- 1Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 2Department of Mental Health and Drug Abuse, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
Objective: People who have been infected by COVID-19 showing persistent symptoms after 4 weeks from recovery are thought to suffer from Long-COVID syndrome (LC). There is uncertainty on the clinical manifestations of LC. We undertook a systematic review to summarize the available evidence about the main psychiatric manifestations of LC.
Method: PubMed (Medline), Scopus, CINHAL, PsycINFO, and EMBASE were searched until May 2022. Studies reporting estimation of emerging psychiatric symptoms and/or psychiatric diagnoses among adult people with LC were included. Pooled prevalence for each psychiatric condition was calculated in absence of control groups to compare with.
Results: Thirty-three reports were included in the final selection, corresponding to 282,711 participants with LC. After 4 weeks from COVID-19 infection recovery, participants reported the following psychiatric symptoms: depression, anxiety, post-traumatic symptoms (PTS), cognitive and sleeping disturbances (i.e., insomnia or hypersomnia). The most common psychiatric manifestation resulted to be sleep disturbances, followed by depression, PTS, anxiety, and cognitive impairment (i.e., attention and memory deficits). However, some estimates were affected by important outlier effect played by one study. If study weight was not considered, the most reported condition was anxiety.
Conclusions: LC may have non-specific psychiatric manifestations. More research is needed to better define LC and to differentiate it from other post-infectious or post-hospitalization syndromes.
Systematic review registration: PROSPERO (CRD42022299408).
Introduction
Long-COVID syndrome (LC) is a condition that can affect people who have recovered from Coronavirus Disease 2019 (COVID-19). This term was introduced to indicate a set of disorders that persist or occur at from 4 weeks after the elimination of the SARS-CoV-2 virus from the body (1). The clinical features of LC are multifaceted; it has been posited that it can affect different organs and systems, causing somatic but also psychological manifestations that impact on quality of life (2).
For most people, mild or moderate COVID-19 lasts for about 2 weeks; in some cases, though, symptoms can persist or develop after healing. Furthermore, also in people with asymptomatic infections later health problems may develop (3–5).
Although progress has been made in the understanding of the clinical and epidemiological features, including the pathogenesis and complications of the acute phase of COVID-19, long-term consequences of the disease remain largely unclear (6).
Additionally, while neuropsychiatric symptoms that manifest acutely during infection, such as depression, post-traumatic symptoms [PTS], sleep and cognitive disturbances or anxiety, have received more attention, the medium- and long-term psychiatric outcomes in COVID-19 patients are still little known and understudied (7, 8).
In the available literature, there are highly heterogeneous research works on this topic, applying widely different sample sizes, inclusion and exclusion criteria, and duration of follow-up. In addition, patient assessment is mainly based on various assessment tools and questionnaires, self-administered in most cases, that do not provide a diagnosis of a condition with definite clinical significance.
Therefore, understanding the medium and long-term impact of COVID-19 is still far from being complete, not only in the context of a multidisciplinary approach, but even more so when focusing on specific areas such as mental health (1).
We undertook this systematic review to summarize the available evidence about the main psychiatric manifestations of LC. A better understanding of the epidemiology of psychopathological manifestations among LC patients is crucial to develop prevention and early interventions.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol of this systematic review was registered with PROSPERO (CRD42022299408).
Data sources and search strategy
We searched the PubMed (Medline), Scopus, CINHAL, PsycINFO, and EMBASE databases until May 2022, using the strategy outlined in the Supplementary Table 1 of the Appendix. In addition, the list of references of the included studies and of other reviews on related topics was screened to identify any other possible study deserving inclusion, and inadvertently missed during the initial literature search. No restrictions regarding language of publication or publication date were set.
Eligibility criteria
We included experimental and observational studies reporting estimation of rates of emerging psychiatric symptoms and/or psychiatric diagnosis among adult people (i.e., ≥18 years old) with LC, without any restriction on other medical comorbidities or setting of enrolment. We excluded studies on participants already suffering from any psychiatric condition, studies assessing the presence of psychiatric symptoms before 4 weeks from COVID-19 recovery, and previous reviews, case-reports, case-series, editorial, and letters to the editor. We only included studies published in peer-reviewed journals, excluding conference abstracts and dissertations. If data from the same sample were published in multiple works, we considered only that study reporting more exhaustive information. Sample overlap was ruled out through a careful check of the registration codes as well as the place and year(s) of sampling.
Where available, outcome data from participants with other inflammatory or infectious diseases, including COVID-19 but without LC, were used as control group.
Terms and definitions
LC was defined as either the presence or the persistence of any symptom that was not present before the infection after 4 weeks from the COVID-19 recovery. Infection from SARS-CoV-2 and recovery from the infection were defined according to the result of the real-time PCR on nasopharyngeal swab sample, or of broncho-alveolar lavage.
Psychiatric symptoms were collected from self-reporting or from validated psychometric tools. Where a psychiatric diagnosis was reported, it had to be defined according to standard operational diagnostic criteria (the Diagnostic and Statistical Manual of Mental Disorders [DSM] or the International Classification of Diseases [ICD]).
Data collection and extraction
Four Reviewers (P.G., V.S., F.R., and F.C.) working independently preliminarily reviewed titles and abstracts of retrieved articles. The initial screening was followed by the analysis of full texts to check compliance with inclusion/exclusion criteria. All disagreements were discussed until consensus, and if consensus was not possible, another member of the team was consulted (M.M.). A standardized form was used for data extraction. Information concerning the year of publication, country, setting, characteristics of study participants (sample size, age, percentages of men and women), LC status, and the presence of psychiatric conditions in the LC groups (and, where available, in the control group) were collected by two authors (P.G. and V.S.) independently. Extraction sheets for each study were cross-checked for consistency and any disagreement was resolved by discussion within the research group.
Statistical analyses
Where possible (i.e., there were at least two studies providing outcome data for LC and controls), quantitative data among studies were summarized using random effects meta-analysis (9). To summarize continuous outcome data (i.e., the scores on a psychometric tool), the pooled Hedges' g standardized mean differences (SMDs) and the corresponding 95% confidence intervals (CIs) were applied, while pooled odds ratios (ORs) and the corresponding 95% CIs (10) were used to report on dichotomous outcome data (i.e., presence/absence of psychiatric diagnosis or psychiatric symptoms).
If meta-analysis was not possible, we calculated the pooled prevalence of psychiatric symptoms and/or psychiatric diagnosis among LC patients. These estimates consisted in weighted-mean prevalence, raw mean prevalence, and median prevalence, with the relative lower and upper ranges across the studies included in the final selection.
The analyses were performed in R (11). Statistical tests were 2-sided and used a significance threshold of p < 0.05.
Risk of bias assessment and the GRADE
Bias risk in the included studies was independently assessed by three reviewers (P.G., V.S., and F.R.), using the Cochrane risk of bias tool (12). All disagreements were discussed until consensus, and if necessary, another member of the team was consulted (M.M.). Each item on the risk of bias assessment was scored as high, low, or unclear, and the GRADE tool was used to assess the overall certainty of evidence (13). Further information is available in the Supplementary material.
