- 1Beijing Key Laboratory of Mental Disorders, National Clinical Research Center for Mental Disorders & National Center for Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China
- 2Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
Objective: Our goal was to review current peer-reviewed articles in which the BDI (Beck Depression Inventory), PHQ-9 (Patient Health Questionnaire), or QIDS-SR16 (16-Item Quick Inventory of Depressive Symptomatology) was used as the primary or secondary outcome measure and to evaluate the quality of PRO (Patient-Reported Outcome) reporting in RCTs (Randomized Controlled Trials) according to the 2013 PRO-specific CONSORT (Consolidated Standards of Reporting Trials) extension.
Methods: We systematically searched in electronic databases. A study would be included if it included patients diagnosed with major depressive disorder according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases, version 10 (ICD-10) as participants, was a randomized controlled trial, included the BDI, PHQ-9, or QIDS-SR16 as the primary or secondary outcome measure, published between 1990 and 2013, and was in English. Two of the authors evaluated the quality of PRO reporting according to the 2013 CONSORT-PRO. Logistic regression were used to evaluate the association between reporting completeness and trial characteristics.
Results: A total of 116 studies were included. These studies were conducted in 25 countries. Sample sizes ranged from 12 to 750. The CONSORT-PRO was not cited in any one of the included studies. Among the 116 studies, 2 (1.72%) studies introduced the rationale for PRO assessment, 60 (51.72%) studies explicitly stated statistical approaches for dealing with missing data, 87 (75.00%) studies reported PRO outcome data at baseline and at subsequent time points. The mean score of reporting completeness was 66.24%. Significantly higher reporting completeness was found for RCTs published after 2013 (OR, 95%CI: 3.81, 1.32–10.99). Studies with a higher sample size were more completely reported than studies with a lower sample size (OR, 95%CI: 1.01, 1.00–1.02).
Conclusion: The CONSORT-PRO guidance was rarely cited. The quality of PRO reporting in depression studies requires improvement. This result may be meaningful for the promotion of PRO reporting in RCTs.
1. Introduction
Patient-reported outcome (PRO), as defined by the US Food and Drug Administration (FDA), is “a measurement of a patient’s health condition that is reported directly by the patient” (1). PRO is increasingly recognized by regulators, clinicians, and patients as a valuable tool to measure treatment benefits in terms of the alleviation of the patients’ symptoms and improvement of their pertinent function (2, 3). Responding to this imperative, PRO endpoints are more commonly incorporated in clinical trial design (4) as the primary or secondary outcome measures (5). Despite this, international reviews indicated that PRO are still underutilized (6). Furthermore, the quality of PRO content in many reports is often suboptimal (7, 8).
To make PRO data from randomized controlled trials (RCTs) meaningful, it is critical to have the study and PRO designed well, analyzed appropriately, and reported in a way that makes the results accessible and useful for the critical appraisal of the study results (9). To address this need, corresponding recommendations have been developed, such as Standard Protocol Items: Recommendations for Interventional Trials-PRO extension (SPIRIT-PRO) (10), Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data (SISAQOL) (11), Consolidated Standards of Reporting Trials Statement-PRO extension (CONSORT-PRO) (12), and the COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures (13). All these guidelines provided good references for good methodological practices that can meaningfully and reliably inform patient safety, treatment choices, and policy decisions through PRO. However, the implementation of these recommendations in RCTs remains suboptimal (14). For instance, a literature review focused on PRO reporting in RCTs evaluating systemic cancer therapy and found that the quality of the reporting was rather low: only 26% of RCTs included a description of the prespecified PRO hypothesis, only 16% of RCTs included methods for PRO data collection, and only 37% of RCTs introduced the statistical approaches for managing missing data (15).
Major depressive disorder (MDD) has been ranked as one of the leading causes of disability worldwide and is projected to cause the heaviest burden by 2030 (16). It is a debilitating disease characterized by depressed mood, diminished interests or pleasure, impaired cognitive function, disturbed sleep or appetite and suicidal ideation (17). MDD is primarily a subjective experience, and the degree of impairment was directly related to symptom severity. Therefore, PROs are increasingly utilized as essential endpoints for clinical studies (18) and may provide clinically important information not accessible through clinician rating scales (19). However, according to a review conducted by Minley et al., the completeness of reporting PROs in RCTs addressing MDD was inadequate. A total of 49 RCTs published between 2016 and 2020 were identified, and the overall mean completion percent for the CONSORT-PRO checklist adaptation was 56.74% (20). The Beck Depression Inventory (BDI), Patient Health Questionnaire-9 (PHQ-9) and Quick Inventory of Depressive Inventory (QIDS-SR16) are frequently used self-report instruments in clinical trials of major depressive disorder (20–22). However, there is limited data regarding the quality of PRO reporting in RCTs of MDD before and after the publish of 2013 PROs-specific CONSORT extension.
Responding to this problem, our goal was to review current peer-reviewed articles in which the BDI, PHQ-9, or QIDS-SR16 was used as the primary or secondary outcome measure and to evaluate the quality of PRO reporting in RCTs according to the 2013 PROs-specific CONSORT extension. By doing so, we hope to comprehensively evaluate the current condition of PRO reporting and explore the impact of PRO-specific CONSORT extension on report quality.
2. Methods
2.1. Study selection
In June 2020, we systematically searched in electronic databases including the Cochrane Library, PubMed, Embase, and Web of Science for articles published in English from January 1990 to June 2020. In September 2023, an update of the literature search was conducted. The search started from 1990, since ICH’s inception in 1990. Then the ICH process has gradually evolved, which symbolizes progress in the development of guidelines on safety, quality and efficacy topics. The search strategy and associated terms were based on the inclusion and exclusion criteria for the patient population, outcomes, and study design: (depress*[title]) and ((“9 item patient health questionnaire”[title/abstract]) or (“nine item patient health questionnaire”[title/abstract]) or (“patient health questionnaire 9”[title/abstract]) or (“phq-9”[title/abstract]) or (“quick inventory of depressive symptomatology self-report”[title/abstract]) or (“qids-sr”[title/abstract]) or (“beck depression inventory”[title/abstract]) or (“bdi”[title/abstract])) and ((randomized controlled trial [pt] or controlled clinical trial [pt] or randomized [tiab] or placebo [tiab] or clinical trials as topic [mesh: noexp] or randomly [tiab] or trial [ti]) not (animals[mh] not humans[mh])) and (1990:2023[pdat]).
