- 1Department of Developmental Pediatrics, The Second Hospital of Jilin University, Changchun, China
- 2Pediatrics Centre, The Second Hospital of Jilin University, Changchun, China
- 3Center for Obsessive-Compulsive Disorder (OCD), Anxiety, and Related Disorders for Children, Division of Child and Adolescent Psychiatry, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Background: Premonitory urges (PUs) have been the focus of recent efforts to assess the severity and develop interventions for tic disorders (TD). We aimed to investigate the PUs in TD and its comorbidities from multiple dimensions, using the Chinese version of the Premonitory Urge for Tics Scale (C-PUTS) and the Chinese version of the Individualized Premonitory Urge for Tics Scale (C-IPUTS), in order to provide perspectives for the diagnosis and management of TD in children and adolescents.
Methods: A total of 123 cases were included in the study. The IPUTS was translated, back-translated, culturally adjusted, and pre-investigated to determine the items of the C-IPUTS. The reliability and validity of the C-IPUTS scale were evaluated by a questionnaire survey on children and adolescents with TD at the Developmental Pediatrics Department of the Second Hospital of Jilin University. Meanwhile, the C-PUTS, which had been evaluated and used in China, Yale Global Tic Severity Scale (YGTSS), Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), Depression Self-Rating Scale (DSRS), Screen for Childhood Anxiety-Related Disorders (SCARED), Achenbach Child Behavior Checklist (CBCL), and Swanson, Nolan and Pelham, Version IV (SNAP-IV), were used to assess the association of PUs with tics and comorbidities of TD.
Results: All dimensions of the C-IPUTS demonstrated good reliability and validity. Our findings suggested that PUs in children and adolescents in China occurred primarily at the head/face and neck/throat. The different dimensions of the C-IPUTS (number, frequency, and intensity) and C-PUTS were positively correlated with the YGTSS total score, while the C-PUTS was positively correlated with the Y-BOCS, SCARED, DSRS, and SNAP-IV scale total scores. The three dimensions of the C-IPUTS demonstrated correlations with anxiety severity and obsessive-compulsive symptoms.
Conclusion: The C-IPUTS can be used to assess PUs reliably and effectively and provide further information for the C-PUTS from various dimensions in a Chinese setting. PUs relate to obsessive-compulsive symptoms, anxiety, attention deficit hyperactivity, and behavioral problems in children and adolescents with TDs. Accordingly, PUs evaluation using the C-IPUTS combined with the PUTS might provide useful information for future therapies for TDs to achieve greater tic reduction.
1. Introduction
Premonitory urges (PUs), often described as aversive or unpleasant sensations (1), are sensory phenomena preceding the onset of tics (2) in tic disorders (TD). PUs are often described by patients as “uncomfortable itching,” “sneezing,” (2) and sensations such as “energy release sensation,” “pressure sensation,” and “itching” or “pain” before tics occur. Leckman et al. (3) pointed out that PUs are the core symptoms of TDs, and can be more painful and/or uncomfortable than tics themselves in some cases. While the nature of tics is multi-factorial, tics are often preceded by PUs and tic expression reportedly temporarily alleviates the discomfort from PUs. Cavanna et al. (4) showed that PUs, and tic severity of TDs can be used as predictors of health-related wellbeing in late adolescence and adulthood. Previous meta-analyses in China showed that PUs have a strong correlation with TDs and play a key role in the expression of tic symptoms (5).
Aside from tics themselves, PUs are associated with attention deficit hyperactivity disorder (ADHD), anxiety, depression, and obsessive-compulsive symptoms (OCS) (6). Similar to the adverse effects of comorbid conditions and coexisting psychopathology on patients’ quality of life, PUs may be more troublesome than tics themselves. In addition, the severity of PUs can predict a reduction in the benefit of behavioral therapy (7), and serves as a potential treatment mechanism underlying tic severity reductions (8, 9). The recommended first-line behavioral interventions for TD, including habit reversal training (HRT) and exposure and defense response (ERP), are based on the perception and habituation to PUs (10). Based on these conditions, it is recommended that PUs could be considered as a target in the assessment and management of tics.
Premonitory urges evaluation has been one of the focuses of the assessment and management of TDs in recent years [see McGuire et al. (11) for the recommendation of evidence-based assessment of TD]. Despite the intrinsic sensation of PUs, multiple TDs, as well as individual differences exist in PUs. One self-report checklist, the Premonitory Urge for Tic Scale (PUTS) (12), has been widely used to measure PUs in patients with TD in the United States (12), China (13), Italy (14), Spain (15), Japan (16), and South Korea (17). Rajagopal et al. (18) found that the PUTS could distinguish between types of PUs associated with simple or complex tics as well as obsessive phenomena. However, the following issues with the PUTS warrant further investigation on PUs. First, the PUTS takes PUs as a whole construct, measured over an uncertain period, and does not enable subjects to identify differences in between specific urges toward different tics. Second, the analysis of the PUTS is limited to an individual dimension of all urges, which does not include evaluation of the frequency and intensity of the urges (19).
An alternative and complementary approach to assessing PUs are necessary. Different individuals and tics have been reported to have different degrees of PUs (20), and individualized urge evaluation might provide crucial supplementary details for the assessment of PUs and provide the opportunity to assess urges from multiple dimensions (21). To this end, McGuire et al. (22) developed the Individualized Premonitory Urge for Tics Scale (I-PUTS) and confirmed that the I-PUTS is a reliable and effective phenomenological measurement of urge that can capture multifarious dimensions of PUs, which provides complete information for PUs and assesses body regions associated with PUs, further advancing understanding of the phenomenology. Nevertheless, the Chinese version of the I-PUTS has not yet been published, and the psychological properties of a Chinese version of the I-PUTS need to be further established.
The comorbid conditions and coexisting psychopathology (e.g., anxiety disorders, OCS, and ADHD) might influence youth with TD (23). Evidence showed correlation of varying strengths between the PUs and OCS (16), overall anxiety symptoms (24), and depressive symptoms (22). As for the associations between ADHD severity and PUs, inconsistent conclusions exist (25). On the other hand, I-PUTS could distinctly capture PUs phenomena, and its ratings were not significantly influenced by co-occurring psychopathology (22). Comparatively, the PUTS total score exhibited a large negative relationship with distress tolerance and a moderate-to-large positive correlation with anxiety severity (22). Therefore, this study will help illustrate the relationship of comorbid conditions and PUs assessment.
This study aimed to assess the psychometric properties of the Chinese version of the I-PUTS (C-IPUTS) and investigate the multiple sites of PUs by combining the self-report PUTS with the clinician-administered C-IPUTS in a Chinese setting. We also explored the network correlation between C-IPUTS dimensions, total C-PUTS score, tics, and other clinical properties (including obsessive-compulsive behavior, ADHD, anxiety, and depression), which reflects a multidimensional and more comprehensive assessment of PUs.
2. Materials and methods
2.1. Participants
This study initially enrolled 160 children and adolescents with TDs (based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-5) aged between 8 and 14 years old. Children and adolescents with infections, intellectual disability, language impairment, autism spectrum disorder, and major psychiatric disorders (such as schizophrenia and bipolar disorder) were excluded. After detailed notification of the protocol, at least one caregiver and their child signed an informed consent. In total, 123 youth ultimately participated in this study. All participants were enrolled from the Department of Developmental Pediatrics of the Second Hospital of Jilin University from September 2021 to August 2022. None of the participant were receiving any tic-influencing medication when enrolled in the study.
2.2. Scales for assessments
2.2.1. Assessment of premonitory urge using C-PUTS and C-IPUTS
The C-PUTS is a self-report question sheet that measures PUs in patients with TDs (12). C-PUTS has demonstrated reliability and validity in assessing PUs in Chinese patients between 6 and 16 years old with TDs (13). The total score varies from 9 to 36 points, and higher scores mean greater severity of PUs.
The I-PUTS (22) is a clinician-administered measurement that assesses the existence, frequency, intensity, and body parts with recognized PUs in the past week. Before performing the I-PUTS translation, we obtained an agreement from Dr. McGuire. We performed an initial and forward-backward translation of the I-PUTS to Chinese in accordance with the guidelines proposed by Beaton et al. (26). The physician asked the participant whether there was a PUs before the tic and rated the corresponding score on a 4-point scale for each recognized tic. Items were scored 0 when tics or PUs are not recognized. Then, the physician requested information about the body parts related to each PUs. Eventually, the data were pooled to obtain the total number, frequency, and intensity of the urges and the site of PUs occurrence.
2.2.2. Yale Global Tic Severity Scale
The Yale Global Tic Severity Scale (YGTSS) (27) is a clinician-completed semi-structured interview rating scale that quantifies the severity and specific nature, including number, frequency, intensity, complexity, and interference, of motor and vocal tics. The YGTSS also provides an impairment score that focuses primarily on the impact of TDs on self-esteem, family life, social acknowledgment, or school in the last week. The maximum total YGTSS score is 100, including a maximum tic severity score of 50 (each half for motor and vocal tics) and the other 50 for the tics impairment. The YGTSS has been demonstrated to have satisfactory reliability and validity and is extensively used internationally and in China.
2.2.3. The Yale-Brown Obsessive Compulsive Scale
The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (28) is a clinician-rated, semi-structured instrument consisting of 10 items to assess the severity of obsessive and compulsive symptoms over the past week. The validity and reliability of this scale have been well documented in many studies and is suitable for children and adults aged 8 years and older (29).
