- 1Department of Psychiatry, Medical Sciences Division, University of Oxford, Oxford, United Kingdom
- 2Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, United Kingdom
- 3NIHR Oxford Health Biomedical Research Centre, Oxford, United Kingdom
- 4Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, United Kingdom
- 5Division of Insurance Medicine, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Postpartum psychosis is defined as a psychotic episode occurring within 4 to 6 weeks of childbirth. While there is robust evidence that adverse life events are associated with the onset and relapse of psychosis outside the postpartum period, the extent to which these contribute to postpartum psychosis is less clear. This systematic review examined whether adverse life events are associated with an increased likelihood of developing postpartum psychosis or subsequent relapse in women diagnosed with postpartum psychosis. The following databases were searched from inception to June 2021: MEDLINE, EMBASE, PsycInfo. Study level data were extracted including setting, number of participants, type of adverse event, and differences between groups. A modified version of the Newcastle-Ottawa Quality Assessments Scale was used to assess risk of bias. In total, 1933 records were identified, of which 17 met the inclusion criteria, comprising nine case–control studies and eight cohort studies. Most studies (16/17) examined the association between adverse life events and the onset of postpartum psychosis, with only in which the outcome was relapse of psychosis. Overall, there were 63 different measures of adversity examined (most of which were examined in a single study only) and 87 associations between these measures and postpartum psychosis tested across the studies. In terms of statistically significant associations with onset/relapse of postpartum psychosis, 15 (17%) were positive (i.e., the adverse event increased the risk of onset/relapse), 4 (5%) were negative, and 68 (78%) were not statistically significant. Our review highlights the diversity of risk factors examined in this field, with few attempts at replication, hence limiting the ability to conclude that any single risk factor is robustly associated with the onset of postpartum psychosis. Further large-scale studies, that attempt to replicate earlier studies, are urgently needed to determine whether adverse life events play a role in the onset and exacerbation of postpartum psychosis.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=260592], identifier [CRD42021260592].
1. Introduction
Postpartum psychosis is a psychiatric emergency (1) that occurs in 0.9 to 2.6 per 1,000 births (2). Variation in the definition of postpartum psychosis across studies has prevented a global estimate of prevalence and meaningful comparison across countries (2). The risk of illness onset is higher among women with an existing diagnosis of bipolar disorder, those who have experienced postpartum psychosis following a previous pregnancy, and in primiparous women (3). Postpartum psychosis typically presents within 10–19 days of birth, with the most common presentation being a combination of affective and psychotic symptoms (4). The nosology of postpartum psychosis is not clear but the disorder is generally considered to fall within the bipolar spectrum (5), with overlapping symptomatology (6). Indeed, bipolar disorder has the highest risk of relapse within the postpartum period (7).
While postpartum psychosis is relatively rare, it is associated with severe outcomes: women with postpartum psychosis are at increased risk of suicide (8), a leading cause of maternal death in the year following delivery (9). With respect to illness course, most women experiencing postpartum psychosis do not have a previous history of mental illness (10), yet more than half later experience relapses that are not associated with childbirth (11). Compared to psychotic episodes that occur during other periods, postpartum psychosis has a clear triggering event, childbirth. However, the reasons why a small subgroup of women are particularly vulnerable to developing an episode of psychosis following birth are poorly understood.
Outside the postpartum period, psychotic disorders are thought to arise through a complex interaction of genetic (12), developmental (13), and environmental risk factors (14). Adverse life events form a category of environmental risk factors, broadly defined as exposure to difficult or unpleasant situations or experiences (15), that can occur during childhood or adulthood. Childhood adverse life events most frequently studied in relation to psychosis include household poverty, parental separation, death of a parent, neglect, abuse, and bullying (15). In adulthood, adverse events may include relationship breakdown, employment difficulties, death of a loved one, and housing problems (16). Some adverse events are specific to the postpartum period, such as complications of pregnancy, complications of childbirth, and complications in the neonate. Although these events are not typically included as ‘adverse events’ in the body of psychosis research, there is evidence that they are psychologically distressing for the mother (17) and might therefore be an important risk factor for postpartum psychosis.
While there is consistent evidence indicating that adverse life events are associated with psychosis onset in both males and females (16) and with illness relapse (18), previous narrative reviews (4, 19, 20), have found limited evidence to suggest that adverse life events or social stress are significant risk factors for postpartum psychosis. In contrast, other postpartum disorders such as depression are robustly associated with adverse life events (21) and perinatal complications (22). Given the contribution of adverse life events to development of (non-postpartum) psychosis and postpartum depression, a systematic appraisal of their role in the onset of postpartum psychosis in warranted. We are aware of one systematic review that examined peripartum complications in relation to first onset postpartum psychosis (23): the authors identified various risk factors from single studies but included case reports and case series (which could introduce bias) and did not utilize a risk of bias tool to assess this in a structured way.
To address the gap in current knowledge, we aimed to systematically review and appraise the quality of evidence for the association of adverse life events with (i) new onset of postpartum psychosis, and (ii) subsequent relapse of psychosis (irrespective of whether relapse occurred following childbirth) in women previously diagnosed with postpartum psychosis. We defined adverse life events as any event that could be considered as a stressor, ranging from individual events occurring in childhood or adulthood, those assessed using life event scales/checklists, and perinatal complications.
2. Materials and methods
PRISMA guidelines for systematic reviews were followed (24), see Supplementary material for checklist, and pre-registered with PROSPERO (CRD42021260592).
2.1. Search strategy
The following databases were searched from inception until June 2021: MEDLINE, EMBASE, PsychInfo. References from a recent review (23) examining associations between obstetric complications and postpartum psychosis were searched. Titles, abstracts, and keywords were searched using the combinations of terms shown below, with the full search strategy outlined in the Supplementary Materials:
• Puerper* OR Postpart* OR Post-part* OR After-pregnan* OR Childbirth* OR Child-birth* OR New-mother* OR Labor OR Labour OR Cesarean OR Delivery OR Deliveries OR Postnatal* OR Post-natal* OR Parturition
• Psychosis* OR Psychotic* OR Psychoses* OR Bipolar OR Mania*
• Life event OR Stress* OR Trauma* OR Advers* OR Abus*
Two authors (ER, VCSB) screened study titles and abstracts of all articles identified in the search and retrieved potentially relevant full-text articles for detailed examination. Full-text publications were then independently reviewed against inclusion criteria by a minimum or two authors (ER, VCSB, TR). Disagreements were resolved by the senior author (AEC).
2.2. Inclusion criteria
Studies were included if they measured the association between adverse life events and first onset of postpartum psychosis or subsequent relapse (either within or outside the postpartum period) among those who had a history of postpartum psychosis. Both prospective and retrospective studies were included, with no restrictions based on language. Studies examining risk factors for first onset postpartum psychosis were excluded if they did not include a control group of healthy women, or if they examined a range of postpartum psychiatric disorders without a clearly defined subgroup with postpartum psychosis. Studies examining factors associated with psychosis relapse among women with postpartum psychosis were excluded if they did not include a non-relapsed comparison group. Case studies, reviews, and publications that had not undergone peer reviewed (dissertations and conference abstracts) were not eligible for inclusion.
First-onset postpartum psychosis was defined as the first psychotic episode occurring during the postpartum period. Given variability in the length of this period across studies, and to maximize the number of studies eligible for inclusion, we considered any definition of ‘postpartum’ used by the original study authors. This could be longer than the definitions applied in the most recent versions of the Diagnostic and Statistical Manual [DSM-5, (25)] or the International Classification of Diseases [ICD-11, (26)] which require onset within 4 and 6 weeks after birth, respectively. Relapse in patients with a prior diagnosis of postpartum psychosis was defined as any subsequent psychotic episode (either within, or outside of, any subsequent postpartum period), as indicated by exacerbation of psychotic symptoms, clinical diagnosis of psychotic episode, readmission to hospital, or a combination of these.
There were no restrictions on the type of adverse life events; these encompassed abuse (physical, emotional, sexual or neglect), stressful life events, trauma, or other adverse experiences. In addition to conventionally defined adverse life events, any complication relating to pregnancy, childbirth, or the neonate was also considered an adverse life event as these complications are known to be psychologically distressing and are associated with postpartum depression.
2.3. Data extraction
Two authors (ER, TJR) extracted study level data independently, with any discrepancies discussed with the senior author (AEC). The following variables were extracted for each study: study setting, number of participants, mean age, length of postpartum period, type of adverse event measured, and study results. For studies using a scale or questionnaire to assess adverse events, the mean and standard deviation (SD) were extracted. For longitudinal studies examining associations between risk factors and outcome, the incidence rate ratio (IRR), relative risk (RR), odds ratio (OR) or hazard ratio (HR) were extracted. For those comparing the number of adverse events between groups, the absolute number, median and interquartile range (IQR) were extracted. For studies with overlapping samples (27, 28), the study with the most complete relevant dataset was included, with authors contacted if this was not clear.