A study would be included if it included patients diagnosed with major depressive disorder according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases, version 10 (ICD-10) as participants, was a randomized controlled trial, included the BDI (Beck Depression Inventory), PHQ-9 (Patient Health Questionnaire), or QIDS-SR16 (16-Item Quick Inventory of Depressive Symptomatology) as the primary or secondary outcome measure, and was in English. A study would be excluded if the full text was unavailable or it is a secondary analysis of RCT. Studies of comorbid MDD in other diseases were not excluded. Two of the authors (JH and HQ) independently screened articles by titles and abstracts and reviewed the full texts of selected articles, any disagreement in the literature selection process was resolved by a consensus and/or a discussion with a senior investigator (JZ).
2.2. Scoring CONSORT-PRO
According to 2013 CONSORT-PRO, there were 52 entries evaluated. The scoring methodology was adapted from Mercieca-Bebber et al. (23) and Minley et al. (20). Item 3b (important changes to methods after trial commencement (such as eligibility criteria), with reasons), 6b (any changes to trial outcomes after the trial commenced, with reasons), and 14b (why the trial ended or was stopped) of CONSORT-PRO were excluded from scoring as it was difficult to verify without checking the trial protocols. Adherence to these items would be only described using frequency of adequately reported (Table 1). Conditional entries are not included in scoring, including 7b (When applicable, explanation of any interim analyses and stopping guidelines), 11a (If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how), and 17b (For binary outcomes, presentation of both absolute and relative effect sizes is recommended). Furthermore, assessment of item 7a was dependent on whether PRO was the primary endpoint for RCTs. Each of the other 45 items was weighted with equal importance. Each item was recorded “yes” and scored 1 if it was adequately reported. The item was labeled “no” and scored 0 if it was not comprehensively reported or not reported at all. The maximum score of RCTs was 45. Reporting score of RCTs was calculated by adding all the items score and dividing by the possible maximum score.
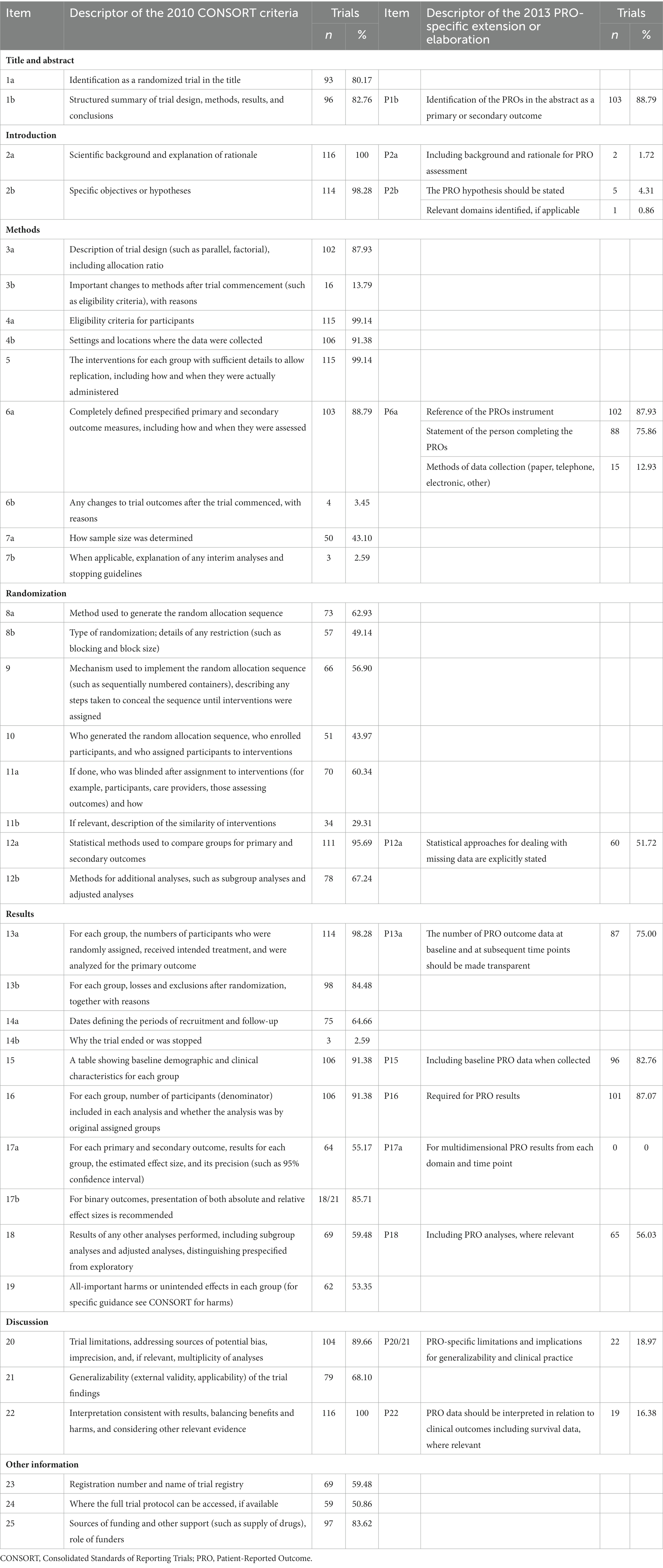
Table 1. Quality of PROs reporting, rated using items of the 2013 extensions of the CONSORT statement (N = 116).
