- 1The Brain Hospital of Guangxi Zhuang Autonomous Region, Liuzhou, China
- 2The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
- 3Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, China
Objective: Intermittent theta-burst stimulation (iTBS), which is a form of repetitive transcranial magnetic stimulation (rTMS), can produce 600 pulses to the left dorsolateral prefrontal cortex (DLPFC) in a stimulation time of just over 3 min. The objective of this systematic review was to compare the safety and efficacy of iTBS and high-frequency (≥ 5 Hz) rTMS (HF-rTMS) for patients with treatment-resistant depression (TRD).
Methods: Randomized controlled trials (RCTs) comparing the efficacy and safety of iTBS and HF-rTMS were identified by searching English and Chinese databases. The primary outcomes were study-defined response and remission.
Results: Two RCTs (n = 474) investigating the efficacy and safety of adjunctive iTBS (n = 239) versus HF-rTMS (n = 235) for adult patients with TRD met the inclusion criteria. Among the two included studies (Jadad score = 5), all were classified as high quality. No group differences were found regarding the overall rates of response (iTBS group: 48.0% versus HF-rTMS group: 45.5%) and remission (iTBS group: 30.0% versus HF-rTMS group: 25.2%; all Ps > 0.05). The rates of discontinuation and adverse events such as headache were similar between the two groups (all Ps > 0.05).
Conclusion: The antidepressant effects and safety of iTBS and HF-rTMS appeared to be similar for patients with TRD, although additional RCTs with rigorous methodology are needed.
Introduction
Depression is a leading cause of disability worldwide and a major contributor to the global burden of disease; it is estimated to be the strongest contributor among developed countries by the end of 2030 (1). Major depressive disorder (MDD) has an estimated lifetime prevalence of 3.4% and a 12-month prevalence of 2.1% according to the latest national epidemiological survey from China (2). Over 700,000 people die by suicide every year, and more than half of these deaths are caused by depression (3). Currently, traditional treatments for MDD include antidepressant medication and psychotherapy, but more than one-third of patients fail to respond to either pharmacotherapy or psychotherapy (4–6). Similarly, up to 30% of patients do not achieve clinical remission (7, 8). In addition, multiple side effects of medication could lead to a poor quality of life and reduced treatment adherence (9). There is still a lack of effective strategies for addressing treatment-resistant depression (TRD). Therefore, new treatment modalities for patients with TRD are urgently needed.
Noninvasive brain stimulation techniques, such as transcranial magnetic stimulation (TMS) (10), transcranial direct current stimulation (tDCS) (11), and transcranial alternating current stimulation (tACS) (12), provide a nonpharmacological alternative for MDD. High-frequency (≥ 5 Hz) repetitive transcranial magnetic stimulation (HF-rTMS) was approved by the Food and Drug Administration (FDA) as a noninvasive brain stimulation technique for TRD in 2008 (13). Evidence for the supremacy of active rTMS over sham stimulation has been accumulating for nearly 20 years (10, 14). A recent study analyzing 81 randomized clinical trials (RCTs) found that active rTMS targeting the left dorsolateral prefrontal cortex (DLPFC) led to a higher rate of clinical remission and response compared to sham stimulation (15). However, a retrospective study found that only 214/730 depressed patients (29.3%) obtained antidepressant response to HF-rTMS, showing that not all patients with MDD could benefit from HF-rTMS (16). In particular, the antidepressant effects of rTMS were not evident in patients with high resistance to prior antidepressant treatments (17). Given that the standard FDA-approved HF-rTMS protocol requires 37.5 min per session and a long treatment course (5 times per week and lasting 4–6 weeks) (18), this approach may increase the daily transport burden and inconvenience for full-time patients, thereby reducing the clinical feasibility of conventional rTMS (19).
New efficient strategies for enhancing the therapeutic efficiency of rTMS are a hot topic in current research and have shown significant clinical value. As a novel and potentially beneficial form of TMS, theta-burst stimulation (TBS) including continuous TBS (cTBS), intermittent TBS (iTBS), bilateral TBS (bTBS), and intermediate TBS (imTBS) have been popularly used in clinical practice (20). Notably, iTBS can produce 600 pulses in a total stimulation time of 3 min 9 s (20), which was also approved by the FDA in 2018 for the treatment of TRD (21). Previous pilot studies have shown that active iTBS is superior to sham stimulation for TRD (22–24). A retrospective study initially investigating the antidepressant outcomes of iTBS versus HF-rTMS over the left DLPFC found that 3-min iTBS protocols may be as effective as HF-rTMS protocols (25). Two randomized controlled studies (RCTs) consistently reported similar antidepressant effects and safety with iTBS and HF-rTMS as an adjunctive treatment for patients with TRD (26, 27). For example, Blumberger et al. carried out a large multicentre RCT that confirmed that iTBS over the left DLPFC as an add-on therapy was noninferior to HF-rTMS as measured by the Hamilton Rating Scale for Depression (HRSD) for the treatment of patients with TRD (26). Similarly, a recently published study showed similar response rates (36.7% versus 33.3%) and remission rates (18.5% versus 14.8%) as evaluated by the Montgomery-Åsberg Depression Rating Scale (MADRS) in patients suffering from TRD treated with iTBS and HF-rTMS (27). The 3-min iTBS protocol seems to be an optimized solution for reducing depressive symptoms, as it saves time and improves acceptability in the treatment of TRD when compared to traditional HF-rTMS.
To date, no systematic review investigating the safety and antidepressant effects of iTBS versus HF-rTMS were published. To fill this gap, we performed this systematic review to evaluate the efficacy and safety of iTBS versus HF-rTMS in the treatment of patients with TRD. Based on the findings of Mutz et al.’s study (28), we hypothesized that iTBS has a similar antidepressant effect as HF-rTMS in adult patients with TRD.
