- 1Department of Anesthesiology, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 2Department of Rehabilitation Medicine, The 3rd Xiangya Hospital, Central South University, Changsha, China
Depression and macrovascular diseases are globally recognized as significant disorders that pose a substantial socioeconomic burden because of their associated disability and mortality. In addition, comorbidities between depression and macrovascular diseases have been widely reported in clinical settings. Patients afflicted with coronary artery disease, cerebrovascular disease or peripheral artery disease exhibit an elevated propensity for depressive symptoms. These symptoms, in turn, augment the risk of macrovascular diseases, thereby reflecting a bidirectional relationship. This review examines the physiological and pathological mechanisms behind comorbidity while also examining the intricate connection between depression and macrovascular diseases. The present mechanisms are significantly impacted by atypical activity in the hypothalamic–pituitary–adrenal axis. Elevated levels of cortisol and other hormones may disrupt normal endothelial cell function, resulting in vascular narrowing. At the same time, proinflammatory cytokines like interleukin-1 and C-reactive protein have been shown to disrupt the normal function of neurons and microglia by affecting blood–brain barrier permeability in the brain, exacerbating depressive symptoms. In addition, platelet hyperactivation or aggregation, endothelial dysfunction, and autonomic nervous system dysfunction are important comorbidity mechanisms. Collectively, these mechanisms provide a plausible physiological basis for the interplay between these two diseases. Interdisciplinary collaboration is crucial for future research aiming to reveal the pathogenesis of comorbidity and develop customised prevention and treatment strategies.
1. Introduction
Because of the rapid progression of society and quick pace of modern life, people of all ages experience enormous amounts of mental stress. Depression, which is a highly prevalent psychiatric disorder (1), is characterized by sustained feelings of sadness, gloom or emptiness; sleep disturbances; increased guilt; feelings of self-reproach, helplessness and anxiety; decreased social and personal efficacy; and weight changes or altered appetites, all of which significantly impact daily life (2, 3). Research shows that 28.48% of college students have clinical depression (4). Similarly, depression also exists in 17–53% of outpatients (5) and in 35.1% of the elderly (6). It is particularly noteworthy that 6% of the population suffers from major depression (7). Depression is characterized by its persistent and recurring nature, which poses a severe hazard to human well-being. Depression is a leading aetiology for disability, and its economic impact on society is considerable (8–10). As time goes on, the economic cost of depression is expected to continue to rise and is projected to double by 2030 (3). Although certain factors, such as neurotransmitter imbalance, immune inflammatory response, and genetics, contribute to depression (11, 12), the specific pathological and physiological mechanisms underlying this disorder remain unclear.
MVD refers mainly to diseases of the large blood vessels, including the coronary arteries, aorta and larger arteries of the brain and limbs (13). MVD is intricately linked to a range of factors, including oxidative stress, inflammatory reactions, genetic predisposition, platelet dysfunction and endothelial impairment (14–18). Elevated cholesterol levels and hyperlipidaemia have been observed to potentially induce endothelial dysfunction through the oxidized low-density lipoprotein, thereby precipitating an imbalance in arterial constriction and dilation before ultimately inciting the development of atherosclerosis (19). Platelet dysfunction may facilitate the advancement of atherosclerosis via the secretion of chemokines and recruitment of leukocytes (receptor–ligand interactions) (20, 21). It is noteworthy that certain genetic factors may also play a role in the aetiology of MVD. To date, a number of genetic loci, including chromosome 9 p21 (chr9p21), alpha 1-3-N-acetylgalactosaminyltransferase and alpha 1-3-galactosyltransferase (ABO), have been identified as being linked to MVD (18). Recent studies have proposed an association between gut microbial dysbiosis (e.g., its metabolites trimethylamine N-oxide, short-chain fatty acids and taurine) and cerebrovascular disease (22). According to clinical practice, the common pathogenic factors of MVD are not single, but instead, they involve a combination of factors (23).
Several investigations have demonstrated a significant correlation between depression and certain MVD, which often manifest as comorbidities (24–28). For instance, research findings indicate that the prevalence rate of depressed patients with peripheral artery disease (PAD) lies between 16 and 35%, and for people with both PAD and depression, the risk of death increases by 24% (29). Similarly, roughly one-third of coronary artery disease (CAD) patients experience depression (30), increasing their cardiovascular mortality risk by 31% (31). In addition, poststroke depression affects 27% of stroke patients and correlates with a 25% mortality rate (32, 33). However, research on the relationship between aortic diseases and depression remains relatively limited. It should be noted that depression and MVD are not unidirectional but often influence each other, showing a bidirectional relationship between them that increase each other’s risks. For instance, the emergence of depression is attributed to vascular risk factors, while the occurrence of MVD is heightened by 30% in individuals experiencing concurrent depression (33–36). Despite significant strides made in unraveling the pathogenesis of depression and MVDs, our understanding of their interplay and underlying mechanisms remains in its infancy. Accordingly, the present review seeks to comprehensively elucidate the potential mechanisms that underscore the interplay between MVD and depression, with the intent of furnishing theoretical underpinnings for optimizing clinical interventions.
2. Risk factors
Comorbidity refers to the aggregation of multiple diseases within specific populations because of their biological, social and environmental interactions, which can result in an overall increase in the disease burden (30). Depression and MVD share similar psychosocial and physical factors in specific populations and manifest in the form of comorbidity (Figure 1). In the complex interaction between depression and MVD, hypertension, hyperlipidaemia, diabetes mellitus (DM), and obesity have been identified as significant mediators (36–39).
