- 1Centre for Neuroscience Studies, Queen’s University, Kingston, ON, Canada
- 2Providence Care Hospital, Kingston, ON, Canada
- 3Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, ON, Canada
- 4Department of Medicine, Queen’s University, Kingston, ON, Canada
- 5Queen’s Cardiopulmonary Unit, Translational Institute of Medicine, Queen’s University, Kingston, ON, Canada
Schizophrenia is a highly heritable, severe psychiatric disorder that involves dysfunctions in thinking, emotions, and behavior, with a profound impact on a person’s ability to function normally in their daily life. Research efforts continue to focus on elucidating possible genetic underlying mechanisms of the disorder. Although the genetic loci identified to date to be significantly associated with schizophrenia risk do not represent disease-causing factors, each one of them could be seen as a possible incremental contributor. Considering the importance of finding new and more efficient pharmacological approaches to target the complex symptomatology of this disorder, in this scoping review, we are focusing on the most recent findings in studies aiming to elucidate the contribution of one of the genetic factors involved – the BDNF gene Val66Met polymorphisms. Here we performed a systematic search in Pubmed, Embase, and Web of Science databases with the search terms: (BDNF gene polymorphism) AND (schizophrenia) for articles published in the last 5 years. To be selected for this review, articles had to report on studies where genotyping for the BDNF Val66Met polymorphism was performed in participants diagnosed with schizophrenia (or schizophrenia spectrum disorders or first-episode psychosis). The search provided 35 results from Pubmed, 134 results from Embase, and 118 results from the Web of Science database. Twenty-two articles were selected to be included in this review, all reporting on studies where an implication of the BDNF Val66Met polymorphisms in the disorder’s pathophysiology was sought to be elucidated. These studies looked at BDNF gene Val66Met polymorphism variants, their interactions with other genes of interest, and different facets of the illness. The Met/Met genotype was found to be associated with higher PANSS positive scores. Furthermore, Met/Met homozygous individuals appear to present with worse cognitive function and lower levels of serum BDNF. In the Val/Val genotype carriers, increased BDNF levels were found to correlate with weight gain under Risperidone treatment. However, due to heterogeneous results, the diversity in study populations and studies’ small sample sizes, generalizations cannot be made. Our findings emphasize the need for further research dedicated to clarifying the role of gene polymorphisms in antipsychotic treatment to enhance specificity and efficacy in the treatment of schizophrenia.
Introduction
Schizophrenia is a severe, chronic mental illness involving thinking, emotions, and behavior dysfunctions, resulting in impaired perception, social withdrawal, and loss of touch with reality. Individuals with schizophrenia often experience hallucinations, delusions, disorganized speech, and abnormal movements or behaviors. Pharmacological treatments available for schizophrenia vary in efficacy, with an estimated 34% of patients meeting the criteria for treatment resistance (1). Considering the profound impact this condition has on a person’s life, requiring long-term treatment and care, research efforts continue to focus on elucidating possible underlying mechanisms of the disorder. Biological, genetic, psychological, and environmental factors have been studied over the years. In the realm of genetic research advances, the genetic loci identified to be significantly associated with schizophrenia risk do not necessarily imply a causality link, but they can be each seen as a possible contributor factor (2). In line with ongoing efforts at exploring pharmacological targets with direct clinical applicability, in this scoping review, we focus on the most recent findings in studies aiming to elucidate the contribution of one of the genetic factors – the BDNF gene Val66Met polymorphisms.
The brain-derived neurotrophic factor (BDNF) is one of the most studied members of the neurotrophin family, which includes neural growth factor (NGF) and neurotrophins 3 and 4, among others (3). BDNF gene expression is highest in the brain (in the hippocampus, amygdala, cerebral cortex, and hypothalamus) as reviewed by Pruunsild (4), but mRNA encoding BDNF is also found in peripheral tissues including the heart, lung, prostate, and bladder (5). The BDNF gene contains 11 exons and is complemented by antiBDNF expression, which has been shown to form dsRNA duplexes in the brain, implying layered regulation of this important factor. BDNF is a secretory polypeptide released at the synapse, affecting synaptic plasticity (6). Binding to a tropomyosin receptor kinase B, also known as tyrosine kinase receptor B (TrkB), BDNF becomes an important signaling factor implicated in regulating proliferation, differentiation, survival, and death of neuronal and glial cells, as well as long-term memory formation, the regulation of long-term potentiation, and hippocampal synaptic plasticity (7, 8). The dysregulation of the TrkB/BDNF pathway has also been implicated in neurological and neurodegenerative conditions as well as stress-related disorders (9–11).
