
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 01 August 2023
Sec. Adolescent and Young Adult Psychiatry
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1200738
This article is part of the Research TopicExploration of Major Depressive Disorder among Children and Adolescents: From Pathogenesis to InterventionView all 15 articles
 Chen-Hui Sun1†
Chen-Hui Sun1† Jian-Xin Mai2†
Jian-Xin Mai2† Zhan-Ming Shi3†
Zhan-Ming Shi3† Wei Zheng4
Wei Zheng4 Wen-Long Jiang5
Wen-Long Jiang5 Ze-Zhi Li2
Ze-Zhi Li2 Xing-Bing Huang2
Xing-Bing Huang2 Xin-Hu Yang2*
Xin-Hu Yang2* Wei Zheng2*
Wei Zheng2*Objective: This meta-analysis of randomized clinical trials (RCTs) was conducted to explore the therapeutic effects, tolerability and safety of repetitive transcranial magnetic stimulation (rTMS) as an adjunct treatment in adolescents with first-episode major depressive disorder (FE-MDD).
Methods: RCTs examining the efficacy, tolerability and safety of adjunctive rTMS for adolescents with FE-MDD were included. Data were extracted by three independent authors and synthesized using RevMan 5.3 software with a random effects model.
Results: A total of six RCTs involving 562 adolescents with FE-MDD were included. Adjunctive rTMS was superior in improving depressive symptoms over the control group [standardized mean difference (SMD) = −1.50, 95% confidence interval (CI): −2.16, −0.84; I2 = 89%, p < 0.00001] in adolescents with FE-MDD. A sensitivity analysis and two subgroup analyses also confirmed the significant findings. Adolescents with FE-MDD treated with rTMS had significantly greater response [risk ratio (RR) = 1.35, 95% CI: 1.04, 1.76; I2 = 56%, p = 0.03] and remission (RR = 1.35, 95% CI: 1.03, 1.77; I2 = 0%, p = 0.03) over the control group. All-cause discontinuations were similar between the two groups (RR = 0.79, 95% CI: 0.32, 1.93; I2 = 0%, p = 0.60). No significant differences were found regarding adverse events, including headache, loss of appetite, dizziness and nausea (p = 0.14–0.82). Four out of six RCTs (66.7%), showed that adjunctive rTMS was more efficacious over the control group in improving neurocognitive function (all p < 0.05).
Conclusion: Adjunctive rTMS appears to be a beneficial strategy in improving depressive symptoms and neurocognitive function in adolescents with FE-MDD. Higher quality RCTs with larger sample sizes and longer follow-up periods are warranted in the future.
As a common mental disorder, major depressive disorder (MDD) affects approximately 5–15% of children and adolescents (1). Depression during adolescence is associated with a high risk of academic failure and behavioral problems (2), suicidal ideation and attempts (3), and adverse mental health consequences (i.e., anxiety disorder and substance use disorder) in the future (4–6). As a result, developments in treating adolescents suffering from MDD may have a positive influence on public health.
The usual treatment modalities for adolescents with MDD, mainly psychotherapy (e.g., cognitive behavioral therapy [CBT]), pharmacotherapy (e.g., selective serotonin reuptake inhibitors [SSRIs]) or both (7, 8), remain limited. Previous studies have found that at least 40% of adolescents with MDD showed unsatisfactory responses to those treatments (9, 10). For instance, psychotherapy may involve substantial time and financial costs, which lead to poor treatment compliance (11), while pharmacotherapy for adolescent patients may be associated with adverse events and even increased suicide risk (12). Therefore, there is an urgent need to explore more efficient and acceptable therapeutics for adolescents with MDD in clinical practice.
Repetitive transcranial magnetic stimulation (rTMS), as a noninvasive physical therapy, can modulate brain network functioning by producing a local magnetic field that acts on the local cerebral cortex and depression-related areas (13). rTMS has received the US Food and Drug Administration (FDA) approval to treat MDD among adults rather than adolescents (9). Accumulating randomized controlled trials (RCTs) have revealed the positive therapeutic effects of rTMS in adult patients suffering from treatment-refractory depression (TRD) (14, 15). Growing evidence has shown that rTMS can also improve drug efficacy in adult patients with first-episode major depressive disorder (FE-MDD) (16). For adolescents with MDD, several open-label studies (17, 18) have shown adjunctive rTMS to be a potentially effective treatment. However, the findings of RCTs (19–24) examining the therapeutic effects and safety of adjunctive rTMS in the treatment of adolescents with FE-MDD were inconsistent.
