
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 07 September 2023
Sec. Addictive Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1167283
This article is part of the Research TopicCommunity Series in Neurobiological Biomarkers for Developing Novel Treatments of Substance and Non-Substance Addiction, volume IIIView all 5 articles
Objectives: Cue-reactivity is a critical step leading to the emergence of addictive psychology and the triggering of addictive behaviors within the framework of addiction theory and is considered a significant risk factor for addiction-related behaviors. However, the effect of cue-reactivity targeted smoking cessation intervention and the cue-reactivity paradigms used in the randomized controlled trials varies, which introduces more heterogeneity and makes a side-by-side comparison of cessation responses difficult. Therefore, the scoping review aims to integrate existing research and identify evidence gaps.
Methods: We searched databases in English (PubMed and Embase) and Chinese (CNKI and Wanfang) using terms synonymous with ‘cue’ and ‘tobacco use disorder (TUD)’ to April 2023, and via hand-searching and reference screening of included studies. Studies were included if they were randomized controlled trials taking cue-reactivity as an indicator for tobacco use disorder (TUD) defined by different kinds of criteria.
Results: Data were extracted on each study’s country, population, methods, timeframes, outcomes, cue-reactivity paradigms, and so on. Of the 2,944 literature were retrieved, 201 studies met the criteria and were selected for full-text screening. Finally, 67 pieces of literature were selected for inclusion and data extraction. The results mainly revealed that non-invasive brain stimulation and exercise therapy showed a trend of greater possibility in reducing subjective craving compared to the remaining therapies, despite variations in the number of research studies conducted in each category. And cue-reactivity paradigms vary in materials and mainly fall into two main categories: behaviorally induced craving paradigm or visually induced craving paradigm.
Conclusion: The current studies are still inadequate in terms of comparability due to their heterogeneity, cue-reactivity can be conducted in the future by constructing a standard library of smoking cue materials. Causal analysis is suggested in order to adequately screen for causes of addiction persistence, and further explore the specific objective cue-reactivity-related indicators of TUD.
“Substance addiction (or drug addiction) is a neuropsychiatric disorder characterized by a recurring desire to continue taking the drug despite harmful consequences.” (1), with tobacco being the most common and well-known addictive substance with a high risk of abuse (2). The World Health Organization’s Eighth Report on the Global Tobacco Epidemic (2021) pointed out that by 2019, the number of smokers over the age of 15 worldwide exceeded 1 billion, and the smoking rate reached 17.5%. Tobacco-induced diseases, such as lung cancer and diabetes, pose a significant threat to human health, causing 8 million yearly deaths worldwide (3).
Recent studies have shown that cue-induced cravings are crucial to address analyzing the physiological and neural processes that make it difficult to tobacco cessation implementation (4–6). And many studies found that cue-targeted interventions are effective in improving cessation outcomes (7–9). However, in regard to randomized controlled trials (RCTs) on tobacco use disorder (TUD), there are still unclearly and incompletely known (1) how many kinds of cue-reactivity targeted cessation interventions, (2) what effects these kinds of interventions have on cue-reactivity, and (3) what are the classification and content of smoking cue-reactivity paradigms. Therefore, an intimate understanding of the above issues will help to review the components of the smoking cessation intervention trials and provide insight into the reasons for trial heterogeneity.
Cue reactivity (CR) is a crucial characteristic of addiction (10). It is referred to “a phenomenon in which exposure to substance cues produces a range of physiological (e.g., alterations in heart rate, respiration, and temperature) and psychological (e.g., substance-related expectations and substance-relevant cognitive biases) responses, which motivates the individual to seek out and administer substances.” (11). In addition, CR is an essential factor in the onset of cravings (12) and may also be an effective predictor of relapse (13). Cue-reactivity in individuals with TUD is associated with tobacco relapse or persistent cessation (14–17). Individuals with TUD after abstinence could potentially relapse due to cravings triggered by re-exposure to smoking situations (18, 19). These situations are not limited to the actual smoking environment of tobacco, tobacco smells, tobacco images, and other scenes of smoking may also trigger a relapse (20, 21).
With advances in research methodology of quantitative cognitive science, an increasing number of researchers are exploring the relationship between measures of addictive behaviors (e.g., self-reported craving, efficacy assessments of tobacco cessation, prediction of relapse and so on) and neuroimaging biomarkers, for example, the activity of specific brain regions (e.g., insula and extended visual system) under cued responses may reflect addictive behaviors to some extent (22, 23) and may serve as the underlying neural basis for cued responses (24). Although functional magnetic resonance imaging (fMRI) brain responses are multiregional (23), and the electroencephalography (EEG) indicators (e.g., P300 and alpha power) from different types of smokers (e.g., early-onset and late-onset smokers) also vary (25–29), these findings can provide benchmarks as the theoretical tools for assessing smoking and formulating as well as improving personal tobacco cessation plans.
Common indicators based on cue-reactivity assessment can be divided into three categories: psychological, physiological and neuroimaging indicators.
Psychological indicators could be subdivided into subjective and objective components, including subjective craving and impulsiveness, objective response inhibition, approach bias and attentional bias (30–38). Most studies have shown that smokers have increased subjective craving (30–32) and impulsiveness (33), as well as decreased inhibitory control (34, 35) and have selective approach bias (36) and attentional bias (37, 38) when exposed to smoking-related cues (SRC) compared to non-smokers.
Physiological indicators mainly include heart rate (HR), blood pressure (BP), sweat gland activity, skin temperature (ST), and skin conductance (SC). Carter et al. (12) found that HR [effect size (ES): d = 0.21] and sweat gland activity (ES: d = 0.44) increased in smokers compared to non-smokers in response to SRC, while ST (ES: d = 0.07) did not show statistically a significant difference between groups in most research among meta-analyzes. Betts et al. found (10) SC (ES: Hedges’ g = 0.19) had significant cue effects and non-significant physiological outcomes included HR, BP, electromyogram, salivation, ST, and startle reflex across studies. Therefore, the above suggests that these physiology-based studies have relatively small effects or no effects.
Studies of brain function primarily include fMRI and EEG indicators used to represent neural responses to SRC. A series of fMRI-based cue response studies found that smokers showed some activation or inhibition in various brain regions during SRC stimuli had been conducted and that there were correlations between specific brain networks, such as the mesolimbic system, medial prefrontal cortex (mPFC), insula, default mode network and salience network (22, 39–41). Engelmann et al. (23) found that smoking cues elicit larger fMRI responses than neutral cues in the extended visual system, precuneus, posterior cingulate gyrus, anterior cingulate gyrus, dorsal and mPFC, insula, and dorsal striatum. EEG-based cue-reactivity studies have shown that smokers exhibit specific changes in the EEG frequency band or event-related potential (ERP) component in response to SRC stimuli, such as the EEG power spectrum showing a significant increase in the alpha band or low-theta band coherence (25, 26). For ERP, the characteristic component is mainly P300. Compared to late smokers (age ≥ 16 years), early smokers (age < 16 years) have more robust P300 responses to smoking-related stimuli (27), and subjective craving is associated with a more substantial P300 amplitude, the higher impulsivity, the higher P300 amplitude (7, 27, 32). Another component is the late positive potential (LPP) of the ERP, which shows a greater LPP in response to smoking-related stimuli (28). The LPP induced by cigarette-related cues in a light smoke group that does not require a long smoking history can produce significant individual differences (29).
Since 1980, cue-reactivity-targeted indicators have been increasingly used to assess the effects of interventions for individuals with TUD (42, 43). The development of indicators based on cue-reactivity paradigms combined with pharmacology, neuroimaging is still an important focus in this field (4, 44). There are significant differences in the efficacy across tobacco cessation intervention studies. However, an overall comprehensive summary of the effects of various treatments and the cue-reactivity paradigms used in RCTs of cue-reactivity-targeted tobacco cessation interventions are still lacking. In this article, we present a scoping review, as being a precursor to systematic reviews, to explore more consistent findings and gaps in current research, to provide a rationale for the development of cue-reactivity-based valuation system for diagnosis and therapy.
The scoping review was conducted according to Arksey and O’Malley’s framework: (1) identifying the research questions; (2) identifying studies; (3) selecting studies; (4) charting the data; and (5) collating, summarizing and reporting the results.
This study specifies the questions for the scoping review: (1) what are the RCTs and the effects of cue-reactivity-targeted tobacco cessation interventions on TUD; (2) outline the cue-reactivity paradigms applied to tobacco cessation interventions on TUD.
The search was conducted by combining subject terms and free words, using the Chinese search terms “smoking, cigarette addiction, tobacco addiction, cue” in the China National Knowledge Infrastructure (CNKI) and WanFang databases, the English search terms “tobacco, nicotine, cue” in the PubMed and EMBase databases. The main search of the database was performed in July 2021, the last update was in May 2022, and the final search of all databases was performed in April 2023. The specific search strategies for the four databases are described in Supplementary Table S1.
1. The study population were individuals with TUD (comprehensively defined through every included literature which mentioned that their research subjects were cigarette or tobacco smokers who had certain score in FagerstrÖm Test for Nicotine Dependence (FTND) or met the criteria of whatever DSM-IV or − 5, or ICD-10, or not gave the detail diagnosis but just reported that the subjects were “nicotine dependence,” or “dependence smokers” or similar terms. See Supplementary Table S2 for detail);
2. The study design was an RCT;
3. The research topic was cue-reactivity as an indicator to evaluate the effects of smoking cessation.
1. Literature other than English or Chinese;
2. Literature for which the full text cannot be obtained;
3. Literature with repeated publications;
4. Literature conducted only on animals or healthy subjects;
5. Literature recruiting subjects with multi-substance use disorder (e.g., cocaine, marijuana, heroin, methamphetamine, alcohol. See Supplementary Table S2 for detail) and/or related physical or mental illnesses (e.g., infectious diseases, cancer, schizophrenia. See Supplementary Table S2 for detail which also concludes the exclusion criteria of medication in each literature);
6. Literature that does not report outcomes in smokers exposed to tobacco-related cues;
7. Comment, research protocols, books or other non-scientific publications, case reports and conference abstracts.
After entering the retrieved literature titles into Endnote X9 for deduplication, a two-step review strategy was adopted: (1) title/abstract level; (2) full-text level. The two authors performed independent screening exercises. Disagreements between two authors (Luo and Gan) that emerged during the literature selection process would be discussed or consulted with a third author for consensus. Data for final inclusion in the literature were extracted and summarized in standardized tables. First author, time of publication, study sample and context, stimulus material, cue-reactivity paradigms, type of intervention, follow-up time, outcome measures and effects were extracted and recorded. The quality of evidence for each study and a formal risk of bias was not assessed. The data were aggregated and reported according to key themes.
In terms of outcome measures, we mainly focused on whether the difference between the treatment and control groups was statistically significant and whether the corresponding effect size was explicitly calculated in the included articles. When inter-group differences do not reach a significant level, we marked them as “NS” (not significant) and the corresponding effect size (ES) would not be shown in the tables, while when the inter-group differences reach a significant level, we would using the up or down arrow to show the change of the treatment compared to control group (s) and the corresponding ES would be shown in the tables. However, if the included articles do not report the ES, we would mark them as “NES” (no effect size). It is notable that a few articles only report the results of intra-group statistics, and in this case, we would provide descriptive comparison results of intra-group statistics.