Alterations in BDNF at both the gene and protein levels during brain development have been implicated in the myriad of abnormalities found in the brains of individuals with schizophrenia (12–14). The genome-wide association study of schizophrenia (GWAS) confirmed the relevance of BDNF, identifying the BDNF genomic locus as enriched in common single nucleotide polymorphisms (SNPs) associated with the disorder, although not reaching the genome-wide statistical threshold (2). However, from the perspective of the neurodevelopmental hypothesis of schizophrenia, which considers the utmost complexity of numerous factors and their interactions in the etiology of this disorder, BDNF has been proposed as a candidate to explain part of the pathogenesis of this disease (13). Located on chromosome 11 in humans, the BDNF gene has a complex structure, encompassing 11 different exons and nine promoters (4). A non-synonymous polymorphism in this gene - rs6265 (C → T, Val → Met), also called Val66Met or G196A polymorphism, is common (15). An amino acid substitution - valine (Val) to methionine (Met) at codon 66 leads to profound impairment in the neuronal activity-dependent secretion of BDNF (16) as the BDNF gene is one of the few genes that are prominently implicated in cognitive functions (14). The exact mechanisms that lead to BDNF alterations in schizophrenia are still largely unknown. However, numerous studies hint at epigenetic changes at the BDNF gene locus, providing a link between genes and environment, particularly stress, childhood adversities, and inflammation (17). The Met allele has been historically considered to confer the disadvantaged phenotypes – at the cellular, structural, physiological, and behavioral levels (6). Episodic memory has been shown to be impaired in healthy individuals carrying the Met allele (18) and substantially impaired in Met/Met carriers – among individuals with schizophrenia, siblings, and healthy controls, compared to other genotypes (16, 19). In schizophrenia, it has been suggested that BDNF signaling may critically affect the structure and functioning of neural circuits involved in modulating neurotransmitter systems, including dopaminergic, serotoninergic, and GABAergic (14, 20). In other studies, individuals carrying the Val/Val allele, initially thought as protective, were also identified as at risk for developing schizophrenia and, in patients, being at risk of experiencing more severe symptoms than patients carrying at least one Met allele (21, 22). An interplay between one allele or the other and environmental factors may explain these mixed, conflicting findings and justify efforts to clarify the current understanding of genetic polymorphism’s impact on schizophrenia.
In this review, we aim to capture the current consensus and divergence in findings relating to the implication of the BDNF Val66Met polymorphisms in the clinical presentation of individuals who have schizophrenia. Data supporting the hypothesis that peripheric BDNF gene expression and protein levels reflect those in the brain hints at possible avenues of finding applicability of genetic polymorphism determination for prognosis, therapy monitoring and therapeutic response in schizophrenia (23).
Methods
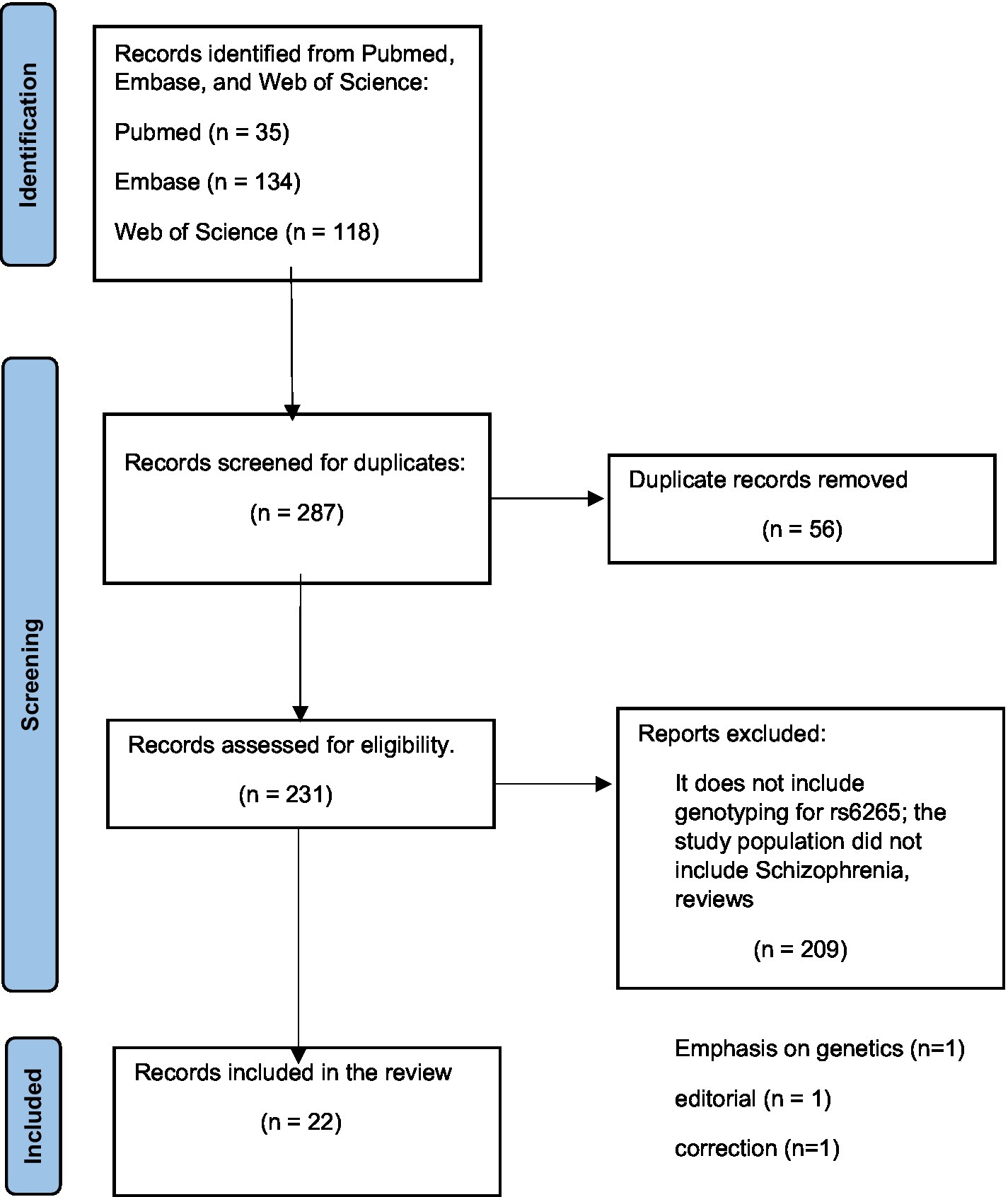
Our team systematically searched Pubmed, Embase, and Web of Science databases with the search terms: (BDNF gene polymorphism) AND (schizophrenia) for articles published in the last 5 years. The search provided 35 results from Pubmed, 134 results from Embase, and 118 results from the Web of Science database. To be selected for this review, articles had to report on studies where genotyping for the BDNF Val66Met polymorphism was performed in participants diagnosed with schizophrenia or schizophrenia spectrum disorders or first-episode psychosis. In addition, these studies also had to aim at analyzing for associations between the Val66Met polymorphism and risk for schizophrenia or with the clinical presentation of participants. After reading abstracts and removing articles that did not fit the criteria, 22 articles were selected to be included in this review seen in the PRISMA diagram (Figure 1).
