are largely cross-sectional, comparing BD individuals as a group to healthy controls or other psychiatric illness groups (2, 4). Individuals with BD in an unstable phase can be challenging to follow up in longitudinal studies due to the fluctuating nature of their condition and often chaotic life situations. Study participants recruited in one condition, e.g., mania, may lose interest in study participation when transitioned to euthymia or depression, and thereby be lost to follow-up. Nonetheless, studying biological changes between different mood states can provide invaluable information on the biology and etiology of bipolar mood states and is needed (4).
The few available studies that have looked at HRV differences between mood states are small and report conflicting findings. A recent within-individual study of inpatient manic males reported significantly decreased HRV in mania compared to euthymia (13). In contrast, another study reported increased HRV in mild mania compared to euthymia in outpatients (14). These conflicting findings indicate a need for more research on HRV changes between bipolar affective states. In addition to the previously mentioned challenges of longitudinal studies in BD, the lack of HRV studies on multiple affective states might be due to the challenges associated with acquiring electrocardiogram (ECG) data in manic patients. This is particularly due to the elevated psychomotor activity and agitation often associated with moderate to severe mania (15). In this study, we addressed this by utilizing wrist-worn photoplethysmography (PPG) devices to measure heart activity in a closed affective ward. Due to ease of use, this approach can also provide the benefit of a more naturalistic observation compared to conventional ECG recordings in a lab setting. Furthermore, HRV data collected unobtrusively through PPG-wearables is increasingly being proposed as a viable biomarker in BD. Adding a layer of passive objective patient data could greatly aid clinicians in diagnostics monitoring.
The aim of this study was to compare HRV within hospitalized manic individuals with their euthymic selves in a naturalistic observational study including both sexes. Given the increased energy and agitation associated with mania (15), we hypothesized lower HRV in the manic state compared to the euthymic state.
Eligible probands were individuals with BD with an ongoing manic episode admitted to a closed affective ward at Haukeland University Hospital in Bergen, Norway from November 2017 up to and including May 2020.
Inclusion criteria were Norwegian-speaking individuals 18 to 70 years old diagnosed with BD by the ICD-10 criteria, able to comply with instructions, and cognitive abilities clinically estimated to correspond to an IQ above 70. Participant diagnoses were set or confirmed by resident physicians or senior consultant psychiatrists at Haukeland University Hospital. Exclusion criteria were participation refusal, previous head trauma requiring hospital treatment, an organic brain disorder, pregnancy, ongoing substance dependence (nicotine permitted), and being in a state of withdrawal.
The Norwegian Regional Medical Research Ethics Committee West approved the study (2017/937). All participants gave written consent in accordance with the Helsinki Declaration.
This study is a within-individual observational case–control study of HRV in bipolar type 1 inpatients. Inpatients were invited to participate upon recommendation from psychiatry residents or senior consultant psychiatrists of closed affective wards at Haukeland University Hospital. No financial compensation or treatment benefits were provided.
We assessed the included patients and obtained overnight multi-sensor wristband recordings at the time of inclusion. Participants were reassessed and recorded prior to discharge from the closed ward or at the open ward they were transferred to, when in remission. The study design is presented in Figure 1A.
