
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 19 May 2023
Sec. Schizophrenia
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1180720
This article is part of the Research TopicPsychoneuroendocrinology of Psychosis Disorders, Volume IIView all 6 articles
 Hua Yu1,2†
Hua Yu1,2† Peiyan Ni3†
Peiyan Ni3† Liansheng Zhao3
Liansheng Zhao3 Yang Tian3
Yang Tian3 Mingli Li3
Mingli Li3 Xiaojing Li1
Xiaojing Li1 Wei Wei1
Wei Wei1 Jinxue Wei3
Jinxue Wei3 Wei Deng1
Wei Deng1 Xiangdong Du4
Xiangdong Du4 Qiang Wang3
Qiang Wang3 Wanjun Guo1
Wanjun Guo1 Xiaohong Ma3
Xiaohong Ma3 Jeremy Coid3
Jeremy Coid3 Tao Li1*
Tao Li1*Background: There is an urgent need to identify differentiating and disease-monitoring biomarkers of schizophrenia, bipolar disorders (BD), and major depressive disorders (MDD) to improve treatment and management.
Methods: We recruited 54 first-episode schizophrenia (FES) patients, 52 BD patients, 35 MDD patients, and 54 healthy controls from inpatient and outpatient clinics. α-Melanocyte Stimulating Hormone (α-MSH), β-endorphin, neurotensin, orexin-A, oxytocin, and substance P were investigated using quantitative multiplex assay method. Psychotic symptoms were measured using the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Syndrome Scale (PANSS), manic symptoms using the Young Mania Rating Scale (YMRS), and depressive symptoms using 17 item-Hamilton Depression Rating Scale (HAMD). We additionally measured cognitive function by using a battery of tests given to all participants.
Results: α-MSH, neurotensin, orexin-A, oxytocin, and substance P were decreased in the three patient groups compared with controls. Neurotensin outperformed all biomarkers in differentiating patient groups from controls. There were no significant differences for 6 neuropeptides in their ability to differentiate between the three patient groups. Higher neurotensin was associated with better executive function across the entire sample. Lower oxytocin and higher substance p were associated with more psychotic symptoms in FES and BD groups. β-endorphin was associated with early morning wakening symptom in all three patient groups.
Conclusion: Our research shows decreased circulating neuropeptides have the potential to differentiate severe mental illnesses from controls. These neuropeptides are promising treatment targets for improving clinical symptoms and cognitive function in FES, BD, and MDD.
Schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorders (MDD) are common and serious mental illnesses associated with substantial morbidity and mortality as well as high personal and societal costs (1). Although these disorders are a severe public health problem (2), our understanding and treatment of mental illnesses have lagged behind the progress of other medical fields (3). The main reason for this lag behind is the lack of understanding of the diseases’ etiopathology. Accumulating data from animal and human studies suggest that the pathophysiology of SCZ, BD and MDD are associated with monoamine abnormality (4–7). The monoamine hypothesis is supported by the clinical efficacy of many drugs for treating mental illness, however, there is usually a time lag between the acute pharmacological effects and clinical improvement (8). Furthermore, their therapeutic effects are limited, with high relapse rates, and a proportion of patients will deteriorate over the life course (9–11). Exploring the neuropathophysiological mechanisms of these disorders will strengthen our understanding of the disease etiology, which will further provide a basis for improving on effectiveness with improved side-effect profile compared to currently available therapies is of considerable importance (12, 13).
Recently, research into the mechanism and drug treatment of mental illness has focused increasingly on neuropeptides, which are distributed throughout the digestive, circulatory, and nervous systems, and can serve as neurotransmitters, neuromodulators, and hormones (11, 14, 15). Neuropeptides are often co-localized and co-released with monoamine neurotransmitters such as dopamine, glutamate, or γ-aminobutyric acid (GABA) (16), and can be detected peripherally. More than 100 neuropeptides have been identified, and most act via one or more of a correspondingly large number of 7-transmembrane, G protein-coupled receptors (GPCRs) (>200) (16). Neuropeptides and their receptors modulate many diverse functions of the central nervous system, including reward, sleep, emotion, and executive function (17–19). Previous studies have indicated β-endorphin, oxytocin, opioid peptides, orexin, and neurotensin (NT), are all implicated in mental illness (18, 20–22). Lower cerebral spinal fluid (CSF) neurotensin concentrations appear to be correlated with greater psychopathology severity, including thought disorder, deficit symptoms, disorganized behavior, and impaired functioning (14). NT deficiency impairs the working memory function (23), and NT genes variances were associated with executive function among healthy participants (19). Plasma oxytocin was reported to be correlated negatively with psychotic symptoms (24). Animal and experimental studies focused on neuropeptides in depression, found α-melanocyte stimulating hormone (MSH), and their receptors might have the potential to be treatment targets in stress-related mood disorders (25, 26). β-endorphin is the most important primary agonist of mu-opioid receptors (27). It is involved in reward-centric and homeostasis-restoring behaviors, which makes it a research target of interest in psychiatric disorders (27, 28).
Despite these findings, neuropeptide studies still offer little insight into either core pathophysiology or treatment options for SCZ, BD, and MDD (29). Precise brain neuropeptide measurement using CSF from lumbar punctures is invasive and not suitable for widespread use (30). In contrast, plasma detection of neuropeptides is less invasive, can accurately detect concentration, and be used widely in clinical research. However, how does the concentration of plasma neuropeptides change, and how does the plasma neuropeptides correlated with clinical symptom as well as cognitive function in these severe mental illnesses are being rarely explored. What’s more, whether these plasma neuropeptides had the potential to discriminate different disease status is still unknown. In this study, we used a new immuno-assay measurement to detect the plasma level of neuropeptides in first-episode schizophrenia (FES), BD, and MDD. Firstly, we explored whether these plasma neuropeptides showed the same change as it was reported in CSF in schizophrenia, BD and MDD, and whether the abnormal level of neuropeptides would be associated with clinical symptoms and cognitive function in patient groups. Finally, we tested whether these neuropeptides can be used as a biomarker to distinguish FES, BD and MDD from controls.
FES, BD, MDD, and healthy control (HC) volunteers were recruited for the current study from in- and outpatient psychiatric facilities at West China Hospital of Sichuan University. We recruited 54 FES (26 male, 28 female), 52 BD (21 male, 31 female), and 35 MDD (15 male, 20 female), diagnosed according to standard operational criteria in the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV). Fifty-four healthy volunteers (23 male, 31 female) were included. Healthy volunteers were screened for major psychiatric disorders using the Structured Clinical Interview for DSM-IV, non-patient edition. We used the Positive and Negative Symptom Rating Scale (PANSS), the Brief Psychiatric Rating Scale (BPRS), the Young Mania Rating Scale (YMRS), and 17-item Hamilton Depression Rating Scale (HAMD) to measure the symptom severity. The clinical rating scales are evaluated by experienced psychiatrists within 3 days after the patients are recruited in the study. Patients were excluded if they met the criteria for alcohol or substance abuse within 1 year of screening, and significant medical illness. All right-handed subjects provided written informed consent. The study was approved by the West China Hospital of Sichuan University Ethics committee, and the first subject was included in March 5th, 2015.