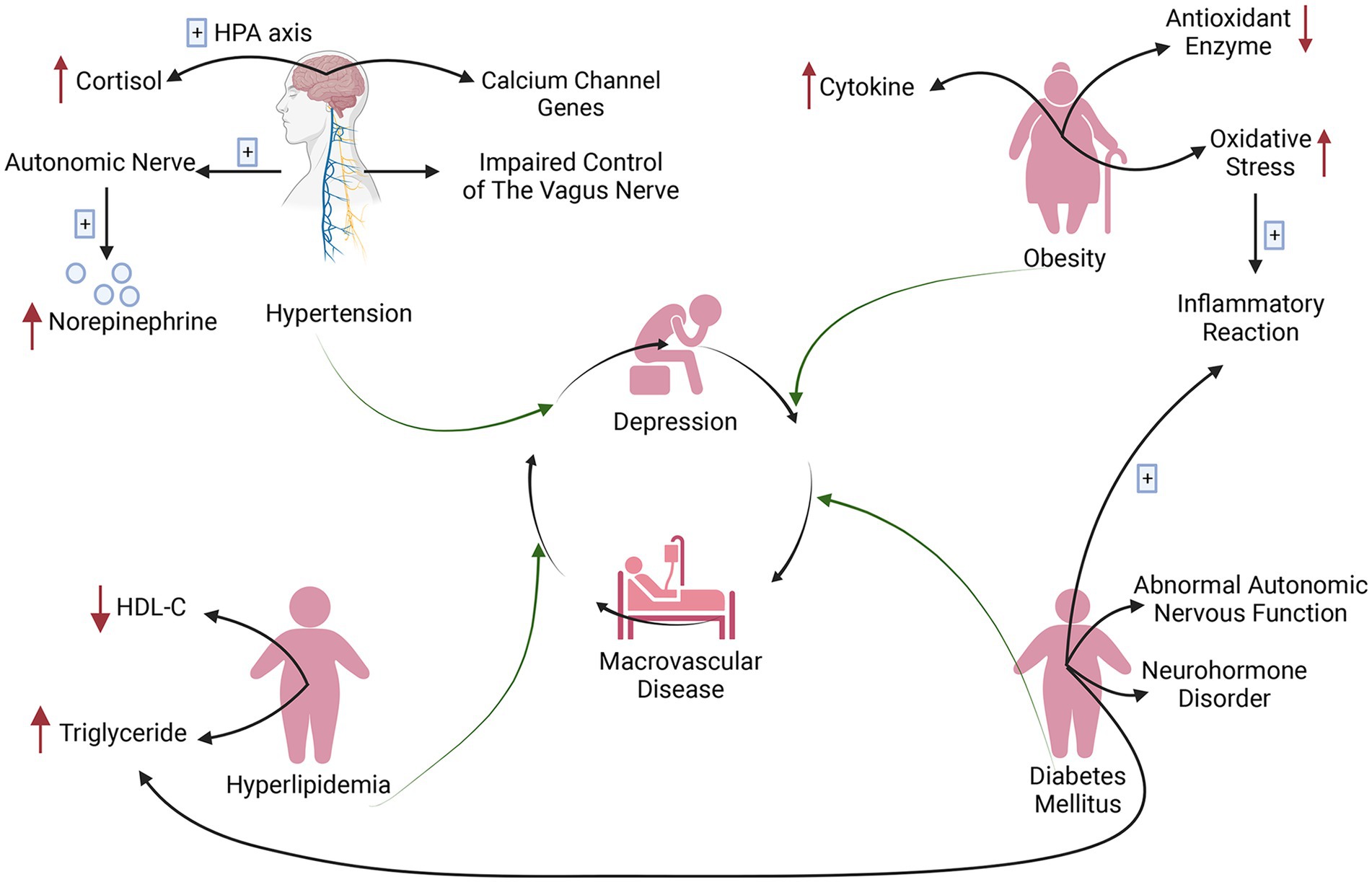
Figure 1. Risk factors for comorbidity of depression and macrovascular disease (created with BioRender.com). Calcium channel genes, autonomic nervous system abnormalities (increased norepinephrine) and increased cortisol (hypothalamic–pituitary–adrenal axis abnormalities) are all associated with macrovascular disease and depression in hypertension, and impaired vagal control is an important factor mediating both diseases. Obese patients amplify oxidative stress to promote an inflammatory response that mediates the development of diabetes mellitus. Neurohormonal dysregulation and abnormal autonomous nerve function in diabetic patients influence the development of depression and macrovascular disease. Diabetic metabolic dysregulation, that is, increased triglycerides and decreased hypothalamic–pituitary–adrenal cholesterol, causes hyperlipidaemia, which further contributes to depression and macrovascular disease because of atherosclerosis. HDL-C, high-density lipoprotein cholesterol; HPA, hypothalamic–pituitary–adrenal.
Adipose tissue secretes cytokines, which are carried to the brain by the blood and affect the neurotransmitter system, ultimately mediating the occurrence of depression (37, 40). Additionally, obesity can significantly exacerbate vascular risk factors such as high blood pressure and lipid abnormalities, thereby resulting in negative effects on vascular structure and function (41). Research has demonstrated that obesity contributes to the development of stroke by amplifying oxidative stress and lowering antioxidant enzyme levels, while oxidative stress induces depressive symptoms by promoting oxidation and inflammation through reactive oxygen species (42).
However, certain investigations have not discovered a correlation between depressive symptoms and clinical factors such as hypertension and hyperlipidaemia (43). A plethora of other research has indicated that hypertension, hyperlipidaemia and DM are not only the traditional risk factors of MVD, but they are also intricately linked to the emergence and advancement of depression. Of type 2 diabetes mellitus (T2DM) patients, 10.6% have comorbid major depression, increasing cardiovascular morbidity and mortality (44). Major depression affects 27% of hypertension patients, heightening their susceptibility to stroke events and heart failure. This link may be attributed to compromised blood pressure regulation (45, 46). Patients suffering from depression and pre-existing CAD may have diminished cardiac regulation, which can result in hypertension (46).
Impaired control of the vagus nerve may mediate the connection between depression and hypertension (47). Studies have shown that calcium channel genes, autonomic nervous system abnormalities (increased norepinephrine) and increased cortisol are all associated with MVD and depression in hypertensive patients (45, 46, 48). Depressed patients frequently exhibit low levels of high-density lipoprotein cholesterol (HDL-C) and elevated triglyceride levels, both of which promote the growth of atherosclerosis, which is a key driver of vascular disease (49). In addition, autonomic nervous dysfunction and neurohormone imbalance, inflammatory reaction and hippocampal structural alterations are all significant factors that contribute to the complex and bidirectional association between DM and depression (40, 50). Elevated triglyceride levels resulting from metabolic dysregulation in DM can cause vascular dysfunction, increase the risk of atherosclerosis and predispose individuals to coronary heart disease (51). Moreover, patients suffering from metabolic syndrome are at an elevated risk of developing depression (52).
3. Pathophysiological mechanism
There has been an upsurge in scholarly studies exploring the bidirectional interplay and fundamental mechanisms linking depression with various vascular diseases including CAD, cerebrovascular disease (CVD) and PAD. Currently, research on their comorbidity mechanisms primarily centers on three aspects (Figure 2): neuroendocrine factors, immune inflammatory responses and platelet dysfunction (53). The mechanisms in question not only precipitate and exacerbate depression but also contribute to the development of numerous complications associated with these vascular diseases.
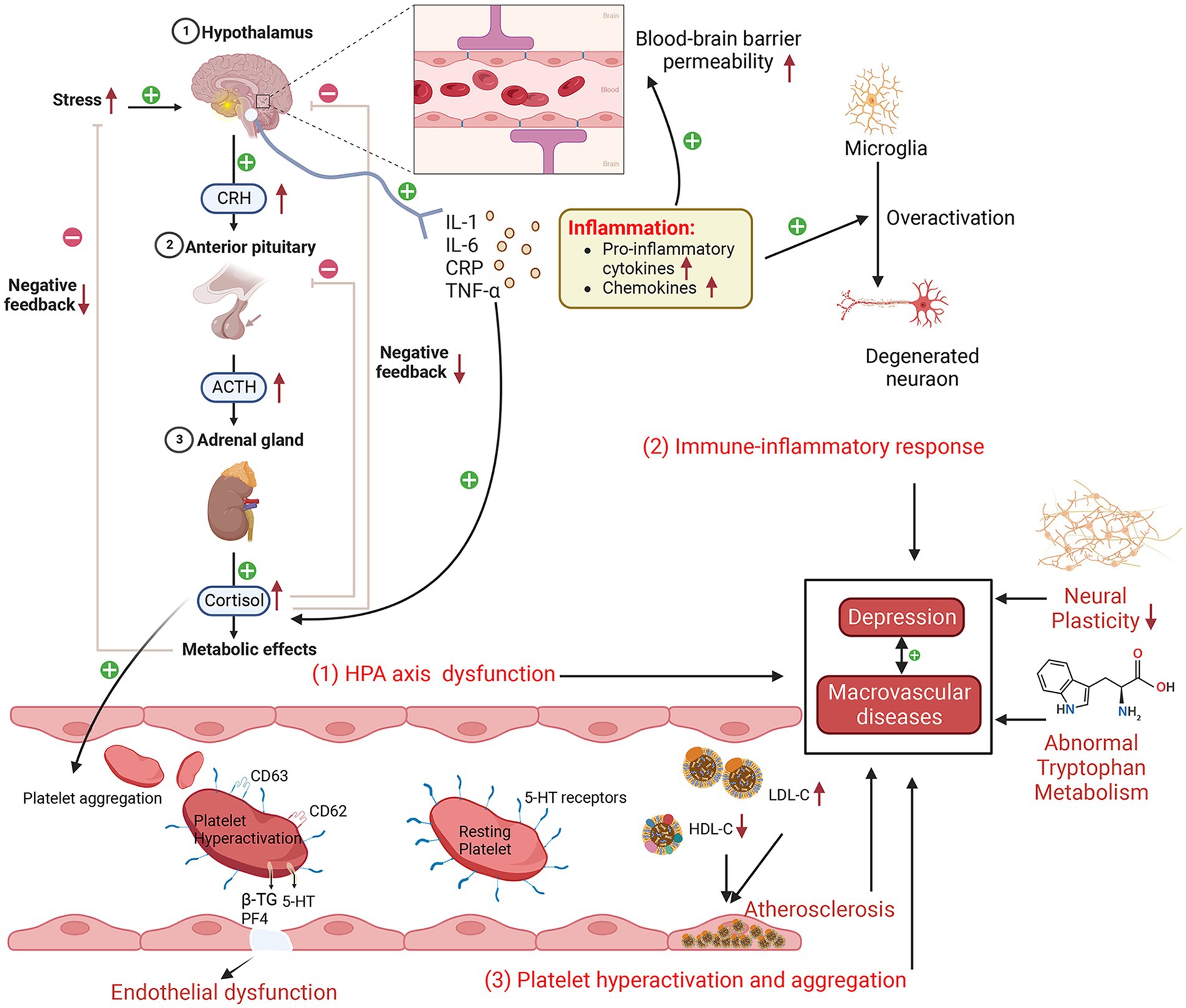
Figure 2. Schematic diagram of the possible comorbidities of depression and macrovascular disease (created with BioRender.com). Depressive episodes are characterized by increased hypothalamic–pituitary–adrenal axis activity, elevated cortisol levels, an enhanced response to corticotropin-releasing hormone, increased adrenocorticotropic hormone, and decreased cortisol feedback inhibition. Subsequently, increased secretion of hormones, such as cortisol, can damage vascular endothelial cells, leading to endothelial dysfunction and an increased inflammatory response. Proinflammatory cytokines affect cerebral blood–brain barrier permeability, disrupt microglia and neuronal function, and promote cortisol secretion, which, in turn, affects hypothalamic–pituitary–adrenal axis function. Platelet hyperactivation and aggregation lead to increased endothelial adhesion, resulting in endothelial dysfunction. In addition, abnormal tryptophan metabolism and reduced neuroplasticity play a role in depression and macrovascular disease. HPA, hypothalamic–pituitary–adrenal; LDL-C, low-density lipoprotein cholesterol; IL-6, interleukin-6; CRP, C-reactive protein; CRH, corticotropin-releasing hormone; IL-1, interleukin-1; TNF-α, tumor necrosis factor-α; 5-HT, serotonin; HDL-C, high-density lipoprotein cholesterol; PF4, platelet factor 4; β-TG, β-thromboglobulin; ACTH, adrenocorticotropic hormone.
