- 1Department of Neuroscience, Biomedicine, and Movement Science, University of Verona, Verona, Italy
- 2Child and Adolescent Neuropsychiatry Unit, Maternal-Child Integrated Care Department, Integrated University Hospital Verona, Verona, Italy
- 3Autism Spectrum Disorders Regional Centre of Verona, Verona, Italy
In autism spectrum disorders (ASDs) in the pediatric population, skin manifestations are generally attributable to the concomitance of allergic forms or to accidental, self-inflicted or abusive lesions. However, clinical evidence has highlighted the presence of an increasing number of abdominal stretch marks, probably caused by the increase in the number of obesity cases in the pediatric population, in general, and therefore also among children with ASD. Stretch marks are often attributed to obesity, as they have an incidence of more than 50% in obese individuals. In the first part of this article we hypothesized that in addition to obesity there are other factors, such as a structural alteration on the skin in people with ASD, which can contribute/aggravate the phenomenon of stretch marks. Despite the high frequency with which stretch marks are found in children with ASD, this aspect has never been studied, the structure of the skin of children with ASD is not known. Furthermore, it is not known whether this structure is different from that of subjects without ASD. In the second part of the article, we hypothesized the mechanisms of the negative impact of simple abdominal stretch marks on the symptomatic picture of children with ASD. The presence of stretch marks, altered tactile perception, altered sensitivity to clothing fabrics can be a combination that influences development and determines negative consequences in the neurological picture of a child with ASD, as it is already known that the altered sensory perception in children with ASD contributes to the deterioration of social behavior. Furthermore, the presence of stretch marks may play a role in the postural and motor defects of children with ASD.
1. Introduction
In the context of autism spectrum disorders (ADS) in children, dermatological manifestations refer mainly to atopic dermatitis, often linked to concomitant allergic forms (1, 2), and to skin injuries (3).
As Slingsby et al. pointed out, the latter must be distinguished into accidental, self-inflicted, and abusive injuries (3). Accidental injuries are found predominantly in the lower legs such as in developing children (4), or in positions similar to those of children with cognitive and motor impairments (e.g., arms, hands, thighs, and buttocks) (5). In the latter type of children, Goldberg et al. specified that accidental injuries may be due to both natural movements, and the normal care maneuvers of caregivers (5). Self-inflicted injuries are found mainly in the hands/wrists and feet (3). Abuse injuries are of different conformation, presenting as bruises with prevalence on the torso, ear and neck for children younger than or equal to 4 years, or bruises in general for children younger than 4 months (6).
Keloids, scars, and post-inflammatory hyper- or hypopigmentation are considered secondary manifestations, often linked to self-injurious behaviors (7, 8). A high incidence of stretch marks (SMs) in the autistic pediatric population is not reported in the literature (8). However, recently, clinically, the authors have identified an increase in this incidence, a data compatible with the values reported in the literature for these manifestations in the entire pediatric population (9, 10). These data may be related to the increased rate of obesity in pediatric subjects (11), as obesity has SMs among its predominant skin manifestations in the areas of greatest fat accumulation (12, 13). The highest risk of obesity among children with ASD is already known (14–17), with causes so far identified mainly in a sedentary lifestyle and incorrect eating habits (18, 19).
An increase in obese children in the pediatric population with ASD may explain the increased evidence of SMs in these subjects. But why is this data so important? Because the authors hypothesize that these skin manifestations can be exacerbated by the alteration of the connective tissue present in the ASD (20). They also hypothesize that even from a neurological point of view there may be an aggravation of the symptomatologic picture, in particular through an overstimulation of an already altered tactile perception. Finally, they hypothesize that the presence of SMs can worsen the motor symptoms and postural instability, already described in the literature (21, 22), because they further weaken an already compromised tissue.
Therefore, in the first part of this article, a characterization of the SMs will be performed, comparing them with a typical pattern of SMs in obese subjects, but without other pathologies. In the second part of this article, the mechanisms by which they are believed to affect a child with ASD will be described.
2. Stretch marks
SMs has long been considered a purely aesthetic skin defect. In recent years they have been reconsidered from a pathological point of view (23).
SMs have different etiologies, because they can depend on mechanical stress of the skin (e.g., rapid weight changes, cachexia, obesity, and surgical sutures) (24–28), hormonal instability (e.g., treatments with corticosteroids, Marphan’s syndrome, and Cushing’s syndrome) (29–33), genetic diseases (e.g., Ehlers-Danson syndrome) (34), or a combination of both mechanical and hormonal changes (e.g., puberty, pregnancy) (10, 35, 36).
SMs assessment is done mainly visually. In a first phase they are defined “rubrae” and are slightly raised streaks of a pink to red erythematous color. This type of SMs is considered treatable, and there are numerous aesthetic options and numerous electromechanical tools available to prevent the degeneration of “rubrae” striae into “albae” striae (37–41). The “albae” striae are the SMs in their final stage. They have a pearly white color and are flat or depressed with respect to the adjacent skin surface. They are considered atrophic lesions of the skin (9).
SMs studies using biopsy samples showed a change in the structure of the extracellular matrix (42–45), with the reorganization of both elastic (42–45) and collagen fibers (45), and hormonal changes (46, 47). In vivo SMs studies, using non-invasive investigation techniques, confirmed changes in the skin’s structure (45, 48–51), and revealed alterations in its mechanical properties (35, 50, 51).
Having noticed the increase of SMs in the abdominal region in young patients with ASD, during normal clinical practice, and given the peculiarity of their shape and their distribution, the authors decided to investigate the problem further.
The literature review revealed that there are no data on SMs in ASD. There is no characterization of these skin changes in individuals with ASD. Finally, there is no comparison of SMs skin texture between subjects with and without ASD.
Randomly considered one of the days in which routine checks were performed at the Children’s Neuropsychiatry Clinic in Verona, with the consent of the parents, the photographic material of the SMs of the first two patients with ASD (of different sex) who arrived, and presented them, was collected.
The data were compared with those already available from a volunteer subject.
Here the authors report the observed results and the first considerations and advanced hypotheses.
3. Cases
The volunteer subject was a 20-year-old man who had had previous evaluations of his SMs (23, 52), and had given written informed consent to the use of his data for the new comparison, respecting his privacy and guaranteeing his anonymity. The previous acquisitions, measurements, and image processing had been performed in accordance with the Declaration of Helsinki. His SMs had been photographed with a simple smart phone, and evaluated using ultrasound and elastography. These last two investigations had been conducted with a MylabTM70 device (Esaote SpA, Genoa, Italy) with a 13 MHz probe. Subsequent processing had been performed using the ImageJ.JS software (National Institute of Mental Health, Bethesda, Maryland, United States).