Intelligence quotient (IQ), verbal IQ, and performance IQ scores of all participants were assessed using the seven-subtest short form of the revised Wechsler Adult Intelligence Scale in Chinese (31). The Cambridge Neuropsychological Test Automated Battery (CANTAB) is a computerized tool used to measure cognitive function in diverse populations (32). Stockings of Cambridge (SOC) is a part of the CANTAB task. Participants are shown two displays, each containing colored balls. Each participant must move the ball in the lower display to copy the pattern shown in the upper display. During the test, participants are asked to make as few moves as possible to match the two patterns. SOC problems solved in minimum moves is a fundamental measure, recording the number of occasions upon which the subject has completed a test problem in the minimum possible number of moves. It included mean moves for 2, 3, 4, and 5-move problems. Here, we used the problems solved in minimum moves for 5-move problems (MM5M). SOC-MM5M is a measure of spatial planning memory and executive function. A lower score indicates better performance.
Not fasted blood samples were collected by venipuncture between 4.00 p.m. and 4.30 p.m. using ethylenediaminetetraacetic acid as an anti-coagulant. Peripheral blood mononuclear cells were removed by refrigerated centrifugation at 1,000 g for 10 min, and the separated plasma was immediately divided into 0.5-mL aliquots and stored at −80°C. Plasma factors were evaluated using MILLIPLEX® MAP kits (Merck KGaA, Darmstadt, Germany). The MILLIPLEX® MAP Human Neuropeptide Magnetic Bead Panel is used for the simultaneous quantification of the following 6 analytes in any combination: α-MSH, β-Endorphin, Neurotensin, Orexin-A, Oxytocin, and Substance P. This kit may be used for the analysis of all or any combination of the above analytes in tissue/cell lysate, culture supernatant samples, and CSF, serum or plasma samples. The immunoassay procedure was followed by the manufacture’s standard procedure and was described by our group elsewhere (15). Standard curves were generated using neuropeptide standards. Data were analyzed using a FLEXMAP 3D® instrument (Luminex, Merck Millipore) operated with xPONENT® software (version 4.0, Luminex). Median fluorescent intensity data were analyzed using a weighted 5-parameter logistic method to calculate the factor concentrations.
Stata 14.0 and SPSS 24.0 were used for the analysis. The distribution of the continuous variables was checked using Shapiro–Wilk’s test. Log10 transformation was used to correct variables that were not normally distributed. Parametric comparisons (t-test or one-way ANOVA with post-hoc Bonferroni analysis). Fisher’s exact test was used to check the statistical significance of between-group differences. Spearman or Pearson correlation was conducted to test the correlation coefficient for categorical variables or continuous variables, respectively. The differences in cognitive function and the plasma neuropeptides among groups were tested by ANCOVA, with age, gender and BMI as covariates, the education year was additionally co-variated out for cognitive function analysis. The post-hoc group comparison was conducted with the alpha value set to 0.05 and with Bonferroni correction, which was a relatively strict method used to correct for multiple comparisons. The results are presented as mean ± standard deviation unless otherwise specified (Table 1). Differentiating performance of plasma markers was assessed using age-gender-BMI adjusted area-under-the-curve (AUC) values from receiver operating characteristic (ROC) analyses (FES vs. HC; BD vs. HC; MDD vs. HC; FES vs. BD; FEP vs. MDD; and BD vs. MDD). The test accuracy, sensitivity, specificity, predictive values and AUC (AUC: 0.9–1.0 = excellent; 0.8–0.9 = good; 0.7–0.8 = fair; 0.6–0.7 = poor; 0.5–0.6 = fail) were measured (33). Differences in AUCs were evaluated using bootstrapping (n = 1,000).
Finally, separate stepwise linear regression analyses were used to test associations between plasma neuropeptides and clinical symptom scores or cognitive function. The linear regression model used age, gender, education, BMI, group status, log10 α-MSH, log10 β-endorphins, log10 neurotensin, log10 orexin A, log10 oxytocin, and log10 substance P as independent variables. The least significant variables were removed one at a time until only significant variables remained. Significance was taken as p < 0.05. Supplementary Table S1 shows the factors excluded, respectively, in the linear regression models.
Demographic, clinical, plasma biomarkers, and cognitive features of patients and healthy controls are presented in Table 1. The FES group was younger than the other three groups. The BD group had a higher body mass index (BMI) compared to the other three groups. There were no gender differences between patients and controls. The three patient groups had lower educational levels than the controls. Compared to FES and BD, the MDD group showed significantly increased HAMD scores. Because few MDD subjects were measured with YMRS, PANSS, and BPRS, we only compared these symptom scores between FES and BD. The YMRS scores were significantly higher in the BD group compared to the FES group. The PANSS total score and BPRS total score were significantly increased in the FES group compared to the BD group. The BD group had a significantly longer illness duration than the FES group (see results in Table 1; Supplementary Table S2).
ANCOVA analysis indicated that there were significant group differences in total IQ, and performance IQ, among the FES, BD, MDD, and HCs. Post hoc analyses revealed decreased total IQ scores, and performance IQ scores in the FES patient group compared to controls. There were no significant differences in SOC-MM5M scores between the patients and control groups (see Table 1).
Controlling for age, gender, and BMI, the log10 α-MSH, log10 neurotensin, log10 orexin A, log10 oxytocin, and log10 substance P level were significantly decreased in the three patient groups compared to controls. In addition, only the BD group showed decreased log10 β-endorphins compared to controls. We did not detect any significant differences between groups for six neuropeptides (see Table 1; Figure 1).
