- 1Kern Medical, Department of Psychiatry, Bakersfield, CA, United States
- 2American Psychiatric Association Substance Abuse and Mental Health Services Administration (SAMHSA) Minority Fellowship, Washington, DC, United States
Background: Among antipsychotics, sialorrhea is most associated with clozapine, and when it occurs, it is uncomfortable, socially stigmatizing, and can contribute to medication non-adherence. Risperidone has a generally negligible muscarinic activity compared to clozapine, and yet, multiple reports of severe sialorrhea associated with risperidone have been reported.
Case presentation: This case report describes risperidone-induced sialorrhea that was unintentionally masked by simultaneous clonidine administration that was intended to treat hypertension. Interestingly, sialorrhea was present but mild when clonidine was present; however, when risperidone was further titrated and clonidine removed, a significant worsening of sialorrhea developed. Sialorrhea did not respond to treatment with anticholinergic medication.
Conclusion: The pathophysiology of antipsychotic-induced sialorrhea is complex and varies between antipsychotics. Risperidone-induced sialorrhea is suspected of having prominent adrenergic pathophysiology that is likely composed of highly viscoelastic saliva (high protein content), differing from the more commonly encountered clozapine-induced sialorrhea. Risperidone-induced sialorrhea is reported as more likely to respond to dose reduction and treatment with α2-adrenergic receptor agonists or β-adrenergic receptor antagonists and less likely to respond to anticholinergic (antimuscarinic) medications.
Background
Sialorrhea is a known potential adverse reaction of antipsychotic medications; however, its incidence varies among antipsychotics, and its pathophysiology is not unanimous. Salivary flow is predominantly under parasympathetic (cholinergic) control, but the sympathetic (adrenergic) system also modulates saliva production (1). Among antipsychotics, sialorrhea is most associated with clozapine, and when it occurs, it is uncomfortable, socially stigmatizing, and can contribute to medication non-adherence (2). Clozapine (including metabolites) has a generally high muscarinic activity compared to the other antipsychotics, which significantly contributes to sialorrhea when it occurs. In contrast, risperidone has a generally negligible muscarinic activity; and yet, multiple reports of severe sialorrhea associated with risperidone have been reported (3–6). This difference demonstrates that sialorrhea as an adverse reaction of antipsychotics has a complex pathophysiology and that the saliva itself in antipsychotic-associated sialorrhea may have different make-up depending on cholinergic vs. adrenergic stimulation proportions. We present a case of risperidone-induced sialorrhea masked by clonidine that demonstrates risperidone's adrenergic properties having a significant contribution to saliva overproduction.
Case report
A 46-year-old Caucasian male with a medical history of hypertension and psychiatric history of schizoaffective disorder, depressed type, presented to the behavioral health unit with paranoid ideation and aggression. He had previously tolerated quetiapine at 800 mg but was medication non-adherent for an unknown duration of time prior to this presentation. On admission, his vitals were normal except for hypertension (154/94 mmHg). Urine toxicology screening was negative, and his labs were unremarkable. On hospital day 1, he was started on risperidone 1 mg twice daily to treat psychosis. On hospital day 2, risperidone was increased to 1.5 mg twice daily. Simultaneously, the patient was started on clonidine 0.1 mg twice daily for ongoing hypertension. Clonidine was chosen for short-term use, as it was unclear if the patient had chronic hypertension in early hospitalization. As hypertension continued, on hospital day 3, amlodipine 10 mg daily was added to his regimen with titration of risperidone to 2 mg twice daily. On hospital day 4, the patient exhibited mild sialorrhea [drooling severity score: 3, moderate (2, 7, 8)] along with mild muscle rigidity and was administered benztropine 2 mg intramuscular for extrapyramidal symptoms that alleviated the rigidity only. Throughout that night, his blood pressure remained elevated, prompting consultation with internal medicine, who recommended discontinuing his clonidine and adding lisinopril 20 mg daily. The final hypertension regimen for hospitalization was lisinopril 20 mg daily and amlodipine 10 mg daily.
