- 1School of Sport and Health, Nanjing Sport Institute, Nanjing, Jiangsu, China
- 2School of Physical Education and Humanities, Nanjing Sport Institute, Nanjing, Jiangsu, China
- 3Department of Sport, Gdansk University of Physical Education and Sport, Gdansk, Poland
- 4Nanjing Foreign Language School Xianlin Campus, Nanjing, Jiangsu, China
Background: Autism spectrum disorder (ASD) is a severe public health concern, and most of the children with ASD experience a substantial delay in FMS. This study aimed to investigate the effectiveness of exercise interventions in improving FMS in children with ASD, and provide evidence to support the scientific use of exercise interventions in practice.
Methods: We searched seven online databases (PubMed, Scopus, Web of Science, Embase, EBSCO, Clinical Trials, and The Cochrane Library) from inception to May 20, 2022. We included randomized control trials of exercise interventions for FMS in children with ASD. The methodological quality of the included studies was assessed using the Physiotherapy Evidence Database Scale. Stata 14.0 software was used for meta-analysis, forest plotting, subgroup analysis, heterogeneity analysis, and meta-regression.
Results: Thirteen studies underwent systematic review (541 participants), of which 10 underwent meta-analysis (297 participants). Overall, exercise interventions significantly improved overall FMS in children with ASD. Regarding the three categories of FMS, exercise interventions significantly improved LMS (SMD = 1.07; 95% CI 0.73 to 1.41, p < 0.001), OCS (SMD = 0.79; 95% CI 0.32 to 1.26, p = 0.001), and SS (SMD = 0.72; 95% CI 0.45 to 0.98, p < 0.0001).
Conclusion: exercise interventions can effectively improve the FMS of children with ASD. The effects on LMS are considered as large effect sizes, while the effects on OCS and SS are considered as moderate effect sizes. These findings can inform clinical practice.
Systematic review registration: https://inplasy.com/inplasy-2022-12-0013/.
1. Introduction
Autism spectrum disorder (ASD) is a complicated neurodevelopmental disorder. The estimated prevalence of ASD is ~1% of the current global population (1). According to the Diagnostic and Statistics Manual of Mental Disorders (DSM-5), individuals with ASD have two core symptoms: severe deficits in social communication behaviors and highly restrictive, repetitive behaviors (2). Previous studies have dominantly focused on these two key areas (3). Studies have also established that individuals with ASD suffer from other health problems or risks, including sleep disturbances (4), obesity (5), executive function deficits (6), physical inactivity (7), and motor dysfunctions (8). While higher levels of physical activity are beneficial for lifespan development and overall health of individuals. Exercise as purposed, structured and repeatedly physical activity can ameliorate health problems. Accumulating evidence has shown that exercise intervention improve a variety of typical impairments in children with ASD, such as social skills (9), stereotypic behaviors (10), and motor competence (11). And exercise intervention is potential for individuals with ASD to adapt their lives, which is engaging a growing area of research interest (12).
Recently, fundamental motor skills (FMS) have been widely regarded as an essential prerequisite for participation in physical activity. They are the essential building blocks for further, more complex motor competency and motor skills (13). FMS generally developed during childhood and subsequently refined into specific skills, including locomotor skills (LMS, e.g., running and hopping), object control skills (OCS, e.g., catching and throwing), and stability skills (SS, e.g., balancing and twisting) (13). Mastery of FMS is reported to contribute to children's physical (14), cognitive (15), and social development (16) and is considered a foundation for an active lifestyle (13, 17, 18). However, a couple of studies have mentioned that children with ASD experience a substantial delay in FMS compared to age-matched children who are typically developing (TD) (19, 20). Pan et al. (21) deployed the Test of Gross Motor Development-Second Edition (TGMD-2) to assess FMS performance in children aged 6–10 years with TD and ASD. They found that LMS and OCS scores of ASD were the lower than TD. Liu et al. (19) evaluated the FMS performance of 30 children with ASD and 30 age-matched children with TD using the Movement Assessment Battery for Children-2 (MABC-2). They found that the FMS performance in children with ASD was lower than that with TD. Although children with ASD generally have deficits in FMS, these deficits are not irreversible (12). Therefore, it is critical to promote FMS in the childhood of children with ASD (13).
With the rapidly increasing studies on exercise intervention to promote FMS in children with ASD, several reviews have begun to summarize the effects of exercise intervention. A systematic review examined the effects of motor and exercise interventions on motor function in children with ASD, and found that exercise interventions improve motor participation, activity, and body functional outcomes (22). Meanwhile, a meta-analysis examined the effects of various exercise-based interventions on gross motor skills in children with ASD. The results demonstrated that exercise interventions had a large effect on overall gross motor function (g = 0.99, p < 0.001) (11). Notably, this study didn't investigate the effect of exercise interventions on the categories of gross motor function. Recently, another systematic review first explored the effects of motor skills interventions on LMS, OCS and SS in children with ASD. Their results found that 86.3% of participants reported significant effects (23). Although studies have confirmed the beneficial effects of exercise interventions on motor competence in children with ASD, there are still some underlying questions. First, previous reviews focused on motor skills or gross motor skills rather than FMS. Secondly, few reviews conducted a meta-analysis on FMS and examined subsequently on LMS, OCS, and SS. Thirdly, most of the studies included in relevant reviews were none randomized controlled trials (RCTs) with low quality. Finally, no review has explored the impact of different FMS measurement tools (which assess different categories of FMS) on the intervention effect.
