
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 25 May 2023
Sec. Anxiety and Stress Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1139992
Introduction: Females in the perimenopausal period are susceptible to mood disorders. Perimenopausal panic disorder (PPD) is characterized by repeated and unpredictable panic attacks during perimenopause, and it impacts the patient's physical and mental health and social function. Pharmacotherapy is limited in the clinic, and its pathological mechanism is unclear. Recent studies have demonstrated that gut microbiota is strongly linked to emotion; however, the relation between PPD and microbiota is limitedly known.
Methods: This study aimed to discover specific microbiota in PPD patients and the intrinsic connection between them. Gut microbiota was analyzed in PPD patients (n = 40) and healthy controls (n = 40) by 16S rRNA sequencing.
Results: The results showed reduced α-diversity (richness) in the gut microbiota of PPD patients. β-diversity indicated that PPD and healthy controls had different intestinal microbiota compositions. At the genus level, 30 species of microbiota abundance had significantly different between the PPD and healthy controls. In addition, HAMA, PDSS, and PASS scales were collected in two groups. It was found that Bacteroides and Alistipes were positively correlated with PASS, PDSS, and HAMA.
Discussion: Bacteroides and Alistipes dysbiosis dominate imbalanced microbiota in PPD patients. This microbial alteration may be a potential pathogenesis and physio-pathological feature of PPD. The distinct gut microbiota can be a potential diagnostic marker and a new therapeutic target for PPD.
Perimenopausal panic disorder (PPD) is characterized by unexpected and repeated panic attacks, severe sensations of impending death, and autonomic nervous system dysfunction, including palpitations, tremors, and sweating (1, 2). A recent cross-national epidemiology study indicates that the panic attack incidence and lifetime prevalence rate of panic disorder are 13.2 and 1.7%, respectively (3). Female reproductive cycles often influence the course of panic disorder (PD) that worsens with menopause (4). Individuals aged between 40 and 49 years have a 3.3% prevalence rate of PD, of which 2.4% are in women (5). As life expectancy rises, the incidence of PPD elevates, causing more medical burdens and lower quality of life. Cognitive behavioral therapy (CBT) combined with first-line drugs has significant clinical short-term effects but limited long-term effects. In addition, several adverse effects and high recurrence rates still exist (6, 7). The pathogenesis of PPD includes brain network function dysfunction, neurotransmitter disturbance, microbiota disorders, and reproductive endocrine dysregulation (8). However, the detailed mechanism remains unclear. Therefore, it is important to explore the pathogenesis of PPD for prevention and treatment strategies.
Recent research implicates the gut microbiota in mental health and mood disorders, including anxiety, depression, bipolar, and stress disorders (9). A disturbed balance of intestinal microbes can cause the loss of several host functions, including neurotransmitter dysbiosis, immunological dysfunction, and increased blood–brain barrier permeability (10). Several studies show that the intestinal microbiota has modulated the central nervous system (CNS), such as the amygdala, hippocampus, and prefrontal cortex (11, 12). These brain areas are the neuroanatomical basis of panic. Additionally, the deficient estrogen alters intestinal microbiota balance during perimenopause (13) and induces anxiety-like behavior (14). Therefore, we believe the intestine microbial imbalances of perimenopause might cause panic attacks.
To the best of the authors' knowledge, there is no study on the gut microbiota of perimenopausal panic disorder. Therefore, this study examined 16S rRNA sequencing results to understand the gut microbiota composition in PPD patients. It was hypothesized that PPD patients had different intestinal microbiota, including microbial diversity, relative abundance, and taxonomic composition, compared to matched healthy controls. Furthermore, the gut microbe and panic symptoms scale were investigated for associations. These results provide evidence for the pathological mechanism, diagnosis, and treatment of PPD.
This study recruited patients with PPD from the Menopausal Syndrome Specialist Clinic in the Bao'an District TCM Hospital (Shenzhen, China). All PD patients aged between 41 and 60 years were diagnosed according to the DSM-5 and their HAMA score was ≥ 14 (2, 15). The exclusion criteria were somatoform disorders or physical diseases causing PD, malignant tumors, or antipsychotic drug use. People who had used probiotics, antibiotics, or other drugs affecting the intestinal microbiota within the last 3 months before sampling were excluded from the study (16, 17). Healthy women matched for age were also engaged in the control group. All patients provided basic demographic information. The Hamilton Anxiety Scale (HAMA), Panic-associated Symptoms Scale (PASS), and Panic Disorder Severity Scale (PDSS) were used to score the test for anxiety and panic symptoms. In general, ~4–6 g fecal samples from PPD patients or healthy controls were collected once at the start of the experiment. Next, the samples were placed in a sterile tube and frozen at −80°C until they were sequenced. Each participant signed an informed consent form, stating that they understood the risks and benefits of having their feces used for scientific research.
The tiny subunit of bacterial ribosomes contains 16S rRNA, a nucleic acid sequence that indicates biological species and is the primary indicator of bacterial phylogeny and taxonomic identification. The 16S rRNA Amplicon Sequencing involves one or more variant regions and utilizes the conserved regions for polymerase chain reaction (PCR) amplification. The sequencing examination and identification of the high variant regions of microorganisms' strains have become an important approach for analyzing their composition and structure. Small fragment libraries are constructed and sequenced using paired-end sequencing on the Illumina MiSeq sequencing platform. The species annotation and relative abundance are determined by splicing and filtering reads and clustering operational taxonomic units (OTU). Analyses of the alpha diversity (α-diversity) and the beta diversity (β-diversity) can investigate the distinctions between samples.
This study extracted the microbial genomic DNA from feces using QIAamp® DNA Stool Mini Kit (Qiagen, German). The OD-1000 spectrophotometer (OneDrop, Nanjing, China) and 1% agarose gel electrophoresis measured DNA concentration and purity. Diluted genomic DNA was used as a template. The gene was amplified efficiently and accurately by PCR with GoTaq® Hot Start Colorless Master Mix (Promega, USA) using the V4 region of 16S rRNA primers with Barcode (515F-806R). The concentrations of PCR products were measured with Pico Green assay and then mixed at the same concentration. PCR Purification Kit (Qiagen) was applied to purify and recover PCR-amplified products. In this study, the purified and recovered products were mixed using Illumina-compatible primers in a second round of PCR amplification. Pico Green fluorescently quantified the second-round products, Agilent 2200 TapeStation, and Illumina MiSeq platform (Illumina, USA), analyzed and sequenced them, respectively.