The two pre-specified primary outcome measures were (i) the development of first-onset postpartum psychosis and (ii) relapse of psychosis among women with a history of postpartum psychosis. We classified adverse life events as: complications relating to childbirth, complications of pregnancy, complications in the neonate, individual adverse events experienced by the mother in both childhood and adulthood, and scales measuring life events.
2.4. Assessment of study quality
Modified versions of the Newcastle-Ottawa Quality Assessment Scale for case–control and cohort studies (29) were used to assess the risk of bias. These scales assess study quality in the domains of selection of participants, comparability of groups, and measurement of outcome. Ratings were performed independently by a minimum of two authors (ER, TJR, AEC) with disagreement resolved by discussion. The maximum possible score was 12 for both case–control and cohort designs.
3. Results
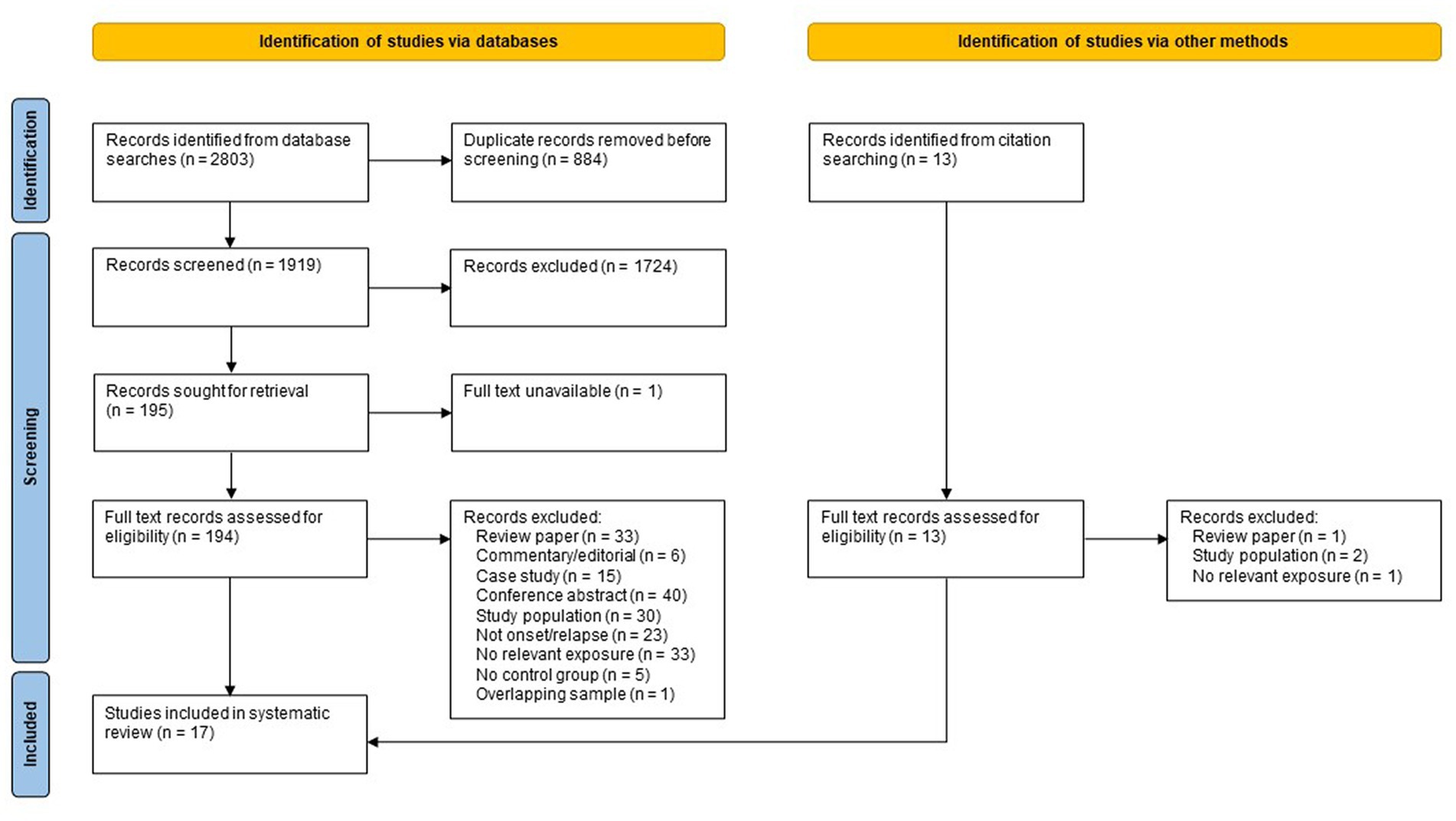
Searching of electronic databases and manual citation searching yielded 1,933 records after deduplication (see Figure 1), of which 17 met the inclusion criteria (27, 30–45), representing 18 independent study samples. Of the 17 included studies, eight were identified via database searching (27, 30, 32, 33, 35, 37, 44, 45), five were identified from a recent systematic review by Nguyen et al. (34, 38, 40, 42, 43) and four studies were found by citation searching of included references (31, 36, 39, 41). Five samples were from Denmark, four from the UK, three from Sweden, three from North America, one from South Africa, one from the Netherlands, and one from India. Nine studies used a case–control design and eight used a cohort design. The earliest study was published in 1960, with most published after 2000. All but one of these studies (16/17) examined the association between adverse life events and new onset of postpartum psychosis; only one study (41) examined the association between adverse life events and subsequent relapse among women previously diagnosed with postpartum psychosis.
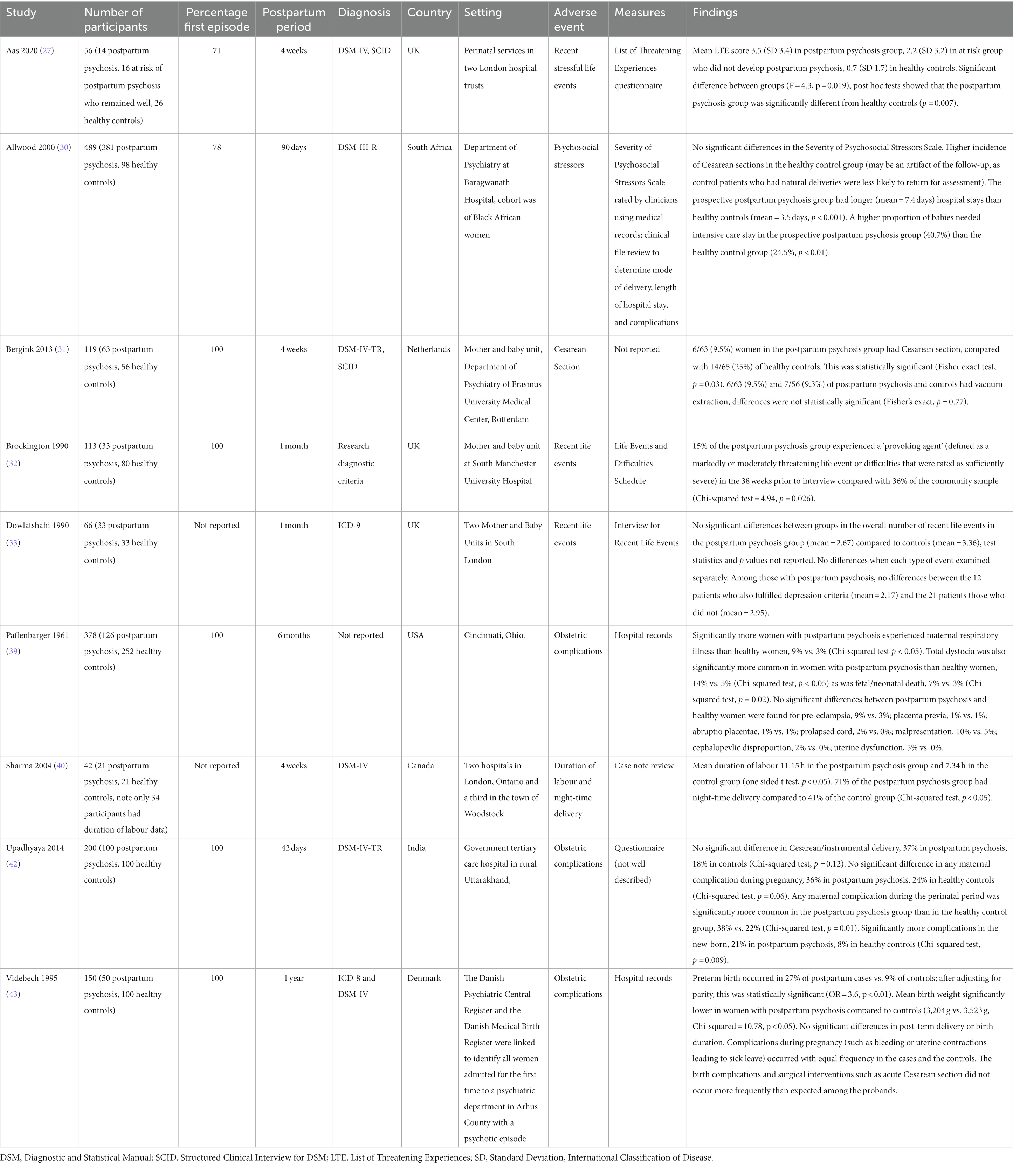
Characteristics and findings of the nine case–control studies are summarized in Table 1. The total sample size of case–control studies was 1,603, with a mean study sample size of 178 (SD = 152). The total number of participants with postpartum psychosis was 821 (mean = 91; SD = 115). Six studies measured exposure to obstetric complications (obtained from medical records), and three used checklists to measure exposure to psychosocial stressors. All studies compared women diagnosed with postpartum psychosis (cases) to healthy postpartum women (controls), while Aas et al. (27) additionally included a group who were at risk of postpartum psychosis (due to history of bipolar disorder or schizoaffective disorder) but remained well in the postpartum period.
