- 1Laboratory of Behavioral Medicine, Neuroscience Institute, Lithuanian University of Health Sciences, Palanga, Lithuania
- 2Department of Neurosciences, Biomedicine and Movement Sciences, Section of Psychiatry, University of Verona, Verona, Italy
- 3Department of Neurosciences and Mental Health, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 4Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy
- 5School of Psychology, University of Galway, Galway, Ireland
Introduction: The dysregulation of psychophysiological responses to mental stressors is a common issue addressed in individuals with psychiatric conditions, while brain circuit abnormalities are often associated with psychiatric conditions and their manifestations. However, to our knowledge, there is no systematic overview that would comprehensively synthesize the literature on psychophysiological responses during laboratory-induced psychosocial stressor and neural correlates in people with mental disorders. Thus, we aimed to systematically review the existing research on psychophysiological response during laboratory-induced stress and its relationship with neural correlates as measured by magnetic resonance imaging techniques in mental disorders.
Methods: The systematic search was performed on PubMed/Medline, EBSCOhost/PsycArticles, Web of Science, and The Cochrane Library databases during November 2021 following the PRISMA guidelines. Risk of bias was evaluated by employing the checklists for cross-sectional and case-control studies from Joanna Briggs Institute (JBI) Reviewers Manual.
Results: Out of 353 de-duplicated publications identified, six studies were included in this review. These studies were identified as representing two research themes: (1) brain anatomy and psychophysiological response to mental stress in individuals with mental disorders, and (2) brain activity and psychophysiological response to mental stress in individuals with mental disorders.
Conclusions: Overall, the evidence from studies exploring the interplay between stress psychophysiology and neural correlates in mental disorders is limited and heterogeneous. Further studies are warranted to better understand the mechanisms of how psychophysiological stress markers interplay with neural correlates in manifestation and progression of psychiatric illnesses.
1. Introduction
Dysregulated psychophysiological responses to psychological stressors is a common feature of people with psychiatric conditions (1–3), signaling the maladaptive coping with daily stressors, which may play a significant role in the etiology of mental disorders (4, 5). The dysregulation of cardiovascular and cortisol stress responses, including hypo- and hyper-reactivity (6) has been extensively studied in laboratory settings in individuals with and without psychiatric conditions, including persons with mood and anxiety disorders (4, 7, 8), trauma- and stressor-related disorders (9), eating disorders (10), personality disorders (11), obsessive-compulsive, and related disorders (12).
Considering the biological mechanisms involved in the development and progression of mental disorders, morpho-functional changes of brain circuits are often linked with psychiatric conditions and their manifestations (13–19), complementing the importance of neuroimaging studies in psychiatry research and clinical application (20). The application of neuroimaging techniques, such as magnetic resonance imaging (MRI) are often used to determine the location and amplitude of morpho-functional changes in the brain linked with mental disorders, ultimately facilitating the identification of potential biomarkers of pathology, to be used to test new therapeutic targets (e.g., neurostimulation techniques, transcranial direct current stimulation, and neurofeedback) (21). Furthermore, mental disorders are also known for functional disruption in neural circuitry underlying emotional processing. For example, both increased and decreased activation of dorsomedial prefrontal cortex during emotional processing of mental image was found in individuals with depression (22) and post-traumatic stress disorder (23). Taking the broader perspective, a recent meta-analysis (24) suggested the dysregulation in the so-called salience network, the ventral striatal and ventromedial prefrontal network as well as lateral orbitofrontal network in those with major mental disorders during emotional processing.
Despite the evident relevance of psychophysiological stress biomarkers and neural correlates in psychiatric conditions, studies examining the interplay between these two groups of biomarkers in clinical populations have been relatively scarce. There have been several narrative reviews on laboratory-induced stress responses and neural correlates in general (25, 26). However, to date, we are not aware of any systematic overview that has mapped or synthesized the body of literature on psychophysiological responses during laboratory-induced mental stressor with that on neural correlates in persons with psychiatric disorders. Examination of such biological variabilities may help bolster our understanding of mechanisms that contribute to the etiology, progression, and manifestation of psychiatric conditions. Thus, the current scoping review aimed to systematically review the existing research on psychophysiological reactions during laboratory-induced stress and their relationship with neural correlates measured by MRI techniques in mental disorders.
2. Methods
2.1. Search strategy
The strategy used in this review was in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (27). PubMed/Medline, EBSCOhost/PsycArticles, Web of Science, and The Cochrane Library were chosen as databases to find publications on the selected topic. We also reviewed additional sources, such as clinicaltrials.gov and the GSK Clinical Study Register. The following search query was employed: (“psychophysiolog*” OR “cardiovascular” OR “cortisol”) AND “stress” AND (“psychological” OR “acute” OR “mental” OR “social” OR “psychosocial”) AND (“response*” OR “reaction*” OR “reactivity”) AND (“magnetic resonance imaging” OR “brain imaging” OR “fmri” OR “DTI”) AND (“psychiatr*” OR “clinical” OR “patient*”).
