
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 15 May 2023
Sec. Psychological Therapy and Psychosomatics
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1135590
This article is part of the Research Topic Psychiatric Comorbidities in Patients with Epilepsy: Diagnosis and treatment. View all 8 articles
Functional seizures (FS) are seizure-like symptoms without electroencephalogram (EEG)-based epileptic activity. Those with FS often show emotion-related dysfunction and disrupted interpersonal relationships, in which posttraumatic stress disorder symptoms (PTS) may play a role. We sought to better understand trauma comorbidities and socioemotional processes in FS, including affectionate touch, a form of social connection linked to emotion regulation and awareness. We administered questionnaires online to a community sample of 89 trauma-exposed FS participants (FS diagnoses were self-reported), 51 with and 38 without clinical-level PTS (FS-PTShi, FS-PTSlo) and 216 seizure-free matched trauma-exposed controls (TCs), 91 with and 125 without clinical-level PTS (TC-PTShi, TC-PTSlo) per the Posttraumatic Stress Disorder Symptom Checklist (PCL). As hypothesized, both FS-PTShi and FS-PTSlo reported more emotional avoidance (Brief Experiential Avoidance Questionnaire), more emotion regulation difficulties (Difficulties in Emotion Regulation Scale), and more perceived stress (Perceived Stress Scale) than PTS-matched counterparts. FS-PTShi also reported less reappraisal (Emotion Regulation Questionnaire), more loneliness (UCLA Loneliness Scale), and less frequent affectionate touch (Physical Affection Scale) during waking and surrounding sleep than TC-PTShi, whereas FS-PTSlo and TC-PTSlo did not differ. Neither FS group differed from PTS-matched controls in emotion suppression (Emotion Regulation Questionnaire) or comfort with social touch (Social Touch Questionnaire). Among FS, FS-PTShi reported more difficulties than FS-PTSlo on nearly all measures (non-significant trend for social support). Findings underscore potential synergistic effects of FS and PTS clinical symptoms in shaping experiences of one’s emotions and social world, suggesting fostering meaningful connections with others, including via affectionate touch, is an important treatment target.
Functional seizures (FS) resemble epileptic seizures behaviorally, but occur without concurrent electroencephalographic (EEG) epileptiform activity (1). This condition, also called psychogenic non-epileptic seizures (PNES), falls under the larger category of functional neurological disorders [FND; (2)], while specifically categorized as a “dissociative neurological symptom disorder” in ICD-11 (3) or “conversion disorder (functional neurological symptom disorder)” in DSM-5 [(4); see also (5, 6)]. As diagnostic and clinical entities, FND are now understood not only in terms of absence of expected neurological indicators, but also as conditions with known and emerging biopsychosocial correlates; for example, emotion processing disruptions are important vulnerabilities, and bodily states may be interpreted or categorized/labeled as somatic rather than affective [models in FND, (7–11); in FS, (12)]. FS is associated with high prevalence of trauma, especially violence exposure and experiences (5, 13, 14); this is a predisposing vulnerability to FS and its social and emotional correlates (see below). Alongside emotion processing-related challenges (15, 16), those with FS may experience conflictual interpersonal relationships (17, 18) and limitations in social functioning due to not working or driving, or concerns about having an FS episode (19). Interpersonal difficulties impact patients’ personal and family life (20), with implications for patient-provider communication (21). Nevertheless, FS individuals describe strong social engagement and desire to be with others (22), reporting larger social networks than individuals with other motor FNDs (23). We investigated experiences in FS of emotion regulation and social connection, including affectionate touch, while aiming to disentangle possible influences of traumatic stress symptoms and mental health comorbidities.
Emotion regulation can be viewed as a multi-faceted, dynamic process, beginning with deliberate or implicit decisions to engage versus avoid emotional situations, to reappraising situations to lessen their emotional impact, through outwardly expressing versus suppressing emotions [i.e., process model of emotion regulation; (24, 25)]. Emotion regulation difficulties can be operationalized as perceived (in)ability to handle feelings of upset (26); difficulties attending to or being aware of feelings is a related but separate facet (27). A review (28) examining studies of FS and emotion through a process model lens found that trouble identifying and describing emotions, and tendency to avoid emotions, were consistent features of FS—perhaps more so than for other FND [e.g., greater alexithymia in FS than functional movement disorder; (29)]—whereas extent of emotion regulation difficulties was less consistent, reflecting heterogeneity among emotion-related domains and within FS [e.g., high and low emotion dysregulation subgroups; (30, 31)].
Emotion regulation in FS in social contexts has received relatively less attention. Because emotions and emotion regulation often unfold within relationships, “co-regulation,” or affect (dys)regulation via other people, is important (32). Furthermore, in both clinical and community samples, feelings of belonging and social connection (or its lack, namely loneliness), can profoundly impact morbidity and mortality (33, 34). In FS, quality relationships (e.g., friendships) predict better prognosis (35) yet may be compromised by FS-related interpersonal distress [e.g., feeling invalidated or dismissed (36)] or struggles processing socio-emotional information (37). The physical component of social connection—affectionate touch—can itself regulate affect and bridge self-and other regulation (38). To our knowledge, this remains unexplored in FS.
As noted above, previous studies of FS have found alterations in socioemotional regulation processes. These FS-linked alterations include greater difficulties with emotional awareness and regulation (39), heightened preconscious attention to social threat (37), greater anxious arousal and other clinical symptoms (40) and related physiological correlates [e.g., lower high-frequency heart rate variability (41, 42), higher cortisol (37)]; yet, these in large part are accounted for in the studies cited by interpersonal trauma and posttraumatic stress reactions. In FS and other FND, adverse life events such as early childhood maltreatment are associated with difficulty forming trusting social relationships (43), and trauma and posttraumatic stress are related to lower social network size [and associated neural correlates; (23)]. Understanding socioemotional processes in FS necessitates considering links with frequently-comorbid conditions (e.g., posttraumatic stress disorder [PTSD]) that also are characterized by socioemotional dysfunction (44–46). In previous research, for example, subgroups of FS individuals with versus without PTSD symptoms showed worse socioemotional and symptom profiles (47–49). In other studies, emotion regulation difficulties were not uniformly greater in FS than in a trauma control group with clinical levels of PTSD symptoms (41, 42). This underscores the need to consider areas of overlap and distinction among potential FS subgroups, in addition to comparing with appropriate control groups.
In the present study, therefore, we investigated whether self-reports of emotion-related processes and interpersonal experiences suggested more problems among FS than controls after accounting for prior trauma exposure and posttraumatic stress reactions; we did this by matching FS groups based on PTS symptoms and overall mental health. We included measures that captured different kinds of affect regulation strategies (24–26, 50) and that reflected self-and co-regulation of affect. We assessed not only emotional avoidance, awareness, and regulation difficulties and tendencies, but also sense of connection to others psychologically and physical connection in social and partner relationships. We were especially interested in affectionate touch (including touch surrounding sleep, or “sleep-touch”), which is linked to affect regulation and relationship closeness (51–53), social and health benefits (38, 54), and emotional and somatic/body awareness (55), which are disrupted in FS (56).
We compared those with FS to seizure-free trauma-exposed controls (TCs) matched in posttraumatic stress symptom (PTS) levels, either at or above (PTShi) versus below (PTSlo) a clinical cut-off. We hypothesized that (H1) FS with high PTS versus controls with high PTS, (H2) FS with low PTS versus controls with low PTS, and (H3) FS with high PTS versus FS with low PTS would report more difficulties on indicators of socioemotional regulation.
Participants were 305 individuals with prior trauma: 89 with FS and 216 TCs. Inclusion requirements were as follows: age 18 or more years; for FS, self-reported diagnosis of FS (see below); for TC, self-reported exposure to traumatic event(s) and no prior seizures/seizure-like symptoms. We separated participants into “PTShi” and “PTSlo” subgroups using PTSD symptom scores (see below), yielding four groups: FS-PTShi (n = 51), FS-PTSlo (n = 38), TC-PTShi (n = 91), and TC-PTSlo (n = 125). Sample characteristics are in Table 1. Trauma characteristics are in Supplementary Tables 1, 2.