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Our research of PubMed and Embase databases in English, as well as CNKI and Wanfang search in Chinese, identified 2,911 possible records. After culling duplicates and checking abstracts and full-text records were confirmed. Finally, 67 records were included in the following analysis. The PRISMA flowchart is given in Figure 1.
The results of the current scoping review identified 67 RCTs covering tobacco cessation therapy, 28 articles of pharmacotherapy, 9 articles of physiotherapy, 11 articles of psychotherapy, 6 articles of exercise therapy and 13 articles of other therapies (primarily combination therapy), respectively. More than half of the included studies were conducted in the United States (one of them is from a multicentre study; n = 42) (8, 9, 45–84), while a minority were conducted in the United Kingdom (n = 6) (85–90), Canada (n = 6) (91–96), China (n = 4) (7, 97–99), Brazil (n = 2) (100, 101), Israel (one of them is from a multicentre study mentioned above; n = 2) (9, 102), Netherlands (n = 2) (103, 104), Chile (n = 1) (105), Korea South (n = 1) (106), Germany (n = 1) (107), and France (n = 1) (108). Basic information from the included literature is shown in Tables 1–5.
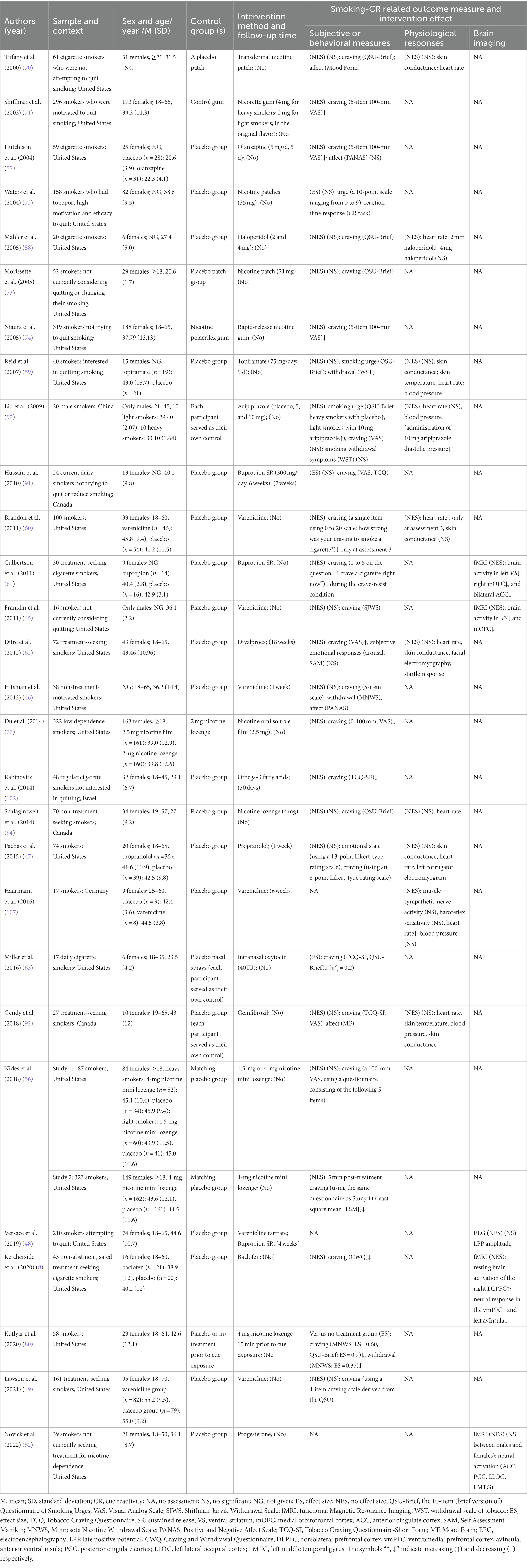
Table 1. Details of 28 included studies that looked at pharmacotherapy that modulates cue reactivity.
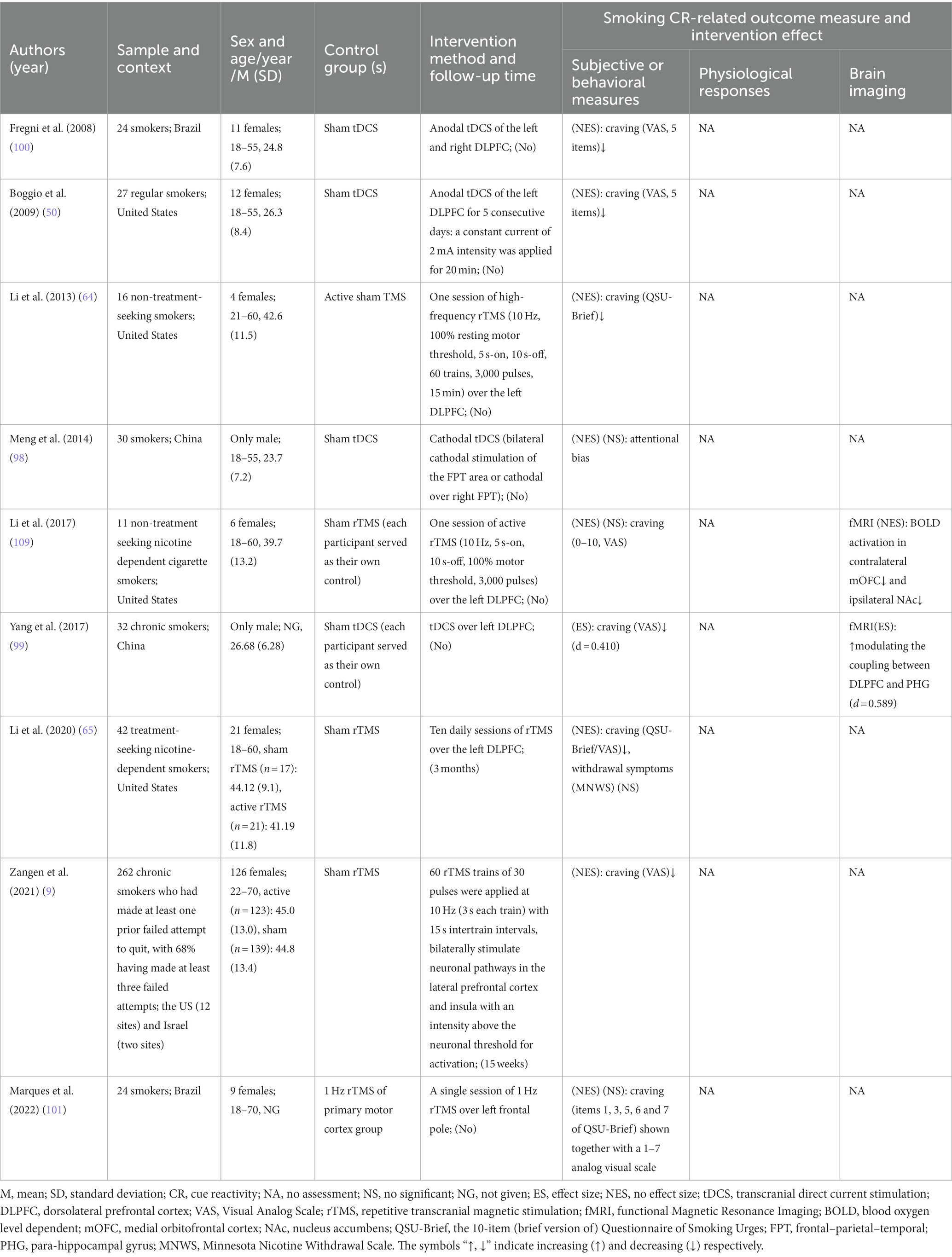
Table 2. Details of 9 included studies that looked at noninvasive brain stimulation that modulates cue reactivity.
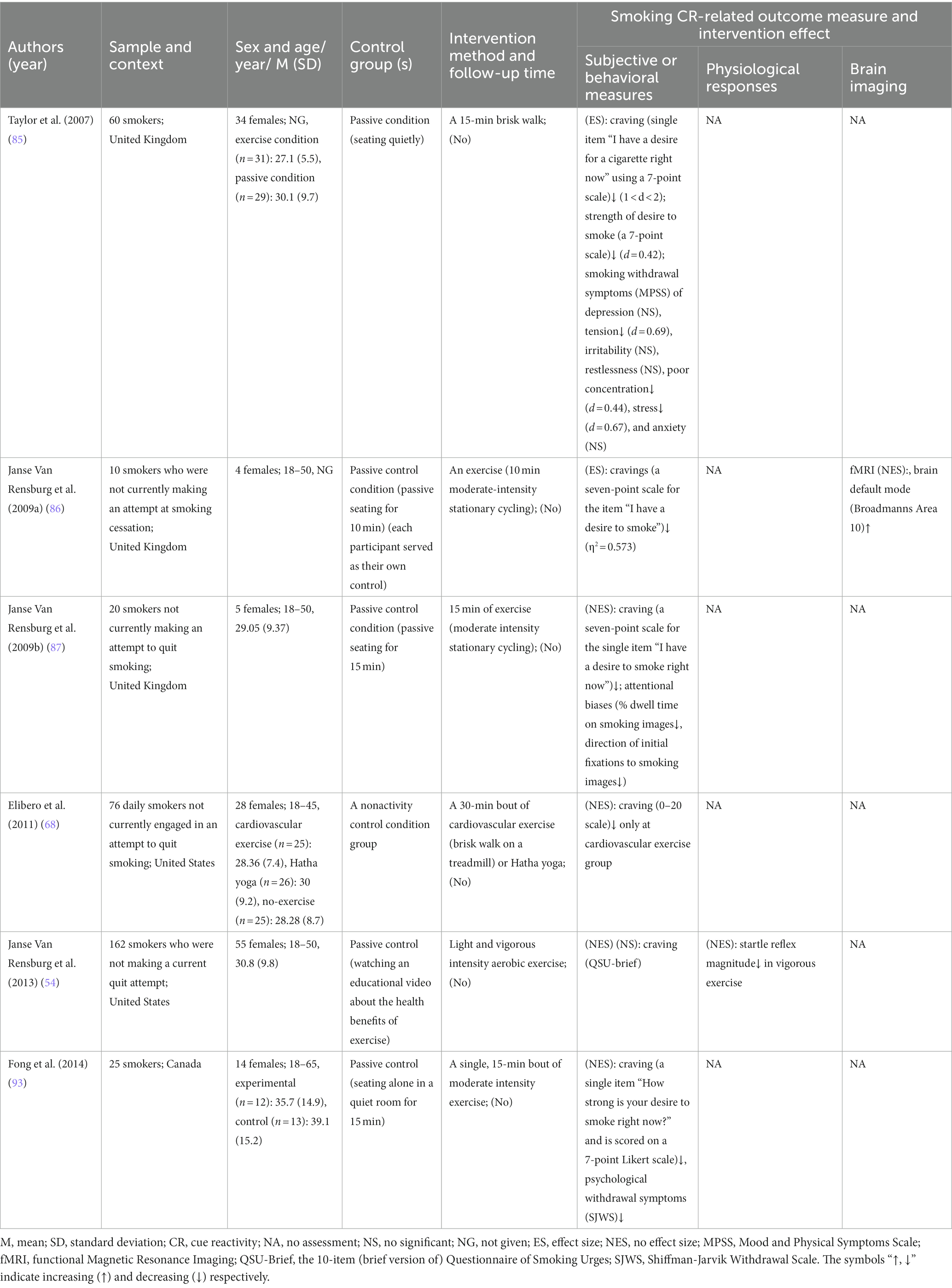
Table 4. Details of 6 included studies that looked at exercise therapy that modulates cue reactivity.