2.2. Selection criteria
The selection criteria were based on PICOS (i.e., population, intervention, comparator, outcomes, study design) approach (28). Publications were considered for inclusion if they (a) were performed in adult in-patient or out-patient psychiatric patients, as defined by any operational defined criteria (e.g., DSM-5, ICD-10) (P); (b) employed standard behavioral method for laboratory-induced mental stress test and structural or functional Magnetic Resonance Imaging (sMRI and fMRI, respectively) for the assessment of morpho-funtional properties of the brain (I); (c) included a healthy control group (C); (d) provided findings on psychophysiological parameters during laboratory-induced stress, such as cortisol response and/or cardiovascular response (e.g., blood pressure, heart rate, heart rate variability, skin conductance etc.) to stress and its association with structural and functional brain indices; (e) were peer-reviewed studies drawing on empirical data (e.g., randomized controlled trials, cross-sectional studies, case-control studies) (S). There were no restrictions to inclusion regarding country of origin, provided that the study was reported in English language.
2.3. Review procedure
Relevant studies were retrieved on November 2021 employing the platform of Rayyan Systems Inc. Selected studies were independently reviewed by two assessors (JGS and MGR). Inclusion and exclusion criteria were determined beforehand. After initial screening of title and abstracts, the assessors reviewed full-text publications, which were retrieved and independently verified for eligibility. Disagreements were acknowledged and resolved through discussions and unified consensus, or with revision of a third senior assessor (MB). Data that was retrieved from publications were analyzed taking a narrative synthesis approach.
2.4. Risk of bias (quality) assessment
Two independent reviewers (JGS and MGR) performed critical appraisal by using Joanna Briggs Institute (JBI) Reviewers Manual (29, 30), a standardized quality assessment tool for clinical (cross-sectional/case-control) studies (31).
3. Results
3.1. Included studies
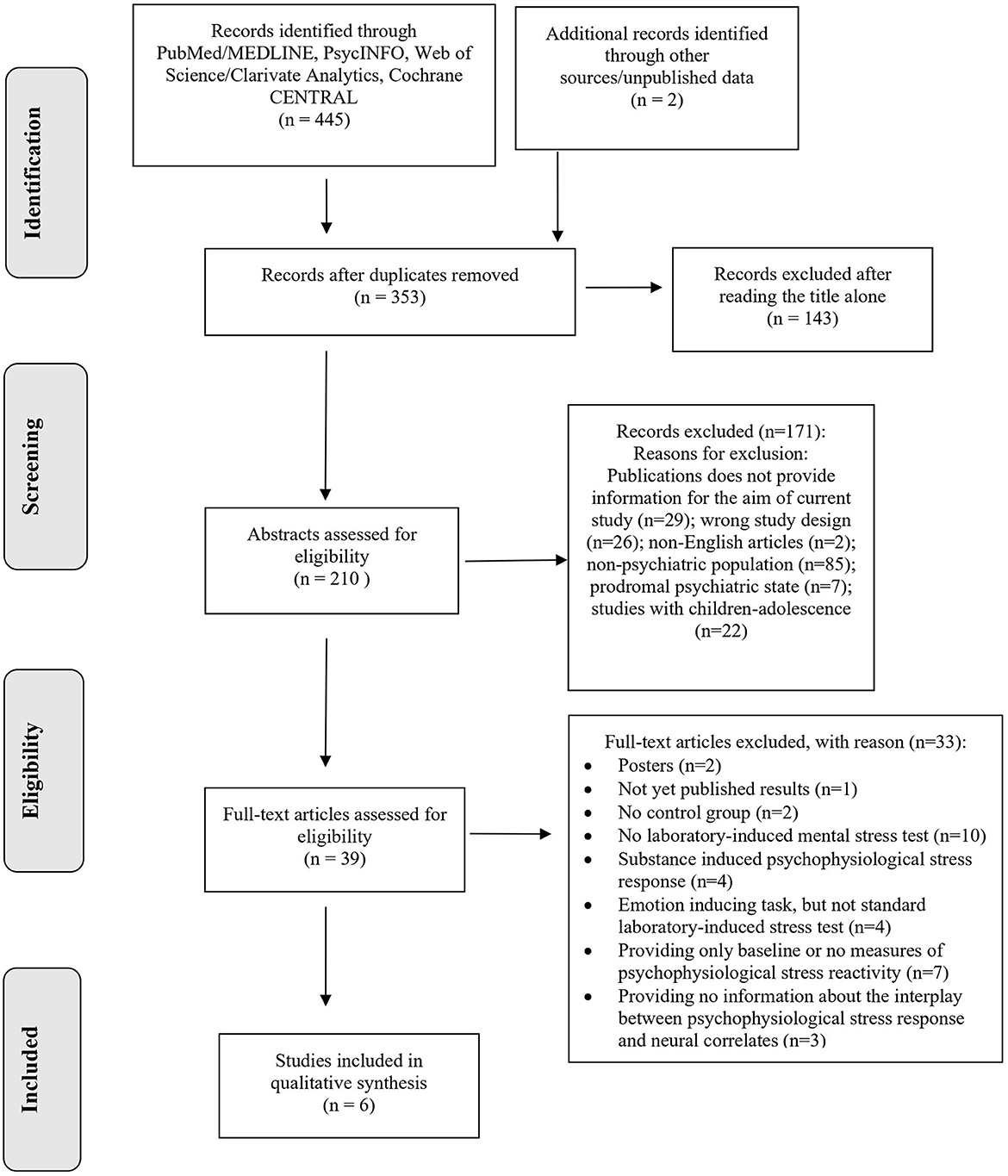
A flow diagram illustrating the studies selection process is presented in Figure 1. The systematic search retrieved 353 de-duplicated records. After title and abstract screening, 314 records were excluded because they clearly did not meet the inclusion criteria. A total of 39 studies underwent for full-text review, after which six studies (32–37), were identified for inclusion in this scoping review and critically appraised in a descriptive manner. Two themes were identified, namely (a) brain anatomy and psychophysiological response to laboratory-induced mental stress in individuals with psychiatric disorders, and (b) brain activity and psychophysiological stress response in individuals with psychiatric disorders.
