- 1Department of Psychiatry, Third People’s Hospital of Fuyang, Fuyang, Anhui, China
- 2Department of Psychiatry, Chaohu Hospital Affiliated to Anhui Medical University, Hefei, Anhui, China
- 3School of Mental Health and Psychological Sciences, Anhui Medical University, Hefei, Anhui, China
Objective: To evaluate the clinical value of systemic immune-inflammation index (SII) based on peripheral blood neutrophil, lymphocyte, and platelet count in evaluating the subtype and severity of depression in patients with depressive disorder.
Methods: This retrospective cohort study was conducted in the Third People’s Hospital of Fuyang City from January 1, 2020 to December 31, 2022. The data included sociodemographic information at admission, clinical data, discharge diagnosis and inflammatory markers. Patients were divided into low SII group and high SII group according to the optimal threshold of SII determined by receiver operating characteristic curve (ROC curve). Binary logistic regression was used to analyze the correlation between moderate/major depression and SII level.
Results: Compared to the low SII group, the high SII group had a higher age level (χ2 = 7.663, p = 0.006), more smokers (χ2 = 9.458, p = 0.002), more moderate/major depression patients (χ2 = 45.645, p < 0.001), and a higher proportion of patients with accompanying somatic symptoms (χ2 = 14.867, p < 0.001). In the final logistic regression model, after controlling for confounding factors, SII at admission was significantly associated with moderate/major depression [β =1.285, p < 0.001; odds ratio (95% confidence intervals) = 3.614 (2.693–4.850)]. Patients with high SII scores were 3.614 times more likely to have moderate/severe depression than those with low SII scores. We propose a cut-off value of SII =540.78 (sensitivity = 36.4% and specificity = 80.3%) according to the maximum Youden index.
Conclusion: Our research indicates that SII may be a useful, repeatable, convenient, and affordable index to identify moderate/major depression in depressive disorder.
1. Introduction
Depression is a frequent illness severely limiting psychosocial functioning and reducing quality of life. In 2008, the World Health Organization ranked depression as the third leading cause of disease burden worldwide and predicts that by 2031, depression will rank first (1). Depressive disorders are heterogeneous syndromes in which individuals affected by them differ significantly in their symptom profile and response to treatment, and the detection and diagnosis of depression often pose a challenge for clinicians. Recognition of this heterogeneity in depression has long led researchers to identify meaningful and valid symptom-based subtypes to guide etiologic research and to help develop more targeted interventions and treatment programs (2). Over the past decade, the use of the 10th edition of the International Classification of Diseases (ICD-10) by most general medical practices and clinicians in China to assign diagnostic categories or subtypes to individuals is clinically necessary to elucidate the heterogeneity and severity of depressive symptoms. Three different forms of depressive episodes are described in ICD-10: mild, moderate, and major. The distinction between the three relies on complex clinical judgments, including the number and type of symptoms and their severity. Approximately 30% of patients do not respond to first-line antidepressants, so there is an urgent need to explore new therapeutic targets (3).
An increasing number of studies suggest the involvement of immune-inflammatory responses as well as neuroinflammation in the pathophysiological processes of depression, while an increasing number of data on the relationship between these processes and classical neurotransmitters, the hypothalamic-pituitary-adrenal axis (HPA) and inflammatory factors have been reported (4–7). Experimental animal studies in animal models of immune inflammation and depression have found that lipopolysaccharide (LPS), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and other pro-inflammatory factors induce inflammatory responses in the body leading to a lack of pleasure as well as depression-like behavior in mice (8, 9). Paroxetine directly affects the macrophage response to inflammatory stimuli, for example, paroxetine significantly inhibits LPS-induced IL-6 production (9), which may be one of the mechanisms of action for its therapeutic effect on depression. Several clinical trials have shown widespread activation of the immune inflammatory response in groups of patients with depressive disorders (10, 11), and meta-analyses have shown that antidepressants reduce some markers of peripheral inflammation, such as significantly reduced levels of peripheral blood interleukin-1β (IL-1β), IL-6, TNF-α and interleukin-10 (IL-10) (10).
The baseline systemic immune-inflammation index (SII) is an indicator of immune response and systemic inflammation based on peripheral blood platelet, lymphocyte, and neutrophil counts, and is a simple way to objectively reflect the balance between inflammatory and immune responses (12, 13). SII calculated using the hematological findings of ischemic stroke patients at the time of admission, has shown good results in predicting post-stroke depression (PSD) at one month (14). Large-scale studies in people recovering from COVID have shown that SII is significantly and positively associated with depression and anxiety scores (15). SII measured in the emergency department, predicts more severe autonomic depression and post-traumatic stress disorder (16). However, there is a paucity of relevant studies on the potential evaluative value of SII for diagnostic subtypes in patients with depressive disorders to date.
The purpose of this study is to explore the clinicopathological characteristics of depressed patients with high SII, to explore the potential of the SII index in the assessment of diagnostic subtypes in patients with depressive disorders, and to provide a simpler diagnostic assessment tool for the clinic from a new perspective.
2. Materials and methods
2.1. Study participants
This is a retrospective cohort study. Patients who were hospitalized and diagnosed with the depressive disorder at the Third People’s Hospital of Fuyang City from January 2020 to December 2022 were consecutively enrolled. Depressive disorders were defined and diagnosed by psychiatrists according to 10th revision Diagnostic Criteria for Mental Behavior Disorders of International Statistical Classification of Diseases and Related Health Problems (ICD-10). The Hamilton Depression Scale (HDRS) is used to assess the severity of depressive symptoms. Hamilton total score was evaluated as follows: 8–16: mild depression; 17–23: moderate depression; and ≥24: major depression (17). The administration of HDRS involves structured interviews with patients, and each item is rated based on patient response. Interviews are conducted by trained psychiatrists, among whom there has been consistent training. Prior to patient assessment using ICD-10 and the HDRS, psychiatrists were trained in consistency to assure consistency among psychiatrists. The inclusion criteria in this study were as follows: (1) meeting the diagnostic criteria of depressive disorder in ICD-10; (2) age ≤ 85 years old. Exclusion criteria were as follows: (1) psychiatric disorders other than depressive disorders; (2) comorbid severe physical diseases; (3) pregnant and lactating women; (4) suffering from systemic or local inflammatory diseases and inflammatory immune diseases.