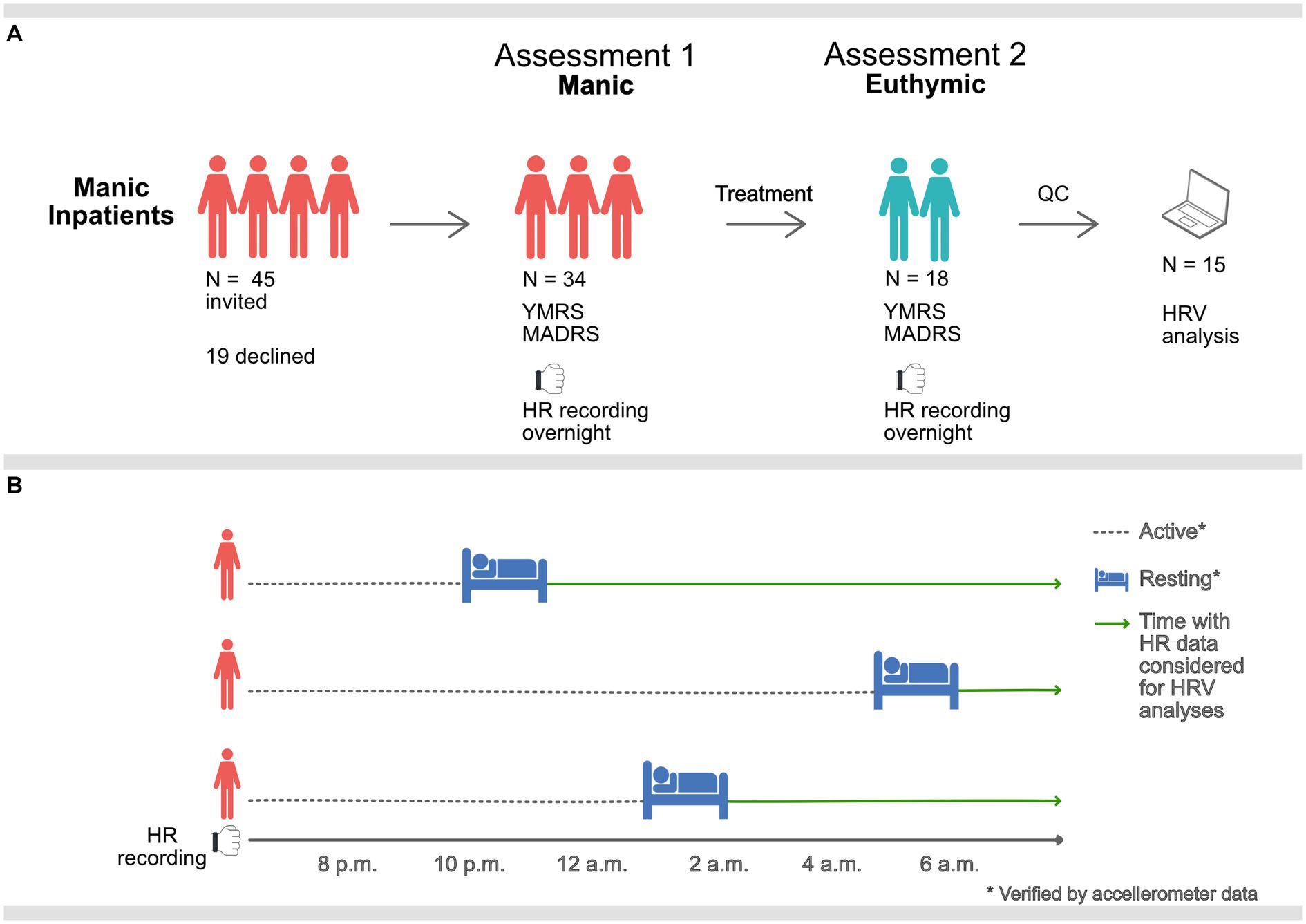
Figure 1. Graphic description of the study procedure. (A) Manic inpatients were assessed and equipped with PPG sensors overnight when manic and when euthymic. 15 participants remained after QC of heart rate (HR) data. (B) Overnight HR data was only considered for HRV analysis during rest, verified by accelerometer data. Sampling times, therefore, vary (mean 11.34 p.m., standard deviation 112-min).
Behavioral challenges associated with manic states (e.g., motor restlessness, agitation, and psychosis) can make accurate heart rate data collection challenging. The use of an ambulatory device for heart rate sampling was selected to facilitate data collection from a clinically realistic sample in a non-laboratory inpatient setting. Ward routines promote rest and sleep at night, providing a time window for better recording conditions for the recording device (16). Five-minute samples were located at night-time after initiation of long-term rest, confirmed by accelerometer data. Hence, HRV metrics were calculated at unique time points corresponding to the individual’s rest initiation time, see Figure 1B. Due to the differences in motor activity and reduced subjective need for sleep, these resting samples ranged from 8.10 p.m. to 5.10 a.m. (mean 11.34 p.m., standard deviation 112-min). There was no relationship between time of sampling and HRV measures, see Supplementary Table S4.
Wrist-worn Empatica E4 devices with photoplethysmography (PPG) sensors, which have been validated against ECG for HRV purposes, were used for pulse detection (16, 17). Heart rate was monitored overnight in a non-laboratory naturalistic hospital setting at a 64 Hz sampling rate. Research personnel secured the device on the participants’ dominant wrist as tightly as tolerated, ideally to a fit allowing one finger under the band, as recommended by the manufacturer. Participants were instructed not to shower or touch the device during the recording. We used Empatica’s online software, E4 Connect, to visualize the loss of sensor contact, as identified by flattened skin conductance and temperature measurements, and to download the inter-beat-intervals (IBI) data as comma-separated value files for post-processing.
