
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 13 July 2023
Sec. Addictive Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1176641
This article is part of the Research Topic Mobile Health Application in Addictive Disorders Therapy View all 8 articles
This study assessed the feasibility and utility of a digital, all-virtual program designed for treatment of methamphetamine use disorder (MUD). Forty-nine adults with moderate- to severe-level MUD (per DSM-5 criteria) commenced the 8-week intervention. All aspects of the program were delivered via smartphone-based app. Intervention components included counseling (cognitive behavioral therapy in group and individual sessions), app-based therapeutic tasks, remote biological drug testing, medical oversight by psychiatrists/nurse practitioners, and contingency management procedures (including rewards for methamphetamine-free saliva drug tests, accomplishing tasks, and engaging in assigned activities). Of the 49 participants who commenced treatment, 27 participants (55%) completed the program. Repeated-measures mixed-model analyses show that participants were more likely to test negative for meth use from week 1 to week 8 (OR = 1.57, 95% CI [1.28, 1.97]; p = 0.034). Well-being and social functioning improved among the majority of participants. These results demonstrate the utility of the all-virtual, digital therapeutic program and its ability to help individuals with MUD to reduce or cease methamphetamine use. The program was efficiently implemented and was well received by participants and clinical personnel, indicating its ability to deliver comprehensive, effective care and to retain the difficult-to-engage population of persons with MUD. Of the 27 completers, 16 responded to a 1-month follow-up survey and reported no meth use in the month since completing the program.
Use of methamphetamine and other illicit stimulants is a major contributor to the extent and severity of the nationwide impacts of substance use disorders (SUDs). Methamphetamine (meth) is increasingly implicated in drug overdose mortality and morbidity across the United States (1), and meth has become prevalent among polydrug users (2, 3). Conservative estimates indicate that more than 1.6 million meth users in the nation are in need of treatment, but fewer than 32% of them receive any formal treatment (4, 5).
Effective treatment for individuals with stimulant use disorder remains an elusive target, and no medication has been approved by the FDA for the treatment of patients with methamphetamine use disorder (MUD). Clinicians rely on behavioral therapies that have only limited effectiveness among the diverse populations of stimulant users, especially for those with MUD. Cognitive behavioral therapy (6), contingency management [CM; (7, 8)], individual/group counseling/motivational interviewing (9, 10), community reinforcement approaches [CRA; (e.g., 11)], and mutual-support groups (12) are the predominant treatment modalities. Most MUD treatment programs include combinations of some or all of these components in different permutations, many based on the Matrix Model (13, 14). Still, no single modality or combination has been consistently effective in retaining individuals with MUD in treatment, partly because traditional models of treatment delivery require frequent in-person attendance at clinical settings, which is a barrier to engagement and can inhibit retention, compromising outcomes.
Retention in care is a particular challenge for MUD treatment programs. A meta-analysis of outcomes across modalities and SUDs from 1965 through 2016 found that an average of 30% of patients did not complete treatment, but meth users dropped out sooner and completed at lower rates than patients with other SUDs (15–17). Recent research using data from the 2019 Treatment Episode Data Set (TEDS) found that only 31.2% of individuals with stimulant use disorder (excluding cocaine) completed traditional outpatient programs (18). Meth use negatively affects retention among patients in treatment for other SUDs (19, 20).
Improvements are necessary in the design and delivery of behavioral therapies that have proven effective for MUD (21). Toward that end, Affect Therapeutics developed and tested a comprehensive, patient-centered, therapeutic program designed specifically for treatment of individuals with MUD. Services are delivered via a HIPAA-compliant app and include several key therapeutic components (CBT, CM, CRA, and group/individual counseling). Digital healthcare using internet-connected devices enables innovative delivery of SUD treatments (22, 23).
Several digital healthcare platforms have shown promise in delivering therapeutic components and in helping patients stay involved in treatment (24, 25), including patients with MUD (26, 27). Among the most broadly applied digital technologies are mobile health applications (apps), which typically operate on a smartphone platform that is ubiquitous, inexpensive, adaptable, and user friendly (28). Apps can efficiently deliver treatment services, including CM (7). CM has been shown to be the most useful modality for retaining patients in outpatient treatment for MUD (29, 30).
This article reports results of a field study that implemented the Affect digital therapeutic program to assess its feasibility and utility for treatment of MUD.
This single-group demonstration study assessed the feasibility and clinical utility of a digital therapeutic program (developed by Affect Therapeutics, Inc.) delivered by HIPAA-compliant smartphone-based app. The app-based program (see details in “Procedures” Intervention below) includes behavioral components—CM, CBT, CRA, group/individual counseling—that are employed in usual care for MUD. Reduction in meth use and cessation of meth use were measured by self-report and remote, video-supervised saliva drug tests twice weekly during the 8-week program. The study protocol was approved by the WCG Institutional Review Board. From the beginning of screening to the end of the intervention period, the study took 6 months.
Primary outcomes were: Retention–measured as program completion—was the primary outcome for establishing the utility and feasibility of the Affect program. Participation–involvement in protocol-specified program activities was measured by participant encounter attendance and interaction with the app during the 8-week intervention (as an additional indicator of feasibility). Effectiveness–preliminary demonstration of effectiveness in terms of reduction or cessation of meth use was measured as intra-participant change in meth use, including duration of days abstinent from meth.