3.1. Neuroendocrine factors
One of the current top research priorities in neuroendocrinology is the aberrant function of the hypothalamic–pituitary–adrenal (HPA) axis, which is a pathogenic mechanism implicated in depression (54), as evidenced by the fact that taking antidepressants can restore the abnormal HPA axis (55). Some scholars believe that the aberrant functioning of the HPA axis may represent a contributory cause for the comorbidity of depression and CAD/CVD (56, 57). Some studies have found a significant upregulation of HPA axis functioning in individuals with depression, which consequently results in overstimulation of the autonomic nervous system. This is clinically manifested by increased basal cortisol levels, an enhanced response to corticotropin-releasing hormone (CRH), increased adrenal cortical hormone (ACTH) secretion, and decreased cortisol feedback inhibition (58–61). In addition, increased secretion of hormones such as cortisol can further damage vascular endothelial cells, leading to abnormal endothelial cell function, increased injury and inflammatory reactions, ultimately causing vascular injury, constriction and narrowing, resulting in the occurrence of MVD (62–65).
3.2. Immune inflammatory reaction
Some studies have demonstrated an elevation in inflammatory markers, including interleukin-1 (IL-1), interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α), among patients afflicted with depression (40, 66–68). These findings provide compelling evidence for the involvement of immune inflammatory reactions in the pathogenesis of depression (69). Moreover, some studies have found that anti-inflammatory treatment can improve depression-like behavior in mice, thereby reinforcing the notion of inflammation as a critical contributor to developing depression (70). The release of proinflammatory cytokines has been found to disturb blood–brain barrier permeability and initiate inflammatory cascades that amplify inflammatory signals. Additionally, these cytokines have been observed to result in functional abnormalities in microglia and neurons, contributing to the exacerbation of depressive symptoms (66, 69, 71–73). Moreover, cytokine activation promotes the upregulation of adhesion molecules on endothelial cells, which then drives the infiltration of monocytes and lymphocytes into the vessel wall. This, in turn, triggers a localized inflammatory response within the vascular lining, ultimately fostering the progression of atherosclerosis and emergence of MVD, such as CVD and CAD (74, 75). Additionally, some cytokines can promote the release of ACTH, CRH and cortisol, thereby affecting the regulatory function of the HPA axis (76, 77). It is worth noting that TNF-α has been identified as a biomarker of inflammatory reactions after brain injury (78). It has also been reported that TNF-α can either indirectly or directly lead to apoptosis and then to vascular calcification and injury (14, 79, 80). IL-6 has been found to be critical in the pathophysiology of brain injury because it can enhance glial cell activation and stimulate endothelial cells to produce adhesion molecules (81). The aforementioned studies indicate that immune inflammatory responses exert a crucial effect on the pathogenesis of depression and MVD. Despite the substantial advancements made in comprehending the link between inflammation and these two disorders, further investigations are warranted to elucidate the precise underlying mechanisms. The aforementioned study outcomes will significantly contribute to our comprehension of the interrelation between these diseases and assist in advancing more potent strategies for their prevention and treatment.
3.3. Platelet dysfunction
Some studies have shown abnormal platelet aggregation and coagulation functions in depression and MVD (16, 82–84). A study based on flow cytometry shows that diabetic patients presenting with concomitant signs of depression exhibited platelet hyperactivation (indicated by platelet activation markers CD 62 and CD 63) compared with those without depression symptoms (85). Moreover, studies have demonstrated that patients affected by depression display increased platelet activity, as assessed via the mean platelet volume, when compared with control groups without depression symptoms (86, 87). This phenomenon may be linked to elevated secretion levels of cortisol and catecholamines in the autonomic nervous system (88, 89). Platelet dysfunction can lead to a series of problems, including increased responsiveness to physiological stress, which, in turn, causes continuous enhancement of platelet activation and aggregation (90). This then causes a rise in platelet adhesion towards the vascular endothelium (91), promoting thrombosis and vascular stenosis, ultimately triggering MVD. During platelet activation, vascular endothelial cells discharge an array of chemokines and cytokines from α-granules, including platelet factor 4 (PF4), β-thromboglobulin (β-TG), and serotonin (5-HT), which enhance inflammatory reaction and thrombosis (83, 92). The pivotal contribution of 5-HT to the onset of depression has been well established (89). As a prominent neurotransmitter, 5-HT is capable of binding with 5-HT receptors located on the platelet surface, augmenting their aggregation process, which requires the participation of the 5-HT transporter protein (83, 93, 94). Therefore, the potential utility of serotonin as a promising pharmacological target for treating depression has been established, notably through the use of selective serotonin reuptake inhibitors (SSRIs) (94–96), which have been confirmed to effectively curtail platelet activation, consequently alleviating the risk of myocardial infarction (97).