IBI data was analyzed in Kubios HRV Premium software version 3.4.2 (18). Data quality was manually screened, and acceptable data were subjected to an automatic artifact correction threshold and the smoothing priors detrending method (λ = 500), using a five-minute window. We set the artifact correction cut-off to the recommended 5% and considered data with a higher correction rate as poor-quality sections. We excluded recordings from three individuals which did not include any 5-min segments with sufficient data quality throughout the night.
We selected commonly used HRV measures, representing the time, frequency, and non-linear domains. Root mean square of the successive differences (RMSSD), representing the time domain, mirrors the variance in time between heartbeats, is widely used for monitoring vagally mediated (i.e., parasympathetic) HRV changes, and is well-suited for use on our 5-min data segments (19). Total, high frequency (HF), low frequency (LF) and very low frequency (VLF) power represented the frequency domain. High frequency (HF) oscillations (ms2/Hz) are often perceived as reflective of predominately parasympathetic influence on the heart and are frequently used in psychology literature (19, 20). This interpretation is controversial, viewed as simplistic, and has received less attention in later years (21). Sample Entropy (SampEn), of the non-linear domain, reflects the complexity and degree of chaos in the heart rate series (19). Finally, high frequency peak values (HF-peak) were used as an indirect measure of respiration frequency (22).
The mood state was evaluated using the Young Mania Rating Scale (YMRS) (23, 24) and Montgomery Asberg Depression Rating Scale (MADRS) (25, 26), two commonly used evaluation scales for mania and depression in clinical and research settings. Euthymia was defined as a total YMRS score < 10 (24, 27). BD diagnosis and other psychiatric comorbidities were confirmed by research personnel trained in the use of Mini-International Neuropsychiatric Interview (M.I.N.I.) when the subjects were euthymic (28).
We used paired two-tailed t-tests to compare HRV between manic and euthymic mood states within the 15 participants. Significance levels were set to p = 0.05. The effect size of change by mood state was calculated using Hedges’ g. Sex and age are customary confounders of HRV (19). We ran a separate linear regression model to examine possible confounding effects. The dependent variable of the linear regression models were the manic-euthymic differences of the three HRV metrics and the independent variables were sex and age. Due to the small number of subjects, we did not include mood state as an independent variable in the linear regression models, using the models purely to examine confounding effects. As respiration may confound HRV metrics, we correlated all applied HRV metrics with HF-peak, a proxy measure of respiration (22). Body mass index (BMI) is also known to influence HRV (29). BMI was, however, not available and was not included in the analysis. This is addressed in the discussion. All analyses were performed in the open-source software R using the packages tidyverse, psych, effectsize, and stats base package (30–33).
We invited 45 inpatients to participate, of which 34 consented. 18 of the 34 completed both assessment points (manic and euthymic). Ultimately, 15 participants provided recordings that passed quality control and were included in the analysis. See Tables 1, 2 for participant demographics and clinical characteristics.

Table 2. Clinical characteristics of n = 15 bipolar type 1 study participants during a manic and euthymic state.
In accordance with the current recommended treatment of mania requiring hospitalization, all participants used psychotropic medication (1). Medication data were retrieved from patient charts and an overview is presented in Table 2. All in all, medication use was stable between measurement times, although we observed between-state medication changes in three of the 15 participants for both psychotropic and somatic medications. A detailed description of medication use is provided in Supplementary material S2.