To the best of our knowledge, no meta-analysis has examined the effect of exercise intervention on FMS and its categories in children with ASD. As a result, to address these underlying issues in the literature and provide theoretical basis for formulating exercise prescriptions, this meta-analysis aims to synthesize published studies to quantify the effect sizes of exercise interventions on FMS and its categories in children with ASD.
2. Materials and methods
The presented study was accomplished in compliance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (24). Furthermore, it was registered in INPLASY (doi: 10.37766/inplasy2022.12.0013).
2.1. Data sources and searches
We searched seven online databases (PubMed, Scopus, Web of Science, Embase, EBSCO, Clinical Trials, and The Cochrane Library) from inception to May 20, 2022. Medical subject headings used were “autism spectrum disorder” AND “children” or “adolescents” AND “fundamental motor skills” AND “physical activity” or “exercise.” Meanwhile, we manually searched the included studies' reference lists, relevant systematic reviews and meta-analyses. In addition, a secondary search was conducted on November 5, 2022, to append the most recent literature. Supplementary Table 1 describes the detailed search strategy.
2.2. Study selection/inclusion criteria
Participants: Children mean age ≤ 18 years who were diagnosed with ASD by the Diagnostic and Statistical Manual of Mental Disorders (DSM-4, or DSM-5), the Autism Diagnostic Interview-Revised (ADI-R), the Autism Diagnostic Observation Schedule (ADOS-2), Gilliam Autism Rating Scale (GARS-2), or other standardized diagnosis criteria.
Interventions: Exercise interventions included structured or unstructured training, exercise, or physical activities.
Control conditions: Treatment as usual, waitlist, or a control group (not treated with any physical activity or exercise).
Outcome measures: The outcome measure was assessed by validated tools, such as the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT-2), TGMD-2, or other standardized tools. Outcome indicators included quantitative data on FMS (LMS, OCS, SS), at least one used to calculate the summary effect size.
Study design: Only RCTs were eligible.
2.3. Exclusion criteria
Studies that meet the following criteria are excluded: the study subject's age was out of the limits; the study data could not be extracted; a publication that was a case study, review, or conference paper; the exercise intervention group contained confounding factors other than exercise, such as behavioral interventions, drugs etc.; studies not published in English.
2.4. Data extraction
After removal of duplicates, two researchers (JYQ and ZZY) independently screened titles, abstracts, and full-texts using Endnote X9 to make an initial assessment. A third authors (TH) was available for mediation throughout this process.
Data were extracted from the included studies according to a predefined protocol by two authors (JYQ and TH) and cross-checked for accuracy by a third author (YQ). The data extracted included: basic details of literature (first author name, year of publication, and country/region); participant characteristics (diagnosis, age range, sex, sample size); intervention components (type, session time, frequency, duration, measurement tools); major findings (LMS, OCS, SS).
2.5. Study quality
Study quality was scored by The Physiotherapy Evidence Database Scale (PEDro) (25). The PEDro is a well-proved scale for assessing the study quality of exercise intervention on children with ASD (26). The PEDro scale includes 11 rating criteria regarding eligibility, randomization, allocation, blinding (subjects and experimenter), intention-to-treat, between-group comparison and point measures. PEDro scale scores range from 0 to 10. Notably, one review stated that blinding might be unrealistic in exercise interventions (27). Thus, blinding was ignored due to the limitations of exercise interventions (28). Three levels ranked the quality of one study: low quality with a score <4, medium quality with a score of 4–5, and high quality with a score ≥6 (29). The quality of included studies were independently assessed by two authors based on PEDro, and a third author resolved any disagreements.
2.6. Data analysis
Stata 14.0 software (Stata, Texas, USA) was used for data analysis. The included data were continuous variables. The effect sizes (ESs) were calculated by Standardized mean difference (SMD) and 95% confidence intervals (CI). In the absence of sufficient data to conduct meta-analyses, the magnitude of ESs was calculated by Hedges' g (30). 0.2, 0.5, and 0.8 represents thresholds for small, medium, and large effects (31). Heterogeneity was determined with the p-value (threshold point of 0.1) and I2 statistic (25, 50, 75% representing thresholds for small, medium, and large ratios of inter-study heterogeneity) (32). We applied the fixed-effect model if no statistical heterogeneity was found across studies (I2 ≤ 50%, p > 0.1). Otherwise, the random-effects model was used (26).