Results
Study characteristics
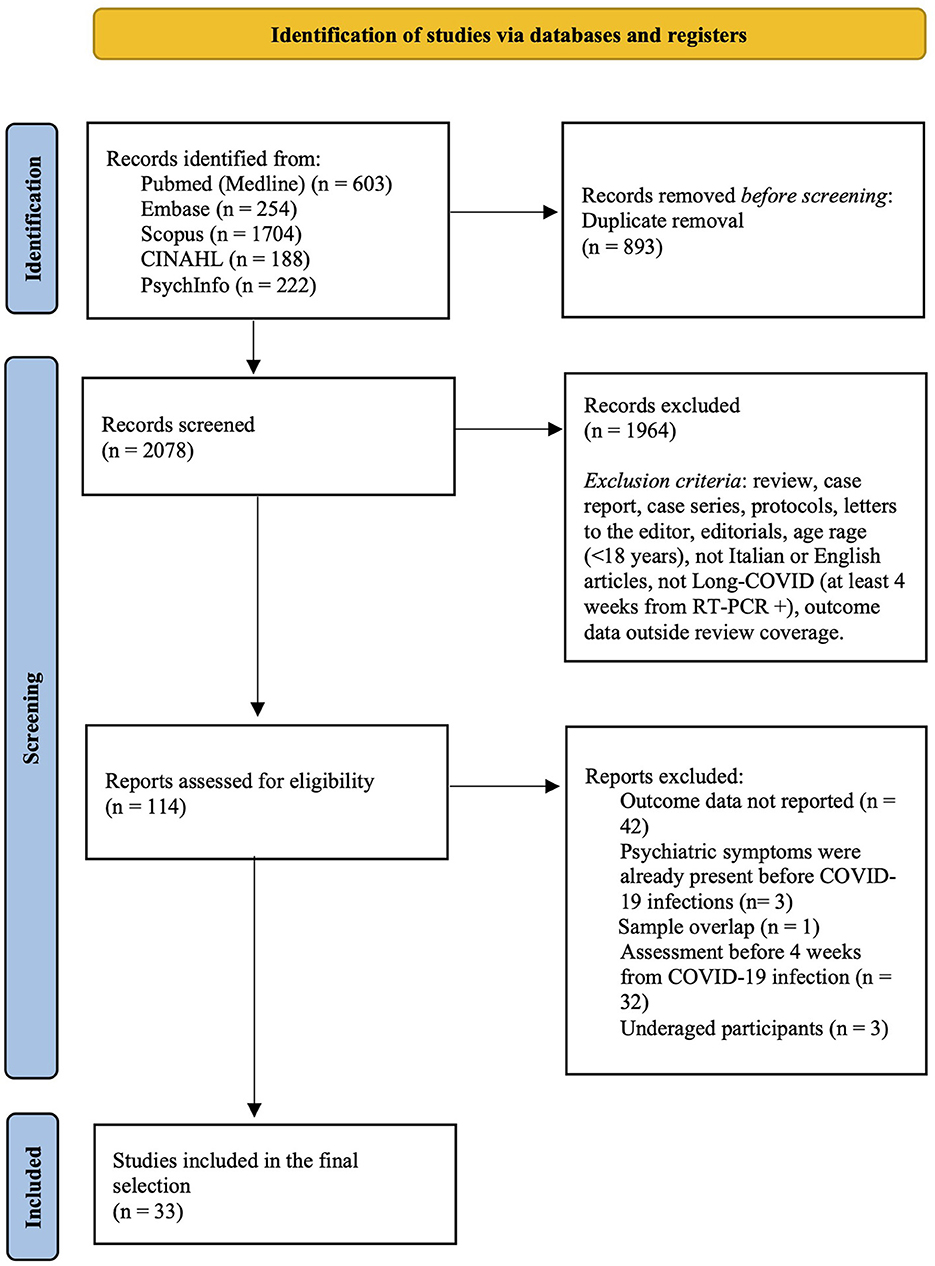
As shown in Figure 1, from 2078 records screened on title and abstract, 114 full texts were analyzed. The review process led to the selection of 33 studies (3, 4, 6–8, 14–41). These studies, referring to 33 different samples and involving a total of 282,711 LC participants, were included in the final selection and quantitative synthesis.
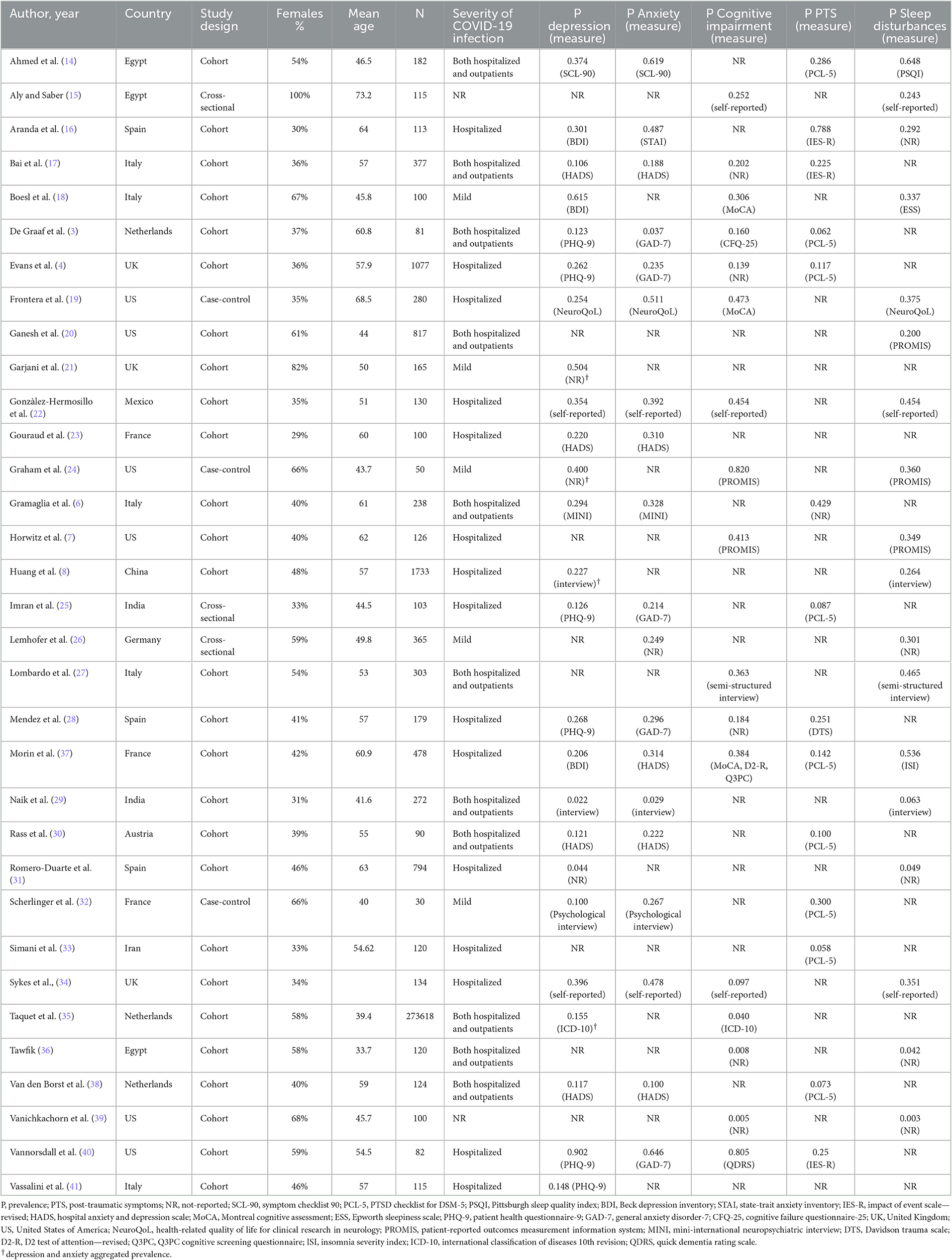
On an average, across the studies, 48% of participants were females (range 23.1–100%). The mean age of participants was 53.2 years (range 33.7–73.2). The selected studies were conducted in 13 countries: US (n = 6; 18.2%); Italy (n = 5; 15.2%); Egypt, France, Netherlands, Spain, UK (each n = 3; 9.1%); India (n = 2; 6.1%); Austria, China, Germany, Iran, Mexico (each n = 1; 3.0%).
All the studies were published in the last 2 years: 2021 (n = 31; 93.9%); 2022 (n = 2; 6.1%).
With respect to the outcomes reported, only 2 studies (6.1%) provided data about psychiatric diagnosis: one study assessed depressive and anxiety disorder (GAD) through a clinical interview, the other study used retrospective screening of the electronic clinical records to investigate prevalence of anxiety and depression, and cognitive impairment, according to the ICD-10 system. The remaining studies (n = 31, 93.9%) used self-reporting or other psychometric tools to measure the level of: depression or anxiety (n = 26, 78.8%), cognitive impairment (n = 16, 48.5%), PTS (n = 13, 39.4%), and sleep disturbances (n = 18, 54.5%). These studies applied dichotomization into positive/negative at the psychometric assessment based on the scales' cut-off for clinical significance, and the estimated prevalence for each study was calculated as the number of participants with score above the cut-off divided to the total number of participants assessed.
Notably, none of the studies included in the final selection applied a control group without LC. Concerning the severity of COVID-19 infection, 15 studies (45.5%) included patients hospitalized due to COVID-19 infection, 5 studies (15.2%) were performed on patients who had mild infection not requiring hospitalization, and 11 studies (33.3%) included both hospitalized and other managed outpatients. Information about infection severity was missing in 2 studies (6.1%).