3.2. Risk of bias within studies
Overall, bias in study methodology was low. However, most of the studies lacked information on confounding variables and/or statistical analysis, while controlling for possible covariates (e.g., sociodemographic, clinical data and/or disorder specific characteristics). Whilst two studies addressed the possible confounding factors (34, 35), the other four studies did not provide the information and statistical results on confounding (32, 33, 36, 37).
3.3. Study synthesis
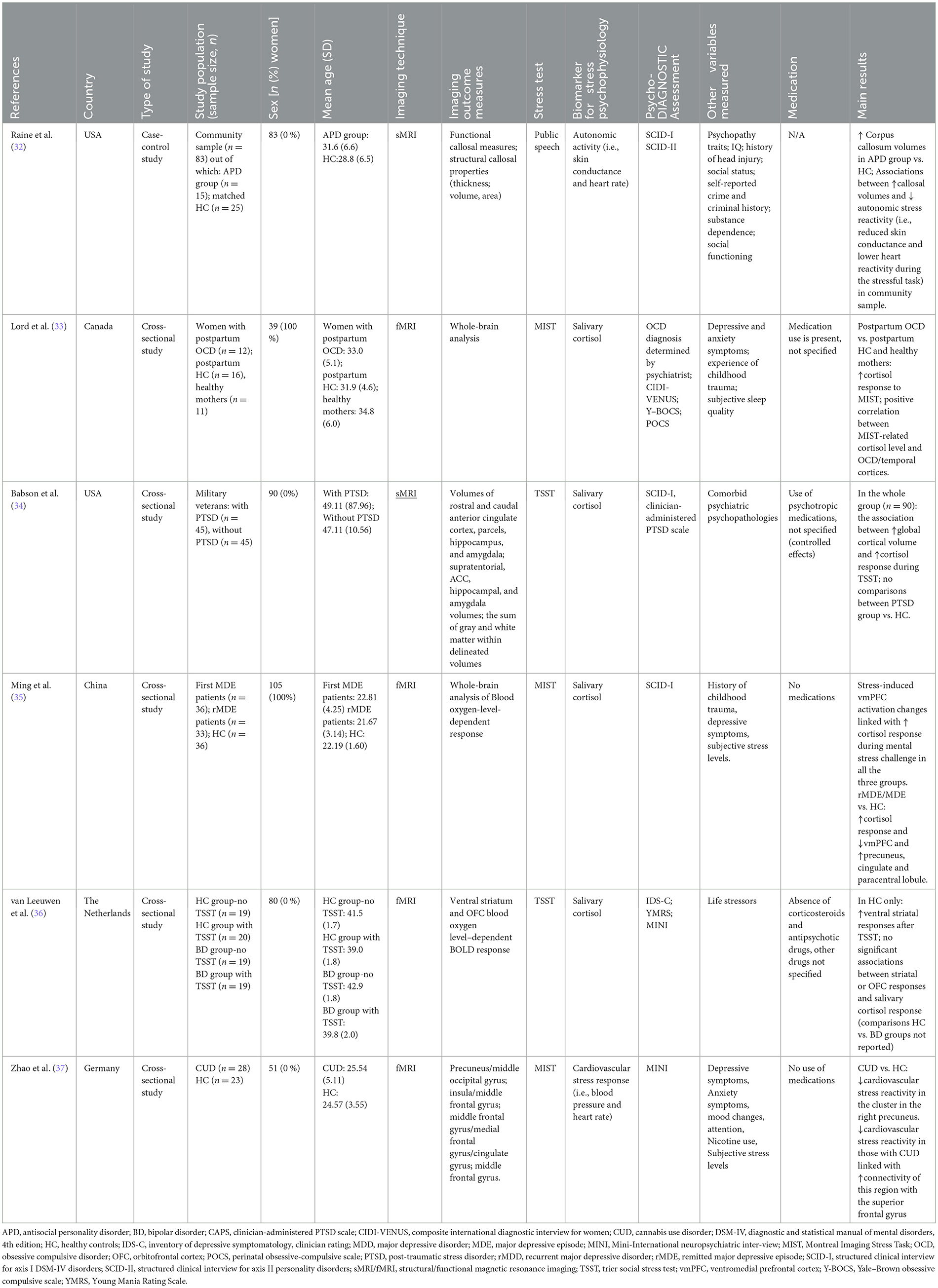
The studies were published between 2003 and 2020, of which three (32–34) were conducted in North America, two in Europe (36, 37), and one in Asia (35). Several mental health conditions were investigated, including antisocial personality disorder (APD) (32), posttraumatic stress disorder (PTSD) (34), major depressive episode (MDE) (35), bipolar disorder (BD) (36), obsessive-compulsive disorder (OCD) (33), and Cannabis Use Disorder (CUD) (37). All samples were single-sex, with the sample sizes ranging from 39 (33) to 105 (35). To measure neural correlates, the majority of the studies (n = 4) used fMRI (33, 35–37), out of which three studies included fMRI scan during the resting period (33, 35, 37). The remaining two out of six studies employed sMRI (32, 34). As a laboratory induced mental stress test, two studies employed Trier Social Stress Test (TSST) (34, 36), which implements public speech and arithmetic tasks as psychological stressors (38). Three studies employed Montreal Imaging Stress Task (MIST) (33, 35, 37), which is derived from TSST, yet contains only computerized mental arithmetic task (39). Lastly, a single study used solely regular public speaking task (32). The detailed characteristics of each study are outlined in Table 1.
3.3.1. Studies of psychophysiological responses to mental stress in individuals with mental disorders that focused on brain anatomy
Two studies examined the interplay between brain structure and psychophysiological stress response (32, 34). Both studies used sMRI, while the measures of psychophysiological response to stress and the employed protocol to observe it considerably differed. The laboratory-induced stress tasks and sMRI were conducted consecutively.