Raw data from the Illumina MiSeq platform provided low-quality information that could impede processing. Consequently, it was preprocessed before the analysis, with the following steps. (i) The raw data from high-throughput Sequencing was isolated from the individual sample data. Then, a quality check was performed following the barcode information and primer sequences. (ii) Each 16S rRNA gene sequence was aligned using the Ribosomal Database Project (RDP) Classifier software (version 2.3) and the Silva database. Next, sequence categorization levels (phylum, class, order, family, and genus) were determined. (iii) Sequences were classified into OTUs based on sequence similarity of 97% using the Mothur software. Then, sequence numbers were used to determine OTU abundance.
This study used Illumina MiSeq to calculate the diversity levels within and between communities (alpha and beta diversities, respectively). MetaboAnalyst software was used to depict and compare the differences in a microbial population with principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA). Statistical analysis was performed using SPSS software (version 23.0). The Shapiro–Wilk test assessed the normality of the data. An unpaired t-test compared between-group differences if the data were a normal distribution. The non-normal data were analyzed by the non-parametric Mann–Whitney U-test. According to the normality of the data, the Pearson or Spearman correlations were conducted to assess the PASS, PDSS, and HAMA Scales with gut microbiota, and p < 0.05 was considered statistically significant. Differentially abundant taxa were analyzed using the LEfSe (α = 0.05, effect size threshold of 2). The non-parametric factorial Kruskal–Wallis sum-rank test determined and identified the taxa whose abundance characteristics differed significantly, and LDA analysis evaluated species abundance on the differential effect.
This study engaged 80 women (40 in each PPD and control group). There were no variations in age (PPD = 49.85 ± 4.12 years vs. Con = 51.65 ± 4.83 years, p = 0.077) and height (PPD = 158.9 ± 3.54 cm vs. Con = 159.4 ± 4.67 cm, p = 0.610) between PPD and healthy control group. However, PPD patients were higher weight (PPD = 56.30 ± 4.45 kg vs. Con = 54.15 ± 3.67 kg, p = 0.021) and body mass index (BMI) (PPD = 22.29 ± 1.48 kg/m2 vs. Con = 21.34 ± 1.50 kg/m2, p = 0.006) than healthy control group. PPD patients had a higher risk of gaining weight, consistent with a previous study on PD (18). The PDSS and PASS scores were 9.20 ± 3.24 and 10.63 ± 3.72 in PPD patients, respectively, and the HAMA scale was different between the two groups (PPD = 28.48 ± 7.15 vs. Con = 5.83 ± 2.34, p < 0.05), indicating that the PPD patients suffered from panic or anxiety (Table 1; Supplementary Figure 1).
Alpha diversity, including the OTU index, ACE, Chao index, Shannon, and Simpson suggested the abundance and diversity of gut microbiota. In this study, alpha diversity was richer and more diverse in gut bacteria species in healthy controls than in PPD patients (Figure 1). PCA and OPLS-DA measured β-diversity (between-habitat diversity) (Figure 2), and the abundance and dispersion of gut microbiota were compared by matrix data. PCA is an unsupervised method that performs ANOVA on several samples and dimensionality reduction in multidimensional data. PCA discovered the directions that best explained the variance in a dataset and represented sample differences on the two-dimensional coordinate map. The principal component 1 (PC1) and principal component 2 (PC2) were 8.5% and 6.8%, respectively (Figure 2A). The OPLS-DA is a supervised modeling method that distinguished variations in a dataset relevant to predicting group labels by dimension reduction. The horizontal axis and the abscissa direction represented the score value of the main component and the difference between groups, respectively. The vertical axis and the ordinate represented the orthogonal component value and the difference within the group, respectively (Figure 2B). A different group of gut microbiota was split into groups and clustered accordingly.
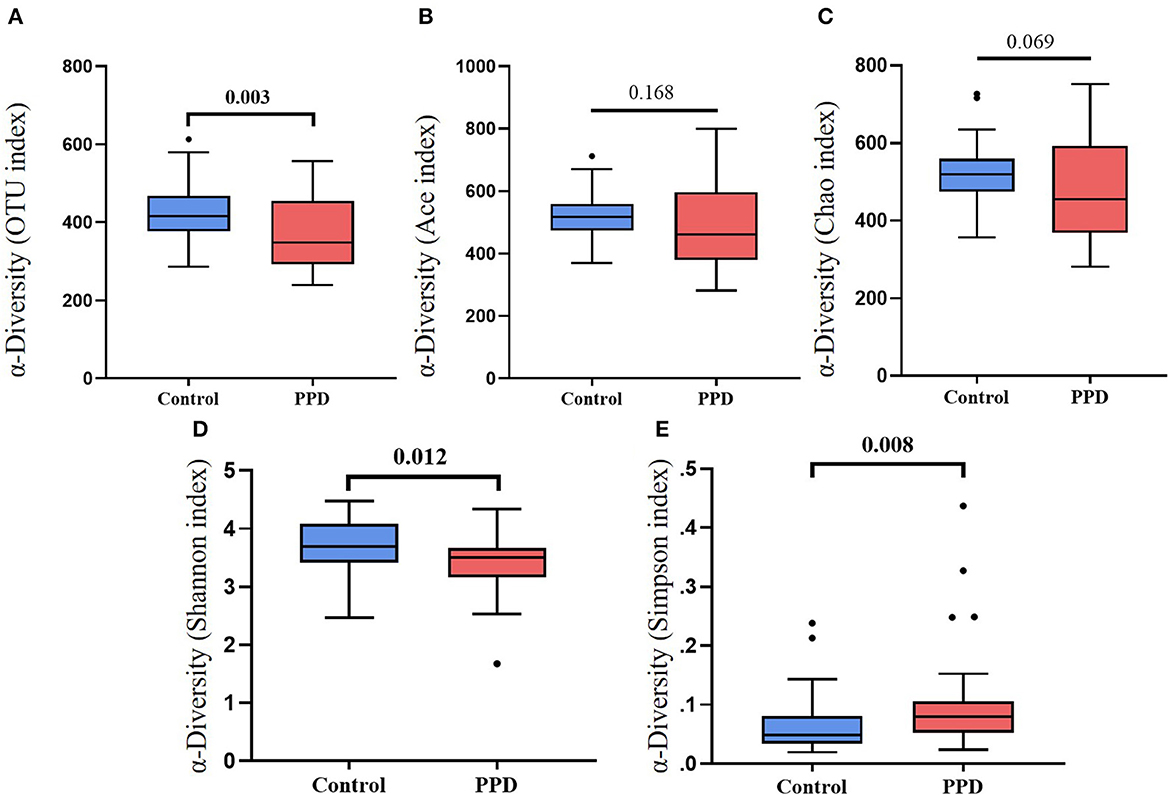
Figure 1. Box plots of α-diversity between PPD patients and healthy controls. (A) OTU index, p = 0.003. (B) ACE index, p = 0.168. (C) Chao index, p = 0.069. (D) Shannon index, p = 0.012. (E) Simpson index, p = 0.008.
