
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 05 May 2023
Sec. Schizophrenia
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1153648
 Abdulrhman Khaity1
Abdulrhman Khaity1 Nada Mostafa Al-dardery2
Nada Mostafa Al-dardery2 Khaled Albakri3
Khaled Albakri3 Omar A. Abdelwahab4
Omar A. Abdelwahab4 Mahmoud Tarek Hefnawy5
Mahmoud Tarek Hefnawy5 Yaman A. S. Yousef1
Yaman A. S. Yousef1 Ruaa E. Taha6
Ruaa E. Taha6 Sarya Swed7
Sarya Swed7 Wael Hafez8,9
Wael Hafez8,9 Rene Hurlemann10,11,12
Rene Hurlemann10,11,12 Mohamed E. G. Elsayed10,13*†
Mohamed E. G. Elsayed10,13*†Aims: We performed this meta-analysis to evaluate the efficacy and safety of glucagon-like peptide-1 receptor-agonists (GLP-1RA) treatment on cardio-metabolic parameters among antipsychotic-treated patients with schizophrenia.
Methods: We searched the Web of Science, Cochrane Central Register of Controlled Trials, PubMed, PsycINFO, and Scopus for relevant Randomized Clinical trials (RCTs) from inception until 1 August 2022. Documents were screened for qualified articles, and all concerned outcomes were pooled as risk ratios (RR) or mean difference (MD) in the meta-analysis models using Review Manager (RevMan version 5.4).
Results: Pooling data from 7 RCTs (398 patients) showed that GLP-1 RA was superior to placebo with regard to body weight [MD = - 4.68, 95% CI (-4.90,−4.46), P < 0.00001], waist circumference [MD = - 3.66, 95% CI (-3.89,−3.44), P < 0.00001], body mass index (BMI) [MD = - 1.09, 95% CI (-1.25,−0.93), P < 0.00001], systolic blood pressure (SBP) [MD = - 3.07, 95% CI (-3.61,−2.53), P < 0.00001], and diastolic blood pressure (DBP) [MD = - 2.02, 95% CI (-2.42,−1.62), P < 0.00001]. The total effect did not favor either of the two groups with respect to insulin and respiratory adverse events {[MD = - 0.06, 95% CI (-0.36, 0.24), p = 0.70], [RR = 0.66, 95% CI (0.31, 1.40), p = 0.28]; respectively}.
Conclusion: Our analysis revealed that GLP-1 RA treatment is safe and effective on cardio-metabolic parameters over control in antipsychotic-treated patients with schizophrenia. Nevertheless, the present evidence is not sufficient to confirm the safety and efficacy of GLP-1RA treatment on insulin and respiratory adverse events. Therefore, further studies are recommended.
Systematic review registration: http://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022333040.
Schizophrenia is one of the mental conditions that affect how individuals think, act, express emotions, interpret reality, and interact with others (1). A combination of antipsychotics and psychosocial therapies has a crucial role in improving a schizophrenia patient's life (2). However, several factors, including genetic susceptibility to diabetes, limited physical exercise, the use of antipsychotic drugs, and malnutrition, raise the risk of cardiometabolic illness in patients with schizophrenia (3–5).
It is worth mentioning that antipsychotic treatments may cause obesity, olanzapine along with clozapine have the highest tendency for weight increase (6). Metabolic syndrome has been detected in half of the schizophrenia patients who received clozapine and one-third of those taking olanzapine (3). The evidence for therapies targeting antipsychotic-associated obesity is scant at present and still does not achieve the target effect (7). This could be elucidated by poor exposure to physical exercise programs. In addition, no positive physical health impacts were determined for dextroamphetamine, famotidine, ranitidine, orlistat, or fluoxetine (8). Moreover, sibutramine was taken off the market due to cardiovascular risks, whereas rimonabant was taken off the market due to an elevated risk of developing depression, anxiety, and suicidal thought (9, 10).
Due to these limitations, attention to glucagon-like peptide-1 receptor agonists (GLP-1RAs) arose to combat the weight increase observed with antipsychotic medication, especially clozapine and olanzapine (11, 12). GLP-1RA has been demonstrated to decrease blood glucose as well as body weight in persons with and without type 2 diabetes (12, 13). Additionally, GLP-1RA therapy reduces the risk of significant adverse cardiovascular consequences (14).
Regarding the mechanism of action, GLP-1RA activate the corresponding receptors in the pancreas, leading to enhanced release of insulin and inhibited glucagon release responses (15, 16). Their action on GLP-1 receptors in the central nervous system and gastrointestinal tract causes reduced appetite and delayed absorption of glucose due to slower gastric emptying (16, 17). In addition, they slow the process of gastric emptying, limiting significant post-meal glycemic levels (15). The short-acting agents (exenatide b.i.d., lixisenatide) have limited effect on fasting and overnight plasma glucose levels, but they maintain their influence on gastric emptying during long-term treatment (15). On the other hand, the long-acting GLP-1 receptor agonists (liraglutide, dulaglutide, exenatide, semaglutide, and albiglutide) have more profound effects on fasting and overnight plasma glucose levels, and HbA1c (15).
Previous studies evaluated the efficacy of GLP-1RAs for antipsychotic-associated cardio-metabolic risk factors. They found that patients with schizophrenia may benefit from GLP-1RA medication for weight control. However, these studies have shown conflicting findings regarding the impact of GLP-1RA on blood pressure, HbA1C, total cholesterol, and serious adverse events (18, 19). Therefore, this meta-analysis was conducted to precisely investigate the efficacy and safety of GLP-1RAs on cardio-metabolic parameters among antipsychotic-treated schizophrenic patients.
We tracked the Cochrane Collaboration recommendations and PRISMA guidelines to prepare this meta-analysis (20). All steps of this study were prespecified and documented at the (PROSPERO): CRD42022333040.
The following conditions were considered for the study:
1) Design: studies that were designated as randomized clinical trials (RCTs).
2) Population: studies that enrolled schizophrenia or schizoaffective disorder patients who were administrated antipsychotic treatments and were overweight or obese or prediabetes.
3) Intervention: studies where the intervention was subcutaneously injection of GLP1-RA whether once-weekly exenatide or once-daily liraglutide. Among the included studies, the tolerability of liraglutide was recorded by dose escalation pattern, up titration of 0.6 mg per week to a daily dose of 3.0 mg. Participants who did not tolerate up titration continued on the highest tolerable dose. Regarding exenatide, the tolerability was measured by monitoring for adverse effects at trial visits.
4) Comparator: studies that used a placebo as a comparator were eligible.