Participants were recruited through multi-media advertising and by word of mouth. Study participant flow is presented in Figure 1. Of the 306 qualified respondents who were remotely screened and assessed for final eligibility, 79 submitted electronically signed consent forms and enrolled. On the intake day required for program involvement, however, 30 individuals did not “attend” the scheduled intake appointment, including assessments and program orientation. Based on the study requirement for enrolled individuals to complete the intake, the 30 who failed to engage were dropped from the study per criteria; thus, the final sample consisted of 49 participants (see Figure 1). Inclusion criteria were: age 18 years or older, able to read and understand English, be an active meth user who met DSM-5 criteria for moderate- to severe-level MUD, affirm an intent to cease meth use, have a smartphone and be capable of using apps, have a mailing address, have medical insurance coverage, and reside in California. Exclusion criteria were: presence of any serious medical or mental health conditions, co-occurring opioid use disorder (moderate to severe level), and involved in any other SUD treatment program. Detailed Inclusion/exclusion criteria appear in Supplemental material/Appendix.
Upon completion of screening and consents, participants were enrolled, were provided the Affect app and orientation (guidance on use of the app and brief refresher on program parameters covered in informed consent), and assigned to a licensed counselor and to a medical provider. Participants were asked to use the app to attend twice-weekly group therapy, once-weekly individual therapy, and one visit (remote) with the medical provider to evaluate overall health and ensure the study did not impose risk to the individual. Additionally, all participants were mailed saliva drug test kits [Medical Disposables (31)] to be self-administered under the supervision of the counselor during the individual therapy sessions each week.
All appointments were managed and accessed via the Affect app and conducted over secure video conference. Individual sessions were 30 min and group counseling sessions were 1 h, all occurring synchronous (live), accessed via the app, and conducted by a licensed addiction counselor. Topics followed a general curriculum derived from the Matrix Model (32). At the conclusion of each appointment, the counselor or medical provider marked attendance in the Affect system and the results of the saliva drug test, if completed. For each appointment marked as attended, the Affect system delivered a small financial reward to the member, who was notified via the app immediately upon delivery. Rewards were redeemable to the participant’s CashApp account. For all negative drug screens, the Affect system calculated the appropriate financial incentive and automatically delivered the reward to the participant via the Affect app. Consecutive streaks of negative drug screens were rewarded with a bonus incentive.
The 8-week Affect program of care for MUD was developed by board-certified addiction psychiatrists and clinicians with extensive expertise in treating patients with MUD and other SUDs. The Affect app delivers all program components, including CM to engage and retain patients while eliciting behavioral change through incentives provided for stimulant-negative results of drug tests and for participation in program activities (e.g., counseling). Participants received app-based prompts for a daily series of behavioral therapeutic tasks and patients engaged with the app and did the incentivized tasks at times convenient to them.
Each day, the Affect app notified the participant of 3–5 new tasks to be completed. These tasks are part of a broader curriculum of content designed to support the participant’s recovery and enable Affect to gather critical participant data necessary to inform care. For example, data collection tasks include basic surveys to gather data regarding daily cravings, substance use, and emotional state. Education tasks include verbal and video content related to the stages of recovery, including topics such as withdrawal management. Motivational tasks include brief verbal or video content coupled with reflection questions, such as reflections on triggers. Skill-building tasks include challenges to promote new, healthy behaviors, such as regular sleep, exercise, and community support group participation.
Program tasks were designed to support behavior change regarding drug avoidance, relapse prevention, prosocial activities, and other lifestyle behaviors that inhibit drug use and enhance life without drugs. Tasks were monitored via the app for satisfactory completion. The timely completion of a task was rewarded at $0.50 each. Behavioral therapy tasks were monitored by the app and by the Care Team for satisfactory completion and verified via direct review of responses in the app (e.g., survey responses). The maximum possible compensation for tasks was $140. Saliva tests were incentivized at a scaled rate (increasing with consecutive tests negative for meth from $10–$30) with $340 maximum possible compensation. Of a total possible compensation of $480 during the intervention period, the average “earned” was <$250.
The app provided a framework to help structure participants’ time in treatment and recovery, offering a user-friendly calendar of upcoming appointments for therapeutic sessions such as group counseling sessions or appointments with psychiatrists and other healthcare providers. Participants engaged in weekly telemedicine sessions with clinical personnel for individual and group therapy.
Meth use was measured by self-report (at intake into the study and twice weekly during the intervention) and by remotely conducted, twice-weekly drug tests during the intervention period using 5-panel saliva-based drug tests [Medical Disposables (31)].
Retention was a binary measure of completion at the end of the 8-week intervention. Participants who withdrew and those who were terminated for unexplained non-response to Affect personnel (or inaction per app-directed activities) for more than seven consecutive days were deemed non-completers.
The Visual Analog Scale (VAS) was used to measure craving for meth (33). Patients were asked to mark their response on a line from “0” for “not at all” to “10” for “extremely high” in response to the following question, “How much do you feel the urge to use meth?” Self-reported craving for meth was measured at baseline (intake), and at least once weekly.