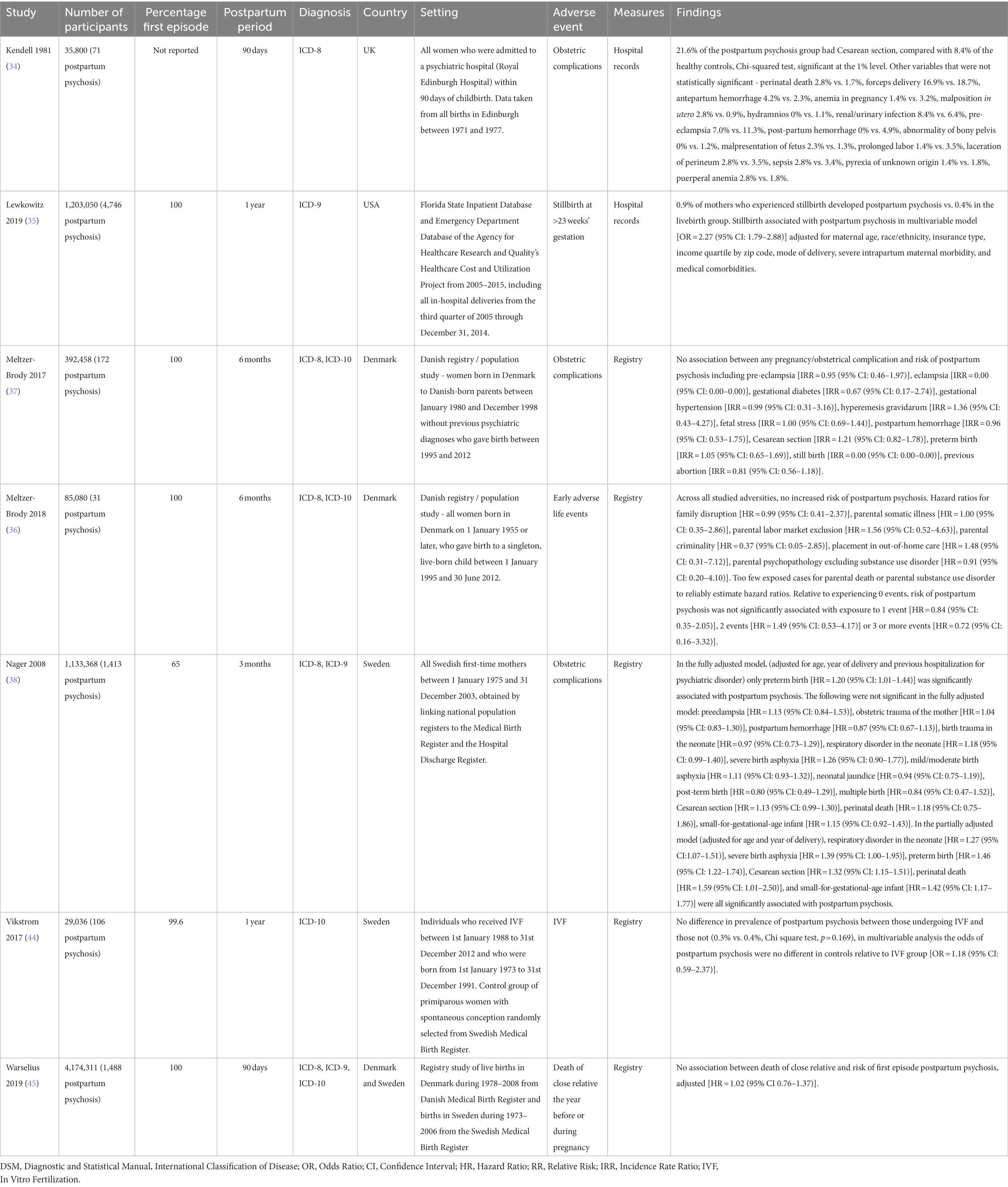
Findings from the seven cohort studies examining onset of postpartum psychosis are summarized in Table 2. Five were population-based registry studies, based in either Denmark or Sweden. The mean sample size across all cohort studies was 1,007,586 (SD = 1,483,998) and the mean number of cases of postpartum psychosis 1,147 (SD = 1,712). There was potential overlap across the study samples, with Meltzer-Brody et al. (37), Meltzer-Brody et al. (36), and Warselius et al. (45) using national registry data from Denmark to measure the association of different exposures. While Nager et al. (38), Vikstrom et al. (44), and Warselius et al. (45) used overlapping samples of the Swedish population. However, as all studies with potentially overlapping samples measured different exposures, all were included in the review. Findings from the single study (41) examining subsequent relapse in women previously diagnosed with postpartum psychosis are summarized in Table 3.
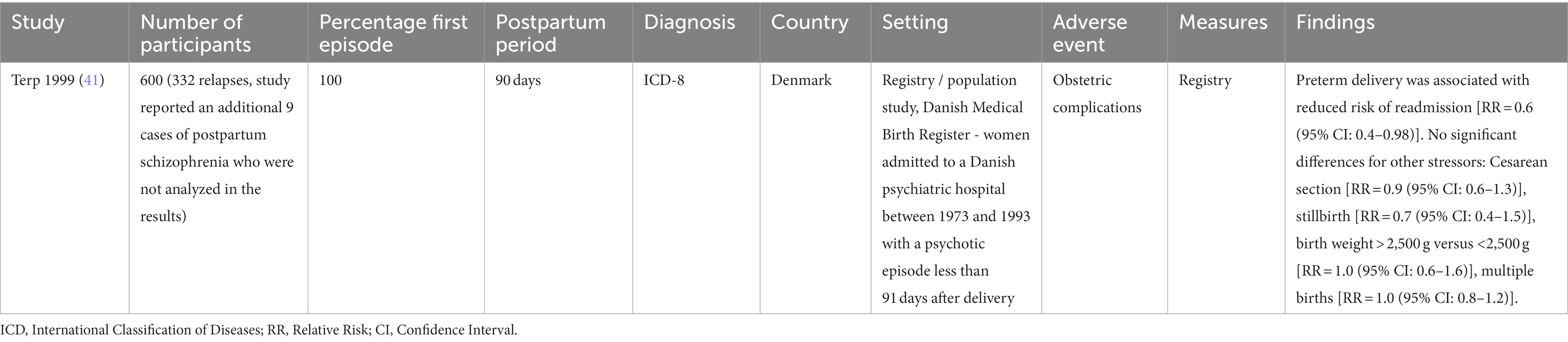
Table 3. Summary of cohort study examining subsequent relapse in women previously diagnosed with postpartum psychosis.
Across studies, a wide range of adverse life events were examined. These were broadly categorized as individual psychosocial stressors experienced by the mother (either in childhood or adulthood), life event checklists, complications of pregnancy, complications of birth, and complications in the neonate.
3.1. Individual psychosocial stressors experienced by the mother
Three individual adult psychosocial stressors were each examined by a single large population-based cohort study: previous abortion (37), in vitro fertilization (44), and death of a close relative in the year before or during pregnancy (45). None showed an association with postpartum psychosis. Meltzer-Brody et al. (37) examined previous abortion in a Danish population-based study of 392,458 women, and found no association with the onset of postpartum psychosis [RR = 0.81 (95% CI: 0.56–1.18)]. Vikstrom et al. (44) compared rates of postpartum psychosis among women who had become pregnant by in vitro fertilization with primiparous women experiencing spontaneous conception who were randomly selected from the Swedish Medical Birth Register. In the in vitro fertilization sample, 0.3% developed postpartum psychosis, compared with 0.4% of the spontaneous conception sample [OR = 1.18 (95% CI: 0.59–2.37)]. In a further registry study using data from Denmark and Sweden, Warselius et al. (45) investigated death of a close relative in the year before or during pregnancy but found no significant association with onset of postpartum psychosis [adjusted HR = 1.02 (95% CI: 0.76–1.37)].
Individual childhood adverse events were examined in one population-based cohort study of 85,080 women in Denmark, of whom 31 developed postpartum psychosis (36). None of the risk factors examined (family disruption, parental somatic illness, parental labour market exclusion, parental criminality, placement in out-of-home care, parental psychopathology) were significantly associated with onset of postpartum psychosis. Moreover, relative to experiencing no adverse events, postpartum psychosis was not associated with experiencing one, two, or more than two adverse events.