Therefore, the main aim of this meta-analysis was to investigate the therapeutic effects, tolerability and safety of adjunctive rTMS for adolescents with FE-MDD. We hypothesized that active rTMS plus antidepressants would be more efficacious than sham rTMS plus antidepressants or antidepressant monotherapy in improving depressive symptoms in FE-MDD patients among adolescents.
Based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (25), three authors (CHS, XHY and ZMS) independently retrieved RCTs examining the efficacy, tolerability and safety of adjunctive rTMS for adolescents with FE-MDD in international (Cochrane Library, PubMed, PsycINFO, and EMBASE) and Chinese (Wan Fang and Chinese Journal Net databases) databases from the establishment of the database to 9 November 2022. The detailed search strategy is presented in Appendix S1. Furthermore, the reference lists of meta-analyses and review articles (1, 9, 26) and the included RCTs (19–24) were searched manually and independently by the same three investigators to identify additional studies.
The inclusion criteria were conducted based on the following PICOS principle. Participants: the study subjects must be adolescent patients (aged ≥12 years and ≤ 18 years) with a diagnosis of FE-MDD based on standardized diagnostic interviews. Following the methodology of a recent systematic review (27), adolescents were defined as those who were 12–18 years old. According to the recommendations of a previous meta-analysis (28), the sample was considered an FE-MDD group if the literature showed explicit characteristic descriptions (e.g., first-episode depression, first-episode depressive disorder, early depression) for the enrolled patients. Intervention: active rTMS plus antidepressants. Comparison: antidepressants plus sham rTMS or antidepressant monotherapy. Outcomes: the primary outcome was the improvement of depressive symptoms at the post-rTMS time point measured with standardized instruments, such as the Hamilton Depression Rating Scale (HAMD) (29). The secondary outcomes were (1) study-defined response (i.e., at least 50% reduction in HAMD scores) and remission (i.e., at least 75% reduction in HAMD scores); (2) discontinuation due to any reason; (3) adverse events; and (4) neurocognitive function. S tudy design: only published RCTs targeting the efficacy and safety of adjunctive active rTMS versus sham rTMS or antidepressant monotherapy for adolescents with FE-MDD were included. Thus, studies examining the efficacy and safety of active rTMS alone versus antidepressants (30) or sham rTMS alone (31) were excluded. Furthermore, studies involving of other inventions such as any kind of psychotherapy were excluded. Case reports/series, non-RCTs and reviews were excluded.
We established a standardized Microsoft Excel table to extract essential information from selected studies. This process was independently conducted by the same authors (XHY, CHS and ZMS). If there were some inconsistencies, they were resolved by discussion within the team or the involvement of a senior investigator WZ (from Guangzhou). If relevant data were missing in the included literature, the first and/or corresponding authors were contacted by email or telephone for accurate information. If the eligible RCT consisted of a mixture of FE-MDD and multiepisode MDD, only data from the FE-MDD group were extracted.
Three authors (XHY, CHS, and ZMS) independently assessed the quality of each RCT using the Cochrane risk of bias (32) and the Jadad scale (33). A Jadad scale score < 3 was rated as ‘low quality’, and a Jadad scale score ≥ 3 was rated as ‘high quality’. The overall evidence level of meta-analyzable outcomes was evaluated by the grading of recommendations assessment, development, and evaluation (GRADE) system (34, 35).
We used Revman software (version 5.3) to compute primary and secondary outcomes through a random effects model (36). For dichotomous data and continuous data, the risk ratio (RR) and standardized mean difference (SMD) and their 95% confidence intervals (CIs) were calculated. Heterogeneity among different studies was determined using Cochrane’s Q and I2 test, with Q < 0.1 or I2 ≥ 50% suggesting significant heterogeneity (37). We conducted a subgroup analysis for the primary outcome: high-frequency (>1 Hz) rTMS (HF-rTMS) targeting the left dorsolateral prefrontal cortex (L-DLPFC) versus low-frequency (≤1 Hz) rTMS (LF-rTMS) targeting the right dorsolateral prefrontal cortex (R-DLPFC). For the primary outcome, a sensitivity analysis was performed to explore the source of heterogeneity by removing one study (23) with an outlying effect size of −2.53. Publication bias was assessed using funnel plots and Egger’s regression interanalyses (38). In all analyses, p < 0.05 was defined as a significant difference (two-sided).