Baseline covariates were collected at study intake. These covariates were used to control the outcomes used in the analyses. The covariates included sociodemographic factors (age, gender, ethnicity/race, employment, sexual orientation, and family structure such as being a parent or currently being involved in a relationship), and substance use severity factors (baseline meth use and meth craving). The sociodemographic and substance use severity factors were collected as part of a battery of baseline measures administered to participants after study enrollment; all participants self-reported current meth use at intake.
Statistical analyses were conducted using SAS 9.4 and STATA 16 software. For all statistical analyses, statistical significance alpha was set at 0.05 using two-tailed hypothesis-testing analyses.
Statistical diagnostics were performed on participant characteristics, including sociodemographic variables. Normality assumptions for continuous variables were examined and tested using the Shapiro–Wilk test for normality. T-tests were used to test for differences in participant characteristics for the continuous variables, which were age and craving score. Chi-square tests were used to test for differences in the categorical variables of gender (males vs. females) and employment (employed vs. not employed), race/ethnicity, sexual orientation, being a parent (yes vs. no), and in a relationship (yes vs. no). Results showed no statistically significant differences in characteristics among participants who finished the 8-week program (N = 27), those who did not complete the program (N = 22), or those who did not “attend” the required intake appointment meeting and were dropped from the study (N = 30; see Table 1).
Comparisons between the 27 completers and 22 non-completers included: number of days in the program, average number of meth-negative drug screens, number of meetings attended, as well as the longest duration of abstinence from meth (consecutive days of no meth use). Normality assumptions for these continuous variables were examined and tested using the Shapiro–Wilk test for normality. Since the distribution of these outcomes was normally distributed, t-test comparisons were made between completers and non-completers.
Meth use was examined in two ways using results from the saliva tests and from self-report. Logistic mixed models analyses were conducted to examine the repeated assessments of meth use using self-reported meth use at study intake (baseline) and twice weekly during the intervention, and by twice-weekly saliva tests from week 1 to week 8. The meth drug test and the self-reports were measured dichotomously (0 = negative meth use, 1 = positive meth use). Next, logistic repeated-measures mixed models were used to examine meth use outcomes (separately for results of twice-weekly saliva tests during the intervention period and of twice-weekly self-reported meth use) at week 1 to week 8 in comparison to meth use at baseline, controlling for age, gender, race/ethnicity, and employment status, in a relationship, being a parent, and sexual orientation. Finally, in order to assess the quality of the statistical models, the Akaike Information Criterion (AIC), Bayesian Information Criterion, and log-likelihoods were examined. The lower the value of the AIC and BIC, the better the model fits the data. We obtained the AIC and BIC for the null model (or sometimes called the unconditional model, which is the model with just the repeated measures and no covariates) and compared this to the final model.
Craving for meth was measured weekly from week 1 to week 8. Linear mixed models were used to assess the repeated measures across 8 weeks controlling for baseline, age, gender, race/ethnicity and employment status, in a relationship, being a parent, and sexual orientation. The craving scores were normally distributed based on the distribution plot and the Shapiro–Wilk test for normality. The quality of the statistical models was also assessed using the Akaike Information Criterion (AIC), Bayesian Information Criterion, and log-likelihood.
Participants who completed the program had a mean age of 44.4 (SD = 9.8) compared with non-completers (mean age = 37.9, SD = 7.9); the dropped individuals who did not engage in the required intake session had a mean age of 41.6 (SD = 12.2). No significant differences were found in terms of age among these three groups (p = 0.09). Almost half of participants were females: 51.9% for completers, 45.5% for non-completers, and 36.7% for the individuals who were dropped due to non-attendance at intake. As shown in Table 1, no significant differences were found in terms of gender (p = 0.78), race/ethnicity (p = 0.86), employment status (p = 0.85), being in a relationship (p = 0.27), being a parent (p = 0.67), or sexual orientation (p = 0.75).
As a preliminary analysis, we examined differences in outcomes between program completers (n = 27) and non-completers (n = 22). The average number of days in the 8-week (56-day) program was 55.1 days (SD = 1.61) for completers compared with 21.9 days for non-completers (t = 12.3, p < 0.001). Significant and more favorable differences were also found between completers vs. non-completers in terms of: (1) the average number of negative drug screens with mean = 5.19, SD = 5.9 for completers vs. a mean = 0.33, SD = 0.79 for non-completers, (t = 3.73, p = 0.003), (2) the average number of meetings attended by completers (mean = 35.3, SD = 11.6) vs. non-completers (mean = 6.86, SD = 3.73), (t = 11.01, p < 0.001), and (3) the longest duration of no meth use (consecutive days) for completers (mean = 10.9 days, SD = 8.4) vs. non-completers (mean = 4.5 days, SD = 1.73), =3.51, p = 0.001 (see Table 2). As shown in Table 2, no significant differences were found in terms of age (p = 0.08), gender (p = 0.72), race/ethnicity (p = 0.54), employment status (p = 0.76), in relationship (p = 0.19), being a parent (p = 0.70), or sexual orientation (p = 0.51).
