
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 20 November 2023
Sec. ADHD
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1236636
This article is part of the Research Topic Chronic insomnia: Treatment and management View all 14 articles
 Feilong Zhu1
Feilong Zhu1 Boya Liu2
Boya Liu2 Dongqing Kuang1
Dongqing Kuang1 Xiaotong Zhu1
Xiaotong Zhu1 Xiaoyu Bi1
Xiaoyu Bi1 Yiqi Song1
Yiqi Song1 Tianshen Quan1
Tianshen Quan1 Yiming Yang1
Yiming Yang1 Yuanchun Ren1*
Yuanchun Ren1*Background: Adults with attention-deficit/hyperactivity disorder (ADHD) may experience sleep problems doubly suffering from the disease and side effects of stimulant medications. Physical activity (PA) is known to produce numerous beneficial effects in adults. However, it was not well-characterized whether PA would still be effective in this situation. The main objective of the current study was to examine the relationship between PA and sleep among adult ADHD patients who were using stimulant medications and quantify the form of this association.
Methods: Adult ADHD participants with stimulant medications use condition from the National Health and Nutrition Examination Survey (NHANES) database between January 1, 2013, and March 2020 (prepandemic) were included in the cross-sectional analysis. Weighted logistic regression was performed to assess the relationship between PA level and sleep. A restricted cubic spline model was used to relax the linear relationship assumptions and investigate the associations between the risk of trouble sleeping and time spent engaging in moderate-to-vigorous PA per week.
Results: A total of 162 eligible adult ADHD participants who reported using stimulant medicines were included. Participants who adhered to the general recommendation of guidelines in the US of 150 min per week of moderate-to-vigorous PA had a significant lower risk of complaining of trouble sleeping (OR: 0.26, 95% CI: 0.10–0.67, p = 0.006), and this association was seen in men (OR: 0.23, 95% CI: 0.09–0.56, p = 0.002), but was not seen in women (OR: 0.71, 95% CI: 0.27–1.88, p = 0.500). Restricted cubic spline analysis showed that the incidence of trouble sleeping gradually decreased after at least 105 min of moderate-intensity PA per week in participants (OR: 1.02, 95% CI: 0.92–1.14). A significant difference appeared after 341 min (OR: 0.87, 95% CI: 0.76–0.99), and the curve leveled after 1,250 min (OR: 0.60, 95% CI: 0.46–0.79).
Conclusion: Our findings observed associations between PA and sleep condition in the adult ADHD patients with stimulant medication use population. Moderate-to-vigorous PA may be beneficial to sleep in adults with ADHD who were using stimulants and thus should be recommended as part of a healthy lifestyle. Gender difference should be considered as an important factor for further studies to examine these associations and explore potential mechanisms.
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental psychiatric disorder in childhood that is characterized by inattention, hyperactivity, and/or impulsivity, which cause functional impairment and lead to difficulties in academic achievement and daily life (1). The prevalence of ADHD in children and adolescents is estimated to be 5.29 to 7.2% around the world, and this number is above 10% in China (2). Furthermore, ADHD is not confined to childhood or adolescence, with nearly 60% of children with ADHD experiencing symptoms into adulthood, causing disruptions at work and in personal relationships (3). The prevalence of ADHD in adults has been shown to be 2.5% in a systematic review of epidemiological studies (4) and 2.8% in the most current World Health Organization (WHO) Mental Health Survey across 10 countries (5). It is worth noting that the incidence of adult ADHD is likely to be underestimated because ADHD is often considered a childhood disorder that improves with age, and the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria is insensitive to the adult population (4, 5). Therefore, adult ADHD should attract much attention.
Executive function impairments are a defining trait among individuals with ADHD and are a current focus of academic research. Additionally, ADHD has been associated with sleep problems, similar to other neurodevelopmental disorders. It is estimated that up to 85% of adults with ADHD have sleep disturbances (6). These disturbances can manifest as long sleep latency, difficulty falling and staying asleep, altered total sleep duration, sleep phase delay syndrome, increased periodic limb movements when sleeping, and drowsiness during the day (7, 8). Fuller-Thomson et al. reported a substantial link between ADHD and insomnia, with those who self-reported having ADHD being five times more likely to suffer from insomnia (OR = 5.18) than those without ADHD (9). Previous studies have reported that sleep deprivation exerts multiple effects on neurobehavioral and cognitive systems, including attention and emotional regulation. As a result, sleep disturbances in ADHD may affect the core psychopathology of the condition. In recent times, there has been an increasing number of adults diagnosed with ADHD and receiving prescribed stimulant medications treatment (10, 11). Methylphenidate, a central nervous system stimulant, has been tested extensively in clinical trials on adults with ADHD, but the overall benefits and risks remain unclear due to concerns about how these trials were designed and how the results were reported. Many previous research results revealed that stimulant medications have been shown to provide marked benefits in symptoms of ADHD, but cause disruptions in circadian rhythms and sleep that may negatively affect mood regulation (12–14). Some researchers have also noted that contrary to sedatives and hypnotics, which reduce brain activity and increase sleepiness, stimulants affect the body and central nervous system, inducing heightened alertness, difficulties falling asleep and delay of circadian rhythmicity (13, 14). In general, adult ADHD patients who take stimulant medicines are particularly vulnerable because they are frequently ignored and also may experience sleep problems doubly suffering from the disease and stimulant medications.