This study was approved by the Ethics Committee of the Third People’s Hospital of Fuyang City (Ethics Approval No.: 2019-340-07) and was performed in accordance with the ethical standards of the Declaration of Helsinki. Patients who met the criteria had signed a written informed consent and were allowed to voluntarily withdraw from this study at any time. A total of 1,478 patients were initially enrolled in this study, of whom 102 met the exclusion criteria and 64 had insufficient baseline clinical information, and 1,312 patients were finally included in the study as study subjects.
2.2. Clinical data collection
Baseline demographic characteristics (gender, age, marital status, education level, smoking history), clinical manifestations (whether accompanied by somatic symptoms, psychiatric symptoms, whether this was a first episode or a relapse), discharge diagnosis, and hematological data on admission (lymphocyte count, neutrophil count, platelet count) were collected from the medical records. Blood samples were obtained from the cephalic vein of participants after fasting for at least 8 h between 6:00 and 8:00 a.m., and were collected in EDTA tubes. The complete blood counts, which included measurements of total white blood cells, neutrophils, lymphocytes, monocytes, and platelets, were analyzed using a Mindray BC-5380/BC-5180 Hematology Analyzer (Shenzhen, China). Subsequently, SII, NLR, MLR, and PLR were calculated using the appropriate formulas. SII = platelet count neutrophil count/lymphocyte count. Neutrophil-to-lymphocyte ratio (NLR) = neutrophil count/lymphocyte count. Platelet-to-lymphocyte ratio (PLR) = platelet count/lymphocyte count. Monocyte-to-lymphocyte ratio (MLR) = monocyte count/lymphocyte count.
2.3. Statistical analysis
All data were statistically analyzed using SPSS 26.0 software. Data are presented as frequencies and percentages for categorical variables and as mean (standard deviation) for numeric variables. Analysis of variance (ANOVA) or chi-square test or Mann–Whitney U test were used to compare demographic and clinicopathological features among each group. A receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off point for biomarkers to detect moderate/major depression. The optimal cut-off point is the point on the ROC curve that corresponds to the