Moreover, according to the CONSORT-PRO scores, studies were categorized into “moderate to good,” or “poor” reporting according to pre-specified thresholds. The RCT was recorded to be “moderate to good” if it addressed more than 60% of the CONSORT-PRO items, and “poor” if ≤60%.
2.3. Trial characteristics and quantitative systems
Characteristics of the trials, such as the year of publication, country, single/multicenter, number of groups, intervention, and sample size, were collected. Two of the authors (JH and HQ) evaluated the quality of PROs reporting according to the 2013 CONSORT-PRO. These two authors examined each article independently. If there was uncertainty in the understanding of an article, the third author (JZ) would resolve it through consensus evaluation. If a PRO was clearly determined as a primary outcome, it would be labeled as primary outcome, otherwise it was considered a secondary outcome. The CONSORT-PRO was published in 2013, a stratified description (1990 to 2012 or 2013 to 2020) of the key evaluations was conducted.
2.4. Statistical analysis
We reported our search results and the frequency of each trial characteristic of the RCTs. Additionally, we reported the frequency of RCTs that cite CONSORT-PRO. Next, we reported the frequency of each CONSORT-PRO item in all RCTs. To determine significant differences between different groups, we used χ2 tests for categorical variables and Wilcoxon Signed Rank Test for continuous variables, respectively. Tests were 2-sided at the 0.05 significance level. Bivariate logistic regression was applied to estimate the odds ratios (ORs) and 95% confidence interval (CI) for associations between study characteristics and quality of PRO reporting.