Results
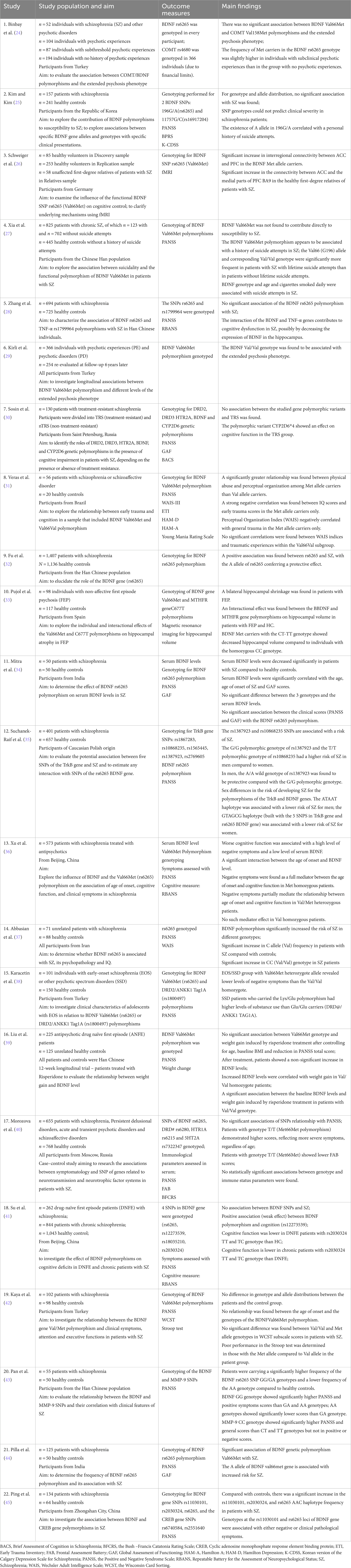
Twenty-two articles were selected for this scoping review, all reporting on studies involving participants with a schizophrenia or schizophrenia-related disorder, where an implication of the BDNF Val66Met polymorphisms in the disorder’s pathophysiology was sought to be elucidated. These studies looked not only at different polymorphism variants but also their interactions with other genes of interest and at different facets of the illness, in some instances highlighting associations with its most difficult symptoms – the negative ones. Table 1 summarizes the 22 studies with their population, aim, outcome measures, and main findings.
Discussion
Since the first study announcing the isolation of BDNF as a new neurotrophic factor in the pig brain in 1982 (46), a consistently increasing number of researchers have continued to explore its role in the physiology and pathology of the human brain. Although studies selected for this review were highly prioritized based on narrow selection criteria that included only the most recent publications (within the past 5-years), they reflect findings from different geographical regions and ethnicities, painting a complex interplay of factors in addition to the BDNF gene, that contribute to the pathophysiology of schizophrenia. Considering that ethnicity has been cited as an important factor explaining divergent findings of the associations between the BDNF Val66Met polymorphism and psychiatric disorders (47, 48), it is worth mentioning that the study populations included here belong to different ethnic backgrounds: eight of the studies were performed in China, 2 in India, 4 in Turkey, 2 in Russia, and 1 in Spain, Brazil, Germany, Iran, South Korea, and Poland, respectively. Significant ethnic differences in the BDNF Val66Met polymorphism were suggested to arise from a natural selection of a particular allele and many environmental factors, further complicating the inferential analysis (49).