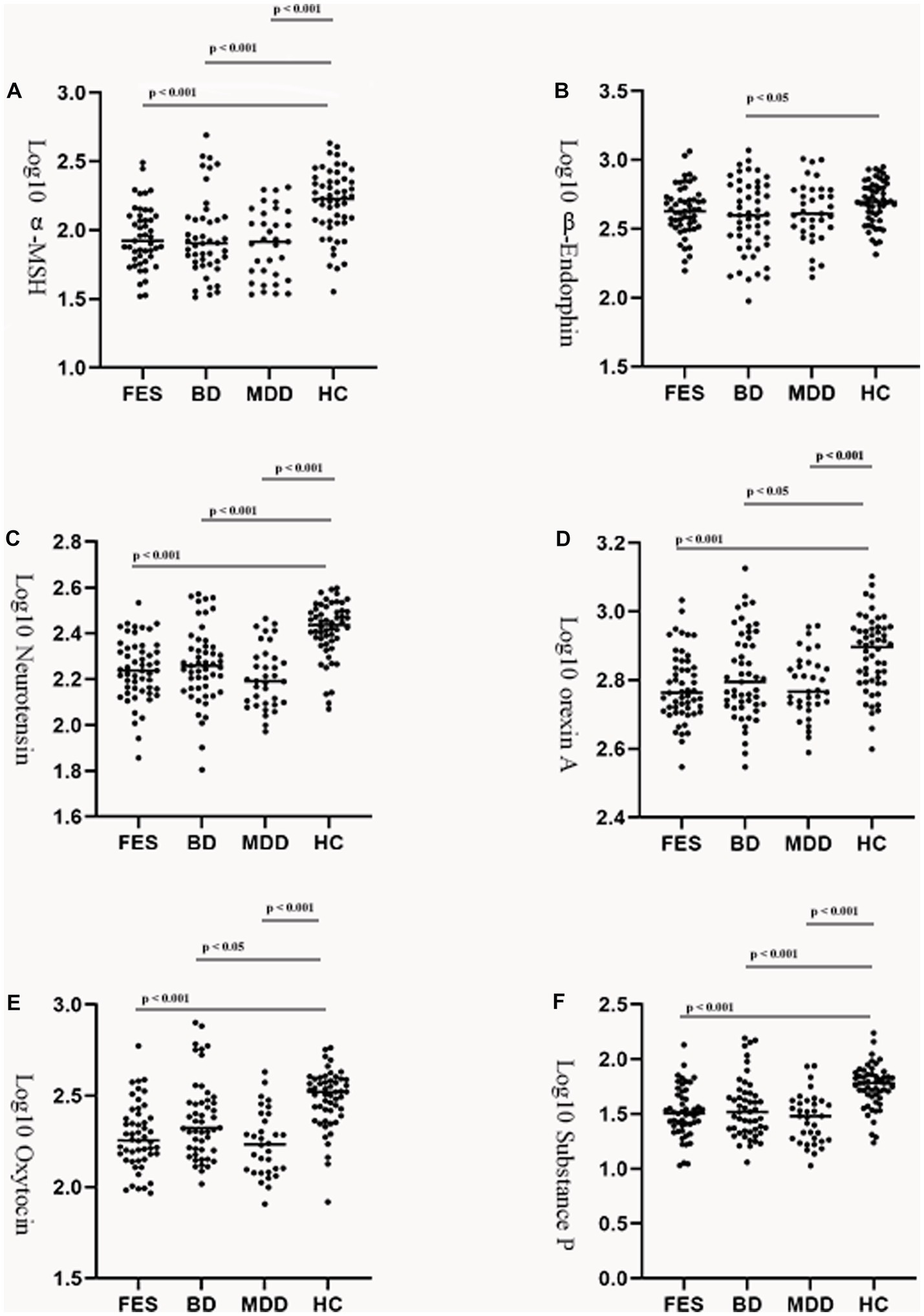
Figure 1. Group comparison of plasma neuropeptides among the patients and control group. Log transformed plasma α-melanocyte-stimulating hormone (A), β-endorphin (B), neurotensin (C), orexin A (D), oxytocin (E), and substance P (F) levels in patients with FES, BD, or MDD and healthy controls. Horizontal lines in dotted plots denote mean values. MSH, melatonin stimulating hormone; FES, first episode schizophrenia; BD, bipolar disorders; MDD, major depressive disorders; HC, healthy controls.
Receiver operating characteristic curves demonstrating the differentiating potential of plasma neuropeptides are shown in Figure 2. Across all comparisons, only patients compared with controls showed a good level of accuracy, and the differentiating performances between patient groups was poor. The highest AUC values were seen for plasma neurotensin when comparing patients with controls; FES versus HC [AUC = 0.83, 95% confidence interval [CI] 0.74–0.91; Figure 2A]; BD versus HC [AUC = 0.80, 95% CI 0.71–0.89; Figure 2B]; and MDD versus HC [AUC = 0.87, 95% CI 0.77–0.94; Figure 2C]. Other AUCs for discriminating patients from controls which showed good levels of discrimination above 0.80 included: oxytocin in FES vs. HC [AUC = 0.80, 95% CI 0.68–0.89; Figure 2C]; oxytocin in MDD vs. HC [AUC = 0.85, 95% CI 0.74–0.92; Figure 2C]; a-MSH in MDD vs. HC [AUC = 0.80, 95% CI 0.68–0.89; Figure 2C]; and substance P in MDD vs. HC [AUC = 0.84, 95% CI 0.74–0.92; Figure 2C]. For contrasts between patient groups, the AUCs are relatively poor (Figures 2D–F).
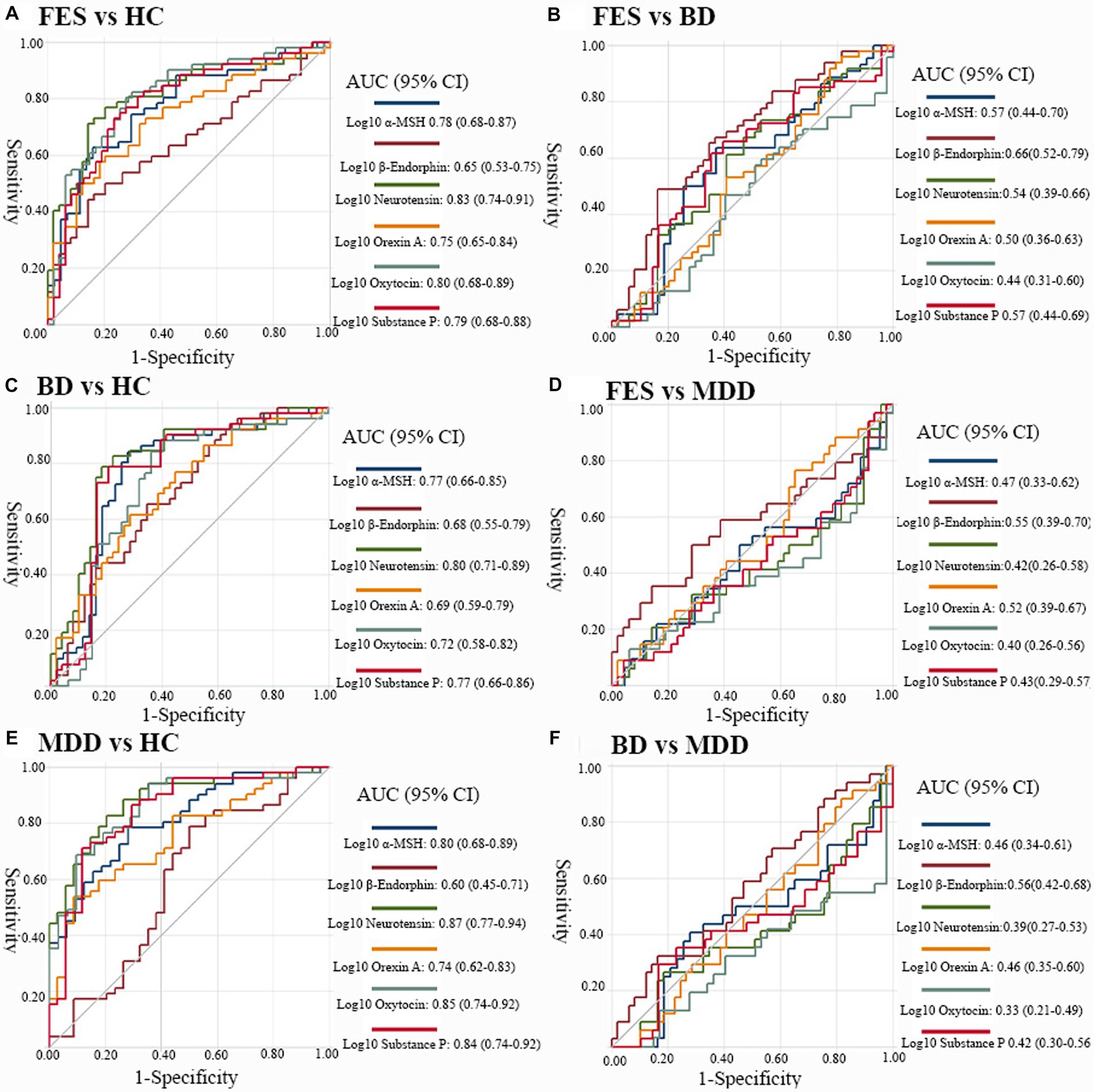
Figure 2. Discriminative performance of biomarkers across diagnostic groups. Receiver-operating characteristics (ROC) curves displaying the performance of plasma α-MSH, β-endorphins, neurotensin, orexin A, oxytocin and substance P to distinguish (A) FES from HCs, (B) BD from HCs, (C) MDD from HCs, (D) individuals with FES from BD, (E) FES versus MDD, and (F) BD versus MDD. MSH, melatonin stimulating hormone; FES, first episode schizophrenia; BD, bipolar disorders; MDD, major depressive disorders; HC, healthy controls; AUC, area under the curve. The AUCs were all covariated out age, gender and body mass index.