3.4. Other mechanisms
The endothelium, a monolayer of cells lining the inner surface of blood vessels, is essential for maintaining vascular function by regulating vessel expansion and contraction, inhibiting platelet aggregation, and preventing leukocyte adhesion (98). Endothelial dysfunction occurs when the physiological function of endothelial cells becomes impaired, which leads to vasoconstriction, elevated cytokine levels and platelet and leukocyte aggregation (99). It is noteworthy that endothelial dysfunction has been regarded as a characteristic marker of depression and atherosclerosis (100, 101), indicating that endothelial dysfunction is one of the pathophysiological factors of depression and MVD (99, 102, 103). Specifically, the flow-mediated dilatation value (which is used to evaluate endothelial function) of depressed patients is lower than that of nondepressed patients (104). This has also being observed in PAD (105).
For the comorbidity mechanism of depression and MVD, some scholars have put forward the vascular depression hypothesis (106). According to this hypothesis, patients affected by MVD frequently manifest depressive features attributed to cerebral white matter lesions, which augment low-density lipoprotein cholesterol levels and decrease high-density lipoprotein cholesterol levels, thereby exacerbating the severity of MVD (107, 108). In addition, other possible mechanisms contribute to the comorbidity of depression and MVD, including decreased neural plasticity (109), autonomic dysfunction (decreased heart rate variability) (110) and abnormal tryptophan metabolism (111).
4. Relationship between specific macrovascular disease and depression
4.1. Coronary artery disease
The marked correlation between CAD and depression has garnered widespread attention. Depression has been determined to be a standalone danger element for CAD (112). According to the findings of a cohort study, individuals exhibiting depression may be at an increased risk of developing ischaemic heart disease. Specifically, those displaying mild symptoms have shown a 1.5-fold higher likelihood than their nondepression counterparts, and those with severe symptoms had an even greater elevated risk of 1.6-fold (110, 113). For patients already burdened with ischaemic heart disease, depressive symptoms lead to an elevated risk of 11% for vascular events and a 23% rise in all-cause mortality (114, 115). Ricardo de Miranda Azevedo et al. (116) suggest that depressive symptoms have demonstrable ramifications for the prognostication of myocardial infarction patients, with notable implications that are contingent on both gender and age. More critically, a retrospective cohort study conducted in China encompassing nationwide heart failure patients indicated that among hospitalized heart failure patients, those with depression were more likely to be readmitted within 30 days (117).
4.2. Cerebrovascular disease
In addition to CAD, an unequivocal correlation between depression and CVD has been repeatedly demonstrated (118). According to a meta-analysis, stroke victims who have poststroke depression have a higher risk of having their strokes reoccur (119), with an incidence rate between 18 and 33% (120, 121). Patients with depression exhibited a 3.18% amplified risk of experiencing transient ischaemic attack (TIA) relative to patients without depression. Furthermore, for patients who have previously undergone TIA, there is a 6.88% likelihood of developing depressive symptoms (122). Patients with CVD who are depressed have a worse prognosis and quality of life. Several investigations have demonstrated that patients who suffer from stroke manifest depressive symptoms, exhibit a comparatively inferior quality of life, have heightened rates of disability, are more likely to attempt suicide, and have elevated rates of mortality relative to those stroke patients absent of depression (39, 123–126), with an all-cause mortality risk ratio of 1.59 (125).
4.3. Peripheral arterial disease
PAD and depression are common comorbidities. Depressive symptoms have been shown to afflict 16–35% of PAD patients, which increases the risk of mortality by 24% (29). According to a meta-analysis covering 20 studies, the prevalence of depression in patients with PAD was estimated to be 13%. This comorbidity has been found to augment the risk of adverse limb consequences, notably intermittent claudication, by 20%; at the same time, these patients are accompanied by more complications such as chest pain and palpitations (127, 128). In addition, PAD patients afflicted with depression are characterized by heightened risks of amputation and mortality, decreased walking function and a lower quality of life (29, 128–130). A nationwide observational study covering 155,647 retired military personnel with PAD revealed that patients suffering from coexisting depression heightened the risk of amputation by 13% and the risk of mortality by 17% in contrast to their counterparts without any comorbid depression (131). These findings indicate that the early identification, evaluation and management of depression in PAD patients are of great significance.
5. Conclusion and prospect
Multiple studies have established a strong link between depression and MVD. Depression affects the prognosis of MVD, while MVD further exacerbates depressive symptoms, indicating a bidirectional relationship between them. This review highlights the complex and diverse pathophysiological mechanisms underlying the comorbidity of depression and MVD, including neuroendocrine factors (HPA axis dysfunction), immune inflammatory responses (proinflammatory cytokines) and platelet dysfunction (hyperactivation and aggregation). In addition, the interaction between them is also influenced by pathological elements including endothelium dysfunction and autonomic nervous system dysfunction. The review has also elucidated the relationships between depression and CAD, CVD, and PAD. These studies on correlations provide a theoretical basis for interdisciplinary collaboration to achieve a better therapeutic effect.
Despite the clinical recognition of comorbidity between depression and MVD, additional studies are required to gain a comprehensive understanding of the underlying pathophysiological mechanisms. Future studies should aim to dive deeper into the mechanisms of interaction between these diseases, including possible pathological, physiological and molecular mechanisms.
Author contributions
SZ and LZ conceived and wrote the article. JY revised and reviewed the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Science and Technology Innovation Program of Hunan Province (No. 2021SK4014) and the Hunan Cancer Hospital Climb Plan.
Acknowledgments
Special thanks to Biorender.com for providing us with scientific mapping tools.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sinyor, M, Rezmovitz, J, and Zaretsky, A. Screen all for depression. BMJ. (2016) 352:i1617. doi: 10.1136/bmj.i1617