2.2.4. Achenbach Child Behavior Checklist
The Achenbach Child Behavior Checklist (CBCL) (30) is a parent-report eight-subscale questionnaire with excellent psychometrics. It is mainly used to screen children and adolescents for social ability and behavioral problems, and the social ability part is only used for those aged 6–18 years; behavioral problems are divided into four age/gender groups, that is, boys and girls aged 4–11 and 12–18 years. The behavior problem part is composed of 113 question items, of which the 56th question includes 8 question items (56a–56h), with a total of 120 questions. Each question item is scored according to a three-level score of 0–2: 0 means no problem, 1 means a mild or occasional problem, and 2 means an obvious or frequent problem. In the present study, the Total Problems, Internalizing Problems (Anxious/Depressed, Withdrawn, and Somatic Complaints), and Externalizing Problems (Rule Breaking Behavior and Aggressive Behavior) scales scores from the CBCL were used to estimate behavioral and emotional problems.
2.2.5. Depression Self-Rating Scale for Children
The Depression Self-Rating Scale for Children (DSRS-C) (31) can be used as a cost-effective screener for depression in children and adolescents aged 8–14, with sound psychometric properties in clinical samples. The 18-item self-rating scale is scored on three levels: none (0), sometimes (1), and often (2). Depression is considered when the total score is 13 or more. Higher scores represent stronger depressive trend.
2.2.6. Screen for Childhood Anxiety-Related Disorders
The 41-item Screen for Childhood Anxiety-Related Disorders (SCARED) (32) is a measure widely used for the assessment of recent anxiety symptoms in those aged 6–18 based on parent and child reports, including SCARED parent and child versions. Participants respond on a 3-point scale of 0, 1, or 2. What is more, five-subscale symptoms generalized anxiety, separation anxiety, social anxiety, panic or somatic symptoms, and school avoidance are included. A total score of 23 or above indicates clinical anxiety.
2.2.7. Swanson, Nolan and Pelham, Version IV
The Swanson, Nolan and Pelham, Version IV (SNAP-IV) parent rating scale consists of 26 items and includes three dimensions: impulsivity/hyperactivity, inattention, and oppositional defiant symptoms. The Chinese SNAP-IV was proved as a reliable and valid tool for assessing ADHD-related symptoms of children and adolescents aged 6–15 years in clinical settings. It has good reliability and validity and psychometric properties in China (33). Each dimension score is divided by the item number which includes 9 for inattention and hyperactivity-impulsivity and 8 for oppositional defiant symptoms. The inattention subset and hyperactivity/impulsivity subset of the parent-rated cut-off are 1.78 and 1.74, respectively.
2.3. Procedures
The study protocol was approved by the ethics committee of the Second Hospital of Jilin University. Two developmental behavioral pediatricians independently conducted the assessments. Informed consent was obtained from all participants and their caregivers prior to participation. Sociodemographic data were collected via questionnaires. Participants and parents completed clinician-administered measures to assess tic severity (YGTSS), PUs phenomenology (C-IPUTS), and obsessive and compulsive symptoms (Y-BOCS). Parents also completed the CBCL, SNAP-IV, and SCARED-P rating scales, while youth completed the PUTS, SCARED-C, and DSRS-C self-report scales. To establish inter-rater reliability, a second rater conducted retest of the C-IPUTS and C-PUTS 1 month after the first assessment.
2.4. Network analysis
Network analysis was used to analyze the relationships among these factors and the structures of scales arising from the recurring associations. A network graph was constructed based on the total sample, incorporating the number, frequency, and intensity dimensions from the C-IPUTS; the impairment and severity of motor and vocal tics from the YGTSS, and C-PUTS, CY-BOCS, SCARED, DSRS, CBCL, and SNAP-IV scales. Solid lines in the graph represent positive correlations, while dashed lines illustrates negative correlations. The thickness of each line correlates with the P-value (thicker lines indicate smaller P-values and greater r values). The line color intensity correlates with the absolute r value. R software (version 3.2.21) and Cytoscape were used to estimate networks and visualize correlations.
2.5. Statistical methods
Statistical analyses were completed using SPSS software (v26, IBM Corp., Armonk, NY, USA). Descriptive statistics characterized the PUs and clinical characteristics based on scales from the I-PUTS and other scales. First, we performed a normality test. Since the I-PUTS dimensions and PUTS total scores were not normally distributed, non-parametric statistics were used. Kruskal–Wallis tests compared differences in C-IPUTS urge number, frequency, and intensity across body regions. Second, we used the frequency and constituent ratio to describe sociodemographic data and the Kruskal–Wallis H test to compare differences in frequency and intensity in each body region. Third, we used reliability analysis (reliability, test-retest reliability) to test the internal consistency and temporal stability of the C-IPUTS. Interclass correlation coefficients (ICCs) were used to evaluate inter-rater reliability for each I-PUTS dimension. Fourth, we used exploratory factor analysis (EFA) to analyze the validity of the C-IPUTS. Fifth, we used Spearman correlation analysis to analyze the correlation between the C-IPUTS (number of urges, frequency, and intensity) and C-PUTS; convergent validity was verified by analyzing each dimension of the C-PUTS, C-IPUTS (number of urges, frequency, and intensity), and YGTSS. Sixth, we used correlation analysis to analyze the relationship between each dimension of the C-IPUTS, C-PUTS, and other comorbidities. Finally, the children and adolescents were divided into two groups: who exhibited concordance on the C-IPUTS and C-PUTS (i.e., reported the consistent presence or absence of PUs on both scales), and those who exhibited discordance (i.e., reported PUs on one scale but not the other). An independent samples t-test was used to assess whether there were statistical differences between youth with concordant versus discordant scores, and to evaluate whether other symptoms were present for those with discordant C-IPUTS and C-PUTS scores.
3. Results
3.1. Clinical characteristics of participants
The age of participants ranged from 8 to 14 years, with a median age of 10 (9, 11), including 99 boys with a median age of 10 (9, 12) and 24 girls with a median age of 9 (8, 10.5). To perform the comparison analysis with the published literature (12), the participants were divided into two groups: 8–10 years (younger age group) (n = 73) and 11–14 years (older age group) (n = 50). There were 55 boys (75.34%) and 18 girls (24.66%) in the younger age group and 45 boys (90%) and 5 girls (10%) in the older age group. The most common site of the first tic was the eyes, accounting for 51.22%. The most common comorbidity was ADHD (n = 48, 39.02%), followed by OCS (n = 25, 20.33%), anxiety disorders (n = 23, 18.70%), depression (n = 19, 15.45%), and behavioral problems (n = 17, 13.82%). Finally, 11.38% of participants revealed a family history of tics.
3.2. Premonitory urges characteristics
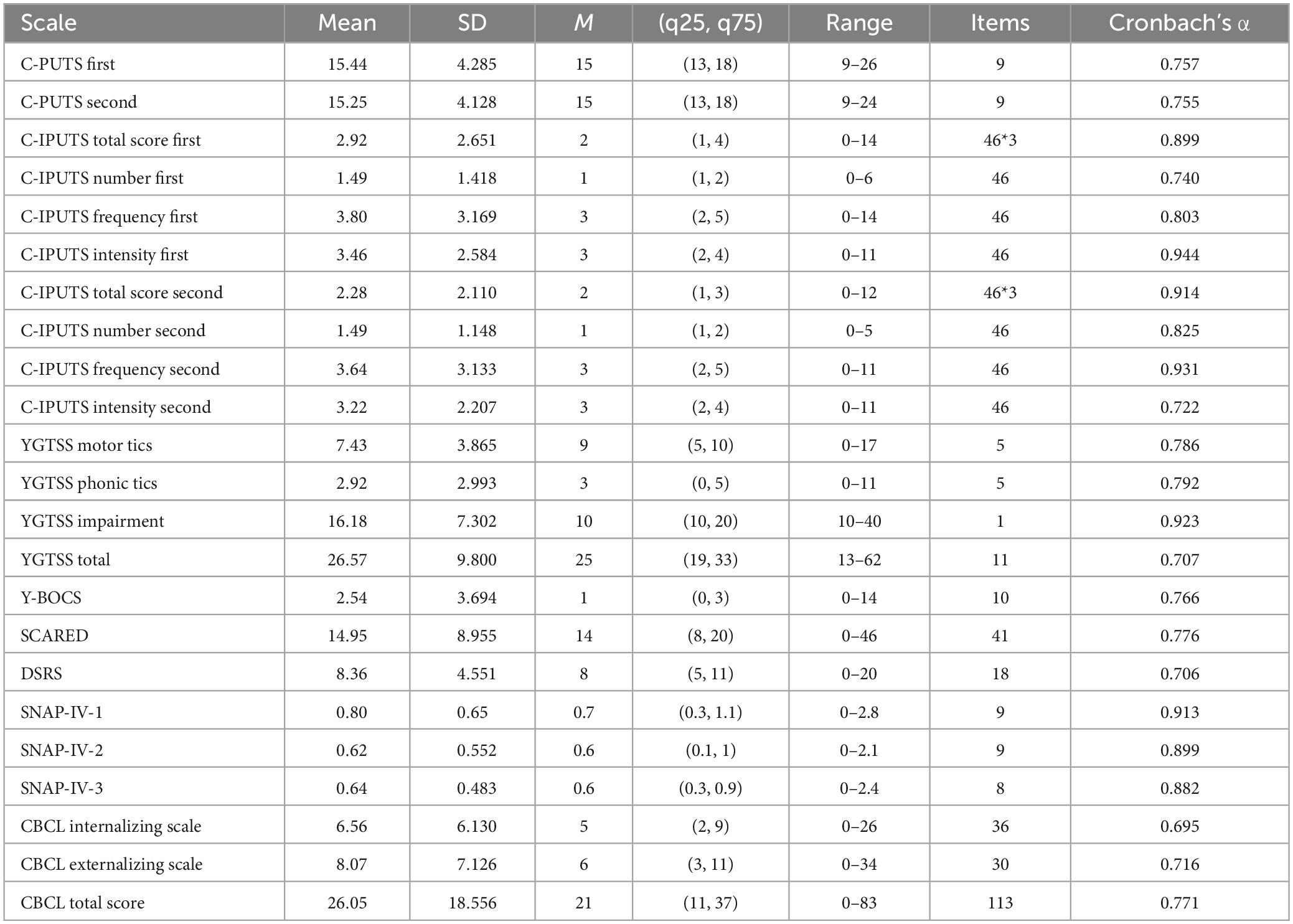
Among the participants, 86.99% (n = 107) cases reported PUs for endorsed tics on the I-PUTS, while 84.55% (n = 104) youth reported PUs on C-PUTS. On average, youth confirmed 7 tics over the past week (Mean = 7.35, SD = 4.25), and experienced PUs 50–75% of the time they had the tic (Mean = 3.70, SD = 2.67), rating the intensity as moderate (Mean = 3.48, SD = 2.36). PUs were predominantly localized in the head/face region (53.3%), neck/throat region (17.3%), arms (7.0%), legs (6.3%), torso (11.0%) and whole body/other regions (5.1%). Kruskal–Wallis H tests revealed significant differences in PUs number (χ2 = 20.67, P = 0.001), frequency (χ2 = 21.34, P = 0.001), and intensity (χ2 = 17.55, P = 0.004) across body regions, with higher intensity ratings for PUs from the neck/throat versus other regions (Table 1).