All studies characteristics are summarized in Table 1.
Prevalence of psychiatric symptoms across the studies
Table 2 summarize the pooled prevalence estimates for each psychiatric symptom across the studies included in this review, and the population prevalence worldwide. Notably, prevalence of depression, anxiety, cognitive impairment, PTS, and sleep disturbances resulted much higher among LC patients than in the general population (42–46).
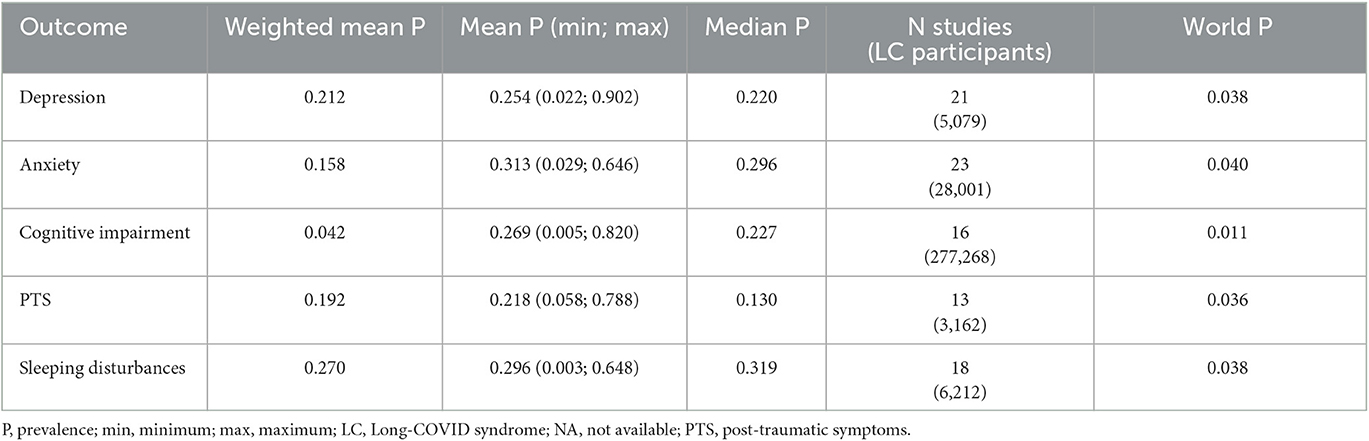
Table 2. Pooled prevalence of psychiatric symptoms across the included studies and worldwide prevalence.
Prevalence of depression
Twenty-one studies (63.3%) provided outcome data for depression among LC patients. The weighted mean prevalence across the studies was 0.212, that is quite similar to the unweighted mean and median prevalence (0.254 [range: 0.022–0.902] and 0.220, respectively), consistent with not significant outlier effect played by any of the study in the pooled estimate. Figure 2 shows comparison of the depression prevalence estimates across the studies, and the weighted mean prevalence.
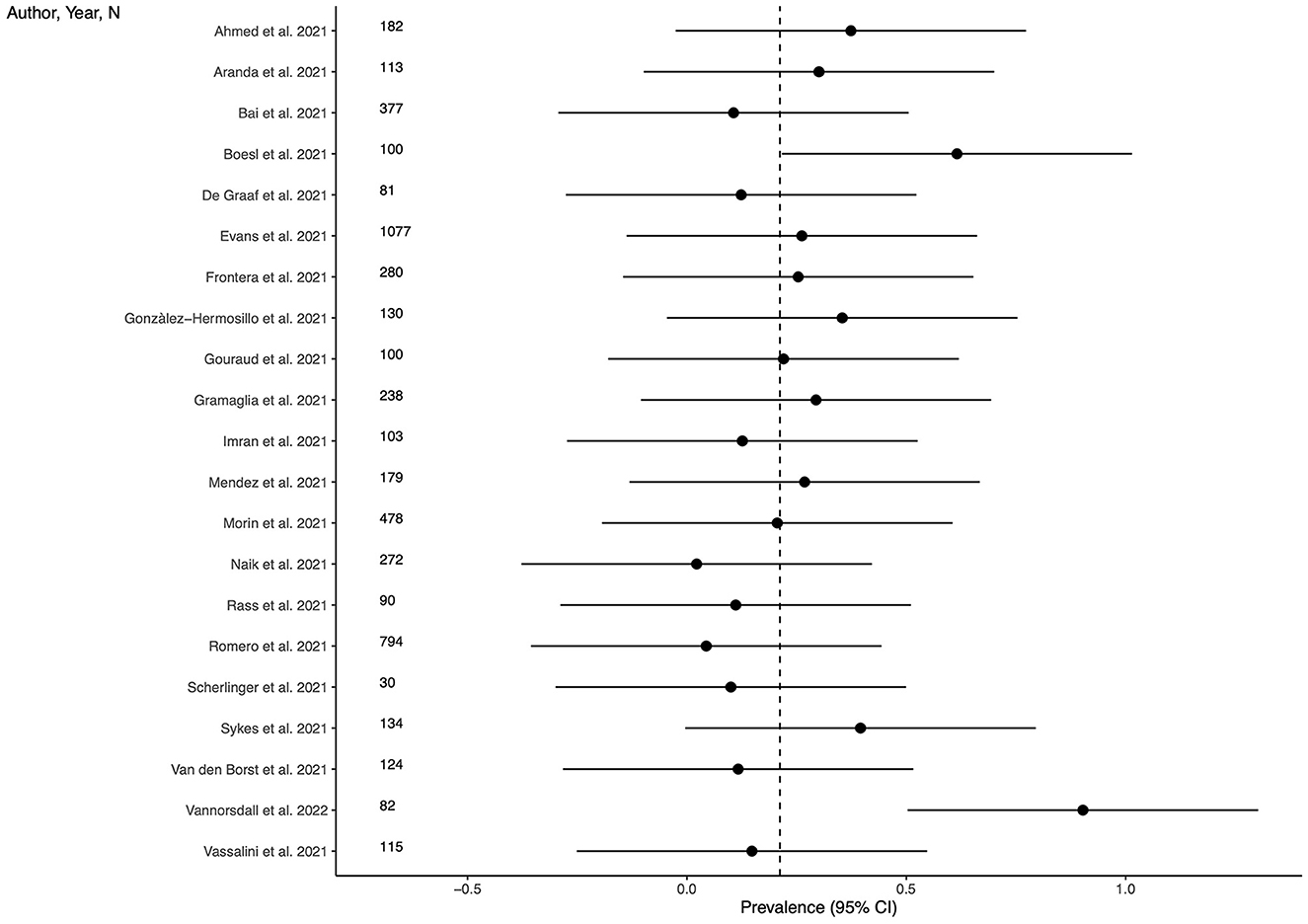
Figure 2. Prevalence of depression across the studies and weighted mean prevalence. The vertical dotted line represents the weighted mean prevalence.
Prevalence of anxiety
Twenty-three studies (69.7%) provided outcome data for anxiety among LC patients. The weighted mean prevalence across the studies was 0.158 and was markedly influenced by the study from Taquet et al. (35) with a far larger sample size. Unweighted mean and median prevalence were 0.313 (range: 0.029–0.646) and 0.296, respectively. Figure 3 shows comparison of the anxiety prevalence estimates across the studies, and the weighted mean prevalence.