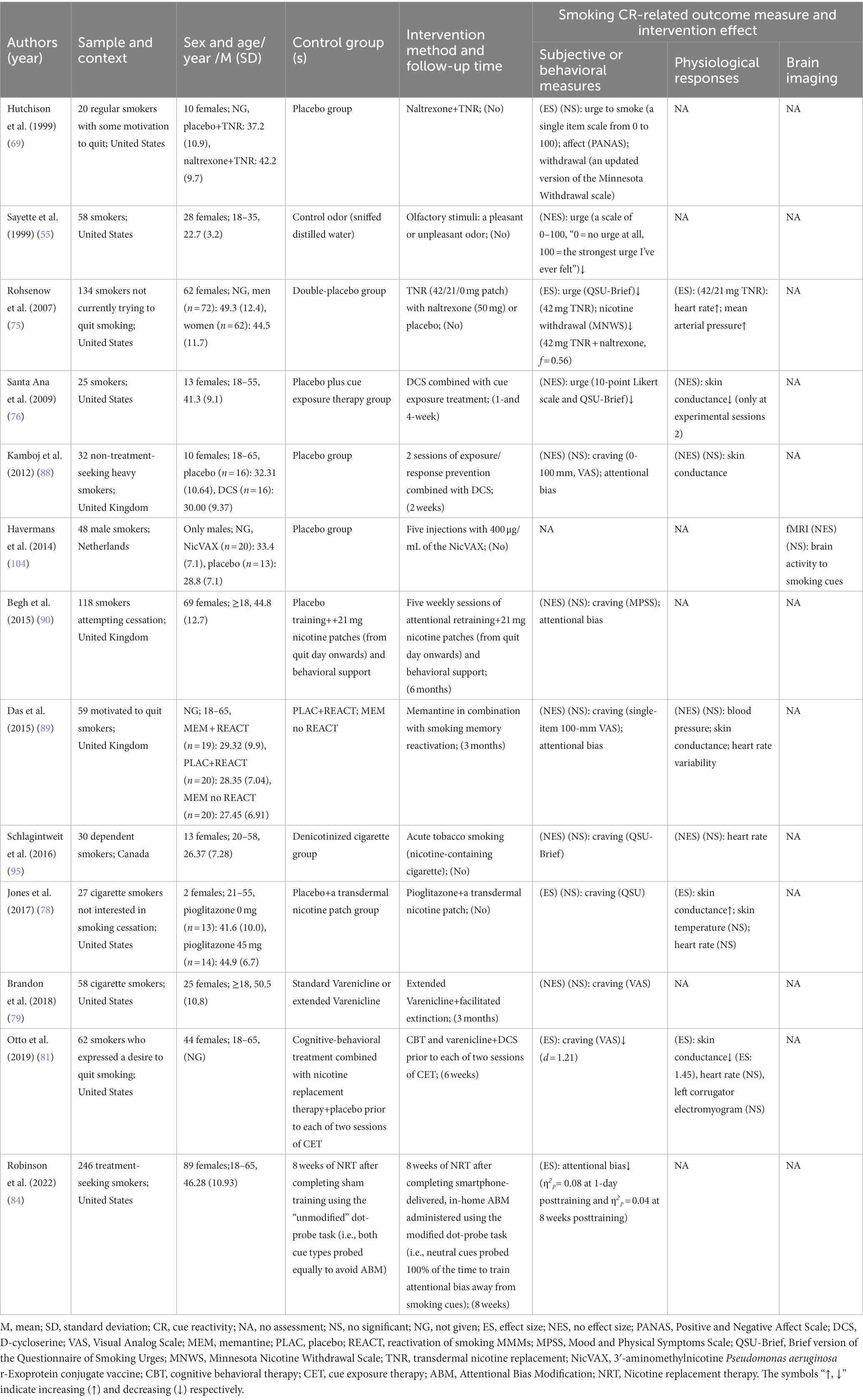
Table 5. Details of 13 included studies that looked at other therapies that modulate cue reactivity.
Notably, 7 of all the included articles only studied male smokers. In terms of age, all subjects were ≥ 18 years old and were generally categorized as youthful to middle-aged (20–50 years). Sample sizes for all studies ranged from 10 to 434, with follow-up ranging from 1 week to 6 months within 22 studies. Of the 67 included, only 6 had no measure of smoking cue-provoked craving, and the rest of the literature contained 29 articles that showed a significant reduction in smoking cue-induced craving, such as aripiprazole (97), baclofen (8), anodal transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC) (50, 100), repetitive transcranial magnetic stimulation (rTMS) (9), physical exercise (85–87, 93), olfactory stimuli (55) and 4-mg nicotine mini-lozenges (56). Twenty papers measured physiological parameters and 9 of them had significant differences between the intervention and the control groups. For example, vigorous exercise (54) reduces startle reflex amplitude, while varenicline (60, 107) reduces heart rate. There are 14 trails on brain function measurements, 11 of which are fMRI, the other 3 trails are EEG. All EEG measurements except LPP and N2 magnitude had statistically significant differences between the groups in P3. Functional MRI revealed brain activity mainly decreased in the medial orbitofrontal cortex (mOFC), ventromedial striatum (VS), ventromedial prefrontal cortex (vmPFC), ventral prefrontal cortex (vPFC), left anterior ventral insula (avInsula), nucleus accumbens (Nac) caudate, while increased in right DLPFC and brain default mode. The measures mentioned above are described in detail under each treatment topic below.
The 28 included TUD-related pharmacotherapy studies, therapeutic agents were nicotine replacement therapy (NRT) which account for the largest proportion, at nearly 1/3, and the others were olanzapine, haloperidol, topiramate, divalproex, omega-3 fatty acids, intranasal oxytocin, propranolol, aripiprazole, bupropion SR, gemfibrozil, baclofen, and varenicline which make up the second proportion. Two of the studies were conducted on male subjects only, and 1 had no sex information. The male-to-female ratio of the remaining studies where approximately 1:2 to 3:1 see Table 1 for details.
For psychological indicators, 26 studies investigated the effect of drugs on cravings induced by smoking cues, resulting in about half of the studies finding no statistically significant differences between groups, while the other studies found that NRT (half of the included NRT-related studies, only one of them has ES which is 0.6 or 0.7, see Table 1 for detail), baclofen, olanzapine, varenicline, bupropion SR, omega-3 fatty acids and intranasal oxytocin (ES: η2 p =0.2) reduced cue-induced craving compared to the control group. For other varenicline-related studies, they all showed no statistically significant differences. Acute varenicline only selectively reduced tonic cravings rather than cue-induced cravings (46), which might be associated with different psychological processes. Divalproex and aripiprazole (light smokers with 10 mg) were reported to enhance cue-induced cravings (62). There is no statistically significant difference between the intervention and control groups in terms of smoking withdrawal symptoms (except that 4 mg nicotine lozenge attenuated it (ES = 0.37)) and affect.
Regarding physiological indicators, varenicline slowed HR but had no significant difference in muscle sympathetic nerve activity, baroreflex sensitivity and BP (107). In contrast, NRT, aripiprazole, propranolol, and gemfibrozil had no significant difference in HR, SC; HR, BP; HR, ST, SC; SC, HR and left corrugator electromyography, respectively.
In terms of brain function metrics, it has found that varenicline related to reduced brain activity of VS and mOFC under fMRI scan (45). Both varenicline and bupropion SR showed no difference in LPP amplitude before or after the intervention (48). It has also found that baclofen enhanced resting brain activation of the right DLPFC and decreased neural response in the vmPFC and left avInsula under fMRI scan (8). Interestingly, Novick and colleagues (82) found that there was not different in the effect of progesterone between males and females in the neural activation of ACC, posterior cingulate cortex (PCC), left lateral occipital cortex (LLOC), and left middle temporal gyrus (LMTG) under fMRI scan.
Of the 9 non-invasive brain stimulation trials included, the two main interventions were tDCS, and rTMS see Table 2 for details. Of these, 4 were tDCS and 3 (2 studies’ stimulated site was the left DLPFC (50, 99) and 1 study’s stimulated site was the left and right DLPFC (100)) of which reduced cue-induced craving while the other one (bilateral cathodal stimulation of the FPT area or cathodal over right FPT (98)) did not assess this indicator, and 5 were rTMS and 3 (2 both stimulated the left DLPFC (64, 65) and 1 bilaterally stimulated neuronal pathways in the lateral prefrontal cortex and insula (9)) of which reduced cue-induced craving. A multicentre, double-blind RCT (9) found that rTMS reduced cue-induced craving, which led to the first clearance by FDA for rTMS as an aid in smoking cessation for adults. Although one session of active rTMS over the left DLPFC did not reduce cue-induced craving, it still reduced blood oxygen level-dependent (BOLD) activation in contralateral mOFC and ipsilateral NAc under pre-and post-intervention fMRI scans. One study reported that tDCS reduced smokers’ craving (ES: d = 0.410) by increasing the coupling between DLPFC and parahippocampal gyrus (ES: d = 0.589) (99).
Of the 11 psychotherapies included, 3 were mindfulness-related interventions, 3 was neurofeedback, 1 was attentional bias modification (ABM), 1 was retrieval-extinction training, 1 was virtual reality cue exposure (VRCE), 1 was augmented reality cue exposure (ARCE), and 1 was stress-based intervention. The subjects of two of the psychotherapy-related studies were both males (see Table 3 for details).
Regarding psychological indicators, compared to the control group, there were no statistically significant differences in mindfulness-related interventions, retrieval-extinction training, VRCE, ARCE and stress-based intervention in cue-induced cravings, while neurofeedback met with mixed results. As for other kinds of psychological indicators, compared to the control group, ABM, retrieval-extinction training, and a brief mindfulness-meditation intervention showed no difference in cognitive biases, negative effect, as well as error rates and reaction times on the smoking Go/NoGo, respectively.
For physiological indicators, retrieval-extinction training and stress-based intervention had nonsignificant difference in HR, BP and HR, BP, SC, respectively. The other psychotherapy-related studies had no measure of physiological indicators.
Under fMRI scan, Mindfulness-Oriented Recovery Enhancement was demonstrated that the decrease in cue-reactivity BOLD (CR-BOLD) response in the VS (ES: d = 1.57) and vPFC (ES: d = 1.7) and the increase in positive emotion regulation BOLD (ER-BOLD) response, as well as the increase in resting-state functional connectivity (rsFC) between rACC and OFC. These manifestations may be related to the facilitation of the reorganization of reward processes, suggesting that they may play a role in the pathophysiology of nicotine addiction (53). Under fMRI scan, neurofeedback has been shown to improve neural activity and functional connectivity between target regions of interest (ROIs; ROIs1: ACC and medial pFC, ROIs2: PCC and precuneus) (106) and reduced craving-related prefrontal cortex (PFC) activation (66). On EEG, a brief mindfulness-meditation intervention reduced P3 amplitude without significant effects on N2 amplitude during the task of NoGo vs. Go (105). Another finding was on neurofeedback training which reduced P300 amplitude with moderate effect size (d = 0.64) (7).