5) Outcomes: studies stated one or more of the following outcomes: body weight, metabolic syndrome parameters [blood pressure (BP), waist circumference, and fasting plasma glucose (FPG)], and adverse drug reaction. In addition, body mass index (BMI), HbA1c, insulin, and bone turnover markers, including Procollagen type I N-terminal propeptide (PINP) level and C-terminal cross-linking telopeptide of type I collagen (CTX) level.
We excluded articles that were not in English, conference abstracts, single-arm, observational, quasi-clinical trials, and studies that used other types of GLP-1 receptor agonists.
A comprehensive literature search was conducted of five electronic databases (Web of Science, PubMed, Cochrane Library for clinical trials in CENTRAL, Scopus, and PsycINFO via Ovid) from inception till 1 August 2022 using the following query: [((exenatide) OR (Liraglutide) OR (Victoza) OR (Saxena) OR (Glucagon-like peptide-1 receptor agonist) OR (GLP-1RA) AND (schizophrenia)) AND (antipsychotic)].
The titles and abstracts of all citations considered for inclusion were reviewed by three authors independently. Then, we extracted the full text of the selected studies to evaluate their applicability and validated them according to our systematic review and meta-analysis standards. Discrepancies were resolved by consensus.
Four reviewers carried out the extraction of data independently using a uniform sheet. From each included trial, the following data were extracted: (1) characteristics of study design, (2) characteristics of the study population, (3) risk of bias scopes (4) study outcomes: body weight, metabolic syndrome parameters [blood pressure (BP), waist circumference, and fasting plasma glucose (FPG)], and adverse drug reaction. In addition, BMI, HbA1c, insulin, and bone turnover markers, including PINP and CTX levels.
The quality of each involved trial was evaluated precisely by two authors independently. We utilized a table of the quality appraisal in the Cochrane handbook (part 2, chapter 8.5). The Cochrane tool for evaluating the possibility of bias comprises the subsequent areas: (1) Random sequence generation (selection bias), (2) allocation concealment (selection bias), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessment (detection bias), (5) incomplete outcome data (attrition bias), (6) selective reporting (reporting bias) and (7) other potential sources of bias. The authors' decision is classified as Unclear risk, Low risk, or High risk of bias. The conflicts were solved by the third author.
Mean changes from baseline for body weight, metabolic syndrome parameters, BMI, HbA1c, and insulin, were pooled as mean difference (MD) between the two groups from baseline to the endpoint in the meta-analysis models utilizing the inverse variance (IV) method. We assumed a fixed-effect model of the MD as the main analysis model. At the same time, relative risk (RR) was used to pool dichotomous data in a fixed-effect model using the Mantel-Haenszel (M-H) method. RevMan software (version 5.4 for Windows) was applied to run the statistical analysis. In addition, we used the Chi-square test (Cochrane Q test) to assess the statistical heterogeneity of the included studies. Significant heterogeneity was reflected by I-square > 50% with a P-value < 0.1.
We performed sensitivity analysis “leave-one-out meta-analysis” for each outcome in the meta-analysis in multiple scenarios to test the strength of the evidence and ensure that the total effect size was not reliant on any certain individual study. In case of significant statistical heterogeneity among included studies (i.e., variability beyond what would be expected by chance), that is may indicate that the studies are too dissimilar to combine and we excluded them from the analysis.
Our search strategy yielded 171 articles. After removing duplication using Endnote X8.0.1., 93 abstracts were evaluated, and 34 articles were suitable for full-text screening. During the full-text evaluation, 27 studies were left out, 12 of them were excluded due to discrepancies in design with our study and eight articles were excluded because they neither meet the primary nor secondary outcomes of this study. Moreover, we excluded seven studies because they involved different interventions. Finally, we included 7 RCTs (398 patients) in this systematic review and meta-analysis (21–27), Four of them used liraglutide and the last three studies rely on exenatide. The PRISMA flow diagram of the study selection process is shown in Figure 1. A summary of the included articles, their design, and their findings are demonstrated in Table 1.
The basic characteristics of the included studies revealed that all ages of participants ranged from 18 to 75 years old (mean age = 42.3 years, SD = 10.7). Most of the participants were male 63.3%. Additionally, the mean BMI of the included population was 36.1 kg/m2 (SD 6.3). All the participants in the included studies experienced schizophrenia. More information regarding the baseline characteristics of the included articles is shown in Table 2.
The quality of included articles ranged from fair to poor quality by the Cochrane Risk of Bias assessment tool for RCTs, Figures 2A, B reveals an overview of the Cochrane Risk of Bias score per item; red color for high-risk, green color for low risk and white for unclear.
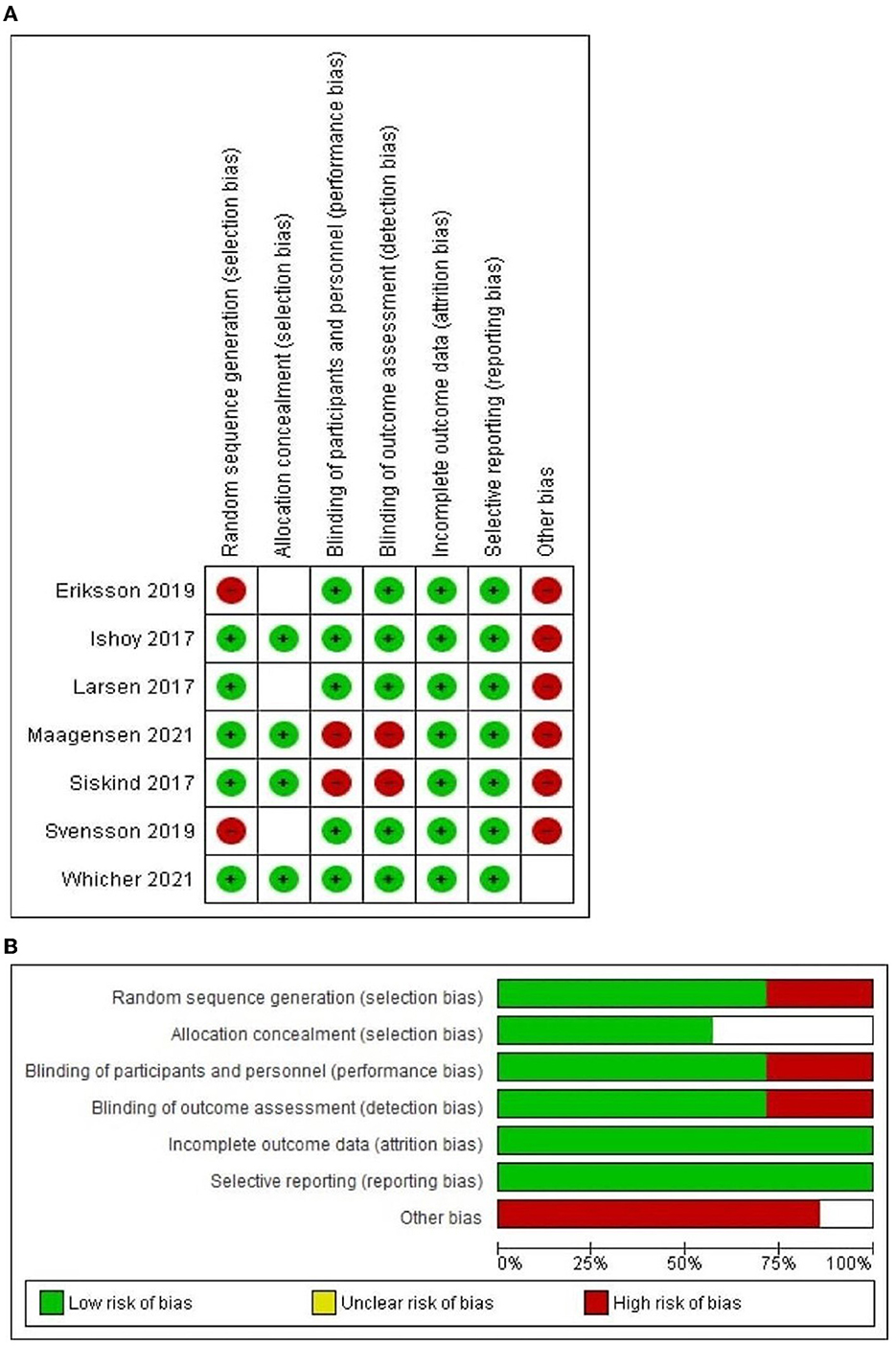
Figure 2. (A) The risk of bias summary graph according to Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB2); (B) The risk of bias traffic light graph according to Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB2).