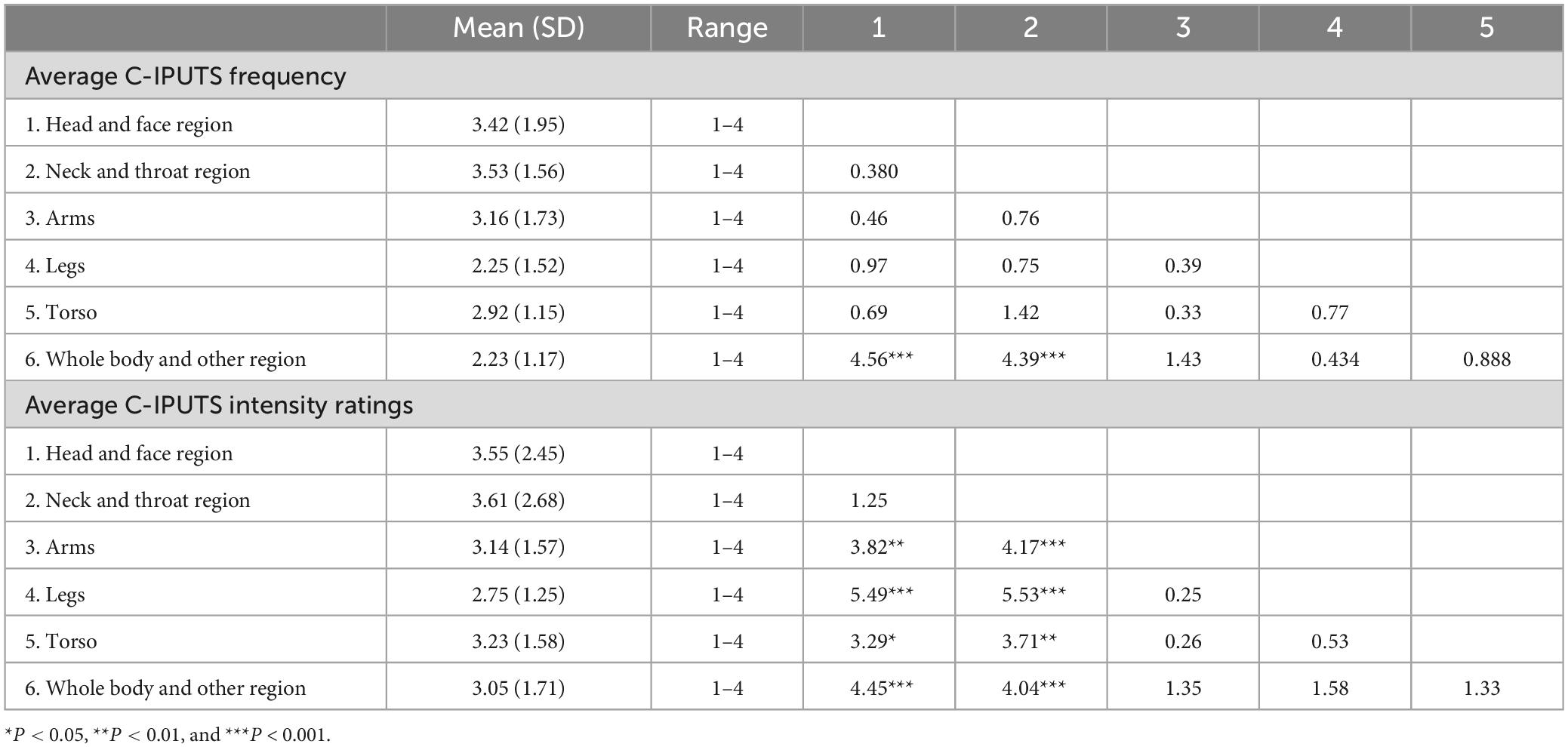
Table 1. Mean, standard deviation, median, and interquartile range for C-IPUTS dimensions by urge body region, and pair-wise comparisons for average frequency and intensity ratings.
3.3. Reliability and validity of the C-IPUTS and other scales
The Cronbach’s α coefficient of the total C-IPUTS scale was 0.899, including 0.899 in the younger age group and 0.902 in the older age group, all of which were greater than 0.70, indicating that the C-IPUTS had good internal consistency. A total of 115 cases (93.50%) completed the 1-month follow-up with the C-IPUTS demonstrating good test-retest reliability (ICC = 0.910). This is consistent with previous findings from McGuire et al. (22). The mean Kaiser–Meyer–Olkin (KMO) score for the 123 participants was 0.784, indicating that the factor analysis model was suitable for this data and factor analysis could be performed. The approximation of Bartlett’s test of sphericity was 458.135, with 3 degrees of freedom (P < 0.001), indicating the applicability of the factor analysis model. The C-IPUTS explained 94.082% of the total variance and showed good construct validity. The Cronbach’s α coefficients for the C-PUTS, YGTSS motor and phonic tic subscales, YGTSS impairment and total scores, Y-BOCS, SCARED, DSRS, SNAP-IV, and CBCL internalizing, externalizing, and total scales were almost all above 0.7, indicating good internal consistency (Table 2). Inter-rater reliability was also good based on interclass correlation coefficients (ICCs) for the I-PUTS Urge Number (0.863), Frequency (0.837), and Intensity (0.694) subscales, as well as the YGTSS (0.981) and Y-BOCS (0.918) total scores.