Of the 6 exercise therapies included, 5 (2 of them have effect size in the range of 0.4–2, see Table 4 for details) of them found that exercise therapy could significantly reduce smoking cue-elicited craving compared with control group, while light and vigorous intensity aerobic exercise had no significant effect on it but reduced startle reflex magnitude in vigorous exercise (54) (see Table 4 for details). In addition, it was found that a 15-min exercise could attenuate withdraw symptoms and attentional biases (85, 87, 93). For neuroimaging indicators, 10 min moderate-intensity stationary cycling was found to activate brain default mode (Broadmanns Area 10) (86).
Of the 13 other tobacco cessation treatments included, 10 were combination treatments, 1 was vaccine (NicVAX), 1 was acute tobacco smoking, and 1 was olfactory stimuli. Among the included studies, 1 (89) found no information on gender (see Table 5 for details). Regarding psychological indicators, olfactory stimuli, either a pleasant or unpleasant odor, reduced cue-evoked craving (55). Interestingly, compared to the control group, over half of combination treatment studies and acute tobacco smoking found no statistically significant differences in cue-induced craving between the groups for either cessation seekers or unmotivated quitters while about half of the combination treatment studies found the treatments reduced craving. As for withdrawal symptoms and attentional bias, they were all showed mixed results in the certain combination treatments. For neuroimaging indicators, only Havermans et al. (104) assessed this indicator and found that NicVAX did not modulate brain activity to smoking cues. Regarding physiological indicators, combination treatments-related studies were inconsistent with each other on SC and HR. And there were no significant differences between groups in BP, heart rate variability, ST, and left corrugator electromyogram, whereas it was found that naltrexone combined with transdermal nicotine replacement could increase mean arterial pressure.
The cue-reactivity paradigms in the 67 included articles were essentially composed of smoking cues and neutral cues, with 2 (48, 62) combining pleasant and unpleasant picture cues in Table 6 for details. Thirty-one trials based on vision (in vitro cues), 20 trials based on behavior (in vivo cues), 8 trials based on behavior and vision (in vivo/vitro cues), 2 trials based on behavior (in vitro cues), 2 trials based on vision and auditory (in vitro cues), 1 trial based on vision (in vivo cues), 1 trial based on behavior, auditory and vision (in vivo/vitro cues), 1 trial based on behavior and vision (in vivo/vitro cues), and 1 trial based on behavior (in vivo/vitro cues; see Supplementary Table S3).
Table 6 gives a description of the smoking cue-reactivity paradigms and their types, as well as stimulus materials in these trials. In terms of types, the cue-reactivity paradigms fall into two main categories: one is the behaviorally induced craving paradigm (containing manipulative behaviors that combine visual and or olfactory sensations or purely imaginative behaviors). Manipulative behaviors are basically that participants were required to watch and smell the lighting of a cigarette (one of their favorite brands) that was placed, and then they were asked to hold the cigarette between their fingers but were not allowed to smoke it and were next instructed to extinguish it. The other category is the visually induced craving paradigm (containing physical objects, pictures, videos, virtual reality and augmented reality). For example, the picture paradigm was basically showing the subjects smoking-related pictures and neutral pictures in a certain way. Based on the results of the 30 included papers, it was found that smoking cues induced greater craving than neutral cues, both behaviorally and visually induced.
The review above summarizes a series of RCTs of CR in tobacco cessation therapy and focuses on a thematic overview of the types of cue-reactivity paradigms used in the trials, with the aim of assessing the effects of various cue-targeted tobacco cessation programs and summarizing the types of cue-reactivity paradigms used to date. Hence, we chose a scoping review to summarize the existing results and exploit the gaps in the current literature.
Overall, these results revealed that non-invasive brain stimulation (6 of 8 related articles) and exercise therapy (5 of 6 related articles) showed a trend of greater possibility in reducing subjective craving, when compared to the remaining therapies (11 of 26 pharmacotherapy-related articles, 2 of 11 psychotherapy-related articles, 4 of 11 other therapies related articles), regardless of variations in the number of studies conducted in each category. But due to more significant heterogeneity of studies across samples, sociodemographic information (gender, age, region), types of cue-reactivity paradigms, outcome measures and other dimensions made comparisons of the efficacy of different interventions, even the same intervention across studies, not sufficiently comparable. Even more identifiable, the measures used to assess subjective craving vary widely across studies, such as the use of the QSU-Brief, CWQ, or various types of VAS (see Tables 1–5), which further make craving in such trials challenging to measure objectively and quantitatively. The above-mentioned heterogeneity of the experimental design and implementation stage makes it challenging to compare the effect of different types of tobacco cessation interventions, further forming the situation of a lack of repetitive research. As a result, the corresponding literature only focused on the development of abstinence methods rather than the exploration of the effects. At the same time, the physiological and brain function indicators accounted for a small proportion of the reviewed articles. The physiological indicators did not show statistically significant differences in more trials. In contrast, studies based on brain function as a measure EEG and fMRI show a quantitative imbalance while their results had their own similarities and differences with non-RCTs.
Pharmacologically, the therapeutic targets under development are the endogenous cannabinoid system, nicotinic acetylcholine α4β2 and α7 subtypes, CB1 receptor neutral antagonists, fatty acid amide hydrolase inhibitors (110) and metabotropic glutamate receptor 5 (111). For example, drugs targeting the endogenous cannabinoid system have been more studied in animal experiments and less in human experiments, currently mainly cannabidiol (112). Although blocking the α4β2, but not α7 subtype has been shown to be effective in reducing nicotine intake in animal studies, blocking the α7, but not α4β2 isoform of the nicotinic acetylcholine receptors reversed cue-triggered nicotine relapse behavior (113). Current studies have developed tobacco cessation medications in addition to those summarized in the results section, such as naloxone (114) which has mostly been found to reduce craving. Franklin et al. (45) found that varenicline diminished smoking cue-elicited ventral striatum and mOFC responses, and Ketcherside et al. (8) found that baclofen mitigates the reward response to smoking cues through an increase in tonic activation of the DLPFC, an executive control region, and the aforementioned altered neural activity correlated with cue-induced craving. However, no clear findings have been made on the pathways by which drugs mediate different manifestations of cue-induced craving, and more drugs with different chemical structures need to be developed. Previous studies need to be repeated to explore the associated addictive mechanisms and ensure the safety of drug treatments and their effectiveness.
Non-invasive brain stimulation was primarily tDCS and rTMS, with rTMS being one of the most effective methods found to reduce cigarette smoking in the intervention group, but neither technique significantly improved outcomes of tobacco cessation rate (115). Based on fMRIs, rTMS (109) and tDCS (116, 117) targeting the DLPFC were found to be the most effective in reducing cravings by reducing activity in the right insula and right thalamus as well as reducing rsFC between the left DLPFC and the mOFC for rTMS. Zangen et al. (9) found rTMS bilaterally stimulating neural pathways in the lateral prefrontal cortex and insula with an intensity above the neuronal threshold for activation can also reduce cigarette craving. Therefore, non-invasive brain stimulation has multiple targets for reducing cue-induced cravings. Further exploration of the mechanism of non-invasive brain stimulation in the treatment of TUD will provide a better basis for improving the reliability and efficiency of treatments.
In psychotherapy, there are mainly mindfulness (118), hypnosis-based treatment (119), cognitive behavioral treatment (120), cue exposure treatment (CET) (81) and psychological paradigm training, which are mainly neurofeedback training (7, 106, 121), retrieval-extinction (67) and ABM (103). These psychotherapies are mainly used to achieve tobacco cessation, or relapse prevention, by reducing smoking cue-induced craving and or the impulsivity to smoke. Although the 6 psychotherapeutic articles included in this scoping review did not find a reduction in craving or modulation of cognitive biases, this does not mean that various psychotherapies are not effective in this regard, when there may be related to individual subjective perception thresholds and different matches with different psychotherapies. On the other hand, Kim et al. (106) and Froeliger et al. (53) found corresponding psychotherapy activity changed in relevant brain regions under fMRI scan, while Andreu et al. (105) found that psychotherapy exhibited different effects on different components of ERP. In summary, psychotherapy can further help to improve substance use disorder (SUD) symptoms and prevent relapse by regulating brain function. This requires future research to strengthen the mechanism of SUD psychotherapy, from brain function and pathophysiological indicators, in order to develop higher physiological and imaging indicators with higher specificity, to compensate for the shortcomings of subjective measures.
For exercise therapy and other treatments, nearly all exercise therapy and approximately half of combination therapies showed the effect of reducing subjective craving, while the other combination therapies were not found to be significantly different from controls in the reviewed literature. However, it is still an integrative treatment approach that has received more attention from researchers and is consistent with the treatment philosophy of the bio-psycho-social medical model. Mondino et al. (122) found that combining transcranial alternating current stimulation and ABM helped smokers wishing to quit smoking reduce craving, attention and impulsive decision-making to smoking cues. Otto et al. (81) found that d-cycloserine enhanced the efficacy of CET in reducing cue-induced craving. In summary, given the variations in the effects of different combinations of treatment modalities for tobacco cessation, further exploration of the interactions and similarities in the mechanisms of multimodal combinations is needed to find more comprehensive and personalized approaches to tobacco cessation.
It is worth mentioning that virtual/augmented reality related treatment is one emerging form of smoking cessation intervention targeting cue-reactivity. To our knowledge, most studies found that virtual/augmented reality related smoking cue-paradigms can provoke cue-reactivity, especially craving (25, 30, 123–126). And the technology of virtual/augmented reality is mainly applied to CET (83, 108, 127). However, many studies, especially virtual reality related studies, aimed at assessing the effects of virtual/augmented reality CET on smoking-related cue-reactivity were quasi-experimental studies without using a control group (128–131), or the RCT study did not report the results of cue-reactivity between groups (132), and most of them found that virtual/augmented reality CET could reduce craving. Notably, the two included articles (83, 108) in our review had no significant difference between groups in craving. Overall, the effect of virtual reality (VR) CET in craving is mixed, which is also reported in a systematic review (127), while there are not enough augmented reality (AR) CET studies to make a similar conclusion. So, the potential of VR-or AR-based smoking cessation intervention is needed to be fully explored.
