- 1Family Psychosomatics, Department of Pediatrics and Neonatology, Justus-Liebig University Giessen, Gießen, Germany
- 2Psychoneuroimmunology Laboratory, Department of Psychosomatic Medicine and Psychotherapy, Justus-Liebig University Giessen, Gießen, Germany
- 3Psychosomatics and Psychotherapy, Charité Center 12 Internal Medicine and Dermatology, Charité—Universitätsmedizin Berlin, Berlin, Germany
Background: Studies measuring hair cortisol concentration (HCC) have been increasingly conducted to document stress-related, endocrine changes aggregated over time. Previous studies have shown that HCC reflects abnormalities in the hypothalamic–pituitary-adrenocortical axis (HPA axis) in the context of somatic diseases, such as Cushing’s syndrome. HCC variations also reveal a corresponding alteration in HPA-axis-function in mental disorders, highlighting its potential role as a biomarker for interventions targeting mental health problems.
Aims: The aim of this study was to investigate the role of HCC in various psychological and neuropsychiatric interventions and to explore the extent to which HCC can serve as a predictive or outcome parameter in such interventions by conducting a PRISMA-compliant review of the literature.
Methods: From May to July 2022, the databases Web of Science, Google Scholar, PsychINFO, and ResearchGate were systematically searched using different combinations of relevant keywords. Studies of different types that examined HCC in the context of a wide range of psychological and neuropsychiatric interventions were included. Studies in languages other than English or German and animal studies were excluded. The MMAT tool was used, to assesses the Risk of bias.
Results: The initial search identified 334 studies. After applying the inclusion and exclusion criteria, 14 publications with a total number of 1,916 participants were identified. An association between HCC and PTSD, depressive disorders, and ongoing social and family stress can be documented. The effect of relaxation techniques, mental training, CBT, or PTSD therapy on HCC has been studied with equivocal results. Some studies found decreased HCC after treatment, while others did not show a clear effect. Baseline HCC appears to be of particular importance. In some studies, higher baseline HCC was associated with increased treatment response, providing a predictive value for HCC.
Discussion: HCC is increasingly being used as a biomarker for the mapping of psychological and neuropsychiatric interventions. However, due to the wide range of study populations and interventions, results are still heterogeneous. Nevertheless, HCC seems to be an encouraging biological parameter to describe the trajectory of different interventions aimed at improving mental health.
Highlights
• HCC is increasingly used as trajectory or predictive parameter in psychological interventions.
• The results do not provide a consistent picture to this point.
• However, HCC appears to be a promising biomarker to illustrate how biological processes can be modulated by psychological and neuropsychiatric interventions.
• The methodological gold standard (large, randomized controlled studies with a standardized and comparable study design) for psychotherapy studies with determination of HCC should be implemented more consistently in the future.
Introduction
Stress has long been recognized as a risk factor for mental and physical health. The hypothalamic–pituitary–adrenal axis (HPA-axis) plays a critical role in the endocrine regulation of stress. Its activation is required for physical adaptation to any stressor and leads to the release of the hormone cortisol (1). To date, blood, saliva, or urine samples have been primarily used to measure cortisol levels. Although these methods are well established and validated, they can only determine current cortisol levels or an integrative averaged daily cortisol level (24-h urine collection). However, none of these provide information about the long-term course of cortisol. In addition, diurnal variations in cortisol production and secretion may be a limiting factor in clinical interpretation (2).
In recent years, there has also been an increase in the investigation of cortisol levels in the context of mental disorders, as shown in a meta-analysis from 2018 (3). The authors demonstrated that the HPA axis appears to be altered in the context of mental disorders and that it may be helpful to consider biomarkers as an adjunct to conventional psychometric measures in psychological intervention studies (3). Biomarkers could be used as a marker of therapeutic progress and disease regression. Cortisol seems to be particularly suitable as a biomarker for mental processes.
As described in another systematic review on the influence of hormones on psychotherapies, it seems plausible that cortisol can influence psycho-affective and emotional functions through physiologically expressed glucocorticoid receptors in the central nervous system. The review attributes some importance to cortisol in the context of psychotherapy (4). Furthermore, it has been shown that cortisol may act as a moderating factor on psychotherapeutic processes (5). However, the extent of these influences require further investigation. The authors claim that the integration of repeated, stress-free hormone measurements, which have little influence on the therapy process, will allow conclusions to be drawn in the future about for whom and why psychotherapy works. They suggest that hair cortisol is a particularly suitable medium for this purpose (4).
The determination of cortisol in scalp hair seems to be a reliable parameter of the HPA axis (2, 6). In contrast to previous methods of cortisol determination, it allows the evaluation of the HPA axis activity over a longer period and represents long-term or continuous hormonal changes. Hair cortisol Concentration (HCC) sampling is non-invasive for the subject and therefore less susceptible to situational confounding, such as an increase in stress level due to the blood sampling (2).
With an average hair growth rate of approximately 1 cm per month, the most proximal centimeter represents the previous month’s HCC. Hair samples should be taken from the posterior vertex as close to the scalp as possible (6, 7). Two techniques are most commonly used to quantify hair cortisol: Liquid chromatography–tandem mass spectrometry (LC–MS/MS), a method for identifying and quantifying chemical compounds based on their fragmentation patterns and molecular masses, and Enzyme Immunoassays (EIA), which allows the detection of small amounts of antigens in a fluid sample based on the binding of the antigen to its specific antibody (6). No accepted standards have yet been established for hair cortisol norms, although several studies have attempted to generate reference data for different age groups as determined by LC–MS/MS or immunoassays (8–10).
As a helpful framework, biomarkers have been classified into diagnostic, prognostic, and intervention-related functions (11). The studies included in this review, used HCC as an intervention-related biomarker to map the effects of an intervention and predict post-intervention symptomatology.
Background
The importance of HCC as a biomarker has been widely documented in various fields such as the measurement of stress or in the context of somatic diseases. The relationship between prolonged stress and HCC was demonstrated in a meta-analysis of chronic stress situations (12). In stress-exposed groups, the mean HCC increased by 22%. A positive correlation between HCC and stress is now widely accepted.
In addition, the measurement of the HCC may be relevant in several somatic diseases. One study showed that mean HCC were significantly increased in patients with Cushing’s syndrome (CS) (13). CS influences HCC, and HCC decreases during effective CS treatment. Increased HCC in patients with cyclic CS overlapped with the symptomatic phases of the disease. A previous study showed consistent results (14).
Studies on the relationship between HCC and overweight or obesity found significantly increased HCC levels in children with obesity (15). Furthermore, there is an increased risk of cardiovascular diseases with elevated HCC (16).
Regarding the association between maternal and infant HCC, several studies have provided interesting and sometimes conflicting results. Studies have shown a positive association between perceived stress and HCC in pregnant women, increased HCC in assisted reproductive technology, and increased HCC in the first trimester when a girl is carried to term (17–19). Regarding the mother–child relationship, one study found increased HCC in mothers with prolonged stress during pregnancy, but decreased HCC in their newborns (20). This interaction was confirmed in another study for the first trimester of pregnancy (21). Other studies showed highly interesting results on hair cortisol in pregnant women, but no clear interaction with fetal hair cortisol could be traced (22, 23).