In the combined samples of the four groups, stepwise linear regression analysis using SOC-MM5M scores as the dependent variable and age, gender, education, BMI, group status, log10 α-MSH, log10 β-endorphins, log10 neurotensin, log10 orexin A, log10 oxytocin and log10 substance P as the independent variables showed that the linear regression model was significant (F1,153 = 8.01, p < 0.01). The adjusted multivariate coefficient of determination (R2) for the model was 0.05 for the predictor. The standardized β coefficient value for neurotensin was −0.22, with a t value of −2.83 (see Table 2; Supplementary Table S1).
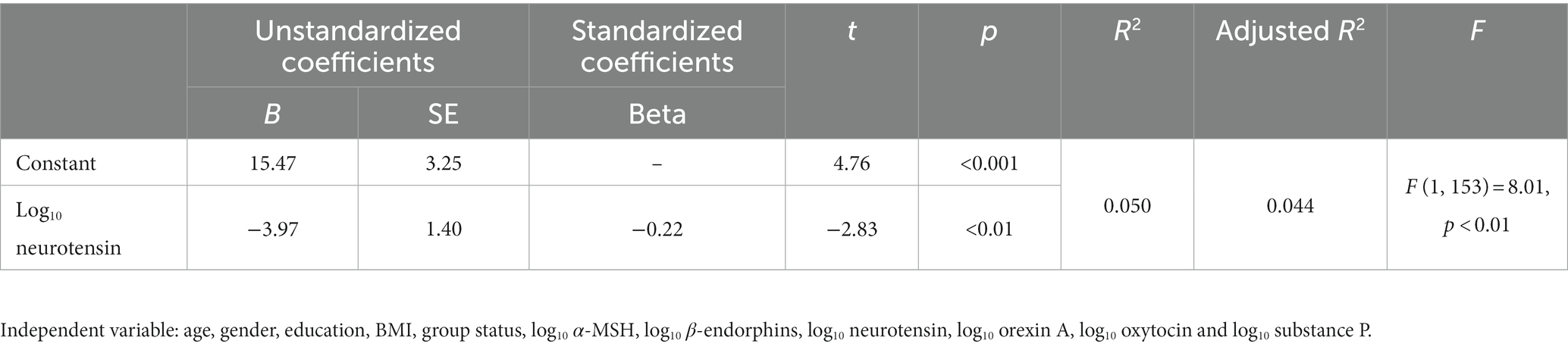
Table 2. Association between neuropeptides with SOC-MM5M in the combined subject groups (step-wise regression).
The summary of linear regression analyses for evaluating the relationships among PANSS subscales and BPRS factor scores within FES and BD groups is presented in Table 3. When using age, gender, education, BMI, log10 α-MSH, log10 β-endorphins, log10 neurotensin, log10 orexin A, log10 oxytocin, and log10 substance P as the independent variables, and P2 (positive symptom scale item 2), N2 (negative symptom scale item 2), N3 (negative symptom scale item 3), and G9 (general pathology symptom scale item 9) as independent variables, we found that the regression models were all significant because all p-values were all lower than 0.05. Oxytocin was the only variable that could predict PANSS subscale symptom scores, with lower oxytocin levels predicting higher PANNS scores. When using the BPRS deficiency energy factor score as the dependent variable, we found that oxytocin level explained a significant amount of the variance in the BPRS deficiency energy factor score in Step 1 (F = 6.40, p < 0.05; R2 = 0.076; β = −0.28). In the second step, the inclusion of substance P (β = 4.33) enhanced the relationship between BPRS deficiency energy factor score and oxytocin level (β = −0.65) based on the magnitude of the standardized beta-coefficient in Step 2 (F = 5.36, p < 0.01; ΔR2 = 0.023). The adjusted multivariate coefficient of determination (R2) for the final model was 0.099 for the predictors. The standardized β coefficient value for oxytocin and substance P were − 0.65 and 4.33, with t values of −3.04 and 2.02 in step 2 (see results in Table 3 and Supplementary Table S1).