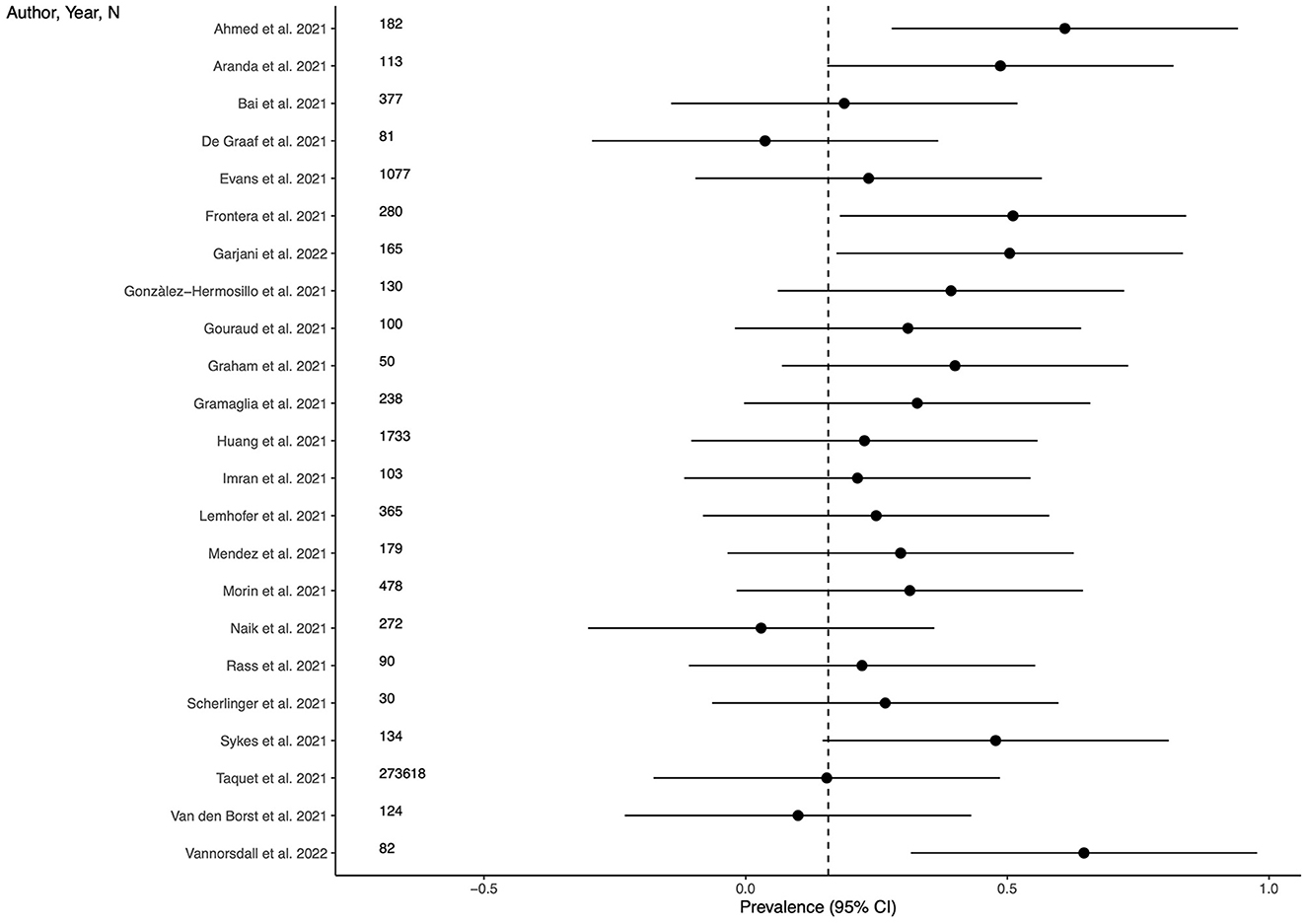
Figure 3. Prevalence of anxiety across the studies and weighted mean prevalence. The vertical dotted line represents the weighted mean prevalence.
Prevalence of cognitive impairment
Sixteen studies (48.5%) provided outcome data for cognitive impairment among LC patients. The weighted mean prevalence across the studies was 0.042 and, again, was markedly influenced by the study from Taquet et al. (35) with the largest sample size and providing among the three lowest estimates of anxiety prevalence. Unweighted mean and median prevalence were 0.269 (range: 0.005–0.820) and 0.227, respectively. Figure 4 shows comparison of the cognitive impairment prevalence estimates across the studies, and the weighted mean prevalence.
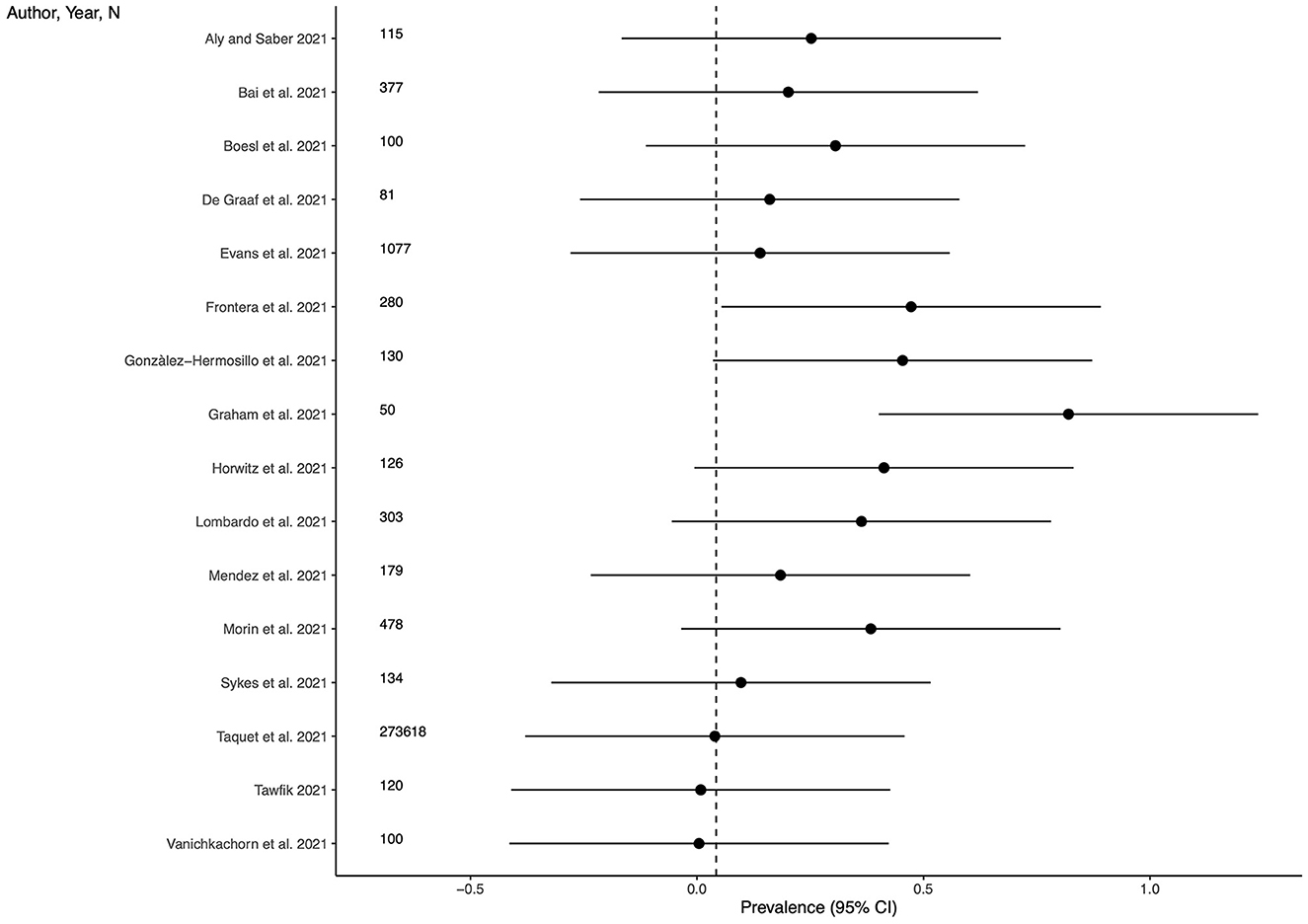
Figure 4. Prevalence of cognitive impairment across the studies and weighted mean prevalence. The vertical dotted line represents the weighted mean prevalence.
Prevalence of PTS
Thirteen studies (39.4%) provided outcome data for PTS among LC patients. The weighted mean prevalence across the studies was 0.192. Unweighted mean and median prevalence were 0.218 (range: 0.058–0.788) and 0.130, respectively. Figure 5 shows comparison of the PTS prevalence estimates across the studies, and the weighted mean prevalence.