Five studies (22, 23, 25–27) reported the efficacy of GLP-1RA on body weight compared to placebo. The findings presented that there was a significant difference between the two groups [MD = −4.7 (-4.91,−4.48), P-value < 0.00001] as shown in Figure 3A. The pooled studies were heterogeneous (P-value < 0.00001, I2 = 96%). After performing the sensitivity analysis and excluding Svensson et al. (26), the heterogeneity was resolved (P-value = 0.8, I2 = 0%). The results are still significant and prefer the experimental group [MD = −5.19 (-5.43,−4.95), P-value < 0.00001] as shown in Figure 3B.
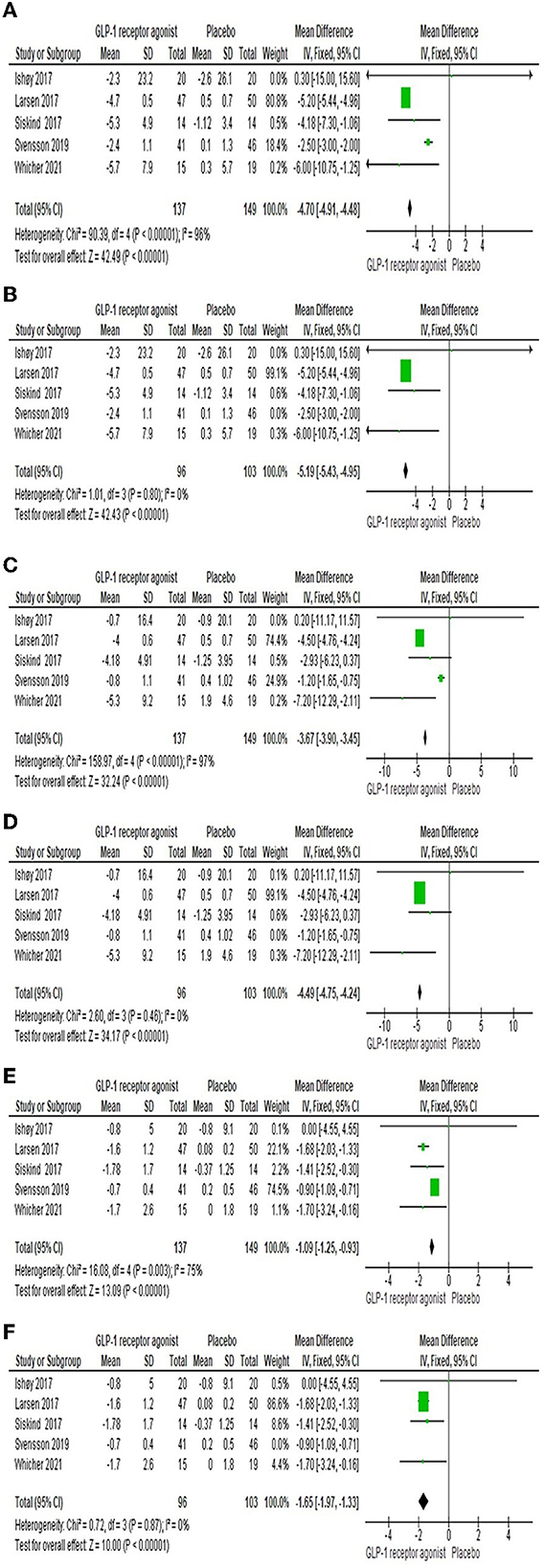
Figure 3. Forest plots of mean difference in (A) body weight, (B) overall body weight after sensitivity analysis, (C) Waist circumference, (D) Waist circumference after sensitivity analysis, (E) BMI, and (F) BMI after sensitivity analysis.
Waist circumference was investigated by five studies (22–26). There was a significant difference among both groups [MD = −3.67 (-3.9,−3.45), P-value < 0.00001], but there was a heterogeneity between the studies (P-value < 0.00001, I2 = 97%) as shown in Figure 3C. However, sensitivity analysis resolved the heterogeneity; we excluded Svensson et al. (26), as shown in Figure 3D.
Five studies (22–26) reported the effect of GLP-1RA on the BMI compared to placebo. The consequences revealed that there was a significant difference among both groups [MD = −1.09 (-1.25,−0.93), P-value < 0.00001] as shown in Figure 3E. The pooled studies were heterogeneous (P-value = 0.003, I2 = 75%). After performing the sensitivity analysis and excluding Svensson et al. (26), the heterogeneity was resolved (P-value = 0.87, I2 = 0%). The results are still significant and prefer the experimental group [MD = −1.65 (-1.97,−1.33), P-value < 0.00001] as shown in Figure 3F.