According to the search strategy, 621 studies were retrieved. After screening the title, abstract and full text, six RCTs (19–24) fulfilled the inclusion criteria and were analyzed in this meta-analysis (Figure 1).
As shown in Table 1, six RCTs were conducted in China covering 562 patients (281 patients in the rTMS group and 281 patients in the control group). The mean age was 15.0 years (range = 12–18 years). Male patients accounted for 36.5% (range = 18.6–51.3%) of the total sample. The use of antidepressants included sertraline (4 RCTs) (19, 21–23) and fluoxetine (1 RCT) (24). Participants underwent 1 Hz frequency rTMS (LF-rTMS) in 2 RCTs (20, 24) and 10 Hz frequency rTMS (HF-rTMS) in 4 RCTs (19, 21–23) (Table 1). The treatment duration of rTMS ranged from 2 to 6 weeks. The detailed treatment parameters of rTMS among the included RCTs are summarized in Table 1.
As displayed in Supplementary Figure 1, four RCTs (4/6, 66.7%) were rated as ‘low risk’ regarding random sequence generation. Three RCTs (3/6, 50.0%) were rated ‘low risk’ regarding the blinding of participants and personnel the blinding of outcome assessment. Selective reporting was rated as ‘low risk’ in all of the included RCTs. The mean Jadad score was 3.7 (range = 2–5), and five out of the six RCTs (5/6, 83.3%) were classified as high-quality studies (Jadad score ≥ 3) (Table 1). Following the GRADE approach (Supplementary Table 2), the overall evidence quality was rated as ‘low’ (2/8, 25%), ‘moderate’ (5/8, 62.5%) and ‘high’ (1/8, 12.5%).
As shown in Figure 2, adjunctive active rTMS outperformed the control group in improving depressive symptoms (5 RCTs, n = 464, SMD = -1.50, 95% CI: −2.16, −0.84; I2 = 89%, p < 0.00001), as measured by the HAMD-24 (3 RCTs) (20, 21, 23) and HAMD-17 (2 RCTs) (19, 24). Similarly, significant findings remained in a sensitivity analysis after excluding one RCT with an outlying effect size (23) (4 RCTs, n = 342, SMD = −1.22, 95% CI: −1.70, −0.73; I2 = 75%, p < 0.00001). In addition, the superiority of adjunctive rTMS was retained when divided into two subgroups by frequency, which included HF-rTMS (3 RCTs, n = 327, SMD = −1.63, 95% CI: −2.67, −0.60; I2 = 94%, p = 0.002) and LF-rTMS (2 RCTs, n = 137, SMD = -1.22, 95% CI: −1.78, −0.65; I2 = 43%, p < 0.0001).
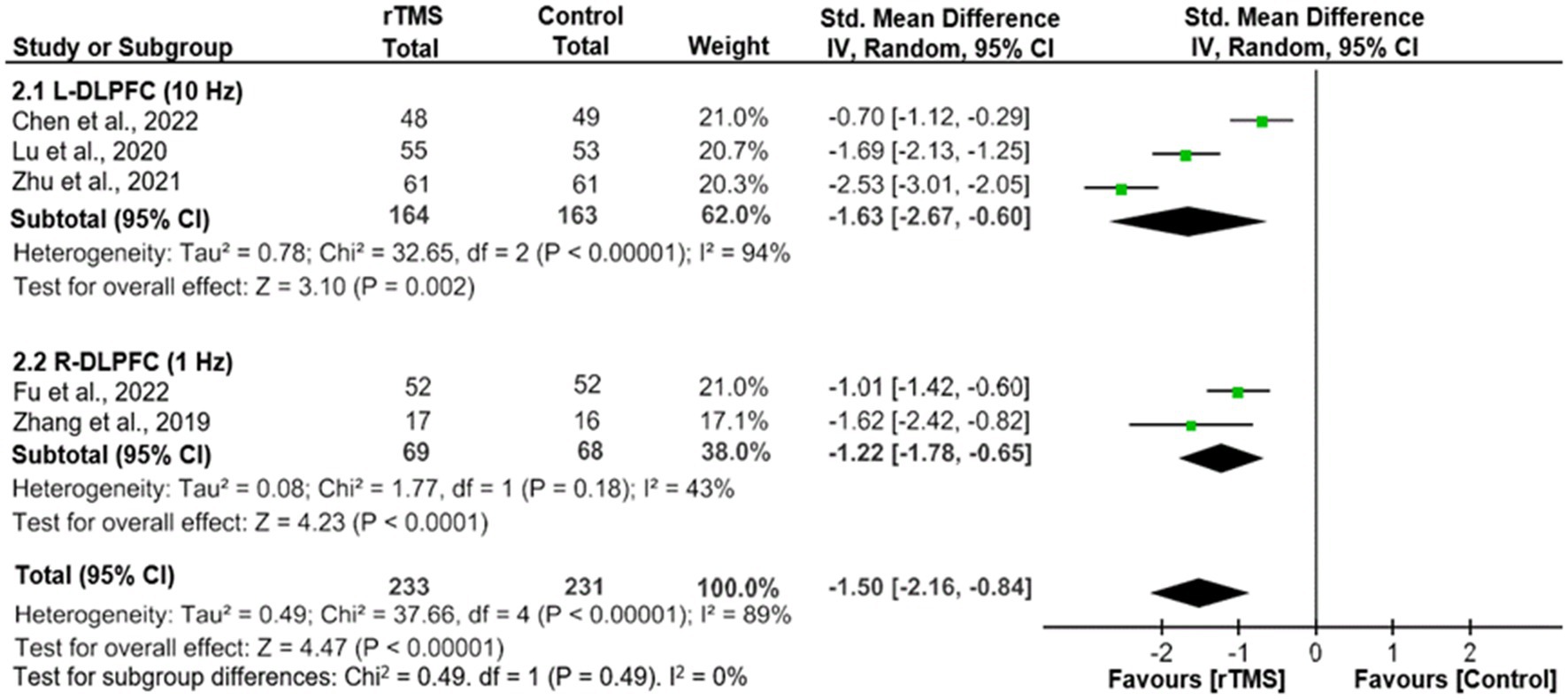