3.2. Life event checklists
Three studies (27, 32, 33) used life event checklists to measure adverse events, these measures included the List of Threatening Experiences (46), Life Events and Difficulties Schedule (47), and Interview for Recent Life Events (48).
Aas et al. (27) reported a positive association between life events and postpartum psychosis in the UK, though this was a small study with a case–control design. This study included women with postpartum psychosis, women at risk of postpartum psychosis (due to a previous diagnosis of bipolar disorder or schizoaffective disorder) who remained well, and healthy controls. Mean scores on the List of Threatening Experiences questionnaire were 3.5 (SD 3.4) in postpartum psychosis group, 2.2 (SD 3.2) in at risk group who did not develop postpartum psychosis, and 0.7 (SD 1.7) in healthy controls. There was a significant effect of group status on scores (F = 4.3, p = 0.019), with pairwise comparisons showing that mean life event scores were statistically different in the postpartum psychosis group and healthy controls only (p = 0.007).
In another small UK case–control study, Brockington et al. (32) found a negative association between life events assessed using the Life Events and Difficulties Schedule, where women with postpartum psychosis were less likely to have experienced severe events or major difficulties in the 38 weeks prior to interview (χ2 = 4.9, p = 0.026). A further UK case–control study conducted by Dowlatshahi and Paykel (33) used the Interview for Recent Life Events (examining events in the past 13 months) and found no statistically significant differences in the number of recent life events experienced by women with postpartum psychosis and healthy controls.
3.3. Complications of pregnancy
Pre-eclampsia was investigated by four studies, none of which showed a significant association with postpartum psychosis. These included two large registry studies in Denmark (37) and Sweden (38), in addition to a cohort study in the UK (34) and a case–control study in the USA (39).
Two case–control studies based in Denmark (43) and India (42) examined any maternal complication during pregnancy, neither of which observed an association with onset of postpartum psychosis. One case–control study in the USA (39) observed that maternal respiratory illnesses were significantly more common among women with postpartum psychosis than healthy controls (p < 0.05). All other complications of pregnancy were examined by single studies and did not show significant associations: antepartum hemorrhage (34), anemia in pregnancy (34), malposition in utero (34), hydramnios (34), renal/urinary infection (34), gestational diabetes (37), gestational hypertension (37), hyperemesis gravidarum (37), fetal stress (37), placenta praevia (39), and abruptio placentae (39).
3.4. Complications of birth
The most common complication of birth was Cesarean section, examined in six studies (two case–control and four cohort). Allwood et al. (30) found a lower incidence of Cesarean section among women with postpartum psychosis compared with healthy controls (neither incidence rates nor statistical tests reported); however, the authors interpreted this as a possible artifact due to the method of recruiting the controls (who were identified from follow-up clinic appointments). In a small (n = 119) case–control study in the Netherlands, Bergink and colleagues (31) similarly observed that Cesarean section was less common in women with postpartum psychosis (9.5%) compared with healthy controls (25%), which was statistically significant (p = 0.03). In contrast, Kendell et al. (34), who examined a cohort including all live births in Edinburgh between 1971 and 1977, reported that Cesarean section was associated with an increased risk of postpartum psychosis: occurring in 21.6% women who went on to develop postpartum psychosis and 8.4% of the control population (p < 0.01). Notably, the two largest studies (37, 38), both nationwide register studies with low risk of bias, observed no significant association between Cesarean section and risk of onset of postpartum psychosis. Non-significant associations between Cesarean section and postpartum psychosis onset were also reported by Nager et al. (38), in a model adjusting for age, year of delivery, and previous hospitalization for psychiatric disorder [adjusted HR = 1.13 (95% CI: 0.99–1.30)] and Meltzer-Brody et al. (37) [RR = 1.21 (95% CI: 0.82–1.78)]. Consistent with these findings, the only study examining relapse of postpartum psychosis (41), found no association with Cesarean section [RR = 0.9 (95% CI: 0.6–1.3)].
Pre-term birth was examined by three studies. Videbech et al. (43), using a case–control study design, found that preterm birth was more common in women with postpartum psychosis (27%) than healthy controls (9%), which remained statistically significant after adjusting for parity (OR = 3.6, p < 0.01). Similarly, a large population cohort study using Swedish registers (38) observed that pre-term birth was significantly associated with the onset of postpartum psychosis after adjusting for age, year of delivery, and previous hospitalization for psychiatric disorder [adjusted HR = 1.20 (95% CI: 1.01–1.44)]. However, a large Danish cohort study failed to replicate this finding (37), instead observing no association between preterm birth and postpartum psychosis onset [HR = 1.05 (95% CI: 0.65–1.69)]. In contrast, Terp et al. (41), reported a negative association between preterm birth and subsequent relapse in women who had been previously diagnosed with first onset postpartum psychosis [RR = 0.6 (95% CI: 0.4–0.98)].
Postpartum hemorrhage was examined by three cohort studies conducted in Denmark (37), Sweden (38), and the UK (34); none found an association with this risk factor and onset of postpartum psychosis. Length of labor was positively associated with postpartum psychosis in a small (n = 38) case–control study (40) (mean duration of labor 11.15 h in the puerperal psychosis group vs. 7.34 h in the control group, one sided t test p < 0.05), but showed no association in another, larger case control study (n = 150) (43). A large registry cohort study based in Sweden (38) and another case–control study in Denmark (43) examined post-term birth, with neither finding an association with postpartum psychosis onset. Two studies examined malpresentation of fetus (34, 39), with neither finding an association.
Most types of birth complication were examined by a single study. Those showing significant associations with onset of postpartum psychosis included longer mean length of hospital stay (7.4 days in cases vs. 3.5 days in controls, p < 0.001) in a study conducted in South Africa (30); total dystocia (occurring in 14% in postpartum psychosis vs. 5% of controls, p < 0.05) in a case–control study in the USA (39); and night-time delivery (occurring in 71% of women with puerperal psychosis vs. 41% of controls, p < 0.05) in one case–control study in Canada (40). When considering any maternal complication during perinatal period, a case–control study in India found this was significantly more common among women with postpartum psychosis than controls (38% vs. 22%, respectively, p = 0.01) (42). The following birth complications were found to show no association with onset of postpartum psychosis: vacuum extraction (31), forceps delivery (34), abnormality of bony pelvis (34), prolonged labor (34), laceration of perineum (34), sepsis (34), pyrexia of unknown origin (34), puerperal anemia (34), eclampsia (37), obstetric trauma of the mother (38), multiple birth (38), prolapsed cord (39), cephalopelvic disproportion (39), uterine dysfunction (39), Cesarean or instrumental delivery (42), any birth complications or surgical interventions (43).
3.5. Complications of neonate
Four studies examined perinatal death (including stillbirth). Paffenbarger et al. (39) reported significantly more fetal or neonatal deaths in women with postpartum psychosis compared to controls (7% vs. 3%, p = 0.02). In a large study comprising all hospital births in Florida state between 2005 and 2014, Lewkowitz et al. (35) observed that subsequent onset of postpartum psychosis was more common among women who experienced stillbirth (0.9%) compared with the livebirth group (0.4%). In this study, stillbirth was significantly associated with postpartum psychosis in a multivariable model adjusted for maternal age, race/ethnicity, insurance type, income quartile, mode of delivery, severe intrapartum maternal morbidity, and medical comorbidities. However, this was not found in other studies. Kendell et al. (34) failed to find an association between perinatal death and onset of postpartum psychosis in a UK cohort (2.8% of the postpartum psychosis group vs. 1.7% of the control group), while a large registry cohort study in Sweden (38) also failed to find an association in a model adjusted for age, year of delivery, and previous hospitalization [adjusted HR = 1.18 (95% CI: 0.75–1.86)].
There was a higher proportion of neonates requiring intensive care (40.7% in postpartum psychosis and 24.5% of healthy controls, p < 0.01) in a single case–control study in South Africa (30). Similarly, a single case–control study in India observed that complications in the neonate were more common among women with postpartum psychosis compared to controls (21% vs. 8%, respectively, p = 0.009) (42). Videbech et al. (43) reported that the mean birthweight was lower in the postpartum psychosis group relative to controls (3,204 g vs. 3,523 g, p < 0.05).
The following complications of the neonate that were examined by single studies were not found to be significantly associated with postpartum psychosis: birth trauma in the neonate (38), respiratory disorder in the neonate (38), severe birth asphyxia (38), mild/moderate birth asphyxia (38), neonatal jaundice (38), and small for gestational age (38).