Changes in HRV were observed from mania to euthymia across time-domain, frequency-domain, and non-linear analysis (Figure 2). RMSSD was significantly higher in the euthymic state compared to the manic state (t (14)=4.29; d = 11.39; 95% CI, 5.70–17.08; p < 0.001). The observed effect was large (g = 1.05; 95% CI, 0.44–1.71). The same was found for all frequency metrics and SampEn: HF-power (t (14)=2.82; d = 0.70; 95% CI, 0.17–1.24; p = 0.01); LF-power (t (14)=5.08; d = 1.4; 95% CI, 0.83–2.04; p < 0.001); VLF-power (t (14)=4.99; d = 1.26; 95% CI, 0.72–1.80; p < 0.001); total power (t (14)=2.65; d = 736.08; 95% CI, 140.64–1331.53; p = 0.02); SampEn (t (14)=3.27; d = 0.19; 95% CI, 0.08–0.31; p < 0.01), with medium to large effect sizes (HF: g = 0.69; 95% CI, 0.14–1.26. LF: g = 1.24; 95% CI, 0.59–1.96. VLF: g = 1.22; 95% CI, 0.57–1.93. Total power: g = 0.65; 95% CI, 0.11–1.21. SampEn: g = 0.80; 95% CI, 0.24–1.40). These findings persisted when excluding participants with medication changes (see Supplementary Table S5). The linear regression models revealed no confounding effects of age and sex on any HRV change scores between mood states (see Supplementary Table S3). There were no significant correlations between respiration frequency (HF-peak) and any of the HRV metrics. Of the total sample, one participant showed an opposite pattern in RMSSD, two participants showed an opposite pattern in HF-power, one in LF and VLF-power, and three in SampEn. These participants resembled the overall sample in age, sex, and mean mania baseline and change scores. Two of the three participants with somatic medication changes were among those showing the opposite pattern in LF and VLF and HF and SampEn, respectively.
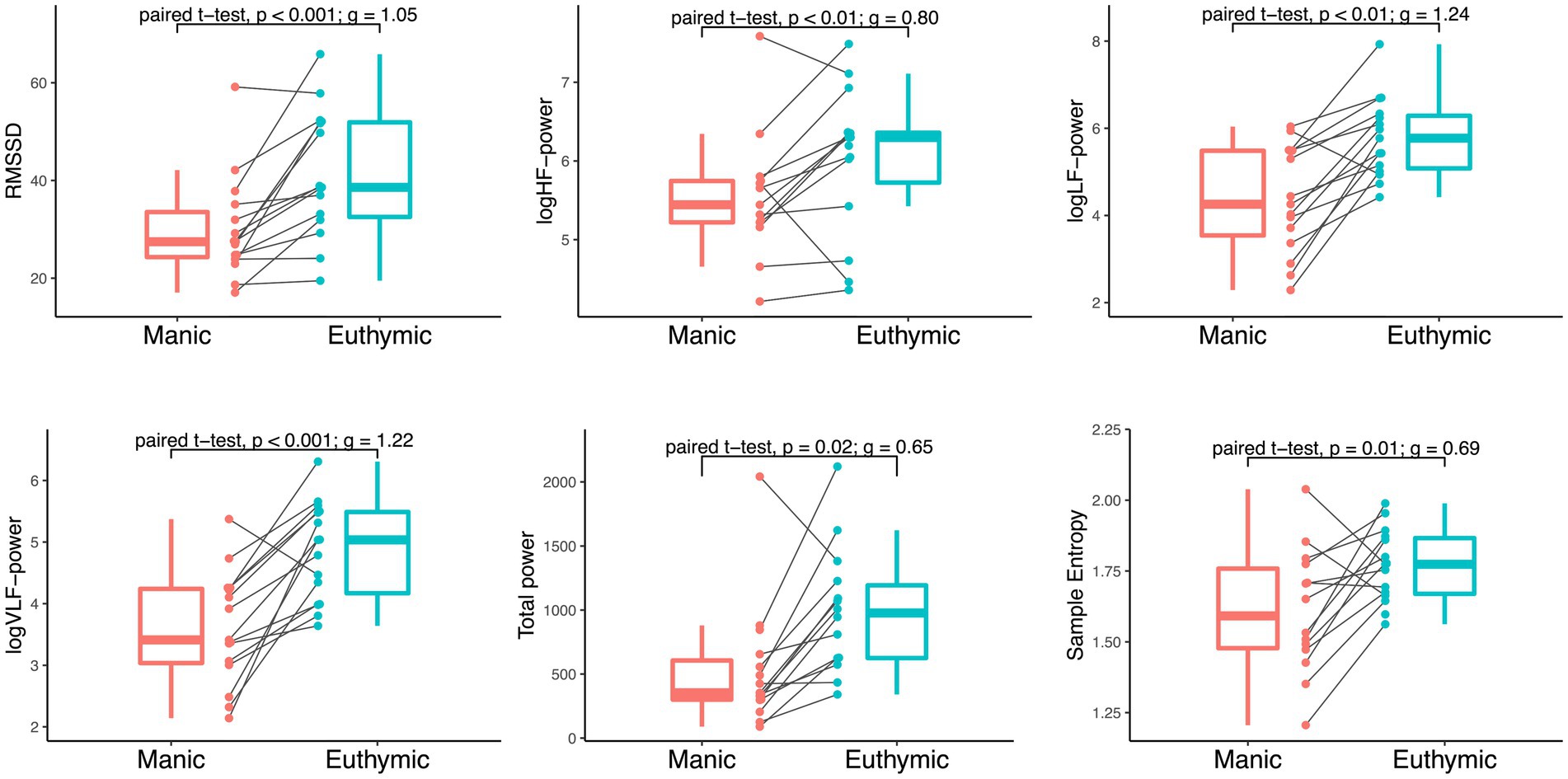
Figure 2. Within-individual comparison of HRV by mood state in 15 bipolar subjects. Paired t-tests of RMSSD (ms2), logHF-power, logLF-power, logVLF-power [log(ms2/Hz)], total power (ms2/Hz), and Sample Entropy during mania and euthymia reveal significant differences in HRV between mood states, p = 0.05 significance level. Boxplots display median, first, and third quartiles with whiskers extending 1.5 times the interquartile range. The spaghetti plots display HRV metric distribution and development between time points for each participant.