Physical activity (PA) has been shown to improve sleep quality for many people. Specifically, moderate-to-vigorous exercise can improve the quality of sleep for adults by speeding up the process of falling asleep and decreasing the amount of time spent awake in bed at night. Moreover, engaging in PA can alleviate daytime sleepiness and reduce the need for sleep medications for some people. The mechanisms by which PA improves sleep include mild body warming after exercise, improvement of vagus nerve function (the vagus nerve mainly plays an inhibitory role in promoting sleep), regulation of cortisol and other endocrine hormone fluctuations, and mood enhancement resulting in relaxation (15). The PA guidelines from the US and WHO recommend that adults engage in moderate-intensity PA for at least 150 min/week or vigorous PA for ≥75 min/week (16). Despite the common perception that people with ADHD are sufficiently active or even hyperactive, they may not engage in optimal movement behaviors for health (17).
Collectively, preliminary findings supported that adults with ADHD may experience sleep disturbs suffering from both the disease and stimulant medications. However, it was not well-characterized whether PA would still be effective in this situation. It remained doubtful that what is the relationship between PA and sleep in the adult ADHD patients with stimulant medication use group. To our knowledge, no public studies have examined the impact of PA on sleep in adult ADHD stimulant users. Therefore, we believe this is an important clinical question worthy of further study for this ‘special group’. In this study, by using a general sample from the National Health and Nutrition Examination Survey (NHANES) in the US, we sought to examine the association between PA and sleep in the adult ADHD patients with stimulant medication use group and quantify the form of this association.
The current study’s data came from the National Health and Nutrition Examination Survey (NHANES), which was a nationally representative complex, stratified, and multistage probability sample of adults and children in the United States. The survey is distinctive in that it combines interviews, physical examinations, and laboratory testing conducted in participants’ homes and in a traveling mobile examination center (MEC). NHANES conducts laboratory tests such as blood tests, urine tests, nutritional assessments, and evaluations for environmental exposures. NHANES data are freely accessible on the website1 for researchers worldwide. The National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board approved the protocol for the original NHANES data collection, and informed consent was acquired from all individual participants.2
The sample with adults over 20 years old included in three cycles (2013–2014, 2015–2016, and 2017-March 2020 prepandemic) of NHANES were chosen for data analysis because they included information about demographics, anthropometrics, prescription medicine use, sleep, physical activity and other covariates used in this study.
Data on medication use were collected through a medication questionnaire conducted by trained interviewers using the Computer-Assisted Personal Interview (CAPI) system. Participants were asked whether they had taken any prescription medications during the month preceding the interview date. Specific questions included “Have you used or taken medication that requires a prescription in the last 30 days? Do not add any prescription vitamins or minerals that you may have already mentioned to me.” In addition, participants were asked to provide the generic name of the drug, how long they had been taking it, their main reason for taking it, and any ICD-10-CM (International Classification of Diseases, Tenth Revision, Clinical Modification) codes related to their reported reasons for use. Data were periodically examined for discrepancies and incorrect entries for quality assurance and control. The interviewer’s list of drugs was compared to the medication names selected from the drug database. Stimulants in this study were defined as methylphenidate, amphetamine, dexmethylphenidate, dextroamphetamine, lisdexamfetamine dimesylate, and levoamphetamine. Based on stimulant medication use and ICD-10-CM codes, participants with ADHD using stimulant medications were identified.
The sleep parameters were derived from the “sleep disorders” dataset comprising questions on sleep habits and disorders in the “NHANES Questionnaire.” Sleep duration was recorded by answering the question “The number of hours that you typically sleep on weekdays or workdays,” and hours were rounded to the closest half-hour (18). Sleep duration was categorized as short (<7 h per night), normal (7–9 h per night) and long (>9 h per night) (19, 20). The answer to the question “Have you ever mentioned your difficulty sleeping to a doctor or other health care provider?” was used to assess trouble sleeping. As shown in Table 1, healthy sleep scores and patterns were established using sleep characteristics such as sleep duration, trouble sleeping, snoring, snorting or stopping breathing, and feeling excessively sleepy during the day. Sleep total scores of 0–1, 2–3, and 4–5 indicated poor, intermediate, or healthy sleep patterns, respectively (21).
The Global Physical Activity Questionnaire (GPAQ) was used to evaluate physical activity. The questionnaire consisted of 6 questions pertaining to leisure time physical activity, which were administered as part of the home interview. Participants were asked if they regularly participated in PA by the following questions: (1) “In a typical week, do you do any vigorous-intensity sports, fitness, or recreational activities that cause large increases in breathing or heart rate, such as running or basketball for at least 10 min continuously?” and (2) “Do you engage in any moderate-intensity sports, fitness, or recreational activities such as brisk walking, bicycling, swimming, or volleyball for at least 10 min continuously during a typical week?” Those who responded “yes” to either question were subsequently queried regarding the number of days and amount of time spent in each activity intensity during a typical week. If a participant completed 150 min per week of moderate-intensity physical activity (MPA), 75 min per week of vigorous-intensity physical activity (VPA), or a combination of MPA and VPA that amounted to 150 min per week (with 1 min of VPA weighted as double that of MPA), they were deemed to have met the physical activity guideline and defined as the active group. On the contrary, participants were defined as the inactive group. This guideline was based on US national physical activity recommendations (22, 23).