3. Results
3.1. Characteristics of selected randomized controlled trials
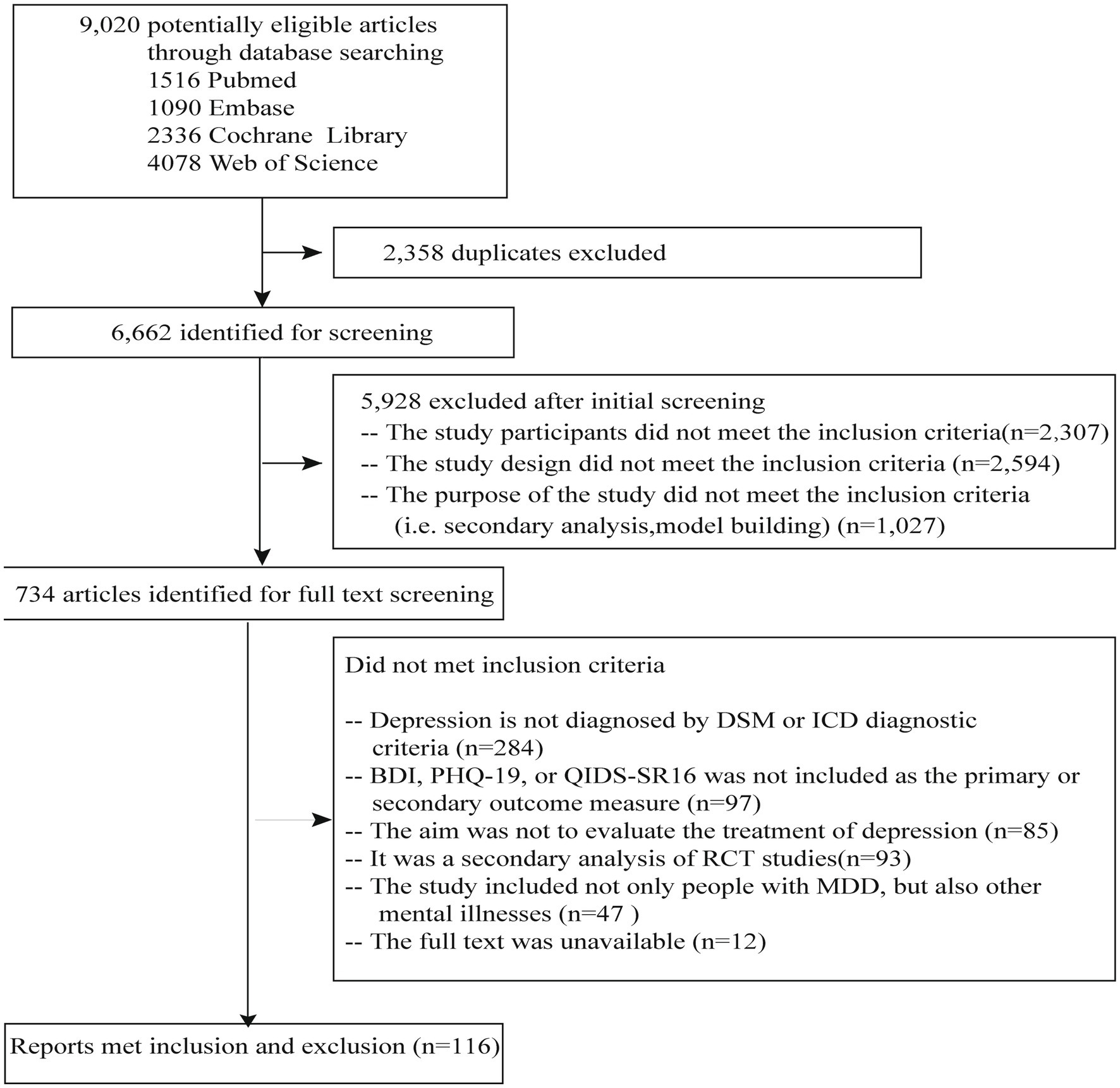
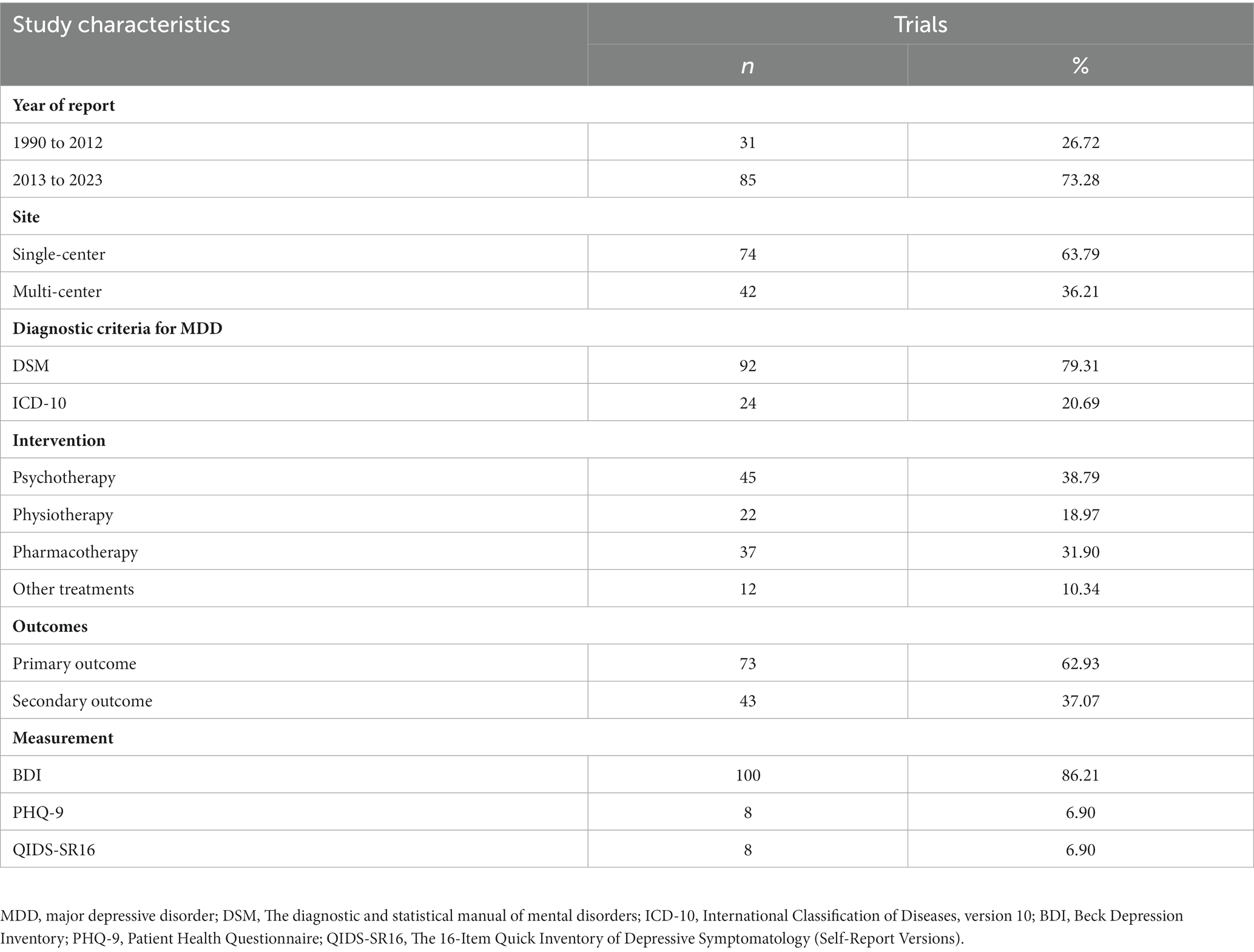
In total, 9,020 studies were found through the database search of studies published from January 1990 to June 2023 (Figure 1). After excluding the duplicates, we screened the articles and excluded the articles without full text or the studies in which the participants were not diagnosed with major depressive disorder; in the end, 116 studies remained (Figure 1). Among them, 31 (26.72%) were published between 1990 and 2012, and 85 (73.28%) were published between 2013 and 2023. These studies were conducted in 25 countries. Of these studies, 74 (73.79%) were single-center and 42 (36.21%) were multicenter. The criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) were used in 92 studies, and the ICD-10 criteria were used in 24 (20.69%) studies. Among all the studies, 87 (75.00%) were two-arm, and 27 (23.28%) were three-arm. The intervention in 45 (38.79%) of the studies was psychotherapy, in 22 (18.97%) was physiotherapy, in 37 (31.90%) was pharmacotherapy, and in 12 (10.34%) were other treatments (e.g., supportive text messages, expressive writing, and measurement-based care). Sample sizes ranged from 12 to 750. The QIDS-SR16 was used as primary or secondary outcomes in eight studies, PHQ-9 was used in eight studies, and BDI was used in 100 (86.21%) studies. Among these studies, patient-reported outcomes (i.e., PHQ-9, QIDS-SR16, and BDI) were included as primary outcomes in 73 (62.93%) and secondary outcomes in 43 (37.07%), respectively (Table 2).