3.4. Correlations between the I-PUTS, PUTS, and clinical data
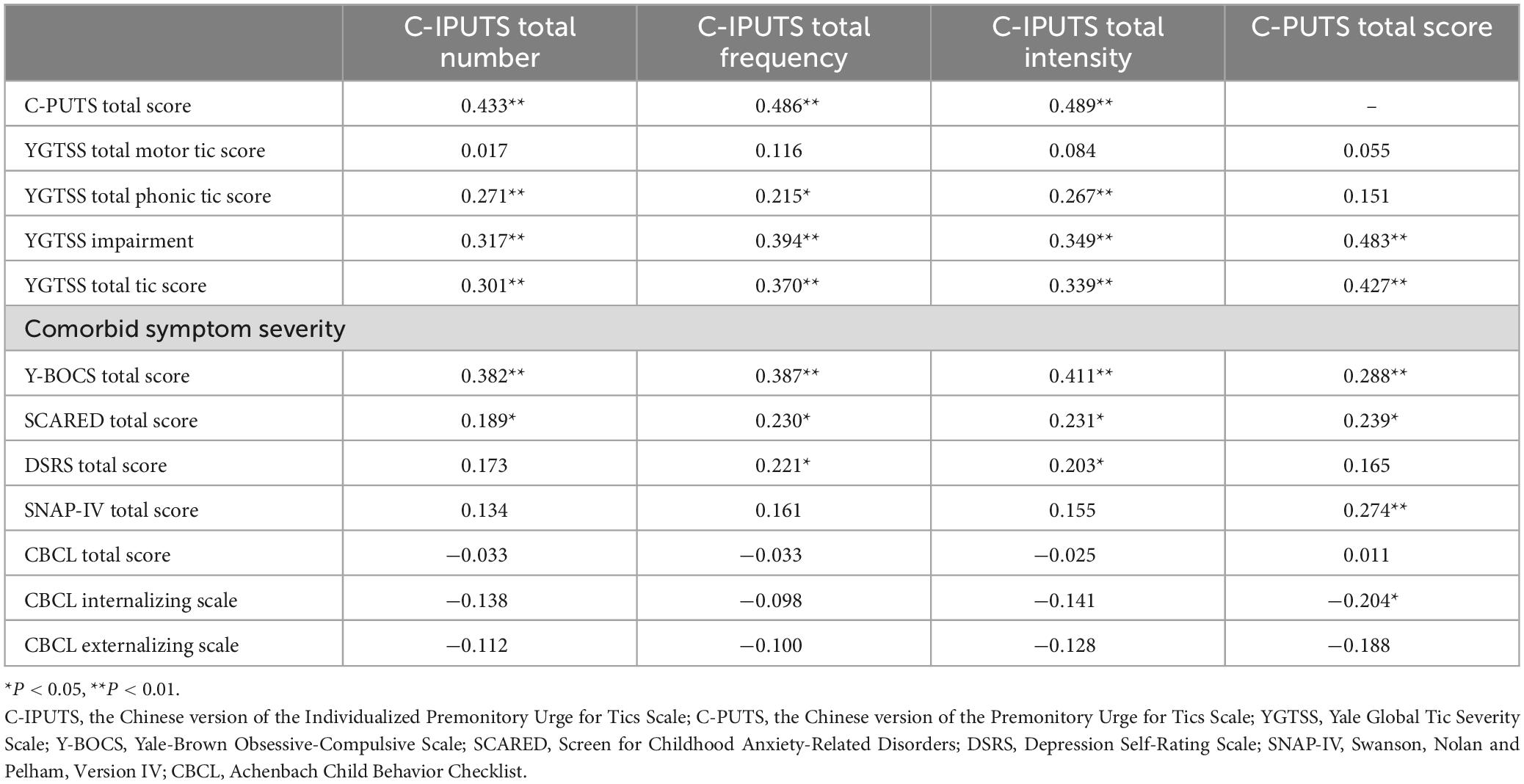
Table 3 and Figure 1 demonstrate correlations between the number, frequency, and intensity of C-IPUTS PUs and the total C-PUTS score (P < 0.05). The C-IPUTS and C-PUTS scales showed a strong positive relationship for premonitory urge symptoms. In particular, robust correlations existed among the three C-IPUTS dimensions assessing PUs. In TD, the number, frequency, and intensity of urges positively correlated with the YGTSS vocal tic scale score and C-IPUTS impairment (P < 0.05). The C-PUTS also positively correlated with the YGTSS total score, vocal tic scale, and impairment (P < 0.05). For TD’s comorbidities, the C-IPUTS better correlated with the Y-BOCS than the SCARED, but showed no correlations with the SNAP-IV, DSRS, or CBCL. The C-PUTS correlated with the Y-BOCS and SCARED, DSRS, and SNAP-IV scales, but not the CBCL.
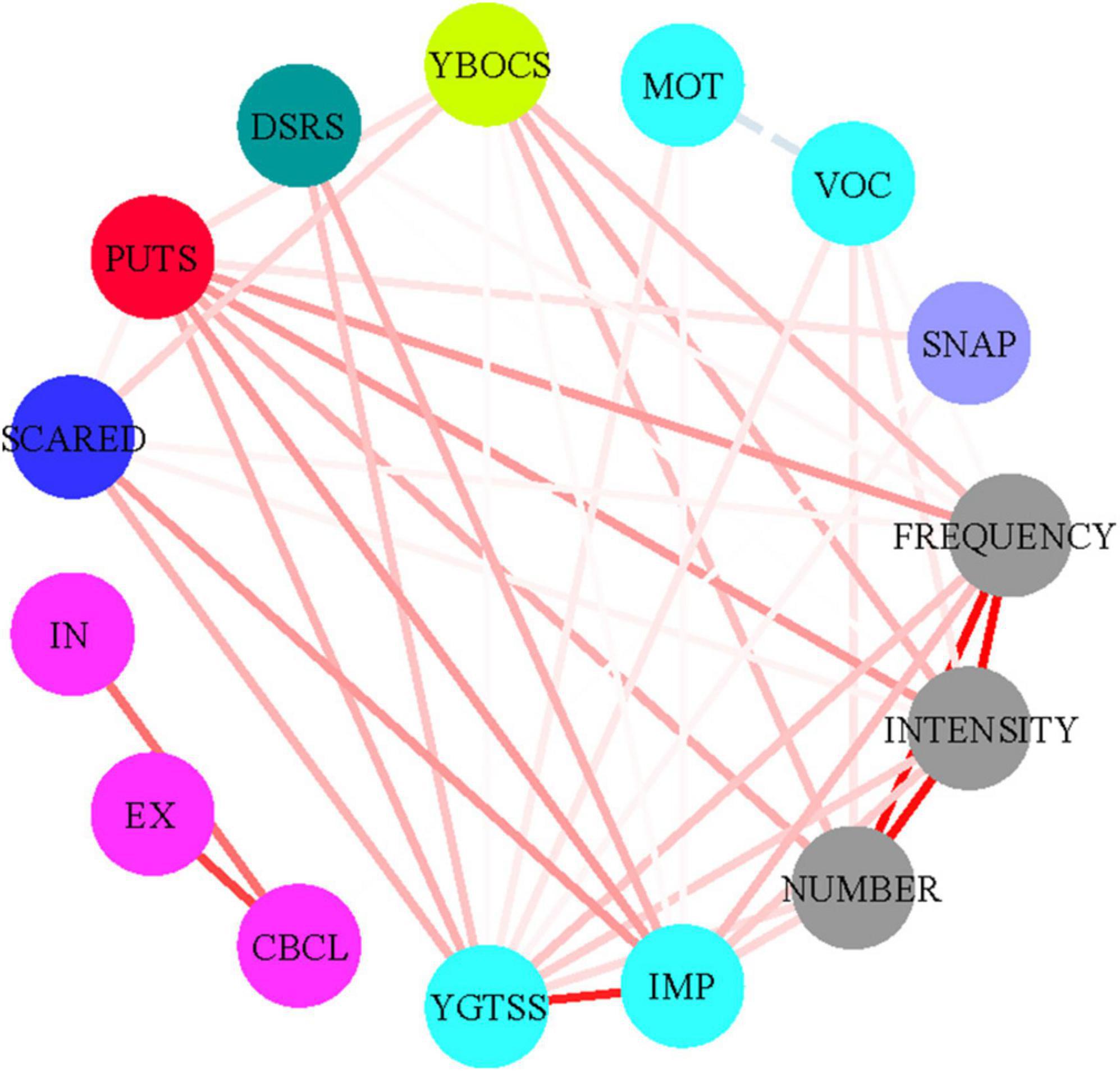
Figure 1. Correlations between the PUTS and I-PUTS scores and clinical constructs. PUTS, Premonitory Urge for Tics Scale; NUMBER, number item of I-PUTS; FREQUENCY, frequency item of I-PUTS; INTENSITY, intensity item of I-PUTS; VOC, vocal subscale of YGTSS; MOT, motor subscale of YGTSS; IMP, impairment item of YGTSS; YGTSS, Yale Global Tic Severity Scale; Y-BOCS, Yale-Brown Obsessive Compulsive Scale; SCARED, Screen for Childhood Anxiety-Related Disorders; DSRS, Depression Self-Rating Scale; SNAP-IV, Swanson, Nolan and Pelham, Version IV; CBCL, Achenbach Child Behavior Checklist; IN, CBCL internalizing scale; EX, CBCL externalizing scale.
In our sample, the male to female ratio was 4.3:1. The mean C-PUTS score was 16 (13, 18) for males and 14 (13, 17) for females. However, rank sum tests indicated these differences were not statistically significant (P > 0.05). Moreover, no significant differences existed between boys and girls for the C-IPUTS number, frequency, or intensity of PUs (P > 0.05). No significant correlations were found between age and C-PUTS or C-IPUTS scores among the total sample.
3.5. Correlation between scales in different age groups
Retrospective reports indicate that PUs typically emerge around the ages 6–7 years (34) but may not be detected until ages 8–10 years (3) as children may lack the ability to recognize and describe urge awareness before age 8 (35). Furthermore, several studies have demonstrated an effect of age using the PUTS and clinician assessments using the I-PUTS (6). To further elucidate this relationship, participants in the current study were divided into two age groups – 8–10 years (younger) and 11–14 years (older).