FS participants were recruited via partnering neurologists/neuropsychologists and websites/social media [e.g., FNDHope.org, NEAD.org (nonepileptic attack disorder), Arizona Epilepsy Foundation, Northeast Regional Epilepsy Group]. FS sample inclusion was based on participants’ self-reported diagnosis of FS or probable FS and questions to corroborate their diagnosis/symptoms (e.g., video-EEG results or physician reports; see Supplementary material). Trauma-exposed controls were recruited from a large university research participation pool “if they had a prior major stressful or traumatic event(s).”
We also created a subsample of FS participants (30 FS-PTShi, 23 FS-PTSlo) including only those who reported both (1) EEG/video-EEG results indicating FS [(see 57, 58)], and (2) an FS diagnosis with which they agreed. We report how results with this more restrictive FS subsample compared with the full sample.
Age, gender, racial/ethnic background, education level, socioeconomic status, and marital/relationship status were recorded.
Seizure frequency was assessed with a single item coded into 3 categories: 1 = seizure-free for 1 year or more, 2 = monthly or less than monthly, and 3 = daily or weekly. Seizure severity was assessed with a single item: “Overall, how severe have your seizures or seizure-like episodes been in the past year?” Options were 1 = very mild, 2 = mild, 3 = severe, and 4 = very severe.
Participants rated 8 items (0 = not at all to 4 = a lot) assessing seizure symptom impact in multiple domains (e.g., work, social relationships) (59). Reliability was excellent: Cronbach’s α = 0.91.
We used an abbreviated Life Events Checklist (LEC; 60). Participants selected from 8 traumatic events which they experienced, and could write in experiences not listed. The LEC comports with PTSD-relevant criteria per diagnostic clinical interviews (60).
Participants rated 20 items (0 = not at all to 4 = extremely) (PCL-5; 61). Thirty-seven early participants completed the 17-item PCL Specific Event version for DSM-IV [PCL-S; (62)], where items are rated from 1 = not at all to 5 = extremely with respect to the event that “stuck with them the most.” We used a crosswalk procedure (63) to convert summed PCL-S scores to summed PCL-5 scores. We used a clinical cut-off of 33, at or above which indicates a probable PTSD diagnosis (64), to create PTShi and PTSlo groups. [α: PCL-5 = 0.95, PCL-S (before crosswalk conversion) = 0.97].
Participants rated 5 items from 1 = none of the time to 6 = all of the time to indicate how much of the time during the last month they felt a certain way (e.g., “been a very nervous person”; “been a happy person,” reverse-scored) (65). Higher MHI-5 scores reliably predict clinical diagnoses, especially depression and anxiety (65). (α = 0.86.)
Participants rated 28 items on a 0% (never) to 100% (always) scale in increments of 10%, which were recoded from 1 (0%) to 11 (100%) for analysis (66). (α = 0.93.)
Eighteen items reflecting six emotion regulation difficulty dimensions were rated using a 5-point Likert scale (1 = almost never to 5 = almost always) (26, 67). Total scores were computed by averaging 15 items (Cronbach’s α = 0.93). The three remaining items from the emotional awareness subscale were examined separately (α = 0.85).
Fifteen items were rated from 1 = strongly disagree to 6 = strongly agree (68). (α = 0.86.)
Six reappraisal subscale items (think about situation differently) and 4 expressive suppression subscale items (hide outward emotional display) were rated from 1 = strongly disagree to 7 = strongly agree (69). (α: reappraisal = 0.91; suppression = 0.80.)
Four items measured perceived stress versus ability to manage it (0 = never to 4 = very often) (70). (α = 0.77.)
Two items each from the appraisal, tangible, and belonging support subscales were rated from 0 = definitely false to 3 = definitely true (71, 72). (α = 0.79.)
Four items are rated from 0 = never to 3 = often; higher scores reflect greater loneliness (73). (α = 0.67.)
We used an 18-item STQ version to measure touch-related attitudes and behaviors (e.g., “I’d be happy to give a neck/shoulder massage to a friend if they are feeling stressed”). Items were rated from 0 = not at all to 4 = extremely; higher scores indicated greater comfort with touch (74, 75). (α =0.88.)
Eight items were rated for frequency (0 = never to 4 = almost daily) of affectionate touch with current partner or, if not presently in a relationship, with most recent partner (38, 76). (α = 0.91.)
Participants rated a single item, “How much do you and your spouse/partner ordinarily touch each other while sleeping in the same bed?” (0 = not at all to 4 = very much) (53).
The study was approved by the university’s institutional review board. All procedures followed APA ethical standards for protection of human subjects. Participants completed measures reported here as part of a larger survey administered via a secure website, www.Surveymonkey.com. A link to the consent form and survey was provided on the recruitment materials. At the end of the survey, FS participants could opt to enter contact information (via a separate link) to receive a $35 giftcard (early participants) or be entered into a $35 giftcard drawing (later participants). Control participants received research participation credit.
We tested our hypotheses comparing FS individuals to trauma-matched controls (FS-PTShi to TC-PTShi, FS-PTSlo to TC-PTSlo) and comparing subgroups within FS (FS-PTShi to FS-PTSlo) with planned comparisons (F-tests) using Bonferroni correction to adjust for multiple comparisons, and with age as a covariate. We report effect size using partial eta squared (ηp2). We conducted parallel analyses with an FS subsample constructed with more restrictive inclusion criteria (see Participants).
The following individuals were excluded before reaching the final 305-participant sample: 12 FS with inconclusive seizure/seizure-like event information; 31 TCs reporting prior seizures; 22 FS reporting no prior trauma; 28 FS and 33 TCs not completing the PCL; 2 FS participants over age 65 (to better age-match groups) and 8 TCs not reporting age; 12 TC-PTShi with the lowest PCL scores to match to the FS-PTShi group; and 42 TC-PTSlo men to better match demographics of FS-PTSlo.
Group comparisons for demographics and clinical characteristics are in Table 1. The four groups did not differ in gender, education, or partner relationship status. Both FS groups were older than TC-PTShi; FS-PTSlo was older than TC-PTSlo. Both FS groups included a lower proportion of non-white participants than TCs. FS-PTShi reported lower income than both TC groups; FS-PTSlo reported lower income than TC-PTSlo.
The two PTS-high groups (FS-PTShi, TC-PTShi) did not differ on the primary matching variable of PTS symptoms, nor in general mental health (MHI-5); both were higher than the two PTS-lo groups, who also did not differ. The same pattern was demonstrated for number of traumatic events (listed in Supplementary Tables 1, 2). FS-PTShi reported more dissociative symptoms than the other three groups, and TC-PTShi reported more dissociative symptoms than FS-PTSlo or TC-PTSlo (the latter two did not differ). Within FS, FS-PTShi reported younger FS onset age, and a non-significant trend toward greater seizure impact than FS-PTSlo. FS-PTSlo reported greater seizure severity than FS-PTShi. There were no group differences in FS condition duration, recency, or symptom frequency.
Spearman correlations among variables are presented in Supplementary Tables 3, 4. Across participants, for most measures better socioemotional regulation was related to fewer clinical symptoms. There were few associations between socioemotional regulation and FS symptoms, with most for FS-PTShi.
For the three hypotheses, those with FS and/or greater symptom load, namely FS-PTShi versus TC-PTShi, FS-PTSlo versus TC-PTSlo, and FS-PTShi versus FS-PTSlo, were expected to report greater difficulties on all socioemotional regulation measures. (Findings for affectionate touch frequency during waking and surrounding sleep were similar when examining only participants with a current partner.)
Hypothesis 1: FS-PTShi versus TC-PTShi (see Figure 1; Supplementary Table 5). For most measures, FS-PTShi reported greater difficulties than TC-PTShi: greater emotional avoidance, greater overall emotion regulation difficulties, less use of reappraisal, greater perceived stress, greater loneliness, and less frequent partner affectionate touch during waking and surrounding sleep (with a non-significant trend toward greater emotional awareness difficulties).