This study identified a within-individual difference in heart rate variability between the manic and euthymic states in bipolar inpatients. As hypothesized, HRV was significantly lower in the manic state compared to the euthymic state. This is largely in accord with existing cross-sectional studies, which indicate dysregulation of vagally mediated parasympathetic activity during moderate to severe bipolar mania (2, 4).
Our finding of reduced HRV, represented by RMSSD, logHF, and sample entropy, during mania, corroborates previous cross-sectional comparisons of BD mania to healthy controls (2). Robust studies by Henry et al. and Chang et al. have reported reduced HRV variance, HF, and entropy measures in inpatients with moderate bipolar mania when compared to healthy controls (9, 34). Chang et al. also demonstrated an inverse relationship between HRV variance and mania severity, which may indicate a state-dependent relationship between HRV and mood state (9). Overall, our findings on longitudinal HRV changes in BD corroborate previous cross-sectional findings – both in terms of an absolute decrease during mania and the state-dependency of HRV changes. Although HRV changes have been sparsely studied longitudinally, one study used a within-individual design similar to the current study (13). Like us, they found lower HRV in manic BD inpatients compared to their euthymic selves. Unlike our study, which applied ambulatory PPG devices, they used ECG to measure heart rate, included solely males, restricted medication use, and excluded patients with a manic state characterized by high motor activity (13). The current study complements this study, demonstrating that these HRV changes also occur across different anti-manic treatment regimes. Furthermore, while HRV, particularly HF-power, typically differs between sexes, our study found that sex did not influence any of the HRV metrics (see Supplementary Table S3) (35). However, it is not possible to draw conclusions regarding sex differences in HRV within the context of BD due to the study’s limited participant count. Restrictions on sex and motor activity may be related to the use of a chest-strap ECG device, exemplifying the downsides of using the gold standard compared to a wristband PPG.
Our findings are, however, opposed by one longitudinal study of 16 BD outpatients (14). They reported higher HRV in mania compared to euthymia and a positive relationship between HRV and mania scores. The seven participants measured in a manic state were characterized as with mild mania, in contrast to our study sample and those presented above studying moderate to severe mania (9, 13, 14, 34). This could indicate a non-linear progression of HRV changes from euthymia via hypomania to mania, i.e., an initial HRV increase followed by a reduction, and help explain the contrasting findings.
Reduced RMSSD, frequency measures, and SampEn may indicate decreased cardiac vagal influence, suggesting ANS dysregulation (19, 36). The ANS and psychiatric disorders are both complex, and their interaction with brain dysfunction and mania likely involve multiple organ systems and brain regions. The neurovisceral integration model proposes bidirectional links between the heart and prefrontal cortex and subcortical circuitries, relating HRV to adaptability; low HRV is associated with maladaptive self-regulation (20, 37). In line with this model, ANS dysfunction has been proposed as integral to bipolar psychopathology via impaired neural-autonomic coordination in cognitive and emotional processes, making ANS dysfunction a possible target for treatment (38). Furthermore, as ANS disruption occurs not only in mania, but both uni- and bipolar depression, it may reflect a general dysregulation associated with psychopathology (20). Yet, the disruption is greater in bipolar compared to unipolar depression, suggesting a larger role played by ANS dysregulation in bipolar psychopathology (39). In light of these previous findings and theories, the current study reinforces HRV as a potential biomarker for BD disease states. In practice, HRV may facilitate monitoring treatment response and mood stability in BD, given adequate technological advances and further studies.