maximum Youden index. The area under the ROC curve (AUC) was performed to determine the predictive value of various indicators for moderate/major depression. A binary logistic regression model (enter) was estimated to determine the independent factors associated with moderate/major depression in patients with depressive disorder. The level of statistical significance was set at α = 0.05 (two-sided).
3. Results
3.1. Demographic characteristics
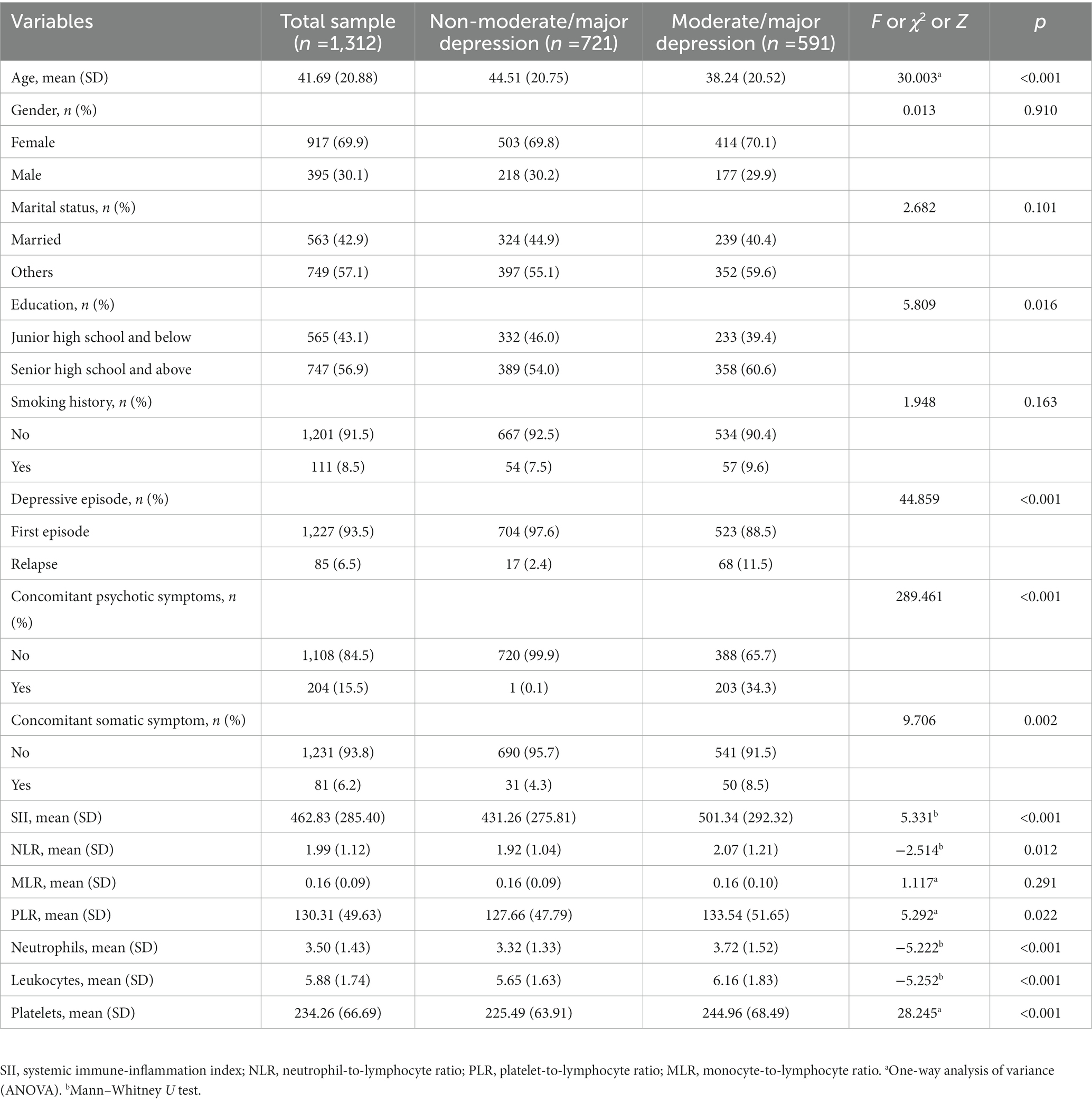
A total of 1,312 patients with depressive disorder were enrolled according to the inclusion and exclusion criteria. On average, the age of the entire sample was 41.69 years old (SD = 20.88; range 9 to 81), most patients were female (917 cases, 91.3%), besides, more than half of patients were unmarried (749 cases, 57%) and most had no history of smoking (1,201 cases, 92%). SII mean was 462.83 (SD = 285.40; Range 68.87 to 1886.67). Among them, 591 patients (45%) were moderate or major depression patients, and 721 patients (55%) were non-moderate or major depression patients, according to the admission diagnosis. Table 1 presents demographic and clinical characteristics by diagnostic group. There were no significant differences in gender, marital status, and smoking history between the two groups. Age, education, depression, concomitant psychotic symptoms, concomitant symptoms, SII, NLR, PLR, SII, neutrophils, leukocytes, and platelets were significantly different among the groups.
3.2. Baseline SII and optimal thresholds
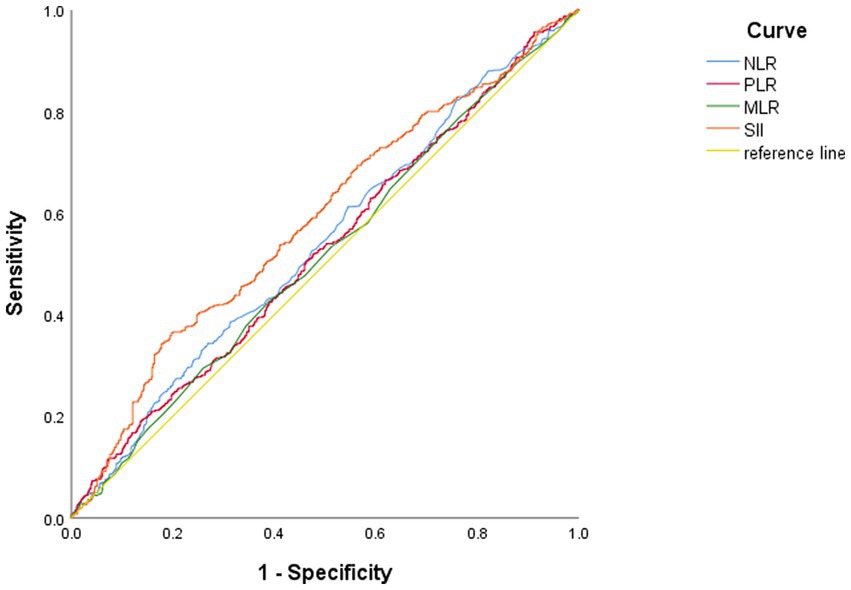
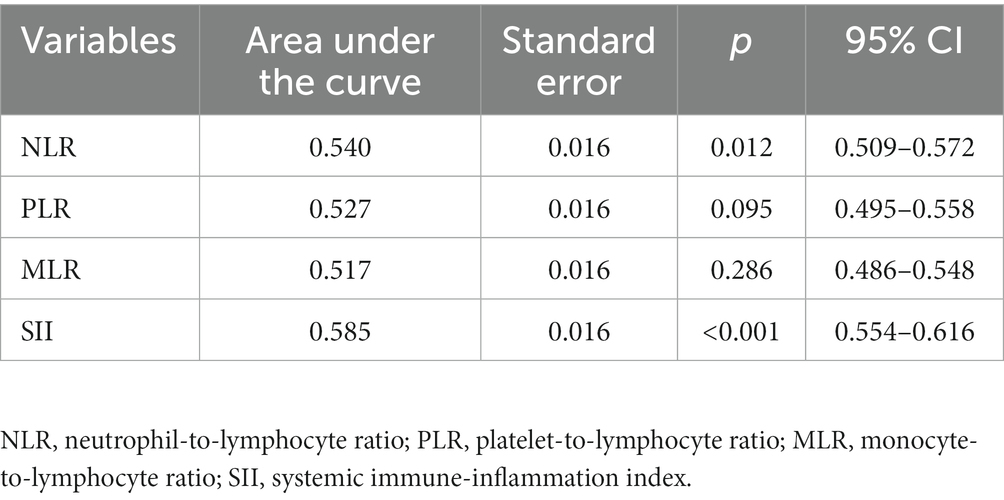
ROC curve (Figure 1 and Table 2) shows that the AUC value is 0.585 (95% CI: 0.554–0.616, p < 0.01). The optimal cut-off point is the point on the ROC curve that corresponds to the maximum Youden index. The optimal cut-off point of SII to determine moderate/major depression in patients with the depressive disorder was 540.78, the sensitivity was 0.364, the specificity was 0.803, and the Youden’s index was 0.167. Patients were grouped according to this optimal cutoff value: 357 (27%) patients in the high SII group (SII ≥ 540.78) and 955 (73%) patients in the low SII group (SII < 540.78). When NLR, MLR and PLR were used for ROC analysis, the areas under the curve were 0.54, 0.517 and 0.527, respectively (Table 2).