Given the hypothesized critical role of prenatal stress in the early mother–child relationship, mediated by stress responses and subsequent neuroendocrinological changes, in contributing to the etiology of mental disorders in general, these findings suggest further investigation of the association between HCC and psychiatric or psychosomatic disorders (24). Previous studies suggest that HCC also plays an important role in mental disorders and underlying etiological relevant processes. For example, one study describes a dysregulation of the HPA axis in children with emotional and behavioral symptoms, which can be reflected by changes in HCC levels. Thus, children with emotional symptoms had significantly lower hair cortisol levels, whereas HCC levels were highest in older children with behavioral symptoms (25). Another study demonstrated that changes in PTSD symptomatology following trauma exposure could be predicted by HCC. A lower baseline HCC was associated with an increase in symptoms following a new trauma (26). A study of PTSD patients (based on a 12-months PTSD diagnosis), trauma patients (based on A1 and A2 criteria of a traumatic event), and healthy controls revealed significantly lower hair cortisol levels in the trauma and PTSD groups (27). In contrast, one study showed elevated HCC in PTSD patients when measured immediately after trauma exposure (28). Studies of the association between depressive disorders, which are highly prevalent in Western societies, and HCC have yielded conflicting results. Elevated hair cortisol levels were associated with persistent depressive symptoms (29). Significantly lower HCC levels have also been reported in adults with major depressive disorder compared to healthy controls (30). No differences in HCC were found between healthy individuals, patients with atypical depression, and patients with nonatypical depression (31). One of the few studies on depression and HCC in children showed that adolescents had higher HCC together with severe depressive symptoms, but only when they had high academic scores (32). This divergent picture of results is also evident in two meta-analyses: one of them concluded that depressive patients tend to have higher HCC than controls whereas PTSD patients show a trend toward lower HCC (33). The other one summarized that in most studies no differences in HCC were found between patients with major depression and healthy controls (34).
Thus, there is now a consensus in research community that environmental and mental stressors, as well as psychological distress, may ultimately be reflected in variations in HCC. The measurement of hair cortisol therefore seems to be a promising method to map chronic cortisol fluctuations that can occur in the context of psychiatric and psychosomatic diseases and thus also their treatment (35).
Research questions
Based on the possible association between mental disorders and HCC, it is reasonable to assume that HCC also plays a role in the evaluation of psychological and neuropsychiatric interventions. However, this is still an evolving area of research, which explains the limited nature of the existing data. To date, there has been no review of the literature that classifies the importance of hair cortisol in the context of psychological or neuropsychiatric interventions. This literature review intends to give a first overview of the current state of research on the role of HCC in the context of psychological and neuropsychiatric interventions.
On the one hand, this review aims to explore the extent to which HCC is used as a trajectory parameter in research investigating the effects of psychological interventions on people with mental health problems. On the other hand, it is intended to answer the question whether the existing literature provides evidence of the extent to which hair cortisol concentration appears to be suitable as an outcome parameter for psychological and neuropsychiatric interventions by summarizing the existing literature in a structured way. The following research questions, which are formulated in a more general way according to the still preliminary character of the existing literature, shall be answered by the present work:
1. To what extent is HCC used as a predictive or trajectory parameter in psychological and neuropsychiatric intervention studies?
2. How do psychological and neuropsychiatric interventions affect HCC?
Methods
This paper is a literature review based on the PRISMA statement (36). Accordingly, the literature research was developed through a qualitative approach and was guided by the PRISMA guidelines (36). A research protocol was developed, based on PRISMA suggestions (36). The review was not registered. By applying this methodology, it was possible to effectively limit the search for literature according to the objectives established. The following steps were employed: (1) definition of the research question, (2) setting up of the search strategy, (3) definition of the inclusion and exclusion criteria, (4) study selection, (5) data analysis.
Search strategy
To address the research questions, the authors systematically searched the databases Web of science, Google Scholar, PsycINFO and ResearchGate for relevant literature from May to July 2022. The primary database utilized in this study was the Web of Science Core Collection. The other databases were employed to fulfill additional search queries, such as retrieving full texts or conducting targeted searches for specific articles. No date limit will be imposed on the search. Studies in languages other than English and German could not be included due to resource limits.
According to the research question, we identified appropriate keywords and linked them by using the Boolean operators “AND” and “OR.” The following combinations were used: (1) hair cortisol AND review or meta-analysis, (2) hair cortisol or hair cortisol concentration or HCC AND psychotherapy or therapy or counseling or psychological therapy or psychosomatic treatment AND response or outcome or predictor or process AND PTSD or posttraumatic stress disorder or depressive disorder or depression or childhood maltreatment or anxiety disorder or somatic symptom disorder or psychosomatic disorder or major depression or fear or borderline disorder or personality disorder.
Through these selected keywords, we aim to cover as broad a field of mental disorders as possible. However, the studies found in this way mainly examined HCC in the context of psychological and neuropsychiatric interventions in the presence of affective disorders. No relevant literature on hair cortisol and psychological or neuropsychiatric interventions in the context of borderline or other personality disorders could be found.
In addition, the reference section of the studies found by automatic search in the databases were manually searched for further relevant publications.
The authors also made a cursory search in gray literature. However, no suitable studies were found.
Inclusion and exclusion criteria
Articles that considered the topic of hair cortisol and psychological or neuropsychiatric interventions in the title or abstract were included. The further criteria for studies to be included will be outlined below.
Study designs
Randomized controlled trials (RCTs), cluster RCTs, controlled clinical trials, controlled before-after studies, prospective, retrospective, and case–control studies as well as other forms of clinical trials were included. Time series analysis and different forms of qualitative research were also initially considered. The authors did not include non-empirical research studies, unpublished master-level dissertations, unpublished conference presentations and articles where no full text is available.
Participants
Studies examining humans of all ages; children and adolescents as well as adults were included. Animal studies were excluded.
Interventions
Different types of psychological interventions were considered for the present work, including widely-applied traditional forms of psychotherapy such as cognitive behavioral therapy (CBT), other forms of behavioral therapy, analytic and psychodynamic therapy, family therapy, or trauma focused therapy, but also interventions from the broader psychotherapeutic or neuropsychiatric spectrum such as stress-reducing therapies, mindfulness-based interventions, and electroconvulsive therapy (ECT). Studies that did not report any form of intervention were excluded.
Comparators
Various comparisons can be of interest for the present study and will be considered in the literature review. Studies that only measured before and after values of HCC are included as well as studies with an untreated control group or other forms of interventions as a control condition.
Outcome
All the included studies need to determine HCC as an outcome parameter. Both ways of quantifying HCC, LC-MS/MS and EIA, will be considered. Studies that did not quantify HCC were excluded.