The next day, on hospital day 5, risperidone was also titrated to 6 mg daily. After the discontinuation of the clonidine, the patient had significantly worsened thick mucinous saliva and was found to have cogwheel rigidity with masked facies (Figure 1). In response, he started on a benztropine 2 mg daily but had no alleviation of severe mucinous sialorrhea and was wearing a towel over his shoulder due to the amount [Drooling severity scale rating: 5, profuse (2, 7, 8)]. The patient utilized atropine 1% ophthalmic drops (administered orally sublingually) but still had minimal relief of sialorrhea despite frequent use. His worsening rigidity and sialorrhea on hospital day 6 prompted medication changes, including discontinuing risperidone and benztropine and starting olanzapine 10 mg daily. After the medication changes, the patient's sialorrhea and rigidity began to subside and eventually resolved. The patient was later transitioned to quetiapine 300 mg prior to discharge and did not experience any further psychotropic-induced adverse reactions.
Discussion
Case analysis and scientific implications
This case report describes risperidone-induced sialorrhea unintentionally masked by simultaneous clonidine administration intended to treat hypertension. Interestingly, sialorrhea was present but mild when clonidine was present; however, when risperidone was further titrated and clonidine removed, significant worsening of sialorrhea developed, and this was scored by the drooling severity scale. There was no relief of sialorrhea with benztropine and minimal relief with atropine. Clonidine, an α2-adrenergic receptor agonist, did alleviate sialorrhea and suggests that risperidone's α2-adrenergic receptor antagonism is a key mechanism in risperidone-induced sialorrhea. We hypothesize that risperidone-induced sialorrhea has higher protein content (higher viscosity) compared to clozapine-induced sialorrhea due to risperidone's adrenergic stimulation mechanism. Although salivary composition analysis was not obtained in this patient, the physical examination did reveal congruent thick mucinous saliva. To investigate this hypothesis further, future cases that suspect risperidone-induced sialorrhea are suggested to obtain saliva composition analysis.
Fundamentals of saliva production
Saliva production is a constant process, but the amount and viscoelasticity (protein content and viscosity) are dependent on multiple factors, including proportions of sympathetic vs. parasympathetic stimulation and circadian rhythm (1). Mastication induces parasympathetic stimulation, predominately by M1 and M3-Muscarinic receptors, which produces copious saliva of low protein concentration (low viscoelasticity) (1, 2). M4-muscarinic receptors stimulation also appears to increase saliva output (2, 9). Sympathetic (adrenergic) stimulation results in saliva production of lower volume but higher protein concentration (high viscoelasticity) (1, 2). α2-adrenergic receptors provide negative feedback for sympathetic stimulation (Figure 2), decreasing salivary flow when stimulated (10). Antagonism of α2-adrenergic receptors increases salivation by disinhibition, leaving α1-adrenergic and β-adrenoreceptors unopposed to induce a protein-rich, catecholamine-concentrated, and increased rate of flow hyper-salivatory state (10). The α2-adrenergic hypersalivation mechanism was demonstrated in multiple studies utilizing yohimbine, a potent α2-adrenergic antagonist (11, 12).