For the collection of the photographic material of the two children with ASD, the parents gave full written informed consent. This material was anonymized before being sent for analysis and processing. Therefore, those who performed the analyzes and elaborations only knew the sex of the children and that they were two teenagers. The data was processed in full compliance with the principles of the Declaration of Helsinki. The only material collected were the photographs of the SMs obtained with a smartphone.
The control subject did not present any pathology, except for a mild obesity, believed to be the cause of the SMs. The children with ASD were obese, and had no other comorbidities. The demographics of the subjects and the characteristics of their stretch marks are summarized in Table 1.
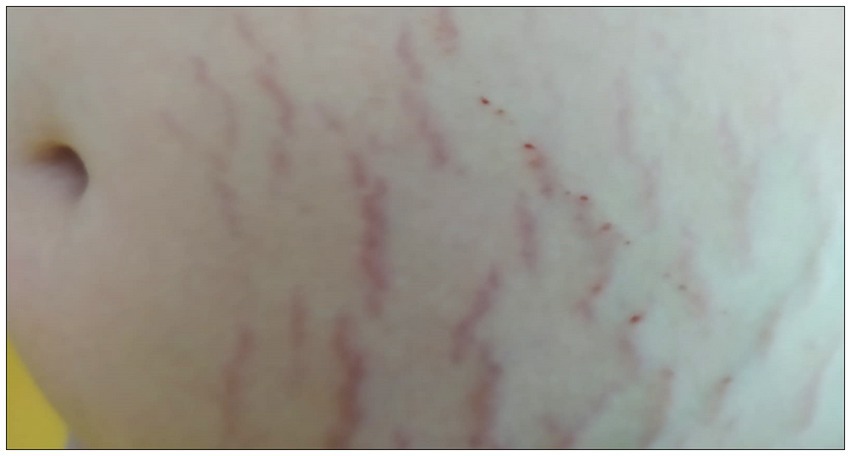
The SMs of the control subject were slightly raised, pink in color, distributed vertically along the entire abdomen (Figures 1A,B). Ultrasound and elastosonographic investigations highlighted different aspects of SMs (Figures 1C,D).The ultrasound showed the presence of areas with greater echogenicity in correspondence with the SMs, which appeared as cylinders that deepened from the dermis to the deep hypodermis (Figures 1C,E,F). Since the echogenicity described referred to connective tissue, a significant alteration in the distribution of collagen fibers was deduced, with thickening compatible with atrophy. Elastosonography revealed a different structural pattern between healthy skin areas and skin areas with SMs (Figure 1D), with the presence of a greater number of rigid (thick connective tissue), and semi-rigid (collagen fibers) components in the area with SMs (Figures 1G–I).
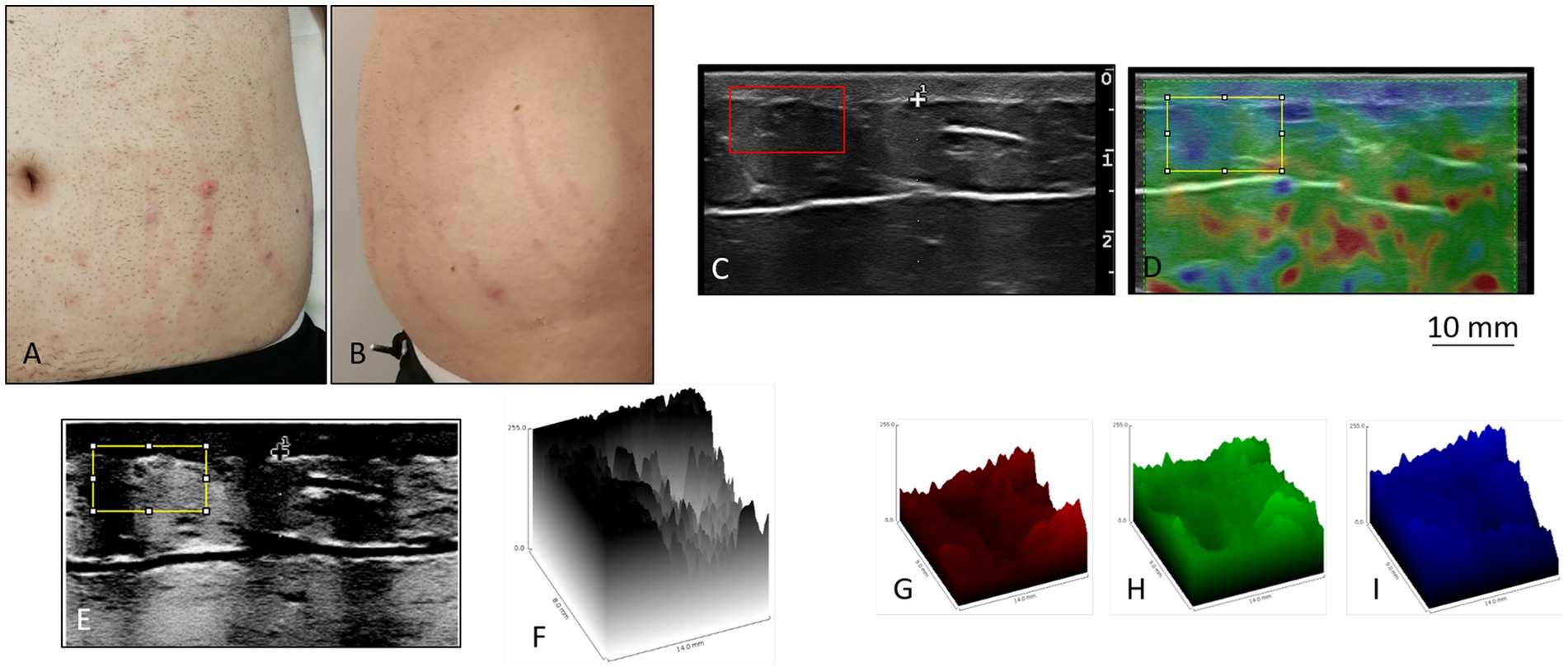
Figure 1. Stretch marks in a 20-year-old man. (A,B) The striae rubrae run vertically along the entire abdomen, from side to side. (C) Detail of the ultrasound. In correspondence of the SMs, columns of highly echogenic collagen fibers are highlighted. Such columns from the dermis deepen into the deep hypodermis. Original image published in Veronese et al. (23, 52). (D) Detail of the elastosonography. The red colour corresponds to rigid tissues, the green colour to semi-rigid tissues, and the blue colour to soft tissues. In correspondence of the SMs there are evident traces that deepen from the dermis to the hypodermis, green in colour at the level of the hypodermis. Areas of the hypodermis not underlying the SMs appear blue. In the yellow box, the left side (blue) corresponds to the intact skin, and the right side (green) corresponds to a SM. Original image published in Veronese et al. (52). (E) Processing of (C) The contrast of the ultrasound figure was increased, and the colours were reversed, in order to highlight the alterations of the connective fibers of the hypodermis in the areas underlying the SMs. (F) Three-dimensional projection of the yellow box in (E). (G–I) Extrapolation of the rigid (red), semi-rigid (green), and soft (blue) components of the box selected in (D). The difference between the area underneath intact skin and the area underneath a SM appears particularly evident in the semi-rigid components.