The study’s demographic covariates of interest included age (20–29 years, 30–44 years, 45–59 years, and ≥ 60 years), sex (female and male), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other), education level (high school or below and above high school), marital status (married/living with partner, widowed/divorced/separated, and never married), and income. Income was measured using the poverty-income ratio (PIR), which is the ratio of household income to the appropriate poverty threshold for household size (24). PIR was categorized as <2 (poor or near poor) and ≥ 2 (middle to high income) (25). Body mass index (BMI) was collected by physical examinations in the Mobile Examination Center, calculated as weight in kilograms divided by height in meters squared (kg/m2), and classified into <25, 25–30, and > 30 kg/m2 categories. Smoking status was determined by the question “Have you ever smoked more than 100 cigarettes in your life?” and classified as ‘yes’ or ‘no’. Daily alcohol intake was assessed from the total nutritional intake (DR1TOT) consumed for the 24 h before the interview, which was then separated into two categories: 0 and > 0 gm. Diabetes and hypertension were identified based on self-reported and medical diagnoses. The Patient Health Questionnaire-9 (PHQ-9) was used to assess the frequency of depressive symptoms over the previous 2 weeks in the NHANES. It is a nine-item depression screening tool with acceptable reliability and validity (26). Response categories for each item were scored on a 0–3 scale (0 = “not at all,” 1 = “several days,” 2 = “more than half the days,” and 3 = “nearly every day”), and the total PHQ-9 score ranged from 0 to 27, with higher PHQ-9 scores indicating a higher probability of severe depression. A total score of ≥10, according to the DSM-IV, was considered clinically relevant depression (26).
Participants were divided into two groups as active and inactive groups. Continuous variables, such as age and BMI, were translated into categorical variables. Baseline data were analyzed using the chi-square test or Fisher’s exact test and expressed as the number (percentage). The odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated using a weighted logistic regression model with adjustment for covariates among baseline characteristics and stratified by gender to examine the associations between physical activity level and sleep in adult ADHD participants with stimulant use. We used a restricted cubic spline model to relax the linear relationship assumptions and investigate the associations between risk of trouble sleeping and time of moderate to vigorous PA (with 1 min of VPA weighted as double that of MPA) per week. The reference category for this analysis was without moderate to vigorous PA every week. R software, version 4.2.1 (The R Foundation for Statistical Computing), was used to execute every statistical analysis. p values less than 0.05 (p < 0.05) indicated statistically significant differences.
A total of 162 eligible ADHD participants who reported stimulant use (53.1% males and 46.9% females, mean (SD) age 39.1 [14.4] years) were included in this analysis. The characteristics of the participants according to physical activity level were presented in Table 2. There were no significant differences in sex, race, household income, smoking, diabetes, depressive symptoms and duration of stimulant use between two groups in the analytic sample. However, age distribution of the active group differed from inactive group, with the active group being younger. Compared to inactive group, the active group were more likely to be highly educated, never married, low obesity rate, alcohol intake, and had a lower prevalence of hypertension. Furthermore, there were significant differences in prevalence of trouble sleeping (p = 0.004) between two groups and no significant differences were found in sleep duration and sleep patterns.
Of the adult ADHD patients with stimulant use, 61 (37.7%) participants met the general recommendation of physical activity guidelines in the US. Patients who met the recommended 150 min/week of moderate to vigorous physical activity had a significantly lower risk of complaining of trouble sleeping (OR: 0.26, 95% CI: 0.10–0.67, p = 0.006). However, no significant differences were observed in sleep duration (OR: 0.61, 95% CI: 0.24–1.52, p = 0.295) or sleep patterns (OR: 0.73, 95% CI: 0.12–4.74, p = 0.727). Additionally, men who met physical activity guidelines reported lower risk of complaining of trouble sleeping (OR: 0.23, 95% CI: 0.09–0.56, p = 0.002) than men did not meet guidelines. However, no significant difference was observed between women (OR: 0.71, 95% CI: 0.27–1.88, p = 0.500). The results were presented in Table 3. In Figure 1, we used restricted cubic splines to flexibly model and visualize the relation of predicting the risk of complaining of trouble sleeping and time of moderate to vigorous PA per week in adults with ADHD who were receiving stimulant medicines. The plot showed a slight increase in the risk below 105 min/week (OR: 1.02, 95% CI: 0.92–1.14) that was not statistically significant and then started to decrease rapidly afterward (P for nonlinearity =0.025), with a substantial reduction in the risk approximately 341 min (OR: 0.87, 95% CI: 0.76–0.99) until approximately 1,250 min/week (OR: 0.60, 95% CI: 0.46–0.79), beyond which the curve leveled.