3.3. Relationship between baseline SII and clinicopathologic features
Compared with the low SII group, the high SII group had a higher age of onset of depressive disorder (χ2 = 7.663, p = 0.006), more smokers (χ2 = 9.458, p = 0.002), more moderate/major depression (χ2 = 45.645, p < 0.001), and a lower number of relapse (χ2 = 9.343, p = 0.002), and the proportion of patients with somatic symptoms was higher (χ2 = 14.867, p < 0.001), the difference was statistically significant. The levels of neutrophils, leukocytes, platelets, SII, NLR, MLR, PLR were significantly higher in the high SII group compared to the low SII group, with statistical significance (all p < 0.05). There were no statistically significant differences in gender, age, education level and whether there were accompanying psychiatric symptoms between the two groups (all p > 0.05), as shown in Table 3.
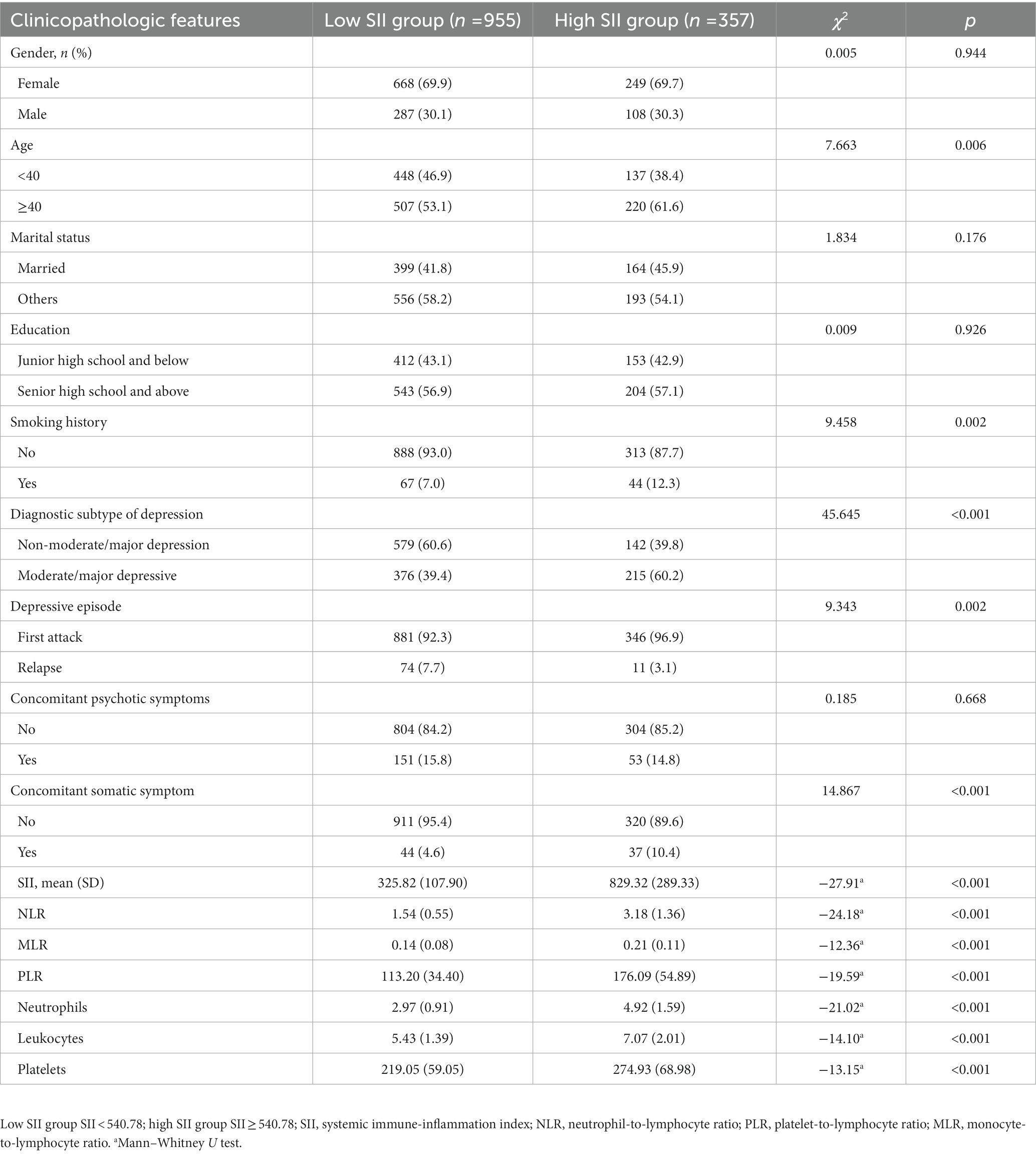
Table 3. Comparison of clinicopathologic features of patients with depressive disorder between the two groups.