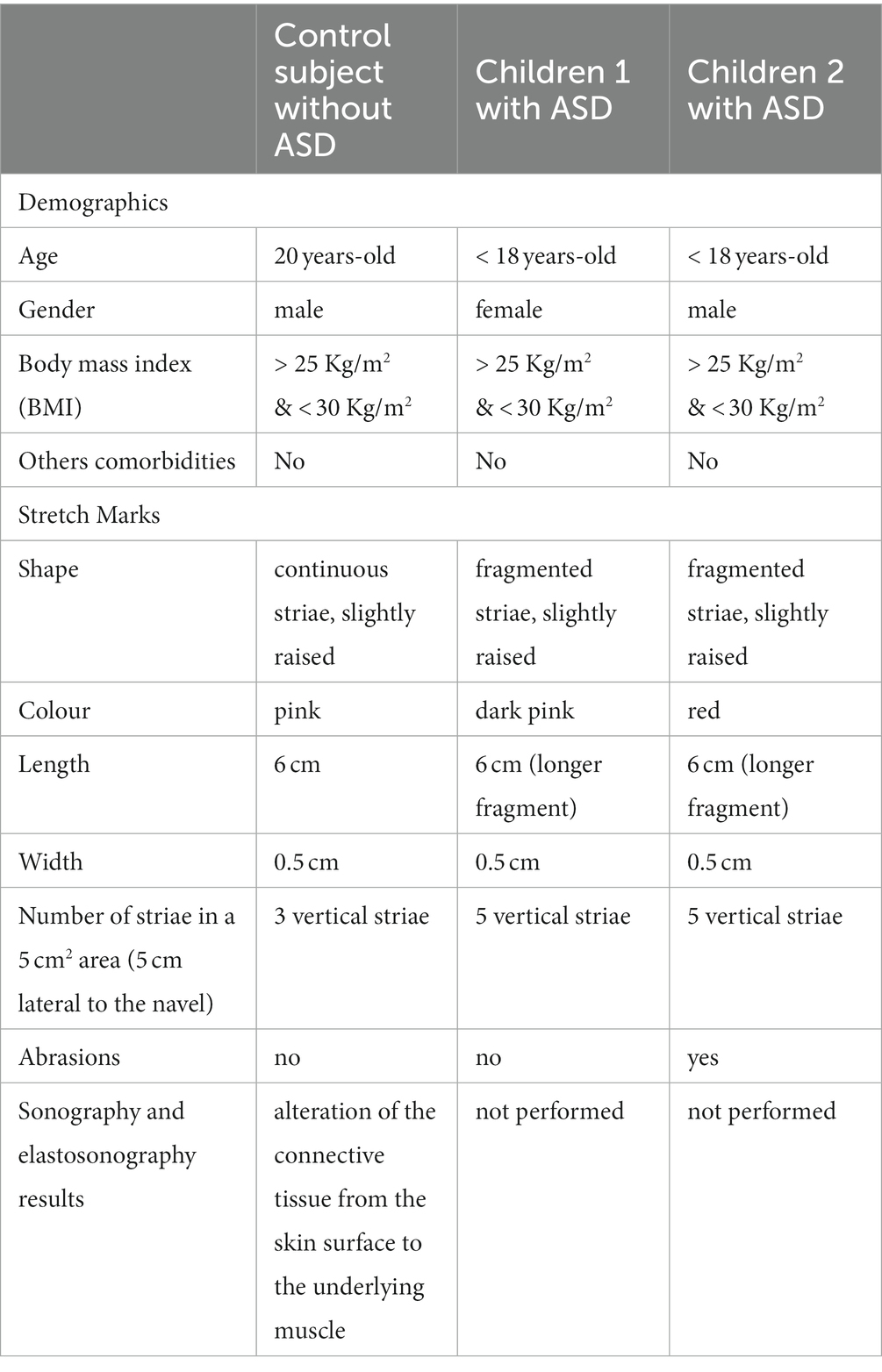
The SMs of the two children with ASD were slightly raised, clearly redder than those of the healthy subject, indicating a more recent formation. They were distributed vertically throughout the abdomen, with a higher frequency than the SMs observed in healthy subjects (Figure 2). This means that the connective tissue alteration observed for the healthy subject was of greater severity in the two children with ASD, being more widespread. There were no apparent differences in the distribution pattern and thickness of the SMs between the two subjects with ASD, thus excluding sexual dimorphism. But it was interesting to observe scratch lesions in correspondence with the SMs of the boy with ASD (Figure 3). It is known that the SMs, in the rubrae phase, can be itchy or painful (39). However, the administration of a specific test to evaluate the presence of pain in the SMs area, and its eventual quantification, were not performed, as they were not considered routine clinical procedures, and were not yet authorized by the competent Ethics Committee.