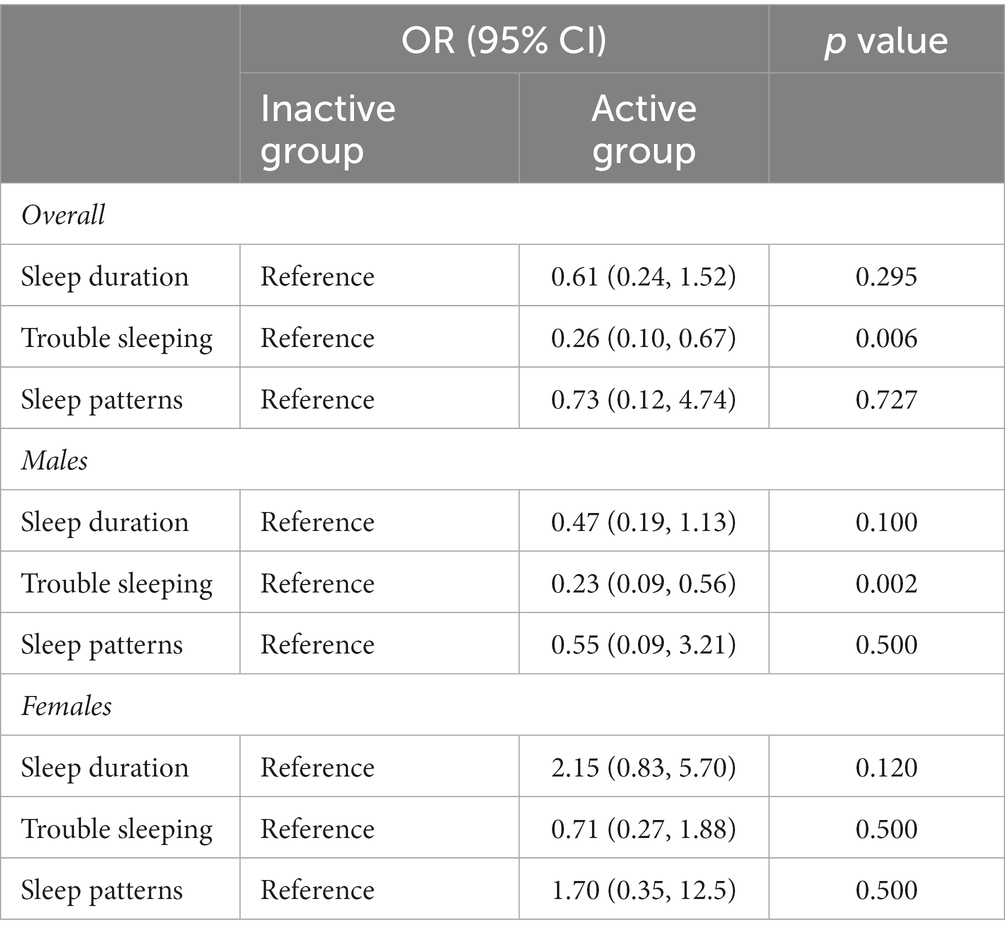
Table 3. Associations between physical activity level and sleep adjusting for covariates in adult ADHD patients with stimulant use.
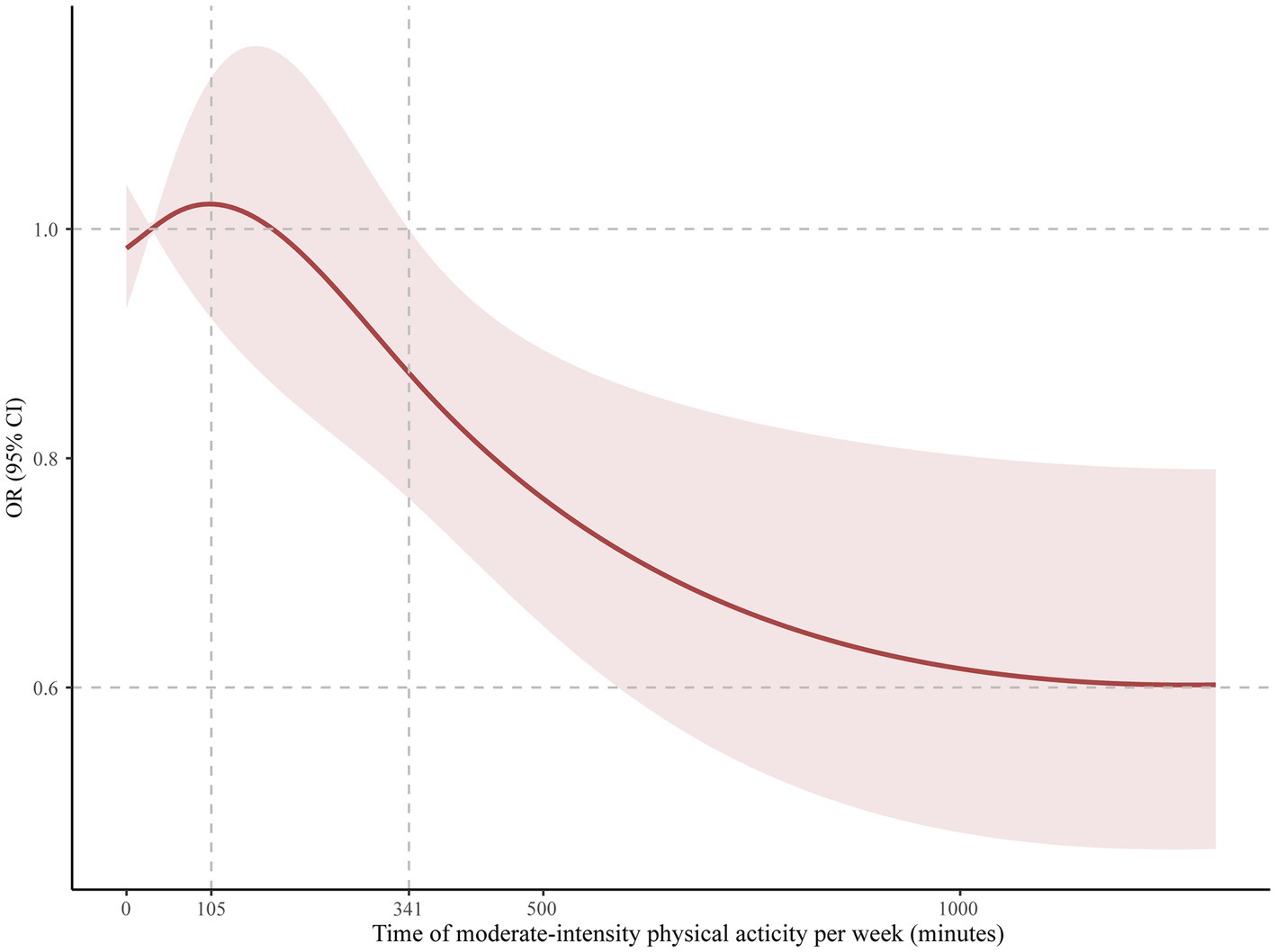
Figure 1. Odds ratio and moderate-intensity physical activity time per week for trouble sleeping in adult ADHD patients on stimulants. The reference group was 0 min/week.
The present study presented a national sample of adults with ADHD who were using stimulant medicines to evaluate the relationship between physical activity and sleep. The motivation for this study was our previous work on this topic among children, but to date, this specific question has not been evaluated extensively in the adult population. Adults with ADHD using stimulant medicines are an especially vulnerable group that are frequently ignored and suffer from both ADHD and stimulants. It was not well-characterized whether physical activity would still be effective in this situation. The results of this study showed that adult ADHD patients on stimulant medications who met the general recommendation of physical activity guidelines (150 min/week of moderate to vigorous physical activity) in the US had a significantly lower risk of complaining of trouble sleeping. Additionally, a nonlinear dose–response relationship was found between physical activity and trouble sleeping, and a significant reduction in the risk of trouble sleeping was seen within the lower range until 341 min.
Adults frequently experience sleep issues, and the prevalence of insomnia disorder in adults is approximately 10–20%, with approximately 50% having a chronic course (27). The relationship between sleep disorders and ADHD is complex, with sleep disorders being both co-occurring conditions and consequences of ADHD. Despite the use of medication and comorbidities, there is abundant evidence that