2. Lu, Y, Tang, C, Liow, CS, Ng, WWN, Ho, CSH, and Ho, RCM. A regressional analysis of maladaptive rumination, illness perception and negative emotional outcomes in Asian patients suffering from depressive disorder. Asian J Psychiatr. (2014) 12:69–76. doi: 10.1016/j.ajp.2014.06.014
3. McCarron, RM, Shapiro, B, Rawles, J, and Luo, J. Depression. Ann Intern Med. (2021) 174:ITC65–80. doi: 10.7326/AITC202105180
4. Gao, L, Xie, Y, Jia, C, and Wang, W. Prevalence of depression among Chinese university students: a systematic review and meta-analysis. Sci Rep. (2020) 10:15897. doi: 10.1038/s41598-020-72998-1
5. Wang, J, Wu, X, Lai, W, Long, E, Zhang, X, Li, W, et al. Prevalence of depression and depressive symptoms among outpatients: a systematic review and meta-analysis. BMJ Open. (2017) 7:e017173. doi: 10.1136/bmjopen-2017-017173
6. Cai, H, Jin, Y, Liu, R, Zhang, Q, Su, Z, Ungvari, GS, et al. Global prevalence of depression in older adults: a systematic review and meta-analysis of epidemiological surveys. Asian J Psychiatr. (2023) 80:103417. doi: 10.1016/j.ajp.2022.103417
7. Malhi, GS, and Mann, JJ. Depression. Lancet. (2018) 392:2299–312. doi: 10.1016/S0140-6736(18)31948-2
8. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
9. Greenberg, PE, Fournier, A-A, Sisitsky, T, Simes, M, Berman, R, Koenigsberg, SH, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). PharmacoEconomics. (2021) 39:653–65. doi: 10.1007/s40273-021-01019-4
10. Friedrich, MJ. Depression is the leading cause of disability around the world. JAMA. (2017) 317:1517. doi: 10.1001/jama.2017.3826
11. Haapakoski, R, Mathieu, J, Ebmeier, KP, Alenius, H, and Kivimäki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. (2015) 49:206–15. doi: 10.1016/j.bbi.2015.06.001
12. Toenders, YJ, Laskaris, L, Davey, CG, Berk, M, Milaneschi, Y, Lamers, F, et al. Inflammation and depression in young people: a systematic review and proposed inflammatory pathways. Mol Psychiatry. (2022) 27:315–27. doi: 10.1038/s41380-021-01306-8
13. Vella, S, and Petrie, JR. Macrovascular disease: pathogenesis and risk assessment. Medicine. (2022) 50:683–90. doi: 10.1016/j.mpmed.2022.08.014
14. Liberale, L, Ministrini, S, Carbone, F, Camici, GG, and Montecucco, F. Cytokines as therapeutic targets for cardio- and cerebrovascular diseases. Basic Res Cardiol. (2021) 116:23. doi: 10.1007/s00395-021-00863-x
15. Kumar, V, Bishayee, K, Park, S, Lee, U, and Kim, J. Oxidative stress in cerebrovascular disease and associated diseases. Front Endocrinol. (2023) 14:1124419. doi: 10.3389/fendo.2023.1124419
16. Smith, NM, Pathansali, R, and Bath, PM. Platelets and stroke. Vasc Med. (1999) 4:165–72. doi: 10.1177/1358836X9900400307
17. Sun, H-J, Wu, Z-Y, Nie, X-W, and Bian, J-S. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. (2020) 10:1568. doi: 10.3389/fphar.2019.01568
18. Chauhan, G, and Debette, S. Genetic risk factors for ischemic and hemorrhagic stroke. Curr Cardiol Rep. (2016) 18:124. doi: 10.1007/s11886-016-0804-z
19. Wilkinson, IB, and Cockcroft, JR. Cholesterol, endothelial function and cardiovascular disease. Curr Opin Lipidol. (1998) 9:237–42. doi: 10.1097/00041433-199806000-00009
20. Lievens, D, and von Hundelshausen, P. Platelets in atherosclerosis. Thromb Haemost. (2011) 106:827–38. doi: 10.1160/TH11-08-0592
21. Nording, HM, Seizer, P, and Langer, HF. Platelets in inflammation and Atherogenesis. Front Immunol. (2015) 6:98. doi: 10.3389/fimmu.2015.00098
22. Zou, X, Wang, L, Xiao, L, Wang, S, and Zhang, L. Gut microbes in cerebrovascular diseases: gut flora imbalance, potential impact mechanisms and promising treatment strategies. Front Immunol. (2022) 13:975921. doi: 10.3389/fimmu.2022.975921
23. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0, 20
24. Scheuer, SH, Kosjerina, V, Lindekilde, N, Pouwer, F, Carstensen, B, Jørgensen, ME, et al. Severe mental illness and the risk of diabetes complications: a Nationwide, register-based cohort study. J Clin Endocrinol Metab. (2022) 107:e3504–14. doi: 10.1210/clinem/dgac204
25. Sierra, P, Cámara, R, Tobella, H, and Livianos, L. What is the real significance and management of major thyroid disorders in bipolar patients? Rev Psiquiatr Salud Ment. (2014) 7:88–95. doi: 10.1016/j.rpsm.2013.07.005
26. Grover, S, Nebhinani, N, Chakrabarti, S, Avasthi, A, Basu, D, Kulhara, P, et al. Cardiovascular risk factors among bipolar disorder patients admitted to an inpatient unit of a tertiary care hospital in India. Asian J Psychiatr. (2014) 10:51–5. doi: 10.1016/j.ajp.2014.03.004
27. Sun, C, Tikellis, G, Klein, R, Steffens, DC, Larsen, EKM, Siscovick, DS, et al. Are microvascular abnormalities in the retina associated with depression symptoms? The cardiovascular health study. Am J Geriatr Psychiatry. (2007) 15:335–43. doi: 10.1097/01.JGP.0000247161.98311.0f
28. Koller, D, Pathak, GA, Wendt, FR, Tylee, DS, Levey, DF, Overstreet, C, et al. Epidemiologic and genetic associations of endometriosis with depression, anxiety, and eating disorders. JAMA Netw Open. (2023) 6:e2251214. doi: 10.1001/jamanetworkopen.2022.51214
29. Dp, B, Ml, P, Aj, S, and Sw, W. Depression in patients with peripheral arterial disease: a systematic review. Eur J Cardiovasc Nurs. (2017) 16:181–93. doi: 10.1177/1474515116687222
30. Akosile, W, Tiyatiye, B, Colquhoun, D, and Young, R. Management of depression in patients with coronary artery disease: a systematic review. Asian J Psychiatr. (2023) 83:103534. doi: 10.1016/j.ajp.2023.103534
31. Wei, J, Hou, R, Zhang, X, Xu, H, Xie, L, Chandrasekar, EK, et al. The association of late-life depression with all-cause and cardiovascular mortality among community-dwelling older adults: systematic review and meta-analysis. Br J Psychiatry. (2019) 215:449–55. doi: 10.1192/bjp.2019.74
32. Liu, L, Xu, M, Marshall, IJ, Wolfe, CD, Wang, Y, and O’Connell, MD. Prevalence and natural history of depression after stroke: a systematic review and meta-analysis of observational studies. PLoS Med. (2023) 20:e1004200. doi: 10.1371/journal.pmed.1004200
33. Daskalopoulou, M, George, J, Walters, K, Osborn, DP, Batty, GD, Stogiannis, D, et al. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PLoS One. (2016) 11:e0153838. doi: 10.1371/journal.pone.0153838
34. Patel, JS, Berntson, J, Polanka, BM, and Stewart, JC. Cardiovascular risk factors as differential predictors of incident atypical and typical major depressive disorder in US adults. Psychosom Med. (2018) 80:508–14. doi: 10.1097/PSY.0000000000000583
35. Li, H, Zheng, D, Li, Z, Wu, Z, Feng, W, Cao, X, et al. Association of Depressive Symptoms with Incident Cardiovascular Diseases in middle-aged and older Chinese adults. JAMA Netw Open. (2019) 2:e1916591. doi: 10.1001/jamanetworkopen.2019.16591