3.6. Summary of evidence across all studies
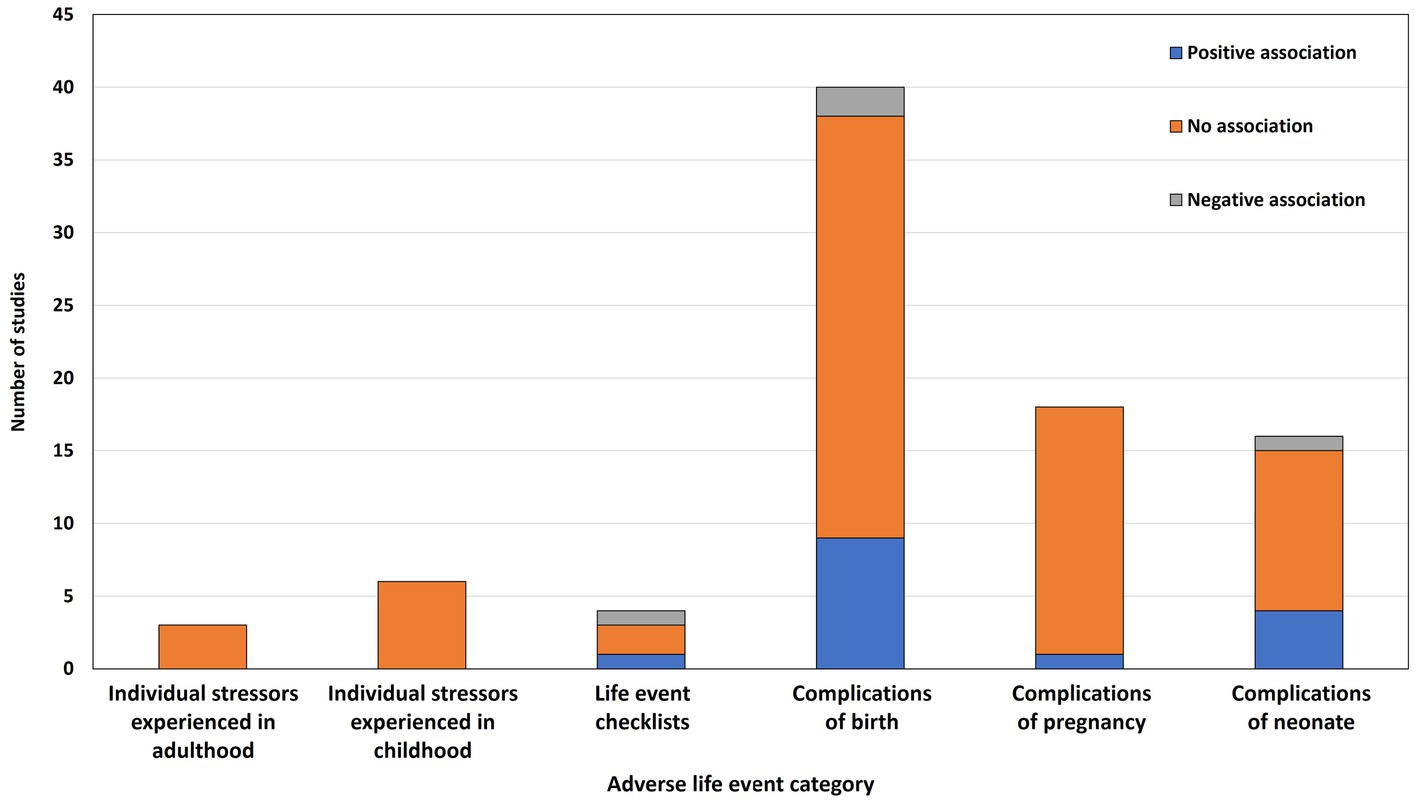
Figure 2 summarizes the results of the reported associations across studies, grouped by category of adverse event. Overall, 63 different measures of adversity were examined and 87 associations between these measures and postpartum psychosis were tested. In terms of statistically significant associations with onset/relapse of postpartum psychosis, 15 (17%) were positive (i.e., the adverse event increased the risk of onset/relapse), 4 (5%) were negative (i.e., the adverse event decreased the risk of onset/relapse), and 68 (78%) were not statistically significant.
3.7. Risk of bias
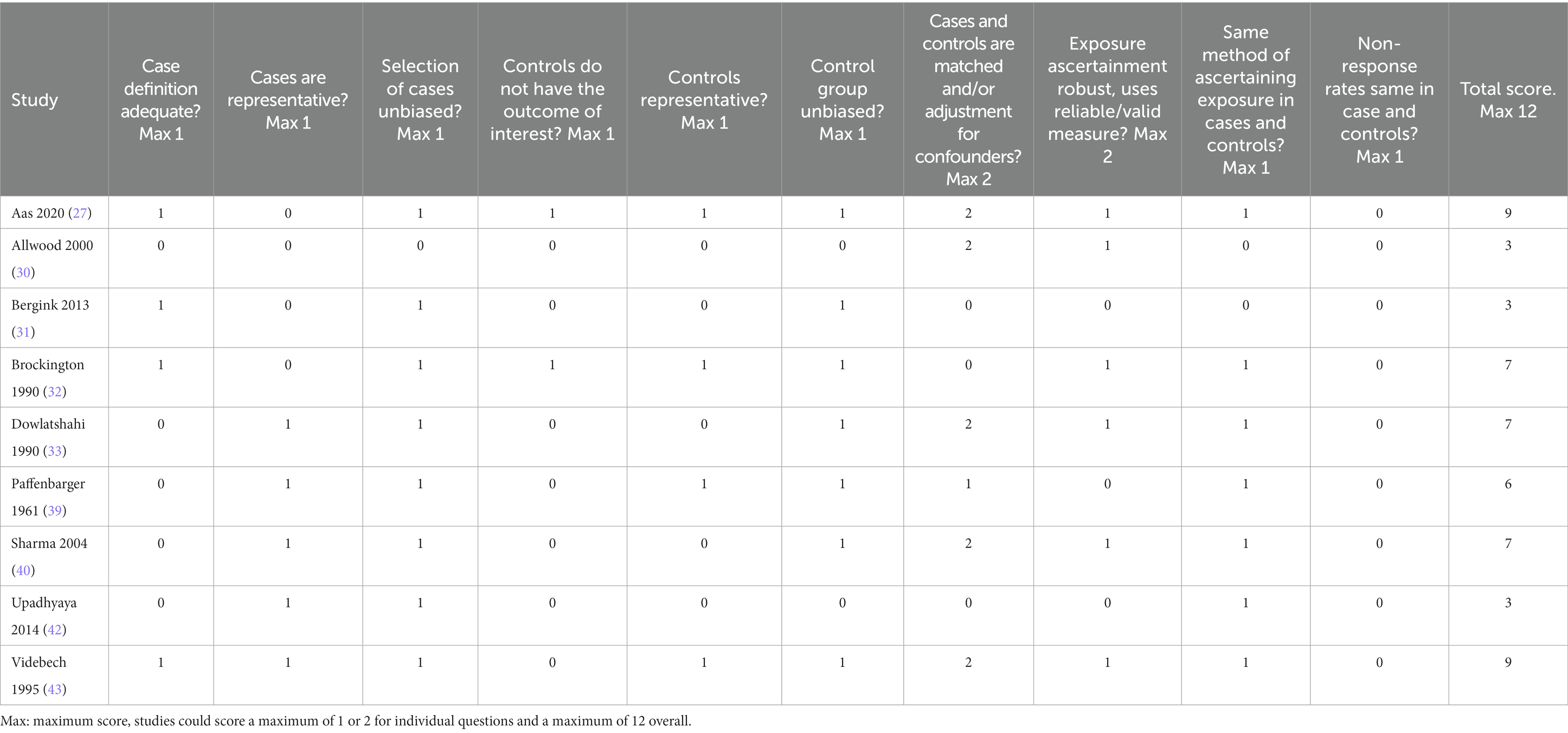
Risk of bias ratings for case–control studies are shown in Table 4. Total scores for case–control studies ranged between 3 and 9, with a median of 7.0 (IQR = 4.0). Only two studies (27, 32) took adequate steps to ensure that controls did not have postpartum psychosis or any other mental disorder. No studies demonstrated that non-response rates were equivalent in cases and controls. Aas et al. (27) and Videbech et al. (43) scored highest with 9, of note, both studies had adequate definition of cases, unbiased selection of cases, selection of representative controls, unbiased selection of controls, adjusted/matched for confounders, and the same method of ascertaining exposure in cases and controls. Three studies (Allwood et al. (30), Bergink et al. (31) and Upadhyaya et al. (42)) obtained low scores (each scoring 3/12). None of these studies took steps to ensure selected controls were representative.