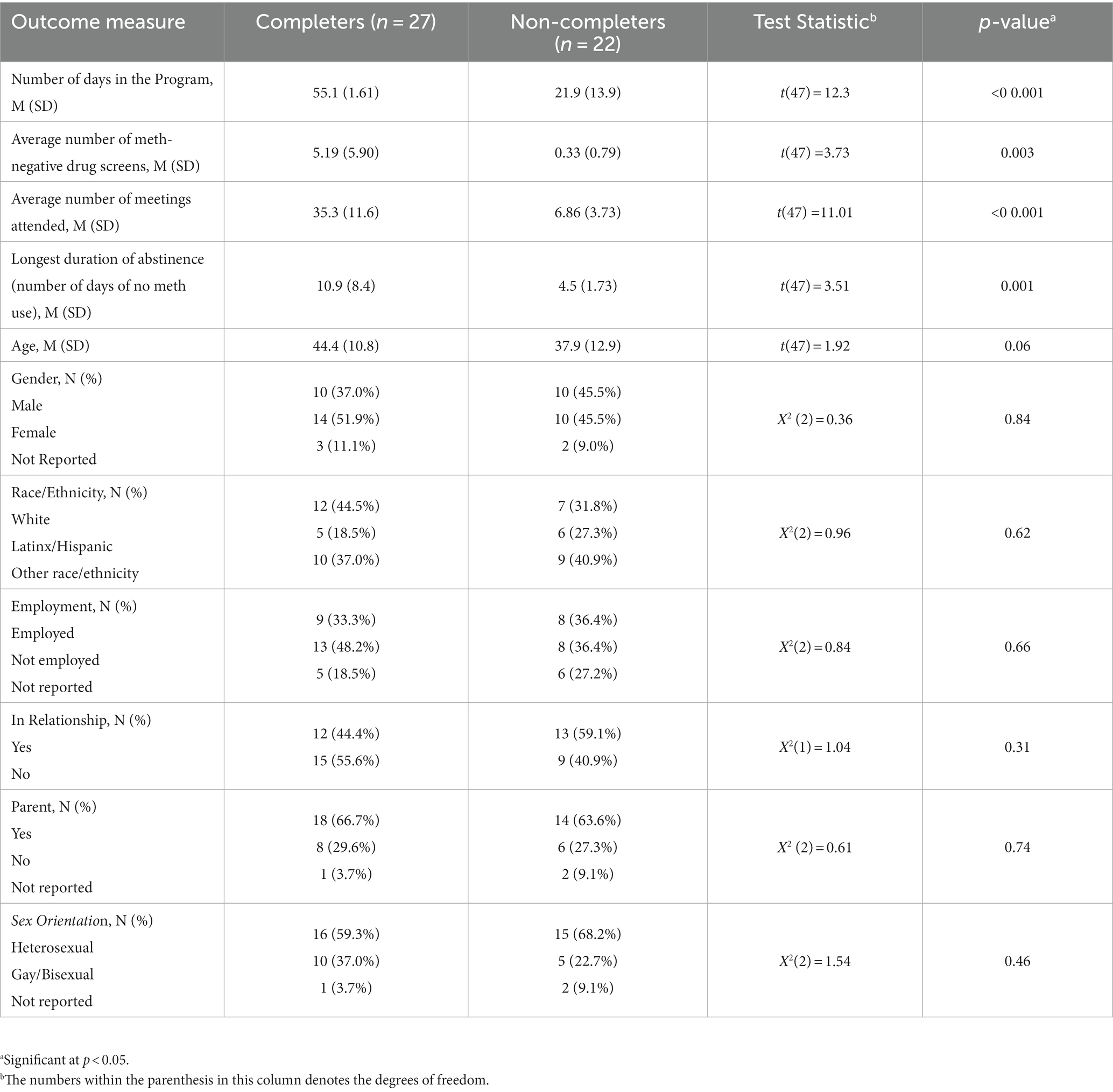
Table 2. Comparison of outcomes between participants who completed the program vs. participants who did not complete the program (N = 49).
Preliminary analyses were performed to examine the percentage differences in meth-negative test results among participants at baseline reference (i.e., initial saliva test), week 1 to week 8. The meth use test results (binary outcome, where 1 = negative for meth use, 0 = positive for meth use) were examined using logistic repeated-measures mixed-model analyses, where we controlled for age, gender, race/ethnicity and employment, in a relationship, being a parent, and sexual orientation. Results indicate that participants were more likely to test negative for meth use at week 1 to week 8 (Odds ratio = 1.57, 95% CI [1.25, 1.97]; p = 0.049) relative to the baseline reference (see Table 3). No significant results were found for age, gender, employment status, in a relationship, being a parent and sexual orientation. A Kaplan-Meir survival plot of the meth-positive saliva screens by week among completers is shown in Figure 2. In the survival plot, the failure parameter is the first positive occurrence for meth after starting in the program.