The case-control study by Raine et al. (32) evaluated 40 men within a community sample of n = 83, of which 15 individuals met the criteria for APD as measured with Structured Clinical Interview for Axis II Personality Disorders (SCID-II), and 25 were matched healthy controls (HC). The primary interest was to evaluate the callosal abnormalities and its manifestation in those with APD. Additionally, the authors also measured how callosal abnormalities were associated with emotional deficits, including reduced autonomic stress responses. To measure autonomic reactivity (i.e., skin conductance and heart rate) to stress, a public speaking task was performed, during which the participants had to prepare (2 min.) and talk (2 min.) about their worst faults (40). Correlational analysis suggested the associations between larger callosal volumes and low autonomic stress reactivity (i.e., reduced skin conductance and lower heart reactivity during the stressful task), providing the results on blunted affect and its interconnectedness with brain structure in APD. Of note, this additional correlational analysis was performed in the larger (n = 83) community sample, in which APD-related characteristics were assessed via the Psychopathy Checklist (41) but the diagnosis of APD was not investigated, while no comparisons between APD and HC were performed. Nevertheless, the major interpretation of the results was that abnormal interhemispheric connectivity might partly account for autonomic blunting during social stressor in those with APD.
Further, the cross-sectional study (34) evaluated 90 military veterans (all men), including 45 individuals with PTSD as measured with Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) and 45 HC. Most individuals with PTSD had a comorbid diagnosis of Major Depressive Disorders. The Trier Social Stress Test (TSST) was employed to measure cortisol response during mental stress challenge. The primary aim was to evaluate regional brain volumes and salivary cortisol measures (i.e., basal, during psychological stress, and DEX-suppressed). The study found that the estimates of global cortical volume but not hippocampal or amygdala volume were moderately linked with cortisol response during the stressful tasks of TSST, while performing the analysis in the whole group (n = 90). The results remained significant even when controlled for PTSD diagnosis, smoking history, alcohol use and study site.
3.3.2. Studies of psychophysiological responses to mental stress in individuals with mental disorders that focused on brain activity
We found four studies (33, 35–37) that evaluated the association between brain activation and psychophysiological stress response (i.e., cortisol and cardiovascular responses) in people with vs. without mental disorders. While one study performed laboratory-induced stressful task followed by fMRI scan (36), others did both tasks simultaneously (33, 35, 37).