3.2. Overall quality of PROs reporting
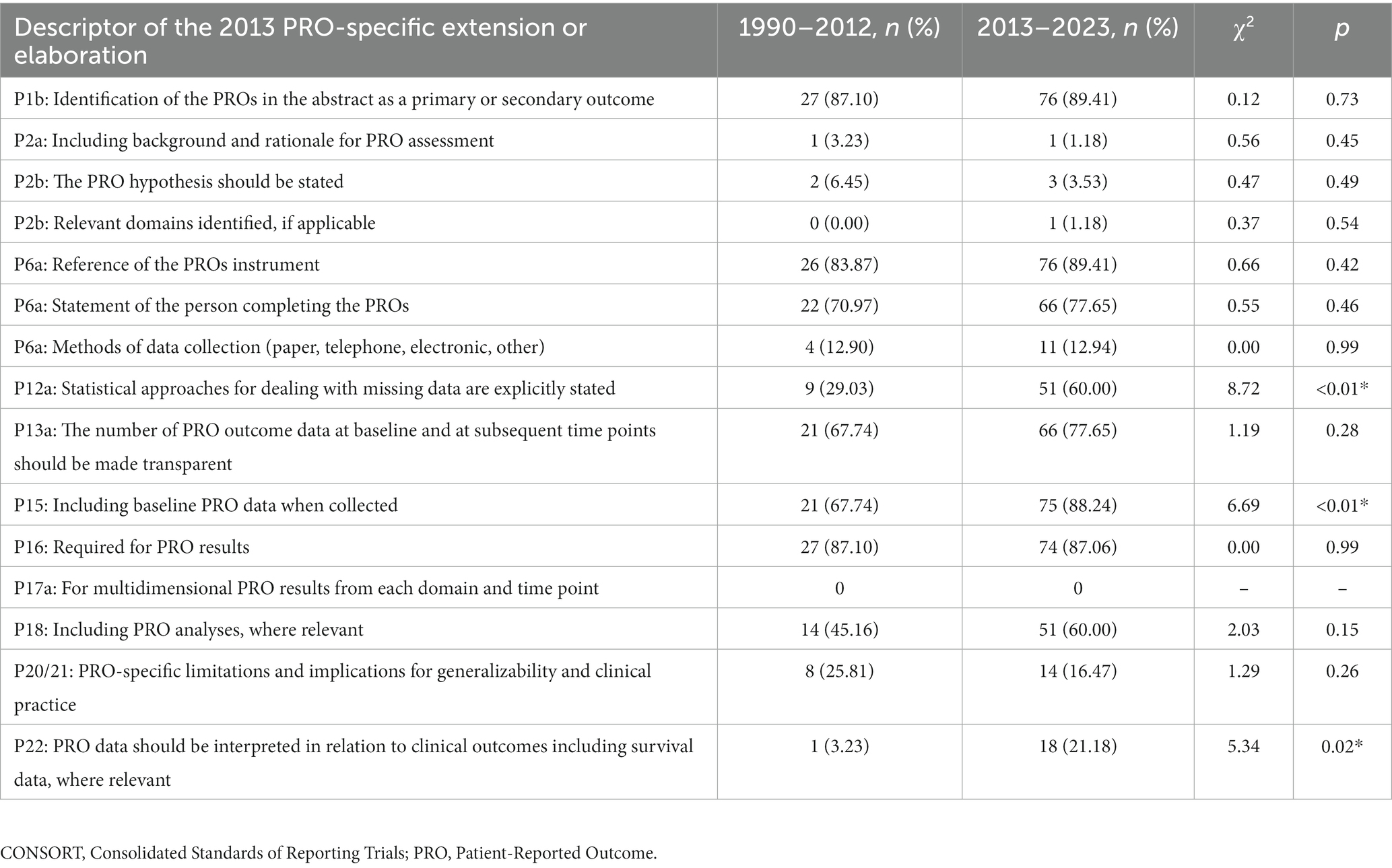
The mean score of reporting was 66.24%. The CONSORT-PRO was not cited in any one of the included studies published after 2013. Among the 116 studies, 2 (1.72%) studies introduced the rationale for PRO assessment, 102 (89.93%) studies included reference of the PROs instrument, 60 (51.72%) studies explicitly stated statistical approaches for dealing with missing data, 87 (75.00%) studies reported PRO outcome data at baseline and at subsequent time points, and 22 (18.97%) studies discussed PRO-specific limitations and implications for generalizability and clinical practice (Table 1).
3.3. Quality of PROs reporting before and after the release of CONSORT-PRO
Among the 116 studies, 31 (26.72%) were published between 1990 and 2012, and 85 (73.28%) were published between 2013 and 2023. Their mean score of reporting was 58.78 and 68.97%, respectively. Significant improvement of reporting completeness was seen in P12a (statistical approaches for dealing with missing data are explicitly stated), P15 (Including baseline PRO data when collected), and P22 (PRO data should be interpreted in relation to clinical outcomes, including survival data, where relevant). Detailed information can be seen in Table 3.
3.4. The associations between study characteristics and quality of PRO reporting
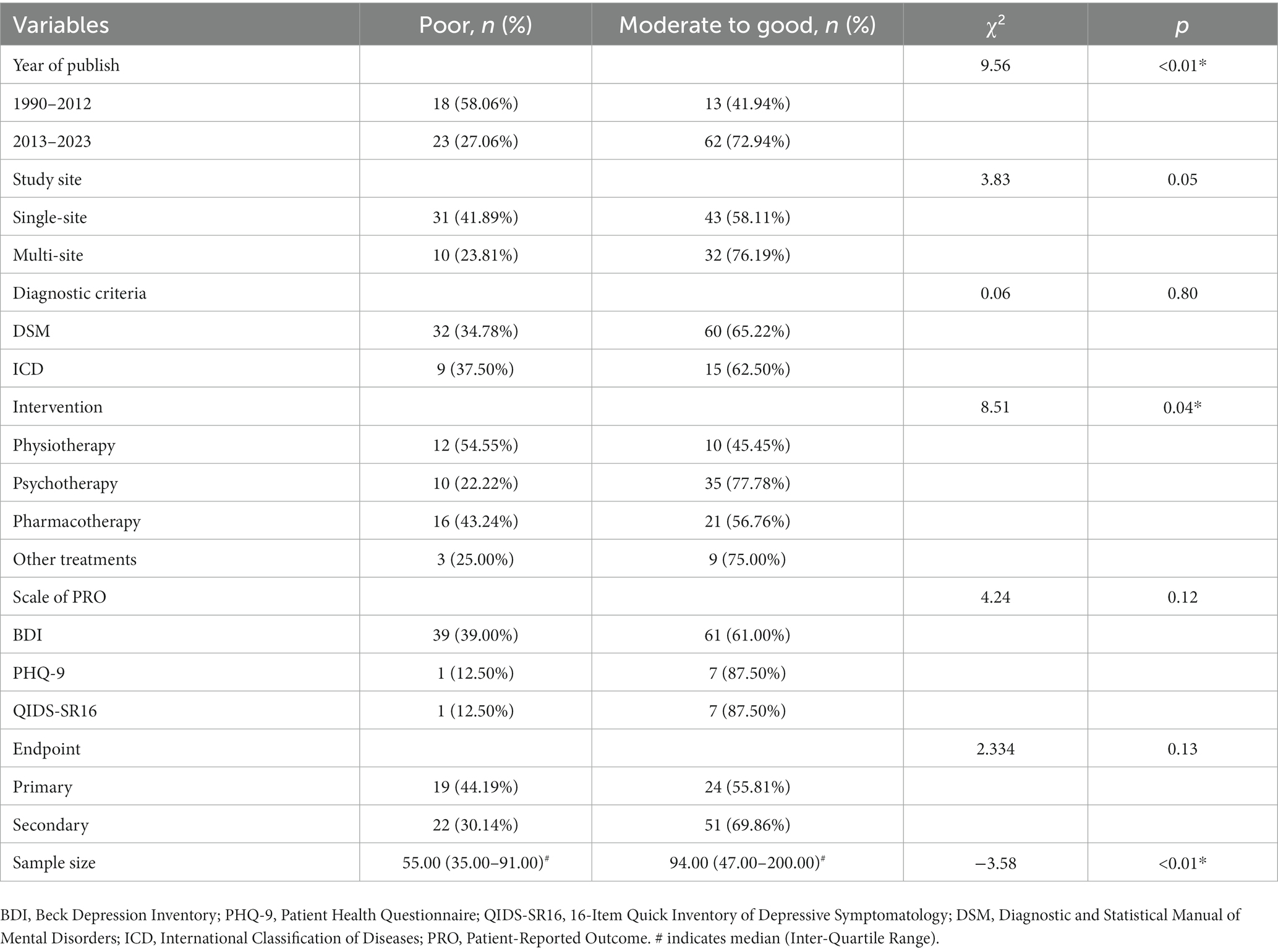
Among the 116 studies, 41 (35.34%) were recorded to be “poor,” and 75 (64.55%) recorded to be “moderate to good.” Our bivariate regression analyses revealed that RCTs published after 2013 were more completely reported than RCTs published between 1990 and 2012 (OR, 95%CI: 3.81, 1.32–10.99). Studies with a higher sample size were more completely reported than studies with a lower sample size (OR, 95%CI: 1.01, 1.00–1.02). Further results of these analyses can be found in Tables 4 5.
4. Discussion
Our study highlighted that the quality of PRO reporting in depression studies requires improvement, even though significant improvement was seen after the release of CONSORT-PRO. This result can provide guidance for reporting information in future studies.
The CONSORT-PRO was published in 2013 and provided an evidence-based list of items recommended for inclusion in trial reports. The CONSORT-PRO facilitates more complete and transparent reporting (23), but it was not cited in any of the included studies published after 2013. A review of the reporting of patient-reported outcomes in elderly patients with hip fractures found that no study has mentioned the CONSORT-PRO or any other PRO-reporting guidelines (24). Between February 2013 and 17 December 2015, only 26 RCTs cited the CONSORT-PRO appropriately, representing a