Figure 2. Adjunctive rTMS for adolescents with FE-MDD: forest plot for the improvement of depressive symptoms assessed by the HAMD. CI, confidence interval; FE-MDD, first-episode major depressive disorder; HAMD, Hamilton Depression Scale; L-DLPFC, left dorsolateral prefrontal cortex; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation.
Adjunctive rTMS was superior to the control group regarding response (4 RCTs, n = 406; RR: 1.35, 95% CI: 1.04, 1.76; I2 = 56%, p = 0.03) and remission (3 RCTs, n = 306; RR: 1.35, 95% CI: 1.03, 1.77; I2 = 0%, p = 0.03) (Table 2).
As shown in Table 2, discontinuation due to any reason was similar between the two groups (6 RCTs, n = 562, RR = 0.79, 95% CI: 0.32, 1.93; I2 = 0%, p = 0.60). The reasons for discontinuation of each included RCT were summarized in Supplementary Table 2.
Four studies (19, 20, 22, 24) reported adverse events. As displayed in Table 2, no significant differences were found regarding adverse events, including headache, loss of appetite, dizziness and nausea (p = 0.14–0.82).
Five out of six RCTs (83.3%, 5/6) (19–21, 23, 24) examine the effect of adjunctive rTMS on neurocognitive function in adolescents with FE-MDD. Among them, 4 RCTs (80.0%, 4/5) (19–21, 23) found that active rTMS group outperformed the comparator in improving neurocognitive function as measured by different measurement tools (Table 3). However, one RCT (20.0%, 1/5) (24) found no significant differences between the two groups.
Given that the number of included RCTs was less than 10, publication bias could not be analyzed as recommended (39).
To the best of our knowledge, this is the first meta-analysis to examine the efficacy, tolerability and safety of rTMS as an adjunct treatment for adolescents (12–18 years) with FE-MDD. Six RCTs (19–24) involving 562 adolescents with FE-MDD were included in this meta-analysis. The main findings are as follows: (1) adjunctive rTMS was superior in improving depressive symptoms over the control group; (2) adolescents with FE-MDD treated with rTMS had a significantly greater response and remission over the control group, suggesting that rTMS may have beneficial effects for adolescents with FE-MDD; (3) rTMS appeared to be safe and tolerable as an adjunct treatment for adolescents with FE-MDD; and (4) adjunctive rTMS appears to be effective in improving neurocognitive function in adolescents with FE-MDD.