The limitations of sample size, PPG recording challenges, and potential confounders necessitate further investigations to enhance the generalizability of the present findings. The naturalistic observational design provided an ecologically valid sample but posed challenges with compliance, data quality, loss to follow-up, and potentially confounding effects due to medication use. Although the use of PPG sensors allowed for the inclusion of severely affected patients, the recordings were susceptible to movement artifacts and sabotage, resulting in participant exclusion due to poor sensor data quality. Given the circadian abnormalities associated with BD, night-day differences in HRV (diurnal variance) should be explored in future studies, especially as more reliable technological solutions become available. Additionally, these studies should consider examining potential confounding factors such as individual sleep–wake patterns, total motor activity, and nutritional factors. Collecting physiological data from a population in an unstable illness phase is time-consuming and resource-intensive, and previous endeavors have had a similar sample size (13, 14). Hence, BMI, somatic comorbidities, and medications were not controlled for in this study. However, a control analysis excluding participants with medication changes yielded similar results.
The use of a within-subject design, where participants acted as their own controls, increases statistical power and addresses some of the limitations of potential confounders of HRV. Still, moderating effects of variables such as medication use on HRV dynamics within an individual may exist. Nevertheless, the study’s medium to large effect sizes of reduction in HRV within individuals transitioning from a manic to euthymic state indicate interesting results warranting replication in larger studies.
In this repeated naturalistic observational study, we compared HRV in hospitalized individuals with BD in manic and euthymic states. We found lower HRV in the manic state compared to the euthymic state, examining RMSSD, HF power, and Sample Entropy. This decrease indicates dysregulation of the ANS. However, existing literature on the association between HRV and mania is somewhat inconsistent. This study provides evidence that HRV is lower and less complex in manic states, but this association may be less clear or reversed in hypomanic states. Studies in larger samples are needed to examine HRV during the progression to mania via hypomania and investigate moderating effects of medications on HRV changes between mood states.
The datasets presented in this article are not readily available because they contain sensitive information on living participants. We have included a minimal dataset of HRV data generated and analyzed in this study, see Supplementary material S1. All data will be made anonymous at the end of the study, November 2025, in accordance with the ethical approval, and be available on reasonable request. Requests to access the datasets should be directed to cGV0dGVyLmpha29ic2VuQGhlbHNlLWJlcmdlbi5ubw==.
The studies involving humans were approved by The Norwegian Regional Medical Research Ethics Committee West (2017/937). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AS, PJ, OBF, BO, JT, TN, and KJO contributed to this manuscript. PJ, OBF, KJO, TN, and JT contributed to the work’s conception, funding, and design. PJ and AS collected and curated the data. AS and BO analyzed and interpreted the data with KJO. AS wrote the manuscript, which was supplemented and revised by PJ, OBF, BO, JT, TN, and KJO. All authors contributed to the article and approved the submitted version.
This work was funded by the Norwegian Research Council (agreement 259293). The funder had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
We would like to thank statistician Christopher Andreas Bartz-Johannessen for statistical consultation. This publication is part of the INTROducing Mental health through Adaptive Technology (INTROMAT) project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1250925/full#supplementary-material
Minimal data set with output from HRV analyses. De-identified and restricted due to ethical and patient privacy concerns. Data contains analysis results of HRV variables from one manic and one euthymic 5-minute sample from each participant (N = 15).
Description of medication use.
Results from linear regression analyses. Investigating confounding effects of age and sex on heart rate variability (logRMSSD). The dependent variable of the linear regression model was the manic-euthymic differences of the HRV metrics, and the independent variables were sex and age.
Results from correlation analyses of sampling time and HRV metrics.
Reults of follow-up paired t-tests of HRV in mania vs euthymia including only participants without medication changes between time points (N = 9).
ANS, Autonomic Nervous System; BD, Bipolar Disorder; BMI, Body Mass Index; ECG, Electrocardiogram; HF-power, High-Frequency power; HRV, Heart Rate Variability; MADRS, Montgomery Asberg Depression Rating Scale; RMSSD, Root Mean Square of Successive Differences; SampEn, Sample Entropy; PPG, Photoplethysmography; YMRS, Young Mania Rating Scale.