Methods
Search strategy and screening criteria
Two researchers (X-JL and Z-JQ) systematically searched the Cochrane Library, PubMed, EMBASE, PsycINFO, Chinese Journal Net, and WanFang databases from inception to 19 November 2022 to identify relevant studies using the following search terms: “(“intermittent theta-burst stimulation” OR (intermittent* AND “theta-burst stimulation”) OR iTBS)” AND (trans-cranial magnetic stimulation OR transcranial magnetic stimulation OR rTMS OR TMS) AND (depress* OR dysphor* OR melanchol* OR antidepress*). Additionally, the references of identified RCTs (26, 27) and relevant articles (29, 30) were manually searched to identify missing studies on the safety and efficacy of iTBS versus HF-rTMS for TRD.
As recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guidelines (31), any published RCTs comparing iTBS and HF-rTMS for TRD were included when they met the following inclusion criteria, which were developed based on the PICOS principles: Participants: adult patients (more than 18 years) with a primary diagnosis of TRD defined by the respective studies; Intervention: treatments as usual (TAU) plus active iTBS; Comparison: TAU plus HF-rTMS (≥ 5 Hz); Outcomes: the primary outcomes of interest were the study-defined response and study-defined remission as measured by HRSD or MADRS; secondary results were the rates of discontinuation and adverse events; Study: only published RCTs comparing the safety and efficacy of iTBS and HF-rTMS for patients with TRD were eligible for inclusion in this systematic review. Numerous studies have found that a standard run of iTBS (600 pulses/session) presents similar or more potent excitatory effects in brain regions than conventional rTMS (32–34). As recommended previously (20, 26), the 3-min protocol of iTBS has a unique advantage in reducing treatment time. Thus, only studies examining daily treatment using a standard dose of 600 pulses of iTBS were included. Studies focusing on other modalities of iTBS, such as accelerated iTBS (≥2 sessions/day) (35) and prolonged iTBS (1800 pulses per session) (36), were excluded. Review articles, meta-analyzes, and case reports or case series were also excluded.
Data extraction
Data extraction for each included RCT was conducted by two independent researchers (X-JL and Z-JQ) using a standardized Microsoft Excel sheet, focusing on the following subjects: study design, participant characteristics, parameters of iTBS and HF-rTMS, and treatment outcomes from the original research. Any differences in data entry between the two researchers (X-JL and Z-JQ) were discussed with a senior author (D-BC), if necessary. For the missing information or clarification, we would contact the author(s) by email or telephone.
Study quality assessment
Two researchers (X-JL and Z-JQ) independently assessed the quality of the included RCTs using the Jadad scale (37) and Cochrane risk of bias tool (38). RCTs with a Jadad score ≥ 3 were considered to be of high quality (39). In addition, the overall evidence level and strength for all primary and secondary outcomes were rated by using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (40).
Results
Literature search
We initially retrieved 959 articles by searching the above databases. Ultimately, 2 RCTs (26, 27) met the inclusion criteria of the present systematic review. The study selection process is presented in Figure 1.
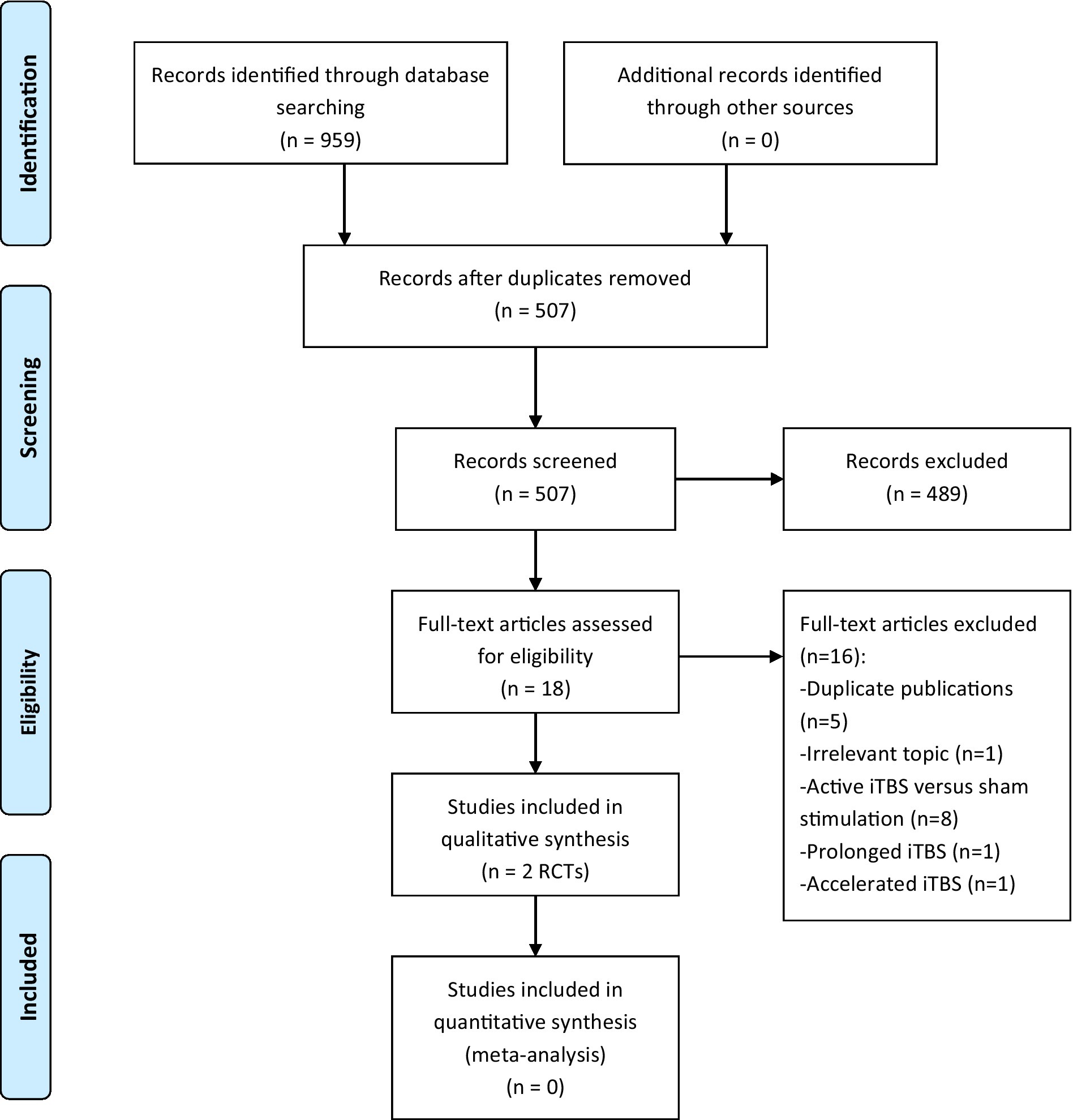
Figure 1. PRISMA flow diagram. iTBS, intermittent theta burst stimulation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyzes; RCTs, randomized controlled trials.