The summary of linear regression analyses for evaluating the relationships among depression severity, measured by the HAMD-17 item 6 (which is a measurement of early morning wakening), in the three patient groups is presented in Table 4. MDD group status explained a significant amount of the variance in early morning wakening severity in Step 1 (F = 41.88, p < 0.001; R2 = 0.30; β = 0.55). The inclusion of log10 endorphin (β = −0.25) enhanced the relationship between early morning awakening severity and MDD group status (β = 0.56) based on the magnitude of the standardized beta-coefficient in Step 2 (F = 27.69, p < 0.001; ΔR2 = 0.06). The adjusted multivariate coefficient of determination (R2) in step 2 was 0.36 for the predictors. The standardized β coefficient value for MDD group status and log10 endorphin were 0.56 and −0.25, with t values of 6.90 and −3.12 (see Table 4; Supplementary Table S1).
We conducted correlation analyses between neuropeptides and illness duration, age of onset, mood episodes, and drug usage in the three patient groups, separately, but did not find any significant correlations in either FES or MDD group. However, in the BD group, we found plasma β-endorphin level was significantly positively correlated with illness duration (r = 0.41, p < 0.01) (see results in Supplementary Table S3).
In this study, we found that plasma α-MSH, orexin-A, oxytocin, neurotensin, and substance P, were all significantly decreased in FES, BD, and MDD groups compared to controls. β-endorphins were only decreased in the BD group compared to controls. In contrast, there were no significant differences observed among the three patient groups for six plasma neuropeptides. Neurotensin was the only plasma biomarker that could provide a relatively high differentiating potential to distinguish FES, BD, and MDD from controls (AUC = 0.83, 0.80, and 0.87 respectively). Furthermore, we have demonstrated that the plasma neuropeptides were differentially associated with specific clinical symptoms and executive function across disease groups. Our results indicate the disease monitoring and disease-categorizing potential of plasma neuropeptides, which could therefore be used in clinical and research settings (34).
Research evidence has already identified the role of neurotensin in the pathophysiology of psychosis and the mechanism of action of antipsychotic drugs (35, 36). Decreased CSF neurotensin concentration has been found in patients with schizophrenia, and improvements in overall psychopathology, including positive and negative symptoms, were correlated with increases in CSF neurotensin concentrations during treatment (14, 35). Our study is consistent with previous research and we find decreased neurotensin in first episode, never treated schizophrenia patients. There has been little previous investigation of the effects of neurotensin in BD and MDD. However, it was reported that neurotensin receptor 2 (NTSR2) was decreased in the anterior cingulate cortex of BD (37). NTSR2 mRNA and NTSR2 binding were reported to be down-regulated in transgenic mice expressing anxiety, stress, and depression (38). Furthermore, neurotensin receptor 1 (Ntsr1) knockout mice also showed increased anxiety and despair behaviors (39). Our results may be consistent with the hypothesis that plasma peptide changes are consistent across the major psychiatric disorders, as these disorders may have transdiagnostic properties (40). In our test of the differentiating performance of plasma neuropeptides, our finding that neurotensin has a good differentiating potential between patient groups and controls, with AUCs above 0.80, is new. Neurotensin is an endogenous tridecapeptide neurotransmitter that is heterogeneously distributed within the mammalian central nervous system (CNS) and has close neuroanatomical, functional associations with the dopamine neurotransmitter system (12, 41).
In our correlation analysis, we found that the measurement of SOC-MM5M was negatively associated with neurotensin. Our results are consistent with the previous study in humans which showed NT genes variances were associated with working memory performance among healthy participants (42). We expanded the association results to a larger spectrum of the human sample, which includes the FES, BD, MDD, and HCs. Evidence has indicated that neurotensin is often co-released with dopamine, and dopamine is widely expressed in the frontal lobe (43). As SOC-MM5M is a measurement of frontal lobe function (44), our results suggested that neurotensin might have the potential to influence frontal lobe function. However, further exploration is needed. Above all, our results support the possibility that plasma neurotensin has a central role in cognitive function, and it has the potential to be the treatment target for improving cognitive deficit in severe mental illness.
We found decreased oxytocin in plasma levels in schizophrenia as well as mood disorder patients. CSF and plasma oxytocin levels were found to be decreased in drug-naive schizophrenia patients and chronic schizophrenia patients and showed increased levels with antipsychotics drug treatment (45). Reduced plasma oxytocin concentrations were observed in patients with MDD and BD compared to controls (24, 46). Our results are consistent with previously published studies that reported decreased plasma oxytocin in schizophrenia, BD, and MDD (24, 45, 46). Furthermore, we found oxytocin was negatively correlated with negative and positive symptom subscales in bipolar and FES patients. Clinical studies have shown that nasal administration of oxytocin improves some symptoms of schizophrenia (47). Our results suggest that oxytocin plays a central role in the pathophysiology of major psychiatric illnesses, and increased oxytocin levels in FES and BD patients may help with the treatment of psychotic symptoms (8).
In our study, we only found decreased β-endorphin in the BD group compared with controls, and there were no differences between FES or MDD and controls. Few studies have explored endorphin levels in BD, although a single electroconvulsive therapy (ECT) study reported that after ECT treatment, the patient group had significantly increased endorphin levels (48). We found that a core symptom of depression, early-morning wakefulness of insomnia, was negatively associated with β-endorphin. Because insomnia is one of the most common symptoms of psychiatric illness, and a previous study has reported that electroacupuncture induced sleep enhancement may be mediated, in part, by increasing the concentrations of β-endorphin (49). Our finding is therefore consistent with β-endorphin having a role in the pathophysiology of insomnia symptoms in major psychiatric illnesses.
We also found decreased Orexin A, α-MSH, and Substance P across the three patient groups, and these three neuropeptides could discriminate patients from controls with modest accuracy (between 0.7 and 0.8). Loss of orexin neurons and decrease of orexin levels in plasma are observed in patients with depression, schizophrenia, and other neurodegenerative diseases (20, 50, 51). Our results may indicate that altered orexin-A signaling was disrupted in schizophrenia, BD, and MDD. α-MSH is one cleavage product of the pituitary hormone pro-opiomelanocortin (POMC), and numerous studies have described the role of POMC in metabolic syndrome (50). Psychopharmacotherapy strongly impacts the metabolic system, in particular in schizophrenia and bipolar disorders (52). As one of POMC’s downstream effector hormones, decreased α-MSH may be a potential risk factor for metabolic syndrome in several mental illnesses. We found that substance P was positively correlated with psychotic symptoms in FES and BD. Based on the fact that SP-containing neurons synapse with dopaminergic neurons in the midbrain, and that application of SP agonists in animal studies lead to increased dopaminergic turnover and locomotor activity (53–55), our results suggested that abnormal SP neurotransmission may be involved in the etiopathology of psychosis.