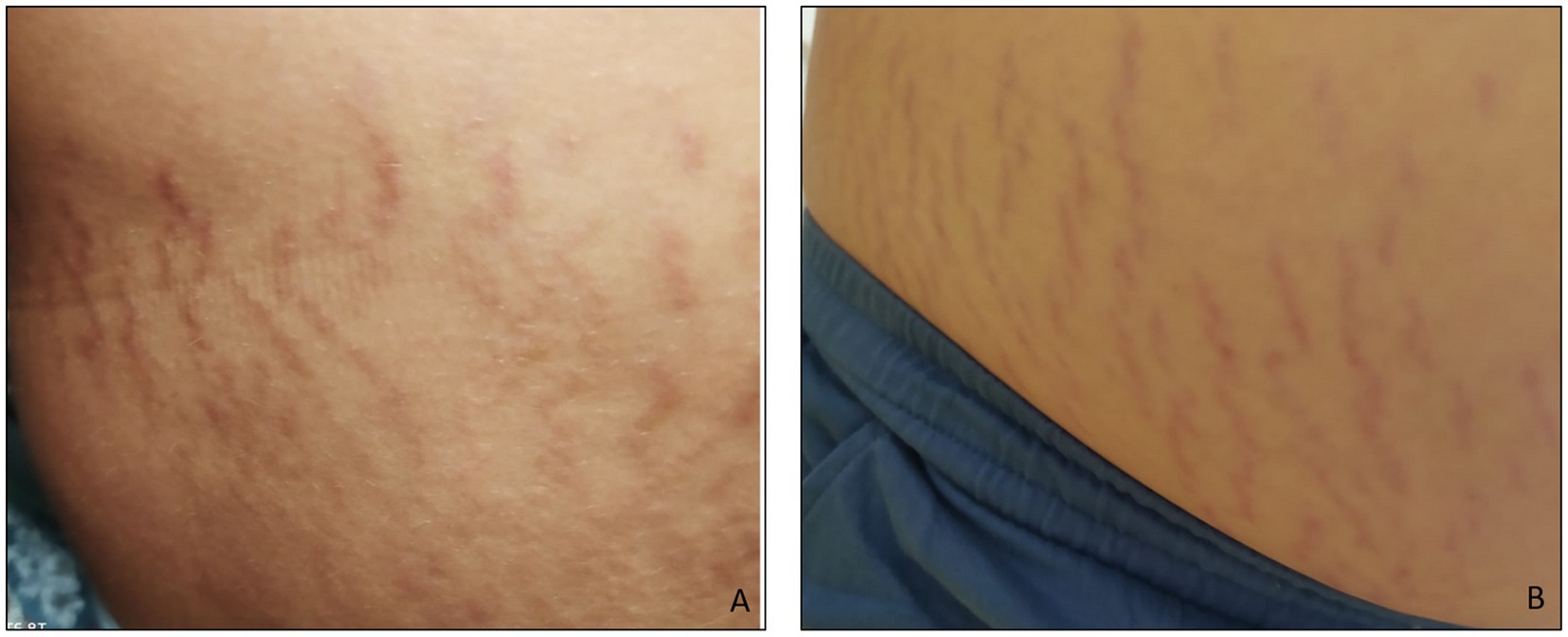
Figure 2. (A) Abdominal stretch marks in a girl and (B) in a boy, both with ASD. The striae rubrae run vertically along the entire abdomen, from side to side. The frequency of occurrence is higher than that observed for the subject in Figure 1, i.e., the number of SMs detected is greater.
Nevertheless, the authors wondered what could be the extent of the annoyance/pain perceived by the subject, and hypothesized the possible consequences of this annoyance/pain. Two aspects were taken into consideration: the alteration of tactile and postural perception.
4. Tactile perception in the ASD
In 1943, in his report on ASD, Kanner described for the first time the altered sensory perception of subjects with ASD (53). In recent years this alteration has been the subject of new studies and in particular it is proving to be an important factor in the process of deterioration of social behavior in ASD (54, 55), and all other aspects of the neurodevelopment, including detail perception, and motor planning (55).
Touch is the first sense to develop (56), and is fundamental as the first communication and attachment system between mother and newborn (56–59). Therefore, it is known that a congenital tactile alteration can considerably impair both the mother–child bond and the subsequent development of a child (55, 60, 61). Perceptual tactile alterations in children with ASD have been observed from early childhood (55, 62, 63). The changes observed by parents or examiners have appeared to be greater than effectively measurable or observed by clinicians (64, 65). Tactile sensitivity has not appeared different between children with and without ASD (66, 67), while the stimulus detection threshold was higher in children of different ages with ASD (66–68). As pointed out by Mikkelsen et al., tactile perception studies in subjects with ASD must be evaluated considering the subjectivity of the clinical evaluation, the heterogeneity of the ASD courts, and the different tactile sensitivity measurements performed (69).
However, there is no doubt that it is present an alteration of tactile perception, although obviously further studies are needed to fully understand it. Hyper and hypo-reactivity to tactile stimuli is well known (70–74). In particular with reference to the perception of vibrations, and thermal pain (71). Depending on whether the response is hyper or hypo, a different correlation with social (72, 73), and communication deterioration (72) was proven, and a different response to therapies was observed (70). The same subject can manifest reactions of different types or reactions not manifested, depending on the type of stimulus applied (75, 76). The presence or absence of response, and the type of response may also depend on the involvement of other sensory organs (75, 76). Furthermore, it should not be underestimated that being subjects with ASD, it is not possible to predict the type of response or to consider a response absent if a child does not react, as has been shown for the perception of pain (77). As suggested by Purpura et al. (55), the same stereotyped behaviors, and repetitive movements of subjects with ASD could be their compensatory systems for the inability to manage stimuli, such as tactile ones, and sensory stimuli in general. Finally, the response could also depend on the skin point stimulated, considering the different neural receptivity to stimulation of different areas of the body (78, 79).
5. Tactile perception of stretch marks in ASD
While SMs can be annoying in a healthy person (41), they can be even more so in a child with ASD. The excoriation presented in Figure 3 demonstrates this. Since the sites of skin lesions in children with ASD do not include the abdomen, be they accidental (4, 5), self-inflicted (3), or due to abuse (5), this excoriation can only be referred to a discomfort induced by the presence of SMs. And the discomfort could be exacerbated by the rubbing/contact of the clothing fabrics on the skin, having recently been established the different tactile pleasantness of some fabrics compared to others in subjects with ASD (80, 81).
Considering the cases presented, the itch that caused the boy’s injury is consequently attributable to the structure of the SMs. Future studies will have to clarify the causes of the alteration of the extracellular collagen matrix, extending from the dermis to the deep hypodermis. It is possible that it may be due to a number of factors combined together in a snowball effect, in primis caused by the general connective tissue disorder present in individuals with ASD, and well described by Zoccante et al. (20).
If in a subject without ASD, a strong weight gain determines the appearance of SMs in more than 50% of the subjects (12), the incidence of SMs in subjects with ASD may be higher, due to the presence of an already structurally different skin, i.e., more easily deformed.
Given that the maturation process of SMs from “rubrae” to the untreatable stage of “albae” is not immediate, one wonders if it is possible to intervene to “heal” the SMs in their first stage and resolve the possible discomfort.
The treatment of “rubrae” SMs is performed with various methods (37–41), more or less invasive, but not always effective. The procedures require more or less long application times and, sometimes, more sessions of treatment. It is not certain that these procedures are applicable to subjects with ASD, both children and adults, given the need for the treated subjects to remain still during the sessions. Also, not all procedures are painless. Therefore, it is not said that they can be tolerated by subjects with ASD, with altered tactile perception.