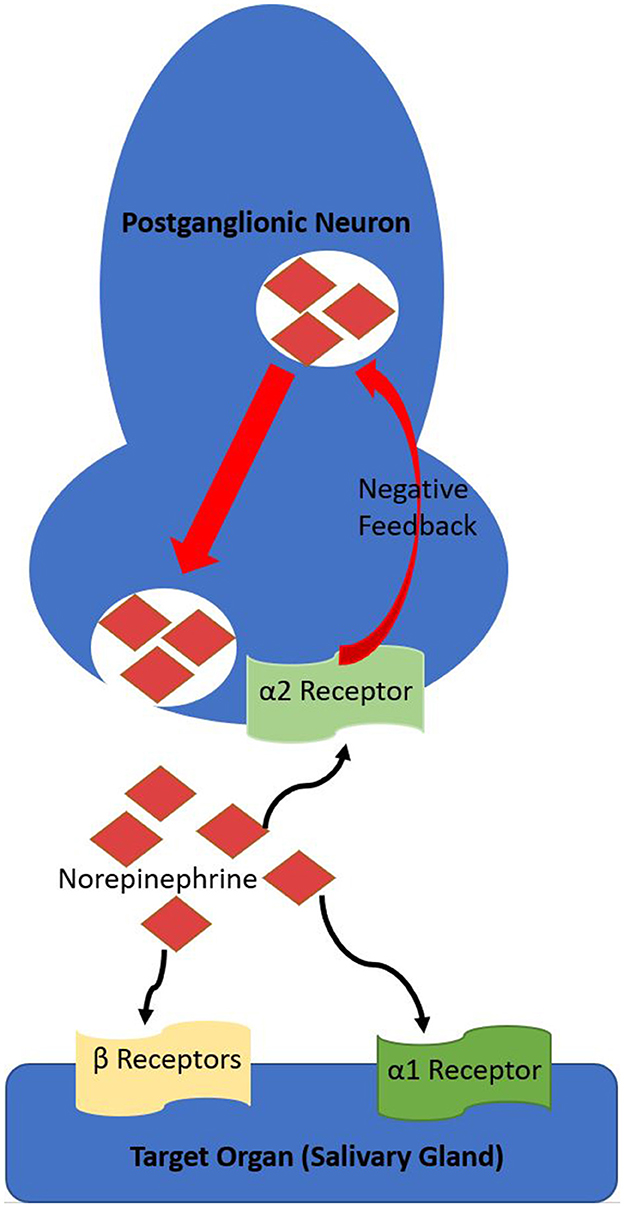
Figure 2. Overview of the sympathetic (adrenergic) pathway and α2-adrenergic receptor role in negative feedback. The α2-adrenergic receptor is a Gi-coupled metabotropic receptor, decreasing intracellular cAMP, thereby decreasing exocytosis.
Hypothesized pathophysiology of risperidone-induced sialorrhea
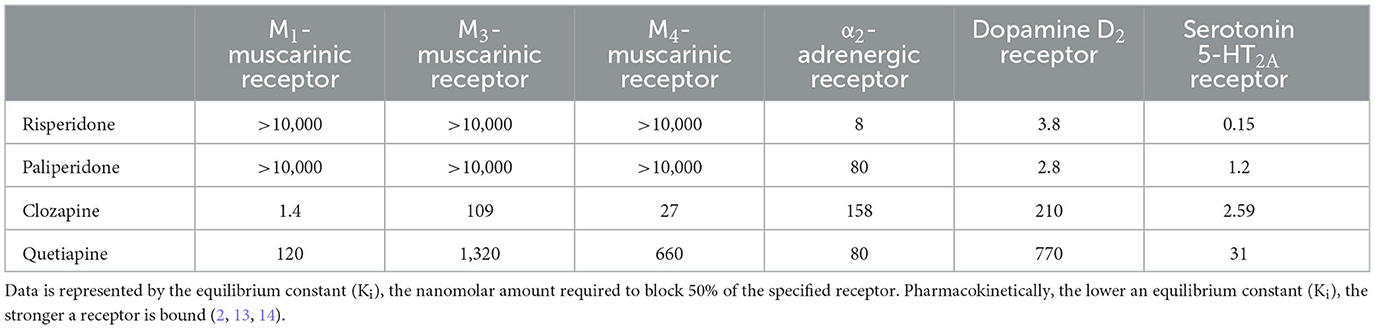
Risperidone and clozapine are both second-generation (atypical) antipsychotics but have differing neuroreceptor affinity across their mechanisms of action (Table 1); these differences are responsible for their differing therapeutic efficacies as well as their most common adverse reactions. We hypothesize that risperidone-induced sialorrhea is likely a result of potent α2-adrenergic antagonism. Risperidone-induced sialorrhea may have some pathophysiological contributions from its antimuscarinic activity; however, these neuroreceptor affinities are far less potent than its α2-adrenergic antagonism.