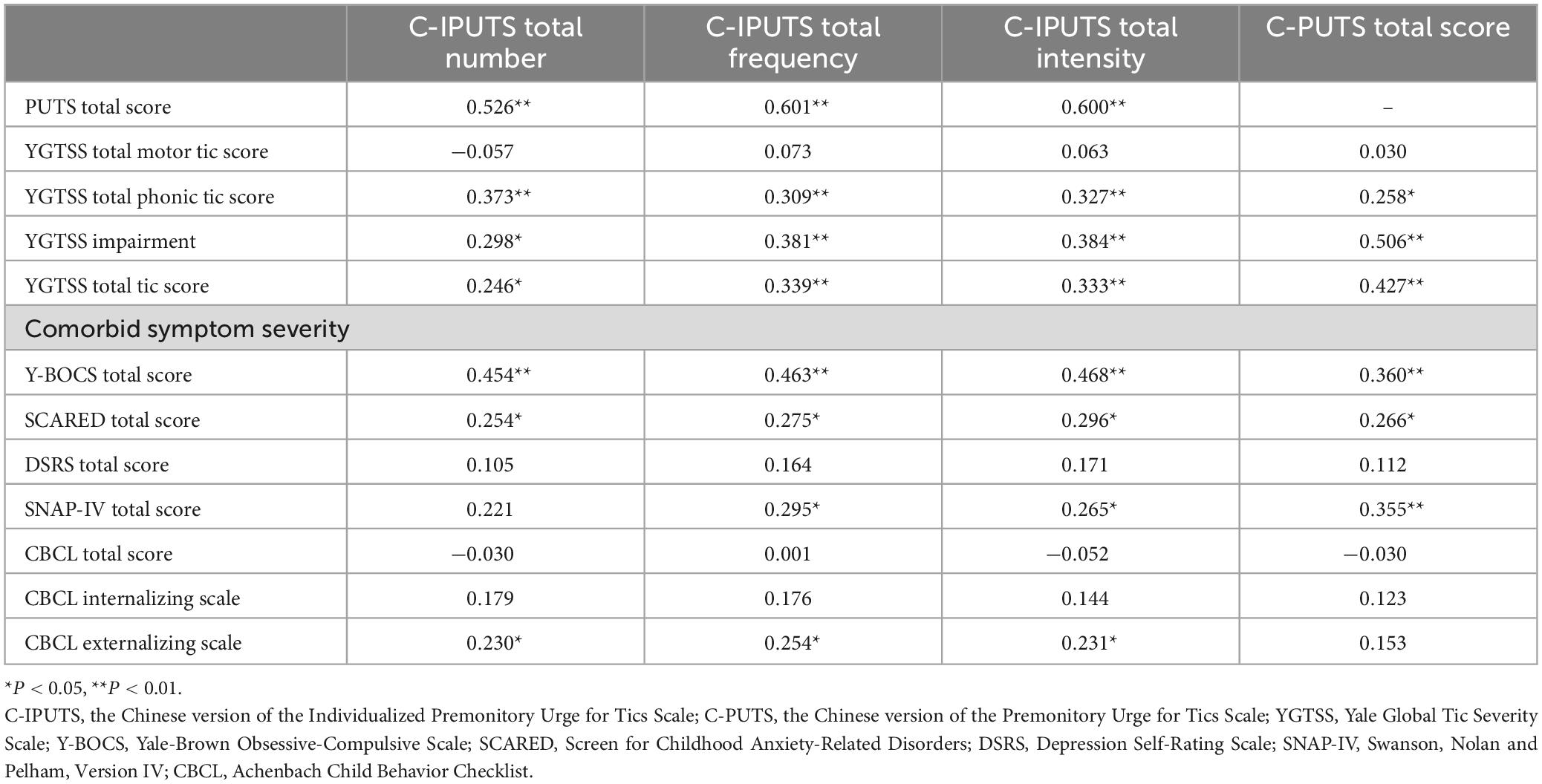
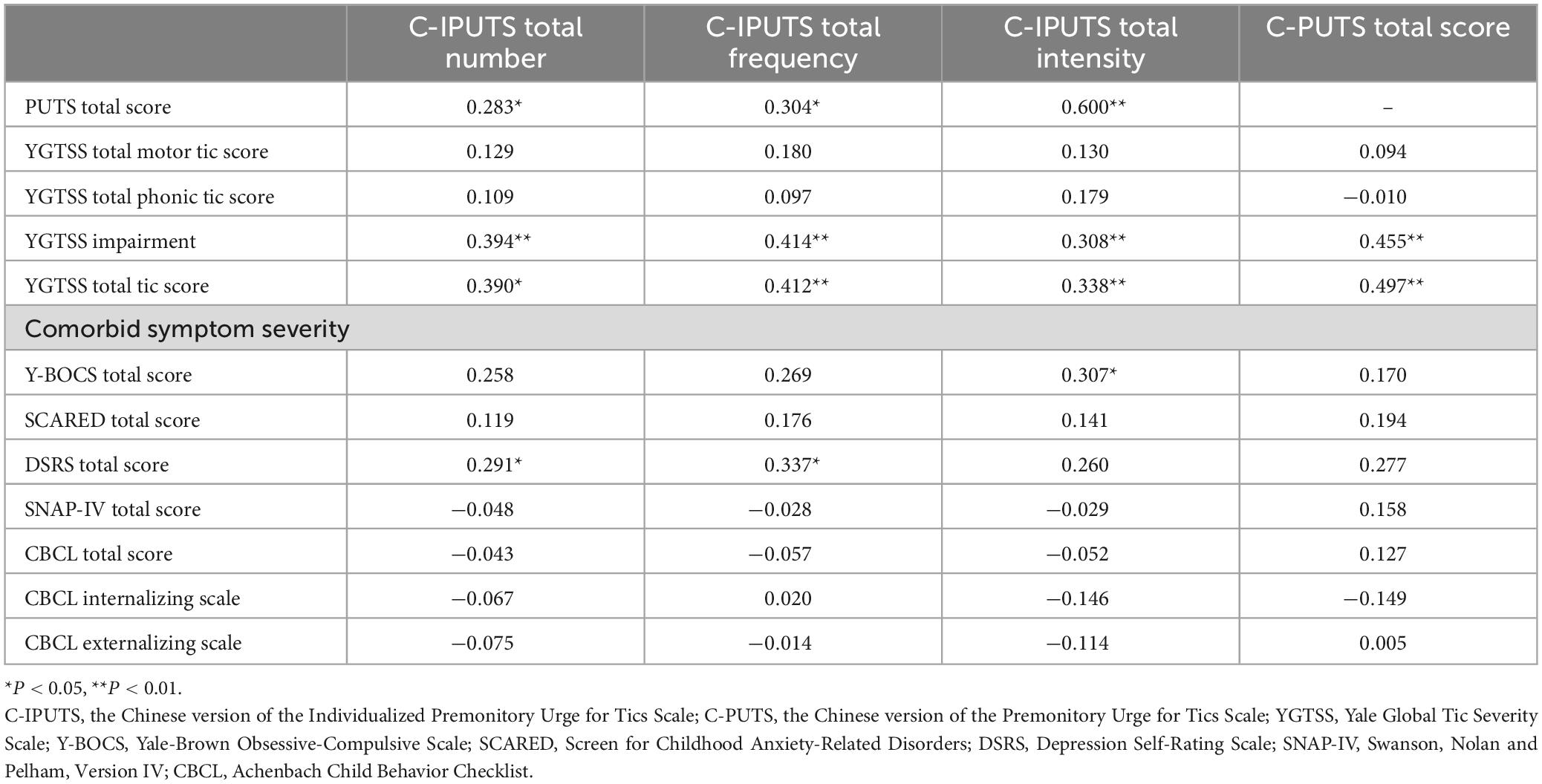
We assessed the correlations between the C-IPUTS, C-PUTS total scores, TD, and comorbidities of TD in different age groups using Spearman correlation analysis. The results showed that there were mild to moderate correlations between the C-IPUTS score and the C-PUTS, YGTSS, Y-BOCS, SCARED, and SNAP-IV scores in the younger age group (P < 0.05). There were no significant correlations between the C-IPUTS/C-PUTS scores and the DSRS or CBCL score (P > 0.05). In the older age group, the C-IPUTS score showed mild correlations with the YGTSS (r = 0.338–0.412, P = 0.003–0.016 < 0.05) and C-PUTS (r = 0.283–0.331, P = 0.028–0.046 < 0.05) scores, but was not significantly associated with any of the other scales (r = 0.028–0.269, P = 0.059–0.857 > 0.05). The C-PUTS score in the younger age group had mild to moderate correlations with the YGTSS, Y-BOCS, SCARED, and SNAP-IV scores (P < 0.05) but had no significant correlations with the DSRS or CBCL score (P > 0.05). In the older age group, C-PUTS was not significantly associated with other scales (r = 0.012–0.277, P = 0.051–0.947 > 0.05), except for YGTSS (r = 0.427, P < 0.01). The correlation analyses in the younger age group and older age group are detailed in Tables 4, 5, respectively.
3.6. Agreement and inconsistency between C-IPUTS and C-PUTS
Several children and adolescents with TDs and PUs had inconsistent results between the C-IPUTS and C-PUTS. For example, 16 children and adolescents reported PUs on the self-reported C-PUTS which were not confirmed on the C-IPUTS. Similarly, 19 children and adolescents did not report PUs on the C-PUTS, but PUs were identified by clinicians using the C-IPUTS. Participants were divided into concordant (n = 100) and discordant (n = 23) groups based on agreement between the C-IPUTS and C-PUTS. Mann–Whitney U tests showed significant differences in YGTSS total score, phonic tic subscale, and Y-BOCS between the two groups. However, no significant differences were found for SCARED, DSRS, SNAP-IV, and CBCL scores (Table 6).