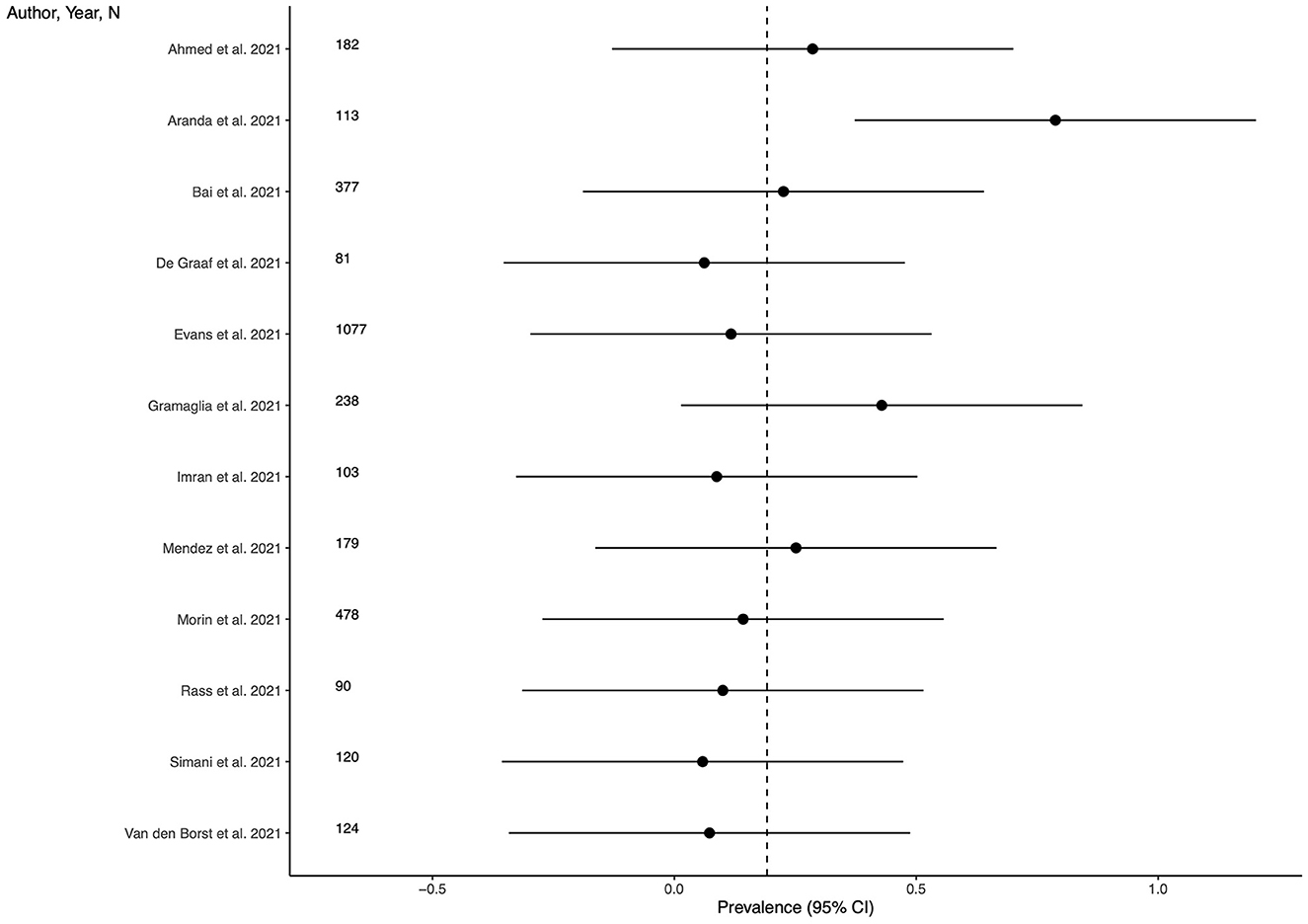
Figure 5. Prevalence of post-traumatic symptoms across the studies and weighted mean prevalence. The vertical dotted line represents the weighted mean prevalence.
Prevalence of sleep disturbances
Eighteen studies (54.5%) provided outcome data for sleep disturbances among LC patients. The weighted mean prevalence across the studies was 0.270. Unweighted mean and median prevalence were 0.296 (range: 0.003–0.648) and 0.319, respectively. Figure 6 shows comparison of the sleep disturbances prevalence estimates across the studies, and the weighted mean prevalence.
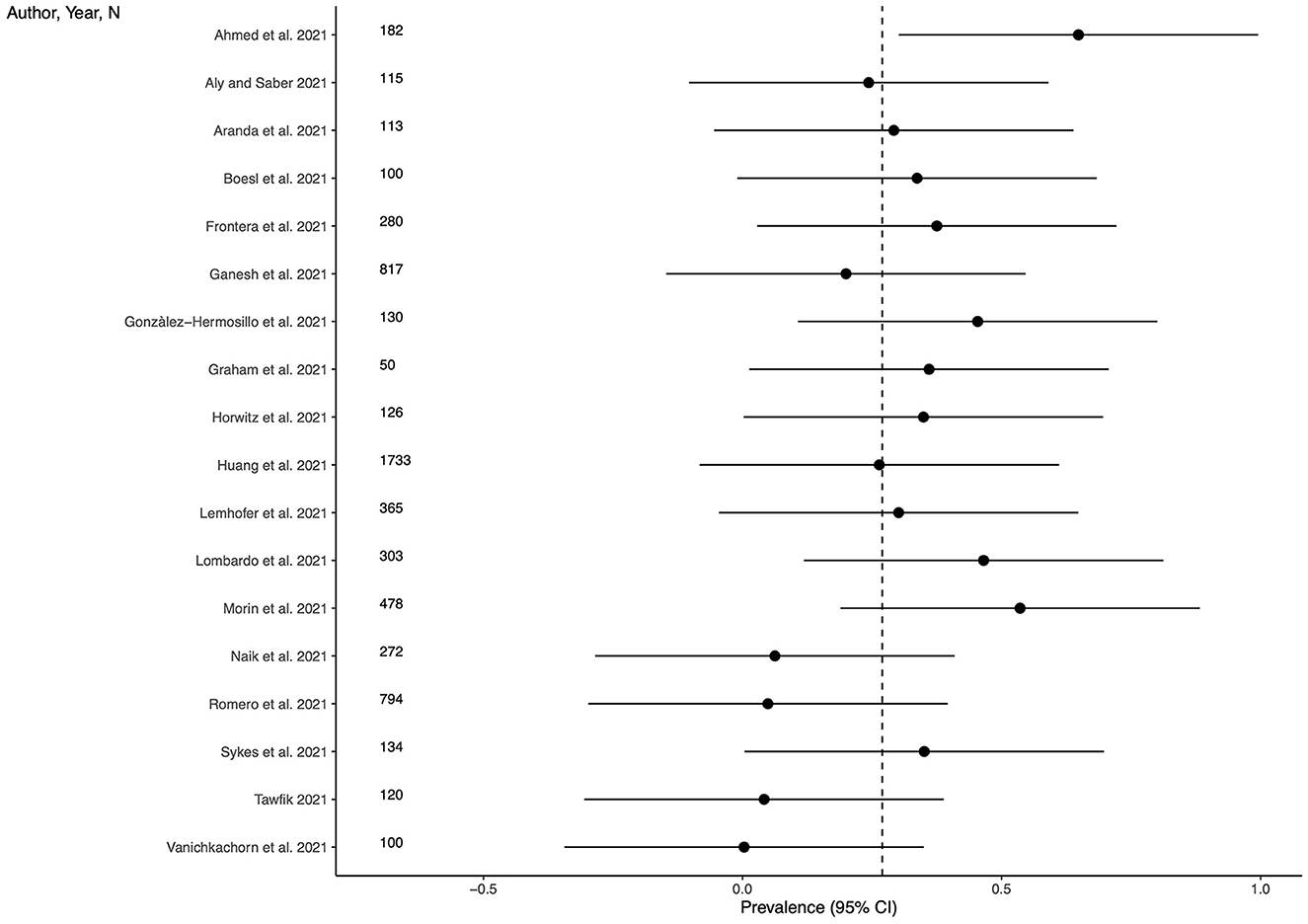
Figure 6. Prevalence of sleep disturbances across the studies and weighted mean prevalence. The vertical dotted line represents the weighted mean prevalence.