1. Grande, I, Berk, M, Birmaher, B, and Vieta, E. Bipolar disorder. Lancet. (2016) 387:1561–72. doi: 10.1016/S0140-6736(15)00241-X
2. Bassett, D. A literature review of heart rate variability in depressive and bipolar disorders. Aust N Z J Psychiatry. (2016) 50:511–9. doi: 10.1177/0004867415622689
3. Alvares, GA, Quintana, DS, Hickie, IB, and Guastella, AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. (2016) 41:89–104. doi: 10.1503/jpn.140217
4. Faurholt-Jepsen, M, Kessing, LV, and Munkholm, K. Heart rate variability in bipolar disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2017) 73:68–80. doi: 10.1016/j.neubiorev.2016.12.007
5. Rajendra Acharya, U, Paul Joseph, K, Kannathal, N, Lim, CM, and Suri, JS. Heart rate variability: a review. Med Biol Eng Comput. (2006) 44:1031–51. doi: 10.1007/s11517-006-0119-0
6. Sandercock, GR, Bromley, PD, and Brodie, DA. The reliability of short-term measurements of heart rate variability. Int J Cardiol. (2005) 103:238–47. doi: 10.1016/j.ijcard.2004.09.013
7. Latalova, K, Prasko, J, Diveky, T, Grambal, A, Kamaradova, D, Velartova, H, et al. Autonomic nervous system in euthymic patients with bipolar affective disorder. Neuro Endocrinol Lett. (2010) 31:829–36.
8. Moon, E, Lee, SH, Kim, DH, and Hwang, B. Comparative study of heart rate variability in patients with schizophrenia, bipolar disorder, post-traumatic stress disorder, or major depressive disorder. Clin Psychopharmacol Neurosci. (2013) 11:137–43. doi: 10.9758/cpn.2013.11.3.137
9. Chang, HA, Chang, CC, Tzeng, NS, Kuo, TB, Lu, RB, and Huang, SY. Heart rate variability in unmedicated patients with bipolar disorder in the manic phase. Psychiatry Clin Neurosci. (2014) 68:674–82. doi: 10.1111/pcn.12178
10. Hage, B, Britton, B, Daniels, D, Heilman, K, Porges, SW, and Halaris, A. Diminution of heart rate variability in bipolar depression. Front Public Health. (2017) 5:312. doi: 10.3389/fpubh.2017.00312
11. Ortiz, A, Bradler, K, Moorti, P, MacLean, S, Husain, MI, Sanches, M, et al. Reduced heart rate variability is associated with higher illness burden in bipolar disorder. J Psychosom Res. (2021) 145:110478. doi: 10.1016/j.jpsychores.2021.110478
12. Benjamin, BR, Valstad, M, Elvsåshagen, T, Jönsson, EG, Moberget, T, Winterton, A, et al. Heart rate variability is associated with disease severity in psychosis spectrum disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. (2021) 111:110108. doi: 10.1016/j.pnpbp.2020.110108
13. Wazen, GLL, Gregorio, ML, Kemp, AH, and Godoy, MF. Heart rate variability in patients with bipolar disorder: from mania to euthymia. J Psychiatr Res. (2018) 99:33–8. doi: 10.1016/j.jpsychires.2018.01.008
14. Faurholt-Jepsen, M, Brage, S, Kessing, LV, and Munkholm, K. State-related differences in heart rate variability in bipolar disorder. J Psychiatr Res. (2017) 84:169–73. doi: 10.1016/j.jpsychires.2016.10.005