adults with ADHD are more prone to sleep problems, indicating that sleep deficits are intrinsic to ADHD. Adult ADHD is associated with longer objectively measured sleep onset latency, disrupted sleep maintenance, and later waking times. Adult ADHD patients with sleep onset problems may complain of daytime tiredness, which is related to a larger incidence of inattention symptoms (14, 28). Although the nature of the connection between ADHD and sleep problems is not fully known, inattention and impulsivity can be caused by sleep disturbances in the general population, suggesting that disturbed sleep may exacerbate ADHD symptoms (29). Conversely, extending sleep benefits to patients with ADHD in terms of attentional and behavioral functioning, emphasizing the importance of sleep as a target for intervention in this population (30), which should be given due weight. Clinically, psychostimulant drugs are commonly used for treating the core symptoms of ADHD by boosting dopamine and norepinephrine neurotransmitters in the brain. Numerous randomized controlled trials and meta-analyses have thoroughly demonstrated the effectiveness of these drugs (31–33). The therapeutic effects of stimulants are most likely related to the enhancement of central nervous system activity, particularly in brain regions that are critical for higher-order cognitive activities, such as the prefrontal cortex. However, many studies point out that stimulants do not improve and may even aggravate sleep problems in adults with ADHD (13, 14). This could be ascribed to the side effects of stimulants, which depend on the specific drug used, dosages, and individual characteristics of the patient. It is critical to realize that stimulant drugs should be used with other management strategies, such as habit modification or anxiety reduction, to counteract the negative effects on sleep.