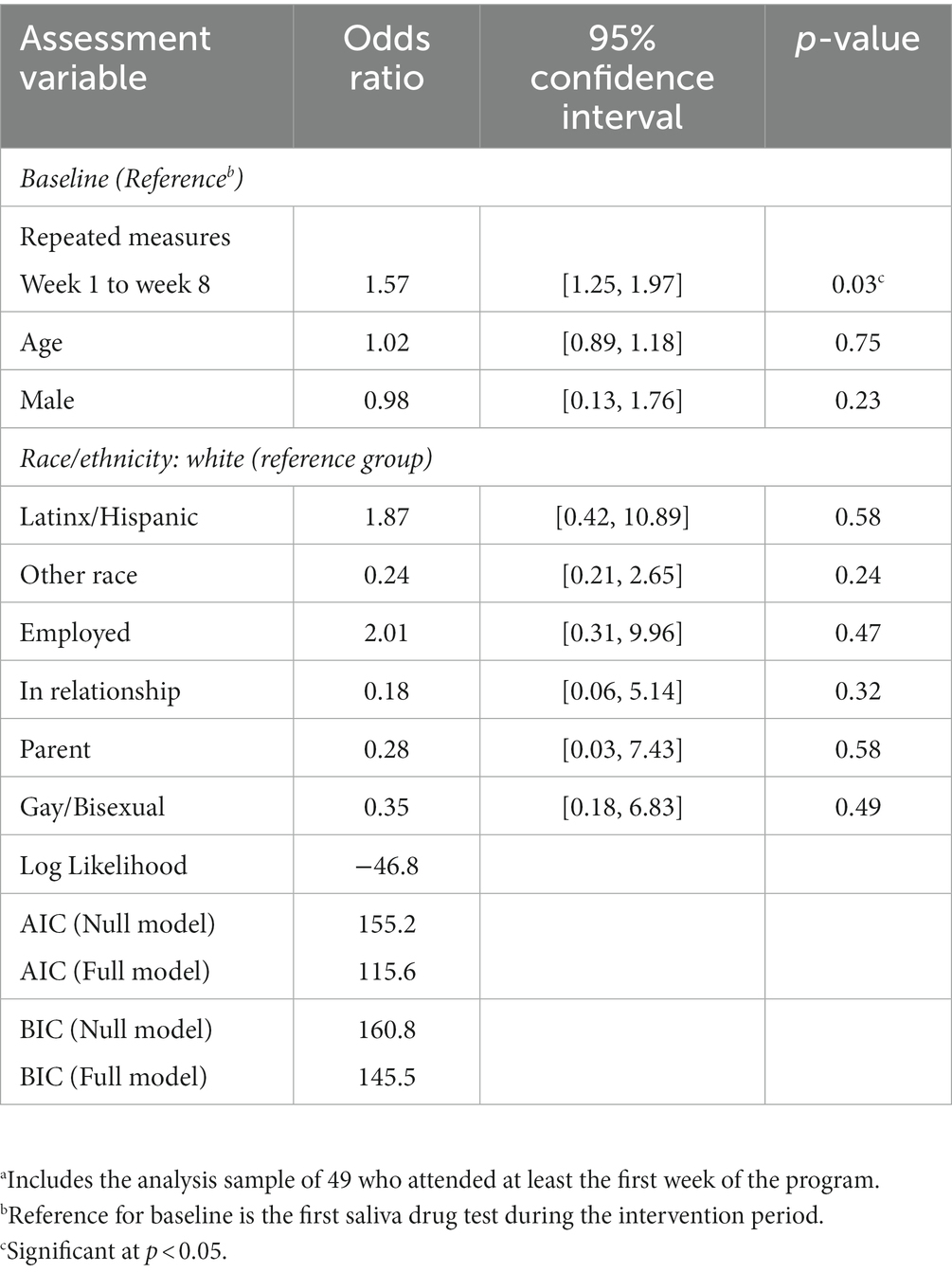
Table 3. Repeated measures meth-negative drug test results (via twice-weekly saliva tests, N = 49)a.
Preliminary analyses were performed to examine the percentage differences in self-reported negative meth use among participants at baseline (at intake, preceding intervention period), week 1 to week 8. Self-reported meth use (binary outcome, where 1 = negative for meth use, 0 = positive meth use) was analyzed using logistic repeated-measures mixed models, where we controlled for age, gender, race/ethnicity and employment, in relationship, being a parent and sexual orientation.
Results indicate that participants were increasingly likely to report no meth use from week 1 to week 8 (Odds ratio = 1.69, 95% CI [1.29, 2.09]; p = 0.024) relative to the baseline reference (at intake) (see Table 4). No significant results were found for age, gender, and employment status, in a relationship, being a parent and sexual orientation.
Preliminary analyses were performed to examine self-reported craving ratings among participants at baseline, week 1 to week 8. At baseline, participants reported an average craving score of 3.69, SD = 1.89. Using linear mixed models repeated measures analyses, results show that the change in craving scores was not significantly different between week 1 to week 8 (t = 1.732, 95% CI [−1.78, 5.24]; p = 0.26) compared to the baseline craving score. No significant results were found for age, gender, and employment status, in a relationship, being a parent and sexual orientation. See Table 5.
This demonstration study occurred during the first 6 months of 2021 when traditional in-clinic SUD treatment was still constrained by COVID pandemic restrictions and limited vaccination. The all-virtual nature of the program eliminated the requirement for patients to frequently appear in person in a clinic setting, which can inhibit initial engagement of individuals with MUD and also imposes a burden that can reduce long-term retention in treatment. That flexibility may have been a factor in the intense interest in the study; 769 persons responded to study announcements, and 306 were screened as potentially eligible candidates. Beyond the planned 48-person sample, we enrolled a total of 79 eligible individuals, all with moderate/severe-level MUD. Although 30 did not follow through with intake and thus never commenced the intervention, there were no significant differences between them and the 49 in the analysis sample. Below we discuss the results in context of the main outcomes.