Selection of the studies
According to the PRISMA guidelines (36) a flow chart was created (Figure 1), to illustrate the selection process of the relevant studies. The Identification phase shows the total number of articles found by database- and snowball-research, according to the keywords used. Before screening, the duplicates were removed. In the Screening phase, we applied the inclusion criteria to titles and abstracts. The exclusion criteria were then applied during the Eligibility phase. The criteria used in the corresponding phase are listed in the flow diagram. Finally, these steps resulted in 14 articles included in this review. The study selection process was conducted by two of the authors (VH, TB) independently.
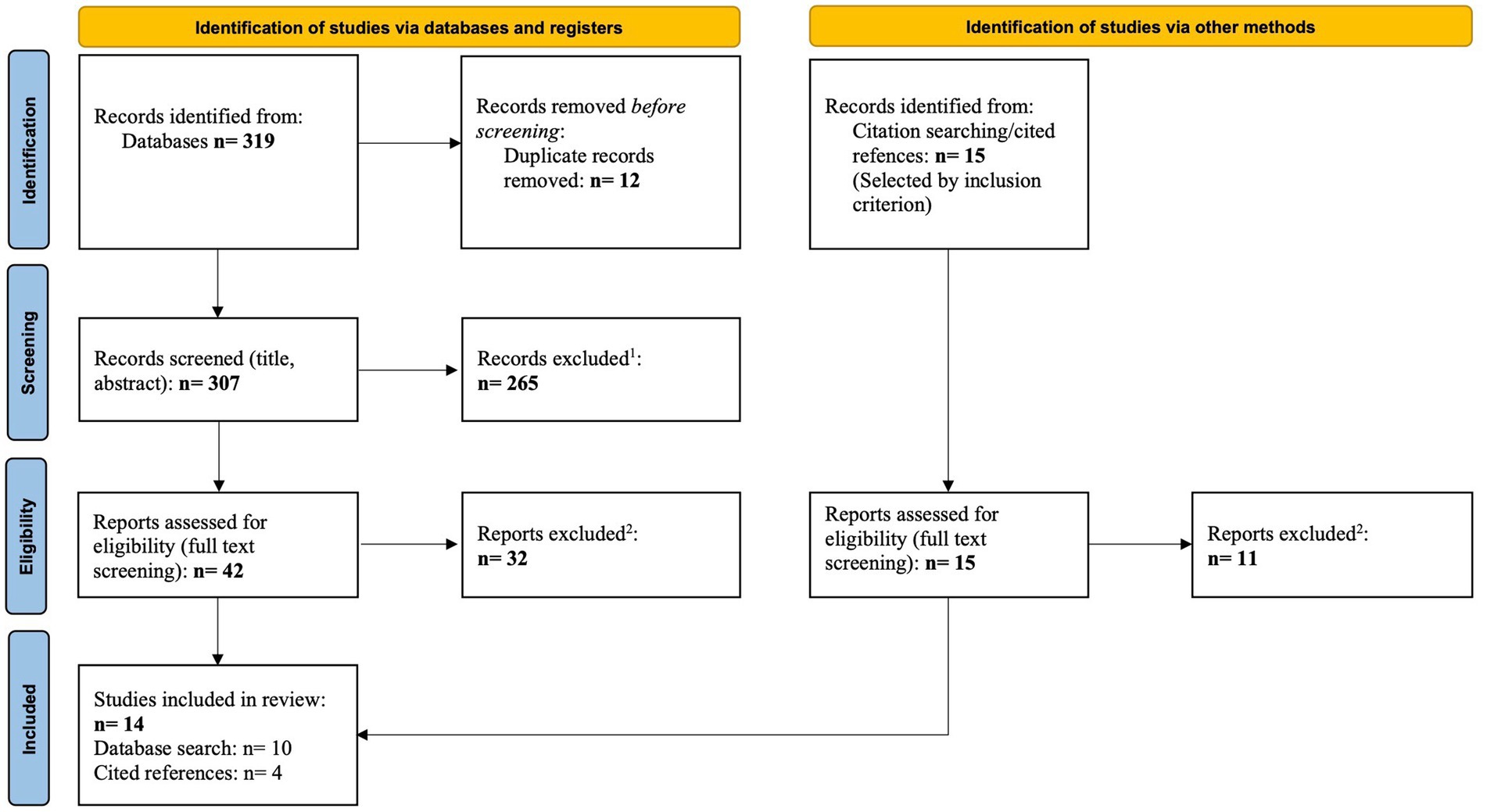
Figure 1. Flow diagram of study selection process [adapted according to PRISMA statement (36)].
Quality assessment and data analysis
Based on the selected studies, we read each article with particular attention to describe the significance of HCC and the potential predictive value of HCC in psychological and neuropsychiatric interventions. Microsoft Excel was used to manage and organize the relevant extracted information from the studies. Subsequently, these were then transferred into a well-structured table containing the most important data and results of the publications considered. If specified in the publications, the individual hair cortisol values measured are also listed. The table is divided into a part with the studies that mainly focus on the longitudinal changes of HCC in the course of a psychological or neuropsychiatric intervention and a second part with the studies on the predictive power of HCC for the treatment outcome (see Tables 1A,B). The studies and their results are moreover presented, summarized, and evaluated in detail in the following.
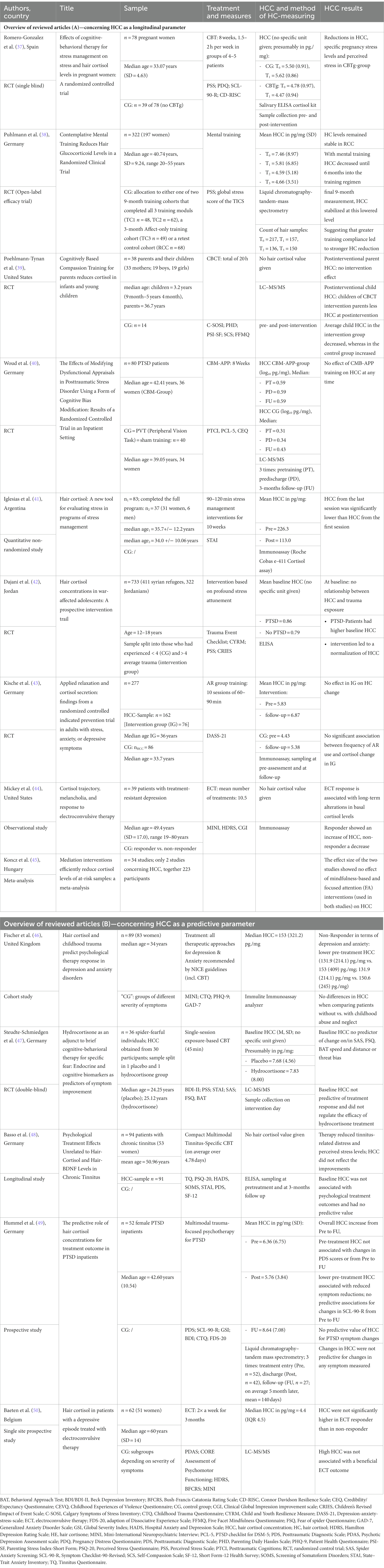
Table 1. Overview of reviewed articles (A)—concerning HCC as a longitudinal parameter and (B)—concerning HCC as a predictive parameter.