Five studies (22–26) assessed the systolic and diastolic blood pressures, and the overall MD showed the GLP-1RA group was significantly lower than control group [MD = −3.09 (-3.63,−2.54), P-value < 0.00001; MD = −2.02 (-2.42,−1.62), P-value < 0.00001], and the pooled articles were homogenous (P-value = 0.16, I2 = 40%; P-value = 0.45, I2 = 0%) as shown in Figures 4A, B, respectively.
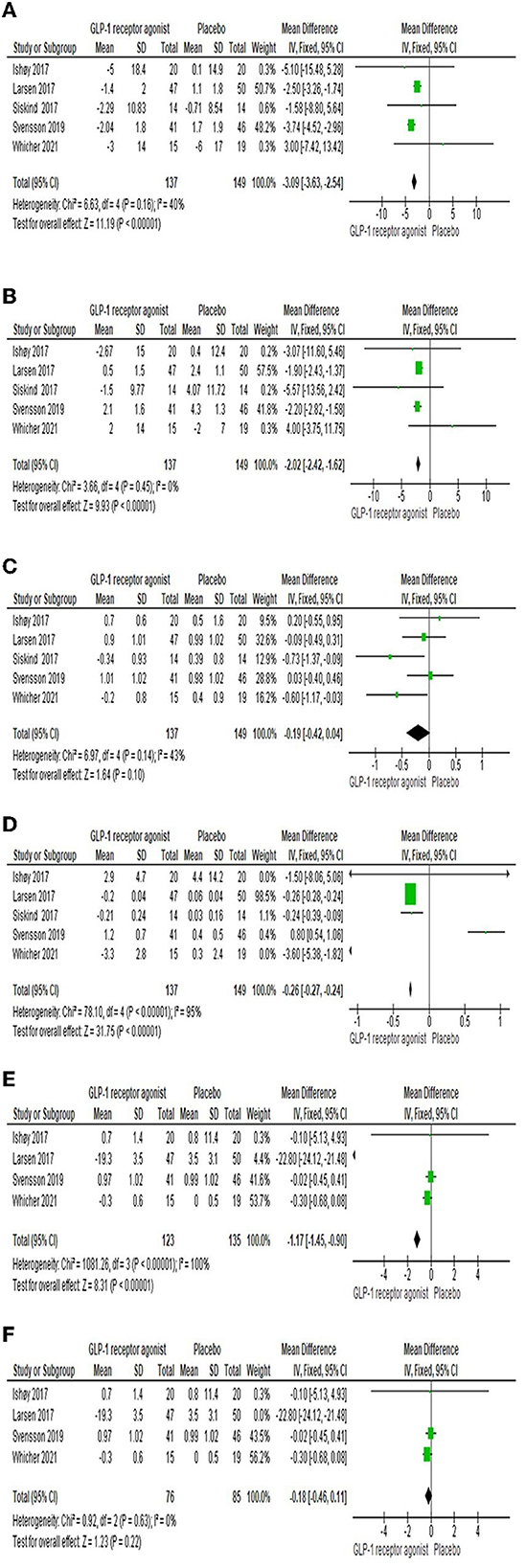
Figure 4. Forest plots of mean difference in (A) systolic blood pressure, (B) diastolic blood pressure, (C) FBG, (D) HbA1c, (E) Total cholesterol, and (F) Total cholesterol after sensitivity analysis.
The impact of the GLP-1RA on fasting plasma glucose was examined by five studies (22–26). Results showed that the total effect did not favor either of two groups [MD = −0.19 (-0.42, 0.04), P-value = 0.1]. The findings of the studies were homogenous (P-value = 0.14, I2 = 43%) as shown in Figure 4C.
Five studies (22–26) investigated the HbA1c, and the overall MD revealed that there was a significant difference among the two groups [MD = −0.26 (-0.27,−0.24), P-value < 0.00001], but there was heterogeneity between the studies (P-value < 0.00001, I2 = 95%) as shown in Figure 4D.
Total cholesterol was investigated by four studies (22–25). There was a significant difference among the two groups [MD = −1.17 (-1.45,−0.9), P-value < 0.00001], but there was a heterogeneity between the studies (P-value < 0.00001, I2 = 100%) as shown in Figure 4E. However, sensitivity analysis resolved the heterogeneity; we excluded Larsen et al. (23), as shown in Figure 4F.
Three studies (23, 25, 26) reported the impact of GLP-1RA on the insulin level compared to Placebo. The findings revealed that there was no significant difference observed among both groups [MD = −0.06 (-0.36, 0.24), P-value = 0.71]. The pooled articles were homogenous (P-value = 0.60, I2 = 0%) as shown in Figure 5A.
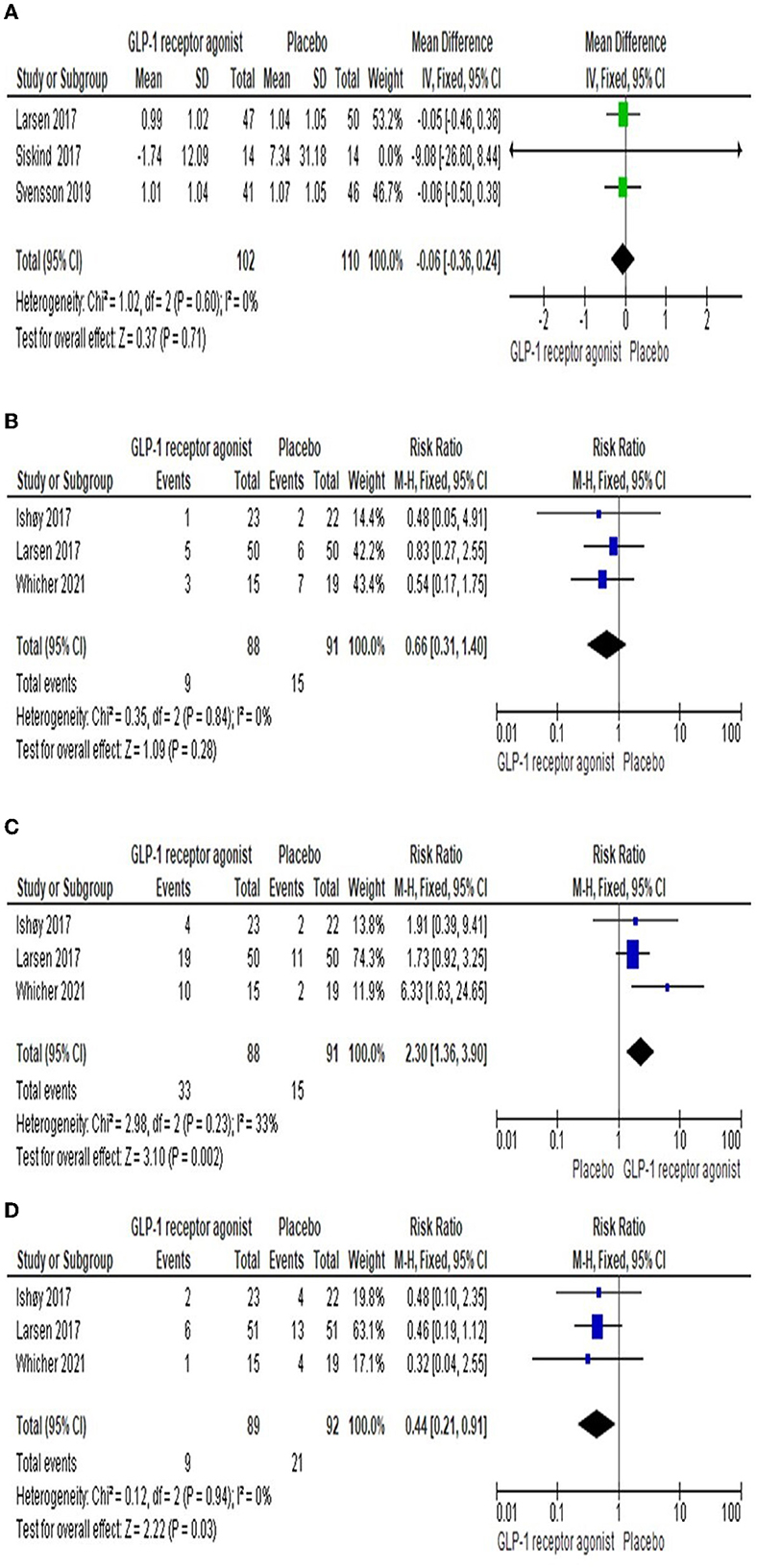
Figure 5. Forest plots of mean difference in (A) Insulin level. In addition, Forest plots of risk ration in (B) Respiratory side effects, (C) Gastrointestinal side effects, and (D) Serious side effects).
Three studies (22, 23, 27) reported the number of cases suffered from respiratory side effects, including upper respiratory tract infection and asthma, in each group. The overall RR demonstrated that there was no significant difference between the two groups [RR = 0.66 (0.31, 1.4), P-value = 0.28]. The pooled studies were homogenous (P-value = 0.84, I2 = 0%) as shown in Figure 5B.
Gastrointestinal adverse events, including nausea, vomiting, constipation, and diarrhea, were observed in three studies (22, 23, 27). The results showed that the number of adverse events among the GLP-1RA group was higher significantly than in the placebo group [RR = 2.3 (1.36, 3.9), P-value = 0.002]. The results of the studies were homogenous (P-value = 0.23, I2 = 33%) as presented in Figure 5C.
Three studies (22, 23, 27) reported serious adverse events, including severe pneumonia and psychiatric admission, in each group. The overall RR revealed that there was a significant difference among the both groups [RR = 0.44 (0.21, 0.91), P-value = 0.03]. The pooled studies were homogenous (P-value = 0.94, I2 = 0%) as presented in Figure 5D.
Two studies (21, 24) assessed the markers of bone turnover through PINP and CTX levels. The influence of exenatide in comparison with placebo was investigated by Eriksson et al. (21). No significant difference was observed between the two groups in terms of both outcomes (p-value = 0.27, p-value = 0.19; respectively). Also, they followed the participants for up to 3 months and the findings did not reflect a significant difference between the baseline and endpoint values regarding both outcomes (p-value = 0.52, p-value = 0.93; respectively) (21). Additionally, Maagensen et al. (24) gave liraglutide to the patients and tracked them for 4 months. According to PINP and CTX values, the changes were not significantly different neither between the control and experimental groups (p-value = 0.2, p-value = 0.07; respectively) nor between start- and end-points in treatment group (p-value = 0.49, p-value= 0.25; respectively) (24).