Recent findings also suggest that gender and age impact how the BDNF Val66Met polymorphism contributes to neurodegenerative disease (50). The study populations included here consist of adults - 18 to 65 years old, except for Karacetin (38). where participants were adolescents with early-onset schizophrenia or other psychotic spectrum disorders. The primary diagnosis of participants encompasses schizophrenia and schizophrenia spectrum disorders, as well as other psychotic spectrum disorders as a subcategory for comparison – as in Karacetin (38). Kirli (29) studied individuals with psychotic experiences and psychotic disorders, including schizophrenia, to investigate longitudinal associations between the BDNF Val66Met polymorphism and different levels of the extended phenotype. Another study compared genotyping differences between healthy volunteers and unaffected first-degree relatives of patients with schizophrenia (26).
Samples varied amply in size, from n = 50 in kumar (34) to n = 1,407 in Fu (32). In most of the studies, the gender variable was predominantly represented by males, with a few exceptions where both genders were matched or females slightly higher in numbers (24, 26, 34). Considering the established understanding that differences in the epidemiology of psychiatric disorders and the persisting tendency to underrepresent women as participants in studies (51), it is worth highlighting that equal recruitment of genders would ensure greater generalizability of findings (52). Although sex as a distinguishing biological factor is crucial in genetic studies, the social construct of gender may further bring clarity, especially in the light of research highlighting more prevalent gender dysphoria in individuals with schizophrenia than in the general population (53). Sex differences were found in the risk of developing schizophrenia by Shuchanek-Raif (35). In this study, the ATAAT haplotype (built with 5 SNPs in the TrKB gene) was associated with a lower risk for schizophrenia in men. In comparison, the GTAGCG haplotype (built with the 5 SNPs in the TrkB gene and rs6265 BDNF gene) was associated with a lower risk of schizophrenia for women.
Most studies included a healthy control group, except those looking for associations between the BDNF Val66Met polymorphism and clinical facets of schizophrenia or the extended psychosis phenotype (29, 36), or evaluating genetic implications in treatment resistance (30).
The clinical severity of the illness extended from the drug-naïve first episode of psychosis to treatment-resistant schizophrenia symptoms and a history of suicide. Clinical presentation is consistently assessed and highlighted in most of the studies. A significant association between the BDNF Val66Met polymorphism and symptoms was found in Pan (43), where the BDNF GG (Met/Met) genotype showed higher Positive and Negative Syndrome Scale (PANSS) positive symptoms score compared to the GA (Met/Val) and AA (Val/Val) genotypes. The AA genotypes showed significantly lower scores than the GA genotype in this study. Negative symptoms were also found to be a full mediator between the age of onset and cognitive function in Met homozygous patients and a partial mediator in Val/Met heterozygous patients by Xu (36). This same study (36) connects worse cognitive function with increased negative symptoms and a low level of serum BDNF. Systematic reviews focusing on peripheral levels of BDNF (54, 55) concluded that moderate reductions of BDNF are observed both in drug-naïve and medicated individuals. Certain BDNF isoforms at low levels were consistently found to correlate with cognitive impairment in individuals with schizophrenia compared to controls (56). In line with these findings, Morozova (40) found that patients with genotype T/T (Met66Met polymorphism) demonstrated higher scores on PANSS, reflecting more severe symptoms, regardless of age. None of the other studies found a significant association between positive and negative PANSS scores and the Val66Met polymorphism, most focusing on the risk for schizophrenia or association with cognitive functions. The PANSS was one of the most utilized clinical assessment tools, followed by BACS, and BFCRS, among others. One of the studies also included the HAM-A and HAM-D to address the affective component of the schizoaffective disorders portion of its sample (31).
Suicidality, a critical issue in schizophrenia, and its association with the BDNF Val66Met polymorphism were explicitly investigated by Xia (27). Their findings show a clear association between this genetic polymorphism and a history of suicide attempts in schizophrenia. Interestingly, the val66 allele and the corresponding Val/Val genotype were more frequent in patients with schizophrenia presenting with lifetime suicide attempts. This study also found an association between the BDNF genotype, age, cigarettes smoked per day, and suicide attempts in patients, highlighting the interaction between genetic and environmental factors in suicidal behavior among patients with schizophrenia.
Cognition was the focus of explorations in five of the studies, depicting poor cognitive performance in patients with the Met allele compared to the Val, in Kaya (42).
A negative correlation between IQ scores and early trauma in the Met allele carriers, as well as a negative correlation between perceptual organization and trauma in the same group, was found by Veras (31). Worse cognitive function was associated, as expected, with a high level of negative symptoms and a low level of serum BDNF in Xu (36), with a mediator effect of the negative symptoms for the interaction age of onset – cognition in Met homozygous patients, as mentioned previously. Looking at the association between the BDNF rs6265 and the TNF-α rs1799964 polymorphism in patients with schizophrenia, Zhang (28) found that the interaction of these two genes contributes to cognitive dysfunction, possibly by decreasing the expression of BDNF in the hippocampus. Only one study – Morozova (40) assessed immunological parameters along with their primary assessments, finding no association between genotype and immune status of patients, contrary to recent literature suggesting the contrary (3). Patients with schizophrenia have been found in numerous studies to present with upregulated genes for inflammatory cytokines and downregulated BDNF gene transcription (57, 58).
Notably, two studies involved fMRI in their design, aiming to delineate morphological and functional changes related to the BDNF Val66Met polymorphism. Schweiger (26) found a significant increase in interregional connectivity between ACC and PFC in the BDNF Met allele carriers. In contrast, Pujol (33) found that BDNF Met carriers with the MTHFR gene CT-TT genotype presented with decreased hippocampal volume.