According to the most recent research, which was published in 2023, adhering to the 24-h movement behavior guidelines, which call for getting enough sleep, doing at least 60 min of moderate-to-vigorous physical activity each day, and limiting recreational screen time to no more than 2 h per day was linked to a lower risk of cognitive and social difficulties in children and adolescents with ADHD (34). This study highlights the importance of physical activity, which appears to have a positive impact on social and neurocognitive function in children and adults. Clinical trials of children with ADHD and preclinical studies on spontaneously hypertensive rats, an animal model of ADHD, have shown that aerobic exercise can be helpful as an adjunct treatment to medication for ADHD. Some studies have also indicated that physical activity can be a helpful tool in treating sleep-related problems in children with ADHD (35), and aerobic exercise may act on catecholamine pathways as a stimulant medication (36). Physical activity can effectively shorten the time it takes to fall asleep, increase total sleep time, and improve sleep quality for many people. Nevertheless, no controlled clinical trials in adults with ADHD have been conducted on the efficacy of physical activity for improving sleep in adults with ADHD; we performed a cross-sectional analysis to demonstrate the efficacy of PA for improving sleep among stimulant users of ADHD. Our results suggest that greater amounts of weekly moderate-to-vigorous activity result in a lower risk of trouble sleeping, indicating that maintaining a certain amount of PA is a protective factor to sleep for adult ADHD with stimulant medication use. This highlights the importance of physical activity in adhering to healthy lifestyle behaviors. Activity duration is a significant parameter in exercise intervention trials. In this study, it was proven that the accumulation of moderate-to-vigorous physical activity within a week for a certain period of time had a positive effect on sleep, as recommended by the guidelines, but we found that a large number of people spent much more than 150 min among the included adult ADHD participants, and the significant effect could be attributed to this.
It was worth mentioning that there was a significant difference in men, but was not significant in women. Possible explanations for this discrepancy were likely physiological differences and hormonal levels (37, 38). There are physiological differences between men and women, including differences in sleep structure and regulatory mechanisms. These differences may contribute to a greater positive response to physical activity in men, while women may exhibit a lesser response to physical activity. In addition, women experience hormonal fluctuations during different physiological cycles, such as the menstrual cycle and menopause. These hormonal changes may influence women’ response to physical activity and the effectiveness of improving their sleep quality. In a detailed dose–response plot, we found that it takes at least 341 min of moderate-to-vigorous physical activity per week to have a significant positive impact on sleep. The results may be used by researchers and clinicians to develop intervention models for individuals with ADHD that focus on the mediating functions of lifestyle factors to enhance sleep quality (39). Moreover, a dose–response plot portrayed an initial surge in the risk of trouble sleeping (0–105 min of physical activity), and it would be beneficial for elaborating on it. When individuals engage in limited or irregular physical activity, their bodies may require adaptation to the new physical demands (40). This adaptive process involves physiological adjustments and repairs, which can momentarily disrupt sleep quality (40). However, this phenomenon is usually temporary, and sleep quality typically improves once the body successfully adapts and adjusts to the new physical activity load. As such, it is crucial to establish a consistent physical activity regimen and allocate adequate time for the body to acclimate to the novel physical demands. However, these findings require confirmation through longitudinal and interventional studies with large sample sizes because there are few controlled clinical trials on sleep problems in people with ADHD. Additionally, more clinical study is also needed on the clinical impact of exercise on sleep problems in adults with ADHD to explore potential shared neurobiological mechanisms.
Our study is significant because it provides clear evidence of an association between sleep and physical activity in adults with ADHD on stimulant medication population. Nevertheless, we must acknowledge several shortcomings and limitations in our study. Firstly, although this article represented one of the first attempts to investigate the role of physical activity with the associations of sleep among adults with ADHD on stimulant medications population, we cannot rule out that some stimulant medications may have positive effects on sleep as various forms of stimulants were used in the current study. Moreover, it was a cross-sectional study and we cannot prove causality or determine whether our findings were due to direct effects of physical activity on sleep. In order to expand on the findings, future studies need to consider these. Secondly, research on sleep in adults with ADHD has recently focused on the role of circadian rhythm and circadian preference (chronotype), and this study did not provide that relevant information, and these outcomes deserve further exploration through sleep monitoring devices in clinical trials. Thirdly, data on sleep and physical activity were subjectively self-reported, which could be improved in future studies by using objective measures such as polysomnography and actigraphy.
Our findings from the NHANES revealed associations and a dose-effect relationship between physical activity and sleep condition in the adult ADHD patients who were using stimulant medications, and strategies to optimize sleep should be recommended for adults with ADHD using stimulants, including increasing physical activity as a healthy lifestyle choice. Gender difference should be considered as an important factor for further studies to examine these associations and explore potential mechanisms. It is important to confirm our findings through additional longitudinal and interventional studies to explore the effect of various exercises on sleep quality in populations with adult ADHD who are taking stimulants.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The study analyzed data downloaded from the National Health and Nutrition Examination Survey public database. The National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board approved the protocol for the original NHANES data collection, and informed consent was acquired from all individual participants (https://www.cdc.gov/nchs/nhanes/irba98.htm).
FZ and YR conceived and designed the study. FZ and DK collected the data and performed the analysis. XZ, BL, YS, TQ, and YY assisted with the investigation. FZ and BL wrote and revised the manuscript. All the authors edited and approved the manuscript.
This research was funded by the Ministry of Education of Humanities and Social Science Foundation (21YJA890025) and Interdisciplinary Research Foundation for Doctoral Candidates of Beijing Normal University (BNUXKJC2212). The funding bodies played no role in the design of the study; collection, analysis, and interpretation of data; or in the writing of the manuscript.
The authors particularly acknowledge Dr. Zhengye Pan for helping with the statistical analysis and AJE for English language editing, including grammar, punctuation, and phrasing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Vol. 5. Washington, DC: American Psychiatric Association (2013).