Two cross-sectional studies focused on affective disorders, studying individuals with current or remitted MDE (MDE and rMDE, respectively) (35) or bipolar disorder (BD) (36). The first study, conducted in China by Ming et al. (35), investigated women with first MDE (n = 36), rMDE (n = 33), and HC (n = 36) with the primary aim to examine state-independent (trait) and state-dependent neural responses to psychological stress in the study participants. Psychiatric diagnosis was confirmed with administration of SCID-I. The MIST was adapted to elicit psychological stress during fMRI. When compared with HC, both rMDE and MDE groups showed higher stress-related cortisol response and similar brain activations, including lower activation of ventromedial prefrontal cortex and greater activation of precuneus, cingulate, and paracentral lobule. Regarding the interplay between neural correlates and psychophysiological stress responses, it was found that stress-induced changes in ventromedial prefrontal cortex activation was inversely associated with higher cortisol response during mental stress in all three groups. According to the authors, the results highlighted the importance of the medial prefrontal cortex in regulation of hypothalamic-pituitary-adrenal axis stress reactivity. These results may possibly explain that the differences found between HC and MDE/rMDE are more attributable to trait effect of depression rather than state or clinical status of onset/remission of depression.
In the second study, conducted in the Netherlands (36), 80 men were studied, of whom 38 had BD as determined by clinician-rated scales (Table 1). The primary aim was to explore ventral striatal and orbitofrontal cortex (OFC) responses during a reward processing task right after TSST in euthymic BD patients vs. HC. Cortisol responses were evaluated during the TSST, while fMRI was conducted together with the reward task. The findings showed no significant associations between striatal or OFC responses and salivary cortisol response during TSST in any of the groups and higher striatal responses after TSST only in HC sample. Comparisons between HC and BD groups were not reported. Overall, the findings suggested altered recovery from psychological stress in individuals with BD regarding striatal reward processing. According to the authors, their findings of reduction of stress-related dynamics in reward processing could partially explain an amplified sensitivity for repeated mood episodes right after acute distressful experiences.
One cross-sectional study (33), conducted in Canada, compared 12 women with postpartum OCD, as determined by study psychiatrists, 16 healthy mothers within their first 6 months postpartum (henceforth labeled as “postpartum HC”) and 11 HC with more than 1 year postpartum (henceforth labeled as “healthy mothers”). The primary aim was to evaluate the cerebral and endocrine measures of psychological stress reactivity during the postpartum period and in the presence of OCD. They all underwent laboratory-induced MIST, while simultaneously being scanned with fMRI. In comparison to postpartum HC, those with postpartum OCD showed heightened cortisol response to mental stress and this was associated with distinct brain activation pattern during MIST, including greater activation in OFC and temporal cortices. The findings suggest that OCD is linked with increased activity within brain regions that are related to alertness, threat detection and emotional liability. It is also suggested that healthy postpartum mothers might have more adapted emotional response to psychological stress due to no activation in OFC in comparison to postpartum women with OCD that showed increased activation of OFC.
Finally, a cross-sectional study conducted in Germany (37) investigated 51 men, including 28 individuals with CUD, as confirmed with the administration of MINI Neuropsychiatric Interview (42) and 23 HC. The authors aimed to identify the integrity of behavioral and neural stress reactivity in those with CUD. Cardiovascular stress responses (i.e., blood pressure and heart rate) were evaluated during MIST and simultaneous fMRI acquisition. The major results suggested that, compared to HC, participants with CUD exhibited decreased cardiovascular stress reactivity in the cluster in the right precuneus. It also suggested that decreased cardiovascular stress reactivity (i.e., blood pressure and heart rate) in those with CUD correlated with increased connectivity between precuneus and the superior frontal gyrus, suggesting the importance of acute stress-induced cognitive performance deficits in those with CUD.
4. Discussion
In this current scoping review, we identified six studies examining psychophysiological response to laboratory-induced stress and neural correlates, measured with MRI. Two themes were identified, including (1) brain anatomy and psychophysiological response to mental stress in individuals with mental disorders, and (2) brain activity and psychophysiological response to mental stress in individuals with mental disorders. With regards to brain anatomy, there is some limited knowledge on callosal abnormalities and autonomic stress reactivity in individuals with APD and regional brain volumes and cortisol stress response in those with PTSD. In terms of brain activity, there is some evidence from observational studies, investigating ventromedial prefrontal cortex activation, ventral striatal responses, and cortisol response in affective disorders as well as cerebral correlates and cortisol stress response in postpartum women with OCD.