minute proportion of RCTs that reported PRO results during that period (23). A review of randomized controlled trials of hematological malignancies reported a similar finding: only 6% (n = 4) of 71 included studies cited the CONSORT-PRO extension explicitly (25). A review of cystic fibrosis randomized controlled trials also found inadequate reporting of patient-reported outcomes using CONSORT-PRO (26). Fifty-nine eligible RCTs were included, and their mean completeness of reporting was 38.38%. There are some potential barriers to citing the CONSORT-PRO, such as lacking endorsement from journals and a widespread lack of awareness of its existence and/or importance. It would be ideal if the journals would set requirements of a reference to the CONSORT-PRO, in order to facilitate more scientific reports and reduce research waste. A failure to cite the CONSORT-PRO may not imply a failure to use it, but it does imply that the extent of awareness remains unsatisfactory overall. We recommend referring to these international criteria in future RCT publications that include PRO data.
Furthermore, we found that the mean score of reporting was 66.24%, which means the overall reporting of PROs was suboptimal in current RCTs of depression. Similar to our result, a review from Minley et al. reported a mean CONSORT-PRO completion score of 56.7%, and also found that training on the application of PRO data in studies of MDD is needed (20). In our review, 5 (4.31%) RCTs report a PRO hypothesis or relevant PRO domains, lower than previous reviews of trials of ovarian cancer (19%) (27). Because PRO data are usually collected at multiple time points, a lack of clear hypotheses may obstruct the accurate evaluation of statistical analyses and research results. Therefore, in the design stage of a study, it is important to establish a PRO-related study hypothesis, specify the interested PRO-related domains, and plan for statistical analysis. Other important PRO criteria that were rarely met include the “interpretation of PRO findings” which was met in only about 14% of all cases. Failure to adequately interpret results can lead to unwarranted conclusions and limit the objectiveness of medical research. PRO-specific limitations and implications for generalizability and clinical practice were well reported in 18.97% of all RCTs of depression included in our study. This proportion was similar to that of the studies on multiple myeloma examined in another review (28) but was much lower than that of the 71 RCTs of hematological malignancies (25). More efforts should be made to improve the quality of reporting because it is helpful for clinicians and patients to assess treatment tolerability and make therapeutic decisions.