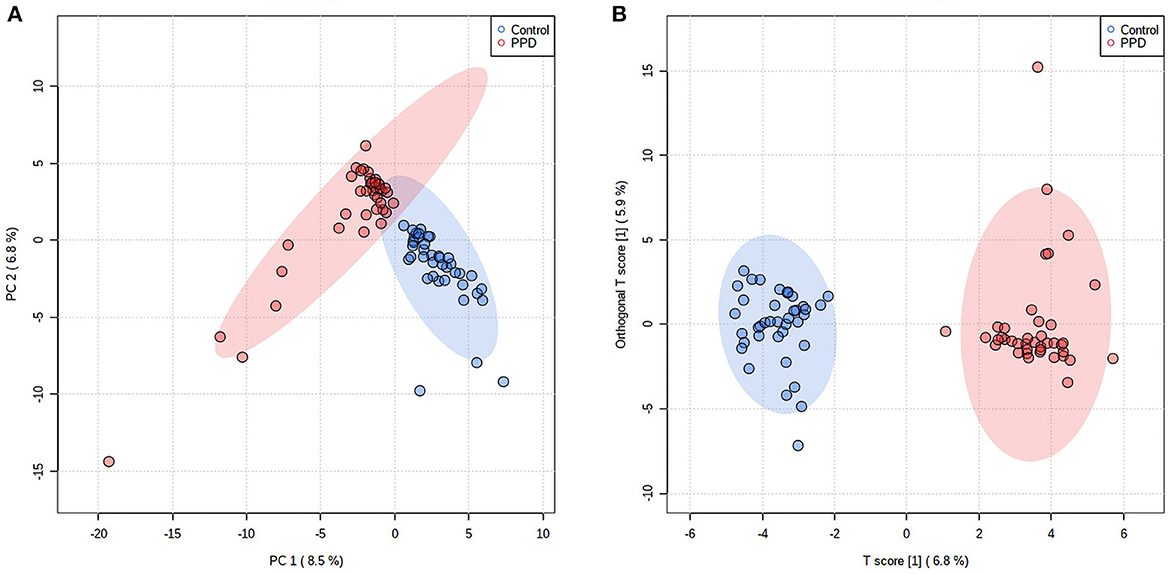
Figure 2. Difference in gut microbiota composition in PPD patients and healthy controls. Each point represents the microbiota of each subject, and each group of subjects was labeled with a different color. The ellipses display 95% confidence regions. (A) PCA, PC1 = 8.5%, PC2 = 6.8%. (B) OPLS-DA, T score = 6.8%, Orthogonal T-score = 5.9%.
The study classified the fecal samples into 1580 OTUs after analysis. A percent stacked column chart was used to visualize gut microbiota abundance. The results showed that the phylum Bacteroidetes and Verrucomicrobia were more abundant, and the Firmicutes and Actinobacteria were scarcer in PPD patients than in healthy controls (Supplementary Figure 2; Supplementary Table 1). The top 60 genera accounted for over 99% of the microbiota among all samples (Supplementary Figure 3; Supplementary Table 2). The study used cluster analysis and heatmaps to determine the relative abundance of genera between samples (Supplementary Figure 4). PPD patients had a microbiota abundance that contained significantly greater amounts of Bacteroides, Phascolarctobacterium, Parabacteroides, Alistipes, Paraprevotella, Sutterella, Akkermansia, Megasphaera, Veillonella, Bilophila, Flavonifractor, Oscillospira, Oscillibacter, Odoribacter, Butyricimonas, and Desulfovibrio than the healthy controls. In contrast, the healthy controls had significantly higher contents of Faecalibacterium, Blautia, Pseudobutyrivibrio, Subdoligranulum, Roseburia, Coprococcus, Bifidobacterium, Clostridium_sensu_stricto_1, Streptococcus, Dorea, Anaerostipes, Anaerotruncus, Collinsella, and Turicibacter than the PPD patients (Figure 3).

Figure 3. Comparison of the mean abundance of the top 30 significant dominant and specific bacterial genera in the PPD patients and healthy controls. Red, healthy controls. Blue, PPD patients.
A Spearman correlation analysis showed that the PASS was positively correlated with Phascolarctobacterium (r = 0.358, p = 0.023), Parabacteroides (r = 0.394, p = 0.012), Alistipes (r = 0.366, p = 0.02), and Akkermansia (r = 0.313, p = 0.049) at the genus level. The Bacteroides (r = 0.377, p = 0.017), Alistipes (r = 0.322, p = 0.043), and Coprococcus (r = -0.402, p = 0.01) were related with PDSS. The HAMA scales were positively correlated with Alistipes (r = 0.466, p = 0.002) and negatively correlated with Faecalibacterium (r = −0.322, p = 0.042). The Phascolarctobacterium (r = 0.363, p = 0.021) was positively correlated with BMI in PPD (Figure 4; Supplementary Table 3). The LEfSe approach identified major gut microbiota species from phylum to genus between healthy control and PPD patients, according to the LDA scores (p < 0.05, LDA score > 2) (Figure 5).