The characteristics of the included studies
Table 1 provides a summary of clinical characteristics and the detailed treatment protocols for each included RCT (26, 27). Two RCTs (n = 474) compared the efficacy and safety of iTBS (n = 239) and HF-rTMS (n = 235) for adult patients with TRD. In the two RCTs, the dose of iTBS (50 Hz) was 600 pulses per session, and the doses of HF-rTMS ranged from 1,600 to 3,000 pulses per session. Participants in iTBS groups experienced a total dose of 12,000 pulses in both RCTs, and the total dose of HF-rTMS varied from 32,000 to 60,000 pulses. Their mean duration of illness ranged from 19.5 to 23.3 months, and the proportion of male patients with TRD was between 31.7% and 40.6%. The treatment duration in both studies was 20 days.
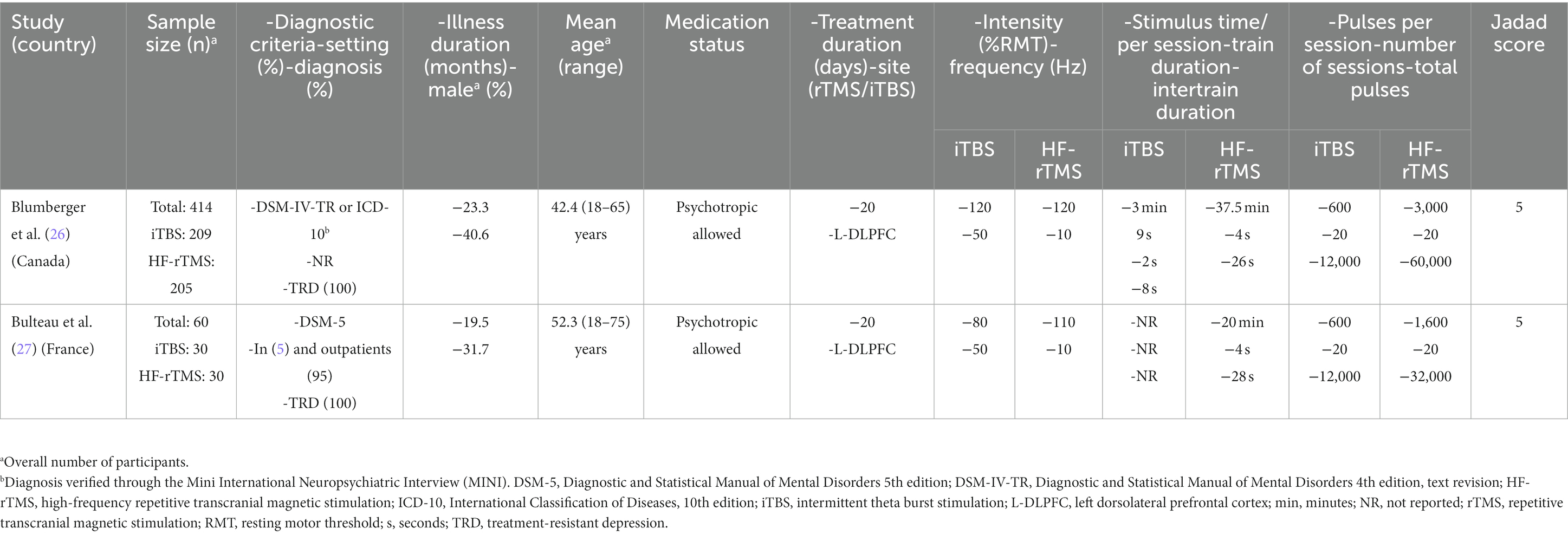
Table 1. Participant characteristics and HF-rTMS/iTBS parameters of each included study in this systematic review.