Treatments of “albae” striae are poorly documented in the literature. A technique that applies simultaneously and in synergy magnetic fields and vacuum (V-EMF therapy) seems to be very promising (82). However, even in this case it is not certain that its application can be extended to subjects with ASD.
The ideal action would be to prevent the onset of SMs. To date there are variable results regarding the topical application of creams and oils by pregnant women using these products against the onset of striae gravidarum (41, 83).
6. Postural and motor repercussions of stretch marks in ASD
The morpho-structural and functional deterioration of the extracellular matrix characteristic of SMs could be related to the motor and postural disturbances present in subjects with ASD (21, 22).
It is well known that childhood overweight and obesity alone primarily determine a postural alteration of the abdomen. This alteration is followed by postural imbalances of the spine, shoulder, leg and foot (84). This means general instability of the core (85).
Some studies in the literature report a correlation between striae and their severity, and prolapse of the pelvic organs (86, 87), and obstetric lesions of the anal sphincter (88). However, it is unclear whether SMs can generally be considered predictive clinical markers of urogenital dystopia (89). These data highlight the possible seriousness of the structural alteration that can be induced by abdominal SMs. Given that the morpho-anatomical alteration related to SMs affects not only the skin, but also the deep layers up to the muscles, it seems inevitable that the concomitance of SMs and the state of obesity aggravates postural alterations and core instability, followed from motor problems, which would derive precisely from these alterations. It has already been studied in children with developmental coordination disorder that improved core stability also leads to improved motor skills (90).
The already altered systemic morphology of the connective tissue present in subjects with ASD (20), alone demonstrates the correlation with postural and motor disorders. The presence of SMs and the state of obesity are aggravating factors.
7. Final remarks and future directions
This article is a preliminary study resulting from the clinical finding of the presence of abdominal SMs in children with ASD, SMs of different texture from the commonly known SMs in subjects without ASD. Literature is scarce on this matter, perhaps because SMs are attributed only to obesity, a known and widespread problem in subjects with ASD.
The ideas resulting from the review of the literature, considering all the possible correlated aspects, have brought out an extremely interesting picture involving both the perceptive and neurological aspects, and the postural and motor aspects.
It is evident that only hypotheses could be formulated and much clinical work needs to be done to test and substantiate them.
We have to study in detail the incidence of appearance, and the texture of SMs in subjects with ASD, in children, but also in adults. The degree of annoyance/pain that these alterations may cause should be assessed. Any behavioral impairments related to the onset and presence of SMs should be evaluated. The correlations between the presence of SMs and postural and motor alterations should be evaluated, too. Therefore, understanding if there is a worsening of alterations already present or if the mere presence of SMs causes alterations. If possible, all of these possible outcomes should be properly quantified.
Furthermore, the authors question whether what appears to be a particular distribution of SMs in children could constitute a phenotypic expression in ASDs. If this were the case, we would have a further tool for the diagnosis of ASD, especially in those borderline subjects, in which the symptoms are vague, and the disturbances are not serious. Unfortunately, given that the appearance of SMs is not premature, and seems to affect only obese children, the use of the particular distribution of SMs as a hallmark of ASD leads to a limited and, in any case, late diagnosis.
However, it should be emphasized that the fact that SMs are much more serious than in obese subjects without ASD is a clear sign of a defect already present in the underlying connective tissue, a defect present since birth. If this relevance were demonstrated, the biopsy analysis of a connective tissue sample could become a tool for early diagnosis.
In any case, the authors wonder whether and how harmful the discomforts possibly induced by SMs could be in a person with ASD. They wonder if, how much and how the structural alteration naturally present in the abdominal connective tissue and in the subcutaneous connective tissue in general can be perceived by subjects with ASD. If, how much and how the continuous contact of the fabrics of the clothes is perceived. Finally, when does this possible discomfort arise, and how neurologically harmful it can be, if present from birth, in a newborn who cannot resolve it in any way, and cannot manifest it, perhaps in any way, except by crying.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
SV, LZ, and AS contributed to conception and design of the study. SV and AS organized the first draft of the manuscript and the first version of the manuscript. SV, LZ, NS, and AS performed the revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor ELC is currently organizing a research topic with the authors LZ and AS.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mostafa, GA, Hamza, RT, and El-Shahawi, H. Allergic manifestations in autistic children: relation to disease severity. J Pediatr Neurol. (2015) 06:115–23. doi: 10.1055/S-0035-1557446
2. Tsai, TY, Chao, YC, Hsieh, CY, and Huang, YC. Association between atopic dermatitis and autism spectrum disorder: a systematic review and meta-analysis. Acta Derm Venereol. (2020) 100:adv00146. doi: 10.2340/00015555-3501
3. Slingsby, B, Yatchmink, Y, and Goldberg, A. Typical skin injuries in children with autism spectrum disorder. Clin Pediatr (Phila). (2017) 56:942–6. doi: 10.1177/0009922817705187
4. Labbé, J, and Caouette, G. Recent skin injuries in normal children. Pediatrics. (2001) 108:271–6. doi: 10.1542/peds.108.2.271