Clozapine-induced sialorrhea is much more common than risperidone-induced sialorrhea, and its pathophysiology is more robustly studied. Clozapine-induced sialorrhea is a result of clozapine's potent M4-muscarinic partial agonism and its metabolite norclozapine having potent muscarinic agonism (15). Clozapine-induced sialorrhea likely has some pathophysiological contribution from α2-adrenergic antagonism, but it is not as potent at this neuroreceptor in comparison to M3 and M4 muscarinic receptors or to risperidone. This likely results in lower viscoelastic saliva compared to risperidone-induced sialorrhea, but no direct comparisons are documented, and it also probably slightly varies from case to case. An additional pathophysiological mechanism of sialorrhea from clozapine results from decreased laryngeal peristalsis and inhibition of swallow reflex from its potent muscarinic modulation (16), which is likely less contributory in risperidone-induced sialorrhea.
Management of risperidone-induced sialorrhea
Sialorrhea is uncomfortable for the individual experiencing it and has significant psychosocial implications. Wet clothing may result in social stigmatization and embarrassment. The need to carry towels and “spit cups” can impair vocational functioning (2). The psychosocial complications of antipsychotic-induced sialorrhea may be a reason for a patient to self-discontinue their medication treatment (17, 18). Therefore, it is paramount to manage this adverse reaction in cases the therapeutic benefit of risperidone is substantial. Further, managing sialorrhea reduces the risk of its medical complications, which can include choking sensation, dysphonia, irritated, and macerated skin in perioral areas, cheilitis, sleep disturbance, and aspiration (2).
Switching antipsychotics or reducing the dose of risperidone would be an optimal first step in managing suspected risperidone-induced sialorrhea. If risperidone-induced sialorrhea only occurs after titrating risperidone to a high dose, tapering down to a previously tolerated lower dose may be considered (3, 4), although this will likely congruently decrease its antipsychotic effectiveness. Many of the therapeutic benefits of risperidone are also due to its conversion to its active metabolite of paliperidone. Paliperidone has a 10-fold weaker affinity for α2-adrenergic antagonism compared to risperidone (Table 2). Therefore, patients who benefit from the antipsychotic effects of risperidone but develop mild-moderate sialorrhea on it may be able to maintain similar treatment efficacy by transitioning to paliperidone and monitoring for a reduction in sialorrhea. However, paliperidone-induced sialorrhea has also been reported (19). Risperidone metabolism utilizes cytochrome P450 enzymes 2D6 and 3A4, and genetically inherited impaired activity of these enzymes may lead to the predisposition of risperidone-induced adverse reactions, including sialorrhea (20).
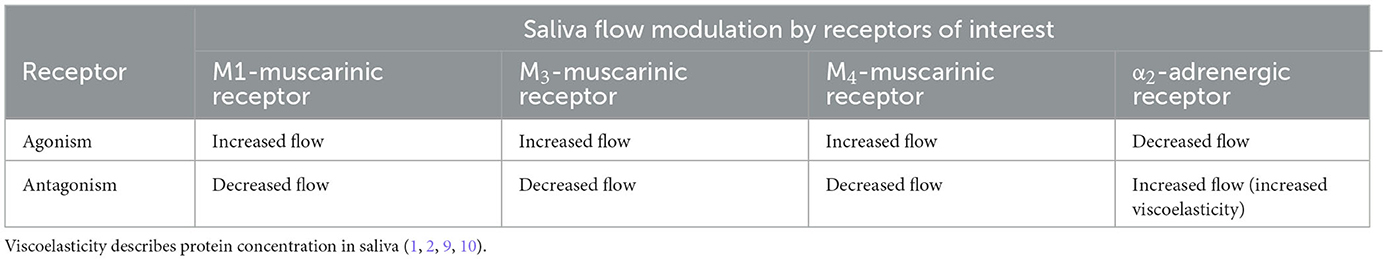
Table 2. Summary of salivary flow by the agonism or antagonism associated with common antipsychotic mechanisms of action.