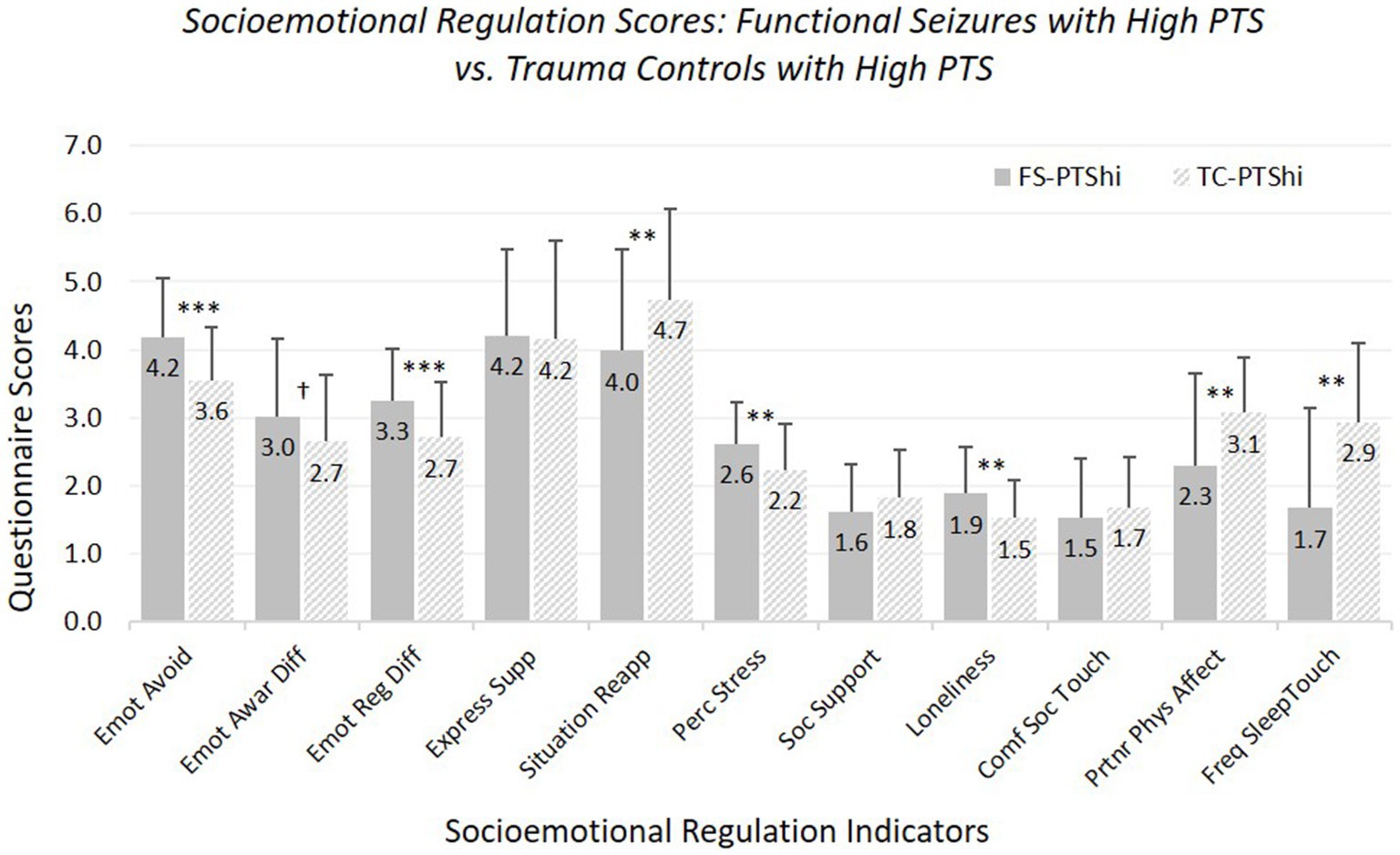
Figure 1. Means and standard deviations of socioemotional regulation characteristics: participants experiencing functional seizures and high posttraumatic stress symptoms versus trauma controls with high posttraumatic stress symptoms. Emot avoid = emotional avoidance per the Brief Experiential Avoidance Questionnaire (1–6 scale); emot awar diff = emotional awareness difficulties per the Difficulties in Emotion Regulation Scale (DERS) – awareness subscale (1–5 scale); emot reg diff = overall emotion regulation difficulties per the DERS without the awareness items (1–5 scale); express supp/situation reapp = expressive emotion suppression and situational reappraisal, the two subscales of the Emotion Regulation Questionnaire (1–7 scale); perc stress = perceived stress per the Perceived Stress Scale (0–4 scale); soc. support = social support per the Interpersonal Support Evaluation List (0–3 scale); loneliness = loneliness measured with the UCLA Loneliness Scale (0–3 scale); comf soc. touch = comfort with social touch per the Social Touch Questionnaire (0–4 scale); partnr phys affect = partner physical affection frequency per the Physical Affection Scale (0–4 scale); freq sleep touch = single item assessing frequency of touch with partner surrounding sleep (0–4 scale). †p < 0.10. *p < 0.05. **p < 0.01. ***p < 0.001.
The two groups did not differ in expressive suppression, perceived social support, or social touch comfort. Age was a significant covariate for perceived social support, affectionate touch frequency, and sleep-touch; older age was associated with less of each.
Findings for FS-PTShi versus TC-PTShi were the same with our strict-inclusion sample, with exceptions that the trend-level difference for difficulties in emotional awareness reached significance, and the significant difference in physical affection with partner during waking became trend-level.
Hypothesis 2: FS-PTSlo versus TC-PTSlo (see Figure 2; Supplementary Table 6). FS-PTSlo reported greater difficulties than TC-PTSlo on several measures (but fewer than for FS-PTShi versus TC-PTShi comparisons). FS-PTSlo reported greater emotional avoidance, emotional awareness difficulties, overall emotion regulation difficulties, and perceived stress, and less social support than TC-PTSlo.