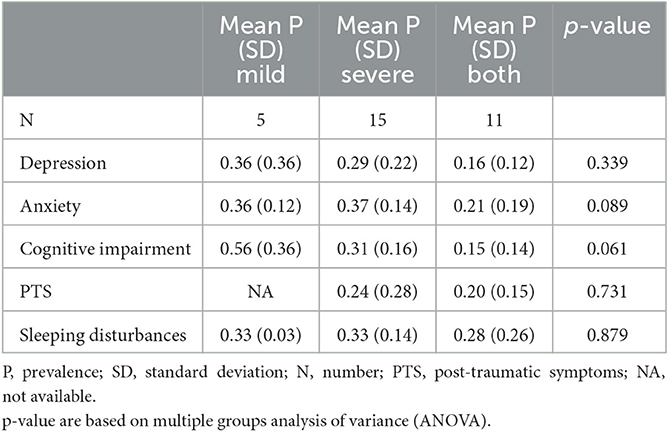
Psychiatric symptoms by COVID-19 infection severity
We examined the potential association between the severity of COVID-19 infection and the occurrence of psychiatric symptoms. For this purpose, we considered hospitalization as a proxy for severe infection, and outpatient management as indicator of mild infection. As shown in Table 3, the comparison of the severe, mild, and both mild and severe groups in terms of average prevalence of psychiatric symptoms did not find any statistically significant difference, suggesting that the severity of the infection is not related to the development of later psychiatric symptoms. Further analysis was conducted to compare only mild and severe patients, with the severe group consisting of studies that included at least one hospitalized patient. This analysis confirmed that there were no statistically significant differences in depression, anxiety, PTS, and sleep disturbances, but found inverse association between the severity of the infection and cognitive complaints (p = 0.048).
Risk of bias and GRADE
A detailed summary on the risk of bias in all 33 trials has been reported in the Appendix (see Supplementary Figures 1, 2), along with an assessment of the quality of the evidence (see Supplementary Table 2). In the GRADE system, the evidence from observational studies is initially set to low, there are then criteria that can be used either to downgrade or upgrade (see further information in the Appendix). The quality of the evidence is rated very low with serious threats related to the risk of bias and inconsistency.
Discussion
This study set out to investigate the prevalence of psychiatric symptoms among LC patients. We found that the symptoms mostly associated with LC were depression, anxiety, cognitive and sleep disturbances, and PTS. The prevalence of these symptoms among LC patients is remarkably higher than that in the general population.
However, it is necessary to underline that, among the studies included in the final selection, there was one study (35) that had a sample size accounting for around 96% of the total number of participants included in this review. That study provided estimates for anxiety and cognitive impairments and the weighted average for these outcomes falls exactly on the value of the prevalence estimated by Taquet et al. (35) by looking at the forest plots of anxiety and cognitive impairment, it can be easily observed that only three studies for anxiety and two studies for cognitive impairment provided estimates smaller than Taquet et al. (35) supporting its influence on the pooled prevalence. Accordingly, for these two outcomes, the raw mean resulted higher than the weighted mean, because the former is not affected by the differences in the sample size across the studies.
Nevertheless, the relatively high pooled prevalence of psychiatric symptoms among LC patients requires a better understanding. Our analyses did not find a significant association between the severity of COVID-19 infection and psychiatric symptoms, except for a potential inverse association with symptoms of cognitive impairment. These findings align with the conclusions drawn from most of the reports included in our systematic review, which examined the relationship between infection severity and psychiatric symptoms (6, 17, 20, 23, 27, 34, 40). Notably, only one study reported an increased risk of depression and anxiety among individuals with the most severe form of infection (8). However, it is important to acknowledge that the confidence in the results is limited by the comparatively small representation of patients with mild infection, leading to low statistical power.
It should be noted that research in the pre-COVID-19 era observed that survivors after intensive care (IC) are at greater risk of developing long-term mental disorders (47). Particularly, anxiety, depression, and PTSD would have occurred in half of this sample of UK patients discharged from IC. Psychiatric symptoms of the post-IC syndrome fall into three broad categories: physical, cognitive, and psychological deterioration. Symptoms of physical deterioration include fatigue and insomnia, while cognitive and psychiatric symptoms include anxiety, depression, memory impairment, and PTSD. Therefore, there seems to be a significant overlap in the experience of some of the LC patients analyzed in this review with post-IC syndrome.
A considerable amount of COVID-related research also focused on the effect on mental health of public health measures (such as quarantine, lock-down, social isolation and other limitations to personal freedom), finding an association with symptoms of depression, anxiety, loneliness, psychosocial distress, and persisting post-traumatic arousal (48, 49). Therefore, another possible explanation for the higher prevalence of psychiatric symptoms among LC patients may be more a consequence of the imposed quarantine and other restrictions in terms of anxiety, fear, anger, and other negative emotions, regardless of specific aspects of the COVID-19 infection such as neuro- or systemic inflammation. Even if the quarantine imposed by a local health authority has not been directly associated with any psychological outcomes (50), it was suggested that belonging to a publicly recognized COVID-19 risk group/community would be associated with increased anxiety, depressive symptoms, self-concern, fear, increased psychosocial distress, and decreased life satisfaction. In addition, loneliness and isolation have been associated with an increased risk for various mental disorders (as well as for various somatic diseases). In the context of the COVID-19 pandemic, loneliness has been found to be predictive of depressive and anxious symptoms during the lockdown measures (51, 52).
COVID-19 is in fact only the most recent of many other infectious diseases that have been associated with chronic sequelae after recovering from the acute phase of infection (53): similarly as with LC, the underlying pathophysiological and etiological mechanisms are far from being clearly understood. The review by Choutka et al. (53) investigated the common characteristics between LC and other chronic infectious syndromes, finding higher prevalence of the following symptoms: intolerance to physical effort, neurocognitive and sensory impairment, persistent flu-like symptoms, disturbed sleep, myalgias, and arthralgias. The greatest analogies are with the post-acute effects described in the SARS epidemic in 2002–2004 (54).
All these elements are probably interrelated with each other and influential in the experience of LC patients.
Limitations
The results of this review should be interpreted considering its limitations. First, the lack of a control group made difficult to draw considerations on the risk of psychiatric symptoms among LC patients, reducing considerably generalizability and reliability of our findings. This translated also in the impossibility to meta-analyze the results of the selected studies to detail the risk of psychiatric symptoms in patients who have had COVID-19. Second, most of the included studies reported measures of psychiatric symptoms instead of assessing psychiatric diagnoses, with a risk for diagnostic overestimation: this was partially attenuated by including only studies applying validated psychometric tools or clinical interviews. Third, there were a marked outlier effect played by one study (35), which implemented a sample size accounting for more than 90% of the total sample size. Even though that study resulted at low risk of bias in the assessment, it may have impacted on the pooled prevalence estimate of the outcomes reported in that study. Fourth, the condition of LC has been assessed only through a temporal criterion, that was 4 weeks after recovery from the infection. The lack of a more comprehensive definition of LC may have increase the heterogeneity in the estimates. Finally, the risk of bias was rated high or unclear in many studies, with serious threats related to the inconsistency in the estimates and to the assessment of confounders.
Implications for research and clinical practice
The overlapping of some clinical features of LC in terms of signs and symptoms with other post-infectious syndromes and with the post-IC syndrome would suggest the involvement of shared pathophysiological pathways. The perspective of identifying a unified etiological model would lead the way toward the implementation of diagnostic markers and tailored treatments (53). At present, however, our understanding of the underlying pathophysiological mechanisms and etiological factors is poor, though promising studies are being conducted (55–57). For example, a recent review advanced the hypothesis that perivascular inflammation serves as the critical pathogenetic factor for LC neuropsychiatric manifestations (58). Indeed, SARS-CoV-2 and other viruses (such as retrovirus) showed the ability to activate brain mast cells and microglia resulting in the release of inflammatory, neurotoxic, and vasoactive mediators impacting neuronal connectivity and signal transmission (59–61). Hopefully, that may also converge to a better definition of functional and psycho-somatic syndromes, such as fibromyalgia and chronic fatigue syndrome, for which the association with viral infections has been previously proposed (62–67).
More research is therefore needed, more clearly comparing different patient groups (e.g., LC patients that were admitted to ICU vs. other ICU patients with or without other infectious diseases; LC patients vs. patients remitting from other infectious diseases) and applying prospective designs, allowing causal considerations, and providing more epidemiological details. Also, qualitative studies investigating the subjective experience of people recovered from COVID-19 are being conducted (68): this approach may also contribute to the understanding of the psychological mechanism contributing to the onset of psychological symptoms. Such different research methods could converge on a better conceptualization and analysis of the symptoms associated with the LC syndrome, as well as supporting the construction of a better defined and unified nomenclature.