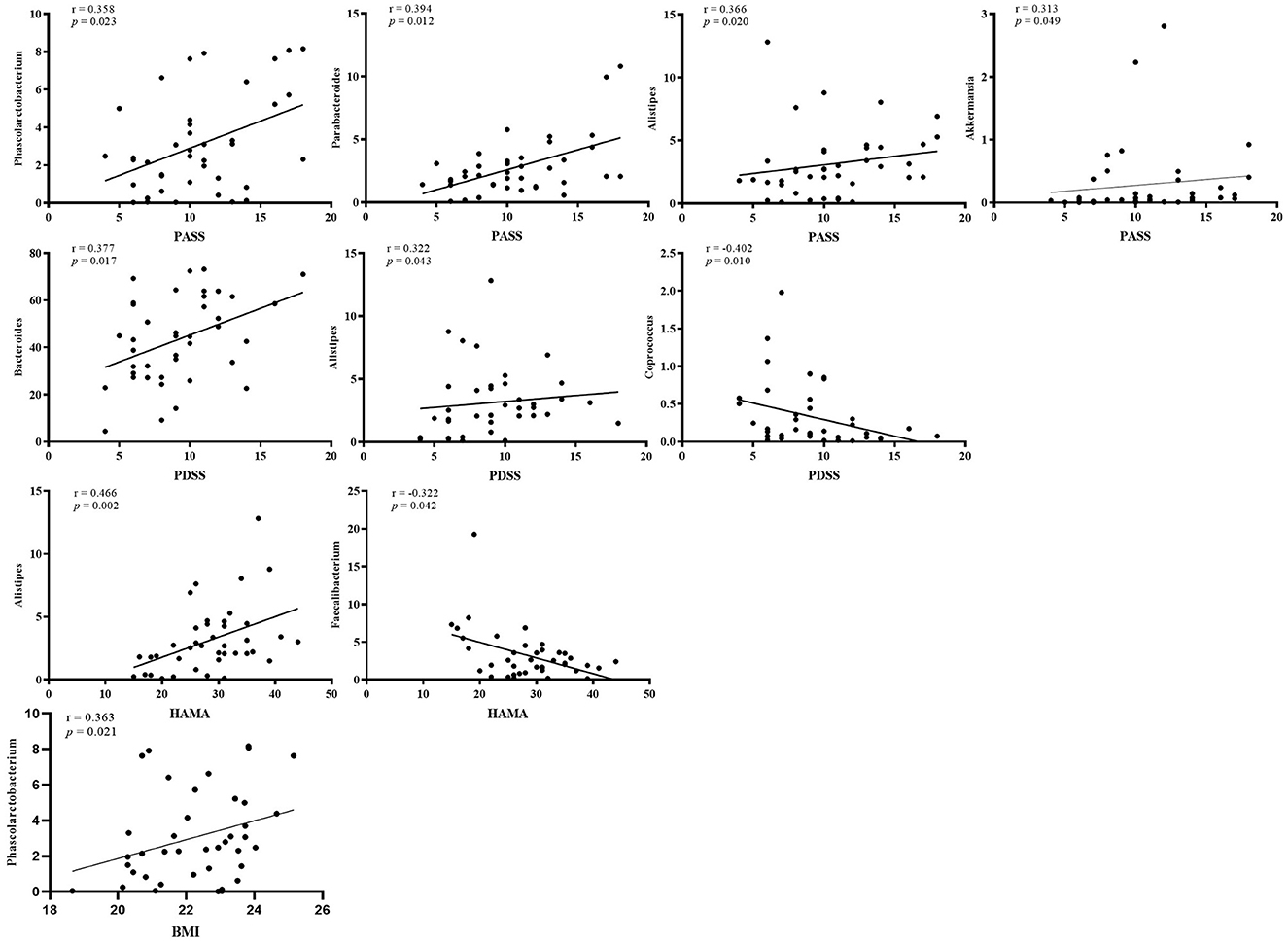
Figure 4. Correlation analysis between microbiota at the genus level and PASS, PDSS, HAMA scores, and BMI in PPD. PASS, panic-associated symptoms scale; PDSS, panic disorder severity scale; HAMA, Hamilton anxiety scale; BMI, body mass index.
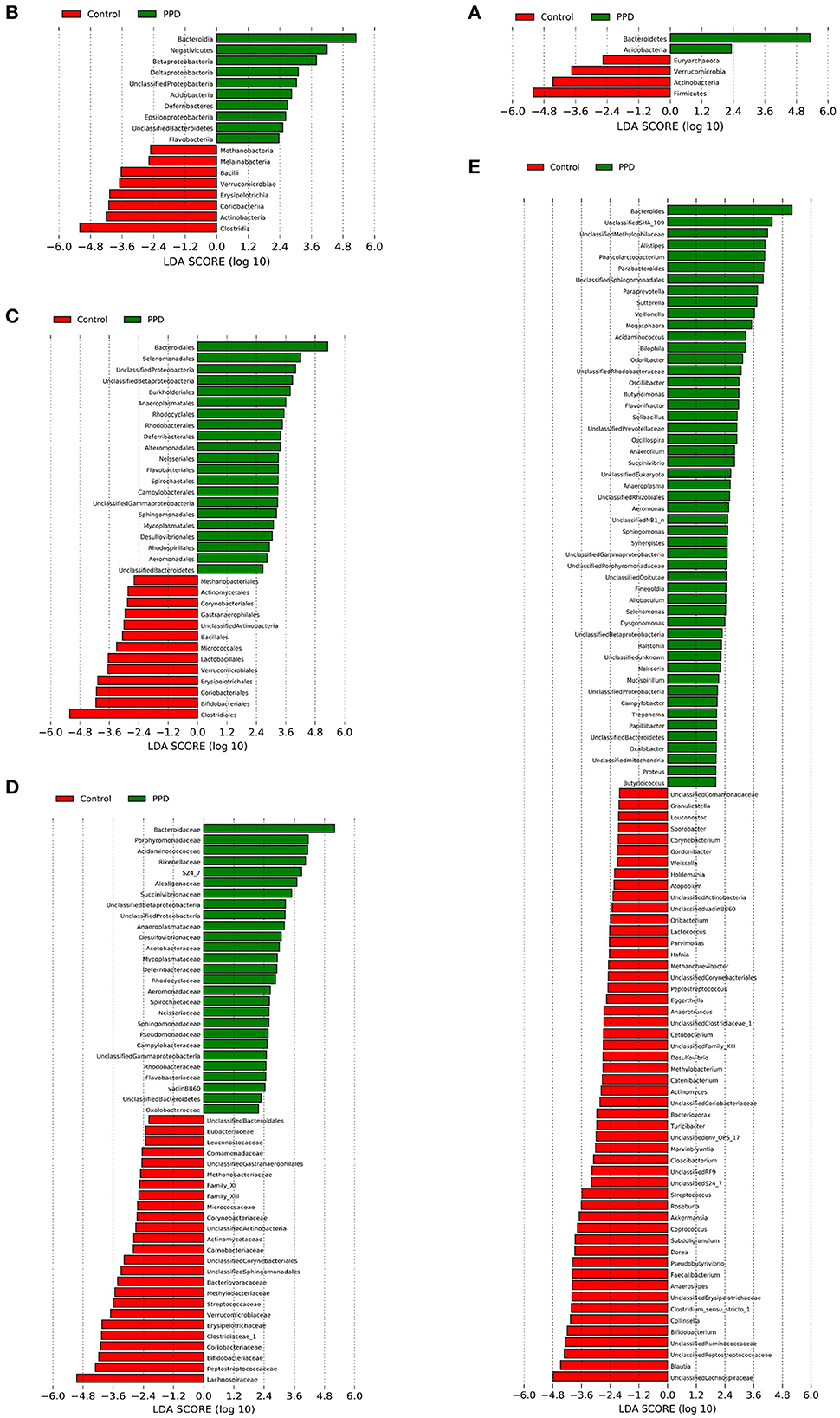
Figure 5. LEfSe analysis revealed diverse intestinal flora taxa and species between PPD patients and healthy controls (LDA score > 2, p < 0.05). (A–E) Classification levels of phylum, class, order, family, and genus. The graph was generated using the LEfSe program. Red, healthy control. Green, PPD patients.