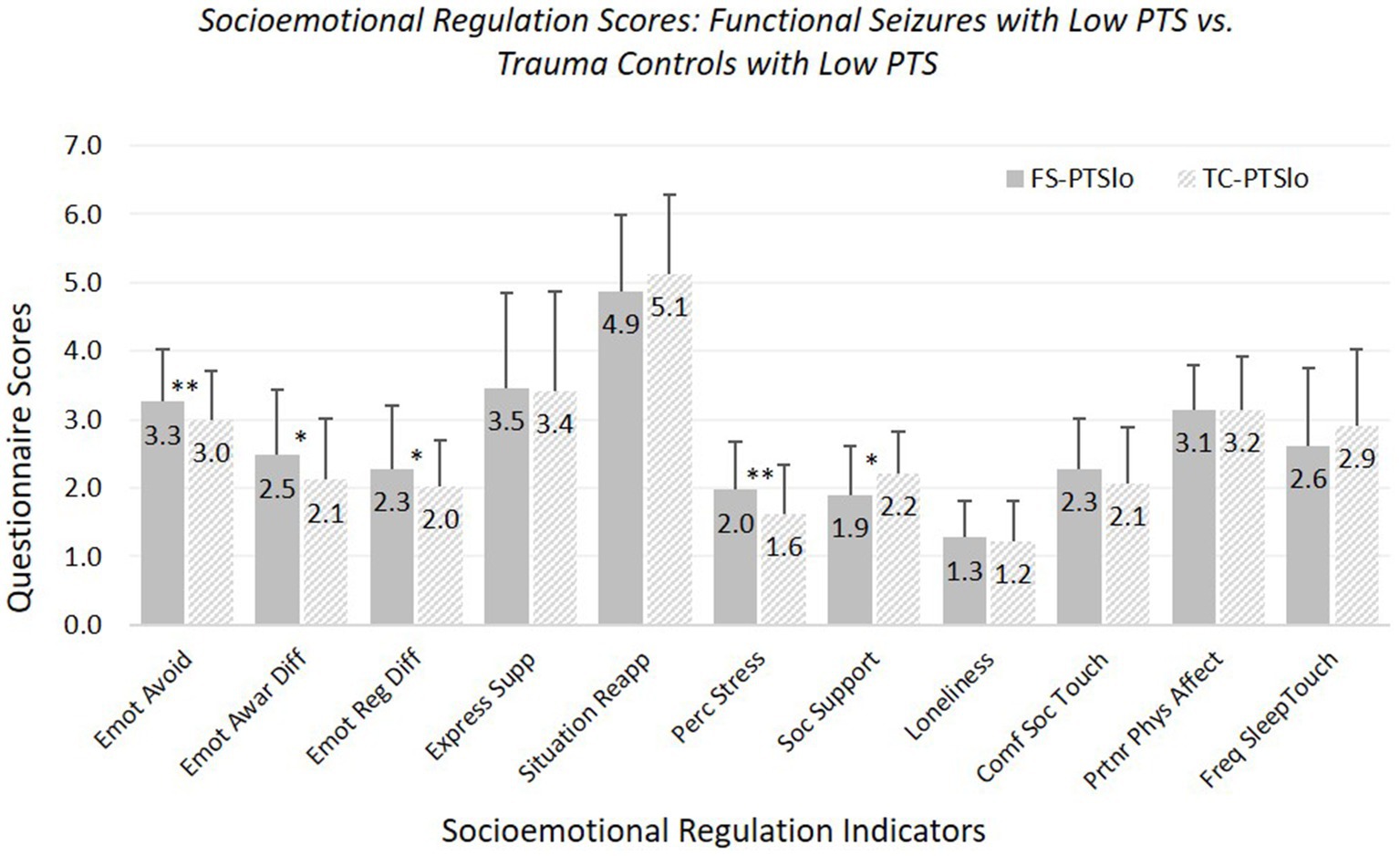
Figure 2. Means and standard deviations of socioemotional regulation characteristics: participants experiencing functional seizures and low posttraumatic stress symptoms versus trauma controls with low posttraumatic stress symptoms. Emot avoid = emotional avoidance per the Brief Experiential Avoidance Questionnaire (1–6 scale); emot awar diff = emotional awareness difficulties per the Difficulties in Emotion Regulation Scale (DERS) – awareness subscale (1–5 scale); emot reg diff = overall emotion regulation difficulties per the DERS without the awareness items (1–5 scale); express supp/situation reapp = expressive emotion suppression and situational reappraisal, the two subscales of the Emotion Regulation Questionnaire (1–7 scale); perc stress = perceived stress per the Perceived Stress Scale (0–4 scale); soc. support = social support per the Interpersonal Support Evaluation List (0–3 scale); loneliness = loneliness measured with the UCLA Loneliness Scale (0–3 scale); comf soc. touch = comfort with social touch per the Social Touch Questionnaire (0–4 scale); partnr phys affect = partner physical affection frequency per the Physical Affection Scale (0–4 scale); freq sleep touch = single item assessing frequency of touch with partner surrounding sleep (0–4 scale). †p < 0.10. *p < 0.05. **p < 0.01.
Groups did not differ in use of expressive suppression or reappraisal, loneliness, comfort with social touch, or frequency of partner affectionate touch during waking or surrounding sleep. Age was a significant covariate for emotional avoidance, emotion regulation difficulties, and perceived stress (all fewer/less with older age), comfort with social touch (more with older age), and partner affectionate touch frequency (less with older age).
With our strict-inclusion sample, FS-PTSlo did not differ significantly from TC-PTSlo in emotional awareness difficulties, and the significant group difference in perceived social support became trend-level; patterns of significance for other measures remained the same.
Hypothesis 3: FS-PTShi versus FS-PTSlo (see Figure 3; Supplementary Table 7). FS-PTShi reported more difficulties than FS-PTSlo on 10 of 11 measures.
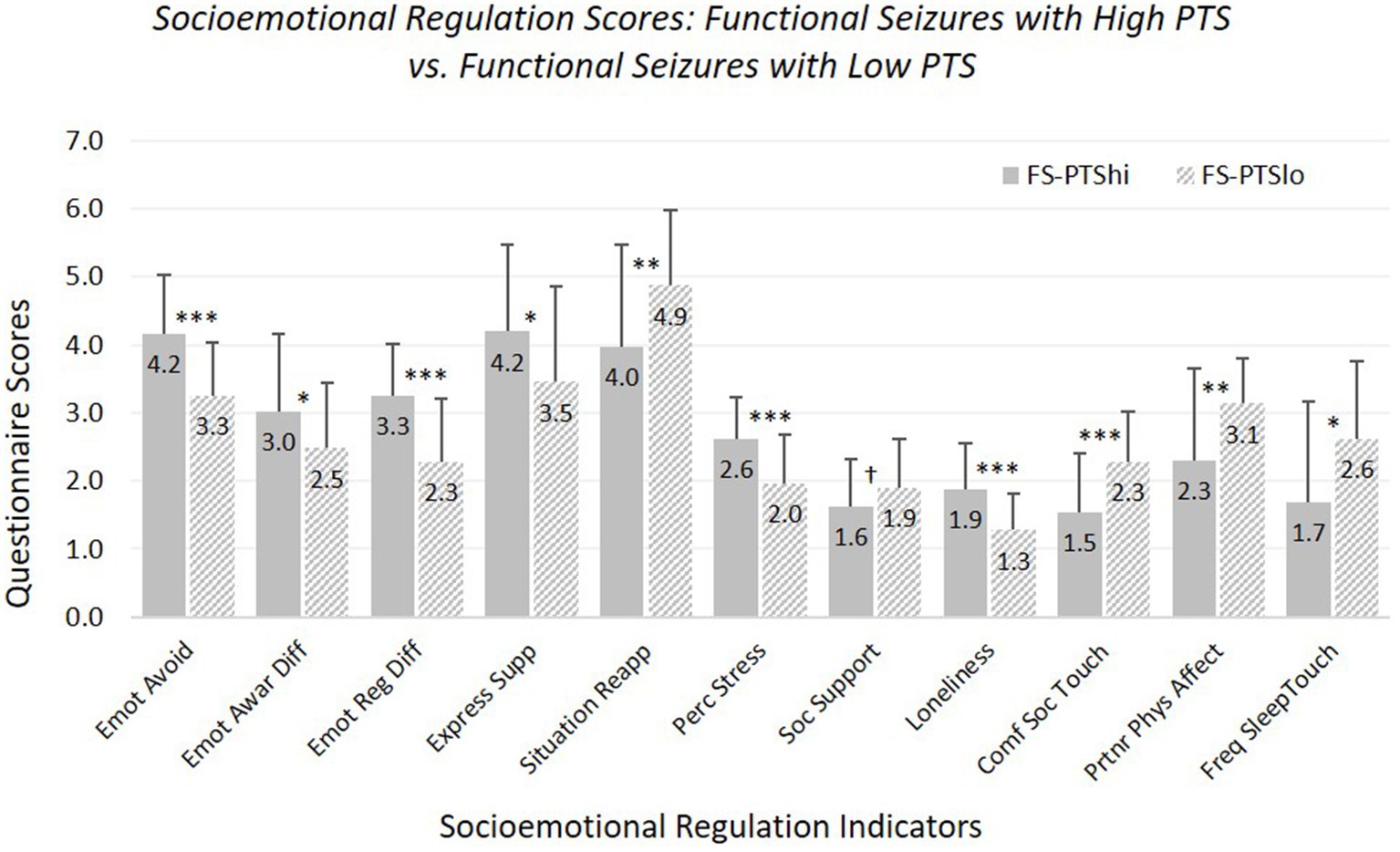
Figure 3. Means and standard deviations of emotional and social characteristics, contrasting participants with functional seizures experiencing high versus low posttraumatic stress symptoms. Emot avoid = emotional avoidance per the Brief Experiential Avoidance Questionnaire (1–6 scale); emot awar diff = emotional awareness difficulties per the Difficulties in Emotion Regulation Scale (DERS) – awareness subscale (1–5 scale); emot reg diff = overall emotion regulation difficulties per the DERS without the awareness items (1–5 scale); express supp/situation reapp = expressive emotion suppression and situational reappraisal, the two subscales of the Emotion Regulation Questionnaire (1–7 scale); perc stress = perceived stress per the Perceived Stress Scale (0–4 scale); soc. support = social support per the Interpersonal Support Evaluation List (0–3 scale); loneliness = loneliness measured with the UCLA Loneliness Scale (0–3 scale); comf soc. touch = comfort with social touch per the Social Touch Questionnaire (0–4 scale); partnr phys affect = partner physical affection frequency per the Physical Affection Scale (0–4 scale); freq sleep touch = single item assessing frequency of touch with partner surrounding sleep (0–4 scale). †p < 0.10. *p < 0.05. **p < 0.01. ***p < 0.001.