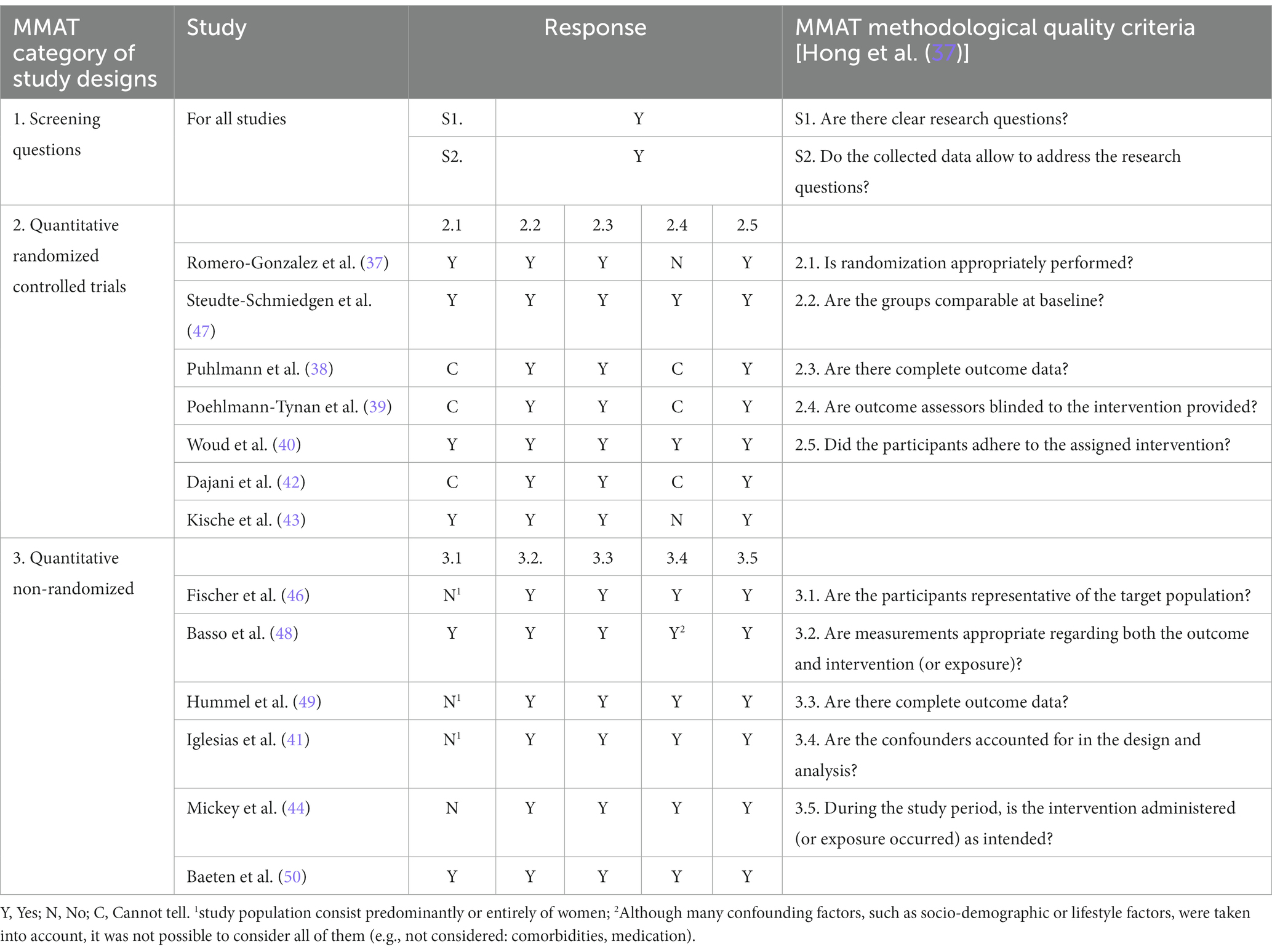
To evaluate the risk of bias and the quality of the studies included, we used the Mixed methods appraisal tool [MMAT; (51)]. The MMAT is specifically designed for systematic mixed studies reviews and was therefore chosen for the present research. As the MMAT appraisal tool [see Table 2; (51)] shows, the methodological quality of the studies, although predominantly high, is heterogeneous. The included studies consist of 13 quantitative trials and one meta-analysis (not assessed by MMAT). Seven of the studies could be identified as randomized controlled trials. The remaining six are different forms of quantitative non-randomized studies such as cohort studies, observational or prospective trials. For all non-randomized studies and for four of the randomized studies, at least four of five questions of the MMAT quality assessment could be answered with “yes.” However, some limitations were observed or described by the respective authors themselves. Among them are a small sample size, determination of HCC at only one or two time points or a short follow-up period. Considering the mostly small number of subjects, it is nevertheless positive to note that the samples of most studies were heterogeneous and thus represented a good cross-section of the underlying population. In the presence of a control group, the subject groups were comparable at baseline in all these studies. Only in three of the non-randomized trials, the study population consisted predominantly or entirely of women. In one, it is explicitly stated that the selection of the participants may not be representative. Therefore, for these studies, the question of the MMAT tool as to whether the sample was representative, had to be answered with “no.”
The non-randomized studies in general must be assessed with a high risk of bias due to the lack of randomization and the absence of a control group in some of them.
In the case of three of the randomized controlled trials, it should be noted negatively that the randomization process was not adequately described. Furthermore, it is not clear whether blinding took place. Another randomized controlled trial did not use blinding of the researchers and participants at all, but this circumstance was clearly described. However, this leads to a high risk of detection bias.
Due to the still small number of studies considered and the heterogeneity of their methodological quality, we decided not to conduct a meta-analysis.
Results
As shown in Figure 1, the initial, automatic search of the above-mentioned databases produced 319 results. First, 12 duplicates were filtered out using the Find Duplicates function in the literature management program EndNote X9. The remaining 307 articles were then screened by title and abstract. In this process, 265 were found to be ineligible for this review. In a further step, the full texts of the remaining 42 studies were screened and evaluated. This resulted in the exclusion of 32 publications.
In addition, as mentioned above, a hand-search was conducted in the reference section of the previously selected studies. Initially, 15 studies were found to be usable. In the full-text screening, 11 of these results had to be excluded. The exclusion criteria can be found in the methods section and in Figure 1. To illustrate the exact selection process, a flowchart was created as described in the methods section (see Figure 1).
The selection process thus resulted in a total number of 14 studies, which are considered and described below.
Overview of included studies
The 14 studies reviewed are summarized in Tables 1A,B. Regarding the research questions, some of the studies address the effects of well-established psychotherapy treatments such as cognitive behavioral therapy (CBT), while others examine the effect of psychological interventions in a broader sense.
The oldest study considered here was published in 2015, the most recent ones are from 2022. The vast majority of the included publications come from Europe (Germany, United Kingdom, Spain, Belgium, Hungary). Two were from the USA and one each from Argentina and Jordan.