The findings of our meta-analysis revealed a significant reduction in body weight, waist circumference, BMI, and serious adverse events among antipsychotic-treated patients with schizophrenia. In addition, the GLP-1RA group was superior to the placebo group in the case of total cholesterol, HbA1c, and blood pressure. However, the overall effect favored placebo over GLP-1RA in terms of GIT adverse events. Though, there was no statistical difference in respiratory side effects, markers of bone turnover, insulin level, and FPG between both groups.
Data from Svensson et al. (26) introduced a significant heterogeneity to the results of body weight, BMI, and waist circumference. Additionally, significant heterogeneity was noticed in the consequences of total cholesterol documented by Larsen et al. (23). This could be elucidated by high variation in the duration of intervention assessment among patients with prediabetes and schizophrenia. The results were not significantly affected after doing the leave-one-out test and excluding the Svensson et al. (26) and Larsen et al. (23) studies from the analysis.
Generally, controlling obesity is a favorable outcome in the management strategy of schizophrenia because it is not only associated with higher risks for insulin resistance, diabetes, and cardiovascular problems but also represents a distressing mental health problem that may reinforce negative self-estimation and treatment compliance (18, 28). The results of this meta-analysis are in the same direction as previous studies' results in terms of body weight, BMI, and waist circumference.
In a previous meta-analysis performed by Zhuo et al. (29) investigated the effect of topiramate and metformin on weight gain in patients treated with antipsychotics. The results of Zhuo et al. (29) showed that topiramate and metformin has significant impact in decreasing body weight and BMI. They reached a conclusion that topiramate had a MD in weight reduction of−3.07 kg by the end of their study, while Metformin's MD was−2.50 kg. As for the BMI, they concluded a result of - 1.59 kg/m2 and 0.61 kg/m2, respectively (29). Compared to our study, we see more potential in our results, as the weight reduction MD is−4.7 and the MD for BMI is−1.65. Interestingly, according to the former studies, GLP-1 RAs were associated with significantly greater weight loss than topiramate or metformin (30–33). Therefore, while topiramate and metformin may be useful adjuncts to lifestyle interventions for weight loss, GLP-1 RAs appear to be more effective at promoting significant and sustained weight loss.
A former meta-analysis conducted by Siskind et al. (18) showed significant lowering effects in FBG in the GLP-1RA group. At the same time, our pooled results along with Wang et al. (19) represented no significant decrease in FBG among the intervention group. This might be explicated by the relatively limited number of included trials Siskind et al. (18). No significant reduction in HbA1c was reported among the GLP-1RA group in the Siskind et al. (18). In contrast, our findings together with Wang et al. (19) showed a significant reduction in HbA1c level in GLP-1RA treated patients. This conflict could be elucidated by variation in baseline characteristics of the population in the included studies in Siskind et al. (18).