3.4. Binary logistic regression analysis of influencing factors of moderate/major depression subtype in patients with depressive disorder
Moderate/major depression were used as the dependent variable (moderate/major depressive was 1, non-moderate/major depressive was 0), marital status (married was 1, other was 0), age classification (≥40 years old: 1, < 40 years old: 0), gender (male was 1, female was 0), education level (high school education or above was 1, Junior high school or below education: 0), smoking (smoking history: 1; no smoking history: 0), somatic symptoms (positive: 1; negative: 0), psychotic symptoms (positive: 1; negative: 0), recurrent depressive disorder (recurrent: 1; first episode: 0), SII (SII ≥ 540.78: 1; SII < 540.78: 0) as independent variable, binary multi-factor logistic regression analysis (Table 3) was performed, and the results showed that concomitant somatic symptoms (p < 0.001, OR = 4.09), concomitant psychotic symptoms (p < 0.001, OR = 574.365), relapse (p < 0.001, OR = 14.312), age (p = 0.022, OR = 0.692), SII level (p < 0.001, OR = 3.614) was significantly associated with moderate/major depression. SII ≥ 540.78 was a risk factor for moderate/major depression in patients with depressive disorder (p < 0.001), as shown in Table 4.
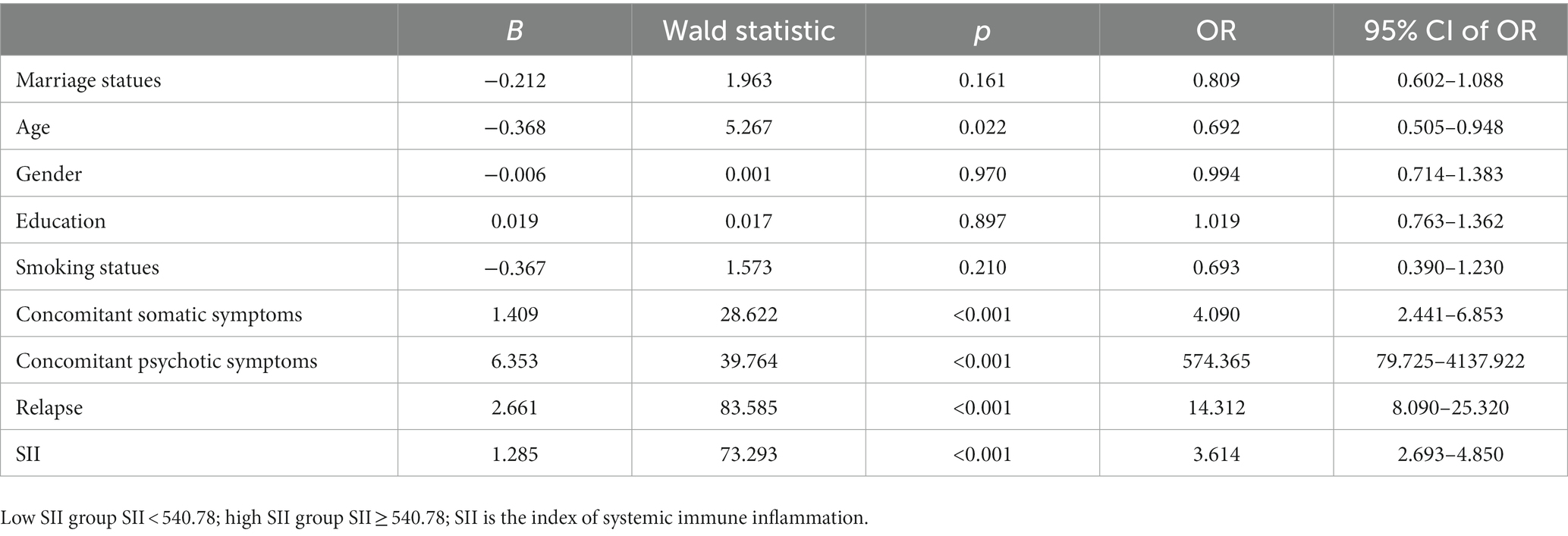
Table 4. Binary logistic regression analysis of influencing factors of moderate/major depression subtypes in patients with depressive disorder.
4. Discussion
Depressive disorder is a disease with high morbidity and disability rate. This study showed that patients with depressive disorder in the high SII group were older, had a higher odds of a history of smoking history, had a higher proportion of moderate/major depression, and had a higher proportion of accompanying somatic symptoms. At the same time, high SII is a risk factor for moderate/major depression in patients with depressive disorder.
This study demonstrated the potential application value of SII index on admission to determine the severity of depression. The SII index objectively reflects the balance between the inflammatory and immune responses of the body. It provides a simple, rapid, and inexpensive way to measure the level of inflammation. The SII index can be calculated by the simple parameters in the blood routine upon admission. For patients with high SII depression, the occurrence of moderate/major depression can be warned at an early stage. The incidence of suicide, somatic, and psychotic symptoms in patients with moderate/major depression are relatively high. Therefore, individualized treatment programs including psychological and physical therapy can be actively adopted in the follow-up treatment to reduce the incidence of suicide and improve the clinical cure rate (1). On the other hand, currently used antidepressants target and promote the neurotransmitter effects of monoamine, yet approximately 30% of patients do not respond to these drugs, so there is an urgent need to explore new therapeutic targets (3). Some clinical studies have reported increased levels of inflammatory cytokines and neutrophils in the blood of patients with major depression, and higher levels of interleukin 6 in the blood of drug-resistant patients (18, 19). A meta-analysis of whole blood gene expression in patients with major depression revealed upregulation of neutrophil-related genes (20). These findings may support the role of neuroinflammation in the etiology of major depression or treatment-resistant depression, and targeting this pathway may have value in the treatment of depression.