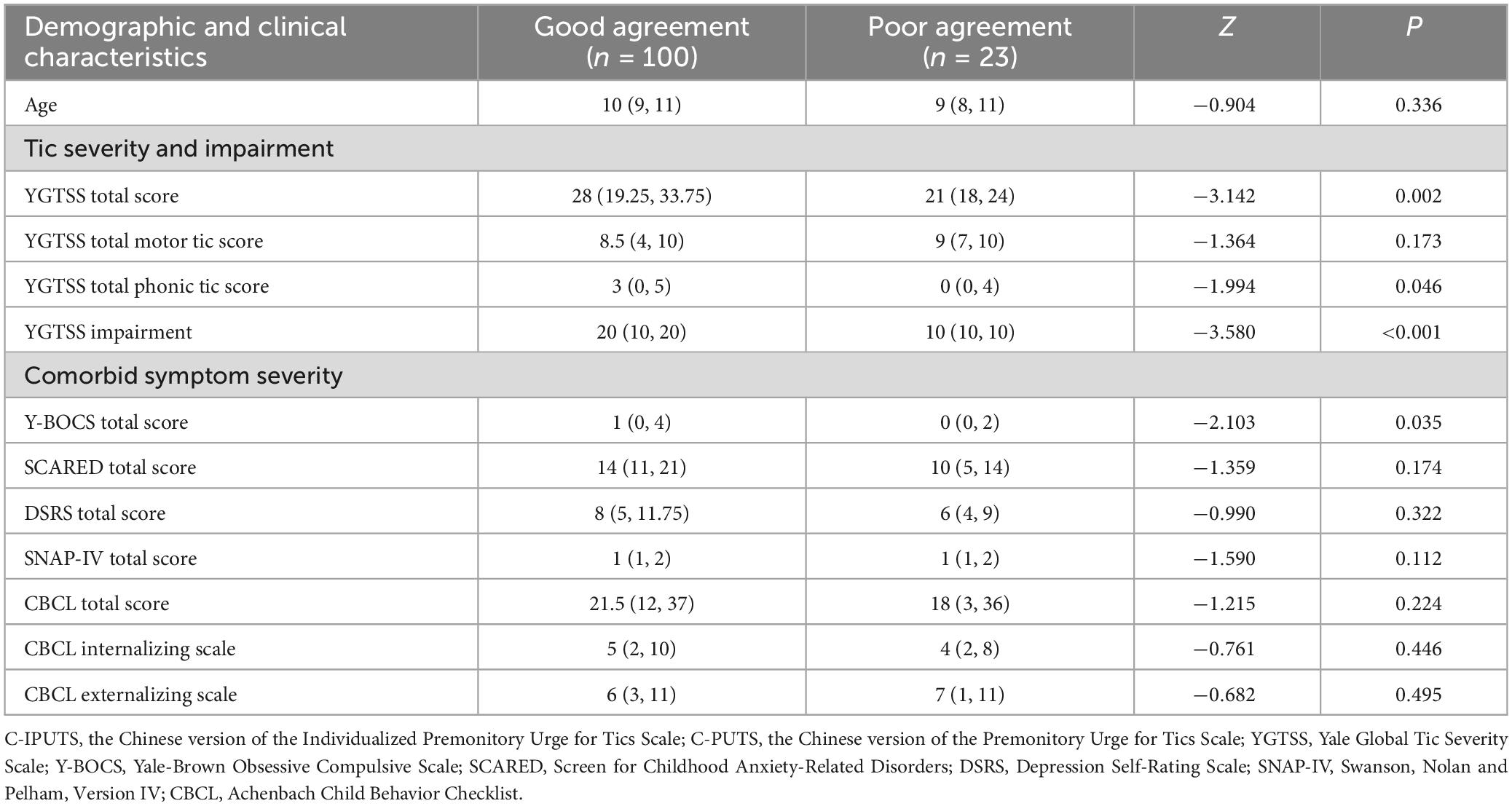
Table 6. Characteristics of concordance (n = 100) and discordance (n = 23) between the C-IPUTS and C-PUTS [M (P25, P75)].
4. Discussion
This study is the first to examine the feasibility of the I-PUTS for assessing children and adolescents’ PUs in a Chinese cultural context. First, we verified the reliability and validity of the C-IPUTS for Chinese children and adolescents, evaluated the PUs, and compared it with the C-PUTS. Second, this study demonstrated the correlation between PUs using the C-PUTS and C-IPUTS with the tics and other comorbidities.
Reliability of the scale refers to the consistency and stability of the measured results. Cronbach’s α coefficient is commonly used to represent the internal consistency of the scale, and retest reliability is used to represent the external consistency of the scale. The results indicate that the C-IPUTS is a highly readable, simple, and understandable PUs assessment tool with good reliability and validity. The total sample (Cronbach’s α = 0.899) and two age groups (Cronbach’s α = 0.902 in the older age group and 0.889 in the younger age group) had good reliability, representing a stable internal consistency across the age span. The retest reliability of the C-IPUTS was 0.910 1 month after the first assessment. It shows that C-IPUTS has good internal consistency and external consistency.
Factor analysis is the most commonly used method to test the scale structure. EFA is used to determine the scale’s structural validity. EFA revealed that the C-IPUTS had good construct validity with loading >0.40 and cumulative variance explained >40%. When one factor was extracted, the cumulative total variance interpretation rate was 94.082%, indicating that the C-IPUTS had good validity.
This study proposed to further the research by Woods et al. (12) and McGuire et al. (22) by appraising the psychometric properties of the C-PUTS and C-IPUTS in the Chinese context. The C-PUTS score reflects the self-rated PUs, while the C-IPUTS score demonstrates the frequency and intensity of PUs assessed by clinicians. The C-PUTS has been demonstrated to have steadfast reliability, validity, and psychometric properties in China, so we directly compared them in this study (36). This study demonstrated a positive correlation between the C-PUTS and various dimensions of the C-IPUTS, indicating the consistency of the evaluation results of the two scales. The C-PUTS is a trustworthy and valid tool for quantifying PUs, and the instrument is internally consistent and temporally stable. It can be used to assess the severity of PUs in children and adolescents with TD. The C-PUTS is concise and can be completed independently by children. However, they have some limitations. First, the C-PUTS has been reported to show reliable validity and reliability in children and adolescents (5). Nevertheless, many TD patients younger than 8 years in China find it challenging to read and understand the self-checklists, which may limit the applicability of the instrument. Second, the regions of the PUs on the body are not evaluated by the C-PUTS, thus confining the application of the C-PUTS in communicating satisfactorily with the patient. Third, the C-PUTS restricts analysis to a single dimension. The C-IPUTS has several considerations that further refine the C-PUTS. First, the C-IPUTS measures the location of PUs, which complements the existing scale. Second, the C-IPUTS measures PUs as a whole frame across a specific period, and the respondent are not capable to distinguish between individual urges for different tics. As different tics and different individuals have changing degrees of PUs, an individualized urge evaluation may offer important supplemental information and provide the opportunity to assess urges from various aspects. Both complement each other and provide good tools for assessing PUs.
In this study, the main sites of PUs were the head/face and throat/neck. Essing et al. (37) revealed that PUs were predominantly localized in the forehead, cheeks, mouth, and throat. Leckman et al. (3) found that PUs can manifest in any part of the human body, and the symptoms may manifest systemically or locally. Nevertheless, the symptoms often appear in the face, neck, shoulders, arms, and midline of the abdomen (38). Leckman et al. (3) reported PUs awareness arises three years after tic onset. This may indicate the subtle and elusive sensory information can enter conscious awareness owing to maturational shift in the cognitive processing of somatosensory experience. Or this is related with a function of the location and type of tics involved, which is consistent with report with regard to the mean age of tic onset involving those anatomical regions closely linked with PUs to a great extent (3). McGuire et al. (22) showed that the neck/throat region may have specific sensory connections to vocal tics that contribute to youth’s awareness of greater urge intensity. In terms of gender, we found that males and females had no significant difference in their self-reported or parent-reported PUs on the C-PUTS and C-IPUTS, which is similar to the findings of Edwards et al. (39). It has been found that the incidence of TD is high in men, and the age of onset is earlier than that in women, but the complexity of tics in women increases with age (40), and this phenomenon has not been found in the present study.
For the entire sample of 123 children and adolescents, we found that the number, frequency, and intensity of the PUs was positively correlated with tic severity ratings, the vocal tics subscale, and impairment, but was not significantly correlated with motor tics, indicating that youngsters with more severe TD also had more evident PUs. Meanwhile, the number, frequency and intensity of PUs in C-IPUTS were correlated with OCS, anxiety severity, and depression. Compared with C-IPUTS, C-PUTS score was significantly correlated with total YGTSS score and impairment, while C-PUTS score was correlated with OCS, anxiety and ADHD severity. The results indicate that the combined use of C-IPUTS and C-PUTS facilitates the evaluation of PUs across multiple domains including the TD comorbidities. A recent meta-analysis (5) suggests that PUs exhibit a robust association with tics, appear integral to tic expression, and may serve as a marker of tic severity. For instance, one study (2) found that 71% of patients with TD reported that in the absence of PUs, their tics would disappear. As such, these urges could indicate not just the presence of tics but also their clinical severity.
In this study, the C-PUTS score demonstrated more correlations with comorbidities (anxiety severity, ADHD, and OCS) in the younger age group compared with the older age group. Similarly, the C-IPUTS score correlated with more comorbidities of TD (anxiety and ADHD) in the younger versus older group. In the older age group, OCS had a mild correlation with the C-IPUTS. This aligns with findings by Woods et al. (12) but differs somewhat from Raines et al. (41) and McGuire et al. (22). McGuire found no significant C-IPUTS and age correlation, yet a C-PUTS and age correlation existed (12). This may be because of difficulty distinguishing PUs from OCS-related sensory phenomena and internal sensory perception, potentially due to clinicians not asking the right questions or children lacking cognition to differentiate these phenomena. Though this study found no significant relationship between PUs and age, further research is still needed to explore potential associations between the C-IPUTS, C-PUTS, and age.