FS-PTShi and FS-PTSlo did not differ in perceived social support, although there was a non-significant trend toward less perceived support for FS-PTShi. Age was not a significant covariate for any measure except sleep-touch (older age associated with less sleep-touch).
Findings were the same with our strict-inclusion sample: differences were significant for all measures except social support.
Functional seizures are complex neuropsychiatric conditions challenging to diagnose and treat. Their ongoing management is complicated by comorbidities that shape patients’ ability to navigate their internal and social world. We found based on an online survey that individuals with self-reported FS and prior trauma exposure reported greater socioemotional regulation difficulties than seizure-free trauma-exposed comparison groups with comparable levels of posttraumatic stress and mental health symptoms. This suggests problems experienced by those with FS were not accounted for by PTS level or poor mental health. Yet, while both FS groups reported more avoidance, emotion regulation difficulties, and stress than respective PTS-matched controls, only FS individuals with clinically-elevated PTS reported more loneliness and less affectionate touch in their partner relationships. Further, FS-PTShi reported more difficulties than FS-PTSlo on nearly all measures. Thus, clinically-elevated PTS may work synergistically with FS to exacerbate difficulties in intra-and interpersonal regulation domains.
Emotional processes have long been thought to underpin FS and other functional disorders (77, 78) in FS/functional movement disorder]. Along with greater perceived stress and difficulties regulating distress, avoidance of inner emotional experience was most relevant in characterizing our FS sample. Emotional awareness findings were less consistent. Our emotional awareness measure assessed attention to one’s own feelings/emotions, which is only weakly related to alexithymia [difficulty identifying and describing feelings (79)], with which FS often is associated (80). FS participants did not suppress outward emotional displays more than TCs, unlike prior research versus healthy controls (15). This prior research used the Emotional Processing Scale, where items potentially combine experiential and expressive suppression [e.g., “bottled up emotions” (81)], whereas our measure [ERQ suppression subscale (69)] assessed only outward suppression. Thus, rather than outward display, profound experiential or emotional avoidance [e.g., of shame (41, 82, 83)]—whether linked to PTSD or intrinsic to FS—may serve as a vulnerability to, and/or proximal trigger for, dissociative and motor responses in FS (84). Further, FS-PTShi reported more dissociative symptoms than TC-PTShi, yet not more PTS nor overall mental health symptoms, echoing dissociation as implicated in FS phenomenology (85) and pathophysiology [(86); in other FND: (87)], particularly with comorbid FS and clinical PTS.
FS-PTShi reported more loneliness and less partner affectionate touch when awake and surrounding sleep than TC-PTShi, despite similar proportions in partner relationships. FS-PTShi and TC-PTShi did not differ, however, in perceived social support or comfort with social touch (nor ability to suppress emotion outwardly, noted above). We speculate social relationships and relationship attitudes more generally are not more compromised in FS, even when coupled with clinical PTS, than among those with clinical PTS alone, similar to prior work finding strong social network engagement in FS (22). Rather, more intimate experiences of physical and emotional connection may be affected in FS individuals with clinical PTS. Improving relationships and increasing affectionate touch during waking and/or surrounding sleep may yield treatment benefits, as physical touch may influence affect regulation and intersect with interoceptive processes (55), which are compromised in FS (88). Sleep-touch may be particularly beneficial, because it often occurs during low- or decreasing-arousal states, with fewer concurrent salient stimuli to distract from touch’s calming effects (53).
FS-PTSlo participants did not report less frequent affectionate touch in their partner relationships than controls. Rather, this important form of co-regulation was spared in this subgroup. FS-PTSlo also did not differ from controls in loneliness, perhaps suggesting a stable sense of social connection despite FS symptoms. The lack of association between socioemotional regulation and FS symptom frequency or severity (which, again, we interpret with caution) also suggests different mechanisms in FS-PTSlo.
Importantly, we relied on participants’ self-reported FS diagnostic history. Although we included participants in our FS groups based on careful examination of their answers to detailed quantitative and qualitative questions, we did not have verified diagnoses based on documentation of evaluations, providers’ direct reports, or clinical interviews. Findings were largely consistent when we re-tested our hypotheses with more strictly-defined diagnostic criteria based on EEG monitoring results, albeit self-reported. Limiting generalizability, our participants were mostly accepting of their diagnoses and able and willing to fill out a lengthy online survey. Participants recruited via FND-related social media sites may have had or desired high social support and connection.
To hold trauma exposure constant, we only included participants reporting prior trauma. Presence or absence of PTSD, and not trauma itself, has differentiated performance on cognitive and clinical measures (48); however, trauma exposure may have been a factor in ways not tested here. We also conceptualized FS as the primary diagnosis and PTSD as a secondary comorbidity; it is possible to conceptualize PTSD as primary in some cases, with FS as an additional feature. Given our small sample, particularly for FS-PTSlo, and unequal sample sizes between FS-PTSlo and TC-PTSlo, findings must be interpreted with caution. We did not consider patient subgroups besides high and low PTS. Given heterogeneity among FS, other subgroups may be fruitful to explore [e.g., high and low emotion dysregulation; (30, 31)].
As with any cross-sectional, self-report online survey, measures reflect participant experience (not behavior), and are colored by participant perceptions. Causality regarding socioemotional patterns and FS or PTS symptoms also cannot be inferred. Additionally, although we collected multiple individual measures that we grouped conceptually as indicators of “socioemotional regulation,” we chose to take a more granular analytical approach by conducting individual pairwise contrasts for each measure (rather than a multivariate analysis), for easier comparison with prior literature [(e.g., 15, 22)].
In prior work we included FS participants with neurologist/epileptologist-verified diagnoses based on careful assessment including video EEG (41, 42); here, we chose to broaden our sample to FS individuals who may not have had access to epilepsy monitoring unit evaluation. Reasonable consistency between present findings and prior work, yet with some differences [e.g., fewer significant correlations between socioemotional processes and symptoms (49)—which also may be due to measure limitations (single items assessing seizure frequency and severity) and small sample size], suggests that a community sample may provide important information, alongside systematic comparisons with diagnostically-verified samples. Such an approach could facilitate understanding FS as a continuum, perhaps with subclinical presentations and normed self-report measures to capture them, akin to studies of depression and other clinical syndromes [(e.g., 89, 90)].
As the field advances its knowledge of FND and PTSD intersections, it becomes both more challenging and more important to understand FS absent PTSD. This challenge is compounded by small samples, and uncertainty about past (traumatic) events when relying on self-report. Our findings nonetheless may have treatment implications for FS with non-clinical PTS. For example, FS-PTSlo were comparable to controls in using cognitive reappraisal as an emotion regulation strategy; this ability to think about a situation differently may help offset otherwise-detrimental effects of emotional avoidance and dysregulation and can be considered a potential relative strength. Concrete, problem-solving versus process-oriented approaches may be warranted, for example, to focus on lowering stress and increasing opportunities for social support. While close connection to others was not reported as problematic, whether those areas are spared, or reflect different views of interpersonal closeness, could be explored further.