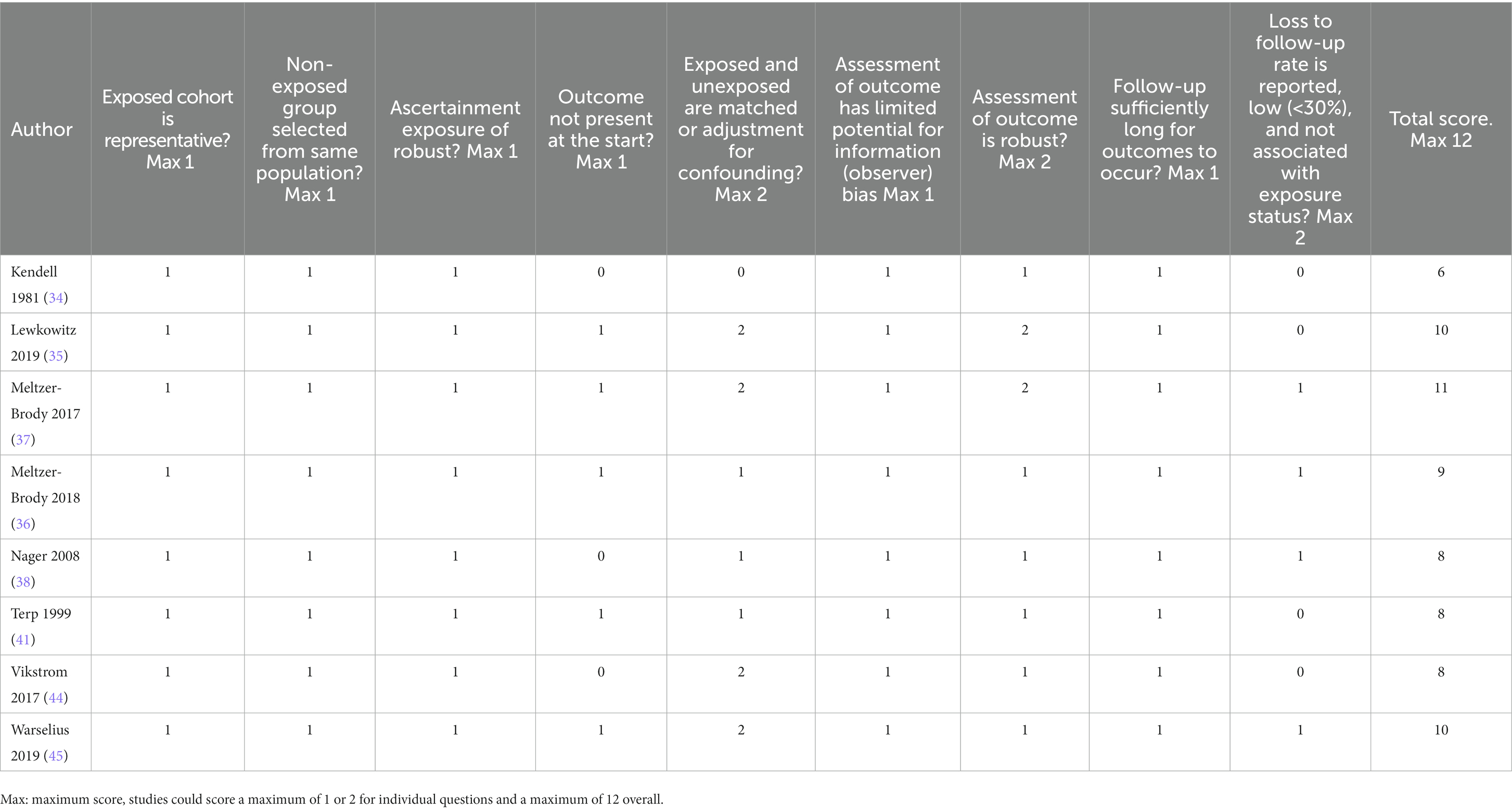
Table 5 shows risk of bias scores for cohort studies. Overall, scores were higher for cohort studies (range: 6–11, median of 8.5, IQR = 4.0) than for the case–control studies (indicating lower risk of bias). All studies were determined to have used a representative cohort and ensured the non-exposed and exposed groups were taken from the same population. Moreover, all employed robust methods to ascertain exposure, had limited risk of observer bias for the outcome, and had a sufficiently long time-period for the outcome to occur. However, none of the studies adequately addressed loss to follow-up in that they failed to report the proportion who were not followed up and whether attrition was associated with exposure status. That said, several studies (36–38, 45) noted that individuals were followed until death or emigration and used appropriate statistical models to account for censoring. The highest scoring study (11/12) was Meltzer-Brody et al. (37), this study examined obstetric complications using a population-based cohort design and was notable as it was one of only two studies that adequately adjusted for potential confounders. The lowest scoring study was Kendell et al. (34), which was also the earliest cohort study and had several limitations including failure to ensure the outcome was not present at the start of the study, failure to adjust for potential confounders, and failure to report loss to follow-up.
4. Discussion
To our knowledge, this is the first systematic review to examine the association between adverse life events and postpartum psychosis. Our review identified 17 studies examining a wide range of adverse life events experienced in childhood and adulthood, as well as those specific to the perinatal period. While these studies failed to convincingly show that adverse life events (experienced at any stage of life) contributed to the onset or relapse of postpartum psychosis, our review identified several major limitations with the current evidence base which prevents us from drawing meaningful conclusions.
One important limitation was that most risk factors (52/63) were examined in a single study only; this was particularly the case for adverse events occurring outside of perinatal period. Although some studies used population-based registry designs (36, 37, 44, 45), the number of participants who developed postpartum psychosis was still relatively low. Furthermore, these record linkage studies defined the postpartum period as ranging from 3 months to 1 year, which is considerably longer than current DSM or ICD definitions of postpartum: 4 and 6 weeks, respectively.
Replications of the results are required before concluding that adverse life events in childhood or adulthood show no association with postpartum psychosis. Indeed, one study that did not meet our inclusion criteria suggested an association is possible: Hazelgrove et al. (28), in a small case–control study, reported that severe childhood maltreatment was associated with postpartum relapse in an at-risk sample. Another study (49) that did not meet inclusion criteria suggested that there was no difference in the rate of childhood adverse events between patients with bipolar disorder who did or did not experience postpartum relapse.
There may also be differences in perceived stress in women vulnerable to postpartum psychosis. Aas et al. (27) observed that women with postpartum psychosis reported higher levels of perceived stress compared with controls, while Allwood et al. (30) also reported increased levels of perceived stress in their postpartum psychosis group. This suggests that while rates of adverse events may be similar among women experiencing postpartum psychosis and healthy controls, women with postpartum psychosis may perceive these events as more stressful and/or uncontrollable.
Perinatal complications are not conventionally considered as adverse life events in psychosis research. These were included to be as broad as possible in our search. Our findings are similar to a previous systematic review of peripartum complications in relation to postpartum psychosis (23), which showed a lack of replication for any single risk factor. Notably this previous systematic review had limited information on included studies, did not formally assess study quality, and included study designs with high risk of bias, such as case reports.
A lack of association between adverse life events and postpartum psychosis has been noted in previous narrative reviews (4, 19, 20), but this is the first systematic review to comprehensively search across a broad range of risk factors. Given the evidence from research published to date, identifying individuals at-risk for postpartum psychosis should focus on well-established risk factors of large effect, such as personal or family history of postpartum psychosis, or personal history of bipolar disorder. The lack of association with adverse life events contrasts with postpartum depression, where adverse life events are among the strongest predictors (21, 50). It also differs from psychotic disorders that onset outside the postpartum period, which have been associated with increased exposure to adverse life events (16). As noted previously, postpartum psychosis is conceptualized as part of the bipolar spectrum, thus, it is notable that childhood adversity is associated with bipolar disorder (51).
Given the absence of evidence supporting a consistent association between adverse life events and postpartum psychosis, other etiological mechanisms beyond the role of adverse events should also be examined in future research. One important risk factor to consider is sleep disturbance, which is a recognized feature of bipolar disorder and schizophrenia (52). Women with bipolar disorder who experience episodes triggered by sleep loss are twice as likely to have experienced an episode of postpartum psychosis compared with women without this trigger (53). Thus, sleep loss associated with childbirth could be a causative factor for some women with this vulnerability.
Female sex hormones that show major fluctuations during the postpartum period might also play a contributing role. Indeed estrogen, which (along with progesterone) increases during pregnancy and then precipitously falls after birth (19), has been hypothesized to be protective against psychosis (54) with withdrawal states associated with psychotic episodes (55). Despite the plausibility of this potential mechanism, few studies have directly measured or manipulated hormones in postpartum psychosis. Of note, a single open label trial of prophylactic estradiol for the prevention of postpartum psychosis in women with a bipolar disorder yielded negative findings (56).
There is some evidence to suggest that genetic predisposition contributes to postpartum psychosis. Women with bipolar disorder who experienced postpartum psychosis were more likely to report a history of affective disorder in first-degree relatives than those with bipolar disorder in the absence of postpartum episodes (57). Moreover, one study suggests that postpartum psychosis shares polygenetic liability with both bipolar disorder and schizophrenia but not major depression (5).
Dysfunction of the immune system has been postulated as a potential mechanism of postpartum psychosis (58). Pregnancy and postpartum are associated with immunomodulation that allows tolerance of the fetus while also providing protection from pathogens. Key inflammatory markers that have been associated with postpartum psychosis are interleukin-8, monocyte chemoattractant protein-1 and c-reactive protein (58). These markers are also associated with psychotic disorders outside the postpartum period (59).