36. Li, GH-Y, Cheung, C-L, Chung, AK-K, Cheung, BM-Y, Wong, IC-K, Fok, MLY, et al. Evaluation of bi-directional causal association between depression and cardiovascular diseases: a Mendelian randomization study. Psychol Med. (2022) 52:1765–76. doi: 10.1017/S0033291720003566
37. Jia, Z, and Li, S. Risk of cardiovascular disease mortality in relation to depression and 14 common risk factors. Int J Gen Med. (2021) 14:441–9. doi: 10.2147/IJGM.S292140
38. Milaneschi, Y, Simmons, WK, van Rossum, EFC, and Penninx, BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
39. Jørgensen, TSH, Wium-Andersen, IK, Wium-Andersen, MK, Jørgensen, MB, Prescott, E, Maartensson, S, et al. Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of Danish patients. JAMA Psychiatry. (2016) 73:1032–40. doi: 10.1001/jamapsychiatry.2016.1932
40. Harsanyi, S, Kupcova, I, Danisovic, L, and Klein, M. Selected biomarkers of depression: what are the effects of cytokines and inflammation? Int J Mol Sci. (2022) 24:578. doi: 10.3390/ijms24010578
41. Lavie, CJ, De Schutter, A, Parto, P, Jahangir, E, Kokkinos, P, Ortega, FB, et al. Obesity and prevalence of cardiovascular diseases and prognosis—the obesity paradox updated. Prog Cardiovasc Dis. (2016) 58:537–47. doi: 10.1016/j.pcad.2016.01.008
42. Lin, D, Wang, L, Yan, S, Zhang, Q, Zhang, JH, and Shao, A. The role of oxidative stress in common risk factors and mechanisms of cardio-cerebrovascular ischemia and depression. Oxidative Med Cell Longev. (2019) 2019:e2491927:1–13. doi: 10.1155/2019/2491927
43. Wiehe, M, Fuchs, SC, Moreira, LB, Moraes, RS, Pereira, GM, Gus, M, et al. Absence of association between depression and hypertension: results of a prospectively designed population-based study. J Hum Hypertens. (2006) 20:434–9. doi: 10.1038/sj.jhh.1002017
44. Zhu, M, Li, Y, Luo, B, Cui, J, Liu, Y, and Liu, Y. Comorbidity of type 2 diabetes mellitus and depression: clinical evidence and rationale for the exacerbation of cardiovascular disease. Front Cardiovasc Med. (2022) 9:861110. doi: 10.3389/fcvm.2022.861110
45. Graham, N, Ward, J, Mackay, D, Pell, JP, Cavanagh, J, Padmanabhan, S, et al. Impact of major depression on cardiovascular outcomes for individuals with hypertension: prospective survival analysis in UK biobank. BMJ Open. (2019) 9:e024433. doi: 10.1136/bmjopen-2018-024433
46. Scalco, AZ, Scalco, MZ, Azul, JBS, and Neto, FL. Hypertension and depression. Clinics. (2005) 60:241–50. doi: 10.1590/S1807-59322005000300010
47. Yang, X, Daches, S, Yaroslavsky, I, George, CJ, and Kovacs, M. Cardiac vagal control mediates the relation between past depression and blood pressure several years later among young adults. Psychophysiology. (2020) 57:e13535. doi: 10.1111/psyp.13535
48. Casamassima, F, Huang, J, Fava, M, Sachs, GS, Smoller, JW, Cassano, GB, et al. Phenotypic effects of a bipolar liability gene among individuals with major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. (2010) 9999B:n/a–309. doi: 10.1002/ajmg.b.30962
49. Sergi, D, Zauli, E, Tisato, V, Secchiero, P, Zauli, G, and Cervellati, C. Lipids at the Nexus between cerebrovascular disease and vascular dementia: the impact of HDL-cholesterol and ceramides. Int J Mol Sci. (2023) 24:4403. doi: 10.3390/ijms24054403
50. Semenkovich, K, Brown, ME, Svrakic, DM, and Lustman, PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. (2015) 75:577–87. doi: 10.1007/s40265-015-0347-4
51. Beckman, JA, Creager, MA, and Libby, P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. (2002) 287:2570–81. doi: 10.1001/jama.287.19.2570
52. Skilton, MR, Moulin, P, Terra, J-L, and Bonnet, F. Associations between anxiety, depression, and the metabolic syndrome. Biol Psychiatry. (2007) 62:1251–7. doi: 10.1016/j.biopsych.2007.01.012
53. Nemeroff, CB, and Goldschmidt-Clermont, PJ. Heartache and heartbreak—the link between depression and cardiovascular disease. Nat Rev Cardiol. (2012) 9:526–39. doi: 10.1038/nrcardio.2012.91
54. Keller, J, Gomez, R, Williams, G, Lembke, A, Lazzeroni, L, Murphy, GM, et al. HPA Axis in major depression: cortisol, clinical symptomatology, and genetic variation predict cognition. Mol Psychiatry. (2017) 22:527–36. doi: 10.1038/mp.2016.120
55. Barden, N, Reul, JMHM, and Holsboer, F. Do antidepressants stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system? Trends Neurosci. (1995) 18:6–11. doi: 10.1016/0166-2236(95)93942-Q
56. Goldston, K, and Baillie, AJ. Depression and coronary heart disease: a review of the epidemiological evidence, explanatory mechanisms and management approaches. Clin Psychol Rev. (2008) 28:288–306. doi: 10.1016/j.cpr.2007.05.005
57. Zhou, L, Wang, T, Yu, Y, Li, M, Sun, X, Song, W, et al. The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed Pharmacother. (2022) 151:113146. doi: 10.1016/j.biopha.2022.113146
58. Dwyer, JB, Aftab, A, Radhakrishnan, R, Widge, A, Rodriguez, CI, Carpenter, LL, et al. Hormonal treatments for major depressive disorder: state of the art. AJP. (2020) 177:686–705. doi: 10.1176/appi.ajp.2020.19080848
59. Stetler, C, and Miller, GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. (2011) 73:114–26. doi: 10.1097/PSY.0b013e31820ad12b
60. Swaab, DF, Bao, A-M, and Lucassen, PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. (2005) 4:141–94. doi: 10.1016/j.arr.2005.03.003
61. Cowen, PJ. Not fade away: the HPA axis and depression. Psychol Med. (2010) 40:1–4. doi: 10.1017/S0033291709005558
62. Reynolds, RM, Ilyas, B, Price, JF, Fowkes, FGR, Newby, DE, Webb, DJ, et al. Circulating plasma cortisol concentrations are not associated with coronary artery disease or peripheral vascular disease. QJM. (2009) 102:469–75. doi: 10.1093/qjmed/hcp057
63. Koertge, J, Al-Khalili, F, Ahnve, S, Janszky, I, Svane, B, and Schenck-Gustafsson, K. Cortisol and vital exhaustion in relation to significant coronary artery stenosis in middle-aged women with acute coronary syndrome. Psychoneuroendocrinology. (2002) 27:893–906. doi: 10.1016/S0306-4530(02)00002-1
64. Alevizaki, M, Cimponeriu, A, Lekakis, J, Papamichael, C, and Chrousos, GP. High anticipatory stress plasma cortisol levels and sensitivity to glucocorticoids predict severity of coronary artery disease in subjects undergoing coronary angiography. Metabolism. (2007) 56:222–6. doi: 10.1016/j.metabol.2006.09.017
65. Johansson, Å, Ahrén, B, Näsman, B, Carlström, K, and Olsson, T. Cortisol axis abnormalities early after stroke – relationships to cytokines and leptin. J Intern Med. (2000) 247:179–87. doi: 10.1046/j.1365-2796.2000.00600.x
66. Goldsmith, DR, Bekhbat, M, Mehta, ND, and Felger, JC. Inflammation-related functional and structural Dysconnectivity as a pathway to psychopathology. Biol Psychiatry. (2023) 93:405–18. doi: 10.1016/j.biopsych.2022.11.003
67. Xia, C-Y, Guo, Y-X, Lian, W-W, Yan, Y, Ma, B-Z, Cheng, Y-C, et al. The NLRP3 inflammasome in depression: potential mechanisms and therapies. Pharmacol Res. (2023) 187:106625. doi: 10.1016/j.phrs.2022.106625
68. Majd, M, Saunders, EFH, and Engeland, CG. Inflammation and the dimensions of depression: a review. Front Neuroendocrinol. (2020) 56:100800. doi: 10.1016/j.yfrne.2019.100800