Although no meta-analysis has investigated the therapeutic effects, tolerability and safety of adjunctive rTMS for adolescents with FE-MDD, several systematic reviews (31, 40, 41) have preliminarily explored the efficacy of adjunctive rTMS for adolescents with MDD. For example, a systematic review (40) found that rTMS could reduce depressive symptoms in adolescents with MDD. However, this systematic review (40) included RCTs consisting of a mixture of FE-MDD and multiepisode MDD. Additionally, a systematic review (41) also suggested that rTMS is an effective and well-tolerated treatment for adolescents with TRD. Therefore, the findings of our study provided further support for the utility of rTMS treatment (either HF-rTMS or LF-rTMS) combined with antidepressants for adolescents with FE-MDD. Importantly, adjunctive rTMS appeared to be safe and tolerable for adolescents or adults with FE-MDD (16).
The underlying mechanism of the effect of rTMS on depressive symptoms may be that it can generate repeated pulses that act on the cerebral cortex and then transform neural functional activities in the brain circuits related to the pathophysiology of depression (42, 43). More specifically, HF-rTMS (> 5 Hz) has an excitatory effect on neural functional activities, and LF-rTMS (≤ 1 Hz) has the opposite effect on depression, which is characterized by reduced neural functional activity in the L-DLPFC and increased neural functional activity in the R-DLPFC (40). Previous research has found that HF-rTMS on L-DLPFC and LF-rTMS on R-DLPFC both have similar mechanisms that induce equivalent functional changes in the brain associated with antidepressant efficiency in MDD patients, including a decrease in brain limbic activity within the left perirhinal cortex (44). In addition, rTMS has a certain potential for modulating pathologic imbalances in GABAergic and glutamatergic neurocircuitry (45, 46), which play an important role in depression (47, 48).
Previous meta-analyses have found that rTMS appears to be effective in improving neurocognitive function in adults with FE-MDD (49, 50). For example, Martin et al. (49) found that rTMS courses administered to the prefrontal cortex for depression may produce modest neurocognitive enhancing effects specific to psychomotor speed, visual scanning, and set-shifting ability. A possible reason is that the improvement of neurocognitive function may be a secondary effect after emotional improvement (49). Although the findings of the neurocognitive effects of adjunctive rTMS for adolescents with FE-MDD are mixed in the included five RCTs (19–21, 23, 24), four out of five RCTs (80.0%) found the significant superiority of adjunctive rTMS over the comparator in improving neurocognitive function after rTMS (19–21, 23). Only one RCT (24) included in this meta-analysis found no deterioration or significant improvement in neurocognitive function with a small sample size (n = 40). Taken together, adjunctive rTMS appears to be effective in improving neurocognitive function in adolescents with FE-MDD, although further studies focusing on adjunctive rTMS on neurocognitive function in adolescents with FE-MDD are warranted.
There are several limitations of this present study. First, the sample size of the meta-analysis was relatively small (n = 562), which might reduce the statistical power. Second, all of the included RCTs had relatively short observation periods (2–6 weeks) and lacked long-term follow-up. Third, all included studies were conducted in China and involved only Chinese adolescents. Thus, the findings of the present study are not generalizable to other countries or populations. Fourth, the confounding effects of antidepressant medications could not be detected due to insufficient information in the included studies. Fifth, the significant heterogeneity for primary outcome (I2 = 89%) remained, even in a sensitivity analysis (I2 = 75%), which may partly attribute to the significant heterogeneity of the rTMS protocols used in the included RCTs.