2. Posner, J, Polanczyk, GV, and Sonuga-Barke, E. Attention-deficit hyperactivity disorder. Lancet. (2020) 395:450–62. doi: 10.1016/S0140-6736(19)33004-1
3. Zhang, H, Yang, B, Peng, G, Zhang, L, and Fang, D. Effects of the DRD4 -521 C/T SNP on local neural activity and functional connectivity in children with ADHD. Front Psych. (2021) 12:785464. doi: 10.3389/fpsyt.2021.785464
4. Magnin, E, and Maurs, C. Attention-deficit/hyperactivity disorder during adulthood. Rev Neurol (Paris). (2017) 173:506–15. doi: 10.1016/j.neurol.2017.07.008
5. Simon, V, Czobor, P, Balint, S, Meszaros, A, and Bitter, I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. (2009) 194:204–11. doi: 10.1192/bjp.bp.107.048827
6. Fadeuilhe, C, Daigre, C, Richarte, V, Grau-Lopez, L, Palma-Alvarez, RF, Corrales, M, et al. Insomnia disorder in adult attention-deficit/hyperactivity disorder patients: clinical, comorbidity, and treatment correlates. Front Psych. (2021) 12:663889. doi: 10.3389/fpsyt.2021.663889
7. Bondopadhyay, U, Diaz-Orueta, U, and Coogan, AN. A systematic review of sleep and circadian rhythms in children with attention deficit hyperactivity disorder. J Atten Disord. (2022) 26:149–224. doi: 10.1177/1087054720978556
8. Krause, AJ, Simon, EB, Mander, BA, Greer, SM, Saletin, JM, Goldstein-Piekarski, AN, et al. The sleep-deprived human brain. Nat Rev Neurosci. (2017) 18:404–18. doi: 10.1038/nrn.2017.55
9. Fuller-Thomson, E, Lewis, DA, and Agbeyaka, SK. Attention-deficit/hyperactivity disorder casts a long shadow: findings from a population-based study of adult women with self-reported ADHD. Child Care Health Dev. (2016) 42:918–27. doi: 10.1111/cch.12380
10. Wynchank, D, Bijlenga, D, Beekman, AT, Kooij, JJS, and Penninx, BW. Adult Attention-Deficit/Hyperactivity Disorder (ADHD) and insomnia: an update of the literature. Curr Psychiatry Rep. (2017) 19:98. doi: 10.1007/s11920-017-0860-0. PMID 29086065
11. Boesen, K, Paludan-Muller, AS, Gotzsche, PC, and Jorgensen, KJ. Extended-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. (2022) 2022:CD012857. doi: 10.1002/14651858.CD012857.pub2
12. Stein, MA, Weiss, M, and Hlavaty, L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. (2012) 9:509–17. doi: 10.1007/s13311-012-0130-0
13. Smith, Yolanda. Stimulants and sleep. News-Medical. Available at: https://www.news-medical.net/health/Stimulants-and-Sleep.aspx (Accessed April 08, 2023)
14. Snitselaar, MA, Smits, MG, van der Heijden, KB, and Spijker, J. Sleep and circadian rhythmicity in adult ADHD and the effect of stimulants. J Atten Disord. (2017) 21:14–26. doi: 10.1177/1087054713479663
15. Huang, BH, Duncan, MJ, Cistulli, PA, Nassar, N, Hamer, M, and Stamatakis, E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med. (2022) 56:718–24. doi: 10.1136/bjsports-2021-104046
16. Ekelund, U, Tarp, J, Fagerland, MW, Johannessen, JS, Hansen, BH, Jefferis, BJ, et al. Joint associations of accelero-meter measured physical activity and sedentary time with all-cause mortality: a harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med. (2020) 54:1499–506. doi: 10.1136/bjsports-2020-103270
17. Tandon, PS, Sasser, T, Gonzalez, ES, Whitlock, KB, Christakis, DA, and Stein, MA. Physical activity, screen time, and sleep in children with ADHD. J Phys Act Health. (2019) 16:416–22. doi: 10.1123/jpah.2018-0215
18. Li, J, and Guo, L. Association between sleep duration and albumin in US adults: a cross-sectional study of NHANES 2015–2018. BMC Public Health. (2022) 22:1102. doi: 10.1186/s12889-022-13524-y
19. Chunnan, L, Shaomei, S, and Wannian, L. The association between sleep and depressive symptoms in US adults: data from the NHANES (2007–2014). Epidemiol Psychiatr Sci. (2022) 31:e63. doi: 10.1017/S20457960220004529
20. Li, C, and Shang, S. Relationship between sleep and hypertension: findings from the NHANES (2007–2014). Int J Environ Res Public Health. (2021) 18:7867. doi: 10.3390/ijerph18157867. PMID 34360157
21. Li, C, Shang, S, and Liang, W. Sleep and risk of hypertension in general American adults: the National Health and Nutrition Examination Surveys (2015–2018). J Hypertens. (2023) 41:63–73. doi: 10.1097/HJH.0000000000003299
22. Carson, V, Adamo, K, and Rhodes, RE. Associations of parenthood with physical activity, sedentary behavior, and sleep. Am J Health Behav. (2018) 42:80–9. doi: 10.5993/AJHB.42.3.8
23. Piercy, KL, Troiano, RP, Ballard, RM, Carlson, SA, Fulton, JE, Galuska, DA, et al. The physical activity guidelines for Americans. JAMA. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854. PMID 30418471