69. Fourrier, C, Sampson, E, Mills, NT, and Baune, BT. Anti-inflammatory treatment of depression: study protocol for a randomised controlled trial of vortioxetine augmented with celecoxib or placebo. Trials. (2018) 19:447. doi: 10.1186/s13063-018-2829-7
70. Correia, AS, Cardoso, A, and Vale, N. Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants. (2023) 12:470. doi: 10.3390/antiox12020470
71. Miller, AH, Maletic, V, and Raison, CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
72. Kiecolt-Glaser, JK, Derry, HM, and Fagundes, CP. Inflammation: depression fans the flames and feasts on the heat. AJP. (2015) 172:1075–91. doi: 10.1176/appi.ajp.2015.15020152
73. Raison, CL, Capuron, L, and Miller, AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. (2006) 27:24–31. doi: 10.1016/j.it.2005.11.006
74. Hayley, S, Hakim, AM, and Albert, PR. Depression, dementia and immune dysregulation. Brain. (2020) 144:746–60. doi: 10.1093/brain/awaa405
75. Gu, H, Tang, C, and Yang, Y. Psychological stress, immune response, and atherosclerosis. Atherosclerosis. (2012) 223:69–77. doi: 10.1016/j.atherosclerosis.2012.01.021
76. Maes, M, Scharpé, S, Meltzer, HY, Bosmans, E, Suy, E, Calabrese, J, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. (1993) 49:11–27. doi: 10.1016/0165-1781(93)90027-e
77. Hou, R, Ye, G, Liu, Y, Chen, X, Pan, M, Zhu, F, et al. Effects of SSRIs on peripheral inflammatory cytokines in patients with generalized anxiety disorder. Brain Behav Immun. (2019) 81:105–10. doi: 10.1016/j.bbi.2019.06.001
78. Maida, CD, Norrito, RL, Daidone, M, Tuttolomondo, A, and Pinto, A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. (2020) 21:6454. doi: 10.3390/ijms21186454
79. Koike, S, Yano, S, Tanaka, S, Sheikh, AM, Nagai, A, and Sugimoto, T. Advanced glycation end-products induce apoptosis of vascular smooth muscle cells: a mechanism for vascular calcification. Int J Mol Sci. (2016) 17:1567. doi: 10.3390/ijms17091567
80. Nash, M, McGrath, JP, Cartland, SP, Patel, S, and Kavurma, MM. Tumour necrosis factor superfamily members in ischaemic vascular diseases. Cardiovasc Res. (2019) 115:713–20. doi: 10.1093/cvr/cvz042
81. Zhu, H, Hu, S, Li, Y, Sun, Y, Xiong, X, Hu, X, et al. Interleukins and ischemic stroke. Front Immunol. (2022) 13:828447. doi: 10.3389/fimmu.2022.828447
82. Kayhan, F, Gündüz, Ş, Ersoy, SA, Kandeğer, A, and Annagür, BB. Relationships of neutrophil-lymphocyte and platelet-lymphocyte ratios with the severity of major depression. Psychiatry Res. (2017) 247:332–5. doi: 10.1016/j.psychres.2016.11.016
83. Ozturk, HM, Ozturk, S, and Yetki̇n E., Linkage between cardiovascular diseases and major depression: contribution of platelet cells. Psychiatry Res. (2020) 287:111026. doi: 10.1016/j.psychres.2017.11.079
84. Ziegelstein, RC, Parakh, K, Sakhuja, A, and Bhat, U. Platelet function in patients with major depression. Intern Med J. (2009) 39:38–43. doi: 10.1111/j.1445-5994.2008.01794.x
85. R, S, Saharia, GK, Patra, S, Bandyopadhyay, D, and Patro, BK. Flow cytometry based platelet activation markers and state of inflammation among subjects with type 2 diabetes with and without depression. Sci Rep. (2022) 12:10039. doi: 10.1038/s41598-022-13037-z
86. Ataoglu, A, and Canan, F. Mean platelet volume in patients with major depression: effect of escitalopram treatment. J Clin Psychopharmacol. (2009) 29:368–71. doi: 10.1097/JCP.0b013e3181abdfd7
87. Izzi, B, Tirozzi, A, Cerletti, C, Donati, MB, De, GG, Hoylaerts, MF, et al. Beyond haemostasis and thrombosis: platelets in depression and its co-morbidities. Int J Mol Sci. (2020) 21:8817. doi: 10.3390/ijms21228817
88. Wang, J-M, Yang, K-D, Wu, S-Y, Zou, X-G, Liao, Y-S, Yang, B, et al. Platelet parameters, C-reactive protein, and depression: an association study. IJGM. (2022) 15:243–51. doi: 10.2147/IJGM.S338558
89. Pivac, N, Jakovljević, M, Mück-Seler, D, and Brzović, Z. Hypothalamic-pituitary-adrenal axis function and platelet serotonin concentrations in depressed patients. Psychiatry Res. (1997) 73:123–32. doi: 10.1016/s0165-1781(97)00120-0
90. Mazereeuw, G, Herrmann, N, Bennett, SAL, Swardfager, W, Xu, H, Valenzuela, N, et al. Platelet activating factors in depression and coronary artery disease: a potential biomarker related to inflammatory mechanisms and neurodegeneration. Neurosci Biobehav Rev. (2013) 37:1611–21. doi: 10.1016/j.neubiorev.2013.06.010
91. Khawaja, IS, Westermeyer, JJ, Gajwani, P, and Feinstein, RE. Depression and coronary artery disease. Psychiatry (Edgmont). (2009) 6:38–51.
92. Nemeroff, CB, and Musselman, DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. (2000) 140:S57–62. doi: 10.1067/mhj.2000.109978
93. Lozano, PA, Alarabi, AB, Garcia, SE, Boakye, ET, Kingbong, HT, Naddour, E, et al. The antidepressant duloxetine inhibits platelet function and protects against thrombosis. Int J Mol Sci. (2022) 23:2587. doi: 10.3390/ijms23052587
94. Grech, J, Chan, MV, Ochin, C, Lachapelle, A, Thibord, F, Schneider, Z, et al. Serotonin-affecting antidepressant use in relation to platelet reactivity. Clin Pharmacol Ther. (2022) 111:909–18. doi: 10.1002/cpt.2517
95. Köhler, CA, Freitas, TH, Stubbs, B, Maes, M, Solmi, M, Veronese, N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. (2018) 55:4195–206. doi: 10.1007/s12035-017-0632-1
96. Gosmann, NP, Costa, MA, Jaeger, MB, Motta, LS, Frozi, J, Spanemberg, L, et al. Selective serotonin reuptake inhibitors, and serotonin and norepinephrine reuptake inhibitors for anxiety, obsessive-compulsive, and stress disorders: a 3-level network meta-analysis. PLoS Med. (2021) 18:e1003664. doi: 10.1371/journal.pmed.1003664
97. Schlienger, RG, Fischer, LM, Jick, H, and Meier, CR. Current use of selective serotonin reuptake inhibitors and risk of acute myocardial infarction. Drug-Safety. (2004) 27:1157–65. doi: 10.2165/00002018-200427140-00006
98. Tonhajzerova, I, Sekaninova, N, Bona Olexova, L, and Visnovcova, Z. Novel insight into Neuroimmune regulatory mechanisms and biomarkers linking major depression and vascular diseases: the dilemma continues. Int J Mol Sci. (2020) 21:2317. doi: 10.3390/ijms21072317
99. Chrysohoou, C, Kollia, N, and Tousoulis, D. The link between depression and atherosclerosis through the pathways of inflammation and endothelium dysfunction. Maturitas. (2018) 109:1–5. doi: 10.1016/j.maturitas.2017.12.001
100. Broadley, AJM, Korszun, A, Jones, CJH, and Frenneaux, MP. Arterial endothelial function is impaired in treated depression. Heart. (2002) 88:521–3. doi: 10.1136/heart.88.5.521
101. Verma, S, Buchanan, MR, and Anderson, TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. (2003) 108:2054–9. doi: 10.1161/01.CIR.0000089191.72957.ED
102. van Dooren, FE, Schram, MT, Schalkwijk, CG, Stehouwer, CD, Henry, RM, Dagnelie, PC, et al. Associations of low grade inflammation and endothelial dysfunction with depression – the Maastricht study. Brain Behav Immun. (2016) 56:390–6. doi: 10.1016/j.bbi.2016.03.004