Consistent with accepted approaches to measure retention in SUD treatment [(e.g., 34, 35)], the outcome was based on completion as a binary measure at the end of the 8-week intervention. Termination or unexplained non-response to Affect personnel (or app-directed activities) for more than seven consecutive days in any period of the 8-week study period equated to “non-completer” status for the purpose of determining retention. The Lappan study (15) reported that the average dropout rate among patients with MUD in all forms of treatment from 1965 to 2016 was 53.5%, inferring a 46.5% completion rate. More recent data from the 2019 Treatment Episode Data Set [TEDS; SAMHSA (18)] show that only 31.2% of individuals were retained in (completed) outpatient programs for stimulant use disorder (other than cocaine), indicating a nearly 68.8% dropout rate. The Affect program completion rate was 55.1%, comparing favorably to 31.2% completion of outpatient programs for MUD shown in the 2019 TEDS data, indicating the Affect dropout rate of 44.9% versus the 68.8% rate in TEDS. The CM component is a strong factor in retention, which is consistent with findings of other literature on MUD programs [(e.g., 29, 36–38)]. Our results confirm the feasibility of the Affect program’s remote management of CM procedures, which were conducted via app, including the delivery of rewards for completion of tasks and activities.
In addition to retention (treatment completion vs. dropout/withdrawal), participation was a construct composed of the number of “attended” therapeutic tasks/activities per the Affect app relative to not-attended events such as: scheduled/planned virtual interactions for program activities (e.g., counseling, group meetings), completed remote drug tests, and kept appointments with referred service providers (e.g., psychiatrists, physicians). Participants who consistently engaged in program activities were more likely to complete the program and reduce meth use according to self-report and drug tests; the 27 completers attended an average of 35 meetings compared to less than 7 attended by non-completers. Overall, more than 41% of all activities and tasks were attended, 8% were not attended due to cause or cancelation, and more than 49% were not attended.
The effectiveness measure is a composite of self-reported meth use (daily Yes/No) and meth-negative saliva test results obtained twice weekly over the course of the program. A participant who provided at least four consecutive meth-negative saliva tests during the final 4 weeks of the 8-week program met criteria for positive reduction in meth use at a significant level, indicating within-participant effectiveness. Reductions in saliva test meth use across the analysis sample were statistically significant. These findings were consistent with self-reported meth abstinence with a Kappa test of concordance statistic of 0.78.
Analyses could not detect significant differences in craving trajectories, but subgroups appeared to have erratic patterns in which early-phase high craving scores were not associated with relapse to use of meth nor with dropout. Notably, participants with higher craving appear to stay in the program longer. The apparent paradox that high craving did not associate with relapse to meth is intriguing but statistically unsubstantiated.
At exit interviews, 12 participants expressed interest in continuing in the Affect program and were offered the opportunity to remain for another 8 weeks but without the CM component. All 12 were retained without CM in the post-study program, which suggests the program’s viability and acceptability to people with MUD. The commercialization of the Affect program for MUD is being explored in a NIDA-funded Small Business Innovation Research project.
General well-being was measured by end-of-treatment administration of the Treatment Effectiveness Assessment (39) using a 1-to-10 scale of responses to questions in four domains related to recovery. Thirty-nine percent of respondents rated their improvement in the drug use domain at a “10” (much better) and 52% rated their improvement in overall health at a “10”.
Preliminary Results of Follow-ups. Of the 27 completers, 16 responded to 1-month follow-up surveys; ~56% of respondents self-reported no meth use in the month since program completion.
The results of the study indicate that in this sample of individuals with MUD, the Affect digital therapeutic program for MUD was feasible to administer, was acceptable to participants, was effective in retaining participants in the program, and helped the majority of program participants to significantly reduce meth use. To thoroughly demonstrate effectiveness of the Affect program for MUD, a larger scale test would compare the Affect program against a research-tested MUD care program [e.g., the Matrix Model; (17)]. Results of this study may be useful to inform the development of and substantiate the practice of dHealth/mHealth-assisted treatment for MUD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The Study involving human participants was reviewed and approved by Western Institutional Review Board (WCG). The patients/participants provided their written informed consent to participate in this study.
KM contributed to planning, conceptualizing, and writing the manuscript. JD helped in conceptualizing and reviewing the manuscript. AA performed the data analysis and helped to write the manuscript. MZ provided comments and reviewed the manuscript. AM assisted in conceptualizing and reviewing the manuscript. BP contributed to conceptualizing and writing the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by Affect Therapeutics, Inc.
The authors would like to thank the staff at Affect Therapeutics and all participants who participated in this study. The authors recognize the valuable work they engage in to provide helpful insights in MUD treatment services.
KM and JD were employed by Affect Therapeutics, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1176641/full#supplementary-material
1. Mattson, CL, Tanz, LJ, Quinn, K, Kariisa, M, Patel, P, and Davis, NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths — United States, 2013–2019. MMWR Morb Mortal Wkly Rep. (2021) 70:202–7. doi: 10.15585/mmwr.mm7006a4externalicon
2. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on drug use and health. (2019). (HHS publication no. PEP19-5068, NSDUH series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf (accessed February 2022).