The findings of this study revealed positive relationships between PUs and symptoms of ADHD, OCS, and anxiety, consistent with previous research (38). Youth with poorer distress tolerance appear particularly prone to more frequent and intense PUs, experiencing them as aversive (22). Indeed, the prevalence of ADHD, anxiety, and OCS over the lifetime is higher among children with TD compared to neurotypical children (42). The most prevalent comorbidity among patients with TD is ADHD. There appears to be significant overlap between PUs and inner restlessness in those with ADHD (43), with severe PUs potentially linked to greater distractibility (44) in this population. A robust association also exists between PUs and OCS. Patients with both TD and OCS engage in repetitive, guilt-driven behaviors until achieving a sense of completeness, reportedly experiencing PUs more intensely during this process compared to neurotypical individuals (8). However, the interrelationship between PUs and compulsive behaviors remains to be clarified. Structural abnormalities in the sensorimotor cortex seem to exist in both OCS (36) and TD (35); nevertheless, it is indefinite whether structural alterations in sensorimotor areas are a cause or a consequence of the urge. In future studies, fMRI and modeling hemodynamic functions (regressors) could be used to find out neural association of urges preceding mental compulsions in TD patients with OCS. In addition, it was reported that about 5% patients with OCS experienced at least one sensory phenomenon (45). Additionally, patients with sensory phenomena were more prone to have comorbid TD than those without sensory phenomena (46).
The three dimensions of the C-IPUTS exhibited correlations with anxiety severity and OCS. Indeed, anxiety and OCS are common co-occurring conditions of TD (47), which are also accompanied by somatosensory sensitivities. This indicates that the I-PUTS substantially captures urge phenomena, and its ratings are not notably affected by co-occurring psychopathology. The co-occurrence of these psychiatric disorders may enhance sensitivity to PUs. Future research should further explore the nature of the PUs experience of TD patients with or without comorbidities and develop tools/methods to assess these experiences in detail.
All 81.30% of participants had concordant results on the clinician-administrated C-IPUTS and self-reported C-PUTS. It is important to note that the assessment timeline for the C-IPUTS is PUs experienced in the past week, while the C-PUTS does not have a well-defined assessment time, and the established association may be greater if the two scales have similar assessment intervals. This may also be responsible for the inconsistency in scores between the two scales. Another reason for the difference between C-IPUTS and C-PUTS scores may be that the children and adolescents in this study had TD and other co-occurring psychiatric symptoms, such as OCS, ADHD, depression, and anxiety, and children and adolescents are liable to have unintentionally confused other uncomfortable somatosensory or somatic anxiety sensations with PUs in their PUTS self-report ratings. Alternatively, children and adolescents may also have unintentionally biased results due to anxiety when completing the C-IPUTS under the guidance of clinicians. The presence of the above problems in children and adolescents may lead to different degrees of deviation in the understanding of the C-PUTS and C-IPUTS, highlighting the importance of using both scales to capture complementary information.
Premonitory urges are a major focus of current research, with many experts positing that they may serve as a marker of TD severity. An Asian study utilized PUTS scales to assess premonitory urge symptoms in adolescents and adults with Tourette’s disorder. After 4 years of treatment, patients’ global functioning was negatively correlated with tics and PUs compared to before treatment. Improvement was associated with reduced severity of OCS and PUs (16). As such, the presence and intensity of PUs across dimensions are critical for effectively treating Tourette’s. When used together, the C-PUTS and C-IPUTS can assess both the occurrence and multidimensional severity of PUs, especially for those with comorbid anxiety and OCS. This represents an invaluable tool for monitoring and managing Tourette’s disorder.
A limitation of this study is that the study population was from a Grade A tertiary hospital in Jilin Province, and the sample was not representative enough; our findings need to be verified further in various regions of the country. Additionally, most of the participants were from urban areas, and the findings may be influenced as such. Finally, this study was a cross-sectional analysis, so the criterion validity could not be tested based on this sample size. Additionally, we only used Pearson correlation for the association analyses due to the small sample size, which precluded conducting multiple regression analyses. Multiple regression analyses would be necessary to draw firm conclusions about the influence of comorbid ADHD, depression, anxiety, and other symptoms. Therefore, we recommend further testing of the C-IPUTS’ reliability and validity in a larger sample to allow for more comprehensive assessment in future research.
In future studies, we can increase the sample size and age range to investigate the differences in various dimensions of PUs at different age levels. It has been shown that the severity of PUs is associated with reduced connectivity between the cerebral cortex and the primary sensorimotor cortex, while the inferior frontal gyrus is associated with the putamen and insula (48). Therefore, longitudinal studies can be performed using the C-IPUTS, the C-PUTS, and both neurophysiological and neuroimaging tools, which may help obtain some of the neurophysiological markers or endophenotypes of PUs. The age span can also be further expanded to investigate the potential dimensions of the neural phenomena of TDs and PUs.
In summary, the C-PUTS is a reliable and valid brief self-report assessment instrument with good psychometric properties for quantifying the PUs phenomenon. The C-IPUTS is measured by clinicians to assess the number, frequency, intensity, and body parts of PUs in the past week, providing more important supplementary information for the C-PUTS. The C-PUTS and C-IPUTS have good correlations with the symptoms of TD, and there is a slight difference between the two in the comorbidities of TD. This study revealed that the combination of the C-PUTS and C-IPUTS is an effective method for the thorough assessment of PUs, providing a strong basis for the diagnosis and treatment of PUs in TD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
GC and WR carried out the study, analyzed the data, and wrote the manuscript with support from all other authors. ZZ and JT contributed to the implementation of the research and data analysis. YZ and JZ performed the assessment of TD comorbidities. PL designed and directed the research and the manuscript. JM provided the critical feedback and helped shape the manuscript. All authors reviewed and agreed on the final manuscript.
Funding
This study was supported by the Scientific Research Foundation from Educational Commission of Jilin Province of China (JJKH20211165kj) and Scientific and Technological Development Plan of Jilin Provincial Science Technology Department (20220401091YY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Bliss J. Sensory experiences of Gilles de la Tourette syndrome. Arch Gen Psychiatry. (1980) 37:1343–7. doi: 10.1001/archpsyc.1980.01780250029002
2. Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette’s syndrome. Mov Disord. (2003) 18:1530–3. doi: 10.1002/mds.10618
3. Leckman J, Walker D, Cohen D. Premonitory urges in Tourette’s syndrome. Am J Psychiatry. (1993) 150:98–102. doi: 10.1176/ajp.150.1.98
4. Cavanna A, David K, Orth M, Robertson M. Predictors during childhood of future health-related quality of life in adults with Gilles de la Tourette syndrome. Eur J Paediatr Neurol. (2012) 16:605–12. doi: 10.1016/j.ejpn.2012.02.004
5. Li Y, Wang F, Liu J, Wen F, Yan C, Zhang J, et al. The Correlation Between the Severity of Premonitory Urges and Tic Symptoms: A Meta-Analysis. J Child Adolesc Psychopharmacol. (2019) 29:652–8. doi: 10.1089/cap.2019.0048
6. Openneer T, Tárnok Z, Bognar E, Benaroya-Milshtein N, Garcia-Delgar B, Morer A, et al. The Premonitory Urge for Tics Scale in a large sample of children and adolescents: psychometric properties in a developmental context. An EMTICS study. Eur Child Adolesc Psychiatry. (2020) 29:1411–24. doi: 10.1007/s00787-019-01450-1
7. Sukhodolsky D, Woods D, Piacentini J, Wilhelm S, Peterson A, Katsovich L, et al. Moderators and predictors of response to behavior therapy for tics in Tourette syndrome. Neurology. (2017) 88:1029–36. doi: 10.1212/wnl.0000000000003710
8. Houghton D, Capriotti M, Scahill L, Wilhelm S, Peterson A, Walkup J, et al. Investigating Habituation to Premonitory Urges in Behavior Therapy for Tic Disorders. Behav Ther. (2017) 48:834–46. doi: 10.1016/j.beth.2017.08.004
9. Essoe J, Ramsey K, Singer H, Grados M, McGuire J. Mechanisms Underlying Behavior Therapy for Tourette’s Disorder. Curr Dev Disord Rep. (2021) 8:161–74. doi: 10.1007/s40474-021-00225-1
10. Pringsheim T, Okun M, Müller-Vahl K, Martino D, Jankovic J, Cavanna A, et al. Practice guideline recommendations summary: Treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:896–906. doi: 10.1212/wnl.0000000000007466
11. McGuire J, Kugler B, Park J, Horng B, Lewin A, Murphy T, et al. Evidence-based assessment of compulsive skin picking, chronic tic disorders and trichotillomania in children. Child Psychiatry Hum Dev. (2012) 43:855–83. doi: 10.1007/s10578-012-0300-7
12. Woods D, Piacentini J, Himle M, Chang S. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. J Dev Behav Pediatr. (2005) 26:397–403. doi: 10.1097/00004703-200512000-00001
13. Li Y, Woods D, Gu Y, Yu L, Yan J, Wen F, et al. Psychometric Properties of the Chinese Version of the Premonitory Urge for Tics Scale: A Preliminary Report. Front Psychol. (2021) 12:573803. doi: 10.3389/fpsyg.2021.573803
14. Gulisano M, Calì P, Palermo F, Robertson M, Rizzo R. Premonitory urges in patients with Gilles de la Tourette syndrome: an Italian translation and a 7-year follow-up. J Child Adolesc Psychopharmacol. (2015) 25:810–6.