Multiple genetic interactions were explored by Su (41), Morozova (40), Zzhang (28), Kim and Kim (25), Sosin (30), Pan (43), Karacetin (38), Ping (45), and Suchanek-Raif (35), revealing various associations or propensity for risk for schizophrenia.
An interesting and pragmatic approach was taken by Liu (39), where the study focused on evaluating the relationship between BDNF levels and the BDNF genotype and weight gain in individuals with schizophrenia treated with Risperidone. While a non-significant increase in BDNF levels correlated with weight gain in Val/Val homozygous patients, these findings raise the importance of examining genetic factors underlying therapeutic response.
A recent review of evidence demonstrating that dysregulation of neurotrophic signaling is common to most neurological and neurodegenerative disorders highlights the importance of restoring the BDNF/TrkB pathway by its fine-tuned activation or suppression (11). The authors discuss and evaluate different strategies directed to increase the availability of BDNF, from systemic BDNF administration and its limitations, nanoparticle-mediated transport, gene therapy with BDNF-encoding viral vectors or transplantation of BDNF-releasing cells, to antidepressant medication and exercise, stressing that in the end the efficiency of such treatments could be limited when receptor stability and function are aberrant, as it is the case in psychiatric disorders.
Considering the wide range of findings in the studies selected for this review, the limitations in generalization, besides study design and methodology variations, are imposed by the complexity of possible factors affecting the BDNF Val66Met polymorphism. Interethnic genetic differences remain significant, and specifying the country of origin may not cover ethnicity. Studies show, for example, that the Met allele frequency is around 50% in the Chinese population but only around 20% in Caucasian subjects (27, 59). In the current globalization era, samples from any geographical region may include different ethnicities. Serum BDNF level was also found to have a diurnal as well as seasonal variation (60), and a diurnal variation in women appears to be correlated with the ovarian function (61).
Further directions may regard longitudinal studies, samples that are representative of the number of participants, sex, and gender (including those who have undergone gender-affirming treatment), as well as ethnicity, in-depth exploration of associations between the BDNF Val66Met polymorphism, its TrkB receptor and immune factors as well as peripheral levels of BDNF, and all this taking into account morphologic changes depicted through imaging investigations as well as the diurnal, seasonal and hormonal BDNF variations, medication interactions, metabolic and lifestyle factors. Identifying BDNF gene variants that lead to effective antipsychotic drug response could have crucial implications for patients and the clinical sector associated costs.
Conclusion
The current review covered a wide range of studies exploring the implications of the BDNF geneVal66Met polymorphism in schizophrenia, published in the last 5 years. The Met/Met genotype was found to be associated with higher PANSS positive scores, the PANSS global scores in these cases also reflecting greater severity of illness. It was also associated with a history of suicide attempts. Consistent with previous studies, Met/Met homozygous individuals present with worse cognitive function and lower levels of serum BDNF.
In the Val/Val genotype carriers, increased BDNF levels were correlated with weight gain under Risperidone treatment. However, due to the study’s small sample size depicting this, generalizations cannot be made. Future studies may take on the challenge of delineating genetic markers that could allow prediction for treatment response in schizophrenia.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the Providence Care Innovation Grant 2019: “Innovative pathways to impactful treatment of chronic schizophrenia: disrupting the status-quo moving toward biological-driven, combined pharmacological and non-pharmacological therapeutic approaches to define markers of therapeutic improvement in cognitive behavioral therapy for psychosis promoted recovery”.
Acknowledgments
We thank the Providence Care Hospital Research Centre for its continuous support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Płaza, O, Gałecki, P, Orzechowska, A, Gałecka, M, Sobolewska-Nowak, J, and Szulc, A. Pharmacogenetics and schizophrenia—can genomics improve the treatment with second-generation antipsychotics? Biomedicine. (2022) 10:3165. doi: 10.3390/biomedicines10123165