Adjunctive rTMS appears to be a beneficial strategy in improving depressive symptoms and neurocognitive function in adolescents with FE-MDD. Higher quality RCTs with larger sample sizes and longer follow-ups are warranted in the future.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
C-HS, Z-MS, J-XM, and X-HY selected studies and extracted the data. WZ from (Guangzhou) reviewed all the data and helped to mediate disagreements. C-HS wrote the first draft. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (82101609), Scientific Research Project of Guangzhou Bureau of Education (202032762), Guangzhou Health Science and Technology Project (20211A011045), Guangzhou Science and Technology Project of traditional Chinese Medicine and integrated traditional Chinese and Western medicine (20212A011018), China International Medical Exchange Foundation (Z-2018-35-2002), Science and Technology Program Project of Guangzhou (202102020658), the Science and Technology Program of Guangzhou (2023A03J0839 and 2023A03J0436), Science and Technology Planning Project of Liwan District of Guangzhou (202201012), The Natural Science Foundation Program of Guangdong (2023A1515011383), Guangzhou Municipal Key Discipline in Medicine (2021−2023), Guangzhou High-level Clinical Key Specialty, and Guangzhou Research-oriented Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1200738/full#supplementary-material
1. Qiu, H, Liang, K, Lu, L, Gao, Y, Li, H, Hu, X, et al. Efficacy and safety of repetitive transcranial magnetic stimulation in children and adolescents with depression: a systematic review and preliminary meta-analysis. J Affect Disord. (2023) 320:305–12. doi: 10.1016/j.jad.2022.09.060
2. Hauenstein, EJ. Depression in adolescence. J Obstet Gynecol Neonatal Nurs. (2003) 32:239–48. doi: 10.1177/0884217503252133
3. Zisook, S, Lesser, I, Stewart, JW, Wisniewski, SR, Balasubramani, GK, Fava, M, et al. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. (2007) 164:1539–46. doi: 10.1176/appi.ajp.2007.06101757
4. Copeland, WE, Shanahan, L, Costello, EJ, and Angold, A. Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Arch Gen Psychiatry. (2009) 66:764–72. doi: 10.1001/archgenpsychiatry.2009.85
5. Fergusson, DM, Horwood, LJ, Ridder, EM, and Beautrais, AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. (2005) 62:66–72. doi: 10.1001/archpsyc.62.1.66
6. Kim-Cohen, J, Caspi, A, Moffitt, TE, Harrington, H, Milne, BJ, and Poulton, R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. (2003) 60:709–17. doi: 10.1001/archpsyc.60.7.709
7. Birmaher, B, Brent, D, AACAP Work Group on Quality Issues, Bernet, W, Bukstein, O, Walter, H, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. (2007) 46:1503–26. doi: 10.1097/chi.0b013e318145ae1c
8. Weisz, JR, McCarty, CA, and Valeri, SM. Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull. (2006) 132:132–49. doi: 10.1037/0033-2909.132.1.132
9. Majumder, P, Balan, S, Gupta, V, Wadhwa, R, and Perera, TD. The safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of major depression among children and adolescents: a systematic review. Cureus. (2021) 13:e14564. doi: 10.7759/cureus.14564
10. Zhou, X, Michael, KD, Liu, Y, Del Giovane, C, Qin, B, Cohen, D, et al. Systematic review of management for treatment-resistant depression in adolescents. BMC Psychiatry. (2014) 14:340. doi: 10.1186/s12888-014-0340-6
11. Vuorilehto, MS, Melartin, TK, Riihimäki, K, and Isometsä, ET. Pharmacological and psychosocial treatment of depression in primary care: low intensity and poor adherence and continuity. J Affect Disord. (2016) 202:145–52. doi: 10.1016/j.jad.2016.05.035
12. Moreno, C, Roche, AM, and Greenhill, LL. Pharmacotherapy of child and adolescent depression. Child Adolesc Psychiatr Clin N Am. (2006) 15:977–98, x. doi: 10.1016/j.chc.2006.05.006
13. Hallett, M. Transcranial magnetic stimulation: a primer. Neuron. (2007) 55:187–99. doi: 10.1016/j.neuron.2007.06.026
14. Levkovitz, Y, Isserles, M, Padberg, F, Lisanby, SH, Bystritsky, A, Xia, G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. (2015) 14:64–73. doi: 10.1002/wps.20199
15. Kauffmann, CD, Cheema, MA, and Miller, BE. Slow right prefrontal transcranial magnetic stimulation as a treatment for medication-resistant depression: a double-blind, placebo-controlled study. Depress Anxiety. (2004) 19:59–62. doi: 10.1002/da.10144
16. Maneeton, B, Maneeton, N, Woottiluk, P, and Likhitsathian, S. Repetitive transcranial magnetic stimulation combined with antidepressants for the first episode of major depressive disorder. Curr Neuropharmacol. (2020) 18:852–60. doi: 10.2174/1570159X18666200221113134
17. Bloch, Y, Grisaru, N, Harel, EV, Beitler, G, Faivel, N, Ratzoni, G, et al. Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT. (2008) 24:156–9. doi: 10.1097/YCT.0b013e318156aa49
18. Wall, CA, Croarkin, PE, Sim, LA, Husain, MM, Janicak, PG, Kozel, FA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. (2011) 72:1263–9. doi: 10.4088/JCP.11m07003
19. Chen, H, Hu, X, Gao, J, Han, H, Wang, X, and Xue, C. Early effects of repetitive transcranial magnetic stimulation combined with sertraline in adolescents with first-episode major depressive disorder. Front Psych. (2022) 13:13. doi: 10.3389/fpsyt.2022.853961
20. Fu, Y, Wang, K, Zhao, Z, Zhao, J, and Tong, Q. Effects of repetitive transcranial magnetic stimulation on cognitive function and serum brain-derived neurotrophic factor levels in adolescents with depressive disorder. J Clin Pathol Res. (2022) 4:042.