Study quality assessment
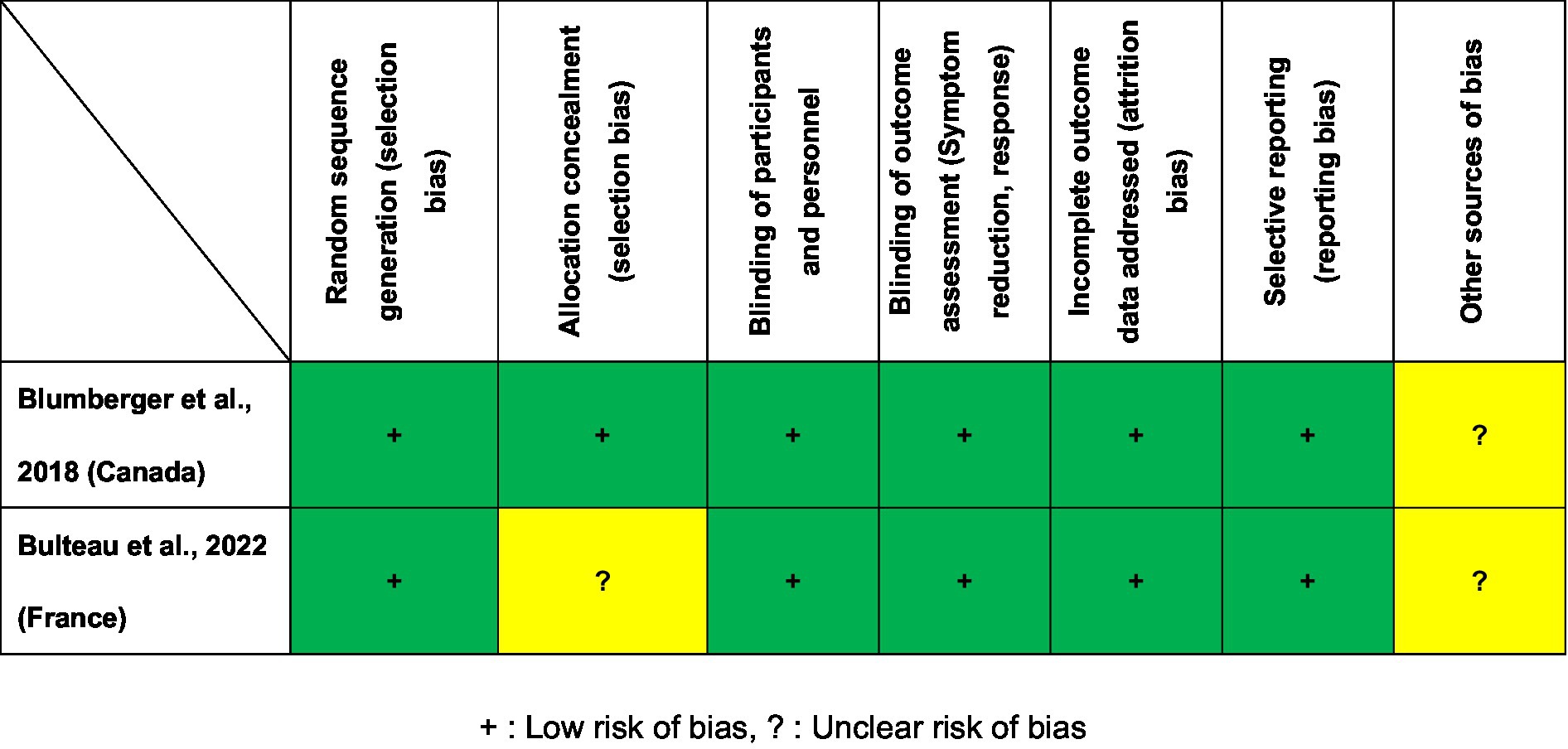
Figure 2 presents the Cochrane risk of bias for the two included RCTs. Two RCTs (26, 27) were judged to be low risk regarding selection bias, blinding, attrition and reporting bias. As shown in Table 1, the Jadad scores of the two studies (26, 27) were 5 points (high quality). On the basis of the GRADE guidelines, the overall evidence level for the 17 primary and secondary outcomes of the two included RCTs (26, 27) ranged from “moderate” (5.9%, 1/17) to “high” (94.1%, 16/17; Supplementary Table S2).
Primary outcomes
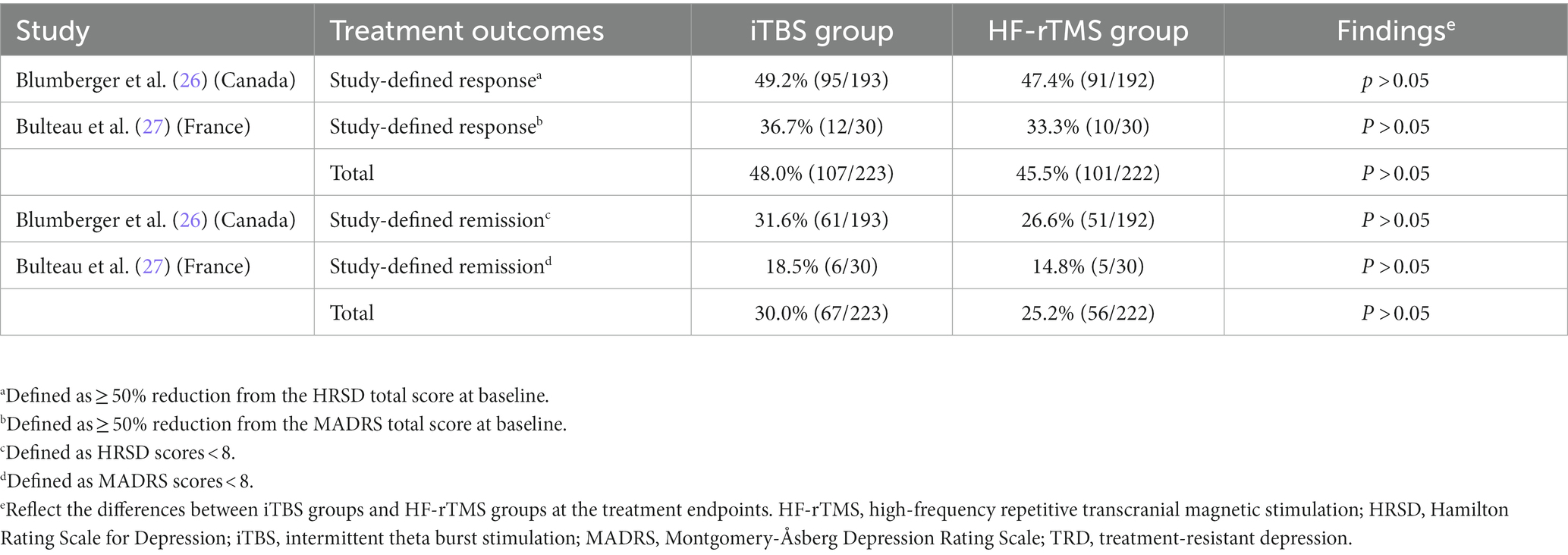
As shown in Table 2, two RCTs (26, 27) reported the rates of study-defined remission and response at the intervention endpoint. Among the two RCTs, no group differences were found regarding the overall rates of response (iTBS group: 48.0% versus HF-rTMS group: 45.5%) and remission (iTBS group: 30.0% versus HF-rTMS group: 25.2%; all Ps > 0.05).