2. Schizophrenia Working Group of the Psychiatric Genomics Consortium . Biological insights from 108 schizophrenia-associated genetic loci. Nature. (2014) 511:421–7. doi: 10.1038/nature13595
3. Lima Giacobbo, B, Doorduin, J, Klein, HC, Dierckx, RA, Bromberg, E, and de Vries, EF. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol. (2019) 56:3295–312. doi: 10.1007/s12035-018-1283-6
4. Pruunsild, P, Kazantseva, A, Aid, T, Palm, K, and Timmusk, T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. (2007) 90:397–406. doi: 10.1016/j.ygeno.2007.05.004
5. Fagerberg, L, Hallström, BM, Oksvold, P, Kampf, C, Djureinovic, D, Odeberg, J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. (2014) 13:397–406. doi: 10.1074/mcp.M113.035600
6. Di Carlo, P, Punzi, G, and Ursini, G. BDNF and schizophrenia. Psychiatr Genet. (2019) 29:200–10. doi: 10.1097/YPG.0000000000000237
7. Minichiello, L . TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. (2009) 10:850–60. doi: 10.1038/nrn2738
8. Pang, PT, and Lu, B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. (2004) 3:407–30. doi: 10.1016/j.arr.2004.07.002
9. Notaras, M, and van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. (2020) 25:2251–74. doi: 10.1038/s41380-019-0639-2
10. Pradhan, J, Noakes, PG, and Bellingham, MC. The role of altered BDNF/TrkB signalling in amyotrophic lateral sclerosis. Front Cell Neurosci. (2019) 13:368. doi: 10.3389/fncel.2019.00368
11. Tejeda, GS, and Díaz-Guerra, M. Integral characterization of defective BDNF/TrkB signalling in neurological and psychiatric disorders leads the way to new therapies. Int J Mol Sci. (2017) 18:268. doi: 10.3390/ijms18020268
12. Millan, MJ, Andrieux, A, Bartzokis, G, Cadenhead, K, Dazzan, P, Fusar-Poli, P, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. (2016) 15:485–515. doi: 10.1038/nrd.2016.28
13. Nieto, R, Kukuljan, M, and Silva, H. BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front Psych. (2013) 4:45. doi: 10.3389/fpsyt.2013.00045
14. Lu, B, and Martinowich, K. Cell biology of BDNF and its relevance to schizophrenia. Novartis Found Symp. (2008) 289:119–35. doi: 10.1002/9780470751251.ch10
15. Tsai, SJ . Critical issues in BDNF Val66Met genetic studies of neuropsychiatric disorders. Front Mol Neurosci. (2018) 11:156. doi: 10.3389/fnmol.2018.00156
16. Egan, MF, Kojima, M, Callicott, JH, Goldberg, TE, Kolachana, BS, Bertolino, A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cells. (2003) 112:257–69. doi: 10.1016/S0092-8674(03)00035-7
17. Frodl, T, Skokauskas, N, Frey, EM, Morris, D, Gill, M, and Carballedo, A. BDNF V al66 M et genotype interacts with childhood adversity and influences the formation of hippocampal subfields. Hum Brain Mapp. (2014) 35:5776–83. doi: 10.1002/hbm.22584
18. Richter-Schmidinger, T, Alexopoulos, P, Horn, M, Maus, S, Reichel, M, Rhein, C, et al. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. (2011) 118:249–57. doi: 10.1007/s00702-010-0539-8
19. Dempster, E, Toulopoulou, T, McDonald, C, Bramon, E, Walshe, M, Filbey, F, et al. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. (2005) 134B:73–5. doi: 10.1002/ajmg.b.30150
20. Baker, SA, Stanford, LE, Brown, RE, and Hagg, T. Maturation but not survival of dopaminergic nigrostriatal neurons is affected in developing and aging BDNF-deficient mice. Brain Res. (2005) 1039:177–88. doi: 10.1016/j.brainres.2005.01.052
21. Ho, BC, Andreasen, NC, Dawson, JD, and Wassink, TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatr. (2007) 164:1890–9. doi: 10.1176/appi.ajp.2007.05111903
22. Chang, HA, Lu, RB, Shy, MJ, Chang, CC, Lee, DPHMS, and Huang, SY. Brain-derived neurotrophic factor Val66Met polymorphism: association with psychopathological symptoms of schizophrenia? J Neuropsychiatry Clin Neurosci. (2009) 21:30–7. doi: 10.1176/jnp.2009.21.1.30
23. Cattaneo, A, Cattane, N, Begni, V, Pariante, CM, and Riva, MA. The human BDNF gene: peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl Psychiatry. (2016) 6:e958–8. doi: 10.1038/tp.2016.214
24. Binbay, T, Kirli, U, Misir, E, Elbi, H, Kayahan, B, Onay, H, et al. The association between the extended psychosis phenotype and COMT val158met and BDNF val66met polymorphisms. Turk J Psychiatry. (2018) 29:221–8. doi: 10.5080/u19426
25. Kim, EJ, and Kim, YK. 196G/a of the brain-derived neurotrophic factor gene polymorphisms predicts suicidal behavior in schizophrenia patients. Psychiatry Investig. (2018) 15:733–8. doi: 10.30773/pi.2018.02.27
26. Schweiger, JI, Bilek, E, Schäfer, A, Braun, U, Moessnang, C, Harneit, A, et al. Effects of BDNF Val66Met genotype and schizophrenia familial risk on a neural functional network for cognitive control in humans. Neuropsychopharmacology. (2019) 44:590–7. doi: 10.1038/s41386-018-0248-9