A better understanding of the LC psycho-pathophysiology is essential to provide and improve treatment. From a therapeutic point of view, in close relation both to the traumatic component of a part of the symptoms found in LC, and to the inflammatory component (initially exerted by the infection and then self-sustained), interventions aimed at reducing the inflammatory process and reducing the excessive activation of the sympathetic nervous system through a relaxation response may be useful. For example, models of intervention involving reconditioning and mindfulness may help patients suffering from LC (69). Future clinical trials on LC patients may be therefore warranted.
Conclusions
People who have recovered from COVID-19 may experience more and persistent psychiatric symptoms. These include depression, anxiety, post-traumatic distress, cognitive and sleeping disturbances. However, there is marked heterogeneity in the literature about how these symptoms are investigated and differentiated from other post-infectious or post-hospitalization conditions. More research, particularly implementing control groups and prospective follow-up, are needed to better define psychopathology related or included into the LC syndrome.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MM, PG, and VS: conceptualization and planning and interpretation of the results. PG, VS, FC, FR, and PM: acquisition. MM: analysis of the data. MM, PG, VS, FC, FR, and PM: drafting. SF, LP, and GG: critical revision of the manuscript. All authors approved the final submitted version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1138389/full#supplementary-material
References
1. Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection. (2021) 49:1163–86. doi: 10.1007/s15010-021-01666-x
2. Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. (2021) 6:e005427. doi: 10.1136/bmjgh-2021-005427
3. De Graaf MA, Antoni ML, Kuile MM, Arbous MS, Duinisveld AJF, Feltkamp MCW, et al. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. eClinicalMedicine. (2021) 32:25. doi: 10.1016/j.eclinm.2021.100731
4. Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. (2021) 9:1275–87. doi: 10.1016/S2213-2600(21)00383-0
5. Fiore G, Ferrari S, Cutino A, Giorgino C, Valeo L, Galeazzi GM, et al. Delirium in COVID-19 and post-liver transplant patients: an observational study. Int J Psychiatry Clin Pract. (2022) 26:343–51. doi: 10.1080/13651501.2022.2026403
6. Gramaglia C, Gambaro E, Bellan M, Balbo PE, Baricich A, Sainaghi PP, et al. Mid-term psychiatric outcomes of patients recovered from COVID-19 from an italian cohort of hospitalized patients. Front Psychiatry. (2021) 12:667385. doi: 10.3389/fpsyt.2021.667385
7. Horwitz LI, Garry K, Prete AM, Sharma S, Mendoza F, Kahan T, et al. Six-month outcomes in patients hospitalized with severe COVID-19. J Gen Intern Med. (2021) 36:3772–7. doi: 10.1007/s11606-021-07032-9
8. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2021) 397:220–32. doi: 10.1016/S0140-6736(20)32656-8
9. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
10. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M. Cochrane Handbook for Systematic Reviews of Interventions. version 6.2. Cochrane (2021). Available online at: www.training.cochrane.org/handbook. (accessed January 28, 2022).
11. RStudio Team. RStudio: Integrated Development Environment for R. (2021) Available online at: http://www.rstudio.com/
12. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Schünemann H, Brozek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence Strength of Recommendations. (2013). Available online at: https://gdt.gradepro.org/app/handbook/handbook.html (accessed July 6, 2022).
14. Ahmed GK, Khedr EM, Hamad DA, Meshref TS, Hashem MM, Aly MM. Long term impact of Covid-19 infection on sleep and mental health: a cross-sectional study. Psychiatry Res. (2021) 305:114243. doi: 10.1016/j.psychres.2021.114243
15. Aly MAEG, Saber HG. Long COVID and chronic fatigue syndrome: a survey of elderly female survivors in Egypt. Int J Clin Pract. (2021) 75:e14886. doi: 10.1111/ijcp.14886
16. Aranda J, Oriol I, Martín M, Feria L, Vázquez N, Rhyman N, et al. Long-term impact of COVID-19 associated acute respiratory distress syndrome. J Infect. (2021) 83:581–8. doi: 10.1016/j.jinf.2021.08.018
17. Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. (2022) 28:611. doi: 10.1016/j.cmi.2021.11.002
18. Boesl F, Audebert H, Endres M, Prüss H, Franke C. A neurological outpatient clinic for patients with post-COVID-19 syndrome—a report on the clinical presentations of the first 100 patients. Front Neurol. (2021) 12:405. doi: 10.3389/fneur.2021.738405
19. Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, Arena V, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. (2021) 426:7486. doi: 10.1016/j.jns.2021.117486
20. Ganesh R, Ghosh AK, Nyman MA, Croghan IT, Grach SL, Anstine CV, et al. Scales for assessment of persistent post-COVID symptoms: a cross sectional study. J Prim Care Community Health. (2021) 12:21501327211030412. doi: 10.1177/21501327211030413
21. Garjani A, Middleton RM, Nicholas R, Evangelou N. Recovery from COVID-19 in multiple sclerosis: a prospective and longitudinal cohort study of the united kingdom multiple sclerosis register. Neurol—Neuroimmunol Neuroinflamm. (2022) 9:1118. doi: 10.1212/NXI.0000000000001118
22. González-Hermosillo JA, Martínez-López JP, Carrillo-Lampón SA, Ruiz-Ojeda D, Herrera-Ramírez S, Amezcua-Guerra LM, et al. del R Post-Acute COVID-19 Symptoms, a Potential Link with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A 6-Month Survey in a Mexican. Cohort Brain Sci. (2021) 11:760. doi: 10.3390/brainsci11060760
23. Gouraud C, Bottemanne H, Lahlou-Laforêt K, Blanchard A, Günther S, Batti SE, et al. association between psychological distress, cognitive complaints, and neuropsychological status after a severe COVID-19 episode: a cross-sectional study. Front Psychiatr. (2021) 12:861. doi: 10.3389/fpsyt.2021.725861
24. Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann Clin Transl Neurol. (2021) 8:1073–85. doi: 10.1002/acn3.51350
25. Imran J, Nasa P, Alexander L, Upadhyay S, Alanduru V. Psychological distress among survivors of moderate-to-critical COVID-19 illness: a multicentric prospective cross-sectional study. Indian J Psychiatry. (2021) 63:285–9. doi: 10.4103/psychiatry.IndianJPsychiatry_1074_20
26. Lemhöfer C, Sturm C, Loudovici-Krug D, Best N, Gutenbrunner C. The impact of Post-COVID-syndrome on functioning—results from a community survey in patients after mild and moderate SARS-CoV-2-infections in Germany. J Occup Med Toxicol. (2021) 16:45. doi: 10.1186/s12995-021-00337-9
27. Lombardo MDM, Foppiani A, Peretti GM, Mangiavini L, Battezzati A, Bertoli S, et al. Long-Term coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open Forum Infect Dis. (2021) 8:ofab384. doi: 10.1093/ofid/ofab384
28. Méndez R, Balanzá-Martínez V, Luperdi SC, Estrada I, Latorre A, González-Jiménez P, et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. (2021) 290:621–31. doi: 10.1111/joim.13262
29. Naik S, Haldar SN, Soneja M, Mundadan NG, Garg P, Mittal A, et al. Post COVID-19 sequelae: a prospective observational study from Northern India. Drug Discov Ther. (2021) 15:254–60. doi: 10.5582/ddt.2021.01093
30. Rass V, Ianosi B-A, Zamarian L, Beer R, Sahanic S, Lindner A, et al. Factors associated with impaired quality of life three months after being diagnosed with COVID-19. Qual Life Res. (2022) 31:1401–14. doi: 10.1007/s11136-021-02998-9
31. Romero-Duarte Á, Rivera-Izquierdo M, Guerrero-Fernández de Alba I, Pérez-Contreras M, Fernández-Martínez NF, Ruiz-Montero R, et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. (2021) 19:129. doi: 10.1186/s12916-021-02003-7
32. Scherlinger M, Felten R, Gallais F, Nazon C, Chatelus E, Pijnenburg L, et al. Refining “Long-COVID” by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection. Infect Dis Ther. (2021) 10:1747–63. doi: 10.1007/s40121-021-00484-w
33. Simani L, Ramezani M, Darazam IA, Sagharichi M, Aalipour MA, Ghorbani F, et al. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol. (2021) 27:154–9. doi: 10.1007/s13365-021-00949-1
34. Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. (2021) 199:113–9. doi: 10.1007/s00408-021-00423-z
35. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. (2021) 18:e1003773. doi: 10.1371/journal.pmed.1003773
36. Tawfik HM, Shaaban HM, Tawfik AM. Post-COVID-19 syndrome in Egyptian healthcare staff: highlighting the carers sufferings. Electron J Gen Med. (2021) 18:em291. doi: 10.29333/ejgm/10838
37. Morin L. The writing committee for the COMEBAC study group: four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. (2021) 325:1525–34. doi: 10.1001/jama.2021.3331
38. van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin Infect Dis Off Publ Infect Dis Soc Am. (2021) 73:e1089–98. doi: 10.1093/cid/ciaa1750
39. Vanichkachorn G, Newcomb R, Cowl CT, Murad MH, Breeher L, Miller S, et al. Post–COVID-19 syndrome (Long Haul Syndrome): description of a multidisciplinary clinic at mayo clinic and characteristics of the initial patient cohort. Mayo Clin Proc. (2021) 96:1782–91. doi: 10.1016/j.mayocp.2021.04.024
40. Vannorsdall TD, Brigham E, Fawzy A, Raju S, Gorgone A, Pletnikova A, et al. Cognitive dysfunction, psychiatric distress, and functional decline after COVID-19. J Acad Consult-Liaison Psychiatry. (2022) 63:133–43. doi: 10.1016/j.jaclp.2021.10.006
41. Vassalini P, Serra R, Tarsitani L, Koukopoulos AE, Borrazzo C, Alessi F, et al. Depressive symptoms among individuals hospitalized with COVID-19: three-month follow-up. Brain Sci. (2021) 11:1175. doi: 10.3390/brainsci11091175
42. CDC C for DC P. Subjective Cognitive Decline—A Public Health Issue. (2019). Available online at: https://www.cdc.gov/aging/data/subjective-cognitive-decline-brief.html (accessed December 22, 2022).