5. Goldberg, AP, Tobin, J, Daigneau, J, Griffith, RT, Reinert, SE, and Jenny, C. Bruising frequency and patterns in children with physical disabilities. Pediatrics. (2009) 124:604–9. doi: 10.1542/peds.2008-2900
6. Pierce, MC, Kaczor, K, Aldridge, S, O'Flynn, J, and Lorenz, DJ. Bruising characteristics discriminating physical child abuse from accidental trauma. Pediatrics. (2010) 125:67–74. doi: 10.1542/peds.2008-3632
7. Accordino, RE, Lucarelli, J, and Yan, AC. Cutaneous disease in autism spectrum disorder: a review. Pediatr Dermatol. (2015) 32:455–60. doi: 10.1111/pde.12582
8. Srebrnik, A, Brenner, S, Holan, A, Stein, D, and Elizur, A. Cutaneous manifestations in an autistic population. J Eur Acad Dermatol Venereol. (1997) 9:118–22. doi: 10.1111/j.1468-3083.1997.tb00248.x
9. Al-Himdani, S, Ud-Din, S, Gilmore, S, and Bayat, A. Striae distensae: a comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br J Dermatol. (2014) 170:527–47. doi: 10.1111/bjd.12681
10. Elsedfy, H. Striae distensae in adolescents: a mini review. Acta Biomed. (2020) 91:176–81. doi: 10.23750/abm.v91i1.9248
11. GBD 2015 Obesity CollaboratorsAfshin, A, Forouzanfar, MH, Reitsma, MB, Sur, P, Estep, K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
12. Nino, M, Franzese, A, Ruggiero Perrino, N, and Balato, N. The effect of obesity on skin disease and epidermal permeability barrier status in children. Pediatr Dermatol. (2012) 29:567–70. doi: 10.1111/j.1525-1470.2012.01738.x
13. Sunkwad, A, and Mahajan, S. Cutaneous manifestations in overweight and obese children and adolescent. Int J Contemp Pediatr. (2021) 8:1662–6. doi: 10.18203/2349-3291.ijcp20213726
14. Sammels, O, Karjalainen, L, Dahlgren, J, and Wentz, E. Autism spectrum disorder and obesity in children: a systematic review and meta-analysis. Obes Facts. (2022) 15:305–20. doi: 10.1159/000523943
15. Kahathuduwa, CN, West, BD, Blume, J, Dharavath, N, Moustaid-Moussa, N, and Mastergeorge, A. The risk of overweight and obesity in children with autism spectrum disorders: a systematic review and meta-analysis. Obes Rev. (2019) 20:1667–79. doi: 10.1111/obr.12933
16. Healy, S, Aigner, CJ, and Haegele, JA. Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism. (2019) 23:1046–50. doi: 10.1177/1362361318791817
17. Hill, AP, Zuckerman, KE, and Fombonne, E. Obesity and autism. Pediatrics. (2015) 136:1051–61. doi: 10.1542/peds.2015-1437
18. Eow, SY, Gan, WY, Lim, PY, Awang, H, and Mohd, SZ. Parental feeding practices and child-related factors are associated with overweight and obesity in children and adolescents with autism spectrum disorder. J Autism Dev Disord. (2021) 52:3655–67. doi: 10.1007/s10803-021-05247-7
19. Canals-Sans, J, Esteban-Figuerola, P, Morales-Hidalgo, P, and Arija, V. Do children with autism spectrum disorders eat differently and less adequately than those with subclinical ASD and typical development? EPINED epidemiological study. J Autism Dev Disord. (2022) 52:361–75. doi: 10.1007/s10803-021-04928-7
20. Zoccante, L, Ciceri, ML, Gozzi, LA, Di Gennaro, G, and Zerman, N. The "Connectivome theory": a new model to understand autism spectrum disorders. Front Psych. (2022) 12:794516. doi: 10.3389/fpsyt.2021.794516
21. Colizzi, M, Ciceri, ML, Di Gennaro, G, Morari, B, Inglese, A, Gandolfi, M, et al. Investigating gait, movement, and coordination in children with neurodevelopmental disorders: is there a role for motor abnormalities in atypical neurodevelopment? Brain Sci. (2020) 10:601. doi: 10.3390/brainsci10090601
22. Zoccante, L, Ciceri, ML, Chamitava, L, Di Gennaro, G, Cazzoletti, L, Zanolin, ME, et al. Postural control in childhood: investigating the neurodevelopmental gradient hypothesis. Int J Environ Res Public Health. (2021) 18:1693. doi: 10.3390/ijerph18041693
23. Veronese, S, Picelli, A, Zoccatelli, A, Zadra, A, Faccioli, N, Smania, N, et al. The pathology under stretch marks? An elastosonography study. J Cosmet Dermatol. (2022) 21:859–64. doi: 10.1111/jocd.14466
24. García, HL. Dermatological complications of obesity. Am J Clin Dermatol. (2002) 3:497–506. doi: 10.2165/00128071-200203070-00006
25. Ono, T, Matsunaga, W, and Yoshimura, K. Striae distensae after tension-requiring skin sutures. J Dermatol. (1991) 18:47–51. doi: 10.1111/j.1346-8138.1991.tb03039.x
26. Strumia, R. Skin signs in anorexia nervosa. Dermatoendocrinol. (2009) 1:268–70. doi: 10.4161/derm.1.5.10193
27. Strumia, R. Eating disorders and the skin. Clin Dermatol. (2013) 31:80–5. doi: 10.1016/j.clindermatol.2011.11.011
28. Yosipovitch, G, DeVore, A, and Dawn, A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. (2007) 56:901–16; quiz 917-920. doi: 10.1016/j.jaad.2006.12.004
29. Amann, J, Wessels, AM, Breitenfeldt, F, Huscher, D, Bijlsma, JWJ, Jacobs, JWG, et al. Quantifying cutaneous adverse effects of systemic glucocorticoids in patients with rheumatoid arthritis: a cross-sectional cohort study. Clin Exp Rheumatol. (2017) 35:471–6.
30. Auchus, RJ. Cushing's syndrome In: MJ Aminoff and RB Daroff, editors. Encyclopedia of the neurological sciences. Second ed. London, UK: Academic Press (2014). 916–8.
31. Nieman, LK. Cushing's syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. (2015) 173:M33–8. doi: 10.1530/EJE-15-0464
32. Salik, I, and Rawla, P. Marfan Syndrome In: StatPearls. National Library of Medicine. Treasure Island, FL: StatPearls Publishing (2021)
33. Stevanović, DV. Corticosteroid-induced atrophy of the skin with telangiectasia. A clinical and experimental study. Br J Dermatol. (1972) 87:548–56. doi: 10.1111/j.1365-2133.1972.tb07444.x
34. Malfait, F, Wenstrup, RJ, and De Paepe, A. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. (2010) 12:597–605. doi: 10.1097/GIM.0b013e3181eed412
35. Henry, F, Piérard-Franchimont, C, Pans, A, and Piérard, GE. Striae distensae of pregnancy. An in vivo biomechanical evaluation. Int J Dermatol. (1997) 36:506–8. doi: 10.1046/j.1365-4362.1997.00041.x
36. Salter, SA, and Kimball, AB. Striae gravidarum. Clin Dermatol. (2006) 24:97–100. doi: 10.1016/j.clindermatol.2005.10.008
37. Abdel-Latif, AM, and Albendary, AS. Treatment of striae distensae with microdermabrasion: a clinical and molecular study. J Egyptian Women Dermatol Soc. (2008) 5:24–30.