As described in this case report, clonidine has been reported as alleviating risperidone-induced sialorrhea, hypothesized due to its properties as a centrally acting α2-adrenergic receptor agonist. Similarly, Gajwani et al. (5) also reported a case of risperidone-induced sialorrhea that did not respond to benztropine but was alleviated with clonidine. Other medications in this class include lofexidine, guanfacine, guanabenz, α-methyldopa, and moxonidine, but have not been reported in the literature as an attempted treatment for risperidone-induced sialorrhea (2). β-adrenergic receptor antagonists such as propranolol are theoretically another treatment option for risperidone-induced sialorrhea. This treatment would aim to halt the unopposed β-adrenergic receptors resulting from risperidone's α2-adrenergic antagonism (Figure 2) (2). β-adrenergic receptor antagonists are known to decrease salivary viscoelasticity but not necessarily the volume of saliva (21).
Anticholinergic (antimuscarinic) medications are commonly prescribed medications for treating clozapine-induced sialorrhea. Atropine ophthalmic drops are frequently used and administered sublingually and on the inside of the cheeks, with many patients reporting relief of sialorrhea (22). We predict that due to likely differences in pathophysiology for clozapine vs. risperidone-induced sialorrhea, selective and non-selective anticholinergic (antimuscarinic) medications are less likely to relieve risperidone-induced sialorrhea, despite some efficacies reported with clozapine-induced sialorrhea. Still, risperidone dose reduction and the addition of diphenhydramine were reported as therapeutic in one case of risperidone-induced sialorrhea (6); and another case was treated with risperidone dose reduction down to a previously tolerated dose and adding biperiden (4).
Conclusions
The pathophysiology of antipsychotic-induced sialorrhea is complex and varies between antipsychotics. Risperidone-induced sialorrhea is suspected of having prominent adrenergic pathophysiology, likely composed of highly viscoelastic saliva (high protein content), differing from the more commonly encountered clozapine-induced sialorrhea. Risperidone-induced sialorrhea is reported to be more likely to respond to dose reduction, treatment with α2-adrenergic receptor agonists or β-adrenergic receptor antagonists, and less likely to respond to anticholinergic (antimuscarinic) medications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Kern Medical Institutional Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
TT and AK formulated the analysis and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Proctor GB. The physiology of salivary secretion. Periodontol 2000. (2016) 70:11–25. doi: 10.1111/prd.12116
2. Praharaj SK, Arora M, Gandotra S. Clozapine-induced sialorrhea: pathophysiology and management strategies. Psychopharmacology. (2006) 185:265–73. doi: 10.1007/s00213-005-0248-4
3. Liang CS, Liao WC, Yang FW, Ho PS. Risperidone-induced sialorrhea: dose-related? Pharmacopsychiatry. (2010) 43:282–3. doi: 10.1055/s-0030-1265197
4. Panagiotidis PT, Fountoulakis KN, Siamouli M, Magiria S, Iacovides A, Kaprinis G. Risperidone-induced sialorrhea responsive to biperiden treatment. Schizophr Res. (2007) 93 410–1. doi: 10.1016/j.schres.2007.03.006
5. Gajwani P, Franco-Bronson K, Tesar GE. Risperidone-induced sialorrhea. Psychosomatics. (2001) 42:276. doi: 10.1176/appi.psy.42.3.276