4.1. Limitations
There are some limitations that should be taken into consideration. We chose to focus on first-episode postpartum psychosis given that a prior postpartum psychosis, bipolar disorder, and schizoaffective disorder are established risk factors, with the potential to confound the relationship. However, in some studies, the samples included patients who were not first episode cases, and in three (33, 34, 40) the number who were experiencing a first episode was unclear. We chose to include a broad range of adverse events, which incorporated both psychosocial stressors and biological factors, such as IVF. We therefore included adverse events that may not be considered as relevant in most studies of psychosis outside the postpartum period.
The studies we examined were predominately from European or North American populations. Only one was from Africa and one from Asia, and there were no studies from South America. The large record-linkage studies which showed a lack of association for a variety of adverse life events in adequately powered samples are from only two countries, Sweden and Denmark. This limits the generalizability of our conclusions beyond Western populations.
We identified only one study that examined the effect of adverse events on relapse following a diagnosis of postpartum psychosis (41), all others examined onset. The definition of ‘postpartum’ varied between studies, with some considering any psychotic episode within 1 year of childbirth. This is at odds with DSM and ICD classifications of the postpartum period, which are defined as 4 and 6 weeks, respectively. Due to the heterogeneity in study design, risk factors and outcomes, we were unable to perform a meta-analysis to pool results across studies.
4.2. Conclusion
In this systematic review, which included studies examining a wide range of risk factors, we were unable to identify robust evidence of an association between adverse life events and onset of postpartum psychosis. Notably, of the 63 potential risk factors studied, only 11 (17%) were examined by more than one study. Further replication, using consistent definitions of postpartum psychosis and risk factors, is required to establish whether there is truly an absence of association.
Studies examining relapse following a diagnosis of postpartum psychosis were also lacking (indeed only one study was identified). Given that more than half of women with first-onset postpartum psychosis will experience further episodes outside the postpartum period (11), and are also at risk of suicide (60), understanding factors that contribute to illness relapse is crucial to improving outcomes for women with this disorder.
Future studies should address the major limitations that we have identified in this review, notably, using a consistent definition of postpartum that corresponds with established diagnostic systems (DSM and ICD). Given the lack of studies examining the contribution of childhood adversity (a well-established risk factor for psychotic disorders generally), we strongly recommend that future studies in this field investigate these measures.
Author contributions
AEC designed and supervised the study. ER conducted the search, screened studies, extracted data, conducted bias ratings, and wrote parts of the manuscript. TJR extracted data, conducted bias ratings, and drafted the manuscript. VCSB screened studies and extracted data. AEC, PM, and PD critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
TJR is supported by an MRC Clinical Research Training Fellowship, MR/W015943/1. AEC is supported by a NARSAD Young Investigator Grant awarded by the Brain & Behavior awarded by the Brain & Behavior Research Foundation, 28336.
Acknowledgments
We thank Katie Hazelgrove for clarifying information regarding the Psychiatry Research and Motherhood study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1154557/full#supplementary-material
References
1. Jones, I, Chandra, PS, Dazzan, P, and Howard, LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. (2014) 384:1789–99. doi: 10.1016/S0140-6736(14)61278-2
2. Vander Kruik, R, Barreix, M, Chou, D, Allen, T, Say, L, Cohen, LS, et al. The global prevalence of postpartum psychosis: a systematic review. BMC Psychiatry. (2017) 17:1–9. doi: 10.1186/s12888-017-1427-7
3. Munk-Olsen, T, Jones, I, and Laursen, TM. Birth order and postpartum psychiatric disorders. Bipolar Disord. (2014) 16:300–7. doi: 10.1111/bdi.12145
4. Perry, A, Gordon-Smith, K, Jones, L, and Jones, I. Phenomenology epidemiology and aetiology of postpartum psychosis: a review. Brain Sci. (2021) 11:1–14. doi: 10.3390/brainsci11010047
5. Di Florio, A, Mei Kay Yang, J, Crawford, K, Bergink, V, Leonenko, G, Pardiñas, AF, et al. Post-partum psychosis and its association with bipolar disorder in the UK: a case-control study using polygenic risk scores. The lancet. Psychiatry. (2021) 8:1045–52. doi: 10.1016/S2215-0366(21)00253-4
6. Kamperman, AM, Veldman-Hoek, MJ, Wesseloo, R, Robertson Blackmore, E, and Bergink, V. Phenotypical characteristics of postpartum psychosis: a clinical cohort study. Bipolar Disord. (2017) 19:450–7. doi: 10.1111/bdi.12523
7. Munk-Olsen, T, Laursen, TM, Mendelson, T, Pedersen, CB, Mors, O, and Mortensen, PB. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. (2009) 66:189–95. doi: 10.1001/archgenpsychiatry.2008.528
8. Appleby, L, Mortensen, PB, and Faragher, EB. Suicide and other causes of mortality after post-partum psychiatric admission. Br J Psychiatry. (1998) 173:209–11. doi: 10.1192/bjp.173.3.209
9. Chin, K, Wendt, A, Bennett, IM, and Bhat, A. Suicide and maternal mortality. Curr Psychiatry Rep. (2022) 24:239–75. doi: 10.1007/s11920-022-01334-3
10. Blackmore, ER, Rubinow, DR, O'Connor, TG, Liu, X, Tang, W, Craddock, N, et al. Reproductive outcomes and risk of subsequent illness in women diagnosed with postpartum psychosis. Bipolar Disord. (2013) 15:394–404. doi: 10.1111/bdi.12071
11. Gilden, J, Kamperman, AM, Munk-Olsen, T, Hoogendijk, WJG, Kushner, SA, and Bergink, V. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. (2020):81. doi: 10.4088/JCP.19r12906
12. Ripke, S, Neale, BM, Corvin, A, Walters, JTR, Farh, KH, Holmans, PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. (2014) 511:421–7. doi: 10.1038/nature13595
13. Murray, RM, Bhavsar, V, Tripoli, G, and Howes, O. 30 years on: how the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. (2017) 43:1190–6. doi: 10.1093/schbul/sbx121
14. Stilo, SA, and Murray, RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. (2019) 21:100. doi: 10.1007/s11920-019-1091-3
15. Morgan, C, and Gayer-Anderson, C. Childhood adversities and psychosis: evidence, challenges, implications. World Psychiatry. (2016) 15:93–102. doi: 10.1002/wps.20330
16. Beards, S, Gayer-Anderson, C, Borges, S, Dewey, ME, Fisher, HL, and Morgan, C. Life events and psychosis: a review and meta-analysis. Schizophr Bull. (2013) 39:740–7. doi: 10.1093/schbul/sbt065
17. Gausia, K, Ryder, D, Ali, M, Fisher, C, Moran, A, and Koblinsky, M. Obstetric complications and psychological well-being: experiences of Bangladeshi women during pregnancy and childbirth. J Health Popul Nutr. (2012) 30:172–80. doi: 10.3329/jhpn.v30i2.11310
18. Martland, N, Martland, R, Cullen, AE, and Bhattacharyya, S. Are adult stressful life events associated with psychotic relapse? A systematic review of 23 studies. Psychol Med. (2020) 50:2302–16. doi: 10.1017/S0033291720003554
19. Bergink, V, Rasgon, N, and Wisner, KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatr. (2016) 173:1179–88. doi: 10.1176/appi.ajp.2016.16040454
20. Davies, W. Understanding the pathophysiology of postpartum psychosis: challenges and new approaches. World J Biol Psychiatry. (2017) 7:77–88. doi: 10.5498/wjp.v7.i2.77
21. Guintivano, J, Manuck, T, and Meltzer-Brody, S. Predictors of postpartum depression: a comprehensive review of the last decade of evidence. Clin Obstet Gynecol. (2018) 61:591–603. doi: 10.1097/GRF.0000000000000368
22. Blom, EA, Jansen, PW, Verhulst, FC, Hofman, A, Raat, H, Jaddoe, VWV, et al. Perinatal complications increase the risk of postpartum depression. The generation R study. BJOG. (2010) 117:1390–8. doi: 10.1111/j.1471-0528.2010.02660.x
23. Nguyen, K, Mukona, LT, Nalbandyan, L, Yar, N, St. Fleur, G, Mukona, L, et al. Peripartum complications as risk factors for postpartum psychosis: a systemic review. Cureus. (2022) 14:1–7. doi: 10.7759/cureus.29224
24. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2020) 2021:372. doi: 10.1136/bmj.n71
25. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association (2013).
26. World Health Organization. International statistical classification of diseases and related health problems. 11th ed. Geneva: WHO (2019).
27. Aas, M, Vecchio, C, Pauls, A, Mehta, M, Williams, S, Hazelgrove, K, et al. Biological stress response in women at risk of postpartum psychosis: the role of life events and inflammation. Psychoneuroendocrinology. (2020) 113:104558. doi: 10.1016/j.psyneuen.2019.104558
28. Hazelgrove, K, Biaggi, A, Waites, F, Fuste, M, Osborne, S, Conroy, S, et al. Risk factors for postpartum relapse in women at risk of postpartum psychosis: the role of psychosocial stress and the biological stress system. Psychoneuroendocrinology. (2021) 128:105218. doi: 10.1016/j.psyneuen.2021.105218
29. Wells, G, Shea, B, O'Connell, D, Peterson, J, Welch, V, Losos, M, et al. (2011). The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed March 23, 2023).