The close relationship between SII and depression has been verified in many patients with other physical diseases complicated with depression, which is consistent with the results of this study, indicating that SII has potential application value in the assessment of depression or anxiety related to physical diseases. For example, among patients with ischemic stroke, SII, platelet/lymphocyte ratio (PLR), and neutrophil/lymphocyte ratio (NLR) increased at admission, in particular, increased SII was significantly associated with the occurrence of post-ischemic stroke depression one month later, which may provide some prognostic clues for early detection of post-ischemic stroke depression (14). In the tuberculosis population, patients with symptoms of depression or anxiety had poorer cellular immune status and stronger inflammatory response than patients without symptoms of anxiety or depression, and a higher SII was significantly associated with symptoms of depression or anxiety (p < 0.05) (21). A study of 2,566 patients with diabetes (including 370 patients diagnosed with comorbidity depression) showed that high SII levels were an independent risk factor for diabetic depression (OR = 1.347, 95%CI: 1.031–1.760, p = 0.02). The researchers further validated the relationship between SII and diabetic depression by using propensity matching analysis, and SII may be an accessible and cost-effective strategy for identifying depression in diabetic patients (22). Data from a follow-up study of persistent psychopathology and cognitive impairment in COVID-19 survivors showed that baseline SII predicted self-rated depressive symptoms and cognitive impairment at three-month follow-up, and changes in SII predicted changes in depression severity during follow-up. Baseline SII can predict neurocognitive impairments associated with the severity of depressive psychopathology, including processing speed, verbal memory and fluency, and psychomotor coordination. The investigators hypothesize that COVID-19 may lead to chronic systemic inflammation, redisplaying patients to persistent depression and related neurocognitive dysfunction (23).
More and more animal models and clinical studies of depression suggest that immunoinflammatory and neuroinflammatory pathways are involved in the pathophysiological processes of depression. Studies on immune inflammation and depression animal models have shown that systemic inflammatory response induced by lipopolysaccharide, tumor necrosis factor α and other pro-inflammatory factors can lead to depression-like behavior in mice (24–26). Compared with healthy controls, the levels of interleukin-4, interleukin-6, and interleukin-10 changed significantly in major depressive disorder patients (27). The role of inflammation in the etiology and exacerbation of depression is further supported by studies showing that increased levels of interleukin-6 in childhood increase the risk of depression later in life (26), while postmortem examination of the brains of depressed patients shows significant neuroinflammation and widespread activation of microglia (28).
Platelets are a specific inflammatory indicator. Platelets are increasingly recognized to play an important role in inflammation and as a putative bridge between mental illness and inflammatory response. Platelet overactivation has been observed in patients with depressive disorders, and platelet parameters have potential predictive power for major depression and bipolar disorder (29). Inflammatory mediators generate activated platelets by activating macrophages. On the one hand, they activate dense particles inside platelets to release serotonin; on the other hand, activating platelets further enhances vascular permeability by releasing pro-inflammatory factors, gathering monocytes, and enhancing inflammatory response (30). Serotonin and pro-inflammatory factors derived from platelet activation are involved in initiating, maintaining, and regulating inflammatory responses, which play an important role in the occurrence and development of depression (30). Thus, platelet activation is involved in systemic inflammatory responses and pathophysiological processes of depression.
Peripheral blood neutrophil/lymphocyte ratio (NLR) is a component of the SII formula and a simple measure to evaluate the inflammatory status and can economically and easily detect the activation of inflammatory systems. More and more studies have shown that the level of NLR in patients with depressive disorder is higher than that in healthy control volunteers, and is closely related to the degree of depression. For example, one study showed significantly higher levels of NLR in patients with affective disorders, and may assess the course of affective disorders (31). Another study also revealed that non-medicated patients with major depressive episodes had higher levels of NLR than healthy controls (32). In male patients with depression, NLR was significantly correlated with depressive symptoms, and the higher the NLR, the more severe the symptoms (33). NLR has become a potential biomarker of inflammation in depressed patients with suicide attempts (34), HAMD scores in depressed patients were positively correlated with NLR levels in depressed patients, with NLR ≥ 1.57 being an independent predictor of severe or very severe depression (35). In the group of adolescents with major depressive disorder, disease severity was positively correlated with NLR, which may support the hypothesis that inflammation plays an important role in the etiology of major depressive disorder in adolescents (36).
The novelty of this study lies in the identification of SII index as a potential predictor for moderate/severe depression. This suggests that monitoring SII levels could help in identifying individuals who are at risk for developing moderate/severe depression, and potentially enable early intervention and treatment. This finding also adds to the growing body of literature on the complex relationship between inflammation and mental health, and highlights the potential role of SII as a novel biomarker for depression risk assessment.
This study has the following deficiencies: (1) there is a lack of information on potential confounders, such as whether the patient was taking any psychiatric medications at the time of admission, especially antidepressants, including the dose and duration of treatment, as previously stated in the literature, antidepressants have anti-inflammatory effects (37, 38). (2) This study was a non-multicenter study; (3) the ability to detect biomarkers reflecting inflammatory and immune responses was insufficient, which could not fully reflect the pathophysiological process of depression; (4) important psychosocial stress situations related to depressive events and pre-admission treatment were not collected, such as negative life events, antidepressant medication or psychotherapy at baseline, etc. These factors may affect the diagnostic subtypes of depressive disorder. (5) SII is only calculated by the blood routine results of patients upon admission, but this indicator has dynamic changes in the course and development of the depressive disorder. In future work, we need to conduct further research on the relationship between the changes of SII at different time points and the occurrence and development of the depressive disorder.