To our knowledge, there was only one study comparing the differences of the plasma neuropeptides between schizophrenia, BD and MDD subjects compared with controls (56). In Hidese et al’s (56) study, the authors reported there was no difference of plasma α-MSH, β-endorphin, neurotensin, oxytocin, and substance P between patient groups and controls in a larger sample size. And they did not find any significant correlation between these above-mentioned plasma neuropeptides with clinical symptom and cognitive function. The reasons for the quite different results might be that there were several differences in our study sample compared with theirs. First, most of their participants are taking psychotropic medication though they adjusted the medication effects in their analysis. However, in our research, we recruited first episode drug naïve schizophrenia patients as well as drug washed MDD subjects, and only BD patients in our research are taking drugs. Second, as they stated in their analysis that they did not record the somatic symptom in their subjects, as the interference of chronic somatic disorders such as diabetes, cardiovascular diseases, endocrinological problems, and their specific pharmacological treatment might have affected plasma neuropeptide levels. In our study, we excluded subjects with somatic symptom such as hypertension, endocrinological problems as well as patients taking any other pharmacological treatment. Finally, it was reported age has effect on the plasma proteins (57), and plasma neuropeptide concentration was found to increase significantly with age (58). Our patients have a relative younger mean age, as most of our subjects are at their 20th, while in Hidese et al’s (56) study, they recruited subjects at a mean age over 30th-40th. All these differences might contribute to the quite different research findings, further longitudinal study is needed.
There are several limitations that need to be addressed. The first limitation of the study is that it remains unclear whether plasma neuropeptide levels correlate with brain levels of neuropeptides because of the blood–brain barrier. In our study, we showed that plasma neuropeptides are correlated with cognitive function, psychotic and depressive symptoms. This may indicate that circulating neuropeptides can reflect the neuropeptide in the central nervous system (24). The second limitation is that drug treatment may confound our results, as BD and MDD groups were previously medicated. Although we also did not detect any significant correlation between drug usage and neuropeptide measurements, further studies must include first episode and drug naïve BD and MDD patients. Finally, the cross-sectional design of this study will not provide evidence of causality, and without longitudinal data, it is not possible to establish a true cause and effect relationship (59). We, therefore suggest that longitudinal observation of changes of neuropeptides in severe mental illness should be conducted in future research.
In conclusion, this study explored the plasma concentration of six neuropeptides, including α-MSH, β-Endorphin, Neurotensin, Orexin-A, Oxytocin, and Substance P in FES, BD, and MDD, and tested their differentiating potential to distinguish patients from controls. We also found there were significant correlation between plasma neuropeptides and psychotic, depressive symptoms, as well as executive function in FES, BD, and MDD groups. If our results are confirmed in further large-scale longitudinal studies, we could conclude plasma neuropeptides might be promising targets for treating clinical symptoms and cognitive deficits.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by West China Hospital of Sichuan University Ethics committee. The patients/participants provided their written informed consent to participate in this study.
HY, PN, JC, and TL developed the study, had full access to all data, and took responsibility for data integrity and accuracy. HY, PN, QW, and WG drafted the manuscript. WW, JW, XD, WD, and XM collected the data. YT, LZ, ML, and XL performed all data analyses. All authors agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of the data and results are appropriately investigated and resolved, and critically revised and approved the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China Key Project (81630030 and 81920108018 to TL), National Natural Science Foundation of China (81871054 and 81501159 to PN, 82101598 to HY), Special Foundation for Brain Research from Science and Technology Program of Guangdong (2018B030334001 to TL), 2021 Project for Hangzhou Medical Disciplines of Excellence and Key Project for Hangzhou Medical Disciplines, 1.3.5 Project for Disciplines of Excellence at West China Hospital of Sichuan University (ZY2016103, ZY2016203, and ZYGD20004 to TL), and Introductory Project of the Suzhou Clinical Expert Team (SZYJTD201715 to XD and TL).
The authors would like to thank all their coworkers at the Affiliated Mental Health Centre and Hangzhou Seventh People’s Hospital, West China Hospital, the State Key Laboratory of Biotherapy in West China Hospital and Suzhou Psychiatry Hospital for their contributions to this research study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1180720/full#supplementary-material
SCZ, schizophrenia; FES, first-episode schizophrenia; BD, bipolar disorder; MDD, major depressive disorder; DSM, Statistical Manual of Mental Disorders; GABA, γ-aminobutyric acid; GPCRs, G protein-coupled receptors; CSF, cerebral spinal fluid; NT, neurotensin; CSF, cerebral spinal fluid; NT, neuotensin; BPRS, Brief Psychiatric Rating Scale; YMRS, Young Mania Rating Scale; HAMD-17, 17-item Hamilton Depression Rating Scale; IQ, Intelligence quotient; CANTAB, Cambridge Neuropsychological Test Automated Battery; SOC, Stockings of Cambridge; MM5M, minimum moves for 5-move problems; AUC, area under the curve; ROC, values from receiver operating characteristic; BMI, body mass index; AIC, Akaike information criterion; SP, substance p; CI, confidence interval; NTSR1, neurotensin receptor 1 gene; MSH, melanocyte-stimulating hormone.
1. Chong, HY, Teoh, SL, Wu, DB, Kotirum, S, Chiou, CF, and Chaiyakunapruk, N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. (2016) 12:357–73. doi: 10.2147/NDT.S96649
2. Walker, ER, McGee, RE, and Druss, BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiat. (2015) 72:334–41. doi: 10.1001/jamapsychiatry.2014.2502
3. Yang, Y, Liu, S, Jiang, X, Yu, H, Ding, S, Lu, Y, et al. Common and specific functional activity features in schizophrenia, major depressive disorder, and bipolar disorder. Front Psych. (2019) 10:52. doi: 10.3389/fpsyt.2019.00052
4. Nakamura, S. Integrated pathophysiology of schizophrenia, major depression, and bipolar disorder as monoamine axon disorder. Front Biosci (Schol Ed). (2022) 14:4. doi: 10.31083/j.fbs1401004
5. Batinic, B, Ristic, I, Zugic, M, and Baldwin, DS. Treatment of symptom clusters in schizophrenia, bipolar disorder and major depressive disorder with the dopamine D3/D2 preferring partial agonist Cariprazine. Front Psych. (2021) 12:784370. doi: 10.3389/fpsyt.2021.784370
6. Zhang, JP, Lencz, T, Zhang, RX, Nitta, M, Maayan, L, John, M, et al. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and Meta-analysis. Schizophr Bull. (2016) 42:1418–37. doi: 10.1093/schbul/sbw058
7. Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. (2002) 4:7–20. doi: 10.31887/DCNS.2002.4.1/bbondy
8. Goh, KK, Chen, C-H, and Lane, H-Y. Oxytocin in schizophrenia: pathophysiology and implications for future treatment. Int J Mol Sci. (2021) 22:2146. doi: 10.3390/ijms22042146
9. Carlsson, A, Waters, N, Holm-Waters, S, Tedroff, J, Nilsson, M, and Carlsson, ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. (2001) 41:237–60. doi: 10.1146/annurev.pharmtox.41.1.237
10. Gitlin, MJ, Swendsen, J, Heller, TL, and Hammen, C. Relapse and impairment in bipolar disorder. Am J Psychiatry. (1995) 152:1635–40. doi: 10.1176/ajp.152.11.1635
11. Barde, S, Rüegg, J, Prud’homme, J, Ekström, TJ, Palkovits, M, Turecki, G, et al. Alterations in the neuropeptide galanin system in major depressive disorder involve levels of transcripts, methylation, and peptide. Proc Natl Acad Sci. (2016) 113:E8472. doi: 10.1073/pnas.1617824113
12. Griebel, G, and Holsboer, F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. (2012) 11:462–78. doi: 10.1038/nrd3702
13. Baldessarini, RJ, Tondo, L, Baethge, CJ, Lepri, B, and Bratti, IM. Effects of treatment latency on response to maintenance treatment in manic-depressive disorders. Bipolar Disord. (2007) 9:386–93. doi: 10.1111/j.1399-5618.2007.00385.x
14. Cáceda, R, Kinkead, B, and Nemeroff, CB. Involvement of neuropeptide Systems in Schizophrenia: human studies In: International review of neurobiology, vol. 78. edn: Academic Press (2007). 327–76.