The cue-reactivity paradigms as the primary means of eliciting smoking craving in experiments shows significant variability in the reviewed articles, reducing the cross-sectional comparability of the effects of various tobacco cessation treatment experiments. The materials used by researchers to stimulate smoking cravings were homemade (7) or modified from other researchers’ galleries (35), from tobacco ads1 (114), queried from google images for ‘positive smoking’ and ‘negative smoking’ (133) or other sources such as the Normative Appetitive Picture System (NAPS) (134) or the International Smoking Image Series (ISIS) (135). Most home-grown stimulated smoking craving images are used for their own experiments, making it difficult to conduct replicated studies. To address these challenges, researchers such as Manoliu (135) generated and validated a large set of individually rated SRC to assess different dimensions of stimulus intensity, including craving, valence and arousal. Thus, they proposed a novel image bank that rates the three dimensions of craving, valence and arousal on a continuous scale, which not only provides a good description of a publicly available rating software but contributes to the scientific field.2 There are only 250 images in the image library, but there are many types of smoking cue materials used in the study, such as pictures, videos, audio, physical cigarettes, virtual or augmented reality simulations of cigarette tools or smoking scenes (25). In addition, the materials used as controls for the study also vary, such as neutral materials, negative emotion materials, positive emotion materials, food materials, stress materials, and aversion materials. Therefore, it is better to expand the smoking and controlled cue material library. Besides that, due to cultural and individual differences, the need for a uniform and standardized database of smoking cue materials has become imperative.
To begin with, the selection of included RCTs and the use of strict inclusion criteria to ensure the relative quality of the review is inevitably biased by the lack of quality control of the included pieces of literature. In addition, the exclusion of literature on TUD with co-morbidities prevents us from demonstrating how CR is affected in the context of comorbidities. However, numerous studies (136–140) suggest that the prevalence of TUD is higher in individuals with associated psychological problems or psychiatric disorders. Most studies (5, 141–144) on the relationship between TUD co-morbidity and CR have shown that individuals with TUD with comorbidity have difficulties quitting and that co-morbidity objectively alters the performance of CR. Therefore, to make tobacco cessation treatment more personalized and comprehensive, comorbidity research should be strengthened to deconstruct the mechanism of regulating brain addiction of TUD with comorbidities, which will be a challenging study. Furthermore, our literature search strategy and limited database selection may have resulted in the omission of literature that met the inclusion criteria, thus preventing this review from providing a comprehensive overview of current advances in smoking cessation therapy based on CR. And we only searched for publications in English and Chinese, which led to missing literature in other languages and further contributed to the abovementioned problems. Finally, there is also a limitation with regards to the differences among the included articles in gender/sex ratio, ethnicity or region or origin or diagnostic criteria of the study participants, sample size of the individual studies, as well as the statistical methods, resulting in significant heterogeneity among various studies. Therefore, we did not statistically test for the overall efficacy, which is also a limitation for a descriptive and comparative approach we adopted here.
This paper reviews the effects of various cue-reactivity-targeted smoking cessation therapies and types of cue-reactivity paradigms to understand the role of cue-reactivity in smoking cessation diagnosis and treatment. It proposes that, given that current studies are still inadequate in terms of homogeneity and lack repeated validation, cue-reactivity can be conducted in the future by constructing a standard library of smoking cue materials and conducting cue-reactivity causal analysis in order to adequately screen for causes of addiction persistence. In summary, the following problems remain: (1) it is still challenging to find specific targets among the factors influencing cue-reactivity, and it cannot be ruled out that they are due to a combination of factors, so causality studies need to be strengthened; (2) the specificity of the indicators can be enhanced by expanding the sample size, strengthening the homogeneity of the sample, standardizing the parameters of the cue-reactivity paradigms, increasing the years of follow-up, and standardizing statistical methods; (3) there is a lack of a unified and standardized database of smoking cues worldwide, and the construction of a database of smoking cues would be a worthwhile endeavor to facilitate repeat trials and the reliability of final scientific findings. Data-driven approaches toward addiction have been increasing in recent years, which could allow for the personalization of big data analysis and the differentiation of responses, such as craving levels between different paradigms, providing practical technical support for the search for a more stable and effective cue-reactivity paradigms.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
ML: Conceptualization; data curation; investigation methodology; project administration; visualization. QG: Conceptualization; supervision; validation. YF: Funding acquisition; supervision. ZC: Conceptualization; funding acquisition; supervision. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (NSFC) (Nos.32060196, 82201597, 31760281, and 81760258) and Yunnan Ten Thousand Talents Plan Young and Elite Talents Project (YNWR-QNBJ-2018-027).
The authors would like to thank Yunxiong Jiang and Kebin Li for their assistance with the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1167283/full#supplementary-material
2. ^The image database and their ratings are available at https://smocuda.github.io/.
1. Zou, Z, Wang, H, d'Oleire Uquillas, F, Wang, X, Ding, J, and Chen, H. Definition of substance and non-substance addiction. Adv Exp Med Biol. (2017) 1010:21–41. doi: 10.1007/978-981-10-5562-1_2
2. LeCocq, MR, Randall, PA, Besheer, J, and Chaudhri, N. Considering drug-associated contexts in substance use disorders and treatment development. Neurotherapeutics. (2020) 17:43–54. doi: 10.1007/s13311-019-00824-2
3. World Health Organization. WHO report on the global tobacco epidemic, Addressing new and emerging products. Geneva: World Health Organization. (2021).
4. Jasinska, AJ, Stein, EA, Kaiser, J, Naumer, MJ, and Yalachkov, Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. (2014) 38:1–16. doi: 10.1016/j.neubiorev.2013.10.013
5. Keyser-Marcus, L, Vassileva, J, Stewart, K, and Johns, S. Impulsivity and cue reactivity in smokers with comorbid depression and anxiety: possible implications for smoking cessation treatment strategies. Am J Drug Alcohol Abuse. (2017) 43:432–41. doi: 10.1080/00952990.2017.1287190
6. Wray, JM, Gass, JC, and Tiffany, ST. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob Res. (2013) 15:1167–82. doi: 10.1093/ntr/nts268
7. Bu, J, Young, KD, Hong, W, Ma, R, Song, H, Wang, Y, et al. Effect of deactivation of activity patterns related to smoking cue reactivity on nicotine addiction. Brain J Neurol. (2019) 142:1827–41. doi: 10.1093/brain/awz114
8. Ketcherside, A, Jagannathan, K, Dolui, S, Hager, N, Spilka, N, Nutor, C, et al. Baclofen-induced changes in the resting brain modulate smoking Cue reactivity: a double-blind placebo-controlled functional magnetic resonance imaging study in cigarette smokers. Clin Psychopharmacol Neurosci. (2020) 18:289–302. doi: 10.9758/cpn.2020.18.2.289
9. Zangen, A, Moshe, H, Martinez, D, Barnea-Ygael, N, Vapnik, T, Bystritsky, A, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry. (2021) 20:397–404. doi: 10.1002/wps.20905
10. Betts, JM, Dowd, AN, Forney, M, Hetelekides, E, and Tiffany, ST. A meta-analysis of Cue reactivity in tobacco cigarette smokers. Nicotine Tob. (2021) 23:249–58. doi: 10.1093/ntr/ntaa147
11. Miller, PM. Principles of addiction: Comprehensive addictive behaviors and disorders. Amsterdam, Boston, MA: Elsevier Academic Press (2013).
12. Carter, BL, and Tiffany, ST. Meta-analysis of cue-reactivity in addiction research. Addiction. (1999) 94:327–40.
13. Niaura, RS, Rohsenow, DJ, Binkoff, JA, Monti, PM, Pedraza, M, and Abrams, DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. (1988) 97:133–52. doi: 10.1037//0021-843x.97.2.133
14. Janes, AC, Gilman, JM, Radoman, M, Pachas, G, Fava, M, and Evins, AE. Revisiting the role of the insula and smoking cue-reactivity in relapse: a replication and extension of neuroimaging findings. Drug Alcohol Depend. (2017) 179:8–12. doi: 10.1016/j.drugalcdep.2017.06.012
15. Luijten, M, Kleinjan, M, and Franken, IH. Event-related potentials reflecting smoking cue reactivity and cognitive control as predictors of smoking relapse and resumption. Psychopharmacology. (2016) 233:2857–68. doi: 10.1007/s00213-016-4332-8
16. Allenby, C, Falcone, M, Wileyto, EP, Cao, W, Bernardo, L, Ashare, RL, et al. Neural cue reactivity during acute abstinence predicts short-term smoking relapse. Addict Biol. (2020) 25:e12733. doi: 10.1111/adb.12733
17. Janes, AC, Pizzagalli, DA, Richardt, S, deB Frederick, B, Chuzi, S, Pachas, G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. (2010) 67:722–9. doi: 10.1016/j.biopsych.2009.12.034
18. Chornock, WM, Stitzer, ML, Gross, J, and Leischow, S. Experimental model of smoking re-exposure: effects on relapse. Psychopharmacology. (1992) 108:495–500. doi: 10.1007/bf02247427
19. Bedi, G, Preston, KL, Epstein, DH, Heishman, SJ, Marrone, GF, Shaham, Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. (2011) 69:708–11. doi: 10.1016/j.biopsych.2010.07.014