Importantly, as an integral part of PRO analysis and interpretation, handling missing data is inevitably, but about half of the articles included in this study did not state their statistical approaches for dealing with missing data completely. A systematic review assessing PRO reporting in studies on multiple myeloma found that only 23.0% reported a statistical plan for handling missing data (28). In another review of PRO reporting in randomized controlled trials of hematological malignancies, the proportion of missing data was reported in 51 (72%) of the 71 RCTs, but approaches used to handle missing data were described in only 26 (37%) trials (25). A review of RCTs on breast cancer published in 2018 indicated that the information about how the missing data were handled was omitted in 48 (73%) studies (29). Similarly, another review of 557 RCTs on cancer showed that the statistical approaches used to deal with missing PRO data was reported in only 20% studies (30). It is commonly known that missing data are common, and sometimes unavoidable, and it can potentially lead to loss of information, biased estimates, and impaired power and interpretability (31). Sensitivity analyses is important to verify the stability and robustness of the findings. Furthermore, transparent reporting of the missing data at each time point, is important for the assessment of potential bias in the PRO results. While deficiencies in reporting were common and there is room for improvement.
In our study, the need for clear, and comprehensive PRO-specific reporting to standardize PRO methodology, improving PRO data quality and minimizing the potential for bias is reinforced. Nonetheless, our study still has limitations. First, we reviewed published articles but not study protocols. Some criteria of the CONSORT-PRO may have been reported in the study protocol. So, our findings should not be interpreted as an appraisal of the overall quality of all PRO studies. However, CONSORT-PRO advised that its criteria should not only be addressed in the study protocol, but also be addressed in the final report. Second, despite our thorough search strategy, our analysis was limited to published studies. Also, only the studies in which BDI, PHQ-19, or QIDS-SR16 was included as the primary or secondary outcome measure were analyzed, and RCTs with other PRO endpoints have been missed. Third, the factors influencing the quality of reporting in the analysis may be not comprehensive, inclusion of more comprehensive factors is needed in future. Despite these limitations, our data may serve as a benchmark to monitor the quality of PRO reporting in future depression studies. It provides a broad overview of the quality of PRO reporting in RCT on MDD and reveals the impact of study characteristic and CONSORT-PRO on report quality.
5. Conclusion
The significant improvement in PRO-reporting was seen after the release of CONSORT-PRO. The quality of PRO reporting in depression studies still requires improvement. More efforts should be made to promote adequate reporting. We believe that increasing the application of the CONSORT-PRO in studies and the endorsement of CONSORT-PRO guidelines by the journals may be meaningful for the promotion of PRO reporting in RCTs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GW: conceptualization, review and editing. JZ, HQ, and JH methodology and formal analysis. JZ: writing—original draft preparation. ZF: review and language editing. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by Beijing Municipal Administration of Hospitals Incubating Program (no. PX2020073 to JZ). The Beijing Hospitals Authority Youth Programme (no. QML20231903 to JZ). The Capital’s Funds for Health Improvement and Research (no. 2020-2-1171 to JZ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1246938/full#supplementary-material
References
1. FDA. Guidance for Industry. Patient-reported Outcome Measures: Use in Medicinal Product Development to Support Labeling Claims (US Department of Health and Human Services Food and Drug Administration). Silver Spring, MD: FDA (2009).
2. Calvert, M, King, M, Mercieca-Bebber, R, Aiyegbusi, O, Kyte, D, Slade, A, et al. SPIRIT-PRO Extension explanation and elaboration: guidelines for inclusion of patient-reported outcomes in protocols of clinical trials. BMJ Open. (2021) 11:e045105. doi: 10.1136/bmjopen-2020-045105
3. Kieffer, CM, Miller, AR, Chacko, B, and Robertson, AS. FDA Reported Use of Patient Experience Data in 2018 Drug Approvals. Ther Innov Regul Sci. (2020) 54:709–16. doi: 10.1007/s43441-019-00106-1
4. Vodicka, E, Kim, K, Devine, EB, Gnanasakthy, A, Scoggins, JF, and Patrick, DL. Inclusion of patient-reported outcome measures in registered clinical trials: Evidence from ClinicalTrials.gov (2007-2013). Contemp Clin Trials. (2015) 43:1–9. doi: 10.1016/j.cct.2015.04.004
5. Silove, D, and Ventevogel, P. Living through interminable adversity: the mental health of the Afghan people. World Psychiatry. (2022) 21:55–6. doi: 10.1002/wps.20955
6. de Bienassis, K, Kristensen, S, Hewlett, E, Roe, D, Mainz, J, and Klazinga, N. Patient-reported indicators in mental health care: towards international standards among members of the OECD. Int J Qual Health Care. (2022) 34:ii7-ii12. doi: 10.1093/intqhc/mzab020
7. Kyte, D, Duffy, H, Fletcher, B, Gheorghe, A, Mercieca-Bebber, R, King, M, et al. Systematic evaluation of the patient-reported outcome (PRO) content of clinical trial protocols. PLoS One. (2014) 9:e110229. doi: 10.1371/journal.pone.0110229
8. Ahmed, K, Kyte, D, Keeley, T, Efficace, F, Armes, J, Brown, JM, et al. Systematic evaluation of patient-reported outcome (PRO) protocol content and reporting in UK cancer clinical trials: the EPiC study protocol. BMJ Open. (2016) 6:e012863. doi: 10.1136/bmjopen-2016-012863
9. Calvert, M, Brundage, M, Jacobsen, PB, Schünemann, HJ, and Efficace, F. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes. (2013) 11:184. doi: 10.1186/1477-7525-11-184