Although this study has some limitations, our study confirms that high SII on admission is an independent risk factor for symptoms reaching moderate/major depression in patients with depressive disorder. In future clinical work, for depressed patients with high SII value upon admission, emphasis should be placed on early depression assessment, early psychological intervention and prevention, which will have a good impact on the prognosis of patients. On the other hand, rapid identification of people with high SII may lead to improvements in subsequent treatments, such as combining conventional treatments with treatments that reduce inflammation levels to treat major depression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Third People’s Hospital of Fuyang City (Ethics Approval No.: 2019-340-07). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
SC, HL, and YL: conception and design. SC, YL, and GY: administrative support. SC, JL, YW, ZL, LiS, and LoS: collection and assembly of data. SC, GY, JL, and HL: data analysis and interpretation. SC, JL, YL, GY, YW, ZL, LiS, LoS, and HL: manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Fuyang Municipal Health Commission Research Project (FY2020xg14) and Clinical Medical Key Specialty Construction Project of Anhui Province of China [(2021)273].
Acknowledgments
The authors want to acknowledge the help and appreciate the efforts of the participating patients and their guardians during data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Malhi, G, and Mann, J. Depression. Lancet. (2018) 392:2299–312. doi: 10.1016/s0140-6736(18)31948-2
2. Harald, B, and Gordon, P. Meta-review of depressive subtyping models. J Affect Disord. (2012) 139:126–40. doi: 10.1016/j.jad.2011.07.015
3. Bessa, J, Carvalho, S, Cunha, I, Fernandes, M, Matos-Pires, A, Neves, R, et al. Treatment-resistant depression in Portugal: perspective from psychiatry experts. Front Psychiatry. (2022) 13:824919. doi: 10.3389/fpsyt.2022.824919
4. Rudzki, L, and Maes, M. From "leaky gut" to impaired glia-neuron communication in depression. Adv Exp Med Biol. (2021) 1305:129–55. doi: 10.1007/978-981-33-6044-0_9
5. Cavaleri, D, Bartoli, F, Capogrosso, CA, Guzzi, P, Moretti, F, Riboldi, I, et al. Blood concentrations of neopterin and biopterin in subjects with depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. (2023) 120:110633. doi: 10.1016/j.pnpbp.2022.110633
6. Troubat, R, Barone, P, Leman, S, Desmidt, T, Cressant, A, Atanasova, B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. (2021) 53:151–71. doi: 10.1111/ejn.14720
7. Mikulska, J, Juszczyk, G, Gawrońska-Grzywacz, M, and Herbet, M. HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. (2021) 11:1298. doi: 10.3390/brainsci11101298
8. Li, R, Zhao, D, Qu, R, Fu, Q, and Ma, S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci Lett. (2015) 594:17–22. doi: 10.1016/j.neulet.2015.03.040
9. Durairaj, H, Steury, M, and Parameswaran, N. Paroxetine differentially modulates LPS-induced TNFα and IL-6 production in mouse macrophages. Int Immunopharmacol. (2015) 25:485–92. doi: 10.1016/j.intimp.2015.02.029
10. Köhler, C, Freitas, T, Stubbs, B, Maes, M, Solmi, M, Veronese, N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. (2018) 55:4195–206. doi: 10.1007/s12035-017-0632-1
11. Hernández, M, Mendieta, D, Martínez-Fong, D, Loría, F, Moreno, J, Estrada, I, et al. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. Eur Neuropsychopharmacol. (2008) 18:917–24. doi: 10.1016/j.euroneuro.2008.08.001
12. Dong, M, Shi, Y, Yang, J, Zhou, Q, Lian, Y, Wang, D, et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. (2020) 12:1758835920937425. doi: 10.1177/1758835920937425
13. Gao, H, Wusiman, L, Cao, B, Wujieke, A, and Zhang, W. The role of preoperative systemic immune-inflammation index in predicting the prognosis of patients with digestive tract cancers: a meta-analysis. Transpl Immunol. (2022) 73:101613. doi: 10.1016/j.trim.2022.101613
14. Hu, J, Wang, L, Fan, K, Ren, W, Wang, Q, Ruan, Y, et al. The association between systemic inflammatory markers and post-stroke depression: a prospective stroke cohort. Clin Interv Aging. (2021) 16:1231–9. doi: 10.2147/cia.S314131
15. Mazza, M, de Lorenzo, R, Conte, C, Poletti, S, Vai, B, Bollettini, I, et al. Anxiety and depression in Covid-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. (2020) 89:594–600. doi: 10.1016/j.bbi.2020.07.037
16. Benedetti, F, Palladini, M, Paolini, M, Melloni, E, Vai, B, de Lorenzo, R, et al. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in Covid-19 survivors: a multimodal magnetic resonance imaging study. Brain Behav Immun Health. (2021) 18:100387. doi: 10.1016/j.bbih.2021.100387
17. Zimmerman, M, Martinez, J, Young, D, Chelminski, I, and Dalrymple, K. Severity classification on the Hamilton depression rating scale. J Affect Disord. (2013) 150:384–8. doi: 10.1016/j.jad.2013.04.028
18. Syed, S, Beurel, E, Loewenstein, D, Lowell, J, Craighead, W, Dunlop, B, et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron. (2018) 99:914–24.e3. doi: 10.1016/j.neuron.2018.08.001
19. O’Brien, S, Scully, P, Fitzgerald, P, Scott, L, and Dinan, T. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. (2007) 41:326–31. doi: 10.1016/j.jpsychires.2006.05.013