21. Lu, GH, and Gao, LH. Effect of sertraline combined with high frequency repetitive transcranial magnetic stimulation on the efficacy and cognitive function of adolescent patients with first-episode depression. Chin J Health Psychol. (2020) 28:663–8.
22. Ma, Q, Shi, F, Li, L, and Shi, Y. Effectiveness of transcranial magnetic stimulation combined with sertraline in adolescent patients with depression. Great Physician. (2021) 6:70–2.
23. Zhu, M. Clinical effect of sertraline combined with repetitive transcranial magnetic stimulation in the treatment of patients with adolescent depression. Clin Res Pract. (2021) 6:83–5. doi: 10.19347/j.cnki.2096-1413.202119025
24. Zhang, C. Effects of rTMS combined with fluoxetine on mood and cognitive function in first-episode depression adolescents. Master's thesis. Zhejiang: Zhejiang University (2019).
25. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
26. Sigrist, C, Vöckel, J, MacMaster, FP, Farzan, F, Croarkin, PE, Galletly, C, et al. Transcranial magnetic stimulation in the treatment of adolescent depression: a systematic review and meta-analysis of aggregated and individual-patient data from uncontrolled studies. Eur Child Adolesc Psychiatry. (2022) 31:1501–25. doi: 10.1007/s00787-022-02021-7
27. Cortese, S, Adamo, N, Del Giovane, C, Mohr-Jensen, C, Hayes, AJ, Carucci, S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2018) 5:727–38. doi: 10.1016/S2215-0366(18)30269-4
28. Fraguas, D, Díaz-Caneja, CM, Ayora, M, Hernández-Álvarez, F, Rodríguez-Quiroga, A, Recio, S, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. (2019) 45:742–51. doi: 10.1093/schbul/sby125
29. Hamilton, M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
30. Feng, H, Xu, J, Qin, G, Gan, J, and Wang, T. Effects of low frequency transcranial magnetic stimulation on cognitive function and life skills in children with depressive disorders. Chin J Gen Pract. (2017) 15:289–91. doi: 10.16766/j.cnki.issn.1674-4152.2017.02.032
31. Zheng, W, Lan, X-J, Qin, Z-J, Yang, X-H, and Shi, Z-M. Low-frequency repetitive transcranial magnetic stimulation for children and adolescents with first-episode and drug-naïve major depressive disorder: a systematic review. Front Psych. (2023) 14:14. doi: 10.3389/fpsyt.2023.1111754
32. Higgins, JP, Altman, DG, Gotzsche, PC, Juni, P, Moher, D, Oxman, AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. Epub 2011/10/20. doi: 10.1136/bmj.d5928
33. Jadad, AR, Moore, RA, Carroll, D, Jenkinson, C, Reynolds, DJ, Gavaghan, DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. Epub 1996/02/01. doi: 10.1016/0197-2456(95)00134-4
34. Balshem, H, Helfand, M, Schunemann, HJ, Oxman, AD, Kunz, R, Brozek, J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. Epub 2011/01/07. doi: 10.1016/j.jclinepi.2010.07.015
35. Atkins, D, Best, D, Briss, PA, Eccles, M, Falck-Ytter, Y, Flottorp, S, et al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
36. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
37. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
38. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. Epub 1997/10/06. doi: 10.1136/bmj.315.7109.629
39. Sterne, JA, Sutton, AJ, Ioannidis, JP, Terrin, N, Jones, DR, Lau, J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. Epub 2011/07/26. doi: 10.1136/bmj.d4002
40. Hett, D, Rogers, J, Humpston, C, and Marwaha, S. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in adolescence: a systematic review. J Affect Disord. (2021) 278:460–9. doi: 10.1016/j.jad.2020.09.058
41. Donaldson, AE, Gordon, MS, Melvin, GA, Barton, DA, and Fitzgerald, PB. Addressing the needs of adolescents with treatment resistant depressive disorders: a systematic review of rTMS. Brain Stimul. (2014) 7:7–12. doi: 10.1016/j.brs.2013.09.012