These 14 studies collectively involved a total of 1,916 participants. The number of participants varied significantly and ranged from 30 to 733. The average age of the study population also varied widely, ranging from adolescents to an average age of 60 years. Mostly, however, it was in the range of 30 to 45 years. Accordingly, studies that included only adolescents/children, only adults, or both age groups as participants were included. Only one study included females only. The other study populations were of mixed gender.
The 14 studies encompassed in this review followed diverse methodological approaches. One is a meta-analysis, and the others are quantitative trials of various types.
Consistent with the defined research questions, the authors included nine studies focusing on longitudinal changes in HCC over the course of a psychological or neuropsychiatric intervention and five studies concentrating on the predictive power of hair cortisol on treatment response.
Results on longitudinal changes of hair cortisol
Regarding longitudinal changes in HCC over the course of psychological or neuropsychiatric interventions, one study investigated the effects of CBT, a well-established form of psychotherapy, on HCC. This randomized controlled trial of 78 pregnant women, divided in an experimental and a control group, examined the efficacy of CBT for stress management in reducing psychological stress and HCC. In the therapy group, a reduction in HCC, specific pregnancy stress scores, and general stress levels (lower PDQ-, PSS-, SCL-90-R-, PSDI-scores) was found. According to the authors, the decrease in these psychological and endocrinological stress parameters reflects the effectiveness of CBT in pregnancy (37).
With regard to less intensive psychological interventions, the effects of different mental training programs on long-term endocrine and psychological stress parameters were investigated. Regardless of the training content, HCC values decreased continuously in 159 participants up to 6 months post-treatment and were significantly reduced compared to the 68 controls. After 9 months, HCC stabilized at a lower level or slightly increased compared with the baseline (38). Similarly, an effect of Cognitively Based Compassion Training (CBCT) on HCC was demonstrated. Therefore, 25 parents undergo an 8-week CBCT program and parental and child HCC was subsequently determined. Interestingly, there was no significant interventional effect on parental HCC. However, the children had lower HCC after their parents’ treatment compared with controls, where concentrations increased (39). Moreover, a Cognitive Bias Modification for Appraisals intervention failed to have any effect on HCC in the study population of 80 PTSD-patients (40).
Two of the three included studies, which focused primarily on the effect of stress-reducing interventions, found an association between treatment and HCC. A 10-week stress management intervention for 83 adults resulted in significantly lower HCC at the last, compared to the first appointment (41). In a group of 411 underage Syrian refugees and 322 Jordanian adolescents, some with PTSD, the influence of a psychosocial, stress-reducing intervention on cortisol production was analyzed. Regardless of gender, origin, or trauma exposure, this form of therapy resulted in an average reduction in HCC of one third across all groups. Looking at the groups individually, a decrease in HCC was evident in the group of adolescents with cortisol hypersecretion and medium secretion, and an increase was measured in adolescents with hyposecretion (42). No association was shown between HCC and an applied relaxation group training in a study with 277 adults with symptoms of stress, anxiety, or depression. Neither for the intervention in general nor for the frequency of therapy significant changes in HCC could be found (43).
Electroconvulsive therapy (ECT) has established itself as a form of neuropsychiatric treatment, especially for severe depression (52). In one study of the effects of ECT on HCC 39 patients with treatment-resistant depression were subjected to an average of 10.5 ECT sessions. In responders, HCC tended to increase, in non-responders it decreased (44).
Finally, a meta-analysis examined the effects of meditation on cortisol. However, only two studies quantified HCC. In both, there was no effect of mindfulness-based and focused attention interventions on HCC (45).
Results on predictive power of hair cortisol on treatment response
Five studies on this topic could be found. Three of them used variants of cognitive behavioral therapy. One of them provided results indicating a predictive value of HCC. In 89 patients with depression and anxiety disorders, it was investigated whether HCC was a predictive parameter for treatment outcome. The treatment included all NICE guideline-recommended treatment approaches for depression and anxiety, including CBT. Non-responders had a lower HCC before the start of therapy (46). In a further study, 36 patients with arachnophobia participated in a single 45-min CBT session. The research group subsequently assessed the value of the basal endogenous glucocorticoid secretion regarding treatment outcome. However, basal HCC did not emerge as a significant predictor of changes in 1-month follow-up scores on the anxiety questionnaires (Self-Rating Anxiety Scale, Fear of Spiders Questionnaire) and therapy response (47). In addition, the effects of tinnitus-specific CBT on HCC and other parameters were examined in 91 patients with chronic tinnitus. HCC did not reflect the positive effects of the CBT on tinnitus-related distress and perceived stress levels. Thus, HCC was not related to with the outcome of this kind of therapy (48).
In terms of less established psychological interventions, a study examined HCC in the context of multimodal trauma-focused inpatient psychotherapy for PTSD patients. Hair samples from 52 female PTSD patients were analyzed at different examination times. HCC measured at admission and discharge did not differ. Lower HCC before therapy could predict less improvement in clinical symptoms, but this relationship was no longer statistically significant after adjustment for baseline dissociative symptoms. Thus, there was no clear predictive value of HCC with respect to changes in PTSD symptomatology (49).
In another study investigating the association between ECT and HCC, 62 patients with varying degrees of major depression were treated with ECT twice a week for 3 months. Here, HCC was not significantly elevated in the responder group. Increased hair cortisol levels therefore do not appear to be associated with better therapy outcome (50).
Discussion
The aim of this review was to summarize the current state of research on the effects of different psychological and neuropsychiatric interventions on HCC and to investigate the extent to which hair cortisol can be altered in such types of interventions. Although only a few studies have been published to date, there has been a general trend toward increased relevance of HCC measures in the context of psychological and neuropsychiatric interventions.
HCC has been used to assess stress-related interventions such as relaxation training and, in fewer studies, for traditional forms of psychotherapeutic interventions such as CBT.
Looking at these publications, the reported study results are still rather inhomogeneous. As CBT is a well-established form of psychotherapy, the results of these studies are of particular interest. Two studies showed different associations between CBT and HCC. One study showed a decrease in HCC in the treatment group (37). In the second study, non-responder had lower baseline HCC before intervention. Thus, reduced HCC appears to be associated with a lower likelihood of treatment success (46). These studies are in contrast to other studies investigating HCC in CBT, which found no predictive value of HCC for treatment outcome and no direct relationship between HCC and therapy results (47, 48). Thus, how and if HCC is affected by this form of therapy cannot yet be adequately demonstrated. Further comparable and well-designed studies are needed to confirm the reported results.
Data on different relaxation methods, mental training, and ECT are also mixed. Half of the included studies found an association between the different interventions and a predictive potential in form of a longitudinal decrease in HCC.
In the context of a multimodal trauma-focused inpatient psychotherapy, there was no predictive value for HCC changes in terms of PTSD symptoms improvement (49). Various mental training interventions in one study and stress-reduction interventions in two studies led to sustained reductions in HCC (38, 41, 42).