43. Dattani S, Ritchie H, Roser M. Mental Health. Our World Data. (2021). Available online at: https://ourworldindata.org/mental-health (accessed December 22, 2022).
44. NIMH NI of MH. Post-Traumatic Stress Disorder (PTSD). Natl Inst Ment Health NIMH. (2022) Available online at: https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd (accessed December 22, 2022).
45. Stickley A, Leinsalu M, DeVylder JE, Inoue Y, Koyanagi A. Sleep problems and depression among 237 023 community-dwelling adults in 46 low- and middle-income countries. Sci Rep. (2019) 9:12011. doi: 10.1038/s41598-019-48334-7
46. WHO WHO. Depression. (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/depression (accessed December 22, 2022).
47. Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, depression and post traumatic stress disorder after critical illness: a UK-wide prospective cohort study. Crit Care Lond Engl. (2018) 22:310. doi: 10.1186/s13054-018-2223-6
48. Benke C, Autenrieth LK, Asselmann E, Pané-Farré CA. Lockdown, quarantine measures, and social distancing: Associations with depression, anxiety and distress at the beginning of the COVID-19 pandemic among adults from Germany. Psychiatry Res. (2020) 293:113462. doi: 10.1016/j.psychres.2020.113462
49. Mastroberardino M, Cuoghi Costantini R, De Novellis AMP, Ferrari S, Filippini C, Longo F, et al. “It's All COVID's Fault!”: symptoms of distress among workers in an italian general hospital during the pandemic. Int J Environ Res Public Health. (2022) 19:7313. doi: 10.3390/ijerph19127313
50. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. (2020) 89:531–42. doi: 10.1016/j.bbi.2020.05.048
51. Loades ME, Chatburn E, Higson-Sweeney N, Reynolds S, Shafran R, Brigden A, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatr. (2020) 59:1218-1239. doi: 10.1016/j.jaac.2020.05.009
52. Marchi M, Magarini FM, Chiarenza A, Galeazzi GM, Paloma V, Garrido R, et al. Experience of discrimination during COVID-19 pandemic: the impact of public health measures and psychological distress among refugees and other migrants in Europe. BMC Public Health. (2022) 22:942. doi: 10.1186/s12889-022-13370-y
53. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. (2022) 28:911–23. doi: 10.1038/s41591-022-01810-6
54. Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. (2020) 7:611–27. doi: 10.1016/S2215-0366(20)30203-0
55. Balcom EF, Nath A, Power C. Acute and chronic neurological disorders in COVID-19: potential mechanisms of disease. Brain J Neurol. (2021) 144:3576–88. doi: 10.1093/brain/awab302
56. Evans RA, Leavy OC, Richardson M, Elneima O, McAuley HJC, Shikotra A, et al. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. (2022) 10:761–75. doi: 10.1016/S2213-2600(22)00127-8
57. Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12:698169. doi: 10.3389/fmicb.2021.698169
58. Theoharides TC, Kempuraj D. Role of SARS-CoV-2 spike-protein-induced activation of microglia and mast cells in the pathogenesis of neuro-COVID. Cells. (2023) 12:688. doi: 10.3390/cells12050688
59. Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. (2021) 112:217–26. doi: 10.1016/j.ijid.2021.09.043
60. Song S-T, Wu M-L, Zhang H-J, Su X, Wang J-H. Mast cell activation triggered by retrovirus promotes acute viral infection. Front Microbiol. (2022) 13:8660. doi: 10.3389/fmicb.2022.798660
61. Abraham SN, John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. (2010) 10:440–52. doi: 10.1038/nri2782
62. Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, et al. Dubbo infection outcomes study group, Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. (2006) 333:575. doi: 10.1136/bmj.38933.585764.AE
63. Hunskar GS, Rortveit G, Litleskare S, Eide GE, Hanevik K, Langeland N, et al. Prevalence of fibromyalgia 10 years after infection with Giardia lamblia: a controlled prospective cohort study. Scand J Pain. (2022) 22:348–55. doi: 10.1515/sjpain-2021-0122
64. Patel H, Sander B, Nelder MP. Long-term sequelae of West Nile virus-related illness: a systematic review. Lancet Infect Dis. (2015) 15:951–9. doi: 10.1016/S1473-3099(15)00134-6
65. Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, Sebastian Hurtado-Zapata J. Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res. (2016) 68:1849–58. doi: 10.1002/acr.22900
66. The PREVAIL III. Study group: a longitudinal study of Ebola Sequelae in Liberia. N Engl J Med. (2019) 380:924–34. doi: 10.1056/NEJMoa1805435
67. White PD, Thomas JM, Amess J, Crawford DH, Grover SA, Kangro HO, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry J Ment Sci. (1998) 173:475–81. doi: 10.1192/bjp.173.6.475
68. Macpherson K, Cooper K, Harbour J, Mahal D, Miller C, Nairn M. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. (2022) 12:e050979. doi: 10.1136/bmjopen-2021-050979
Keywords: Long-COVID syndrome, COVID-19, mental health, depression, anxiety, posttraumatic stress
Citation: Marchi M, Grenzi P, Serafini V, Capoccia F, Rossi F, Marrino P, Pingani L, Galeazzi GM and Ferrari S (2023) Psychiatric symptoms in Long-COVID patients: a systematic review. Front. Psychiatry 14:1138389. doi: 10.3389/fpsyt.2023.1138389
Received: 05 January 2023; Accepted: 29 May 2023;
Published: 21 June 2023.
Edited by:
Zonglin He, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaReviewed by:
Theoharis Constantin Theoharides, Nova Southeastern University, United StatesRenata Kochhann, Hospital Moinhos de Vento, Brazil
Māris Taube, Riga Stradinš University, Latvia
Alberto Spalice, Sapienza University of Rome, Italy
Copyright © 2023 Marchi, Grenzi, Serafini, Capoccia, Rossi, Marrino, Pingani, Galeazzi and Ferrari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gian Maria Galeazzi, Z2lhbm1hcmlhLmdhbGVhenppQGF1c2wucmUuaXQ=
 Mattia Marchi
Mattia Marchi Pietro Grenzi1
Pietro Grenzi1 Luca Pingani
Luca Pingani Gian Maria Galeazzi
Gian Maria Galeazzi Silvia Ferrari
Silvia Ferrari