42. Iwabuchi, SJ, Peng, D, Fang, Y, Jiang, K, Liddle, EB, Liddle, PF, et al. Alterations in effective connectivity anchored on the insula in major depressive disorder. Eur Neuropsychopharmacol. (2014) 24:1784–92. doi: 10.1016/j.euroneuro.2014.08.005
43. Liston, C, Chen, AC, Zebley, BD, Drysdale, AT, Gordon, R, Leuchter, B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. (2014) 76:517–26. doi: 10.1016/j.biopsych.2014.01.023
44. Richieri, R, Boyer, L, Padovani, R, Adida, M, Colavolpe, C, Mundler, O, et al. Equivalent brain SPECT perfusion changes underlying therapeutic efficiency in pharmacoresistant depression using either high-frequency left or low-frequency right prefrontal rTMS. Prog Neuro-Psychopharmacol Biol Psychiatry. (2012) 39:364–70. doi: 10.1016/j.pnpbp.2012.07.012
45. Croarkin, PE, Nakonezny, PA, Wall, CA, Murphy, LL, Sampson, SM, Frye, MA, et al. Transcranial magnetic stimulation potentiates glutamatergic neurotransmission in depressed adolescents. Psychiatry Res Neuroimag. (2016) 247:25–33. doi: 10.1016/j.pscychresns.2015.11.005
46. Dubin, MJ, Mao, X, Banerjee, S, Goodman, Z, Lapidus, KA, Kang, G, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci. (2016) 41:E37–45. doi: 10.1503/jpn.150223
47. Croarkin, PE, Levinson, AJ, and Daskalakis, ZJ. Evidence for GA‑BAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev. (2011) 35:818–25. doi: 10.1016/j.neubiorev.2010.10.002
48. Sanacora, G, Treccani, G, and Popoli, M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. (2012) 62:63–77. doi: 10.1016/j.neuropharm.2011.07.036
49. Martin, DM, McClintock, SM, Forster, JJ, Lo, TY, and Loo, CK. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress Anxiety. (2017) 34:1029–39. doi: 10.1002/da.22658
50. Patel, R, Silla, F, Pierce, S, Theule, J, and Girard, TA. Cognitive functioning before and after repetitive transcranial magnetic stimulation (rTMS): a quantitative meta-analysis in healthy adults. Neuropsychologia. (2020) 141:107395. doi: 10.1016/j.neuropsychologia.2020.107395
(“Transcranial Magnetic Stimulation”[MeSH] OR Transcranial Magnetic Stimulation OR rtms OR tms) AND (“depression”[MeSH] OR depression OR depressive OR depressed OR melancholia) AND (child OR childhood OR children OR adolescent OR adolescents OR puberty OR pubertal OR juvenile OR teen* OR youth OR preschool OR preschool child OR school age OR high school OR student OR pediatric* OR paediatric* OR minors OR boys OR boy OR girl*) AND (first episode OR early phase OR early-phase OR FEP OR recent onset OR untreated OR unmedicated OR non medicated OR undiagnosed OR first diagnosed OR first diagnosis OR drug-free OR antidepressant-free OR medication-free OR drug-naïve OR antidepressant-naïve OR medication-naïve OR treatment-naïve OR never-medicated) AND (random OR random* OR control OR RCT).
Keywords: repetitive transcranial magnetic stimulation, adolescent, depression, first episode, meta-analysis
Citation: Sun C-H, Mai J-X, Shi Z-M, Zheng W, Jiang W-L, Li Z-Z, Huang X-B, Yang X-H and Zheng W (2023) Adjunctive repetitive transcranial magnetic stimulation for adolescents with first-episode major depressive disorder: a meta-analysis. Front. Psychiatry. 14:1200738. doi: 10.3389/fpsyt.2023.1200738
Received: 05 April 2023; Accepted: 18 July 2023;
Published: 01 August 2023.
Edited by:
Huanzhong Liu, Chaohu Hospital of Anhui Medical University, ChinaReviewed by:
Gerasimos N. Konstantinou, University of Toronto, CanadaCopyright © 2023 Sun, Mai, Shi, Zheng, Jiang, Li, Huang, Yang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zheng, emhlbmd3ZWkwNzAyQDE2My5jb20=; Xin-Hu Yang, eW91bmd4aW5odUBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.