15. Ni, P, Tian, Y, Gu, X, Yang, L, Wei, J, Wang, Y, et al. Plasma neuropeptides as circulating biomarkers of multifactorial schizophrenia. Compr Psychiatry. (2019) 94:152114. doi: 10.1016/j.comppsych.2019.152114
16. Hökfelt, T, Barde, S, Xu, Z-QD, Kuteeva, E, Rüegg, J, Le Maitre, E, et al. Neuropeptide and small transmitter coexistence: fundamental studies and relevance to mental illness. Front Neural Circuits. (2018) 12:106. doi: 10.3389/fncir.2018.00106
17. Nestler, EJ, Hyman, SE, Holtzman, DM, and Malenka, RC. Neuropeptides. In: Molecular neuropharmacology: A Foundation for Clinical Neuroscience, 3Edn. McGraw-Hill Education: New York, NY (2015).
18. de Wied, D, and Sigling, HO. Neuropeptides involved in the pathophysiology of schizophrenia and major depression. Neurotox Res. (2002) 4:453–68. doi: 10.1080/10298420290031432
19. Wang, M, Ma, H, Huang, YL, Zhu, G, and Zhao, JP. Association of Neurotensin receptor 1 gene polymorphisms with processing speed in healthy Chinese-Han subjects. J Molecul Neurosci. (2014) 54:787–9. doi: 10.1007/s12031-014-0404-6
20. LaCrosse, AL, and Olive, MF. Neuropeptide systems and schizophrenia. CNS Neurol Disord Drug Targets. (2013) 12:619–32. doi: 10.2174/1871527311312050010
21. Beckmann, H, Lang, RE, and Gattaz, WF. Vasopressin–oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Netherlands: Elsevier Science. (1985) 10:187–91.
22. Glovinsky, D, Kalogeras, KT, Kirch, DG, Suddath, R, and Wyatt, RJ. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res. (1994) 11:273–6. doi: 10.1016/0920-9964(94)90021-3
23. Pedersen, CA, Gibson, CM, Rau, SW, Salimi, K, Smedley, KL, Casey, RL, et al. Intranasal oxytocin reduces psychotic symptoms and improves theory of mind and social perception in schizophrenia. Schizophr Res. (2011) 132:50–3. doi: 10.1016/j.schres.2011.07.027
24. Cochran, DM, Fallon, D, Hill, M, and Frazier, JA. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry. (2013) 21:219–47. doi: 10.1097/HRP.0b013e3182a75b7d
25. Kormos, V, and Gaszner, B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides. (2013) 47:401–19. doi: 10.1016/j.npep.2013.10.014
26. Kokare, DM, Dandekar, MP, Singru, PS, Gupta, GL, and Subhedar, NK. Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology. (2010) 58:1009–18. doi: 10.1016/j.neuropharm.2010.01.006
27. Pilozzi, A, Carro, C, and Huang, X. Roles of β-endorphin in stress, behavior, neuroinflammation, and brain energy metabolism. Int J Mol Sci. (2020) 22:338. doi: 10.3390/ijms22010338
28. Hegadoren, KM, O'Donnell, T, Lanius, R, Coupland, NJ, and Lacaze-Masmonteil, N. The role of beta-endorphin in the pathophysiology of major depression. Neuropeptides. (2009) 43:341–53. doi: 10.1016/j.npep.2009.06.004
29. Nemeroff, CB, and Bissette, G. Neuropeptides, dopamine, and schizophrenia. Ann N Y Acad Sci. (1988) 537:273–91. doi: 10.1111/j.1749-6632.1988.tb42113.x
30. Simrén, J, Leuzy, A, Karikari, TK, Hye, A, Benedet, AL, Lantero-Rodriguez, J, et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer's disease. Alzheimers Dement. (2021) 17:1145–56. doi: 10.1002/alz.12283
31. Wechsler, D. Manual for the Wechsler adult intelligence scale, Revised. New York: Psychological Corporation (1981).
32. Green, R, Till, C, Al-Hakeem, H, Cribbie, R, Téllez-Rojo, MM, Osorio, E, et al. Assessment of neuropsychological performance in Mexico City youth using the Cambridge neuropsychological test automated battery (CANTAB). J Clin Exp Neuropsychol. (2019) 41:246–56. doi: 10.1080/13803395.2018.1529229
33. Chan, MK, Krebs, MO, Cox, D, Guest, PC, Yolken, RH, Rahmoune, H, et al. Development of a blood-based molecular biomarker test for identification of schizophrenia before disease onset. Transl Psychiatry. (2015) 5:e601–1. doi: 10.1038/tp.2015.91
34. Demjaha, A, Morgan, K, Morgan, C, Landau, S, Dean, K, Reichenberg, A, et al. Combining dimensional and categorical representation of psychosis: the way forward for DSM-V and ICD-11? Psychol Med. (2009) 39:1943–55. doi: 10.1017/S0033291709990651
35. Sharma, RP, Janicak, PG, Bissette, G, and Nemeroff, CB. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. Am J Psychiatry. (1997) 154:1019–21. doi: 10.1176/ajp.154.7.1019
36. Widerlöv, E, Lindström, LH, Besev, G, Manberg, PJ, Nemeroff, CB, Breese, GR, et al. Subnormal CSF levels of neurotensin in a subgroup of schizophrenic patients: normalization after neuroleptic treatment. Am J Psychiatry. (1982) 139:1122–6. doi: 10.1176/ajp.139.9.1122
37. Tomita, H, Ziegler, ME, Kim, HB, Evans, SJ, Choudary, PV, Li, JZ, et al. G protein-linked signaling pathways in bipolar and major depressive disorders. Front Genet. (2013) 4:297. doi: 10.3389/fgene.2013.00297
38. Peeters, PJ, Fierens, FL, van den Wyngaert, I, Goehlmann, HW, Swagemakers, SM, Kass, SU, et al. Gene expression profiles highlight adaptive brain mechanisms in corticotropin releasing factor overexpressing mice. Brain Res Mol Brain Res. (2004) 129:135–50. doi: 10.1016/j.molbrainres.2004.06.038
39. Fitzpatrick, K, Winrow, CJ, Gotter, AL, Millstein, J, Arbuzova, J, Brunner, J, et al. Altered sleep and affect in the neurotensin receptor 1 knockout mouse. Sleep. (2012) 35:949–56. doi: 10.5665/sleep.1958
40. Zhu, Y, Womer, FY, Leng, H, Chang, M, Yin, Z, Wei, Y, et al. The relationship between cognitive dysfunction and symptom dimensions across schizophrenia, bipolar disorder, and major depressive disorder. Front Psych. (2019) 10:253. doi: 10.3389/fpsyt.2019.00253
41. Panksepp, J, and Harro, J. Future of neuropeptides in biological psychiatry and emotional psychopharmacology: goals and strategies In ed. Jaak Panksepp. Textbook of biological psychiatry. New York, NY: Wiley-Liss (2004). 627–59.