4.1. Appraisal of the identified themes by the present research
The first two studies (32, 34) investigated brain anatomy and its interplay with either autonomic nervous system activation of hypothalamic pituitary-adrenal (HPA) axis activation during mental stress challenge in either men with APD (32) or PTSD (34). In the first study (32) larger callosal volumes were associated with lower autonomic stress reactivity in those with APD, while in the second study (34) the presence of PTSD did not play a role in the interplay between neural and endocrine correlates during psychological stress. The latter study somewhat contradicted the results found in healthy young men (43) where larger hippocampal volume was significantly linked with higher cortisol response during TSST. However, in the study exploring individuals with PTSD (34) the possible underlying reasons were not discussed, which might be linked with reduced hippocampal volume in individuals with PTSD (44). Unfortunately, even though both studies presented the results on psychophysiology of stress (i.e., skin conductance, heart rate and cortisol responses) and neural correlates (i.e., callosal abnormalities and regional brain volumes) corresponding to the brain anatomy, the methodological differences between the studies limits the extent to which their results can be meaningfully compared. In addition, Raine et al. (32) conducted their correlational analysis not specifically on participants who met criteria for APD (n = 15), but on the wider community-based sample from which this target group was drawn (n = 83). Thus, attributing those results to clinical APD should be done cautiously. It is also important to note that the sample of both studies included men only.
Additional four studies found associations between brain activity (e.g., ventromedial prefrontal cortex activation, ventral striatal responses or cerebral correlates or precuneus activity) and psychophysiological responses (e.g., cortisol or cardiovascular response) to stress in women with remitted MDE (35), men with BD (36), women with postpartum OCD (33), and men with CUD (37). In most of the studies (three out of four) stress-induction paradigms and fMRI were performed simultaneously (33, 35, 37), while showing the significance of acute stress on different brain activity measures in mental disorders. However, differences between studies in the methodological approach prevent from a direct comparisons making them highly heterogeneous and lacking common biological mechanisms.
Regarding further studies, based on the results of this scoping review, it is evident that there is a need for more interdisciplinary research of stress psychophysiology and brain imaging in people with mental disorders. Most mental disorders are currently still under-represented within the research of neuroimaging and stress psychophysiology, even though brain changes and psychobiology of stress in mental disorders are known to be altered relative to healthy populations (1, 13–16). One of the reasons for the scarcity of studies could be the need for relatively expensive and complex scientific investigations. Secondly, all the studies have employed rather small sample sizes that are homogeneous in terms of gender. Thus, further studies, investigating larger samples and examining sex differences could help to provide a better understanding of any role of gender that might be attributed to the interplay between stress psychophysiology and brain anatomy or activity, given that sex/gender often plays a cardinal role in psychiatric psychopathology (45). Finally, longitudinal and/or experimental studies need to be conducted, as we did not find any studies that could definitively assess the causal relationship between psychophysiological stress responses and neural correlates.
4.2. Limitations
The limitations of this scoping review are worth mentioning. We found a small number of studies, precluding us from making systematic comparisons across the research or from providing a broader map on the existing literature within this topic. This overview was also limited to only English-language articles, thus excluding potentially relevant studies. Further, due to high heterogeneity of the studies, the comparison between studies were challenging. Specifically, it was difficult to disentangle what findings were determined by disorder specific reasons and which one depicted the unique and transdiagnostic interplay between neural and cardiovascular/endocrine correlates. In fact, high levels of heterogeneity could be observed not only regarding the mental disorders, but also types of stress test (e.g., MIST, TSST), biomarkers (e.g., cardiovascular measures, salivary cortisol, skin conductance), sMRI brain structures (e.g., white matter, gray matter) or fMRI modalities (resting fMRI, reward task). However, in terms of the strengths, this review used transparent, systematic, and rigorous methods throughout the entire process, employing a broad search of the literature with four different databases, and ensuring a good quality of the selected studies with additional quality assessment.
5. Conclusion
Our scoping review provided a critical systematic overview of the existing empirical research on psychophysiology of laboratory induced-stress and neurobiological measures during magnetic resonance imaging in psychiatric in-patient and out-patient populations. The selected studies provided further knowledge of the importance to study neural correlates and its interplay with stress psychophysiology in individuals with mental disorder. However, many limitations exist related to the methodology and heterogeneity of the studies. Further research may concentrate on larger study samples, employing more diversity in terms of mental disorders and sex. Also, longitudinal studies are still warranted, allowing to draw the causal relationship between stress psychophysiology and brain circuit abnormalities.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JG-S: conceptualization and writing—original draft. MGR: conceptualization and writing—review and editing. PB and MB: conceptualization and supervision. BH and NM: writing—review and editing and supervision. All authors contributed to the article and approved the submitted version.
Funding
This paper was prepared within the context of JG-S research internship granted by University of Milan program Young Investigator Training Program. PB was partially supported by the Italian Ministry of Health (Ricerca Corrente 2023).