15. American, PA. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association (2013).
16. Menghini, L, Gianfranchi, E, Cellini, N, Patron, E, Tagliabue, M, and Sarlo, M. Stressing the accuracy: wrist-worn wearable sensor validation over different conditions. Psychophysiology. (2019) 56:e13441. doi: 10.1111/psyp.13441
17. Schuurmans, AAT, de Looff, P, Nijhof, KS, Rosada, C, Scholte, RHJ, Popma, A, et al. Validity of the Empatica E4 wristband to measure heart rate variability (HRV) parameters: a comparison to electrocardiography (ECG). J Med Syst. (2020) 44:190. doi: 10.1007/s10916-020-01648-w
18. Tarvainen, MP, Niskanen, JP, Lipponen, JA, Ranta-Aho, PO, and Karjalainen, PA. Kubios HRV--heart rate variability analysis software. Comput Methods Prog Biomed. (2014) 113:210–20. doi: 10.1016/j.cmpb.2013.07.024
19. Shaffer, F, and Ginsberg, JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
20. Beauchaine, TP, and Thayer, JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol. (2015) 98:338–50. doi: 10.1016/j.ijpsycho.2015.08.004
21. Hayano, J, and Yuda, E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol. (2019) 38:3. doi: 10.1186/s40101-019-0193-2
22. Thayer, JF, Sollers, JJ 3rd, Ruiz-Padial, E, and Vila, J. Estimating respiratory frequency from autoregressive spectral analysis of heart period. IEEE Eng Med Biol Mag. (2002) 21:41–5. doi: 10.1109/MEMB.2002.1032638
23. Young, RC, Biggs, JT, Ziegler, VE, and Meyer, DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
24. Lukasiewicz, M, Gerard, S, Besnard, A, Falissard, B, Perrin, E, Sapin, H, et al. Young Mania Rating Scale: how to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. (2013) 22:46–58. doi: 10.1002/mpr.1379
25. Montgomery, SA, and Asberg, M. A new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
26. Müller, MJ, Himmerich, H, Kienzle, B, and Szegedi, A. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). J Affect Disord. (2003) 77:255–60. doi: 10.1016/S0165-0327(02)00120-9
27. Malhi, GS, Ivanovski, B, Hadzi-Pavlovic, D, Mitchell, PB, Vieta, E, and Sachdev, P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. (2007) 9:114–25. doi: 10.1111/j.1399-5618.2007.00324.x
28. Sheehan, DV, Lecrubier, Y, Sheehan, KH, Amorim, P, Janavs, J, Weiller, E, et al. The Mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59:22–33.
29. Thayer, JF, Yamamoto, SS, and Brosschot, JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. (2010) 141:122–31. doi: 10.1016/j.ijcard.2009.09.543
30. R Core Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2020).
31. Wickham, H, Averick, M, Bryan, J, Chang, W, McGowan, LDA, François, R, et al. Welcome to the tidyverse. J Open Source Softw. (2019) 4:1686. doi: 10.21105/joss.01686
32. Revelle, W. Psych: Procedures for psychological, psychometric, and personality research. R package version 2.3.3 ed. Evanston, Illinois: Northwestern University (2023).
33. Ben-Shachar, M, Lüdecke, D, and Makowski, D. Effectsize: estimation of effect size indices and standardized parameters. J Open Source Softw. (2020) 5:2815. doi: 10.21105/joss.02815
34. Henry, BL, Minassian, A, Paulus, MP, Geyer, MA, and Perry, W. Heart rate variability in bipolar mania and schizophrenia. J Psychiatr Res. (2010) 44:168–76. doi: 10.1016/j.jpsychires.2009.07.011
35. Koenig, J, and Thayer, JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. (2016) 64:288–310. doi: 10.1016/j.neubiorev.2016.03.007
36. Thayer, JF, and Sternberg, E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. (2006) 1088:361–72. doi: 10.1196/annals.1366.014
37. Thayer, JF, and Lane, RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. (2000) 61:201–16. doi: 10.1016/S0165-0327(00)00338-4
38. Outhred, T, Kemp, AH, and Malhi, GS. Physiological correlates of bipolar Spectrum disorders and their treatment In: V Kumari, P Bob, and NN Boutros, editors. Electrophysiology and psychophysiology in psychiatry and psychopharmacology. Cham: Springer International Publishing (2014). 47–102.
Keywords: bipolar disorder, mania, manic state, heart rate variability, HRV, PPG (photoplethysmography), autonomous nervous system (ANS), vagal activity
Citation: Stautland A, Jakobsen P, Fasmer OB, Osnes B, Torresen J, Nordgreen T and Oedegaard KJ (2023) Reduced heart rate variability during mania in a repeated naturalistic observational study. Front. Psychiatry. 14:1250925. doi: 10.3389/fpsyt.2023.1250925
Received: 10 July 2023; Accepted: 21 August 2023;
Published: 07 September 2023.
Edited by:
Heinz Grunze, Paracelsus Medical Private University, Nuremberg, GermanyReviewed by:
Nadja Freund, Ruhr University Bochum, GermanyCopyright © 2023 Stautland, Jakobsen, Fasmer, Osnes, Torresen, Nordgreen and Oedegaard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Stautland, YW5kcmVhLnN0YXV0bGFuZEB1aWIubm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.