20. Wittenberg, G, Greene, J, Vértes, P, Drevets, W, and Bullmore, E. Major depressive disorder is associated with differential expression of innate immune and neutrophil-related gene networks in peripheral blood: a quantitative review of whole-genome transcriptional data from case-control studies. Biol Psychiatry. (2020) 88:625–37. doi: 10.1016/j.biopsych.2020.05.006
21. Liu, X, Bai, X, Ren, R, Tan, L, Zhang, Y, Lan, H, et al. Association between depression or anxiety symptoms and immune-inflammatory characteristics in in-patients with tuberculosis: a cross-sectional study. Front Psychiatry. (2022) 13:985823. doi: 10.3389/fpsyt.2022.985823
22. Wang, J, Zhou, D, Dai, Z, and Li, X. Association between systemic immune-inflammation index and diabetic depression. Clin Interv Aging. (2021) 16:97–105. doi: 10.2147/cia.S285000
23. Mazza, M, Palladini, M, de Lorenzo, R, Magnaghi, C, Poletti, S, Furlan, R, et al. Persistent psychopathology and neurocognitive impairment in Covid-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. (2021) 94:138–47. doi: 10.1016/j.bbi.2021.02.021
24. Bollen, J, Trick, L, Llewellyn, D, and Dickens, C. The effects of acute inflammation on cognitive functioning and emotional processing in humans: a systematic review of experimental studies. J Psychosom Res. (2017) 94:47–55. doi: 10.1016/j.jpsychores.2017.01.002
25. Miller, A, and Raison, C. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. doi: 10.1038/nri.2015.5
26. Setiawan, E, Wilson, A, Mizrahi, R, Rusjan, P, Miler, L, Rajkowska, G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiat. (2015) 72:268–75. doi: 10.1001/jamapsychiatry.2014.2427
27. Chen, S, Yin, Y, Yue, Y, Li, Y, Zhang, Y, Jiang, W, et al. Integrating functional neuroimaging and serum proteins improves the diagnosis of major depressive disorder. J Affect Disord. (2023) 325:421–8. doi: 10.1016/j.jad.2023.01.034
28. Leonard, B. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. (2018) 30:1–16. doi: 10.1017/neu.2016.69
29. Wei, Y, Feng, J, Ma, J, Chen, D, Xu, H, Yin, L, et al. Characteristics of platelet-associated parameters and their predictive values in Chinese patients with affective disorders. BMC Psychiatry. (2022) 22:150. doi: 10.1186/s12888-022-03775-9
30. Dietrich-Muszalska, A, and Wachowicz, B. Platelet haemostatic function in psychiatric disorders: effects of antidepressants and antipsychotic drugs. World J Biol Psychiatry. (2017) 18:564–74. doi: 10.3109/15622975.2016.1155748
31. Marazziti, D, Torrigiani, S, Carbone, M, Mucci, F, Flamini, W, Ivaldi, T, et al. Neutrophil/lymphocyte, platelet/lymphocyte, and monocyte/lymphocyte ratios in mood disorders. Curr Med Chem. (2022) 29:5758–81. doi: 10.2174/0929867328666210922160116
32. Demir, S, Atli, A, Bulut, M, İbiloğlu, A, Güneş, M, Kaya, M, et al. Neutrophil-lymphocyte ratio in patients with major depressive disorder undergoing no pharmacological therapy. Neuropsychiatr Dis Treat. (2015) 11:2253–8. doi: 10.2147/ndt.S89470
33. Kinoshita, H, Takekawa, D, Kudo, T, Sawada, K, Mikami, T, and Hirota, K. Higher neutrophil-lymphocyte ratio is associated with depressive symptoms in Japanese general male population. Sci Rep. (2022) 12:9268. doi: 10.1038/s41598-022-13562-x
34. Kumar, K, Srivastava, S, Sharma, B, Avasthi, R, and Kotru, M. Comparison between inflammatory biomarkers (high-sensitivity C-reactive protein and neutrophil-lymphocyte ratio) and psychological morbidity in suicide attempt survivors brought to medicine emergency. Cureus. (2021) 13:e17459. doi: 10.7759/cureus.17459
35. Aydin Sunbul, E, Sunbul, M, Yanartas, O, Cengiz, F, Bozbay, M, Sari, I, et al. Increased neutrophil/lymphocyte ratio in patients with depression is correlated with the severity of depression and cardiovascular risk factors. Psychiatry Investig. (2016) 13:121–6. doi: 10.4306/pi.2016.13.1.121
36. Özyurt, G, and Binici, N. Increased neutrophil-lymphocyte ratios in depressive adolescents is correlated with the severity of depression. Psychiatry Res. (2018) 268:426–31. doi: 10.1016/j.psychres.2018.08.007
37. Creeden, J, Imami, A, Eby, H, Gillman, C, Becker, K, Reigle, J, et al. Fluoxetine as an anti-inflammatory therapy in Sars-Cov-2 infection. Biomed Pharmacother. (2021) 138:111437. doi: 10.1016/j.biopha.2021.111437
38. Tomaz, V, Chaves Filho, A, Cordeiro, R, Jucá, P, Soares, M, Barroso, P, et al. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J Affect Disord. (2020) 268:188–200. doi: 10.1016/j.jad.2020.03.022
Keywords: depression disorder, systemic immune-inflammation index, moderate/major depression, trait marker, risk factors
Citation: Cui S, Li J, Liu Y, Yao G, Wu Y, Liu Z, Sun L, Sun L and Liu H (2023) Correlation of systemic immune-inflammation index and moderate/major depression in patients with depressive disorders: a large sample cross-sectional study. Front. Psychiatry. 14:1159889. doi: 10.3389/fpsyt.2023.1159889
Edited by:
Hikaru Hori, Fukuoka University, JapanReviewed by:
Angelos Halaris, Loyola University Chicago, United StatesXiaochu Zhang, University of Science and Technology of China, China
Copyright © 2023 Cui, Li, Liu, Yao, Wu, Liu, Sun, Sun and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Cui, sophiacui1988@163.com; Huanzhong Liu, huanzhongliu@ahmu.edu.cn
 Shu Cui
Shu Cui Juanjuan Li1
Juanjuan Li1 Gaofeng Yao
Gaofeng Yao Zhiwei Liu
Zhiwei Liu Huanzhong Liu
Huanzhong Liu