27. Xia, H, Zhang, G, Du, X, Zhang, Y, Yin, G, Dai, J, et al. Suicide attempt, clinical correlates, and BDNF Val66Met polymorphism in chronic patients with schizophrenia. Neuropsychology. (2018) 32:199–205. doi: 10.1037/neu0000383
28. Zhang, Y, Fang, X, Fan, W, Tang, W, Cai, J, Song, L, et al. Interaction between BDNF and TNF-α genes in schizophrenia. Psychoneuroendocrinology. (2018) 89:1–6. doi: 10.1016/j.psyneuen.2017.12.024
29. Kirli, U, Binbay, T, Drukker, M, Elbi, H, Kayahan, B, Gökçelli, DK, et al. Is BDNF-Val66Met polymorphism associated with psychotic experiences and psychotic disorder outcome? Evidence from a 6 years prospective population-based cohort study. Am J Med Genet B Neuropsychiatr Genet. (2019) 180:113–21. doi: 10.1002/ajmg.b.32641
30. Sosin, D, Ivashchenko, D, Sozaeva, Z, Ryzhikova, K, Fadeeva, V, Chomskaya, V, et al. Cognitive impairment in patients with treatment resistant schizophrenia: associations with DRD2, DRD3, HTR2A, BDNF and CYP2D6 genetic polymorphisms. Neurol Psychiatry Brain Res. (2019) 33:48–55. doi: 10.1016/j.npbr.2019.06.003
31. Veras, AB, Chao, MV, Getz, M, Goetz, R, Cheniaux, E, Lopes, FL, et al. Traumatic experiences and cognitive profiles of schizophrenia cases influenced by the BDNF Val66met polymorphism. Psychiatry Res. (2019) 271:111–3. doi: 10.1016/j.psychres.2018.11.029
32. Fu, X, Wang, J, Du, J, Sun, J, Baranova, A, and Zhang, F. BDNF gene's role in schizophrenia: from risk allele to methylation implications. Front Psych. (2020) 11:564277. doi: 10.3389/fpsyt.2020.564277
33. Pujol, N, Mané, A, Bergé, D, Mezquida, G, Amoretti, S, Pérez, L, et al. Influence of BDNF and MTHFR polymorphisms on hippocampal volume in first-episode psychosis. Schizophr Res. (2020) 223:345–52. doi: 10.1016/j.schres.2020.08.002
34. Mitra, P, Ghosh, R, Sharma, S, Nebhinani, N, and Sharma, P. Association of circulating BDNF levels with BDNF rs6265 polymorphism in schizophrenia. Behav Brain Res. (2020) 394:112832. doi: 10.1016/j.bbr.2020.112832
35. Suchanek-Raif, R, Raif, P, Kowalczyk, M, Paul-Samojedny, M, Zielińska, A, Kucia, K, et al. An analysis of five TrkB gene polymorphisms in schizophrenia and the interaction of its haplotype with rs6265 BDNF gene polymorphism. Dis Markers. (2020) 2020:1–7. doi: 10.1155/2020/4789806
36. Xu, H, Wang, J, Zhou, Y, Chen, D, Xiu, M, Wang, L, et al. BDNF affects the mediating effect of negative symptoms on the relationship between age of onset and cognition in patients with chronic schizophrenia. Psychoneuroendocrinology. (2021) 125:105121. doi: 10.1016/j.psyneuen.2020.105121
37. Abbasian, S, Yoosefee, S, and Shahsavand-Ananloo, E. Association between brain-derived neurotropic factor gene variant (rs6265; C> T) and schizophrenia, its psychopathology and intelligence. Eur J Psychiatry. (2021) 35:207–15. doi: 10.1016/j.ejpsy.2021.04.004
38. Karaçetin, G, Bayoglu, B, Soylemez, TE, Topal, M, Koc, EB, Tekden, M, et al. BDNF Val66Met polymorphism is associated with negative symptoms in early-onset schizophrenia spectrum and other psychotic disorders. Eur J Psychiatry. (2022) 36:26–34. doi: 10.1016/j.ejpsy.2021.04.002
39. Liu, J, Wang, P, Sun, L, Guan, X, Xiu, M, and Zhang, X. The association between BDNF levels and risperidone-induced weight gain is dependent on the BDNF Val66Met polymorphism in antipsychotic-naive first episode schizophrenia patients: a 12-week prospective study. Transl Psychiatry. (2021) 11:458. doi: 10.1038/s41398-021-01585-3
40. Morozova, A, Zorkina, Y, Pavlov, K, Pavlova, O, Abramova, O, Ushakova, V, et al. Associations of genetic polymorphisms and neuroimmune markers with some parameters of frontal lobe dysfunction in schizophrenia. Front Psych. (2021) 12:655178. doi: 10.3389/fpsyt.2021.655178
41. Su, X, Qiao, L, Liu, Q, Shang, Y, Guan, X, Xiu, M, et al. Genetic polymorphisms of BDNF on cognitive functions in drug-naive first episode patients with schizophrenia. Sci Rep. (2021) 11:20057. doi: 10.1038/s41598-021-99510-7
42. Kaya, ÖB, Hasan, KA, Kahve, AC, Darçin, AE, Çavuş, RS, and Dilbaz, N. Association of BDNF gene Val66Met polymorphism with suicide attempts, focused attention and response inhibition in patients with schizophrenia. Noro Psychiatrist Ars. (2022) 59:91. doi: 10.29399/npa.27647
43. Pan, L, Cao, Z, Chen, L, Qian, M, and Yan, Y. Association of BDNF and MMP-9 single-nucleotide polymorphisms with the clinical phenotype of schizophrenia. Front Psychiatry. (2022) 13:13. doi: 10.3389/fpsyt.2022.941973