This research is the first to identify significant distinctions in gut microbiome between perimenopausal panic disorder (PPD) patients and healthy women (healthy controls).
Gut microbiota has been reported extensively in mood disorders. However, research on it related to PPD is limited. Recent research has confirmed that gut microbial imbalance is one of the etiologies in psychiatric diseases (19, 20). Studies on animals have found that microbiota alteration can influence anxiety-like and despair behavior in stress-induced mice (21, 22). PD patients have a higher abundance of Bacteroidetes in oral microbiota than healthy controls (23). This study found that PPD patients and healthy controls had distinct significant differences in gut microbiota. Community richness (ACE Index and Chao Index) had no difference between PPD and healthy women, while evenness (Simpson Index) was higher in PPD. The β-diversity showed different microbiota communities in PPD patients compared to healthy control, and the microbial composition of PPD had changed, as reported in prior literature (24, 25). In addition, PPD patients had higher Bacteroidetes and the ratio of Bacteroidetes/Firmicutes was increased. The same alteration in gut microbiota was also found in generalized anxiety disorder (GAD) patients (25). These results suggest that the gut microbial balance of PPD patients was disorder. Dysbiosis of gut microbiota can induce anxiety or fear in the central nervous system (CNS) through aberrant immunological responses (26, 27). A study has shown that pro-inflammatory cytokines are also high levels in panic disorder (28). Estrogen inhibits inflammatory mediator production in the brain (29). Estrogen deficiency in perimenopause increases the pro-inflammatory cytokines that affect neurological systems (30, 31). The reduced pro-inflammatory cytokines by probiotic therapy can alleviate stress and anxiety (32–34). Therefore, the perimenopausal panic states might be induced by microbiota imbalance, causing an inflammatory immune response.
16S rRNA is important for assessing the physio pathology characteristics of PPD patients' gut microbiota. Although an interaction between microbiota and anxiety is recognized, there is less evidence of the specific microbiota of panic and their interactions. Prevotella and Veillonella are higher in panic oral microbiota and might influence CNS by increased inflammation (23). In the present research, PPD patients had more abundances of Bacteroides and Alistipes in the intestinal tract than healthy controls, and the two bacteria were positively correlated with panic and anxiety symptoms. In the literature, Bacteroides and Alistipes are positively correlated with anxiety in GAD patients (24, 25). In addition, Alistipes can influence the tryptophan metabolism and cause serotonergic system imbalance (35, 36). This imbalance can modify neurological impulses related to anxiety (37). Low tryptophan availability can inhibit the generation and release of serotonin in the brain, causing panic or anxiety (38).
Excessive Bacteroides and Alitipes can cause inflammation (35, 39). The pro-inflammatory cytokine can activate indolamine 2,3-dioxygenase (IDO), transforming tryptophan into kynurenine (40). Kynurenine can cross the blood–brain barrier to the brain easily and produces catabolite, Quinolinic acid, and Kynurenic acid (41). The Quinolinic acid has excitability and neurotoxicity (42) and induces an anxiogenic effect (43, 44), while the Kynurenic acid has neuroprotective effects (45). Moreover, IDO enzymes are more susceptible to immune activation in females than males and increase anxiogenic tryptophan catabolite levels (46). Variations of tryptophan pathway metabolism cause Bacteroides and Alistipes to influence PPD (47–49). Therefore, the Bacteroides and Alistipes can be biomarkers in PPD and may influence panic symptoms by regulating the immune–tryptophan–kynurenine pathway.
Furthermore, the gut microbiota can influence small molecule metabolites. Indoles and short-chain fatty acids (SCFAs) are important immune response modifiers and can exert anti-inflammatory effects (50). The aryl hydrocarbon receptor can be activated by indole metabolites, which can induce regulatory T-cell differentiation and release anti-inflammatory cytokines (51). In addition, SCFAs are endogenous and decrease IL-1β, IL-6, and TNF-α levels during host inflammatory response (52). SCFAs mediate systemic inflammation and central neuroimmune function. SCFAs have neuroactive properties and may promote serotonin biosynthesis in the brain, alleviate panic disorder (53, 54). Butyrate, as an anti-inflammatory SCFAs, can inhibit inflammatory responses (55) and indirectly mediate brain functions by stimulating the vagus nerve and regulating immune responses (56). The current study demonstrated that the Faecalibacterium, Coprococcus, and Roseburia are higher in healthy controls than in PPD patients. These three colonies of microbiota can produce butyrate (57, 58). Faecalibacterium as a novel probiotic therapy plays a role in low-grade inflammation (59). In addition, PPD patients have higher Phascolarctobacterium and Parabacteroides, which can metabolize amino acids and proteins, produce toxic substances, such as ammonia, putrescine, and phenol (60). Similar outcomes have been observed in major depression disorder (61). Although there is a significant difference in BMI values between the groups, the majority of both PPD patients and healthy controls fall within the normal range of BMI (< 24.9) in our study. Hence, we propose that the increased abundance of Phascolarctobacterium in PPD patients may be a potential factor contributing to their higher BMI. Additionally, increasing Roseburia and decreasing Parabacteroides are correlated with healthier eating behavior (62), which supports demographic results that PPD patients have higher BMI and weight. Therefore, the small molecule metabolites modulated by microbiota may further influence anxiety and panic symptoms. The gut microbiota may impact perimenopausal panic symptoms by regulating host immunity, metabolism, and inflammation.