38. Bleve, M, Capra, P, Pavanetto, F, and Perugini, P. Ultrasound and 3D skin imaging: methods to evaluate efficacy of striae distensae treatment. Dermatol Res Pract. (2012) 2012:673706. doi: 10.1155/2012/673706
39. Jiménez, GP, Flores, F, Berman, B, and Gunja-Smith, Z. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. (2003) 29:362–5. doi: 10.1046/j.1524-4725.2003.29086.x
40. Shokeir, H, El Bedewi, A, Sayed, S, and El Khalafawy, G. Efficacy of pulsed dye laser versus intense pulsed light in the treatment of striae distensae. Dermatol Surg. (2014) 40:632–40. doi: 10.1111/dsu.0000000000000007
41. Ud-Din, S, McGeorge, D, and Bayat, A. Topical management of striae distensae (stretch marks): prevention and therapy of striae rubrae and albae. J Eur Acad Dermatol Venereol. (2016) 30:211–22. doi: 10.1111/jdv.13223
42. Watson, RE, Parry, EJ, Humphries, JD, Jones, CJ, Polson, DW, Kielty, CM, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. (1998) 138:931–7. doi: 10.1046/j.1365-2133.1998.02257.x
43. Piérard, GE, Nizet, JL, Adant, JP, Camacho, MA, Pans, A, and Fissette, J. Tensile properties of relaxed excised skin exhibiting striae distensae. J Med Eng Technol. (1999) 23:69–72. doi: 10.1080/030919099294311
44. Perez-Aso, M, Roca, A, Bosch, J, and Martínez-Teipel, B. Striae reconstructed, a full thickness skin model that recapitulates the pathology behind stretch marks. Int J Cosmet Sci. (2019) 41:311–9. doi: 10.1111/ics.12538
45. Schuck, DC, de Carvalho, CM, Sousa, MPJ, Fávero, PP, Martin, AA, Lorencini, M, et al. Unraveling the molecular and cellular mechanisms of stretch marks. J Cosmet Dermatol. (2020) 19:190–8. doi: 10.1111/jocd.12974
46. Cordeiro, RC, Zecchin, KG, and de Moraes, AM. Expression of estrogen, androgen, and glucocorticoid receptors in recent striae distensae. Int J Dermatol. (2010) 49:30–2. doi: 10.1111/j.1365-4632.2008.04005.x
47. Youssef, SES, El-Khateeb, EA, Aly, DG, and Moussa, MH. Striae distensae: immunohistochemical assessment of hormone receptors in multigravida and nulligravida. J Cosmet Dermatol. (2017) 16:279–86. doi: 10.1111/jocd.12337
48. Rolfe, H, Wurm, E, and Gilmore, S. An investigation of striae distensae using reflectance confocal microscopy. Australas J Dermatol. (2012) 53:181–5. doi: 10.1111/j.1440-0960.2012.00884.x
49. Bertin, C, Lopes-DaCunha, A, Nkengne, A, Roure, R, and Stamatas, GN. Striae distensae are characterized by distinct microstructural features as measured by non-invasive methods in vivo. Skin Res Technol. (2014) 20:81–6. doi: 10.1111/srt.12088
50. Stamatas, GN, Lopes-DaCunha, A, Nkengne, A, and Bertin, C. Biophysical properties of striae distensae evaluated in vivo using non-invasive assays. Skin Res Technol. (2015) 21:254–8. doi: 10.1111/srt.12186
51. Cho, C, Cho, E, Kim, N, Shin, J, Woo, S, Lee, J, et al. Biophysical properties of striae rubra and striae alba in human skin: comparison with normal skin. Skin Res Technol. (2019) 25:283–8. doi: 10.1111/srt.12645
52. Veronese, S, Picelli, A, Smania, N, and Sbarbati, A. Hypodermis involvement in skin disorders: imaging and functional imaging diagnostic tools. Skin Res Technol. (2021) 27:641–3. doi: 10.1111/srt.12990
54. Thye, MD, Bednarz, HM, Herringshaw, AJ, Sartin, EB, and Kana, RK. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev Cogn Neurosci. (2018) 29:151–67. doi: 10.1016/j.dcn.2017.04.010
55. Purpura, G, Cerroni, F, Carotenuto, M, Nacinovich, R, and Tagliabue, L. Behavioural differences in sensorimotor profiles: a comparison of preschool-aged children with sensory processing disorder and autism Spectrum disorders. Children (Basel). (2022) 9:408–18. doi: 10.3390/children9030408
56. Pleger, B, and Villringer, A. The human somatosensory system: from perception to decision making. Prog Neurobiol. (2013) 103:76–97. doi: 10.1016/j.pneurobio.2012.10.002
57. Moszkowski, RJ, and Stack, DM. Infant touching behaviour during mother–infant face-to-face interactions. Inf Child Develop. (2007) 16:307–19. doi: 10.1002/icd.510
58. Hertenstein, MJ. Touch: its communicative functions in infancy. Hum Dev. (2002) 45:70–94. doi: 10.1159/000048154
59. Jean, AD, and Stack, DM. Functions of maternal touch and infants' affect during face-to-face interactions: new directions for the still-face. Infant Behav Dev. (2009) 32:123–8. doi: 10.1016/j.infbeh.2008.09.008
60. Field, T. Touch for socioemotional and physical well-being: a review. Dev Rev. (2010) 30:367–83. doi: 10.1016/j.dr.2011.01.001
61. Cascio, CJ. Somatosensory processing in neurodevelopmental disorders. J Neurodev Disord. (2010) 2:62–9. doi: 10.1007/s11689-010-9046-3
62. Blakemore, SJ, Tavassoli, T, Calò, S, Thomas, RM, Catmur, C, Frith, U, et al. Tactile sensitivity in Asperger syndrome. Brain Cogn. (2006) 61:5–13. doi: 10.1016/j.bandc.2005.12.013
63. Mammen, MA, Moore, GA, Scaramella, LV, Reiss, D, Ganiban, JM, Shaw, DS, et al. Infant avoidance during a tactile task predicts autism spectrum behaviors in toddlerhood. Infant Ment Health J. (2015) 36:575–87. doi: 10.1002/imhj.21539
64. Tomchek, SD, and Dunn, W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther. (2007) 61:190–200. doi: 10.5014/ajot.61.2.190
65. Ben-Sasson, A, Cermak, SA, Orsmond, GI, Tager-Flusberg, H, Carter, AS, Kadlec, MB, et al. Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. Am J Occup Ther. (2007) 61:584–92. doi: 10.5014/ajot.61.5.584
66. Demopoulos, C, Brandes-Aitken, AN, Desai, SS, Hill, SS, Antovich, AD, Harris, J, et al. Shared and divergent auditory and tactile processing in children with autism and children with sensory processing dysfunction relative to typically developing peers. J Int Neuropsychol Soc. (2015) 21:444–54. doi: 10.1017/S1355617715000387