20. McClernon, FJ, Conklin, CA, Kozink, RV, Adcock, RA, Sweitzer, MM, Addicott, MA, et al. Hippocampal and insular response to smoking-related environments: neuroimaging evidence for drug-context effects in nicotine dependence. Neuropsychopharmacology: official publication of the American college of. Neuropsychopharmacology. (2016) 41:877–85. doi: 10.1038/npp.2015.214
21. Van Gucht, D, Beckers, T, Van den Bergh, O, and Vansteenwegen, D. Does exposure to habitual smoking contexts before smoking cessation reduce relapse? Results from a pilot study. Behav Chang. (2010) 27:19–28. doi: 10.1375/bech.27.1.19
22. Janes, AC, Krantz, NL, Nickerson, LD, Frederick, BB, and Lukas, SE. Craving and Cue reactivity in nicotine-dependent tobacco smokers is associated with different insula networks. Biol Psychiatry Cogn Neurosci Neuroimaging. (2020) 5:76–83. doi: 10.1016/j.bpsc.2019.09.005
23. Engelmann, JM, Versace, F, Robinson, JD, Minnix, JA, Lam, CY, Cui, Y, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. (2012) 60:252–62. doi: 10.1016/j.neuroimage.2011.12.024
24. Hayashi, T, Ko, JH, Strafella, AP, and Dagher, A. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci U S A. (2013) 110:4422–7. doi: 10.1073/pnas.1212185110
25. Tamburin, S, Dal Lago, D, Armani, F, Turatti, M, Saccà, R, Campagnari, S, et al. Smoking-related cue reactivity in a virtual reality setting: association between craving and EEG measures. Psychopharmacology. (2021) 238:1363–71. doi: 10.1007/s00213-020-05733-3
26. Bu, J, Ma, R, Fan, C, Sun, S, Cheng, Y, Piao, Y, et al. Low-Theta electroencephalography coherence predicts cigarette craving in nicotine addiction. Front Psych. (2019) 10:296. doi: 10.3389/fpsyt.2019.00296
27. Mashhoon, Y, Betts, J, Farmer, SL, and Lukas, SE. Early onset cigarette smokers exhibit greater P 300 reactivity to smoking-related stimuli and report greater craving. Brain Res. (2018) 1687:173–84. doi: 10.1016/j.brainres.2018.02.037
28. Deweese, MM, Robinson, JD, Cinciripini, PM, and Versace, F. Conditioned cortical reactivity to cues predicting cigarette-related or pleasant images. Int J Psychophysiol. (2016) 101:59–68. doi: 10.1016/j.ijpsycho.2016.01.007
29. Engelmann, JM, Versace, F, Gewirtz, JC, and Cinciripini, PM. Individual differences in brain responses to cigarette-related cues and pleasant stimuli in young smokers. Drug Alcohol Depend. (2016) 163:229–35. doi: 10.1016/j.drugalcdep.2016.04.025
30. Pericot-Valverde, I, Germeroth, LJ, and Tiffany, ST. The use of virtual reality in the production of Cue-specific craving for cigarettes: a meta-analysis. Nicotine Tob Res. (2016) 18:538–46. doi: 10.1093/ntr/ntv216
31. Gass, JC, and Tiffany, ST. Craving and tobacco use: development of the choice behavior under cued conditions (CBUCC) procedure. Psychol Addict Behav. (2017) 31:276–83. doi: 10.1037/adb0000259
32. Piasecki, TM, Fleming, KA, Trela, CJ, and Bartholow, BD. P3 event-related potential reactivity to smoking cues: relations with craving, tobacco dependence, and alcohol sensitivity in young adult smokers. Psychol Addict Behav. (2017) 31:61–72. doi: 10.1037/adb0000233
33. Martinez, S, Jones, JD, Vadhan, NP, Brandt, L, Comer, SD, and Bisaga, A. The acute and repeated effects of cigarette smoking and smoking-related cues on impulsivity. Drug Alcohol Rev. (2021) 40:864–8. doi: 10.1111/dar.13206
34. Kräplin, A, Scherbaum, S, Bühringer, G, and Goschke, T. Decision-making and inhibitory control after smoking-related priming in nicotine dependent smokers and never-smokers. Addict Behav. (2019) 88:114–21. doi: 10.1016/j.addbeh.2018.08.020
35. Zhao, H, Turel, O, Brevers, D, Bechara, A, and He, Q. Smoking cues impair monitoring but not stopping during response inhibition in abstinent male smokers. Behav Brain Res. (2020) 386:112605. doi: 10.1016/j.bbr.2020.112605
36. Zlomuzica, A, Lange, M, Reher, S, Machulska, A, and Rinck, M. The effects of psychological stress on approach tendencies for smoking-related cues in smokers. Eur J Neurosci. (2022) 55:2581–91. doi: 10.1111/ejn.15295
37. Masiero, M, Lucchiari, C, Maisonneuve, P, Pravettoni, G, Veronesi, G, and Mazzocco, K. The attentional Bias in current and former smokers. Front Behav Neurosci. (2019) 13:154. doi: 10.3389/fnbeh.2019.00154
38. Rehme, AK, Bey, K, Frommann, I, Mogg, K, Bradley, BP, Bludau, J, et al. Selective attention to smoking cues in former smokers. Eur Neuropsychopharmacol. (2018) 28:276–84. doi: 10.1016/j.euroneuro.2017.12.003
39. Kim, JI, Lee, JD, Hwang, HJ, Ki, SW, Park, IH, and Park, TY. Altered subcallosal and posterior cingulate cortex-based functional connectivity during smoking cue and mental simulation processing in smokers. Prog Neuro-Psychopharmacol Biol Psychiatry. (2020) 97:109772. doi: 10.1016/j.pnpbp.2019.109772
40. Ghahremani, DG, Faulkner, P, Cox, MC, and London, ED. Behavioral and neural markers of cigarette-craving regulation in young-adult smokers during abstinence and after smoking. Neuropsychopharmacology: official publication of the American college of. Neuropsychopharmacology. (2018) 43:1616–22. doi: 10.1038/s41386-018-0019-7
41. Janes, AC, Betts, J, Jensen, JE, and Lukas, SE. Dorsal anterior cingulate glutamate is associated with engagement of the default mode network during exposure to smoking cues. Drug Alcohol Depend. (2016) 167:75–81. doi: 10.1016/j.drugalcdep.2016.07.021
42. Lowe, MR, Green, L, Kurtz, SM, Ashenberg, ZS, and Fisher, EB Jr. Self-initiated, cue extinction, and covert sensitization procedures in smoking cessation. J Behav Med. (1980) 3:357–72. doi: 10.1007/bf00845290
43. Raw, M, and Russell, MA. Rapid smoking, cue exposure and support in the modification of smoking. Behav Res Ther. (1980) 18:363–72. doi: 10.1016/0005-7967(80)90001-7
44. Falcone, M, Cao, W, Bernardo, L, Tyndale, RF, Loughead, J, and Lerman, C. Brain responses to smoking cues differ based on nicotine metabolism rate. Biol Psychiatry. (2016) 80:190–7. doi: 10.1016/j.biopsych.2015.11.015
45. Franklin, T, Wang, Z, Suh, JJ, Hazan, R, Cruz, J, Li, Y, et al. Effects of varenicline on smoking cue–triggered neural and craving responses. Arch Gen Psychiatry. (2011) 68:516–26. doi: 10.1001/archgenpsychiatry.2010.190
46. Hitsman, B, Hogarth, L, Tseng, LJ, Teige, JC, Shadel, WG, DiBenedetti, DB, et al. Dissociable effect of acute varenicline on tonic versus cue-provoked craving in non-treatment-motivated heavy smokers. Drug Alcohol Depend. (2013) 130:135–41. doi: 10.1016/j.drugalcdep.2012.10.021
47. Pachas, GN, Gilman, J, Orr, SP, Hoeppner, B, Carlini, SV, Grasser, EB, et al. Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology. (2015) 232:1619–28. doi: 10.1007/s00213-014-3797-6
48. Versace, F, Stevens, EM, Robinson, JD, Cui, Y, Deweese, MM, Engelmann, JM, et al. Brain responses to cigarette-related and emotional images in smokers during smoking cessation: no effect of Varenicline or bupropion on the late positive potential. Nicotine Tob Res. (2019) 21:234–40. doi: 10.1093/ntr/ntx264
49. Lawson, SC, Gass, JC, Cooper, RK, Tonkin, SS, Colder, CR, Mahoney, MC, et al. The impact of three weeks of pre-quit varenicline on reinforcing value and craving for cigarettes in a laboratory choice procedure. Psychopharmacology. (2021) 238:599–609. doi: 10.1007/s00213-020-05713-7
50. Boggio, PS, Liguori, P, Sultani, N, Rezende, L, Fecteau, S, and Fregni, F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett. (2009) 463:82–6. doi: 10.1016/j.neulet.2009.07.041
51. Wise, T, Marwood, L, Perkins, AM, Herane-Vives, A, Joules, R, Lythgoe, DJ, et al. Instability of default mode network connectivity in major depression: a two-sample confirmation study. Transl Psychiatry. (2017) 7:e1105. doi: 10.1038/tp.2017.40
52. Bowen, S, and Marlatt, A. Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychol Addict Behav. (2009) 23:666–71. doi: 10.1037/a0017127
53. Froeliger, B, Mathew, AR, McConnell, PA, Eichberg, C, Saladin, ME, Carpenter, MJ, et al. Restructuring reward mechanisms in nicotine addiction: a pilot fMRI study of mindfulness-oriented recovery enhancement for cigarette smokers. Evid Based Complement Alternat Med. (2017) 2017:7018010–4. doi: 10.1155/2017/7018014
54. Janse Van Rensburg, K, Elibero, A, Kilpatrick, M, and Drobes, DJ. Impact of aerobic exercise intensity on craving and reactivity to smoking cues. Exp Clin Psychopharmacol. (2013) 21:196–203. doi: 10.1037/a0032768
55. Sayette, MA, and Parrott, DJ. Effects of olfactory stimuli on urge reduction in smokers. Exp Clin Psychopharmacol. (1999) 7:151–9. doi: 10.1037//1064-1297.7.2.151
56. Nides, M, Shanga, GM, Bishop, A, and Becker, WD. Nicotine lozenges in the relief of behaviorally provoked craving. Am J Health Behav. (2018) 42:69–80. doi: 10.5993/ajhb.42.3.7
57. Hutchison, KE, Rutter, MC, Niaura, R, Swift, RM, Pickworth, WB, and Sobik, L. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology. (2004) 175:407–13. doi: 10.1007/s00213-004-1837-3
58. Mahler, SV, and De Wit, H. Effects of haloperidol on reactions to smoking cues in humans. Behav Pharmacol. (2005) 16:123–6. doi: 10.1097/00008877-200503000-00008
59. Reid, MS, Palamar, J, Raghavan, S, and Flammino, F. Effects of topiramate on cue-induced cigarette craving and the response to a smoked cigarette in briefly abstinent smokers. Psychopharmacology. (2007) 192:147–58. doi: 10.1007/s00213-007-0755-6
60. Brandon, TH, Drobes, DJ, Unrod, M, Heckman, BW, Oliver, JA, Roetzheim, RC, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology. (2011) 218:391–403. doi: 10.1007/s00213-011-2327-z
61. Culbertson, CS, Bramen, J, Cohen, MS, London, ED, Olmstead, RE, Gan, JJ, et al. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. (2011) 68:505–15. doi: 10.1001/archgenpsychiatry.2010.193
62. Ditre, JW, Oliver, JA, Myrick, H, Henderson, S, Saladin, ME, and Drobes, DJ. Effects of divalproex on smoking cue reactivity and cessation outcomes among smokers achieving initial abstinence. Exp Clin Psychopharmacol. (2012) 20:293–301. doi: 10.1037/a0027789
63. Miller, MA, Bershad, A, King, AC, Lee, R, and De Wit, H. Intranasal oxytocin dampens cue-elicited cigarette craving in daily smokers: a pilot study. Behav Pharmacol. (2016) 27:697–703. doi: 10.1097/fbp.0000000000000260