Nevertheless, our study is in line with previous reviews in terms of insulin levels between the two groups. The outcomes of glycemic indexes should be taken into consideration because schizophrenic patients are at high risk for growing type 2 diabetes mellitus, they are at 2–5-fold more significant risk for developing diabetes than the general public, though, the exact prevalence varies across studies. Moreover, schizophrenia itself is proposed as the causal factor for DM, and this can be supported by the higher prevalence of diabetes in newly diagnosed young patients even before administering antipsychotic drugs such as clozapine which also rises the chance of increasing type 2 diabetes (34–36).
Although Wang et al. (19) stated superior results in the GLP-1RA group in terms of diastolic blood pressure, the results were not statistically significant in systolic blood pressure. Additionally, the findings of Siskind et al. (18) demonstrated that the total impact did not favor both groups in the case of diastolic and systolic blood pressure. However, our pooled results revealed a significant statistical decrease in systolic and diastolic blood pressure among the GLP-1RA group. This discrepancy might be attributed to the quality of the involved trials and some variations in the population characteristics of the comprised articles.
Regarding total cholesterol, our results showed that GLP-1RA has a significantly favorable role in controlling the total cholesterol level, whereas Wang et al. (19) did not demonstrate a significant difference among both groups. The authors of the Siskind et al. (18) found that the total impact did not favor GLP-1RA over placebo in case of adverse events. Despite there being no statistical difference among both groups in terms of respiratory adverse events, including upper respiratory tract infection and asthma, our findings together with Wang et al. (19) recorded that GLP-1RA was significantly superior to placebo concerning serious adverse events, including severe pneumonia and psychiatric admission. Therefore, the GLP-1RA could be the better choice for alleviating side effects among antipsychotic-treated patients with schizophrenia.
Along with positive and negative symptoms, schizophrenia is characterized by cognitive impairment contributing to the long-term burden associated with the disease and reducing the quality of life (37). Various cognitive domains are impaired in schizophrenia, such as verbal learning, visual learning, memory, problem-solving, attention, and reasoning (38). Therapeutic options for cognitive impairment are still limited as first and second-generation antipsychotics have limited effectiveness in treating cognition (39). Horska et al. (16) showed that the use of GLP-1RAs induces a proposed pro-cognitive effect through improvement of central nervous system deficits which might be also beneficial in schizophrenia patients. GLP-1Rs have binding sites within the cerebral cortex, thalamus, hypothalamus, substantia nigra, circumventricular organ, hippocampus, cerebellum, and brainstem nucleus (40–42). The direct modulatory effects of GLP-1RAs on insulin signaling pathways are associated with improved cognition, as GLP-1RAs activate cyclic adenosine monophosphate (cAMP) (43) which promotes neuroprotection, neuronal development, attenuating oxidative stress and neuroinflammation (44–46). GLP-1RAs might even restore alterations in neurotransmitter function and normalize insulin signaling within the brain, stimulating neuroplasticity (47) also through stimulating the release of brain-derived neurotrophic factor (BDNF), an important factor for learning and memory, low levels of BDNF were previously associated with low cognitive performance in individuals with schizophrenia (48).
Generally, the antipsychotic treatment has been linked to bone fractures risk and osteoporosis (21, 49). Circulating bone turnover markers (BTMs), including PINP and CTX, can be used to evaluate changes in bone formation and resorption (21). According to the former study, GLP-1RA, liraglutide, has been demonstrated to escalate bone formation in body weight-reduced obese women when compared to placebo (50). However, the efficacy of GLP-1RA whether exenatide or liraglutide on bone formation status through markers of bone turnover was still unclear in prediabetes patients with schizophrenia who received clozapine or olanzapine (21, 24). It is essential to highlight those limitations in sample size, the discrepancy in the population characteristics, and the short duration that was conducted to assess the intervention have a critical impact on these findings. Accordingly, we could not pool the results that assessed the influence of GLP-1RA on markers of bone turnover in the meta-analysis model.
GLP-1RA for cardio-metabolic parameters in schizophrenia patients looks promising. Our findings have shown that liraglutide and exenatide can improve glucose metabolism, reduce weight gain, and improve lipid profiles in patients with schizophrenia who are at risk for developing metabolic disorders. Currently, GLP-1RA is administered via subcutaneous injection (51). While this method has been effective in managing diabetes, it can be inconvenient and uncomfortable for patients (51, 52). Therefore, researchers are exploring the possibility of developing an oral form of GLP-1RA (53). This would be more convenient for patients and could potentially improve adherence to treatment. However, there are challenges associated with developing an oral form of GLP-1RA (52–54). The medication would need to survive the acidic environment of the stomach and be absorbed into the bloodstream in a way that maintains its effectiveness (54). Overall, while an oral form of GLP-1RA would be beneficial for patients with type 2 diabetes, more research is needed to develop a safe and effective formulation (53, 54).
On the other hand, it is still unknown if the potential addition of GLP-1RA to second-generation antipsychotic therapies (such as Olanzapine and Clozapine) would eventually positively affect the patient's compliance with the antipsychotic treatment. Metabolic side effects and weight gain are the leading cause of mal-compliance (55). Schizophrenia patients commonly lack insight into various aspects of their illness and the necessary antipsychotic treatment (56), which is an important clinical issue and can be challenging for psychiatrists when patients with psychosis discontinue their antipsychotic treatment due to weight gain, which often leads to new relapse (57) and often new inpatient admission, with resulting healthcare costs (58). In addition, previous studies documented that GLP-1RA, liraglutide, may have potential association with suicidal ideation (59, 60). Therefore, further studies with large sample sizes would be helpful, under careful monitoring of potential suicidal risk.
The major limitations in this study included: (1) a limited number of included trials that assessed the role of GLP-1RA on bone turnover markers in schizophrenia patients, and (2) we observed a marked heterogeneity in some outcomes, which can be accredited to the discrepancy in the period of intervention. Therefore, we recommend further well-designed and high-quality studies with an increased sample size to enhance the possibility of providing level 1 evidence using meta-analysis investigating the efficacy of GLP-1RA on cardiometabolic parameters and bone turnover markers in schizophrenic patients who underwent antipsychotic therapy.
Nevertheless, the strengths of our study are as follows: (1) our meta-analysis represented the last updated evidence assessing the efficacy and safety of GLP-1RA among schizophrenic patients, (2) we provided a more comprehensive analysis in an attempt to solve the previous conflicting findings, (3) we complied the PRISMA checklist when representing this manuscript and conducted all steps as stated in the Cochrane Handbook in our review.