The variability of HCC in ECT appears to be puzzling. Therapy responders showed increased HCC in one study (44). However, this result could not be confirmed, and a high HCC was not associated with a positive treatment outcome in another, more recent study (50).
In summary, there is a trend toward a reduction in HCC with psychological and neuropsychiatric interventions, as no study showed a clear increase in HCC after the intervention, but several found a reduction in cortisol levels. As stress, as reflected by the cortisol levels, is an important underlying factor in the development of mental disorders, a reduction of cortisol levels seems to be of particular importance in alleviating the symptoms of disorders such as depression or PTSD. This highlights the importance of establishing a standard biomarker to measure the impact of psychological interventions.
However, the results are still rather inconclusive. The explanation may be complex. One important aspect to consider seems to be that the effects of psychotherapy are not linear. Psychotherapy can often be upsetting and challenging within a given process. Thus, it may have an immediate stress-inducing effect, with the relieving effect coming later. The immediate effect of psychotherapy may also vary depending on the type of therapy and the invasiveness of the therapeutic interventions. The collection of catamnestic data may therefore be of particular importance in mapping the long-term effects of interventions. The lack of effects in some of the reported studies may be explained by the fact that they involved low-intensity stress management training and the like, which did not produce profound changes in the patients that resonated down to the biological level.
The most important reason for the heterogeneous data may be the methodology used in the included studies and their varying quality. Due to the quality criteria, only a small number of publications could be included, which underlines the novelty of the research field. Hair cortisol as a biomarker for psychological and neuropsychiatric interventions should be considered as little established in a still evolving research field, which required the inclusion of studies of heterogeneous quality.
Some methodological limitations of HCC measurement must be kept in mind, which limit the validity and significance of the study results presented above. The comparability of all included studies is limited due to differences in age structure, numbers of participants and type of intervention. In addition, the reported divergent results may be due to different study designs. Additionally, gender differences in HCC, with higher values in adult men, may influence the discrepancy (8). Age per se, and hormonal changes, such as pregnancy, influence HCC. Higher hair cortisol levels have been found in young children and older adults (53). Thus, when evaluating the effect of therapy on hair cortisol or when assessing the predictive power of HCC, the age of the participants is an important potential confounding factor.
Furthermore, the different measurement methods of HCC limit the comparability of the reviewed studies. It could be shown that the two methods of measurement were highly correlated with each other, but that immunoassays showed a constant tendency toward higher values (54). In addition, there is inter-laboratory variation in HCC detection (12), which explains why it has not been possible to establish generally accepted reference values. Nevertheless, the establishment of normative HCC data appears to be of critical importance as long as the reported variance in the treatment-related outcomes may be due to different methodological approaches to HCC determination. Normative values could thus contribute to a better comparability of the results.
Nevertheless, a possible effect of various psychological interventions on HCC as well as some predictive value of hair cortisol in the context of such treatment modalities cannot be excluded. However, these results are still inconclusive and need to be investigated and replicated in further studies.
Conclusion
In summary, HCC may be a valuable parameter for monitoring psychological and neuropsychiatric interventions in psychotherapy research. HCC seems to be particularly suitable for this purpose because it consists of aggregated cortisol levels over a defined period of time and represents a stress-related aspect of any psychological intervention or neuropsychiatric treatment. At present, the extent to which HCC can be used for these purposes cannot be clearly determined, not least because of the inconsistent data situation. Thus, the current state of research reflects only a fundamental orientation. However, it indicates that the documentation of changes in HCC during the therapeutic process seems promising and most authors consider it useful to include psychoendocrine biomarkers in psychological and neuropsychiatric intervention studies. Nevertheless, the potential of HCC for true psychological or neuropsychiatric investigations needs to be solidified by further studies with greater consideration of the parameters and study design requirements mentioned above. In the long run, this will hopefully define a clearer direction on the impact of psychological interventions on HCC and thus contribute to the relevance of hair cortisol as a standard psychobiological parameter. Psychopharmacological studies and larger epidemiological surveys of stress in a general population would benefit as well.
The psychoendocrine approach to psychotherapy and psychological interventions may help to compare the effects of different psychotherapeutic methods and to establish differential indications for psychosomatic/psychotherapeutic treatment modalities. Especially for the psychosomatic field, the combination of psychological and somatic parameters to measure the therapeutic effect seems intuitive and highly relevant.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TB, BB, and EP contributed to conception and design of the study. VH contributed to literature research and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank “KroKi Verein für chronisch kranke Kinder” for their financial support for future studies and laboratory investigations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Herman, JP , McKlveen, JM , Ghosal, S , Kopp, B , Wulsin, A , Makinson, R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. (2016) 6:603–21. doi: 10.1002/cphy.c150015
2. Stalder, T , and Kirschbaum, C . Analysis of cortisol in hair—state of the art and future directions. Brain Behav Immun. (2012) 26:1019–29. doi: 10.1016/j.bbi.2012.02.002