In the present study, we identified social and emotion regulation challenges and strengths in FS individuals compared with PTS-matched controls. Those with FS indicated feeling comfortable with social touch at levels comparable to controls matched in PTS/mental health, but deriving a sense of physical and emotional closeness from intimate relationships appeared more challenging when posttraumatic stress symptoms were clinically elevated. Thus, PTSD-related disruptions in co-regulation may intensify self-regulation difficulties and avoidance tendencies in FS. Attention to social/interpersonal strategies for co-regulation alongside self-focused affect regulation may be an important avenue for understanding affective processes in FS and enhancing patients’ well-being.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Arizona State University’s Institutional Review Board. The participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
We acknowledge the Institute for Mental Health Research, the Arizona State University Institute for Social Science Research, and the Arizona State University Graduate and Professional Association for supporting this research. We also thank FND Hope, NEAD.org, and the Northeast Regional Epilepsy Group, and Lorna Myers, Robin Garrett, and Cornelia Drees, for facilitating participant recruitment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1135590/full#supplementary-material
1. Devinsky, O, Gazzola, D, and LaFrance, WC Jr. Differentiating between nonepileptic and epileptic seizures. Nat Rev Neurol. (2011) 7:210–20. doi: 10.1038/nrneurol.2011.24
2. Hallett, M, Aybek, S, Dworetzky, BA, McWhirter, L, Staab, JP, and Stone, J. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. (2022) 21:537–50. doi: 10.1016/s1474-4422(21)00422-1
3. World Health Organization. ICD-11: International classification of diseases. (2022). Available at: https://icd.who.int/
4. American Psychiatric Association. (ed.). Diagnostic and statistical manual of mental disorders. 5th edn. Washington, DC: (2013).
5. Beghi, M, Cornaggia, I, Magaudda, A, Perin, C, Peroni, F, and Cornaggia, CM. Childhood trauma and psychogenic nonepileptic seizures: a review of findings with speculations on the underlying mechanisms. Epilepsy Behav. (2015) 52:169–73. doi: 10.1016/j.yebeh.2015.09.007
6. Beghi, M, Zhang, L, Beghi, E, Giussani, G, Erba, G, Longinetti, E, et al. History of violence/maltreatment and psychogenic non-epileptic seizures. Seizure. (2020) 81:8–12. doi: 10.1016/j.seizure.2020.07.012
7. Drane, DL, Fani, N, Hallett, M, Khalsa, SS, Perez, DL, and Roberts, NA. A framework for understanding the pathophysiology of functional neurological disorder. CNS Spectr. (2021) 26:555–561. doi: 10.1017/S1092852920001789
8. Pick, S, Goldstein, LH, Perez, DL, and Nicholson, TR. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry. (2019) 90:704–11. doi: 10.1136/jnnp-2018-319201
9. Perez, DL, Edwards, MJ, Nielsen, G, Kozlowska, K, Hallett, M, and LaFrance, WC Jr. Decade of Progress in motor functional neurological disorder: continuing the momentum. J Neurol Neurosurg Psychiatry. (2021) 92:668–77. doi: 10.1136/jnnp-2020-323953
10. Jungilligens, J, Paredes-Echeverri, S, Popkirov, S, Barrett, LF, and Perez, DL. A new science of emotion: implications for functional neurological disorder. Brain. (2022) 145:2648–63. doi: 10.1093/brain/awac204
11. Sojka, P, Bareš, M, Kašpárek, T, and Světlák, M. Processing of emotion in functional neurological disorder. Front Psych. (2018) 9:9. doi: 10.3389/fpsyt.2018.00479
12. Brown, RJ, and Reuber, M. Psychological and psychiatric aspects of psychogenic non-epileptic seizures (Pnes): a systematic review. Clin Psychol Rev. (2016) 45:157–82. doi: 10.1016/j.cpr.2016.01.003
13. Asadi-Pooya, AA, Beghi, M, and Baslet, G. Is sexual trauma a risk factor for functional (psychogenic) seizures? Neurosci Biobehav Rev. (2021) 128:58–63. doi: 10.1016/j.neubiorev.2021.06.019
14. Ludwig, L, Pasman, JA, Nicholson, T, Aybek, S, David, AS, Tuck, S, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and Meta-analysis of case-control studies. Lancet Psychiatry. (2018) 5:307–20. doi: 10.1016/S2215-0366(18)30051-8
15. Novakova, B, Howlett, S, Baker, R, and Reuber, M. Emotion processing and psychogenic non-epileptic seizures: a cross-sectional comparison of patients and healthy controls. Seizure. (2015) 29:4–10. doi: 10.1016/j.seizure.2015.03.007
16. Pick, S, Mellers, JDC, Goldstein, LH, and Bias, A. Implicit for facial emotion in dissociative seizures: additional evidence. Epilepsy Behav. (2018) 80:80:296–302. doi: 10.1016/j.yebeh.2018.01.004
17. Green, B, Norman, P, and Reuber, M. Attachment style, relationship quality, and psychological distress in patients with psychogenic non-epileptic seizures versus epilepsy. Epilepsy Behav. (2017) 66:120–6. doi: 10.1016/j.yebeh.2016.10.015
18. Krawetz, P, Fleisher, W, Pillay, N, Staley, D, Arnett, J, and Maher, J. Family functioning in subjects with Pseudoseizures and epilepsy. J Nerv Ment Dis. (2001) 189:38–43. doi: 10.1097/00005053-200101000-00007
19. Asadi-Pooya, AA, Alsaadi, T, Gigineishvili, D, Hingray, C, Hosny, H, Karakis, I, et al. Social aspects of life in patients with functional (psychogenic nonepileptic) seizures: an international study. Epilepsy Behav. (2020) 113:107534. doi: 10.1016/j.yebeh.2020.107534
20. LaFrance, WC Jr, Alosco, ML, Davis, JD, Tremont, G, Ryan, CE, Keitner, GI, et al. Impact of family functioning on quality of life in patients with psychogenic nonepileptic seizures versus epilepsy. Epilepsia. (2011) 52:292–300. doi: 10.1111/j.1528-1167.2010.02765.x
21. Reuber, M, and Mayor, R. Recent Progress in the understanding and treatment of nonepileptic seizures. Curr Opin Psychiatry. (2012) 25:244–50. doi: 10.1097/YCO.0b013e3283523db6
22. Vaidya-Mathur, U, Myers, L, Laban-Grant, O, Lancman, M, Lancman, M, and Jones, J. Socialization characteristics in patients with psychogenic nonepileptic seizures (PNES). Epilepsy Behav. (2016) 56:59–65. doi: 10.1016/j.yebeh.2015.12.032
23. Ospina, JP, Larson, AG, Jalilianhasanpour, R, Williams, B, Diez, I, Dhand, A, et al. Individual differences in social network size linked to nucleus Accumbens and hippocampal volumes in functional neurological disorder: a pilot study. J Affect Disord. (2019) 258:50–4. doi: 10.1016/j.jad.2019.07.061
24. Gross, JJ . The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. (1998) 2:271–99. doi: 10.1037/1089-2680.2.3.271
25. Gross, JJ . Emotion regulation: current status and future prospects. Psychol Inq. (2015) 26:1–26. doi: 10.1080/1047840X.2014.940781
26. Gratz, KL, and Roemer, L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. (2004) 26:41–54. doi: 10.1023/B:JOBA.0000007455.08539.94
27. Bardeen, JR, Fergus, TA, and Orcutt, HK. An examination of the latent structure of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. (2012) 34:382–92. doi: 10.1007/s10862-012-9280-y
28. Williams, IA, Levita, L, and Reuber, M. Emotion dysregulation in patients with psychogenic nonepileptic seizures: a systematic review based on the extended process model. Epilepsy Behav. (2018) 86:37–48. doi: 10.1016/j.yebeh.2018.06.049
29. Ekanayake, V, Kranick, S, LaFaver, K, Naz, A, Frank Webb, A, LaFrance, WC Jr, et al. Personality traits in psychogenic nonepileptic seizures (PNES) and psychogenic movement disorder (Pmd): neuroticism and perfectionism. J Psychosom Res. (2017) 97:23–9. doi: 10.1016/j.jpsychores.2017.03.018