67. Espenhahn, S, Godfrey, KJ, Kaur, S, McMorris, C, Murias, K, Tommerdahl, M, et al. Atypical tactile perception in early childhood autism. J Autism Dev Disord. (2022). doi: 10.1007/s10803-022-05570-7
68. Tavassoli, T, Bellesheim, K, Tommerdahl, M, Holden, JM, Kolevzon, A, and Buxbaum, JD. Altered tactile processing in children with autism spectrum disorder. Autism Res. (2016) 9:616–20. doi: 10.1002/aur.1563
69. Mikkelsen, M, Wodka, EL, Mostofsky, SH, and Puts, NAJ. Autism spectrum disorder in the scope of tactile processing. Dev Cogn Neurosci. (2018) 29:140–50. doi: 10.1016/j.dcn.2016.12.005
70. Ayres, AJ, and Tickle, LS. Hyper-responsivity to touch and vestibular stimuli as a predictor of positive response to sensory integration procedures by autistic children. Am J Occup Ther. (1980) 34:375–81. doi: 10.5014/ajot.34.6.375
71. Cascio, C, McGlone, F, Folger, S, Tannan, V, Baranek, G, Pelphrey, KA, et al. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord. (2008) 38:127–37. doi: 10.1007/s10803-007-0370-8
72. Foss-Feig, JH, Heacock, JL, and Cascio, CJ. Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Res Autism Spectr Disord. (2012) 6:337–44. doi: 10.1016/j.rasd.2011.06.007
73. Helga, OM, Sampaio, A, Martínez-Regueiro, R, Gómez-Guerrero, L, López-Dóriga, CG, Gómez, S, et al. Touch processing and social behavior in ASD. J Autism Dev Disord. (2017) 47:2425–33. doi: 10.1007/s10803-017-3163-8
74. Cascio, CJ, Gu, C, Schauder, KB, Key, AP, and Yoder, P. Somatosensory event-related potentials and association with tactile behavioral responsiveness patterns in children with ASD. Brain Topogr. (2015) 28:895–903. doi: 10.1007/s10548-015-0439-1
75. Lane, AE, Dennis, SJ, and Geraghty, ME. Brief report: further evidence of sensory subtypes in autism. J Autism Dev Disord. (2011) 41:826–31. doi: 10.1007/s10803-010-1103-y
76. Scheerer, NE, Curcin, K, Stojanoski, B, Anagnostou, E, Nicolson, R, Kelley, E, et al. Exploring sensory phenotypes in autism spectrum disorder. Mol Autism. (2021) 12:67. doi: 10.1186/s13229-021-00471-5
77. Allely, CS. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. ScientificWorldJournal. (2013) 2013:916178. doi: 10.1155/2013/916178
78. Willoughby, WR, Thoenes, K, and Bolding, M. Somatotopic arrangement of the human primary somatosensory cortex derived from functional magnetic resonance imaging. Front Neurosci. (2021) 14:598482. doi: 10.3389/fnins.2020.598482
79. Saadon-Grosman, N, Loewenstein, Y, and Arzy, S. The 'creatures' of the human cortical somatosensory system. Brain Commun. (2020) 2:fcaa003. doi: 10.1093/braincomms/fcaa003
80. Kyriacou, C, Forrester-Jones, R, and Triantafyllopoulou, P. Clothes, sensory experiences and autism: is wearing the right fabric important? J Autism Dev Disord. (2021) 53:1495–508. doi: 10.1007/s10803-021-05140-3
81. Haigh, SM, Minshew, N, Heeger, DJ, Dinstein, I, and Behrmann, M. Over-responsiveness and greater variability in roughness perception in autism. Autism Res. (2016) 9:393–402. doi: 10.1002/aur.1505
82. Scarano, A, Sbarbati, A, Amore, R, Iorio, EL, Ferraro, G, Lorusso, F, et al. A new treatment for stretch marks and skin ptosis with electromagnetic fields and negative pressure: a clinical and histological study. J Cutan Aesthet Surg. (2021) 14:222–8. doi: 10.4103/JCAS.JCAS_122_20
83. Korgavkar, K, and Wang, F. Stretch marks during pregnancy: a review of topical prevention. Br J Dermatol. (2015) 172:606–15. doi: 10.1111/bjd.13426
84. Maciałczyk-Paprocka, K, Stawińska-Witoszyńska, B, Kotwicki, T, Sowińska, A, Krzyżaniak, A, Walkowiak, J, et al. Prevalence of incorrect body posture in children and adolescents with overweight and obesity. Eur J Pediatr. (2017) 176:563–72. doi: 10.1007/s00431-017-2873-4
85. Key, J. 'The core': understanding it, and retraining its dysfunction. J Bodyw Mov Ther. (2013) 17:541–59. doi: 10.1016/j.jbmt.2013.03.012
86. Ahmed, AA, Taha, OT, and Elprince, M. Evaluation of the severity of striae gravidarum in women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. (2020) 253:21–4. doi: 10.1016/j.ejogrb.2020.07.029
87. Miranne, JM, Kramer, ME, Mete, M, and Iglesia, CB. The association of abdominal striae with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. (2019) 25:305–8. doi: 10.1097/SPV.0000000000000548
88. Halperin, O, Noble, A, Balachsan, S, Klug, E, and Liebergall-Wischnitzer, M. Association between severities of striae gravidarum and obstetric anal sphincter injuries (OASIS). Midwifery. (2017) 54:25–8. doi: 10.1016/j.midw.2017.07.019
89. Cunha, PR, Mattos, CB, and Salai, AF. Striae as a predictive clinical marker of urogenital dystopia and preventive treatment proposal. J Eur Acad Dermatol Venereol. (2017) 31:e236–7. doi: 10.1111/jdv.14017
90. Au, MK, Chan, WM, Lee, L, Chen, TM, Chau, RM, and Pang, MY. Core stability exercise is as effective as task-oriented motor training in improving motor proficiency in children with developmental coordination disorder: a randomized controlled pilot study. Clin Rehabil. (2014) 28:992–1003. doi: 10.1177/0269215514527596
Keywords: stretch marks, autism spectrum disorder, connective tissue, tactile alteration, connectivome
Citation: Veronese S, Zoccante L, Smania N and Sbarbati A (2023) Stretch marks: a visible expression of connective’s involvement in autism spectrum disorders. Front. Psychiatry. 14:1155854. doi: 10.3389/fpsyt.2023.1155854
Edited by:
Emily L. Casanova, Loyola University New Orleans, United StatesReviewed by:
Giulia Purpura, University of Milano Bicocca, ItalyStefano Marini, Independent Researcher, Termoli, Italy
Copyright © 2023 Veronese, Zoccante, Smania and Sbarbati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheila Veronese, c2hlaWxhLnZlcm9uZXNlQHVuaXZyLml0
 Sheila Veronese
Sheila Veronese Leonardo Zoccante
Leonardo Zoccante Nicola Smania1
Nicola Smania1 Andrea Sbarbati
Andrea Sbarbati