3. Laufer, S , Engel, S , Knaevelsrud, C , and Schumacher, S . Cortisol and alpha-amylase assessment in psychotherapeutic intervention studies: a systematic review. Neurosci Biobehav Rev. (2018) 95:235–62. doi: 10.1016/j.neubiorev.2018.09.023
4. Fischer, S , and Zilcha-Mano, S . Why does psychotherapy work and for whom? Hormonal answers. Biomedicine. (2022) 10:1361. doi: 10.3390/biomedicines10061361
5. Meuret, AE , Trueba, AF , Abelson, JL , Liberzon, I , Auchus, R , Bhaskara, L, et al. High cortisol awakening response and cortisol levels moderate exposure-based psychotherapy success. Psychoneuroendocrinology. (2015) 51:331–40. doi: 10.1016/j.psyneuen.2014.10.008
6. Hodes, A , Meyer, J , Lodish, MB , Stratakis, CA , and Zilbermint, M . Mini-review of hair cortisol concentration for evaluation of Cushing syndrome. Expert Rev Endocrinol Metab. (2018) 13:225–31. doi: 10.1080/17446651.2018.1517043
7. Wennig, R . Potential problems with the interpretation of hair analysis results. Forensic Sci Int. (2000) 107:5–12. doi: 10.1016/s0379-0738(99)00146-2
8. Binz, TM , Rietschel, L , Streit, F , Hofmann, M , Gehrke, J , Herdener, M, et al. Endogenous cortisol in keratinized matrices: systematic determination of baseline cortisol levels in hair and the influence of sex, age and hair color. Forensic Sci Int. (2018) 284:33–8. doi: 10.1016/j.forsciint.2017.12.032
9. Noppe, G , van Rossum, EFC , Koper, JW , Manenschijn, L , Bruining, GJ , de Rijke, YB, et al. Validation and reference ranges of hair cortisol measurement in healthy children. Horm Res Paediatr. (2014) 82:97–102. doi: 10.1159/000362519
10. Sauve, B , Koren, G , Walsh, G , Tokmakejian, S , and Van Uum, SHM . Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. (2007) 30:E183–91. doi: 10.25011/cim.v30i5.2894
11. Engel, S , Klusmann, H , Laufer, S , Kapp, C , Schumacher, S , and Knaevelsrud, C . Biological markers in clinical psychological research—a systematic framework applied to Hpa Axis regulation in Ptsd. Compr Psychoneuroendocrinol. (2022) 11:100148. doi: 10.1016/j.cpnec.2022.100148
12. Stalder, T , Steudte-Schmiedgen, S , Alexander, N , Klucken, T , Vater, A , Wichmann, S, et al. Stress-related and basic determinants of hair cortisol in humans: a Meta-analysis. Psychoneuroendocrinology. (2017) 77:261–74. doi: 10.1016/j.psyneuen.2016.12.017
13. Manenschijn, L , Koper, JW , van den Akker, ELT , de Heide, LJM , Geerdink, EAM , de Jong, FH, et al. A novel tool in the diagnosis and follow-up of (cyclic) Cushing's syndrome: measurement of long-term cortisol in scalp hair. J Clin Endocrinol Metab. (2012) 97:E1836–43. doi: 10.1210/jc.2012-1852
14. Thomson, S , Koren, G , Fraser, LA , Rieder, M , Friedman, TC , and Van Uum, SHM . Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp Clin Endocrinol Diabetes. (2010) 118:133–8. doi: 10.1055/s-0029-1220771
15. Veldhorst, MAB , Noppe, G , Jongejan, M , Kok, CBM , Mekic, S , Koper, JW, et al. Increased scalp hair cortisol concentrations in obese children. J Clin Endocrinol Metab. (2014) 99:285–90. doi: 10.1210/jc.2013-2924
16. Iob, E , and Steptoe, A . Cardiovascular disease and hair cortisol: a novel biomarker of chronic stress. Curr Cardiol Rep. (2019) 21:116. doi: 10.1007/s11886-019-1208-7
17. Kalra, S , Einarson, A , Karaskov, T , Van Uum, S , and Koren, G . The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. (2007) 30:E103–7. doi: 10.25011/cim.v30i2.986
18. Caparros-Gonzalez, RA , Romero-Gonzalez, B , Quesada-Soto, JM , Gonzalez-Perez, R , Marinas-Lirola, JC , and Peralta-Ramirez, MI . Maternal hair cortisol levels affect neonatal development among women conceiving with assisted reproductive technology. J Reprod Infant Psychol. (2019) 37:480–98. doi: 10.1080/02646838.2019.1578949
19. Romero-Gonzalez, B , Puertas-Gonzalez, JA , Gonzalez-Perez, R , Davila, M , and Peralta-Ramirez, MI . Hair cortisol levels in pregnancy as a possible determinant of fetal sex: a longitudinal study. J Dev Orig Health Dis. (2021) 12:902–7. doi: 10.1017/s2040174420001300
20. van der Voorn, B , Hollanders, JJ , Kieviet, N , Dolman, KM , de Rijke, YB , van Rossum, EFC, et al. Maternal stress during pregnancy is associated with decreased cortisol and cortisone levels in neonatal hair. Horm Res Paediatr. (2018) 90:299–307. doi: 10.1159/000495007
21. Romero-Gonzalez, B , Caparros-Gonzalez, RA , Gonzalez-Perez, R , Delgado-Puertas, P , and Peralta-Ramirez, MI . Newborn Infants' hair cortisol levels reflect chronic maternal stress during pregnancy. PLoS One. (2018) 13:e0200279. doi: 10.1371/journal.pone.0200279
22. Hoffman, MC , D'Anna-Hernandez, K , Benitez, P , Ross, RG , and Laudenslager, ML . Cortisol during human fetal life: characterization of a method for processing small quantities of newborn hair from 26 to 42 weeks gestation. Dev Psychobiol. (2017) 59:123–7. doi: 10.1002/dev.21433
23. Hollanders, JJ , van der Voorn, B , Kieviet, N , Dolman, KM , de Rijke, YB , van den Akker, ELT, et al. Interpretation of glucocorticoids in neonatal hair: a reflection of intrauterine glucocorticoid regulation? Endocr Connect. (2017) 6:692–9. doi: 10.1530/ec-17-0179
24. Kendler, KS , Karkowski, LM , and Prescott, CA . Causal relationship between stressful life events and the onset of major depression. Am J Psychiatr. (1999) 156:837–41. doi: 10.1176/ajp.156.6.837
25. Golub, Y , Kuitunen-Paul, S , Panaseth, K , Stonawski, V , Frey, S , Steigleder, R, et al. Salivary and hair cortisol as biomarkers of emotional and behavioral symptoms in 6–9 year old children. Physiol Behav. (2019) 209:112584. doi: 10.1016/j.physbeh.2019.112584
26. Steudte-Schmiedgen, S , Stalder, T , Schönfeld, S , Wittchen, HU , Trautmann, S , Alexander, N, et al. Hair cortisol concentrations and cortisol stress reactivity predict Ptsd symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology. (2015) 59:123–33. doi: 10.1016/j.psyneuen.2015.05.007
27. Steudte, S , Kirschbaum, C , Gao, W , Alexander, N , Schönfeld, S , Hoyer, J, et al. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. (2013) 74:639–46. doi: 10.1016/j.biopsych.2013.03.011
28. Steudte, S , Kolassa, IT , Stalder, T , Pfeiffer, A , Kirschbaum, C , and Elbert, T . Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with Ptsd. Psychoneuroendocrinology. (2011) 36:1193–200. doi: 10.1016/j.psyneuen.2011.02.012
29. Iob, E , Kirschbaum, C , and Steptoe, A . Persistent depressive symptoms, Hpa-Axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry. (2020) 25:1130–40. doi: 10.1038/s41380-019-0501-6
30. Pochigaeva, K , Druzhkova, T , Yakovlev, A , Onufriev, M , Grishkina, M , Chepelev, A, et al. Hair cortisol as a marker of hypothalamic-pituitary-adrenal Axis activity in female patients with major depressive disorder. Metab Brain Dis. (2017) 32:577–83. doi: 10.1007/s11011-017-9952-0