30. Brown, RJ, Bouska, JF, Frow, A, Kirkby, A, Baker, GA, Kemp, S, et al. Emotional dysregulation, alexithymia, and attachment in psychogenic nonepileptic seizures. Epilepsy Behav. (2013) 29:178–83. doi: 10.1016/j.yebeh.2013.07.019
31. Uliaszek, AA, Prensky, E, and Baslet, G. Emotion regulation profiles in psychogenic non-epileptic seizures. Epilepsy Behav. (2012) 23:364–9. doi: 10.1016/j.yebeh.2012.01.009
32. Sbarra, DA, and Hazan, C. Coregulation, dysregulation, self-regulation: an integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Personal Soc Psychol Rev. (2008) 12:141–67. doi: 10.1177/1088868308315702
33. Hawkley, LC, and Cacioppo, JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. (2010) 40:218–27. doi: 10.1007/s12160-010-9210-8
34. Holt-Lunstad, J . Social connection as a public health issue: the evidence and a systemic framework for prioritizing the “social” in social determinants of health. Annu Rev Public Health. (2022) 43:193–213. doi: 10.1146/annurev-publhealth-052020-110732
35. Ettinger, AB, Dhoon, A, Weisbrot, DM, and Devinsky, O. Predictive factors for outcome of nonepileptic seizures after diagnosis. J Neuropsychiatry Clin Neurosci. (1999) 11:458–63. doi: 10.1176/jnp.11.4.458
36. Fairclough, G, Fox, J, Mercer, G, Reuber, M, and Brown, RJ. Understanding the perceived treatment needs of patients with psychogenic nonepileptic seizures. Epilepsy Behav. (2014) 31:295–303. doi: 10.1016/j.yebeh.2013.10.025
37. Bakvis, P, Roelofs, K, Kuyk, J, Edelbroek, PM, Swinkels, WA, and Spinhoven, P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia. (2009) 50:1001–11. doi: 10.1111/j.1528-1167.2008.01862.x
38. Burleson, MH, Roberts, NA, Munson, AA, Duncan, CJ, Randall, AK, Ha, T, et al. Feeling the absence of touch: distancing, distress, regulation, and relationships in the context of Covid-19. J Soc Pers Relat. (2022) 39:56–79. doi: 10.1177/02654075211052696
39. Zeng, R, Myers, L, and Lancman, M. Post-traumatic stress and relationships to coping and alexithymia in patients with psychogenic non-epileptic seizures. Seizure. (2018) 57:70–5. doi: 10.1016/j.seizure.2018.03.011
40. Myers, L, Matzner, B, Lancman, M, Perrine, K, and Lancman, M. Prevalence of alexithymia in patients with psychogenic non-epileptic seizures and epileptic seizures and predictors in psychogenic non-epileptic seizures. Epilepsy Behav. (2013) 26:153–7. doi: 10.1016/j.yebeh.2012.11.054
41. Roberts, NA, Burleson, MH, Torres, DL, Parkhurst, DK, Garrett, R, Mitchell, LB, et al. Emotional reactivity as a vulnerability for psychogenic nonepileptic seizures? Responses while reliving specific emotions. J Neuropsychiatry Clin Neurosci. (2020) 32:95–100. doi: 10.1176/appi.neuropsych.19040084
42. Roberts, NA, Burleson, MH, Weber, DJ, Larson, A, Sergeant, K, Devine, MJ, et al. Emotion in psychogenic nonepileptic seizures: responses to affective pictures. Epilepsy Behav. (2012) 24:107–15. doi: 10.1016/j.yebeh.2012.03.018
43. Williams, B, Ospina, JP, Jalilianhasanpour, R, Fricchione, GL, and Perez, DL. Fearful attachment linked to childhood abuse, alexithymia, and depression in motor functional neurological disorders. J Neuropsychiatry Clin Neurosci. (2019) 31:65–9. doi: 10.1176/appi.neuropsych.18040095
44. Beauchaine, TP, and Thayer, JF. Heart rate variability as a Transdiagnostic biomarker of psychopathology. Int J Psychophysiol. (2015) 98:338–50. doi: 10.1016/j.ijpsycho.2015.08.004
45. Christ, NM, Elhai, JD, Forbes, CN, Gratz, KL, and Tull, MT. A machine learning approach to modeling PTSD and difficulties in emotion regulation. Psychiatry Res. (2021) 297:113712. doi: 10.1016/j.psychres.2021.113712
46. Hyland, P, Shevlin, M, Cloitre, M, Karatzias, T, Vallières, F, McGinty, G, et al. Quality not quantity: loneliness subtypes, psychological trauma, and mental health in the us adult population. Soc Psychiatry Psychiatr Epidemiol. (2019) 54:1089–99. doi: 10.1007/s00127-018-1597-8
47. Myers, L, Perrine, K, Lancman, M, Fleming, M, and Lancman, M. Psychological trauma in patients with psychogenic nonepileptic seizures: trauma characteristics and those who develop Ptsd. Epilepsy Behav. (2013) 28:121–6. doi: 10.1016/j.yebeh.2013.03.033
48. Myers, L, Zeng, R, Perrine, K, Lancman, M, and Lancman, M. Cognitive differences between patients who have psychogenic nonepileptic seizures (Pness) and posttraumatic stress disorder (Ptsd) and patients who have PNESs without PTSD. Epilepsy Behav. (2014) 37:82–6. doi: 10.1016/j.yebeh.2014.06.009
49. Paredes-Echeverri, S, Guthrie, AJ, and Perez, DL. Toward a possible trauma subtype of functional neurological disorder: impact on symptom severity and physical health. Front Psych. (2022) 13:13. doi: 10.3389/fpsyt.2022.1040911
50. Dixon-Gordon, KL, Aldao, A, and De Los, RA. Repertoires of emotion regulation: a person-centered approach to assessing emotion regulation strategies and links to psychopathology. Cogn Emot. (2015) 29:1314–25. doi: 10.1080/02699931.2014.983046
51. Murphy, MLM, Janicki-Deverts, D, and Cohen, S. Receiving a hug is associated with the attenuation of negative mood that occurs on days with interpersonal conflict. PLoS One. (2018) 13:e0203522. doi: 10.1371/journal.pone.0203522
52. Jolink, TA, Chang, YP, and Algoe, SB. Perceived partner responsiveness forecasts behavioral intimacy as measured by affectionate touch. Personal Soc Psychol Bull. (2022) 48:203–21. doi: 10.1177/0146167221993349
53. Roberts, NA, Burleson, MH, Pituch, K, Flores, M, Woodward, C, Shahid, S, et al. Affective experience and regulation via sleep, touch, and "sleep-touch" among couples. Affect Sci. (2022) 3:353–69. doi: 10.1007/s42761-021-00093-3
54. Jakubiak, BK, and Feeney, BC. Affectionate touch to promote relational, psychological, and physical well-being in adulthood: a theoretical model and review of the research. Personal Soc Psychol Rev. (2017) 21:228–52. doi: 10.1177/1088868316650307
55. Burleson, MH, and Quigley, KS. Social Interoception and social Allostasis through touch: legacy of the Somatovisceral Afference model of emotion. Soc Neurosci. (2021) 16:92–102. doi: 10.1080/17470919.2019.1702095
56. Ricciardi, L, Demartini, B, Crucianelli, L, Krahé, C, Edwards, MJ, and Fotopoulou, A. Interoceptive awareness in patients with functional neurological symptoms. Biol Psychol. (2016) 113:68–74. doi: 10.1016/j.biopsycho.2015.10.009
57. LaFrance, WC Jr, Baker, GA, Duncan, R, Goldstein, LH, and Reuber, M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the international league against epilepsy nonepileptic seizures task force. Epilepsia. (2013) 54:2005–18. doi: 10.1111/epi.12356
58. Baslet, G, Bajestan, SN, Aybek, S, Modirrousta, M, DCP, JP, Cavanna, A, et al. Evidence-based practice for the clinical assessment of psychogenic nonepileptic seizures: a report from the American neuropsychiatric association committee on research. J Neuropsychiatry Clin Neurosci. (2021) 33:27–42. doi: 10.1176/appi.neuropsych.19120354
59. Jacoby, A, Baker, G, Smith, D, Dewey, M, and Chadwick, D. Measuring the impact of epilepsy: the development of a novel scale. Epilepsy Res. (1993) 16:83–8. doi: 10.1016/0920-1211(93)90042-6
60. Gray, MJ, Litz, BT, Hsu, JL, and Lombardo, TW. Psychometric properties of the life events checklist. Assessment. (2004) 11:330–41. doi: 10.1177/1073191104269954
61. Blevins, CA, Weathers, FW, Davis, MT, Witte, TK, and Domino, JL. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. (2015) 28:489–98. doi: 10.1002/jts.22059
62. Weathers, F, Litz, B, Herman, D, Huska, JA, and Keane, T. The PTSD checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the annual convention of the international society for traumatic stress studies. (1993).