Secondary outcomes
No group differences were found in terms of discontinuation rates (iTBS group: 7.9% versus HF-rTMS group: 6.8%) or adverse events (e.g., headache, dizziness, nausea, and fatigue) in the two included RCTs (26, 27) (all Ps > 0.05). Details are presented in Supplementary Table S1.
Discussion
To the best of our knowledge, this study is the first systematic review of RCTs to investigate the efficacy and safety of iTBS versus HF-rTMS for patients suffering from TRD. As a result, only two RCTs (26, 27) involving 474 subjects were included. The two included RCTs were published within the last 5 years, suggesting that iTBS and HF-rTMS for subjects suffering from TRD is a new and clinically important topic. The following two major findings of this systematic review included: (1) the antidepressant effects of iTBS and HF-rTMS for patients with TRD were equivalent, and (2) iTBS using 600 pulses per session for patients with TRD among adults was relatively safe and well tolerated.
As reported in this systematic review, the two included RCTs (26, 27) used a standard operation of 600 pulses of unilateral iTBS over the left DLPFC for adult patients with TRD and achieved a similar rate of antidepressant response and remission when compared to HF-rTMS. One RCT (27) examining the long-term effectiveness of iTBS versus HF-rTMS in patients with TRD found that both groups had a similar significant improvement of depressive symptoms at 6 months. Similarly, a large network meta-analysis (113 trials, 6,750 participants) found that iTBS was superior to sham stimulation and had similar antidepressant effects as conventional rTMS (including HF-rTMS, low-frequency rTMS, and bilateral rTMS) (28). Interestingly, a similar antidepressant efficacy between intensive/accelerated iTBS and HF-rTMS for the treatments of patients with TRD were reported by Fitzgerald et al.’s study (41). Taken together, these findings provide initial support for the role of iTBS as a potential treatment with greater capacity in a shorter stimulation duration for patients with TRD.
As a new form of rTMS, the high-frequency stimulation of iTBS uses 50-Hz triplet bursts that mimic endogenous theta rhythms and influence brain synaptic plasticity more quickly and with longer-lasting effects (42). Previous preclinical studies suggested that the antidepressant effects of iTBS may be related to neuroplasticity (20). Lazzaro et al. (32) found that a 3-min iTBS treatment protocol with 600 pulses per session achieves a similar effect on neural plasticity as the 37.5-min HF-rTMS treatment. Although the recommended iTBS parameters for motor cortex experiments were 600 pulses per session (20), whether it is the optimal dosing strategy for the treatment of TRD is currently unclear. A previous study suggested that increasing the total pulses per session or the number of daily sessions of rTMS may achieve larger antidepressant efficacy (43). In contrast to the standard dose of 600 pulses of iTBS, Li et al. (36) found that a 2-week prolonged iTBS (piTBS) monotherapy with 1800 pulses per session showed the same antidepressant efficacy within a shorter treatment time when compared to the conventional 4–6 week rTMS strategy. However, an exploratory study discovered that doubling the number of iTBS pulses did not enhance the excitatory effect and may have an inhibitory effect (44). Blumberger et al. (45) compared once-daily iTBS and twice-daily iTBS for patients with TRD, finding that using more than 600 iTBS pulses or administering over multiple sessions per day did not produce additional benefits. Interestingly, a recent meta-analysis (5 RCTs, 239 participants) found that active accelerated iTBS (applied 2–10 sessions of iTBS daily treatment with 24,000–90,000 total pulses) achieved a larger response rate in treating major depressive episodes when compared to sham stimulation (46). To date, the heterogeneity of iTBS stimulation parameters such as treatment pulses (600–1800 pulses per session) and stimulation sessions (1–10 sessions per day) has caused some confusion in the clinical practice. Additionally, it is worth noting that prolonging the duration of iTBS stimulation or increasing the number of treatment sessions per day in a patient will be somewhat challenging for the clinical agency. Nevertheless, there is a lack of head-to-head studies comparing the safety and antidepressant effects of iTBS (daily treatment of 600 pulses) with either piTBS or accelerated iTBS for patients with TRD. Thus, further RCTs with high quality are warranted to explore the optimum protocol of iTBS in treating MDD.
Apart from the antidepressant effects, adjunctive TBS may improve the neurocognitive function of psychiatric disorders (47, 48), which has important clinical therapeutic significance. A recent meta-analysis found that iTBS shown a positive effect in enhancing neurocognitive function in healthy adults (49). The findings were consistent with a recent systematic review investigating adjunctive iTBS for neurocognitive dysfunction in elderly patients with schizophrenia (50). However, data on the neurocognitive effects of iTBS versus HF-rTMS were not reported in the two included RCTs (26, 27).