64. Li, X, Hartwell, KJ, Owens, M, Lematty, T, Borckardt, JJ, Hanlon, CA, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. (2013) 73:714–20. doi: 10.1016/j.biopsych.2013.01.003
65. Li, X, Hartwell, KJ, Henderson, S, Badran, BW, Brady, KT, and George, MS. Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: a double-blind, sham-controlled, randomized clinical trial. Brain Stimul. (2020) 13:1271–9. doi: 10.1016/j.brs.2020.06.007
66. Hartwell, KJ, Hanlon, CA, Li, X, Borckardt, JJ, Canterberry, M, Prisciandaro, JJ, et al. Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. J Psychiatry Neurosci. (2016) 41:48–55. doi: 10.1503/jpn.140200
67. Germeroth, LJ, Carpenter, MJ, Baker, NL, Froeliger, B, LaRowe, SD, and Saladin, ME. Effect of a brief memory updating intervention on smoking behavior: a randomized clinical trial. JAMA Psychiat. (2017) 74:214–23. doi: 10.1001/jamapsychiatry.2016.3148
68. Elibero, A, Janse Van Rensburg, K, and Drobes, DJ. Acute effects of aerobic exercise and hatha yoga on craving to smoke. Nicotine Tob Res. (2011) 13:1140–8. doi: 10.1093/ntr/ntr163
69. Hutchison, KE, Monti, PM, Rohsenow, DJ, Swift, RM, Colby, SM, Gnys, M, et al. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology. (1999) 142:139–43. doi: 10.1007/s002130050872
70. Tiffany, ST, Cox, LS, and Elash, CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. (2000) 68:233–40. doi: 10.1037//0022-006x.68.2.233
71. Shiffman, S, Shadel, WG, Niaura, R, Khayrallah, MA, Jorenby, DE, Ryan, CF, et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology. (2003) 166:343–50. doi: 10.1007/s00213-002-1338-1
72. Waters, AJ, Shiffman, S, Sayette, MA, Paty, JA, Gwaltney, CJ, and Balabanis, MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. (2004) 72:1136–43. doi: 10.1037/0022-006x.72.6.1136
73. Morissette, SB, Palfai, TP, Gulliver, SB, Spiegel, DA, and Barlow, DH. Effects of transdermal nicotine during imaginal exposure to anxiety and smoking cues in college smokers. Psychol Addict Behav. (2005) 19:192–8. doi: 10.1037/0893-164x.19.2.192
74. Niaura, R, Sayette, M, Shiffman, S, Glover, ED, Nides, M, Shelanski, M, et al. Comparative efficacy of rapid-release nicotine gum versus nicotine polacrilex gum in relieving smoking cue-provoked craving. Addiction. (2005) 100:1720–30. doi: 10.1111/j.1360-0443.2005.01218.x
75. Rohsenow, DJ, Monti, PM, Hutchison, KE, Swift, RM, Mac Kinnon, SV, Sirota, AD, et al. High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Exp Clin Psychopharmacol. (2007) 15:81–92. doi: 10.1037/1064-1297.15.1.81
76. Santa Ana, EJ, Rounsaville, BJ, Frankforter, TL, Nich, C, Babuscio, T, Poling, J, et al. D-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. (2009) 104:220–7. doi: 10.1016/j.drugalcdep.2009.04.023
77. Du, D, Nides, M, Borders, J, Selmani, A, and Waverczak, W. Comparison of nicotine oral soluble film and nicotine lozenge on efficacy in relief of smoking cue-provoked acute craving after a single dose of treatment in low dependence smokers. Psychopharmacology. (2014) 231:4383–91. doi: 10.1007/s00213-014-3586-2
78. Jones, JD, Comer, SD, Metz, VE, Manubay, JM, Mogali, S, Ciccocioppo, R, et al. Pioglitazone, a PPARγ agonist, reduces nicotine craving in humans, with marginal effects on abuse potential. Pharmacol Biochem Behav. (2017) 163:90–100. doi: 10.1016/j.pbb.2017.10.002
79. Brandon, TH, Unrod, M, Drobes, DJ, Sutton, SK, Hawk, LW, Simmons, VN, et al. Facilitated extinction training to improve pharmacotherapy for smoking cessation: a pilot feasibility trial. Nicotine Tob Res. (2018) 20:1189–97. doi: 10.1093/ntr/ntx203
80. Kotlyar, M, Vogel, RI, Dufresne, SR, Mills, AM, and Vuchetich, JP. Effect of nicotine lozenge use prior to smoking cue presentation on craving and withdrawal symptom severity. Drug Alcohol Depend. (2020) 206:107706. doi: 10.1016/j.drugalcdep.2019.107706
81. Otto, MW, Pachas, GN, Cather, C, Hoeppner, SS, Moshier, SJ, Hearon, BA, et al. A placebo-controlled randomized trial of D-cycloserine augmentation of cue exposure therapy for smoking cessation. Cogn Behav Ther. (2019) 48:65–76. doi: 10.1080/16506073.2018.1476908
82. Novick, AM, Duffy, KA, Johnson, RL, Sammel, MD, Cao, W, Strasser, AA, et al. Effect of progesterone administration in male and female smokers on nicotine withdrawal and neural response to smoking cues: role of progesterone conversion to allopregnanolone. Biol Sex Differ. (2022) 13:60. doi: 10.1186/s13293-022-00472-w
83. Yang, MJ, Brandon, KO, Sutton, SK, Kleinjan, M, Hernandez, LM, Sawyer, LE, et al. Augmented reality for extinction of cue-provoked urges to smoke: proof of concept. Psychol Addict Behav. (2022) 36:990–8. doi: 10.1037/adb0000868
84. Robinson, JD, Cui, Y, Linares Abrego, P, Engelmann, JM, Prokhorov, AV, Vidrine, DJ, et al. Sustained reduction of attentional bias to smoking cues by smartphone-delivered attentional bias modification training for smokers. Psychol Addict Behav. (2022) 36:906–19. doi: 10.1037/adb0000805
85. Taylor, A, and Katomeri, M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob Res. (2007) 9:1183–90. doi: 10.1080/14622200701648896
86. Janse Van Rensburg, K, Taylor, A, Hodgson, T, and Benattayallah, A. Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: an fMRI study. Psychopharmacology. (2009) 203:589–98. doi: 10.1007/s00213-008-1405-3
87. Van Rensburg, KJ, Taylor, A, and Hodgson, T. The effects of acute exercise on attentional bias towards smoking-related stimuli during temporary abstinence from smoking. Addiction. (2009) 104:1910–7. doi: 10.1111/j.1360-0443.2009.02692.x
88. Kamboj, SK, Joye, A, Das, RK, Gibson, AJ, Morgan, CJ, and Curran, HV. Cue exposure and response prevention with heavy smokers: a laboratory-based randomised placebo-controlled trial examining the effects of D-cycloserine on cue reactivity and attentional bias. Psychopharmacology. (2012) 221:273–84. doi: 10.1007/s00213-011-2571-2
89. Das, RK, Hindocha, C, Freeman, TP, Lazzarino, AI, Curran, HV, and Kamboj, SK. Assessing the translational feasibility of pharmacological drug memory reconsolidation blockade with memantine in quitting smokers. Psychopharmacology. (2015) 232:3363–74. doi: 10.1007/s00213-015-3990-2
90. Begh, R, Munafò, MR, Shiffman, S, Ferguson, SG, Nichols, L, Mohammed, MA, et al. Lack of attentional retraining effects in cigarette smokers attempting cessation: a proof of concept double-blind randomised controlled trial. Drug Alcohol Depend. (2015) 149:158–65. doi: 10.1016/j.drugalcdep.2015.01.041
91. Hussain, S, Zawertailo, L, Busto, U, Zack, M, Farvolden, P, and Selby, P. The impact of chronic bupropion on plasma cotinine and on the subjective effects of ad lib smoking: a randomized controlled trial in unmotivated smokers. Addict Behav. (2010) 35:164–7. doi: 10.1016/j.addbeh.2009.09.004
92. Gendy, MNS, Di Ciano, P, Kowalczyk, WJ, Barrett, SP, George, TP, Heishman, S, et al. Testing the PPAR hypothesis of tobacco use disorder in humans: a randomized trial of the impact of gemfibrozil (a partial PPARα agonist) in smokers. PLoS One. (2018) 13:e0201512. doi: 10.1371/journal.pone.0201512
93. Fong, AJ, De Jesus, S, Bray, SR, and Prapavessis, H. Effect of exercise on cigarette cravings and ad libitum smoking following concurrent stressors. Addict Behav. (2014) 39:1516–21. doi: 10.1016/j.addbeh.2014.05.027
94. Schlagintweit, HE, Good, KP, and Barrett, SP. The impact of nicotine lozenges and stimulus expectancies on cigarette craving. J Psychopharmacol. (2014) 28:773–9. doi: 10.1177/0269881113519508
95. Schlagintweit, HE, and Barrett, SP. Does acute tobacco smoking prevent cue-induced craving? J Psychopharmacol. (2016) 30:468–73. doi: 10.1177/0269881116639288
96. Barnabe, A, Gamache, K, de Camargo, JVP, Allen-Flanagan, E, Rioux, M, Pruessner, J, et al. A novel stress-based intervention reduces cigarette use in non-treatment seeking smokers. Neuropsychopharmacology: official publication of the American college of. Neuropsychopharmacology. (2023) 48:308–16. doi: 10.1038/s41386-022-01455-6
97. Liu, Y, Sun, HQ, Bao, YP, Li, SX, Beveridge, TJ, Di, XL, et al. Subjective, cognitive/psychomotor, and physiological effects of aripiprazole in Chinese light and heavy smokers. Drug Alcohol Depend. (2009) 101:42–52. doi: 10.1016/j.drugalcdep.2008.10.024
98. Meng, Z, Liu, C, Yu, C, and Ma, Y. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates smoking behavior. J Psychiatr Res. (2014) 54:19–25. doi: 10.1016/j.jpsychires.2014.03.007
99. Yang, LZ, Shi, B, Li, H, Zhang, W, Liu, Y, Wang, H, et al. Electrical stimulation reduces smokers' craving by modulating the coupling between dorsal lateral prefrontal cortex and parahippocampal gyrus. Soc Cogn Affect Neurosci. (2017) 12:1296–302. doi: 10.1093/scan/nsx055
100. Fregni, F, Liguori, P, Fecteau, S, Nitsche, MA, Pascual-Leone, A, and Boggio, PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry. (2008) 69:32–40. doi: 10.4088/jcp.v69n0105
101. Marques, RC, Marques, D, Vieira, L, and Cantilino, A. Left frontal pole repetitive transcranial magnetic stimulation reduces cigarette cue-reactivity in correlation with verbal memory performance. Drug Alcohol Depend. (2022) 235:109450. doi: 10.1016/j.drugalcdep.2022.109450
102. Rabinovitz, S. Effects of omega-3 fatty acids on tobacco craving in cigarette smokers: a double-blind, randomized, placebo-controlled pilot study. J Psychopharmacol. (2014) 28:804–9. doi: 10.1177/0269881114536477
103. Elfeddali, I, de Vries, H, Bolman, C, Pronk, T, and Wiers, RW. A randomized controlled trial of web-based attentional Bias modification to help smokers quit. Health Psychol. (2016) 35:870–80. doi: 10.1037/hea0000346
104. Havermans, A, Vuurman, EF, van den Hurk, J, Hoogsteder, P, and van Schayck, OC. Treatment with a nicotine vaccine does not lead to changes in brain activity during smoking cue exposure or a working memory task. Addiction. (2014) 109:1260–7. doi: 10.1111/add.12577
105. Andreu, CI, Cosmelli, D, Slagter, HA, and Franken, IHA. Effects of a brief mindfulness-meditation intervention on neural measures of response inhibition in cigarette smokers. PLoS One. (2018) 13:e0191661. doi: 10.1371/journal.pone.0191661