63. Moshier, SJ, Lee, DJ, Bovin, MJ, Gauthier, G, Zax, A, Rosen, RC, et al. An empirical crosswalk for the PTSD checklist: translating DSM-IV to DSM-5 using a veteran sample. J Trauma Stress. (2019) 32:799–805. doi: 10.1002/jts.22438
64. Bovin, MJ, Marx, BP, Weathers, FW, Gallagher, MW, Rodriguez, P, Schnurr, PP, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. (2016) 28:1379–91. doi: 10.1037/pas0000254
65. Berwick, DM, Murphy, JM, Goldman, PA, Ware, JE Jr, Barsky, AJ, and Weinstein, MC. Performance of a five-item mental health screening test. Med Care. (1991) 29:169–76. doi: 10.1097/00005650-199102000-00008
66. Zingrone, NL, and Alvarado, CS. The dissociative experiences scale-ii: descriptive statistics, factor analysis, and frequency of experiences. Imagin Cogn Pers. (2001) 21:145–57. doi: 10.2190/k48d-xaw3-b2kc-ubb7
67. Victor, SE, and Klonsky, ED. Validation of a brief version of the difficulties in emotion regulation scale (DERS-18) in five samples. J Psychopathol Behav Assess. (2016) 38:582–9. doi: 10.1007/s10862-016-9547-9
68. Gámez, W, Chmielewski, M, Kotov, R, Ruggero, C, Suzuki, N, and Watson, D. The brief experiential avoidance questionnaire: development and initial validation. Psychol Assess. (2014) 26:35–45. doi: 10.1037/a0034473
69. Gross, JJ, and John, OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. (2003) 85:348–62. doi: 10.1037/0022-3514.85.2.348
70. Cohen, S, Kamarck, T, and Mermelstein, R. A global measure of perceived stress. J Health Soc Behav. (1983) 24:385–96. doi: 10.2307/2136404
71. Cohen, S, and Hoberman, HM. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. (1983) 13:99–125. doi: 10.1111/j.1559-1816.1983.tb02325.x
72. Newsom, JT, and Schulz, R. Social support as a mediator in the relation between functional status and quality of life in older adults. Psychol Aging. (1996) 11:34–44. doi: 10.1037/0882-7974.11.1.34
73. Russell, D, Peplau, LA, and Cutrona, CE. The revised Ucla loneliness scale: concurrent and discriminant validity evidence. J Pers Soc Psychol. (1980) 39:472–80. doi: 10.1037//0022-3514.39.3.472
74. Wilhelm, FH, Kochar, AS, Roth, WT, and Gross, JJ. Social anxiety and response to touch: incongruence between self-evaluative and physiological reactions. Biol Psychol. (2001) 58:181–202. doi: 10.1016/s0301-0511(01)00113-2
75. Burleson, MH, Roberts, NA, Coon, DW, and Soto, JA. Perceived cultural acceptability and comfort with affectionate touch: differences between Mexican Americans and European Americans. J Soc Pers Relat. (2019) 36:1000–22. doi: 10.1177/0265407517750005
76. Diamond, M, and Passionate, L. Friendships among adolescent sexual-minority women. J Res Adolesc. (2000) 10:191–209. doi: 10.1207/SJRA1002_4
77. Lesser, RP . Treatment and outcome of psychogenic nonepileptic seizures. Epilepsy Curr. (2003) 3:198–200. doi: 10.1046/j.1535-7597.2003.03601.x
78. Paola, LD, Marchetti, RL, Teive, HA, and LaFrance, WC Jr. Psychogenic nonepileptic seizures and psychogenic movement disorders: two sides of the same coin? Arq Neuropsiquiatr. (2014) 72:793–802. doi: 10.1590/0004-282x20140111
79. Maroti, D, Lilliengren, P, and Bileviciute-Ljungar, I. The relationship between alexithymia and emotional awareness: a Meta-analytic review of the correlation between TAS-20 and LEAS. Front Psychol. (2018) 9:9:453. doi: 10.3389/fpsyg.2018.00453
80. Gürsoy, SC, Ergün, S, Midi, İ, and Topçuoğlu, V. Theory of mind and its relationship with alexithymia and quality of life in patients with psychogenic nonepileptic seizures: comparisons with generalised epilepsy and healthy controls. Seizure. (2021) 91:251–7. doi: 10.1016/j.seizure.2021.06.032
81. Baker, R, Thomas, S, Thomas, PW, Gower, P, Santonastaso, M, and Whittlesea, A. The emotional processing scale: scale refinement and abridgement (Eps-25). J Psychosom Res. (2010) 68:83–8. doi: 10.1016/j.jpsychores.2009.07.007
82. Myers, L, Gray, C, Roberts, N, Levita, L, and Reuber, M. Shame in the treatment of patients with psychogenic nonepileptic seizures: the elephant in the room. Seizure. (2022) 94:176–82. doi: 10.1016/j.seizure.2021.10.018
83. Reuber, M, Roberts, NA, Levita, L, Gray, C, and Myers, L. Shame in patients with psychogenic nonepileptic seizure: a narrative review. Seizure. (2022) 94:94:165–75. doi: 10.1016/j.seizure.2021.10.017
84. Dimaro, LV, Dawson, DL, Roberts, NA, Brown, I, Moghaddam, NG, and Reuber, M. Anxiety and avoidance in psychogenic nonepileptic seizures: the role of implicit and explicit anxiety. Epilepsy Behav. (2014) 33:33:77–86. doi: 10.1016/j.yebeh.2014.02.016
85. Roberts, NA, and Reuber, M. Alterations of consciousness in psychogenic nonepileptic seizures: emotion, emotion regulation and dissociation. Epilepsy Behav. (2014) 30:30:43–9. doi: 10.1016/j.yebeh.2013.09.035
86. van der Kruijs, SJM, Jagannathan, SR, Bodde, NMG, Besseling, RMH, Lazeron, RHC, Vonck, KEJ, et al. Resting-state networks and dissociation in psychogenic non-epileptic seizures. J Psychiatr Res. (2014) 54:126–33. doi: 10.1016/j.jpsychires.2014.03.010
87. Perez, DL, Matin, N, Williams, B, Tanev, K, Makris, N, LaFrance, WC Jr, et al. Cortical thickness alterations linked to somatoform and psychological dissociation in functional neurological disorders. Hum Brain Mapp. (2018) 39:428–39. doi: 10.1002/hbm.23853
88. Koreki, A, Garfkinel, SN, Mula, M, Agrawal, N, Cope, S, Eilon, T, et al. Trait and state interoceptive abnormalities are associated with dissociation and seizure frequency in patients with functional seizures. Epilepsia. (2020) 61:1156–65. doi: 10.1111/epi.16532
89. Gillis, MM, Haaga, DAF, and Ford, GT. Normative values for the Beck anxiety inventory, fear questionnaire, Penn State worry questionnaire, and social phobia and anxiety inventory. Psychol Assess. (1995) 7:450–5. doi: 10.1037/1040-3590.7.4.450
Keywords: functional seizures, psychogenic nonepileptic seizures, emotion regulation, posttraumatic stress disorder, affectionate touch, social connection
Citation: Roberts NA, Villarreal LD and Burleson MH (2023) Socioemotional self- and co-regulation in functional seizures: comparing high and low posttraumatic stress. Front. Psychiatry. 14:1135590. doi: 10.3389/fpsyt.2023.1135590
Received: 01 January 2023; Accepted: 13 April 2023;
Published: 15 May 2023.
Edited by:
Mercedes Sarudiansky, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Johannes Jungilligens, Ruhr University Bochum, GermanyCopyright © 2023 Roberts, Villarreal and Burleson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole A. Roberts, Tmljb2xlLkEuUm9iZXJ0c0Bhc3UuZWR1
†Present address: Lucia Dayana Villarreal,Department of Psychology, The University of Tulsa, Tulsa, OK, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.