31. Herane-Vives, A , de Angel, V , Papadopoulos, A , Wise, T , Chua, KC , Strawbridge, R, et al. Short-term and long-term measures of cortisol in saliva and hair in atypical and non-atypical depression. Acta Psychiatr Scand. (2018) 137:216–30. doi: 10.1111/acps.12852
32. Xu, YY , Liu, YP , Chen, Z , Zhang, J , Deng, HH , and Gu, JX . Interaction effects of life events and hair cortisol on perceived stress, anxiety, and depressive symptoms among Chinese adolescents: testing the differential susceptibility and diathesis-stress models. Front Psychol. (2019) 10:297. doi: 10.3389/fpsyg.2019.00297
33. Koumantarou Malisiova, E , Mourikis, I , Darviri, C , Nicolaides, NC , Zervas, IM , Papageorgiou, C, et al. Hair cortisol concentrations in mental disorders: a systematic review. Physiol Behav. (2021) 229:113244. doi: 10.1016/j.physbeh.2020.113244
34. Psarraki, EE , Kokka, I , Bacopoulou, F , Chrousos, GP , Artemiadis, A , and Darviri, C . Is there a relation between major depression and hair cortisol? A systematic review and Meta-analysis. Psychoneuroendocrinology. (2021) 124:105098. doi: 10.1016/j.psyneuen.2020.105098
35. Wester, VL , and van Rossum, EFC . Clinical applications of cortisol measurements in hair. Eur J Endocrinol. (2015) 173:M1–M10. doi: 10.1530/eje-15-0313
36. Page, MJ , McKenzie, JE , Bossuyt, PM , Boutron, I , Hoffmann, TC , Mulrow, CD, et al. The Prisma 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
37. Romero-Gonzalez, B , Puertas-Gonzalez, JA , Strivens-Vilchez, H , Gonzalez-Perez, R , and Peralta-Ramirez, MI . Effects of cognitive-Behavioural therapy for stress management on stress and hair cortisol levels in pregnant women: a randomised controlled trial. J Psychosom Res. (2020) 135:162. doi: 10.1016/j.jpsychores.2020.110162
38. Puhlmann, LMC , Vrtička, P , Linz, R , Stalder, T , Kirschbaum, C , Engert, V, et al. Contemplative mental training reduces hair glucocorticoid levels in a randomized clinical trial. Psychosom Med. (2021) 83:894–905. doi: 10.1097/psy.0000000000000970
39. Poehlmann-Tynan, J , Engbretson, A , Vigna, AB , Weymouth, LA , Burnson, C , Zahn-Waxler, C, et al. Cognitively-based compassion training for parents reduces cortisol in infants and Young children. Infant Ment Health J. (2020) 41:126–44. doi: 10.1002/imhj.21831
40. Woud, ML , Blackwell, SE , Shkreli, L , Würtz, F , Cwik, JC , Margraf, J, et al. The effects of modifying dysfunctional appraisals in posttraumatic stress disorder using a form of cognitive Bias modification: results of a randomized controlled trial in an inpatient setting. Psychother Psychosom. (2021) 90:386–402. doi: 10.1159/000514166
41. Iglesias, S , Jacobsen, D , Gonzalez, D , Azzara, S , Repetto, EM , Jamardo, J, et al. Hair cortisol: a new tool for evaluating stress in programs of stress management. Life Sci. (2015) 141:188–92. doi: 10.1016/j.lfs.2015.10.006
42. Dajani, R , Hadfield, K , van Uum, S , Greff, M , and Panter-Brick, C . Hair cortisol concentrations in war-affected adolescents: a prospective intervention trial. Psychoneuroendocrinology. (2018) 89:138–46. doi: 10.1016/j.psyneuen.2017.12.012
43. Kische, H , Zenker, M , Pieper, L , Beesdo-Baum, K , and Asselmann, E . Applied relaxation and cortisol secretion: findings from a randomized controlled indicated prevention trial in adults with stress, anxiety, or depressive symptoms. Stress. (2022) 25:122–33. doi: 10.1080/10253890.2022.2045939
44. Mickey, BJ , Ginsburg, Y , Sitzmann, AF , Grayhack, C , Sen, S , Kirschbaum, C, et al. Cortisol trajectory, melancholia, and response to electroconvulsive therapy. J Psychiatr Res. (2018) 103:46–53. doi: 10.1016/j.jpsychires.2018.05.007
45. Koncz, A , Demetrovics, Z , and Takacs, ZK . Meditation interventions efficiently reduce cortisol levels of at-risk samples: a Meta-analysis. Health Psychol Rev. (2021) 15:56–84. doi: 10.1080/17437199.2020.1760727
46. Fischer, S , King, S , Papadopoulos, A , Hotopf, M , Young, AH , and Cleare, AJ . Hair cortisol and childhood trauma predict psychological therapy response in depression and anxiety disorders. Acta Psychiatr Scand. (2018) 138:526–35. doi: 10.1111/acps.12970
47. Steudte-Schmiedgen, S , Fay, E , Capitao, L , Kirschbaum, C , and Reinecke, A . Hydrocortisone as an adjunct to brief cognitive-Behavioural therapy for specific fear: endocrine and cognitive biomarkers as predictors of symptom improvement. J Psychopharmacol. (2021) 35:641–51. doi: 10.1177/02698811211001087
48. Basso, L , Boecking, B , Neff, P , Brueggemann, P , Mazurek, B , and Peters, EMJ . Psychological treatment effects unrelated to hair-cortisol and hair-Bdnf levels in chronic tinnitus. Frontiers. Psychiatry. (2022) 13:13. doi: 10.3389/fpsyt.2022.764368
49. Hummel, KV , Schellong, J , Trautmann, S , Kummer, S , Hürrig, S , Klose, M, et al. The predictive role of hair cortisol concentrations for treatment outcome in Ptsd inpatients. Psychoneuroendocrinology. (2021) 131:105326. doi: 10.1016/j.psyneuen.2021.105326
50. Baeten, RF , van Rossum, EFC , de Rijke, YB , Sabbe, BGC , van der Mast, RC , Belge, JB, et al. Hair cortisol in patients with a depressive episode treated with electroconvulsive therapy. J Affect Disord. (2020) 274:784–91. doi: 10.1016/j.jad.2020.05.042
51. Hong, QN , Fàbregues, S , Bartlett, G , Boardman, F , Cargo, M , Dagenais, P, et al. The mixed methods appraisal tool (Mmat) version 2018 for information professionals and researchers. Educ Inf. (2018) 34:285–91. doi: 10.3233/EFI-180221
52. van Diermen, L , van den Ameele, S , Kamperman, AM , Sabbe, BC , Vermeulen, T , Schrijvers, D, et al. Prediction of electroconvulsive therapy response and remission in major depression: Meta-analysis. Br J Psychiatry. (2018) 212:71–80. doi: 10.1192/bjp.2017.28
53. Dettenborn, L , Tietze, A , Kirschbaum, C , and Stalder, T . The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress. (2012) 15:578–88. doi: 10.3109/10253890.2012.654479
Keywords: hair cortisol, hair cortisol concentration (HCC), psychotherapy research, psychological interventions, outcome, psycho-neuroendocrinology, structured review
Citation: Botschek T, Hußlein V, Peters EMJ and Brosig B (2023) Hair cortisol as outcome parameter for psychological and neuropsychiatric interventions—a literature review. Front. Psychiatry. 14:1227153. doi: 10.3389/fpsyt.2023.1227153
Edited by:
Béla Birkás, University of Pécs, HungaryReviewed by:
Yulia Golub, University of Oldenburg, GermanyMichael Kellner, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2023 Botschek, Hußlein, Peters and Brosig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Burkhard Brosig, YnVya2hhcmQuYnJvc2lnQHBzeWNoby5tZWQudW5pLWdpZXNzZW4uZGU=
†These authors have contributed equally to this work and share first authorship
 Tim Botschek
Tim Botschek Vincent Hußlein
Vincent Hußlein Eva M. J. Peters
Eva M. J. Peters Burkhard Brosig
Burkhard Brosig