3. Twillman, RK, Dawson, E, LaRue, L, Guevara, MG, Whitley, P, and Huskey, A. Evaluation of trends of near-real-time urine drug test results for methamphetamine, cocaine, heroin, and fentanyl. JAMA Netw Open. (2020) 3:e1918514. doi: 10.1001/jamanetworkopen.2019.18514
4. LaRue, L, Twillman, RK, Dawson, E, Whitley, P, Frasco, MA, Huskey, A, et al. Rate of fentanyl positivity among urine drug test results positive for cocaine or methamphetamine. JAMA Netw Open. (2019) 2:e192851. doi: 10.1001/jamanetworkopen.2019.2851
5. Jones, CM, Compton, WM, and Mustaquim, D. Patterns and characteristics of methamphetamine use among adults—United States, 2015–2018. MMWR Morb Mortal Wkly Rep. (2020) 69:317–3. doi: 10.15585/mmwr.mm6912a1
6. Rawson, RA, Rataemane, S, Rataemane, L, Ntlhe, N, Fox, RS, McCuller, J, et al. Dissemination and implementation of cognitive behavioral therapy for stimulant dependence: a randomized trial comparison of 3 approaches. Subst Abus. (2013) 34:108–7. doi: 10.1080/08897077.2012.691445
7. McPherson, SM, Burduli, E, Smith, CL, Herron, J, Oluwoye, O, Hirchak, K, et al. A review of contingency management for the treatment of substance-use disorders: adaptation for underserved populations, use of experimental technologies, and personalized optimization strategies. Subst Abuse Rehabil. (2018) 9:43–57. doi: 10.2147/SAR.S138439
8. Roll, JM, Chudzynski, J, Cameron, JM, Howell, DN, and McPherson, S. Duration effects in contingency management treatment of methamphetamine disorders. Addict Behav. (2013) 38:2455–62. doi: 10.1016/j.addbeh.2013.03.018
9. AshaRani, PV, Hombali, A, Seow, E, Ong, WJ, Tan, JH, and Subramaniam, M. Non-pharmacological interventions for methamphetamine use disorder: a systematic review. Drug Alcohol Depend. (2020) 212:108060. doi: 10.1016/j.drugalcdep.2020.108060
10. Parsons, JT, John, SA, Millar, BM, and Starks, TJ. Testing the efficacy of combined motivational interviewing and cognitive behavioral skills training to reduce methamphetamine use and improve HIV medication adherence among HIV-positive gay and bisexual men. AIDS Behav. (2018) 22:2674–86. doi: 10.1007/s10461-018-2086-5
11. Roozen, HG, Boulogne, JJ, van Tulder, MW, van den Brink, W, De Jong, CA, and Kerkhof, AJ. A systematic review of the effectiveness of the community reinforcement approach in alcohol, cocaine and opioid addiction. Drug Alcohol Depend. (2004) 74:1–13. doi: 10.1016/j.drugalcdep.2003.12.006
12. Humphreys, K, Barreto, NB, Alessi, SM, Carroll, KM, Crits-Christoph, P, Donovan, DM, et al. Impact of 12 step mutual help groups on drug use disorder patients across six clinical trials. Drug Alcohol Depend. (2020) 215:108213. doi: 10.1016/j.drugalcdep.2020.108213
13. Rawson, RA, Marinelli-Casey, P, Anglin, MD, Dickow, A, Frazier, Y, Gallagher, C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. (2004) 99:708–7. doi: 10.1111/j.1360-0443.2004.00707.x
14. Rawson, RA, Gonzales, R, Greenwell, L, and Chalk, M. Process-of-care measures as predictors of client outcome among a methamphetamine-dependent sample at 12- and 36-month follow-ups. J Psychoactive Drugs. (2012) 44:342–9. doi: 10.1080/02791072.2012.718653
15. Lappan, S, Brown, A, and Hendricks, P. Dropout rates of in-person psychosocial substance use disorder treatments: a systematic review and meta-analysis. Addiction. (2020) 115:201–7. doi: 10.1111/add.14793
16. Maglione, M, Chao, B, and Anglin, MD. Correlates of outpatient drug treatment drop-out among methamphetamine users. J Psychoactive Drugs. (2000) 32:221–8. doi: 10.1080/02791072.2000.10400232
17. Rawson, RA, McCann, MJ, Flammino, F, Shoptaw, S, Miotto, K, Reiber, C, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. (2006) 101:267–4. doi: 10.1111/j.1360-0443.2006.01312.x
18. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment episode data set (TEDS): 2019. Admissions to and discharges from publicly funded substance use treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration (2021).
19. Brecht, ML, Greenwell, L, and Anglin, MD. Methamphetamine treatment: trends and predictors of retention and completion in a large state treatment system (1992-2002). J Subst Abus Treat. (2005) 29:295–6. doi: 10.1016/j.jsat.2005.08.012.
20. Wang, L, Min, JE, Krebs, E, Evans, E, Huang, D, Liu, L, et al. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy. (2017) 49:32–40. doi: 10.1016/j.drugpo.2017.07.009
21. De Crescenzo, F, Ciabattini, M, D'Alò, GL, De Giorgi, R, Del Giovane, C, Cassar, C, et al. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med. (2018) 15:e1002715. doi: 10.1371/journal.pmed.1002715
22. Muench, F, van Stolk-Cooke, K, Kuerbis, A, Stadler, G, Baumel, A, Shao, S, et al. A randomized controlled pilot trial of different Mobile messaging interventions for problem drinking compared to weekly drink tracking. PLoS One. (2017) 12:e0167900. doi: 10.1371/journal.pone.0167900
23. Zhang, L, Li, N, Li, Y, Zhang, T, Li, D, Liu, Y, et al. Preliminary efficacy of a digital therapeutics smartphone application for methamphetamine use disorder: an experimental study. Front Psychol. (2022) 13:1027695. doi: 10.3389/fpsyt.2022.1027695.