Ultimately, GLP-1RA appears to be a promising therapeutic candidate, along with their additional neuroprotective effects, through improving insulin signaling, neurotransmission, neuroinflammation, and synaptic plasticity. Nonetheless, the present evidence is not enough to verify the efficacy of GLP-1RA on bone formation status. Accordingly, more trials with an increased sample size are recommended.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
AK, NM, KA, OA, MH, YY, RT, and SS: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft preparation, and writing—review and editing. WH: conceptualization and methodology. ME: supervision, writing—review and editing, proofreading, and visualization. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1153648/full#supplementary-material
Supplementary Figure S1. Graphical abstract.
1. Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. (2013) 150:3–10. doi: 10.1016/j.schres.2013.05.028
2. Reichenberg A, Feo C, Prestia D, Bowie CR, Patterson TL, Harvey PD. The course and correlates of everyday functioning in schizophrenia. Schizophr Res Cogn. (2015) 1:1–15. doi: 10.1016/j.scog.2014.03.001
3. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. (2015) 14:339–47. doi: 10.1002/wps.20252
4. Stubbs B, Koyanagi A, Veronese N, Vancampfort D, Solmi M, Gaughran F, et al. Physical multimorbidity and psychosis: comprehensive cross sectional analysis including 242,952 people across 48 low- and middle-income countries. BMC Med. (2016) 14:189. doi: 10.1186/s12916-016-0734-z
5. Vancampfort D, Wampers M, Mitchell AJ, Correll CU, De Herdt A, Probst M, et al. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry. (2013) 12:240–50. doi: 10.1002/wps.20069
6. Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. (2013) 382:951–62. doi: 10.1016/S0140-6736(13)60733-3
7. Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res. (2012) 140:159–68. doi: 10.1016/j.schres.2012.03.017
8. Vancampfort D, Firth J, Correll CU, Solmi M, Siskind D, De Hert M, et al. The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry. (2019) 18:53–66. doi: 10.1002/wps.20614
9. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. (2007) 370:1706–13. doi: 10.1016/S0140-6736(07)61721-8
10. James WPT, Caterson ID, Coutinho W, Finer N, van Gaal LF, Maggioni AP et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. (2011) 363:905–17. doi: 10.1056/NEJMoa1003114
11. Ebdrup BH, Knop FK, Ishøy PL, Rostrup E, Fagerlund B, Lublin H, et al. Glucagon-like peptide-1 analogs against antipsychotic-induced weight gain: potential physiological benefits. BMC Med. (2012) 10:92. doi: 10.1186/1741-7015-10-92
12. Mayfield K, Siskind D, Winckel K, Russell AW, Kisely S, Smith G, et al. Glucagon-like peptide-1 agonists combating clozapine-associated obesity and diabetes. J Psychopharmacol. (2016) 30:227–36. doi: 10.1177/0269881115625496
13. Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. (2010) 33:1173–5. doi: 10.2337/dc09-1203
14. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. (2018) 6:105–13. doi: 10.1016/S2213-8587(17)30412-6
15. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab. (2021) 46:101102. doi: 10.1016/j.molmet.2020.101102
16. Horska K, Ruda-Kucerova J, Skrede S. GLP-1 agonists: superior for mind and body in antipsychotic-treated patients? Trends Endocrinol Metab. (2022) 33:628–38. doi: 10.1016/j.tem.2022.06.005
17. Shaefer CF, Kushner P, Aguilar R. User's guide to mechanism of action and clinical use of GLP-1 receptor agonists. Postgrad Med. (2015) 127:818–26. doi: 10.1080/00325481.2015.1090295
18. Siskind D, Hahn M, Correll CU, Fink-Jensen A, Russell AW, Bak N, et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes, Obes Metab. (2019) 21:293–302. doi: 10.1111/dom.13522
19. Wang Y, Wang D, Cheng J, Fang X, Chen Y, Yu L, et al. Efficacy and tolerability of pharmacological interventions on metabolic disturbance induced by atypical antipsychotics in adults: a systematic review and network meta-analysis. J Psychopharmacol. (2021) 35:1111–9. doi: 10.1177/02698811211035391
20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:332–6. doi: 10.1136/bmj.b2535
21. Eriksson R, Broberg BV, Ishøy PL, Bak N, Andersen UB, Jørgensen NR, et al. Bone status in obese, non-diabetic, antipsychotic-treated patients, and effects of the glucagon-like peptide-1 receptor agonist exenatide on bone turnover markers and bone mineral density. Front Psychiatry. (2019) 9:781. doi: 10.3389/fpsyt.2018.00781
22. Ishøy PL, Knop FK, Broberg BV, Bak N, Andersen UB, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes Obes Metab. (2017) 19:162–71. doi: 10.1111/dom.12795
23. Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. (2017) 74:719–28. doi: 10.1001/jamapsychiatry.2017.1220
24. Maagensen H, Larsen JR, Jørgensen NR, Fink-Jensen A, Vilsbøll T. Liraglutide does not change bone turnover in clozapine- and olanzapine-treated schizophrenia overweight patients with prediabetes – randomized controlled trial. Psychiatry Res. (2021) 296:2020–2. doi: 10.1016/j.psychres.2020.113670
25. Siskind DJ, Russell AW, Gamble C, Winckel K, Mayfield K, Hollingworth S, et al. Treatment of clozapine-associated obesity and diabetes with exenatide (CODEX) in adults with schizophrenia: a randomised controlled trial. Diabetes Obes Metab. (2018) 20:1050–55. doi: 10.1111/dom.13167
26. Svensson CK, Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, et al. One-year follow-up on liraglutide treatment for prediabetes and overweight/obesity in clozapine- or olanzapine-treated patients. Acta Psychiatr Scand. (2019) 139:26–36. doi: 10.1111/acps.12982
27. Whicher CA, Price HC, Phiri P, Rathod S, Barnard-Kelly K, Ngianga K, et al. The use of liraglutide 3. 0 mg daily in the management of overweight and obesity in people with schizophrenia, schizoaffective disorder and first episode psychosis: Results of a pilot randomized, double-blind, placebo-controlled trial Diabetes. Obes Metab. (2021) 23:1262–71. doi: 10.1111/dom.14334
28. Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. (2012) 308:1150–9. doi: 10.1001/2012.jama.11132
29. Zhuo C, Xu Y, Liu S, Li J, Zheng Q, Gao X, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. (2018) 9:1–10. doi: 10.3389/fphar.2018.01393
30. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the scale diabetes randomized clinical trial. JAMA. (2015) 314:687–99. doi: 10.1001/jama.2015.9676
31. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. (2012) 95:297–308. doi: 10.3945/ajcn.111.024927
32. Bohula EA, Scirica BM, Inzucchi SE, McGuire DK, Keech AC, Smith SR, et al. Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial. Lancet. (2018) 392:2269–79. doi: 10.1016/S0140-6736(18)32328-6
33. Apovian CM, Okemah J, O'Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. (2019) 36:44–58. doi: 10.1007/s12325-018-0824-8
34. Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry. (2000) 157:975–81. doi: 10.1176/appi.ajp.157.6.975
35. Suvisaari J, Keinänen J, Eskelinen S, Mantere O. Diabetes and schizophrenia. Curr Diab Rep. (2016) 16:1–10. doi: 10.1007/s11892-015-0704-4
36. Mamakou V, Thanopoulou A, Gonidakis F, Tentolouris N, Kontaxakis V. Schizophrenia and type 2 diabetes mellitus. Psychiatrike. (2018) 29:64–73. doi: 10.22365/jpsych.2018.291.64
37. Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. (2002) 159:1395–402. doi: 10.1176/appi.ajp.159.8.1395
38. Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, et al. The MCCB impairment profile for schizophrenia outpatients:results from the matrics psychometric and standardization study robert. Schizophr Res. (2011) 126:124–31. doi: 10.1016/j.schres.2010.11.008
39. Hsu WY, Lane HY, Lin CH. Medications used for cognitive enhancement in patients with schizophrenia, bipolar disorder, Alzheimer's disease, and Parkinson's disease. Front Psychiatry. (2018) 9:91. doi: 10.3389/fpsyt.2018.00091
40. Alvarez E, Martínez MD, Roncero I, Chowen JA, García-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. (2005) 92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x
41. Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo- controlle. Diabetologia. (2016) 59:954–65. doi: 10.1007/s00125-016-3874-y
42. Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. (1995) 7:2294–300. doi: 10.1111/j.1460-9568.1995.tb00650.x
43. Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol. (2008) 132:329–38. doi: 10.1085/jgp.200810044
44. Holscher C. Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer's disease. Recent Pat CNS Drug Discov. (2010) 5:109–17. doi: 10.2174/157488910791213130
45. Hölscher C, Li L. New roles for insulin-like hormones in neuronal signalling and protection: new hopes for novel treatments of Alzheimer's disease? Neurobiol Aging. (2010) 31:1495–502. doi: 10.1016/j.neurobiolaging.2008.08.023
46. McClean PL, Gault VA, Harriott P, Hölscher C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer's disease. Eur J Pharmacol. (2010) 630:158–62. doi: 10.1016/j.ejphar.2009.12.023
47. Salles GN, Calió ML, Hölscher C, Pacheco-Soares C, Porcionatto M, Lobo AO. Neuroprotective and restorative properties of the GLP-1/GIP dual agonist DA-JC1 compared with a GLP-1 single agonist in Alzheimer's disease. Neuropharmacology. (2020) 162:107813. doi: 10.1016/j.neuropharm.2019.107813
48. Zhang XY, Liang J, Chen DC, Xiu MH, Yang F De, Kosten TA, et al. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology. (2012) 222:277–84. doi: 10.1007/s00213-012-2643-y
49. Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. (2015) 14:119–36. doi: 10.1002/wps.20204
50. Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jørgensen NR, et al. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. (2015) 100:2909–17. doi: 10.1210/jc.2015-1176
51. Ji L, Dong X, Li Y, Li Y, Lim S, Liu M, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: a 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab. (2021) 23:404–14. doi: 10.1111/dom.14232
52. Fineman M, Flanagan S, Taylor K, Aisporna M, Shen LZ, Mace KF, et al. Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing. Clin Pharmacokinet. (2011) 50:65–74. doi: 10.2165/11585880-000000000-00000
53. Yabe D, Deenadayalan S, Horio H, Kaneto H, Jensen TB, Terauchi Y, et al. Efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes: a subgroup analysis by baseline variables in the PIONEER 9 and PIONEER 10 trials. J Diabetes Investig. (2022) 13:975–85. doi: 10.1111/jdi.13764
54. Drucker DJ. Advances in oral peptide therapeutics. Nat Rev Drug Discov. (2020) 19:277–89. doi: 10.1038/s41573-019-0053-0
55. Tschoner A, Engl J, Laimer M, Kaser S, Rettenbacher M, Fleischhacker WW, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pract. (2007) 61:1356–70. doi: 10.1111/j.1742-1241.2007.01416.x
56. Buckley PF, Wirshing DA, Bhushan P, Pierre JM, Resnick SA, Wirshing WC. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. (2007) 21:129–41. doi: 10.2165/00023210-200721020-00004
57. Di Capite S, Upthegrove R, Mallikarjun P. The relapse rate and predictors of relapse in patients with first-episode psychosis following discontinuation of antipsychotic medication. Early Interv Psychiatry. (2018) 12:893–9. doi: 10.1111/eip.12385
58. Stürup AE, Nordentoft M, Jimenez-Solem E, Osler M, Davy JW, Christensen TN, et al. Discontinuation of antipsychotics in individuals with first-episode schizophrenia and its association to functional outcomes, hospitalization and death: a register-based nationwide follow-up study. Psychol Med. (2022) 12:1–9. doi: 10.1017/S0033291722002021
59. Nakanishi R, Hirose T, Tamura Y, Fujitani Y, Watada H. Attempted suicide with liraglutide overdose did not induce hypoglycemia. Diabetes Res Clin Pract. (2013) 99:e3–4. doi: 10.1016/j.diabres.2012.10.017
Keywords: schizophrenia, systematic review, meta-analysis, GLP-1 receptor agonists, treatment
Citation: Khaity A, Mostafa Al-dardery N, Albakri K, Abdelwahab OA, Hefnawy MT, Yousef YAS, Taha RE, Swed S, Hafez W, Hurlemann R and Elsayed MEG (2023) Glucagon-like peptide-1 receptor-agonists treatment for cardio-metabolic parameters in schizophrenia patients: a systematic review and meta-analysis. Front. Psychiatry 14:1153648. doi: 10.3389/fpsyt.2023.1153648
Received: 29 January 2023; Accepted: 12 April 2023;
Published: 05 May 2023.
Edited by:
Sarah Tosato, University of Verona, ItalyReviewed by:
Najmeh Shahini, Golestan University of Medical Sciences, IranCopyright © 2023 Khaity, Mostafa Al-dardery, Albakri, Abdelwahab, Hefnawy, Yousef, Taha, Swed, Hafez, Hurlemann and Elsayed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed E. G. Elsayed, bW9oYW1lZC5lbHNheWVkMUB1bmktb2xkZW5idXJnLmRl
†ORCID: Mohamed E. G. Elsayed orcid.org/0000-0002-0011-7837
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.