24. Hoge, C, Bowling, CB, Lim, SS, Drenkard, C, and Plantinga, LC. Association of poverty income ratio with physical functioning in a cohort of patients with systemic lupus erythematosus. J Rheumatol. (2020) 47:983–90. doi: 10.3899/jrheum.190991
25. Hansen, AR, Pritchard, T, Melnic, I, and Zhang, J. Physical activity, screen time, and school absenteeism: self-reports from NHANES 2005–2008. Curr Med Res Opin. (2016) 32:651–9. doi: 10.1185/03007995.2015.1135112
26. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
28. Mahajan, N, Hong, N, Wigal, TL, and Gehricke, JG. Hyperactive-impulsive symptoms associated with self-reported sleep quality in nonmedicated adults with ADHD. J Atten Disord. (2010) 14:132–7. doi: 10.1177/1087054709347170
29. Coogan, AN, Schenk, M, Palm, D, Uzoni, A, Grube, J, Tsang, AH, et al. Impact of adult attention deficit hyperactivity disorder and medication status on sleep/wake behavior and molecular circadian rhythms. Neuropsychopharmacology. (2019) 44:1198–206. doi: 10.1038/s41386-019-0327-6
30. Becker, SP, Epstein, JN, Tamm, L, Tilford, AA, Tischner, CM, Isaacson, PA, et al. Shortened sleep duration causes sleepiness, inattention, and oppositionality in adolescents with attention-deficit/hyperactivity disorder: findings from a crossover sleep restriction/extension study. J Am Acad Child Adolesc Psychiatry. (2019) 58:433–42. doi: 10.1016/j.jaac.2018.09.439
31. Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactive DisorderSteering Committee on Quality Improvement and ManagementWolraich, M, Brown, L, Brown, RT, Du Paul, G, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. (2011) 128:1007–22. doi: 10.1542/peds.2011-2654
32. Wolraich, ML, Hagan, JF Jr, Allan, C, Chan, E, Davison, D, Earls, M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. (2019) 144:e20192528. doi: 10.1542/peds.2019-2528
33. Adler, LA, Goodman, D, Weisler, R, Hamdani, M, and Roth, T. Effect of lisdexamfetamine dimesylate on sleep in adults with attention-deficit/hyperactivity disorder. Behav Brain Funct. (2009) 5:34. doi: 10.1186/1744-9081-5-34
34. Taylor, A, Kong, C, Zhang, Z, Herold, F, Ludyga, S, Healy, S, et al. Associations of meeting 24-h movement behavior guidelines with cognitive difficulty and social relationships in children and adolescents with attention deficit/hyperactive disorder. Child Adolesc Psychiatry Ment Health. (2023) 17:42. doi: 10.1186/s13034-023-00588-w
35. de Lira, CAB, Andrade, MS, de Mello, MT, and Vancini, RL. Physical exercise to manage sleep problems in pediatric patients with epilepsy and ADHD. Epilepsy Behav. (2017) 75:271–2. doi: 10.1016/j.yebeh.2017.06.025
36. Klil-Drori, S, and Hechtman, L. Potential social and neurocognitive benefits of aerobic exercise as adjunct treatment for patients with ADHD. J Atten Disord. (2020) 24:795–809. doi: 10.1177/1087054716652617
37. Liang, X, Li, R, Wong, SHS, Sum, RKW, Wang, P, Yang, B, et al. Physical activity and executive function in children with ADHD: the mediating role of sleep. Front Pediatr. (2021) 9:775589. doi: 10.3389/fped.2021.775589
38. Kario, K, Schwartz, JE, Davidson, KW, and Pickering, TG. Gender differences in associations of diurnal blood pressure variation, awake physical activity, and sleep quality with negative affect: the work site blood pressure study. Hypertension. (2001) 38:997–1002. doi: 10.1161/hy1101.095009
39. Hong, GCC, Conduit, R, Wong, J, Di Benedetto, M, and Lee, E. Diet, physical activity, and screen time to sleep better: multiple mediation analysis of lifestyle factors in school-aged children with and without attention deficit hyperactivity disorder. J Atten Disord. (2021) 25:1847–58. doi: 10.1177/1087054720940417
Keywords: attention-deficit/hyperactivity disorder (ADHD), stimulants, sleep, physical activity, NHANES
Citation: Zhu F, Liu B, Kuang D, Zhu X, Bi X, Song Y, Quan T, Yang Y and Ren Y (2023) The association between physical activity and sleep in adult ADHD patients with stimulant medication use. Front. Psychiatry. 14:1236636. doi: 10.3389/fpsyt.2023.1236636
Received: 08 June 2023; Accepted: 31 October 2023;
Published: 20 November 2023.
Edited by:
Shazia M. Jamil, Scripps Clinic, United StatesReviewed by:
Wan-Chun Su, National Institutes of Health (NIH), United StatesCopyright © 2023 Zhu, Liu, Kuang, Zhu, Bi, Song, Quan, Yang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanchun Ren, eXVhbmNodW4tcmVuQGJudS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.