106. Kim, DY, Yoo, SS, Tegethoff, M, Meinlschmidt, G, and Lee, JH. The inclusion of functional connectivity information into fMRI-based neurofeedback improves its efficacy in the reduction of cigarette cravings. J Cogn Neurosci. (2015) 27:1552–72. doi: 10.1162/jocn_a_00802
107. Haarmann, H, Gossler, A, Herrmann, P, Bonev, S, Nguyen, XP, Hasenfuß, G, et al. Effects of varenicline on sympatho-vagal balance and cue reactivity during smoking withdrawal: a randomised placebo-controlled trial. Tob Induc Dis. (2016) 14:26. doi: 10.1186/s12971-016-0091-x
108. Malbos, E, Borwell, B, Einig-Iscain, M, Korchia, T, Cantalupi, R, Boyer, L, et al. Virtual reality cue exposure therapy for tobacco relapse prevention: a comparative study with standard intervention. Psychol Med. (2022):1–11. doi: 10.1017/s0033291722002070
109. Li, X, Du, L, Sahlem, GL, Badran, BW, Henderson, S, and George, MS. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend. (2017) 174:98–105. doi: 10.1016/j.drugalcdep.2017.02.002
110. Butler, K, and Le Foll, B. Novel therapeutic and drug development strategies for tobacco use disorder: endocannabinoid modulation. Expert Opin Drug Discovery. (2020) 15:1065–80. doi: 10.1080/17460441.2020.1767581
111. Chiamulera, C, Marzo, CM, and Balfour, DJK. Metabotropic glutamate receptor 5 as a potential target for smoking cessation. Psychopharmacology. (2017) 234:1357–70. doi: 10.1007/s00213-016-4487-3
112. Hindocha, C, Freeman, TP, Grabski, M, Stroud, JB, Crudgington, H, Davies, AC, et al. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction. (2018) 113:1696–705. doi: 10.1111/add.14243
113. Ramachandran Nair, L, and Liu, X. Targeting the α4β2-and α7-subtypes of nicotinic acetylcholine receptors for smoking cessation medication development. J Addict Res Ther. (2019) 10:381
114. Krause, D, Warnecke, M, Schuetz, CG, Soyka, M, Manz, KM, Proebstl, L, et al. The impact of the opioid antagonist naloxone on experimentally induced craving in nicotine-dependent individuals. Eur Addict Res. (2018) 24:255–65. doi: 10.1159/000494346
115. Wing, VC, Barr, MS, Wass, CE, Lipsman, N, Lozano, AM, Daskalakis, ZJ, et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. (2013) 6:221–30. doi: 10.1016/j.brs.2012.06.008
116. Falcone, M, Bernardo, L, Ashare, RL, Hamilton, R, Faseyitan, O, McKee, SA, et al. Transcranial direct current brain stimulation increases ability to resist smoking. Brain Stimul. (2016) 9:191–6. doi: 10.1016/j.brs.2015.10.004
117. Mondino, M, Luck, D, Grot, S, Januel, D, Suaud-Chagny, MF, Poulet, E, et al. Effects of repeated transcranial direct current stimulation on smoking, craving and brain reactivity to smoking cues. Sci Rep. (2018) 8:8724. doi: 10.1038/s41598-018-27057-1
118. Kragel, EA, Sweitzer, MM, and Davis, JM. The effect of brief mindfulness training on brain reactivity to food cues during nicotine withdrawal: a pilot functional imaging study. Mindfulness. (2019) 10:2272–6. doi: 10.1007/s12671-019-01201-y
119. Li, X, Chen, L, Ma, R, Wang, H, Wan, L, Wang, Y, et al. The top-down regulation from the prefrontal cortex to insula via hypnotic aversion suggestions reduces smoking craving. Hum Brain Mapp. (2019) 40:1718–28. doi: 10.1002/hbm.24483
120. González-Roz, A, Secades-Villa, R, Pericot-Valverde, I, Weidberg, S, and Alonso-Pérez, F. Effects of delay discounting and other predictors on smoking relapse. Span J Psychol. (2019) 22:E9. doi: 10.1017/sjp.2019.11
121. Karch, S, Paolini, M, Gschwendtner, S, Jeanty, H, Reckenfelderbäumer, A, Yaseen, O, et al. Real-time fMRI neurofeedback in patients with tobacco use disorder during smoking cessation: functional differences and implications of the first training session in regard to future abstinence or relapse. Front Hum Neurosci. (2019) 13:65. doi: 10.3389/fnhum.2019.00065
122. Mondino, M, Lenglos, C, Cinti, A, Renauld, E, and Fecteau, S. Eye tracking of smoking-related stimuli in tobacco use disorder: a proof-of-concept study combining attention bias modification with alpha-transcranial alternating current stimulation. Drug Alcohol Depend. (2020) 214:108152. doi: 10.1016/j.drugalcdep.2020.108152
123. Zamboni, L, Campagnari, S, Giordano, R, Fusina, F, Carli, S, Congiu, A, et al. A virtual reality craving study in tobacco addiction: the role of non-pharmacological support in tobacco detox therapy. Front Psych. (2022) 13:940100. doi: 10.3389/fpsyt.2022.940100
124. De Bruijn, GJ, De Vries, J, Bolman, C, and Wiers, R. (no) escape from reality? Cigarette craving in virtual smoking environments. J Behav Med. (2021) 44:138–43. doi: 10.1007/s10865-020-00170-1
125. Brandon, KO, Vinci, C, Kleinjan, M, Hernandez, LM, Sawyer, LE, Sutton, SK, et al. Testing augmented reality for eliciting Cue-provoked urges to smoke: toward moving Cue-exposure into the real world. Nicotine Tob Res. (2021) 23:861–5. doi: 10.1093/ntr/ntaa259
126. Vinci, C, Brandon, KO, Kleinjan, M, Hernandez, LM, Sawyer, LE, Haneke, J, et al. Augmented reality for smoking cessation: development and usability study. JMIR Mhealth Uhealth. (2020) 8:e21643. doi: 10.2196/21643
127. Keijsers, M, Vega-Corredor, MC, Tomintz, M, and Hoermann, S. Virtual reality technology use in cigarette craving and smoking interventions (I "virtually" quit): systematic review. J Med Internet Res. (2021) 23:e24307. doi: 10.2196/24307
128. Moon, J, and Lee, JH. Cue exposure treatment in a virtual environment to reduce nicotine craving: a functional MRI study. Cycberpsychol Behav. (2009) 12:43–5. doi: 10.1089/cpb.2008.0032
129. Lee, J, Lim, Y, Graham, SJ, Kim, G, Wiederhold, BK, Wiederhold, MD, et al. Nicotine craving and cue exposure therapy by using virtual environments. Cycberpsychol Behav. (2004) 7:705–13. doi: 10.1089/cpb.2004.7.705
130. Choi, JS, Park, S, Lee, JY, Jung, HY, Lee, HW, Jin, CH, et al. The effect of repeated virtual nicotine cue exposure therapy on the psychophysiological responses: a preliminary study. Psychiatry Investig. (2011) 8:155–60. doi: 10.4306/pi.2011.8.2.155
131. Pericot-Valverde, I, Secades-Villa, R, Gutiérrez-Maldonado, J, and García-Rodríguez, O. Effects of systematic cue exposure through virtual reality on cigarette craving. Nicotine Tob Res. (2014) 16:1470–7. doi: 10.1093/ntr/ntu104
132. Pericot-Valverde, I, Secades-Villa, R, and Gutiérrez-Maldonado, J. A randomized clinical trial of cue exposure treatment through virtual reality for smoking cessation. J Subst Abus Treat. (2019) 96:26–32. doi: 10.1016/j.jsat.2018.10.003
133. Hong, JS, Kim, SM, Jung, HY, Kang, KD, Min, KJ, and Han, DH. Cognitive avoidance and aversive cues related to tobacco in male smokers. Addict Behav. (2017) 73:158–64. doi: 10.1016/j.addbeh.2017.05.003
134. Stritzke, WG, Breiner, MJ, Curtin, JJ, and Lang, AR. Assessment of substance cue reactivity: advances in reliability, specificity, and validity. Psychol Addict Behav. (2004) 18:148–59. doi: 10.1037/0893-164x.18.2.148
135. Manoliu, A, Haugg, A, Sladky, R, Hulka, L, Kirschner, M, Brühl, AB, et al. Smo CuDa: a validated smoking Cue database to reliably induce craving in tobacco use disorder. Eur Addict Res. (2021) 27:107–14. doi: 10.1159/000509758
136. Grant, BF, Hasin, DS, Blanco, C, Stinson, FS, Chou, SP, Goldstein, RB, et al. The epidemiology of social anxiety disorder in the United States: results from the National Epidemiologic Survey on alcohol and related conditions. J Clin Psychiatry. (2005) 66:1351–61. doi: 10.4088/jcp.v66n1102
137. Hasin, D, and Kilcoyne, B. Comorbidity of psychiatric and substance use disorders in the United States: current issues and findings from the NESARC. Curr Opin Psychiatry. (2012) 25:165–71. doi: 10.1097/YCO.0b013e3283523dcc
138. Grant, BF, Saha, TD, Ruan, WJ, Goldstein, RB, Chou, SP, Jung, J, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on alcohol and related conditions-III. JAMA Psychiat. (2016) 73:39–47. doi: 10.1001/jamapsychiatry.2015.2132
139. Hasin, DS, Sarvet, AL, Meyers, JL, Saha, TD, Ruan, WJ, Stohl, M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. (2018) 75:336–46. doi: 10.1001/jamapsychiatry.2017.4602
140. Evans-Polce, RJ, Kcomt, L, Veliz, PT, Boyd, CJ, and McCabe, SE. Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates. Am J Psychiatry. (2020) 177:1073–81. doi: 10.1176/appi.ajp.2020.20010005
141. Potvin, S, Dugré, JR, Fahim, C, and Dumais, A. Increased connectivity between the nucleus Accumbens and the default mode network in patients with schizophrenia during cigarette cravings. J Dual Diagn. (2019) 15:8–15. doi: 10.1080/15504263.2018.1526432
142. Janes, AC, Gilman, JM, Frederick, BB, Radoman, M, Pachas, G, Fava, M, et al. Salience network coupling is linked to both tobacco smoking and symptoms of attention deficit hyperactivity disorder (ADHD). Drug Alcohol Depend. (2018) 182:93–7. doi: 10.1016/j.drugalcdep.2017.11.005
143. Ely, AV, Jagannathan, K, Hager, N, Ketcherside, A, Franklin, TR, and Wetherill, RR. Double jeopardy: comorbid obesity and cigarette smoking are linked to neurobiological alterations in inhibitory control during smoking cue exposure. Addict Biol. (2020) 25:e12750. doi: 10.1111/adb.12750
Keywords: tobacco use disorder, cue-reactivity, smoking cessation, intervention, cue-reactivity paradigms, scoping review
Citation: Luo M, Gan Q, Fu Y and Chen Z (2023) Cue-reactivity targeted smoking cessation intervention in individuals with tobacco use disorder: a scoping review. Front. Psychiatry. 14:1167283. doi: 10.3389/fpsyt.2023.1167283
Received: 16 February 2023; Accepted: 18 August 2023;
Published: 07 September 2023.
Edited by:
Yasser Khazaal, Université de Lausanne, SwitzerlandReviewed by:
Sarah Gerhardt, University of Heidelberg, GermanyCopyright © 2023 Luo, Gan, Fu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuangfei Chen, Y2hlbi56aGZAb3V0bG9vay5jb20=
†These authors have contributed equally to this work and share first authorship
‡Quan Gan is officially working at International Agency for Research on Cancer of the World Health Organization since October 2022. This work was completed during his study at the Université Paris-Saclay
#ORCID: Miaoling Luo, https://orcid.org/0000-0002-2528-8990
Quan Gan, https://orcid.org/0000-0001-7869-1348
Yu Fu, https://orcid.org/0000-0002-6038-3766
Zhuangfei Chen, https://orcid.org/0000-0001-5777-8954
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.