Women are twice likely to develop affective disorders than males. Anxiety symptoms are observed more frequently in the menopause transition (63). A systematic review indicated that the estradiol variation could cause different gut microbiota between premenopausal and postmenopausal states (64). Female hormones play an important role in gut microbiota composition (13). Postmenopausal women treated with estrogen-like compounds isoflavones can suppress Clostridiaceae which is related to inflammation (65, 66). The gut microbiota impacts estrogen levels by secretion of β-glucuronidase deconjugating estrogen (67). Reduced gut microbiota diversity can decrease estrogen metabolism (68), decrease the glucocorticoid neutralization effect, and make women more vulnerable to stress (69). A study on animals documented that female mice with gut microbiota variations were more susceptible to stress (70). It is because menopause women are more susceptible to exogenous stress, which can activate the HPA axis to increase cortisol and change in gut microbiota (71, 72). Therefore, further study should be conducted on the mechanism of intricate intestinal microbial interactions with perimenopausal panic disorder.
This study focused on the difference in the gut microbiota between PPD patients and healthy controls. The Bacteroides and Alistipes abundances are highly correlated with perimenopausal panic symptoms and can represent diagnostic biomarkers for PPD patients. A microbiota imbalance can induce neuroinflammation, neurometabolic dysregulation, and neuroimmune dysfunction, leading to a panic attack.
The current study has some limitations. This study recruited PPD patients from only one city, Shenzhen (China). The result might vary if respondents with different dietary, climates, and geography are studied. Geographical factors more significantly impact microbial variance than dietary and urbanization factors (73). Second, this study's sample is relatively small and, thus, does not strongly represent diversity well. Future studies need to consider larger datasets with greater geographical coverage. Lastly, this study lacks the results of gut microbiota in female reproductive cycles except for the perimenopausal period. The effect of estrogen on intestinal microbiota is unclear and can be improved in subsequent studies. This study indicated that gut microbiota in PPD is important to immune response. A follow-up study will be conducted to investigate the effects further.
We discovered that intestinal flora is different between PPD and healthy women. Bacteroides and Alistipes dysbiosis dominate imbalanced microbe in PPD. The gut microbiota can influence PPD through immune inflammation and tryptophan–kynurenine pathway metabolism. This microbial alteration may be a potential pathogenesis and physio-pathological feature of PPD. The findings of this research will serve as a supporting evidence for the clinical diagnosis and therapy of PPD. Future study should investigate the in-depth interaction between PPD and gut microbiota and optimize the treatment ideas.
The original contributions presented in the study are included in the Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Affiliated Bao'an TCM Hospital of Guangzhou University of Chinese Medicine (No. 20140227-2) and the Chinese Clinical Trial Registry, ChiCTR-INR-16009724. The patients/participants provided their written informed consent to participate in this study.
YX and GC obtained funding, conceptualized the study, and designed the protocol. SL supervised recruitment, analyzed data, and drafted the manuscript. HW, JQ, and ML recruited and coordinated the trial and manuscript revision. EG completed sample sequencing and reviewed the manuscript. XW supported recruitment and sample collection. All authors provided a revision of the manuscript and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Nos: 81574064 and 81473755), the Natural Science Foundation of Guangdong Province (No: 2023A1515011123), the Shenzhen Science and Technology Innovation Committee (No: JCYJ20210324124613037), and the Shenzhen Bao'an Traditional Chinese Medicine Development Foundation (No: 2022KJCX-ZJZL-9).
All patients and healthy participants who provided stool samples in this study are thanked by the authors. The authors also thank Dr. Shiyi Qi, Dr. Runjin Zhou, and Dr. Yingxin Peng for their great help in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1139992/full#supplementary-material
Supplementary Figure 1. Subject inclusion flow chart.
Supplementary Figure 2. Stacked bar plots showing the distribution of taxa in PPD patients and healthy controls. Relative abundance at the phylum level.
Supplementary Figure 3. Stacked bar plots showing the distribution of taxa in PPD patients and healthy controls. Relative abundance at the genus level.
Supplementary Figure 4. Cluster analysis and heatmap of top 50 species abundance at the genus level in PPD patients and healthy controls. The clustering result is shown in the form of a dendrogram and heatmap. Metaboanalyst (version 5.0) was used for clustering. Each column represents the analyzed samples, and each row represents one of the top 50 microbiota genera. On top of the heatmap: red squares, healthy controls; green squares, PPD patients. The dendrogram for samples is shown on top of the heatmap, and the microbiome dendrogram is on the left side. Dark blue to dark red color gradient denotes lower to higher expression.
Supplementary Table 1. Top five phyla microbiota between PPD patients and healthy controls. Median (IQR: 25, IQR: 75).
Supplementary Table 2. Top 60 genera microbiota between PPD patients and healthy controls.
Supplementary Table 3. Correlation analysis between BMI, PASS, PDSS, HAMA scales, and fecal microbiota.