In this systematic review, similar rates of discontinuation and adverse events were observed in the two groups, indicating high clinical acceptability and feasibility of iTBS in the treatment of patients suffering from TRD. This result was consistent with a previous review that reported that iTBS as an add-on therapy was relatively safe for psychiatric disorders and found no serious adverse events except for mild side effects (e.g., headache, dizziness, nausea, and discomfort) (48). Oberman et al. (51) conducted a study focusing on the safety of TBS for the general population and found that only a few subjects suffered from mild adverse events. Similarly, studies focused on other modalities of iTBS, such as piTBS or accelerated iTBS, which were also confirmed to be safe and well tolerated in treating patients with MDD (22, 35, 36).
Overall, the primary strength of this systematic review is that two included RCTs (Jadad score = 5) were classified as high quality. However, there were several limitations in this systematic review that should be noted. First, although a comprehensive systematic search was conducted, only a relatively small number of studies (2 RCTs) met the inclusion criteria for qualitative synthesis. Second, a meta-analysis could not be conducted due to the significant heterogeneity between each included RCT. Third, a medication effect cannot be ruled out because patients remain on their ongoing pharmacological treatment. Fourth, this systematic review only included studies that used the standard dosage of 600 pulses of iTBS for daily treatment, excluding other patterns of iTBS, such as prolonged iTBS and accelerated iTBS. Fifth, all patients in the two included RCTs suffered from treatment-resistant unipolar depression, suggesting that our findings may not be generalizable to treatment-resistant bipolar depression. Finally, this systematic review has not been registered.
Conclusion
The antidepressant efficacy and safety of iTBS and HF-rTMS appeared to be similar for patients with TRD, although further RCTs with rigorous methodology are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
X-JL, Q-ML, and Z-JQ selected studies. X-JL and Z-JQ extracted the data. D-BC reviewed all the data and helped mediate disagreements. X-JL and X-HY wrote the first draft. WZ edited the manuscript. All authors contributed to the interpretation of data and approved the last manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (82101609), Scientific Research Project of Guangzhou Bureau of Education (202032762), Guangzhou Health Science and Technology Project (20211A011045), Guangzhou Science and Technology Project of traditional Chinese Medicine and integrated traditional Chinese and Western medicine (20212A011018), China International Medical Exchange Foundation (Z-2018-35-2002), Science and Technology Program Project of Guangzhou (202102020658), the Science and Technology Program of Guangzhou (2023A03J0839 and 2023A03J0436), Science and Technology Planning Project of Liwan District of Guangzhou (202201012), The Natural Science Foundation Program of Guangdong (2023A1515011383), Guangzhou Municipal Key Discipline in Medicine (2021-2023), Guangzhou High-level Clinical Key Specialty, and Guangzhou Research-oriented Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1244289/full#supplementary-material
References
1. World Health Organization. Depression and other common mental disorders: global health estimates. Geneva: World Health Organization. (2017). Available at: https://www.who.int/key-messages#.
2. Huang, Y, Wang, Y, Wang, H, Liu, Z, Yu, X, Yan, J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24. doi: 10.1016/S2215-0366(18)30511-X
3. World Health Organization. Suicide worldwide in 2019: global health estimates. Geneva: World Health Organization. (2021). Available at: https://www.who.int/publications/i/item/9789240026643.
4. Cuijpers, P, Stringaris, A, and Wolpert, M. Treatment outcomes for depression: challenges and opportunities. Lancet Psychiatry. (2020) 7:925–7. doi: 10.1016/S2215-0366(20)30036-5
5. Rush, AJ, Kraemer, HC, Sackeim, HA, Fava, M, Trivedi, MH, Frank, E, et al. Report by the acnp task force on response and remission in major depressive disorder. Neuropsychopharmacology. (2006) 31:1841–53. doi: 10.1038/sj.npp.1301131
6. Khan, A, Faucett, J, Lichtenberg, P, Kirsch, I, and Brown, WA. A systematic review of comparative efficacy of treatments and controls for depression. PLoS One. (2012) 7:e41778. doi: 10.1371/journal.pone.0041778
7. Berlim, MT, and Turecki, G. What is the meaning of treatment resistant/refractory major depression (trd)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. (2007) 17:696–707. doi: 10.1016/j.euroneuro.2007.03.009
8. Rush, AJ, Trivedi, MH, Wisniewski, SR, Nierenberg, AA, Stewart, JW, Warden, D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a star*d report. Am J Psychiatry. (2006) 163:1905–17. doi: 10.1176/ajp.2006.163.11.1905
9. Solmi, M, Fornaro, M, Ostinelli, EG, Zangani, C, Croatto, G, Monaco, F, et al. Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry. (2020) 19:214–32. doi: 10.1002/wps.20765
10. George, MS, Lisanby, SH, Avery, D, McDonald, WM, Durkalski, V, Pavlicova, M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. (2010) 67:507–16. doi: 10.1001/archgenpsychiatry.2010.46
11. Meron, D, Hedger, N, Garner, M, and Baldwin, DS. Transcranial direct current stimulation (tdcs) in the treatment of depression: systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev. (2015) 57:46–62. doi: 10.1016/j.neubiorev.2015.07.012
12. Zheng, W, Cai, D-B, Nie, S, Chen, J-H, Huang, X-B, Goerigk, S, et al. Adjunctive transcranial alternating current stimulation for patients with major depressive disorder: a systematic review and meta-analysis. Front Psych. (2023) 14:1154354. doi: 10.3389/fpsyt.2023.1154354
13. Holtzheimer, PE, McDonald, WM, Mufti, M, Kelley, ME, Quinn, S, Corso, G, et al. Accelerated repetitive transcranial magnetic stimulation for treatment-resistant depression. Depress Anxiety. (2010) 27:960–3. doi: 10.1002/da.20731