6. Usta MG, Tufan AE, Cüceloglu EA. Diphenhydramine use in the treatment of risperidone-induced sialorrhea. J Child Adolesc Psychopharmacol. (2012) 22:254–5. doi: 10.1089/cap.2011.0097
7. Suskind DL, Tilton A. Clinical study of botulinum-A toxin in the treatment of sialorrhea in children with cerebral palsy. Laryngoscope. (2002) 112:73–81. doi: 10.1097/00005537-200201000-00014
8. Jongerius PH, van Limbeek J, Rotteveel JJ. Assessment of salivary flow rate: biologic variation and measure error. Laryngoscope. (2004) 114:1801–4. doi: 10.1097/00005537-200410000-00023
9. Bai YM, Lin CC, Chen JY, Liu WC. Therapeutic effect of pirenzepine for clozapine-induced hypersalivation: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Psychopharmacol. (2001) 21:608–11. doi: 10.1097/00004714-200112000-00012
10. Rogers D, Shramko J. Therapeutic options in the treatment of clozapine-induced sialorrhoea. Pharmacotherapy. (2000) 20:1092–5. doi: 10.1592/phco.20.13.1092.35036
11. Chatelut E, Rispail Y, Berlan M, Montastruc JL. Yohimbine increases human salivary secretion. Br J Clin Pharmacol. (1989) 28:366–8. doi: 10.1111/j.1365-2125.1989.tb05440.x
12. Ehlert U, Erni K, Hebisch G, Nater U. Salivary alpha-amylase levels after yohimbine challenge in healthy men. J Clin Endocrinol Metab. (2006) 91:5130–3. doi: 10.1210/jc.2006-0461
13. Martin A, Scahill L, Kratochvil C. Pediatric Psychopharmacology, Principles and Practice, 2nd Edn. Oxford: Oxford University Press (2010). p. 314.
14. Roth. NIH Psychoactive Drug Screening Program. Available online at: http://pdsp.med.unc.edu
15. Gray L, McOmish C, Scarr E, Dean B, Hannan AJ. Role of muscarinic receptors in the activity of N-desmethylclozapine: reversal of hyperactivity in the phospholipase C knockout mouse. Behav Pharmacol. (2008) 19:543–7. doi: 10.1097/FBP.0b013e32830c3669
16. Pearlman C. Clozapine, nocturnal sialorrhea, and choking. J Clin Psychopharmacol. (1994) 14:283.
17. Grabowski J. Clonidine treatment of clozapine-induced hypersalivation. J Clin Psychopharmacol. (1992). 12:69–70.
18. Lieberman JA, Safferman AZ. Clinical profile of clozapine: adverse reactions and agranulocytosis. Psychiatr Q. (1992)63:51–70.
19. Burk BG, Donaldson V, Jackson CW, Cates ME, Birur B. Paliperidone-associated sialorrhea: a case report with review of current literature. J Clin Psychopharmacol. (2022) 42:480–4. doi: 10.1097/JCP.0000000000001588
20. Berecz R, Dorado P, De La Rubia A, Cáceres MC, Degrell I, LLerena A. The role of cytochrome P450 enzymes in the metabolism of risperidone and its clinical relevance for drug interactions. Curr Drug Targets. (2004) 5:573–9. doi: 10.2174/1389450043345263
21. Newall AR, Orser R, Hunt M. The control of oral secretions in bulbar ALS/MND. J Neurol Sci. (1996) 139(Suppl.):43–4.
Keywords: antipsychotics, adverse reactions, psychopharmacology, second-generation antipsychotics, schizophrenia, iloperidone
Citation: Torrico T and Kahlon A (2023) Pathophysiology and management of risperidone-induced sialorrhea: case report. Front. Psychiatry 14:1185750. doi: 10.3389/fpsyt.2023.1185750
Received: 13 March 2023; Accepted: 27 June 2023;
Published: 13 July 2023.
Edited by:
Roberto Ciccocioppo, University of Camerino, ItalyReviewed by:
Andrej Janzic, Health Insurance Institute of Slovenia, SloveniaGerasimos N. Konstantinou, University of Toronto, Canada
Copyright © 2023 Torrico and Kahlon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tyler Torrico, tylertorrico@gmail.com
 Tyler Torrico
Tyler Torrico