24. Rubenis, AJ, Baker, AL, and Arunogiri, S. Methamphetamine use and technology-mediated psychosocial interventions: a mini-review. Addict Behav. (2021) 121:106881. doi: 10.1016/j.addbeh.2021.106881
25. Bergman, BG, and Kelly, JF. Online digital recovery support services: an overview of the science and their potential to help individuals with substance use disorder during COVID-19 and beyond. J Subst Abus Treat. (2021) 120:108152. doi: 10.1016/j.jsat.2020.108152
26. Reback, CJ, Fletcher, JB, Swendeman, DA, and Metzner, M. Theory-based text-messaging to reduce methamphetamine use and HIV sexual risk behaviors among men who have sex with men: automated unidirectional delivery outperforms bidirectional peer interactive delivery. AIDS Behav. (2019) 23:37–47. doi: 10.1007/s10461-018-2225-z
27. Reback, CJ, Fletcher, JB, and Mata, RP. A theory-based mHealth intervention (getting off) for methamphetamine-using men who have sex with men: protocol for a randomized controlled trial. JMIR Res Protoc. (2021) 10:e22572. doi: 10.2196/22572
28. Sweeney, MM, Holtyn, AF, Stitzer, ML, and Gastfriend, DR. Practical technology for expanding and improving substance use disorder treatment: telehealth, remote monitoring, and digital health interventions. Psychiatr Clin North Am. (2022) 45:515–8. doi: 10.1016/j.psc.2022.05.006
29. Roll, J. Contingency management: an evidence-based component of methamphetamine use disorder treatments. R Addiction. (2007) 102:114–10. doi: 10.1111/j.1360-0443.2006.01774.x
30. Brown, HD, and DeFulio, A. Contingency management for the treatment of methamphetamine use disorder: a systematic review. Drug Alcohol Depend. (2020) 216:108307. doi: 10.1016/j.drugalcdep.2020.108307
31. Medical Disposables. Saliva drug test: mouth swab tests technologies (2021). Available at: https://www.medicaldisposables.us/oral-saliva-drug-test-mouth-swab-thc-s/1824.htm (Accessed May 2022).
32. CSAT Center for Substance Abuse Treatment. Counselor's treatment manual: Matrix intensive outpatient treatment for people with stimulant use disorders. DHHS publication no. (SMA) 06–4152. Rockville, MD: Substance Abuse and Mental Health Services Administration (2006).
33. Nakama, H, Chang, L, Cloak, C, Jiang, C, Alicata, D, and Haning, W. Association between psychiatric symptoms and craving in methamphetamine users. Am. J. Addict. (2008) 17:441–6. doi: 10.1080/10550490802268462
34. Hser, YI, Evans, E, Huang, D, and Anglin, DM. Relationship between drug treatment services, retention, and outcomes. Psychiatr Serv. (2004) 55:767–4. doi: 10.1176/appi.ps.55.7.767
35. McLellan, AT. What we need is a system: creating a responsive and effective substance abuse treatment system In: WR Miller and KM Carroll, editors. Rethinking substance abuse: What the science shows, and what we should do about it. New York: The Guilford Press (2006)
36. Petry, NM, Peirce, JM, Stitzer, ML, Blaine, J, Roll, JM, Cohen, A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a National Drug Abuse Treatment Clinical Trials Network Study. Arch Gen Psychiatry. (2005) 62:1148–56. doi: 10.1001/archpsyc.62.10.1148
37. Jiang, H, Du, J, Wu, F, Wang, Z, Fan, S, Li, Z, et al. Efficacy of contingency management in improving retention and compliance to methadone maintenance treatment: a random controlled study. Shanghai Arch Psychiatry. (2012) 24:11–9. doi: 10.3969/j.issn.1002-0829.2012.01.002
38. Miguel, AQ, Madruga, CS, Cogo-Moreira, H, Yamauchi, R, Simões, V, da Silva, CJ, et al. Contingency management is effective in promoting abstinence and retention in treatment among crack cocaine users in Brazil: a randomized controlled trial. Psychol Addict Behav. (2016) 30:536–3. doi: 10.1037/adb0000192
Keywords: methamphetamine, digital health, app, craving, outcomes, contingency management, substance use disorder
Citation: Muhlner K, DeFlavio J, Ang A, Zito M, McLellan AT and Perrochet B (2023) Demonstration of an app-delivered digital therapeutic program for methamphetamine use disorder. Front. Psychiatry. 14:1176641. doi: 10.3389/fpsyt.2023.1176641
Received: 28 February 2023; Accepted: 27 June 2023;
Published: 13 July 2023.
Edited by:
Saeideh Valizadeh-Haghi, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Eric Andrew Woodcock, Wayne State University, United StatesCopyright © 2023 Muhlner, DeFlavio, Ang, Zito, McLellan and Perrochet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristin Muhlner, a3Jpc3RpbkBhZmZlY3R0aGVyYXBldXRpY3MuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.