14. O'Reardon, JP, Solvason, HB, Janicak, PG, Sampson, S, Isenberg, KE, Nahas, Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. (2007) 62:1208–16. doi: 10.1016/j.biopsych.2007.01.018
15. Brunoni, AR, Chaimani, A, Moffa, AH, Razza, LB, Gattaz, WF, Daskalakis, ZJ, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiat. (2017) 74:143–52. doi: 10.1001/jamapsychiatry.2016.3644
16. Berlim, MT, van den Eynde, F, Tovar-Perdomo, S, and Daskalakis, ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rtms) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. (2014) 44:225–39. doi: 10.1017/S0033291713000512
17. Hsu, JH, Downar, J, Vila-Rodriguez, F, Daskalakis, ZJ, and Blumberger, DM. Impact of prior treatment on remission with intermittent theta burst versus high-frequency repetitive transcranial magnetic stimulation in treatment resistant depression. Brain Stimul. (2019) 12:1553–5. doi: 10.1016/j.brs.2019.07.011
18. Cheng, C-M, Li, C-T, and Tsai, S-J. Current updates on newer forms of transcranial magnetic stimulation in major depression. Adv Exp Med Biol. (2021) 1305:333–49. doi: 10.1007/978-981-33-6044-0_18
19. Frey, J, Najib, U, Lilly, C, and Adcock, A. Novel tms for stroke and depression (notsad): accelerated repetitive transcranial magnetic stimulation as a safe and effective treatment for post-stroke depression. Front Neurol. (2020) 11:788. doi: 10.3389/fneur.2020.00788
20. Huang, Y-Z, Edwards, MJ, Rounis, E, Bhatia, KP, and Rothwell, JC. Theta burst stimulation of the human motor cortex. Neuron. (2005) 45:201–6. doi: 10.1016/j.neuron.2004.12.033
21. Caulfield, KA. Is accelerated, high-dose theta burst stimulation a panacea for treatment-resistant depression? J Neurophysiol. (2020) 123:1–3. doi: 10.1152/jn.00537.2019
22. Li, C-T, Chen, M-H, Juan, C-H, Huang, H-H, Chen, L-F, Hsieh, J-C, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain: a. J Neurol. (2014) 137:2088–98. doi: 10.1093/brain/awu109
23. Cheng, C-M, Juan, C-H, Chen, M-H, Chang, C-F, Lu, HJ, Su, T-P, et al. Different forms of prefrontal theta burst stimulation for executive function of medication-resistant depression: evidence from a randomized sham-controlled study. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 66:35–40. doi: 10.1016/j.pnpbp.2015.11.009
24. Chistyakov, AV, Rubicsek, O, Kaplan, B, Zaaroor, M, and Klein, E. Safety, tolerability and preliminary evidence for antidepressant efficacy of theta-burst transcranial magnetic stimulation in patients with major depression. Int J Neuropsychopharmacol. (2010) 13:387–93. doi: 10.1017/S1461145710000027
25. Bakker, N, Shahab, S, Giacobbe, P, Blumberger, DM, Daskalakis, ZJ, Kennedy, SH, et al. RTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. (2015) 8:208–15. doi: 10.1016/j.brs.2014.11.002
26. Blumberger, DM, Vila-Rodriguez, F, Thorpe, KE, Feffer, K, Noda, Y, Giacobbe, P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (three-d): a randomised non-inferiority trial. Lancet (London, England). (2018) 391:1683–92. doi: 10.1016/s0140-6736(18)30295-2
27. Bulteau, S, Laurin, A, Pere, M, Fayet, G, Thomas-Ollivier, V, Deschamps, T, et al. Intermittent theta burst stimulation (itbs) versus 10 Hz high-frequency repetitive transcranial magnetic stimulation (rtms) to alleviate treatment-resistant unipolar depression: a randomized controlled trial (theta-dep). Brain Stimul. (2022) 15:870–80. doi: 10.1016/j.brs.2022.05.011
28. Mutz, J, Vipulananthan, V, Carter, B, Hurlemann, R, Fu, CHY, and Young, AH. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. (2019) 364:l1079. doi: 10.1136/bmj.l1079
29. Blumberger, DM, Mulsant, BH, Thorpe, KE, McClintock, SM, Konstantinou, GN, Lee, HH, et al. Effectiveness of standard sequential bilateral repetitive transcranial magnetic stimulation vs bilateral theta burst stimulation in older adults with depression: the four-d randomized noninferiority clinical trial. JAMA Psychiat. (2022) 79:1065–73. doi: 10.1001/jamapsychiatry.2022.2862
30. Voigt, JD, Leuchter, AF, and Carpenter, LL. Theta burst stimulation for the acute treatment of major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. (2021) 11:330. doi: 10.1038/s41398-021-01441-4
31. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
32. Di Lazzaro, V, Dileone, M, Pilato, F, Capone, F, Musumeci, G, Ranieri, F, et al. Modulation of motor cortex neuronal networks by rtms: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. (2011) 105:2150–6. doi: 10.1152/jn.00781.2010
33. Kaster, TS, Downar, J, Vila-Rodriguez, F, Thorpe, KE, Feffer, K, Noda, Y, et al. Trajectories of response to dorsolateral prefrontal rtms in major depression: a three-d study. Am J Psychiatry. (2019) 176:367–75. doi: 10.1176/appi.ajp.2018.18091096
34. Si, Y, Wu, X, Li, F, Zhang, L, Duan, K, Li, P, et al. Different decision-making responses occupy different brain networks for information processing: a study based on eeg and tms. Cereb Cortex. (2019) 29:4119–29. doi: 10.1093/cercor/bhy294
35. Cole, EJ, Phillips, AL, Bentzley, BS, Stimpson, KH, Nejad, R, Barmak, F, et al. Stanford neuromodulation therapy (snt): a double-blind randomized controlled trial. Am J Psychiatry. (2022) 179:132–41. doi: 10.1176/appi.ajp.2021.20101429
36. Li, C-T, Cheng, C-M, Chen, M-H, Juan, C-H, Tu, P-C, Bai, Y-M, et al. Antidepressant efficacy of prolonged intermittent theta burst stimulation monotherapy for recurrent depression and comparison of methods for coil positioning: a randomized, double-blind, sham-controlled study. Biol Psychiatry. (2020) 87:443–50. doi: 10.1016/j.biopsych.2019.07.031
37. Jadad, AR, Moore, RA, Carroll, D, Jenkinson, C, Reynolds, DJ, Gavaghan, DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
38. Higgins, JPT, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
39. Linde, K, Clausius, N, Ramirez, G, Melchart, D, Eitel, F, Hedges, LV, et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet (London, England). (1997) 350:834–43.