42. Li, J, Chen, C, Chen, C, He, Q, Li, H, Li, J, et al. Neurotensin receptor 1 gene (NTSR1) polymorphism is associated with working memory. PLoS One. (2011) 6:e17365. doi: 10.1371/journal.pone.0017365
43. Petrie, KA, Schmidt, D, Bubser, M, Fadel, J, Carraway, RE, and Deutch, AY. Neurotensin activates GABAergic interneurons in the prefrontal cortex. J Neurosci. (2005) 25:1629–36. doi: 10.1523/JNEUROSCI.3579-04.2005
44. Robbins, TW, James, M, Owen, AM, Sahakian, BJ, Lawrence, AD, McInnes, L, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge neuropsychological test automated battery. J Int Neuropsychol Soc. (1998) 4:474–90. doi: 10.1017/S1355617798455073
45. Sasayama, D, Hattori, K, Teraishi, T, Hori, H, Ota, M, Yoshida, S, et al. Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophr Res. (2012) 139:201–6. doi: 10.1016/j.schres.2012.06.016
46. Ozsoy, S, Esel, E, and Kula, M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. (2009) 169:249–52. doi: 10.1016/j.psychres.2008.06.034
47. Matsuzaki, M, Matsushita, H, Tomizawa, K, and Matsui, H. Oxytocin: a therapeutic target for mental disorders. J Physiol Sci. (2012) 62:441–4. doi: 10.1007/s12576-012-0232-9
48. Chaudhry, HR, Hofmann, P, Loimer, N, Kotter, M, Quehenberger, F, and Fueger, G. Prolactin and beta-endorphin serum elevations after ECT in manic patients. Acta Psychiatr Scand. (2000) 102:386–9. doi: 10.1034/j.1600-0447.2000.102005386.x
49. Cheng, CH, Yi, PL, Lin, JG, and Chang, FC. Endogenous opiates in the nucleus tractus solitarius mediate electroacupuncture-induced sleep activities in rats. Evid Based Complement Alternat Med. (2011) 2011:159209:1–11. doi: 10.1093/ecam/nep132
50. Tsuchimine, S, Hattori, K, Ota, M, Hidese, S, Teraishi, T, Sasayama, D, et al. Reduced plasma orexin-a levels in patients with bipolar disorder. Neuropsychiatr Dis Treat. (2019) 15:2221–30. doi: 10.2147/NDT.S209023
51. Chen, Q, de Lecea, L, Hu, Z, and Gao, D. The hypocretin/orexin system: an increasingly important role in neuropsychiatry. Med Res Rev. (2015) 35:152–97. doi: 10.1002/med.21326
52. Raue, S, Wedekind, D, Wiltfang, J, and Schmidt, U. The role of proopiomelanocortin and α-melanocyte-stimulating hormone in the metabolic syndrome in psychiatric disorders: a narrative Mini-review. Front Psych. (2019) 10:834. doi: 10.3389/fpsyt.2019.00834
53. Blomeley, C, and Bracci, E. Substance P depolarizes striatal projection neurons and facilitates their glutamatergic inputs. J Physiol. (2008) 586:2143–55. doi: 10.1113/jphysiol.2007.148965
54. Roberts, GW, Ferrier, IN, Lee, Y, Crow, TJ, Johnstone, EC, Owens, DG, et al. Peptides, the limbic lobe and schizophrenia. Brain Res. (1983) 288:199–211. doi: 10.1016/0006-8993(83)90095-1
55. Trépanier, MO, Hopperton, KE, Mizrahi, R, Mechawar, N, and Bazinet, RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. (2016) 21:1009–26. doi: 10.1038/mp.2016.90
56. Hidese, S, Yoshida, F, Ishida, I, Matsuo, J, Hattori, K, and Kunugi, H. Plasma neuropeptide levels in patients with schizophrenia, bipolar disorder, or major depressive disorder and healthy controls: a multiplex immunoassay study. Neuropsychopharmacol Rep. (2023) 43:57–68. doi: 10.1002/npr2.12304
57. Ignjatovic, V, Lai, C, Summerhayes, R, Mathesius, U, Tawfilis, S, Perugini, MA, et al. Age-related differences in plasma proteins: how plasma proteins change from neonates to adults. PLoS One. (2011) 6:e17213. doi: 10.1371/journal.pone.0017213
58. Lin, LC, Lin, HS, and Yang, RC. Neuropeptide Y gene polymorphism and plasma neuropeptide Y level in febrile seizure patients in Taiwan. Kaohsiung J Med Sci. (2007) 23:560–5. doi: 10.1016/S1607-551X(08)70003-2
Keywords: schizophrenia, bipolar disorder, major depressive disorder, neuropeptide, disease monitoring
Citation: Yu H, Ni P, Zhao L, Tian Y, Li M, Li X, Wei W, Wei J, Deng W, Du X, Wang Q, Guo W, Ma X, Coid J and Li T (2023) Decreased plasma neuropeptides in first-episode schizophrenia, bipolar disorder, major depressive disorder: associations with clinical symptoms and cognitive function. Front. Psychiatry. 14:1180720. doi: 10.3389/fpsyt.2023.1180720
Received: 06 March 2023; Accepted: 02 May 2023;
Published: 19 May 2023.
Edited by:
Mary V. Seeman, University of Toronto, CanadaReviewed by:
Changwei Wei, Capital Medical University, ChinaCopyright © 2023 Yu, Ni, Zhao, Tian, Li, Li, Wei, Wei, Deng, Du, Wang, Guo, Ma, Coid and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Li, bGl0YW96anVzY0B6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.