1. Rapkin AJ, Mikacich JA, Moatakef-Imani B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. (2002) 4:419–28. doi: 10.1007/s11920-002-0069-7
3. de Jonge P, Roest AM, Lim CC. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. (2016) 33:1155–77. doi: 10.1002/da.22572
4. Claudia P, Andrea C, Chiara C, Stefano L, Giuseppe M, Vincenzo DL, et al. Panic disorder in menopause: a case control study. Maturitas. (2004) 48:147–54. doi: 10.1016/j.maturitas.2003.08.003
5. Olaya B, Moneta MV, Miret M, Ayuso-Mateos JL, Haro JM. Epidemiology of panic attacks, panic disorder and the moderating role of age: results from a population-based study. J Affect Disord. (2018) 241:627–33. doi: 10.1016/j.jad.2018.08.069
6. Ziffra M. Panic disorder: a review of treatment options. Ann Clin Psychiatry. (2021) 33:124–33. doi: 10.12788/acp.0014
7. Pompoli A, Furukawa TA, Efthimiou O, Imai H, Tajika A, Salanti G. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. (2018) 48:1945–53. doi: 10.1017/S0033291717003919
8. Johnson PL, Federici LM, Shekhar A. Etiology, triggers and neurochemical circuits associated with unexpected, expected, and laboratory-induced panic attacks. Neurosci Biobehav Rev. (2014) 46 Pt 3:429–54. doi: 10.1016/j.neubiorev.2014.07.027
9. Jarbrink-Sehgal E, Andreasson A. The gut microbiota and mental health in adults. Curr Opin Neurobiol. (2020) 62:102–14. doi: 10.1016/j.conb.2020.01.016
10. Rutsch A, Kantsjo JB, Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. (2020) 20:11. doi: 10.3389/fimmu.2020.604179
11. Hoban AE, Stilling RM, Moloney GM, Moloney RD, Shanahan F, Dinan TG, et al. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome. (2017) 5:102. doi: 10.1186/s40168-017-0321-3
12. Darch HT, Collins MK, O'Riordan KJ, Cryan JF. Microbial memories: sex-dependent impact of the gut microbiome on hippocampal plasticity. Eur J Neurosci. (2021) 54:5235–44. doi: 10.1111/ejn.15119
13. Vieira AT, Castelo PM, Ribeiro DA, Ferreira CM. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. (2017) 8:1884. doi: 10.3389/fmicb.2017.01884
14. Puga-Olguin A, Rodriguez-Landa JF, Rovirosa-Hernandez MJ, et al. Long-term ovariectomy increases anxiety- and despair-like behaviors associated with lower Fos immunoreactivity in the lateral septal nucleus in rats. Behav Brain Res. (2019) 360:185–95. doi: 10.1016/j.bbr.2018.12.017
15. Chen G, Wang X, Zhang S, Xu X, Liang J, Xu Y. In vivo investigation on bio-markers of perimenopausal panic disorder and catgut embedding acupoints mechanism. Medicine (Baltimore). (2020) 99:e19909. doi: 10.1097/MD.0000000000019909
16. Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. (2014) 5:3654. doi: 10.1038/ncomms4654
17. Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. (2018) 6:3. doi: 10.1186/s40168-018-0515-3
18. Guenzel N, Schober DJ. Psychiatric comorbidities and bmi: an exploratory analysis. Issues Ment Health Nurs. (2017) 38:698–704. doi: 10.1080/01612840.2017.1341588
19. Wagner-Skacel J, Dalkner N, Moerkl S, Kreuzer K, Farzi A, Lackner S, et al. Sleep and microbiome in psychiatric diseases. Nutrients. (2020) 12:8. doi: 10.3390/nu12082198
20. Lach G, Schellekens H, Dinan TG. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. (2018) 15:36–59. doi: 10.1007/s13311-017-0585-0
21. Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiat. (2017) 82:472–87. doi: 10.1016/j.biopsych.2016.12.031
22. Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep-Uk. (2017) 7:59. doi: 10.1038/srep43859
23. Xie Z, Jiang W, Deng M, Wang W, Xie X, Feng X, et al. Alterations of oral microbiota in patients with panic disorder. Bioengineered. (2021) 12:9103–12. doi: 10.1080/21655979.2021.1994738
24. Chen Y-H, Bai J, Wu D, Yu S-F, Qiang X-L, Bai H, et al. Association between fecal microbiota and generalized anxiety disorder: severity and early treatment response. J Affect Disorders. (2019) 259:56–66. doi: 10.1016/j.jad.2019.08.014
25. Jiang H-Y, Zhang X, Yu Z-H, Zhang Z, Deng M, Zhao J-H, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. (2018) 104:130–6. doi: 10.1016/j.jpsychires.2018.07.007
26. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
27. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
28. Quagliato LA, Nardi AE. Cytokine alterations in panic disorder: a systematic review. J Affect Disord. (2018) 228:91–6. doi: 10.1016/j.jad.2017.11.094
29. Xu Y, Sheng H, Tang Z, Lu J, Ni X. Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav Brain Res. (2015) 288:71–8. doi: 10.1016/j.bbr.2015.04.017
30. McCarthy M, Raval AP. The peri-menopause in a woman's life: a systemic inflammatory phase that enables later neurodegenerative disease. J Neuroinflamm. (2020) 17:1. doi: 10.1186/s12974-020-01998-9
31. Gameiro CM, Romao F, Castelo-Branco C. Menopause and aging: changes in the immune system–a review. Maturitas. (2010) 67:316–20. doi: 10.1016/j.maturitas.2010.08.003
32. Tran N, Zhebrak M, Yacoub C, Pelletier J, Hawley D. The gut-brain relationship: investigating the effect of multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. J Affect Disord. (2019) 252:271–7. doi: 10.1016/j.jad.2019.04.043
33. Lew L-C, Hor Y-Y, Yusoff NAA, Choi S-B, Yusoff MSB, Roslan NS, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin Nutr. (2019) 38:2053–64. doi: 10.1016/j.clnu.2018.09.010
34. Chong HX, Yusoff NAA, Hor Y-Y, Lew L-C, Jaafar MH, Choi S-B, et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef Microbes. (2019) 10:355–73. doi: 10.3920/BM2018.0135
35. Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The genus alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol. (2020) 11:906. doi: 10.3389/fimmu.2020.00906
36. Dhaliwal GK. Alistipes: The influence of a commensal on anxiety and depression. Catalyst: Facets Biochemistr Biomed Sci. (2019) 3:2–10. Available online at: https://journals.mcmaster.ca/catalyst/article/view/1933
37. Haleem DJ, Mahmood K. Brain serotonin in high-fat diet-induced weight gain, anxiety and spatial memory in rats. Nutr Neurosci. (2021) 24:226–235. doi: 10.1080/1028415X.2019.1619983
38. Russo S, Kema IP, Bosker F, Haavik J, Korf J. Tryptophan as an evolutionarily conserved signal to brain serotonin: molecular evidence and psychiatric implications. World J Biol Psychia. (2009) 10:258–68. doi: 10.3109/15622970701513764
39. Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. (2007) 20:593–621. doi: 10.1128/CMR.00008-07
40. Kaiser H, Yu K, Pandya C. Kynurenine, a tryptophan metabolite that increases with age, induces muscle atrophy and lipid peroxidation. Oxid Med Cell Longev. (2019) 20:19. doi: 10.1155/2019/9894238
41. Fukui S, Schwarcz R, Rapoport SI. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. (1991) 56:2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x
42. Lapin IP. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J Neural Transm. (1978) 42:37–43. doi: 10.1007/BF01262727
43. Lapin IP, Mutovkina LG, Ryzov IV, Mirzaev S. Anxiogenic activity of quinolinic acid and kynurenine in the social interaction test in mice. J Psychopharmacol. (1996) 10:246–9. doi: 10.1177/026988119601000312
44. Butler MI, Long-Smith C, Moloney GM, Morkl S, O'Mahony SM, Cryan JF, et al. The immune-kynurenine pathway in social anxiety disorder. Brain Behav Immun. (2022) 99:317–26. doi: 10.1016/j.bbi.2021.10.020
45. Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. (1984) 48:273–8. doi: 10.1016/0304-3940(84)90050-8
46. Songtachalert T, Roomruangwong C, Carvalho AF, Bourin M, Maes M. Anxiety disorders: sex differences in serotonin and tryptophan metabolism. Curr Top Med Chem. (2018) 18:1704–15. doi: 10.2174/1568026618666181115093136
47. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
48. Schopman SME, Bosman RC, Muntingh ADT, van Balkom AJLM, Batelaan NM. Effects of tryptophan depletion on anxiety, a systematic review. Transl Psychiatry. (2021) 11:118. doi: 10.1038/s41398-021-01219-8
49. Zhang Y, Fan Q, Hou Y, Zhang X, Yin Z, Cai X, et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain Behav Immun. (2022) 102:11–22. doi: 10.1016/j.bbi.2022.02.007
50. Zhang SS, Tan QW, Guan LP. Antioxidant, anti-inflammatory, antibacterial, and analgesic activities and mechanisms of quinolines, indoles and related derivatives. Mini-Rev Med Chem. (2021) 21:2285–99. doi: 10.2174/1389557521666210111145011
51. Ala M. Tryptophan metabolites modulate inflammatory bowel disease and colorectal cancer by affecting immune system. Int Rev Immunol. (2022) 41:326–45. doi: 10.1080/08830185.2021.1954638
52. Sam QH, Ling H, Yew WS, Tan Z, Ravikumar S, Chang MW, et al. The divergent immunomodulatory effects of short chain fatty acids and medium chain fatty acids. Int J Mol Sci. (2021) 22:12. doi: 10.3390/ijms22126453
53. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
54. Maron E, Shlik J, Nutt DJ. Tryptophan research in panic disorder. Int J Tryptophan Res. (2008) 1:3–12. doi: 10.4137/IJTR.S929
55. Segain JP, Blétière DRd, Bourreille A, Leray V, Gervois N, Rosales C, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. (2000) 47:397–403. doi: 10.1136/gut.47.3.397
56. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
57. Jalanka J, Major G, Murray K, Singh G, Nowak A, Kurtz C, et al. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int J Mol Sci. (2019) 20:2. doi: 10.3390/ijms20020433
58. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. (2016) 7:979. doi: 10.3389/fmicb.2016.00979
59. Martín R, Miquel S, Chain F, Natividad JM, Jury J, Lu J, et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. (2015) 15:67. doi: 10.1186/s12866-015-0400-1
60. Kaur H, Das C, Mande SS. In silico analysis of putrefaction pathways in bacteria and its implication in colorectal cancer. Front Microbiol. (2017) 8:2166. doi: 10.3389/fmicb.2017.02166
61. Cheung SG, Goldenthal AR, Uhlemann A-C, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psychiatry. (2019) 10:34. doi: 10.3389/fpsyt.2019.00034
62. Medawar E, Haange S-B, Rolle-Kampczyk U, Engelmann B, Dietrich A, Thieleking R, et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl Psychiatry. (2021) 11:500. doi: 10.1038/s41398-021-01620-3
63. Tangen T, Mykletun A. Depression and anxiety through the climacteric period: an epidemiological study (HUNT-II). J Psychosom Obst Gyn. (2008) 29:125–131. doi: 10.1080/01674820701733945
64. Yang M, Wen S, Zhang J, Peng J, Shen X, Xu L. Systematic review and meta-analysis: changes of gut microbiota before and after menopause. Disease Markers. (2022) 22:73. doi: 10.1155/2022/3767373
65. Frankenfeld CL, Atkinson C, Wähälä K, Lampe JW. Obesity prevalence in relation to gut microbial environments capable of producing equol or O-desmethylangolensin from the isoflavone daidzein. Eur J Clin Nutr. (2014) 68:526–30. doi: 10.1038/ejcn.2014.23
66. Nakatsu CH, Armstrong A, Clavijo AP, Martin BR, Barnes S, Weaver CM. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS One. (2014) 9:e108924. doi: 10.1371/journal.pone.0108924
67. Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. (2011) 10:324–335. doi: 10.1016/j.chom.2011.10.003
68. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
69. Faravelli C, Scarpato MA, Castellini G, Sauro CL. Gender differences in depression and anxiety: the role of age. Psychiatry Res. (2013) 210:1301–3. doi: 10.1016/j.psychres.2013.09.027
70. Bridgewater LC, Zhang C, Wu Y. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci Rep. (2017) 7:10776. doi: 10.1038/s41598-017-11069-4
71. Frankiensztajn LM, Elliott E, Koren O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr Opin Neurobiol. (2020) 62:76–82. doi: 10.1016/j.conb.2019.12.003
72. Mezzullo M, Gambineri A, Di Dalmazi G, Fazzini A, Magagnoli M, Baccini M, et al. Steroid reference intervals in women: influence of menopause, age and metabolism. Eur J Endocrinol. (2021) 184:395–407. doi: 10.1530/EJE-20-1147
Keywords: perimenopause, panic disorder, gut microbiota, 16S rRNA, inflammation, tryptophan
Citation: Lin S, Wang H, Qiu J, Li M, Gao E, Wu X, Xu Y and Chen G (2023) Altered gut microbiota profile in patients with perimenopausal panic disorder. Front. Psychiatry 14:1139992. doi: 10.3389/fpsyt.2023.1139992
Received: 08 January 2023; Accepted: 02 May 2023;
Published: 25 May 2023.
Edited by:
Joaquim Radua, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), SpainReviewed by:
Carla Masala, University of Cagliari, ItalyCopyright © 2023 Lin, Wang, Qiu, Li, Gao, Wu, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxiang Xu, eHV5eDE5NjhAMTYzLmNvbQ==; Guizhen Chen, Y2d6aGVuMjAwMEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.