44. Pilla, VSNKK, Mitra, P, Sharma, S, Nebhinani, N, and Sharma, P. W129 a study on BDNF polymorphism (RS6265) in schizophrenia. Clin Chim Acta. (2022) 530:S340. doi: 10.1016/j.cca.2022.04.884
45. Ping, J, Zhang, J, Wan, J, Huang, C, Luo, J, Du, B, et al. A polymorphism in the BDNF gene (rs11030101) is associated with negative symptoms in Chinese Han patients with schizophrenia. Front Genet. (2022) 13:849227. doi: 10.3389/fgene.2022.849227
46. Barde, YA, Edgar, D, and Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. (1982) 1:549–53. doi: 10.1002/j.1460-2075.1982.tb01207.x
47. Pivac, N, Nikolac, M, Nedic, G, Mustapic, M, Borovecki, F, Hajnsek, S, et al. Brain derived neurotrophic factor Val66Met polymorphism and psychotic symptoms in Alzheimer's disease. Prog Neuro-Psychopharmacol Biol Psychiatry. (2011) 35:356–62. doi: 10.1016/j.pnpbp.2010.10.020
48. Shimizu, E, Hashimoto, K, and Iyo, M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. (2004) 126B:122–3. doi: 10.1002/ajmg.b.20118
49. Gratacòs, M, González, JR, Mercader, JM, de Cid, R, Urretavizcaya, M, and Estivill, X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: a meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. (2007) 61:911–22. doi: 10.1016/j.biopsych.2006.08.025
50. Barha, CK, Liu-Ambrose, T, Best, JR, Yaffe, K, and Rosano, C, Study BC. Sex-dependent effect of the BDNF Val66Met polymorphism on executive functioning and processing speed in older adults: evidence from the health ABC study. Neurobiol Aging. (2019) 74:161–70. doi: 10.1016/j.neurobiolaging.2018.10.021
51. Pedersen, SL, Lindstrom, R, Powe, PM, Louie, K, and Escobar-Viera, C. Lack of representation in psychiatric research: a data-driven example from scientific articles published in 2019 and 2020 in the American journal of psychiatry. Am J Psychiatr. (2022) 179:388–92. doi: 10.1176/appi.ajp.21070758
52. Howard, LM, Ehrlich, AM, Gamlen, F, and Oram, S. Gender-neutral mental health research is sex and gender biased. Lancet Psychiatry. (2017) 4:9–11. doi: 10.1016/S2215-0366(16)30209-7
53. Rajkumar, RP . Gender identity disorder and schizophrenia: neurodevelopmental disorders with common causal mechanisms? Schizophr Res Treat. (2014) 2014:1–8. doi: 10.1155/2014/463757
54. Fernandes, BS, Steiner, J, Berk, M, Molendijk, ML, Gonzalez-Pinto, A, Turck, CW, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry. (2015) 20:1108–19. doi: 10.1038/mp.2014.117
55. Green, MJ, Matheson, SL, Shepherd, A, Weickert, CS, and Carr, VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. (2011) 16:960–72. doi: 10.1038/mp.2010.88
56. Carlino, D, De Vanna, M, and Tongiorgi, E. Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunctions? Neuroscientist. (2013) 19:345–53. doi: 10.1177/1073858412469444
57. Kordi-Tamandani, DM, Sahranavard, R, and Torkamanzehi, A. DNA methylation and expression profiles of the brain-derived neurotrophic factor (BDNF) and dopamine transporter (DAT1) genes in patients with schizophrenia. Mol Biol Rep. (2012) 39:10889–93. doi: 10.1007/s11033-012-1986-0
58. Mill, J, Tang, T, Kaminsky, Z, Khare, T, Yazdanpanah, S, Bouchard, L, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. (2008) 82:696–711. doi: 10.1016/j.ajhg.2008.01.008
59. Varnäs, K, Lawyer, G, Jönsson, EG, Kulle, B, Nesvåg, R, Hall, H, et al. Brain-derived neurotrophic factor polymorphisms and frontal cortex morphology in schizophrenia. Psychiatr Genet. (2008) 18:177–83. doi: 10.1097/YPG.0b013e3283050a94
60. Molendijk, ML, Haffmans, JP, Bus, BA, Spinhoven, P, Penninx, BW, Prickaerts, J, et al. Serum BDNF concentrations show strong seasonal variation and correlations with the amount of ambient sunlight. PLoS One. (2012) 7:e48046. doi: 10.1371/journal.pone.0048046
61. Pluchino, N, Cubeddu, A, Begliuomini, S, Merlini, S, Giannini, A, Bucci, F, et al. Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Hum Reprod. (2009) 24:2303–9. doi: 10.1093/humrep/dep119
Keywords: schizophrenia, BDNF, gene polymorphism, neurotrophins, genotyping
Citation: Farcas A, Hindmarch C and Iftene F (2023) BDNF gene Val66Met polymorphisms as a predictor for clinical presentation in schizophrenia – recent findings. Front. Psychiatry. 14:1234220. doi: 10.3389/fpsyt.2023.1234220
Edited by:
Luca De Peri, Cantonal Sociopsychiatric Organization, SwitzerlandReviewed by:
Harpreet Kaur, Cleveland Clinic, United StatesReiji Yoshimura, University of Occupational and Environmental Health Japan, Japan
Eliyahu Dremencov, Slovak Academy of Sciences, Slovakia
Copyright © 2023 Farcas, Hindmarch and Iftene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Farcas, NmFtZkBxdWVlbnN1LmNh
 Adriana Farcas
Adriana Farcas Charles Hindmarch
Charles Hindmarch Felicia Iftene1,2
Felicia Iftene1,2