10. Calvert, M, Kyte, D, Mercieca-Bebber, R, Slade, A, Chan, A-W, King, MT, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols. JAMA. (2018) 319:483. doi: 10.1001/jama.2017.21903
11. Coens, C, Pe, M, Dueck, AC, Sloan, J, Basch, E, Calvert, M, et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. (2020) 21:e83–96. doi: 10.1016/S1470-2045(19)30790-9
12. Calvert, M, Blazeby, J, Altman, DG, Revicki, DA, Moher, D, Brundage, MD, et al. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. (2013) 309:814–22. doi: 10.1001/jama.2013.879
13. Gagnier, JJ, Lai, J, Mokkink, LB, and Terwee, CB. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Qual Life Res. (2021) 30:2197–218. doi: 10.1007/s11136-021-02822-4
14. Cella, D. In our patient-centered era, it is time we gave patient-reported outcomes their due. Cancer. (2020) 126:2592–3. doi: 10.1002/cncr.32763
15. Bylicki, O, Gan, HK, Joly, F, Maillet, D, You, B, and Peron, J. Poor patient-reported outcomes reporting according to CONSORT guidelines in randomized clinical trials evaluating systemic cancer therapy. Ann Oncol. (2015) 26:231–7. doi: 10.1093/annonc/mdu489
16. Bains, N, and Abdijadid, S. Major depressive disorder In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC (2022)
17. Otte, C, Gold, SM, Penninx, BW, Pariante, CM, Etkin, A, Fava, M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
18. IsHak, WW, Mirocha, J, Pi, S, Tobia, G, Becker, B, Peselow, ED, et al. Patient-reported outcomes before and after treatment of major depressive disorder. Dialogues Clin Neurosci. (2014) 16:171–83. doi: 10.31887/DCNS.2014.16.2/rcohen
19. Uher, R, Perlis, RH, Placentino, A, Dernovsek, MZ, Henigsberg, N, Mors, O, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. (2012) 29:1043–9. doi: 10.1002/da.21993
20. Minley, K, Smith, CA, Batioja, K, Andriana Peña, BS, Shepard, S, Heigle, B, et al. The evaluation of reporting of patient-reported outcomes in MDD: A meta-epidemiological study of clinical trials. J Psychiatr Res. (2022) 150:79–86. doi: 10.1016/j.jpsychires.2022.03.028
21. Cameron, I, Crawford, J, Lawton, K, Sharma, S, Dutoit, S, Hay, S, et al. Assessing the validity of the PHQ-9, HADS, BDI-II and QIDS-SR16 in measuring severity of depression in a UK sample of primary care patients with a diagnosis of depression: Study protocol. Prim Care Commun. Psychiatry. (2008) 13:67–71. doi: 10.1080/17468840802067486
22. Titov, N, Dear, BF, McMillan, D, Anderson, T, Zou, J, and Sunderland, M. Psychometric comparison of the PHQ-9 and BDI-II for measuring response during treatment of depression. Cogn Behav Ther. (2011) 40:126–36. doi: 10.1080/16506073.2010.550059
23. Mercieca-Bebber, R, Rouette, J, Calvert, M, King, MT, McLeod, L, Holch, P, et al. International society for quality of life research best practice for, preliminary evidence on the uptake, use and benefits of the CONSORT-PRO extension. Qual Life Res. (2017) 26:1427–37. doi: 10.1007/s11136-017-1508-6
24. van der Vet, P, Wilson, S, Houwert, RM, Verleisdonk, EJ, and Heng, M. Quality and reporting of patient-reported outcomes in elderly patients with hip fracture: a systematic review. BMJ Open. (2022) 12:e058197. doi: 10.1136/bmjopen-2021-058197
25. Chakraborty, R, Cannella, L, Cottone, F, and Efficace, F. Quality of patient-reported outcome reporting in randomised controlled trials of haematological malignancies according to international quality standards: a systematic review. Lancet Haematol. (2020) 7:e892–901. doi: 10.1016/S2352-3026(20)30292-1
26. Moore, T, Nees, D, Hightower, B, Brock, L, Kee, M, Wise, A, et al. Underreporting of patient-reported outcomes in cystic fibrosis randomized controlled trials using CONSORT-PRO and RoB 2.0. Respir Med Res. (2023) 83:100962. doi: 10.1016/j.resmer.2022.100962
27. Mercieca-Bebber, R, Friedlander, M, Kok, PS, Calvert, M, Kyte, D, Stockler, M, et al. The patient-reported outcome content of international ovarian cancer randomised controlled trial protocols. Qual Life Res. (2016) 25:2457–65. doi: 10.1007/s11136-016-1339-x
28. Brock, L, Hightower, B, Moore, T, Nees, D, Heigle, B, Shepard, S, et al. Reporting of patient-reported outcome measures in randomized controlled trials on shoulder rotator cuff injuries is suboptimal and requires standardization. Arthrosc Sports Med Rehabil. (2022) 4:e1429–36. doi: 10.1016/j.asmr.2022.04.032
29. Pe, M, Dorme, L, Coens, C, Basch, E, Calvert, M, Campbell, A, et al. Statistical analysis of patient-reported outcome data in randomised controlled trials of locally advanced and metastatic breast cancer: a systematic review. Lancet Oncol. (2018) 19:e459–69. doi: 10.1016/S1470-2045(18)30418-2
30. Efficace, F, Fayers, P, Pusic, A, Cemal, Y, Yanagawa, J, Jacobs, M, et al. Quality of patient-reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient-reported outcome extension: A pooled analysis of 557 trials. Cancer. (2015) 121:3335–42. doi: 10.1002/cncr.29489
31. Mercieca-Bebber, R, Palmer, MJ, Brundage, M, Calvert, M, Stockler, MR, and King, MT. Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: a systematic review. BMJ Open. (2016) 6:e010938. doi: 10.1136/bmjopen-2015-010938
Keywords: patient-reported outcome, major depressive disorder, randomized controlled trials, meta-epidemiological review, reporting quality evaluation
Citation: Zhou J, Qi H, Hu J, Feng Z and Wang G (2023) Low-quality of patient-reported outcome reporting in randomized clinical trials of major depressive disorder—a meta-epidemiological review. Front. Psychiatry. 14:1246938. doi: 10.3389/fpsyt.2023.1246938
Edited by:
Renerio Fraguas, University of São Paulo, BrazilReviewed by:
Erika Baumbach, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG), GermanyXiao-min Zhu, Suzhou Guangji Hospital, China
Copyright © 2023 Zhou, Qi, Hu, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wang, Z2FuZ3dhbmdkb2NAY2NtdS5lZHUuY24=
 Jia Zhou
Jia Zhou Han Qi
Han Qi Jia Hu1,2
Jia Hu1,2