103. Ramirez, JL, Drudi, LM, and Grenon, SM. Review of biologic and behavioral risk factors linking depression and peripheral artery disease. Vasc Med. (2018) 23:478–88. doi: 10.1177/1358863X18773161
104. Aydin Sunbul, E, Sunbul, M, and Gulec, H. The impact of major depression on heart rate variability and endothelial dysfunction in patients with stable coronary artery disease. Gen Hosp Psychiatry. (2017) 44:4–9. doi: 10.1016/j.genhosppsych.2016.10.006
105. Brevetti, G, Schiano, V, and Chiariello, M. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis. (2008) 197:1–11. doi: 10.1016/j.atherosclerosis.2007.11.002
106. Taylor, WD, Aizenstein, HJ, and Alexopoulos, GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. (2013) 18:963–74. doi: 10.1038/mp.2013.20
107. Yong-Ku KimHan, K-M. Neural substrates for late-life depression: A selective review of structural neuroimaging studies. Prog Neuro-Psychopharmacol Biol Psychiatry. (2021) 104:110010. doi: 10.1016/j.pnpbp.2020.110010
108. Łucka, A, Arabska, J, Fife, E, Kroc, Ł, Sołtysik, BK, Kłoszewska, I, et al. Atherogenic indices are increased in elderly patients with unipolar depression—case–control analysis. Metab Syndr Relat Disord. (2017) 15:291–5. doi: 10.1089/met.2017.0008
109. Dean, J, and Keshavan, M. The neurobiology of depression: an integrated view. Asian J Psychiatr. (2017) 27:101–11. doi: 10.1016/j.ajp.2017.01.025
110. Stein, PK, Carney, RM, Freedland, KE, Skala, JA, Jaffe, AS, Kleiger, RE, et al. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res. (2000) 48:493–500. doi: 10.1016/s0022-3999(99)00085-9
111. Huang, P, Yan, L, Li, Z, Zhao, S, Feng, Y, Zeng, J, et al. Potential shared gene signatures and molecular mechanisms between atherosclerosis and depression: evidence from transcriptome data. Comput Biol Med. (2023) 152:106450. doi: 10.1016/j.compbiomed.2022.106450
112. Zellweger, M. Coronary artery disease and depression. Eur Heart J. (2004) 25:3–9. doi: 10.1016/j.ehj.2003.09.009
113. Anda, R, Williamson, D, Jones, D, Macera, C, Eaker, E, Glassman, A, et al. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. (1993) 4:285–94. doi: 10.1097/00001648-199307000-00003
114. Meijer, A, Conradi, HJ, Bos, EH, Anselmino, M, Carney, RM, Denollet, J, et al. Adjusted prognostic association of depression following myocardial infarction with mortality and cardiovascular events: individual patient data meta-analysis. Br J Psychiatry. (2013) 203:90–102. doi: 10.1192/bjp.bp.112.111195
115. Summers, KM, Martin, KE, and Watson, K. Impact and clinical Management of Depression in patients with coronary artery disease. Pharmacotherapy. (2010) 30:304–22. doi: 10.1592/phco.30.3.304
116. de Miranda, AR, Roest, AM, Carney, RM, Freedland, KE, Lane, DA, Parakh, K, et al. Individual depressive symptoms and all-cause mortality in 6673 patients with myocardial infarction: heterogeneity across age and sex subgroups. J Affect Disord. (2018) 228:178–85. doi: 10.1016/j.jad.2017.11.025
117. Neelkumar PatelChakraborty, S, Bandyopadhyay, D, Amgai, B, Hajra, A, Atti, V, et al. Association between depression and readmission of heart failure: A national representative database study. Prog Cardiovasc Dis. (2020) 63:585–90. doi: 10.1016/j.pcad.2020.03.014
118. Göthe, F, Enache, D, Wahlund, LO, Winblad, B, Crisby, M, Lökk, J, et al. Cerebrovascular diseases and depression: epidemiology, mechanisms and treatment. Panminerva Med. (2012) 54:161–70.
119. Wu, Q, Zhou, A, Han, Y, Liu, Y, Yang, Y, Wang, X, et al. Poststroke depression and risk of recurrent stroke: a meta-analysis of prospective studies. Medicine. (2019) 98:e17235. doi: 10.1097/MD.0000000000017235
120. López-Espuela, F, Roncero-Martín, R, Canal-Macías, ML, Moran, JM, Vera, V, Gomez-Luque, A, et al. Depressed mood after stroke: predictive factors at six months follow-up. Int J Environ Res Public Health. (2020) 17:9542. doi: 10.3390/ijerph17249542
121. Rg, R, and Re, J. Post-stroke depression: a review. Am J Psychiatry. (2016) 173:221–31. doi: 10.1176/appi.ajp.2015.15030363
122. Kahlon, CK, and Nasrallah, HA. Bidirectional relationship between transient ischemic attacks and depression: a review. Ann Clin Psychiatry. (2019) 31:214–20.
123. Mitchell, AJ, Sheth, B, Gill, J, Yadegarfar, M, Stubbs, B, Yadegarfar, M, et al. Prevalence and predictors of post-stroke mood disorders: a meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry. (2017) 47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001
124. Ayerbe, L, Ayis, S, Wolfe, CDA, and Rudd, AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:14–21. doi: 10.1192/bjp.bp.111.107664
125. Cai, W, Mueller, C, Li, Y-J, Shen, W-D, and Stewart, R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. (2019) 50:102–9. doi: 10.1016/j.arr.2019.01.013
126. Eriksson, M, Glader, E-L, Norrving, B, and Asplund, K. Poststroke suicide attempts and completed suicides: a socioeconomic and nationwide perspective. Neurology. (2015) 84:1732–8. doi: 10.1212/WNL.0000000000001514
127. Abi-Jaoudé, JG, Naiem, AA, Edwards, T, Lukaszewski, M-A, Obrand, DI, Steinmetz, OK, et al. Comorbid depression is associated with increased major adverse limb events in peripheral arterial disease: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2022) 64:101–10. doi: 10.1016/j.ejvs.2022.04.020
128. Rezvani, F, Pelt, M, Härter, M, and Dirmaier, J. Effects of walking impairment on mental health burden, health risk behavior and quality of life in patients with intermittent claudication: a cross-sectional path analysis. PLoS One. (2022) 17:e0273747. doi: 10.1371/journal.pone.0273747
129. Kim G.E. SmolderenAquarius, AE, de Vries, J, Smith, ORF, Hamming, JF, and Denollet, J. Depressive symptoms in peripheral arterial disease: A follow-up study on prevalence, stability, and risk factors. J Affect Disord. (2008) 110:27–35. doi: 10.1016/j.jad.2007.12.238
130. Scierka, LE, Mena-Hurtado, C, Ahmed, ZV, Yousef, S, Arham, A, Grimshaw, AA, et al. The association of depression with mortality and major adverse limb event outcomes in patients with peripheral artery disease: a systematic review and meta-analysis. J Affect Disord. (2023) 320:169–77. doi: 10.1016/j.jad.2022.09.098
Keywords: depression, macrovascular disease, cerebrovascular disease, coronary artery disease, platelet dysfunction, proinflammatory cytokines
Citation: Zhao S, Zhu L and Yang J (2023) Association between depression and macrovascular disease: a mini review. Front. Psychiatry. 14:1215173. doi: 10.3389/fpsyt.2023.1215173
Edited by:
Sen Li, Beijing University of Chinese Medicine, ChinaReviewed by:
Lina Zhou, Xi'an Jiaotong University, ChinaJane Elizabeth Persons, The University of Iowa, United States
Copyright © 2023 Zhao, Zhu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinfeng Yang, eWFuZ2ppbmZlbmdAaG5jYS5vcmcuY24=
 Shuwu Zhao
Shuwu Zhao Liping Zhu
Liping Zhu Jinfeng Yang
Jinfeng Yang