30. Allwood, CW, Berk, M, and Bodemer, W. An investigation into puerperal psychoses in black women admitted to Baragwanath hospital. S Afr Med J. (2000) 90:518–20.
31. Bergink, V, Burgerhout, KM, Weigelt, K, Pop, VJ, De Wit, H, Drexhage, RC, et al. Immune system dysregulation in first-onset postpartum psychosis. Biol Psychiatry. (2013) 73:1000–7. doi: 10.1016/j.biopsych.2012.11.006
32. Brockington, IF, Martin, C, Brown, GW, Goldberg, D, and Margison, F. Stress and puerperal psychosis. Br J Psychiatry. (1990) 157:331–4. doi: 10.1192/bjp.157.3.331
33. Dowlatshahi, D, and Paykel, ES. Life events and social stress in puerperal psychoses: absence of effect. Psychol Med. (1990) 20:655–62. doi: 10.1017/S0033291700017177
34. Kendell, RE, Rennie, D, Clarke, JA, and Dean, C. The social and obstetric correlates of psychiatric admission in the puerperium. Psychol Med. (1981) 11:341–50. doi: 10.1017/S0033291700052156
35. Lewkowitz, AK, Rosenbloom, JI, Keller, M, López, JD, Macones, GA, Olsen, MA, et al. Association between stillbirth ≥23 weeks gestation and acute psychiatric illness within 1 year of delivery. Am J Obstet Gynecol. (2019) 221:491.e1-.e22–491.e22. doi: 10.1016/j.ajog.2019.06.027
36. Meltzer-Brody, S, Larsen, JT, Petersen, L, Guintivano, J, Florio, AD, Miller, WC, et al. Adverse life events increase risk for postpartum psychiatric episodes: a population-based epidemiologic study. Depress Anxiety. (2018) 35:160–7. doi: 10.1002/da.22697
37. Meltzer-Brody, S, Maegbaek, ML, Medland, SE, Miller, WC, Sullivan, P, and Munk-Olsen, T. Obstetrical pregnancy and socio-economic predictors for new-onset severe postpartum psychiatric disorders in primiparous women. Psychol Med. (2017) 47:1427–41. doi: 10.1017/S0033291716003020
38. Nager, A, Sundquist, K, Ramírez-León, V, and Johansson, LM. Obstetric complications and postpartum psychosis: a follow-up study of 1.1 million first-time mothers between 1975 and 2003 in Sweden. Acta Psychiatr Scand. (2008) 117:12–9. doi: 10.1111/j.1600-0447.2007.01096.x
39. Paffenbarger, RS Jr, Steinmetz, CH, Pooler, BG, and Hyde, RT. The picture puzzle of the postpartum psychoses. J Chronic Dis. (1961) 13:161–73. doi: 10.1016/0021-9681(61)90149-7
40. Sharma, V, Smith, A, and Khan, M. The relationship between duration of labour, time of delivery, and puerperal psychosis. J Affect Disord. (2004) 83:215–20. doi: 10.1016/j.jad.2004.04.014
41. Terp, IM, Engholm, G, Møller, H, and Mortensen, PB. A follow-up study of postpartum psychoses: prognosis and risk factors for readmission. Acta Psychiatr Scand. (1999) 100:40–6. doi: 10.1111/j.1600-0447.1999.tb10912.x
42. Upadhyaya, SK, Sharma, A, and Raval, CM. Postpartum psychosis: risk factors identification. N Am J Med Sci. (2014) 6:274–7. doi: 10.4103/1947-2714.134373
43. Videbech, P, and Gouliaev, G. First admission with puerperal psychosis: 7–14 years of follow-up. Acta Psychiatr Scand. (1995) 91:167–73. doi: 10.1111/j.1600-0447.1995.tb09761.x
44. Vikström, J, Josefsson, A, Hammar, M, Bladh, M, and Sydsjö, G. Risk of postpartum psychosis after IVF treatment: a nationwide case-control study. Hum Reprod. (2017) 32:139–46. doi: 10.1093/humrep/dew302
45. Warselius, P, Cnattingius, S, Li, J, Wei, D, Valdimarsdottir, UA, Kosidou, K, et al. Maternal bereavement shortly before or during pregnancy and risk of postpartum psychotic illness: a population-based study from Denmark and Sweden. Clin Epidemiol. (2019) 11:285–98. doi: 10.2147/CLEP.S195741
46. Brugha, T, Bebbington, P, Tennant, C, and Hurry, J. The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med. (1985) 15:189–94. doi: 10.1017/S003329170002105X
47. Brown, GW, and Birley, JL. Crises and life changes and the onset of schizophrenia. J Health Soc Behav. (1968) 9:203–14. doi: 10.2307/2948405
48. Paykel, ES. Methodological aspects of life events research. J Psychosom Res. (1983) 27:341–52. doi: 10.1016/0022-3999(83)90065-X
49. Perry, A, Gordon-Smith, K, Di Florio, A, Forty, L, Craddock, N, Jones, L, et al. Adverse childhood life events and postpartum psychosis in bipolar disorder. J Affect Disord. (2016) 205:69–72. doi: 10.1016/j.jad.2016.06.061
50. Biaggi, A, Conroy, S, Pawlby, S, and Pariante, CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. (2016) 191:62–77. doi: 10.1016/j.jad.2015.11.014
51. Palmier-Claus, JE, Berry, K, Bucci, S, Mansell, W, and Varese, F. Relationship between childhood adversity and bipolar affective disorder: systematic review and meta-analysis. Br J Psychiatry. (2016) 209:454–9. doi: 10.1192/bjp.bp.115.179655
52. Meyer, N, Faulkner, SM, McCutcheon, RA, Pillinger, T, Dijk, DJ, and MacCabe, JH. Sleep and circadian rhythm disturbance in remitted schizophrenia and bipolar disorder: a systematic review and meta-analysis. Schizophr Bull. (2020) 46:1126–43. doi: 10.1093/schbul/sbaa024
53. Lewis, KJS, Di Florio, A, Forty, L, Gordon-Smith, K, Perry, A, Craddock, N, et al. Mania triggered by sleep loss and risk of postpartum psychosis in women with bipolar disorder. J Affect Disord. (2018) 225:624–9. doi: 10.1016/j.jad.2017.08.054
54. Riecher-Rössler, A. Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. Lancet Psychiatry. (2017) 4:63–72. doi: 10.1016/S2215-0366(16)30379-0
55. Mahé, V, and Dumaine, A. Oestrogen withdrawal associated psychoses. Acta Psychiatr Scand. (2001) 104:323–31. doi: 10.1111/j.1600-0447.2001.00288.x
56. Kumar, C, McIvor, R, Davies, T, Brown, N, Papadopoulos, A, Wieck, A, et al. Estrogen administration does not reduce the rate of recurrence of affective psychosis after childbirth. J Clin Psychiatry. (2003) 64:112–8. doi: 10.4088/JCP.v64n0202
57. Jones, I, and Craddock, N. Do puerperal psychotic episodes identify a more familial subtype of bipolar disorder? Results of a family history study. Psychiatr Genet. (2002) 12:177–80. doi: 10.1097/00041444-200209000-00011
58. Hazelgrove, K. The role of the immune system in postpartum psychosis. Brain, Behav Immun Health. (2021) 18:100359. doi: 10.1016/j.bbih.2021.100359
59. Pillinger, T, Osimo, EF, Brugger, S, Mondelli, V, McCutcheon, RA, and Howes, OD. A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr Bull. (2019) 45:1120–33. doi: 10.1093/schbul/sby160
Keywords: psychotic disorders, schizophrenia, postpartum, perinatal, childbirth, adverse childhood experiences, stress, trauma
Citation: Reilly TJ, Roberts E, Sagnay De La Bastida VC, McGuire P, Dazzan P and Cullen AE (2023) Systematic review of the association between adverse life events and the onset and relapse of postpartum psychosis. Front. Psychiatry. 14:1154557. doi: 10.3389/fpsyt.2023.1154557
Edited by:
Teresa Sanchez-Gutierrez, International University of La Rioja, SpainReviewed by:
Hiroyoshi Takeuchi, Keio University, JapanJessie Lin, Hong Kong Polytechnic University, Hong Kong SAR, China
Copyright © 2023 Reilly, Roberts, Sagnay De La Bastida, McGuire, Dazzan and Cullen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexis E. Cullen, YWxleGlzLmN1bGxlbkBwc3ljaC5veC5hYy51aw==
†These authors have contributed equally to this work and share first authorship
 Thomas J. Reilly1,2†
Thomas J. Reilly1,2† Emma Roberts
Emma Roberts Paola Dazzan
Paola Dazzan Alexis E. Cullen
Alexis E. Cullen