15. Yang C, Zhang L, Zhu P, Zhu C, Guo Q. The prevalence of tic disorders for children in China: A systematic review and meta-analysis. Medicine. (2016) 95:e4354. doi: 10.1097/md.0000000000004354
16. Kano Y, Fujio M, Kaji N, Matsuda N, Nonaka M, Kono T. Changes in sensory phenomena, tics, obsessive-compulsive symptoms, and global functioning of tourette syndrome: a follow-up after four years. Front Psychiatry. (2020) 11:619. doi: 10.3389/fpsyt.2020.00619
17. Kim M, Chung S, Yang J, Park J, Nam S, Park T. Development of the Korean Form of the Premonitory Urge for Tics Scale: A Reliability and Validity Study. Soa Chongsonyon Chongsin Uihak. (2020) 31:146–53. doi: 10.5765/jkacap.200013
18. Rajagopal S, Cavanna A. Premonitory urges and repetitive behaviours in adult patients with Tourette syndrome. Neurol Sci. (2014) 35:969–71. doi: 10.1007/s10072-014-1706-8
19. Reese H, Scahill L, Peterson A, Crowe K, Woods D, Piacentini J, et al. The premonitory urge to tic: measurement, characteristics, and correlates in older adolescents and adults. Behav Ther. (2014) 45:177–86. doi: 10.1016/j.beth.2013.09.002
20. Schubert L, Verrel J, Behm A, Bäumer T, Beste C, Münchau A. Inter-individual differences in urge-tic associations in Tourette syndrome. Cortex. (2021) 143:80–91. doi: 10.1016/j.cortex.2021.06.017
21. Brabson L, Brown J, Capriotti M, Ramanujam K, Himle M, Nicotra C, et al. Patterned changes in urge ratings with tic suppression in youth with chronic tic disorders. J Behav Ther Exp Psychiatry. (2016) 50:162–70. doi: 10.1016/j.jbtep.2015.07.004
22. McGuire J, McBride N, Piacentini J, Johnco C, Lewin A, Murphy T, et al. The premonitory urge revisited: An individualized premonitory urge for tics scale. J Psychiatr Res. (2016) 83:176–83. doi: 10.1016/j.jpsychires.2016.09.007
23. Leisman G, Sheldon D. Tics and Emotions. Brain Sci. (2022) 12:242. doi: 10.3390/brainsci12020242
24. Rozenman M, Johnson O, Chang S, Woods D, Walkup J, Wilhelm S, et al. Relationships Between Premonitory Urge and Anxiety in Youth With Chronic Tic Disorders. Child Health Care. (2015) 44:235–48. doi: 10.1080/02739615.2014.986328
25. Hagstrom J, Spang K, Vangkilde S, Maigaard K, Skov L, Pagsberg A, et al. An observational study of emotion regulation in children with Tourette syndrome. J Child Psychol Psychiatry. (2021) 62:790–7. doi: 10.1111/jcpp.13375
26. Beaton D, Bombardier C, Guillemin F, Ferraz M. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. (2000) 25:3186–91. doi: 10.1097/00007632-200012150-00014
27. Wen F, Gu Y, Yan J, Liu J, Wang F, Yu L, et al. Revisiting the structure of the Yale Global Tic Severity Scale (YGTSS) in a sample of Chinese children with tic disorders. BMC Psychiatry. (2021) 21:394. doi: 10.1186/s12888-021-03399-5
28. Goodman W, Price L, Rasmussen S, Mazure C, Fleischmann R, Hill C, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. (1989) 46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007
29. Chen X, Chen G, Wang J, Huang J. Reliability and validity of the self-report Chinese version of the Yale-brown obsessive-compulsive scale modified for body Dysmorphic disorder (BDD-YBOCS) in patients undergoing plastic surgery. Aesthetic Plast Surg. (2022) 46:2023–30. doi: 10.1007/s00266-022-02784-z
30. Bordin I, Rocha M, Paula C, Teixeira M, Achenbach T, Rescorla L, et al. Child Behavior Checklist (CBCL),Youth Self-Report (YSR) and Teacher’s Report Form(TRF): an overview of the development of the original and Brazilian versions. Cad Saude Publica. (2013) 29:13–28. doi: 10.1590/s0102-311x2013000100004
31. Sonnby K, Skordas K, Vadlin S, Olofsdotter S, Nilsson K, Ramklint M. Psychometric validation of two versions of the adolescent Depression Self-Rating Scale (DSRS-A and DSRS-A Screener). Nord J Psychiatry. (2022) 76:233–42. doi: 10.1080/08039488.2021.1956583
32. Ang C. Anxiety in Malaysian children and adolescents: validation of the Screen for Child Anxiety Related Emotional Disorders (SCARED). Trends Psychiatry Psychother. (2020) 42:7–15. doi: 10.1590/2237-6089-2018-0109
33. Gau S, Lin C, Hu F, Shang C, Swanson J, Liu Y, et al. Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, Version IV Scale-Teacher Form. J Pediatr Psychol. (2009) 34:850–61. doi: 10.1093/jpepsy/jsn133
34. Bloch M, Leckman J. Clinical course of Tourette syndrome. J Psychosom Res. (2009) 67:497–501. doi: 10.1016/j.jpsychores.2009.09.002
35. Draper A, Jackson G, Morgan P, Jackson S. Premonitory urges are associated with decreased grey matter thickness within the insula and sensorimotor cortex in young people with Tourette syndrome. J Neuropsychol. (2016) 10:143–53. doi: 10.1111/jnp.12089
36. Li Y, Yan J, Cui Y. Clinical characteristics of pediatric patients with treatment-refractory Tourette syndrome: An evidence-based survey in a Chinese population. World J Psychiatry. (2022) 12:958–69. doi: 10.5498/wjp.v12.i7.958
37. Essing J, Jakubovski E, Psathakis N, Cevirme S, Leckman J, Müller-Vahl K. Premonitory Urges Reconsidered: Urge Location Corresponds to Tic Location in Patients With Primary Tic Disorders. J Mov Disord. (2022) 15:43–52. doi: 10.14802/jmd.21045
38. Nam S, Park J, Park T. Clinical Aspects of Premonitory Urges in Patients with Tourette’s Disorder. Soa Chongsonyon Chongsin Uihak. (2019) 30:50–6. doi: 10.5765/jkacap.180025
39. Edwards K, Raines J, Winnick J, Sherman M, Higginson C, Navin K, et al. Sex and psychiatric comorbidity correlates of the premonitory urge for tic scale in youth with persistent tic disorders. J Neural Transm. (2020) 127:977–85. doi: 10.1007/s00702-020-02151-9
40. Baizabal-Carvallo J, Jankovic J. Sex differences in patients with Tourette syndrome. CNS Spectr. (2022) doi: 10.1017/s1092852922000074 [Epub ahead of print].
41. Raines J, Edwards K, Sherman M, Higginson C, Winnick J, Navin K, et al. Premonitory Urge for Tics Scale (PUTS): replication and extension of psychometric properties in youth with chronic tic disorders (CTDs). J Neural Transm. (2018) 125:727–34. doi: 10.1007/s00702-017-1818-4
42. Lowe T, Capriotti M, McBurnett K. Long-term follow-up of patients with Tourette’s syndrome. Mov Disord Clin Pract. (2019) 6:40–5. doi: 10.1002/mdc3.12696
43. Eddy C, Cavanna A. Premonitory urges in adults with complicated and uncomplicated Tourette syndrome. Behav Modif. (2014) 38:264–75. doi: 10.1177/0145445513504432
44. Peterson B, Skudlarski P, Anderson A, Zhang H, Gatenby J, Lacadie C, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. (1998) 55:326–33. doi: 10.1001/archpsyc.55.4.326
45. Ferrão Y, Shavitt R, Prado H, Fontenelle L, Malavazzi D, de Mathis M, et al. Sensory phenomena associated with repetitive behaviors in obsessive-compulsive disorder: an exploratory study of 1001 patients. Psychiatry Res. (2012) 197:253–8. doi: 10.1016/j.psychres.2011.09.017
46. Martino D, Madhusudan N, Zis P, Cavanna A. An introduction to the clinical phenomenology of Tourette syndrome. Int Rev Neurobiol. (2013) 112:1–33. doi: 10.1016/b978-0-12-411546-0.00001-9
47. Specht M, Woods D, Piacentini J, Scahill L, Wilhelm S, Peterson A, et al. Clinical characteristics of children and adolescents with a primary tic disorder. J Dev Phys Disabil. (2011) 23:15–31. doi: 10.1007/s10882-010-9223-z
Keywords: tic disorders, premonitory urges, Premonitory Urge for Tics Scale, Individualized Premonitory Urge for Tics Scale, reliability, validity
Citation: Che G, Ren W, McGuire JF, Li P, Zhao Z, Tian J, Zhang J and Zhang Y (2023) Clinical evaluation of premonitory urges in children and adolescents using the Chinese version of Individualized Premonitory Urge for Tics Scale. Front. Psychiatry 14:1224825. doi: 10.3389/fpsyt.2023.1224825
Received: 18 May 2023; Accepted: 10 October 2023;
Published: 16 November 2023.
Edited by:
Tjhin Wiguna, University of Indonesia, IndonesiaCopyright © 2023 Che, Ren, McGuire, Li, Zhao, Tian, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, bF9waW5nQGpsdS5lZHUuY24=
 Guanghua Che1,2
Guanghua Che1,2 Joseph F. McGuire
Joseph F. McGuire Ping Li
Ping Li