40. Balshem, H, Helfand, M, Schünemann, HJ, Oxman, AD, Kunz, R, Brozek, J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
41. Fitzgerald, PB, Chen, L, Richardson, K, Daskalakis, ZJ, and Hoy, KE. A pilot investigation of an intensive theta burst stimulation protocol for patients with treatment resistant depression. Brain Stimul. (2020) 13:137–44. doi: 10.1016/j.brs.2019.08.013
42. Suppa, A, Huang, YZ, Funke, K, Ridding, MC, Cheeran, B, Di Lazzaro, V, et al. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul. (2016) 9:323–35. doi: 10.1016/j.brs.2016.01.006
43. Teng, S, Guo, Z, Peng, H, Xing, G, Chen, H, He, B, et al. High-frequency repetitive transcranial magnetic stimulation over the left dlpfc for major depression: session-dependent efficacy: a meta-analysis. Eur Psychiatry. (2017) 41:75–84. doi: 10.1016/j.eurpsy.2016.11.002
44. Gamboa, OL, Antal, A, Moliadze, V, and Paulus, W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. (2010) 204:181–7. doi: 10.1007/s00221-010-2293-4
45. Blumberger, DM, Vila-Rodriguez, F, Wang, W, Knyahnytska, Y, Butterfield, M, Noda, Y, et al. A randomized sham controlled comparison of once vs twice-daily intermittent theta burst stimulation in depression: a Canadian rTMS treatment and biomarker network in depression (cartbind) study. Brain Stimul. (2021) 14:1447–55. doi: 10.1016/j.brs.2021.09.003
46. Cai, D-B, Qin, Z-J, Lan, X-J, Liu, Q-M, Qin, X-D, Wang, J-J, et al. Accelerated intermittent theta burst stimulation for major depressive disorder or bipolar depression: a systematic review and meta-analysis. Asian J Psychiatr. (2023) 85:103618. doi: 10.1016/j.ajp.2023.103618
47. Zheng, LN, Guo, Q, Li, H, Li, CB, and Wang, JJ. Effects of repetitive transcranial magnetic stimulation with different paradigms on the cognitive function and psychotic symptoms of schizophrenia patients. Beijing Da Xue Xue Bao. (2012) 44:732–6. doi: 10.3969/j.issn.1671-167X.2012.05.013
48. Rachid, F. Safety and efficacy of theta-burst stimulation in the treatment of psychiatric disorders: a review of the literature. J Nerv Ment Dis. (2017) 205:823–39. doi: 10.1097/NMD.0000000000000742
49. Pabst, A, Proksch, S, Médé, B, Comstock, DC, Ross, JM, and Balasubramaniam, R. A systematic review and meta-analysis of the efficacy of intermittent theta burst stimulation (itbs) on cognitive enhancement. Neurosci Biobehav Rev. (2022) 135:104587. doi: 10.1016/j.neubiorev.2022.104587
50. Zhang, X, Yang, X, Shi, Z, Xu, R, Tan, J, Yang, J, et al. A systematic review of intermittent theta burst stimulation for neurocognitive dysfunction in older adults with schizophrenia. J Pers Med. (2023) 13:485. doi: 10.3390/jpm13030485
Keywords: intermittent theta burst stimulation, high-frequency rTMS, treatment-resistant depression, systematic review, response
Citation: Lan X-J, Yang X-H, Qin Z-J, Cai D-B, Liu Q-M, Mai J-X, Deng C-j, Huang X-B and Zheng W (2023) Efficacy and safety of intermittent theta burst stimulation versus high-frequency repetitive transcranial magnetic stimulation for patients with treatment-resistant depression: a systematic review. Front. Psychiatry. 14:1244289. doi: 10.3389/fpsyt.2023.1244289
Edited by:
Xiaochu Zhang, University of Science and Technology of China, ChinaReviewed by:
Shi-Bin Wang, Guangdong Mental Health Center, ChinaShen Li, Tianjin Medical University, China
Wen-Wang Rao, McGill University, Canada
Copyright © 2023 Lan, Yang, Qin, Cai, Liu, Mai, Deng, Huang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zheng, emhlbmd3ZWkwNzAyQDE2My5jb20=
†These authors have contributed equally to this work
 Xian-Jun Lan
Xian-Jun Lan Xin-Hu Yang
Xin-Hu Yang Zhen-Juan Qin1†
Zhen-Juan Qin1† Dong-Bin Cai
Dong-Bin Cai Can-jin Deng
Can-jin Deng Xing-Bing Huang
Xing-Bing Huang Wei Zheng
Wei Zheng