Conflict of interest
JG-S serves as a consultant at FACITtrans.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. (2013) 38:1220–35. doi: 10.1016/j.psyneuen.2012.11.015
2. Monteleone AM, Ruzzi V, Patriciello G, Cascino G, Pellegrino F, Vece A, et al. Emotional reactivity and eating disorder related attitudes in response to the trier social stress test: an experimental study in people with anorexia nervosa and with bulimia nervosa. J Affect Disord. (2020) 274:23–30. doi: 10.1016/j.jad.2020.05.051
3. Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J Affect Disord. (2012) 143:223–30. doi: 10.1016/j.jad.2012.05.059
4. Umeoka EHL, van Leeuwen JMC, Vinkers CH, Joëls M. The role of stress in bipolar disorder. Curr Top Behav Neurosci. (2021) 48:21–39. doi: 10.1007/7854_2020_151
5. Guest FL, Guest PC. Developmental origins of stress and psychiatric disorders. Investig Early Nutr Effects Long Term Health. (2018) 1735:47–58. doi: 10.1007/978-1-4939-7614-0_3
6. Phillips AC, Ginty AT, Hughes BM. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int J Psychophysiol. (2013) 90:1–7. doi: 10.1016/j.ijpsycho.2013.02.002
7. Schiweck C, Piette D. Heart rate and high frequency heart rate variability during stress as biomarker for clinical depression. A systematic review. Psychol Med. (2019) 49:200–11. doi: 10.1017/S0033291718001988
8. Hek K, Direk N, Newson RS, Hofman A, Hoogendijk WJ, Mulder CL, et al. Anxiety disorders and salivary cortisol levels in older adults: a population-based study. Psychoneuroendocrinology. (2013) 38:300–5. doi: 10.1016/j.psyneuen.2012.06.006
9. Steudte-Schmiedgen S, Kirschbaum C, Alexander N, Stalder T. An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: insight from recent hair cortisol findings. Neurosci Biobehav Rev. (2016) 69:124–35. doi: 10.1016/j.neubiorev.2016.07.015
10. Ginty AT, Phillips AC, Higgs S, Heaney JL, Carroll D. Disordered eating behaviour is associated with blunted cortisol and cardiovascular reactions to acute psychological stress. Psychoneuroendocrinology. (2012) 37:715–24. doi: 10.1016/j.psyneuen.2011.09.004
11. Aleknaviciute J, Tulen JH, Kamperman AM, de Rijke YB, Kooiman CG, Kushner SA. Borderline and cluster C personality disorders manifest distinct physiological responses to psychosocial stress. Psychoneuroendocrinology. (2016) 72:131–8. doi: 10.1016/j.psyneuen.2016.06.010
12. Gustafsson PE, Gustafsson PA, Ivarsson T, Nelson N. Diurnal cortisol levels and cortisol response in youths with obsessive-compulsive disorder. Neuropsychobiology. (2008) 57:14–21. doi: 10.1159/000123117
13. Lotfinia S, Soorgi Z, Mertens Y, Daniels J. Structural and functional brain alterations in psychiatric patients with dissociative experiences: a systematic review of magnetic resonance imaging studies. J Psychiatr Res. (2020) 128:5–15. doi: 10.1016/j.jpsychires.2020.05.006
14. Enneking V, Leehr EJ, Dannlowski U, Redlich R. Brain structural effects of treatments for depression and biomarkers of response: a systematic review of neuroimaging studies. Psychol Med. (2020) 50:187–209. doi: 10.1017/S0033291719003660
15. Rossetti MG, Mackey S, Patalay P, Allen NB, Batalla A, Bellani M, et al. Sex and dependence related neuroanatomical differences in regular cannabis users: findings from the ENIGMA Addiction Working Group. Transl Psychiatry. (2021) 11:272. doi: 10.1038/s41398-021-01382-y
16. Zuliani R, Delvecchio G, Bonivento C, Cattarinussi G, Perlini C, Bellani M, et al. Increased gyrification in schizophrenia and non affective first episode of psychosis. Schizophr Res. (2018) 193:269–75. doi: 10.1016/j.schres.2017.06.060
17. Calvo A, Delvecchio G, Altamura AC, Soares JC, Brambilla P. Gray matter differences between affective and non-affective first episode psychosis: A review of Magnetic Resonance Imaging studies: Special Section on “Translational and Neuroscience Studies in Affective Disorders” Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J Affect Disord. (2019) 243:564–74. doi: 10.1016/j.jad.2018.03.008
18. Cattarinussi G, Delvecchio G, Maggioni E, Bressi C, Brambilla P. Ultra-high field imaging in major depressive disorder: a review of structural and functional studies. J Affect Disord. (2021) 290:65–73. doi: 10.1016/j.jad.2021.04.056
19. Dusi N, De Carlo V, Delvecchio G, Bellani M, Soares JC, Brambilla P, et al. features of clinical outcome in bipolar disorder: a selected review: Special Section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J Affect Disord. (2019) 243:559–63. doi: 10.1016/j.jad.2018.05.066
20. Walton E, Turner JA, Ehrlich S. Neuroimaging as a potential biomarker to optimize psychiatric research and treatment. Int Rev Psychiatry. (2013) 25:619–31. doi: 10.3109/09540261.2013.816659
21. Brammer M. The role of neuroimaging in diagnosis and personalized medicine–current position and likely future directions. Dialog Clin Neurosci. (2009) 11:389–96. doi: 10.31887/DCNS.2009.11.4/mbrammer
22. Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. (2013) 7:666. doi: 10.3389/fnhum.2013.00666
23. Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. (2010) 167:640–7. doi: 10.1176/appi.ajp.2009.09081168
24. McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry. (2020) 177:411–21. doi: 10.1176/appi.ajp.2019.18111271
25. Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage. (2009) 47:922–36. doi: 10.1016/j.neuroimage.2009.04.073
26. Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci Biobehav Rev. (2017) 77:74–86. doi: 10.1016/j.neubiorev.2017.02.025
27. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
28. Akers J, Aguiar-Ibáñez R, Baba-Akbari A. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York (2009).
29. Peters M, Godfrey C, McInerney P, Soares CB, Khalil H, Parker D. Methodology for JBI Scoping Reviews. The Joanna Briggs Institute Reviewers Manual 2015. Joanna Briggs Institute, The University of Adelaide (2015). p. 3–24.
30. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evid Implement. (2015) 13:141–6. doi: 10.1097/XEB.0000000000000050
31. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
32. Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, Lacasse L, et al. Corpus callosum abnormalities in psychopathic antisocial individuals. Arch Gen Psychiatry. (2003) 60:1134–42. doi: 10.1001/archpsyc.60.11.1134
33. Lord C, Steiner M, Soares CN, Carew CL, Hall GB. Stress response in postpartum women with and without obsessive–compulsive symptoms: an fMRI study. J Psychiatry Neurosci. (2012) 37:78. doi: 10.1503/jpn.110005
34. Babson KA, Woodward SH, Schaer M, Sephton SE, Kaloupek DG. Salivary cortisol and regional brain volumes among veterans with and without posttraumatic stress disorder. Biol Psychiatry. (2017) 2:372–9. doi: 10.1016/j.bpsc.2016.11.007
35. Ming Q, Zhong X, Zhang X, Pu W, Dong D, Jiang Y, et al. State-independent and dependent neural responses to psychosocial stress in current and remitted depression. Am J Psychiatry. (2017) 174:971–9. doi: 10.1176/appi.ajp.2017.16080974
36. van Leeuwen JMC, Vink M, Joëls M, Kahn RS, Hermans EJ, Vinkers CH. Reward-related striatal responses following stress in healthy individuals and patients with bipolar disorder. Biol Psychiatry. (2019) 4:966–74. doi: 10.1016/j.bpsc.2019.06.014
37. Zhao W, Zimmermann K, Zhou X, Zhou F, Fu M, Dernbach C, et al. Impaired cognitive performance under psychosocial stress in cannabis-dependent men is associated with attenuated precuneus activity. J Psychiatry Neurosci. (2020) 45:88. doi: 10.1503/jpn.190039
38. Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test'–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. (1993) 28:76–81. doi: 10.1159/000119004
39. Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. (2005) 30:319–25.
40. Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. (2000) 57:119–27. doi: 10.1001/archpsyc.57.2.119
41. Hare RD, Harpur TJ, Hakstian AR, Forth AE, Hart SD, Newman JP. The revised psychopathy checklist: reliability and factor structure. Psychol Assess. (1990) 2:338. doi: 10.1037/1040-3590.2.3.338
42. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59(Suppl 20):22–33. doi: 10.1037/t18597-000
43. Pruessner M, Pruessner JC, Hellhammer DH, Bruce Pike G, Lupien SJ. The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatry Res. (2007) 155:1–10. doi: 10.1016/j.pscychresns.2006.12.007
44. Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC, et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. (2008) 69:1087–91. doi: 10.4088/JCP.v69n0707
Keywords: stress, psychophysiology, brain imaging, MRI, psychiatry, review, scoping review, mental disorders
Citation: Gecaite-Stonciene J, Rossetti MG, Brambilla P, Hughes BM, Mickuviene N and Bellani M (2023) Psychophysiological responses to psychological stress exposure and neural correlates in adults with mental disorders: a scoping review. Front. Psychiatry 14:1191007. doi: 10.3389/fpsyt.2023.1191007
Received: 04 April 2023; Accepted: 11 July 2023;
Published: 26 July 2023.
Edited by:
Marianna Mazza, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Marie-Laure Paillere Martinot, Hôpitaux Universitaires Pitié Salpêtrière, FranceLi Wang, Children's National Hospital, United States
Copyright © 2023 Gecaite-Stonciene, Rossetti, Brambilla, Hughes, Mickuviene and Bellani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julija Gecaite-Stonciene, anVsaWphLmdlY2FpdGUmI3gwMDA0MDtsc211Lmx0
 Julija Gecaite-Stonciene
Julija Gecaite-Stonciene Maria G. Rossetti
Maria G. Rossetti Paolo Brambilla
Paolo Brambilla Brian M. Hughes5
Brian M. Hughes5 Narseta Mickuviene
Narseta Mickuviene Marcella Bellani
Marcella Bellani