Due to different frameworks among several measurement tools, the overall outcomes are hardly measured based on a consistent standard. Therefore, we analyzed the effect on the three sub-categories (LMS/OCS/SS) of the FMS (13). Considering that the ESs may be influenced by heterogeneity factors (FMS measurements, intervention type, intervention time, intervention frequency, intervention duration), Subgroup analyses were conducted. Furthermore, regression analyses were also performed for measurements, type, time, frequency, and duration. Since fewer than ten studies were included in each analysis, publication bias was not investigated. Sensitivity analysis was performed by excluding individual studies one by one using Stata 14.0 software.
2.7. Evidence certainty assessment
Two independent researchers assessed the quality of the evidence for each outcome using the Grading Recommendations to Assess Development and Evaluation System (GRADE), which is one of the international standards for quality of evidence and the classification of recommendation strength. The quality of the evidence can be classified to four levels: I (high); II (moderate); III (low); IV (very low) (33). Five factors can decrease the quality of the evidence: (1) risk of bias; (2) imprecision; (3) inconsistency; (4) indirectness; and (5) publication bias. Three factors can increase the quality of the evidence: (1) effect size; (2) dose-response gradient; and (3) control of confounding variables.
3. Results
3.1. Literature search results
The initial search found 2,696 articles from seven databases. After removing 649 duplicated articles, 2,047 were screened by titles and abstracts. Subsequently, full-text screening was retrieved for 44 articles, of which 13 were eligible for inclusion. Finally, 13 articles were included in the systematic review, and ten were included in the meta-analysis. Three articles were excluded from the meta-analysis as they only reported the FMS total score (34–36). Figure 1 shows the screening process for the study and the reasons for exclusion (37).
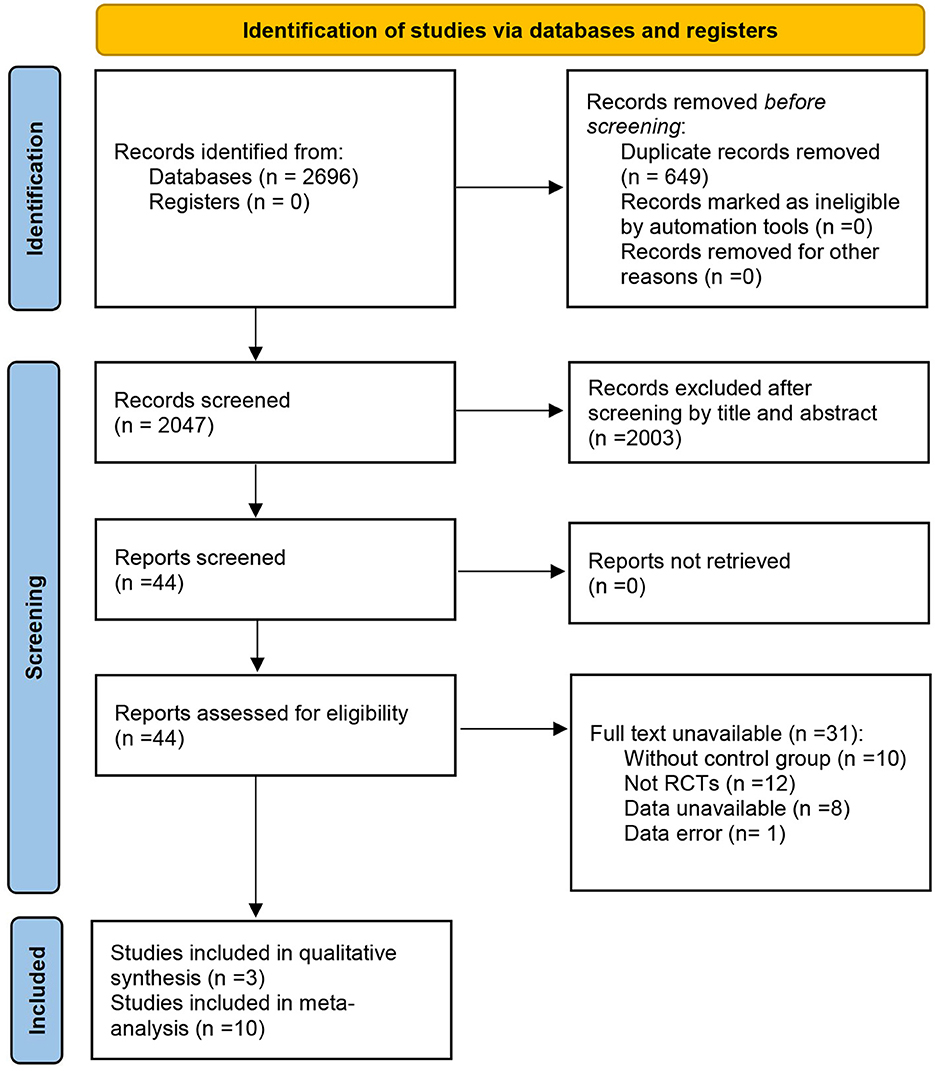
Figure 1. PRISMA flow diagram of the selection studies (37).
3.2. Characteristics of included trials
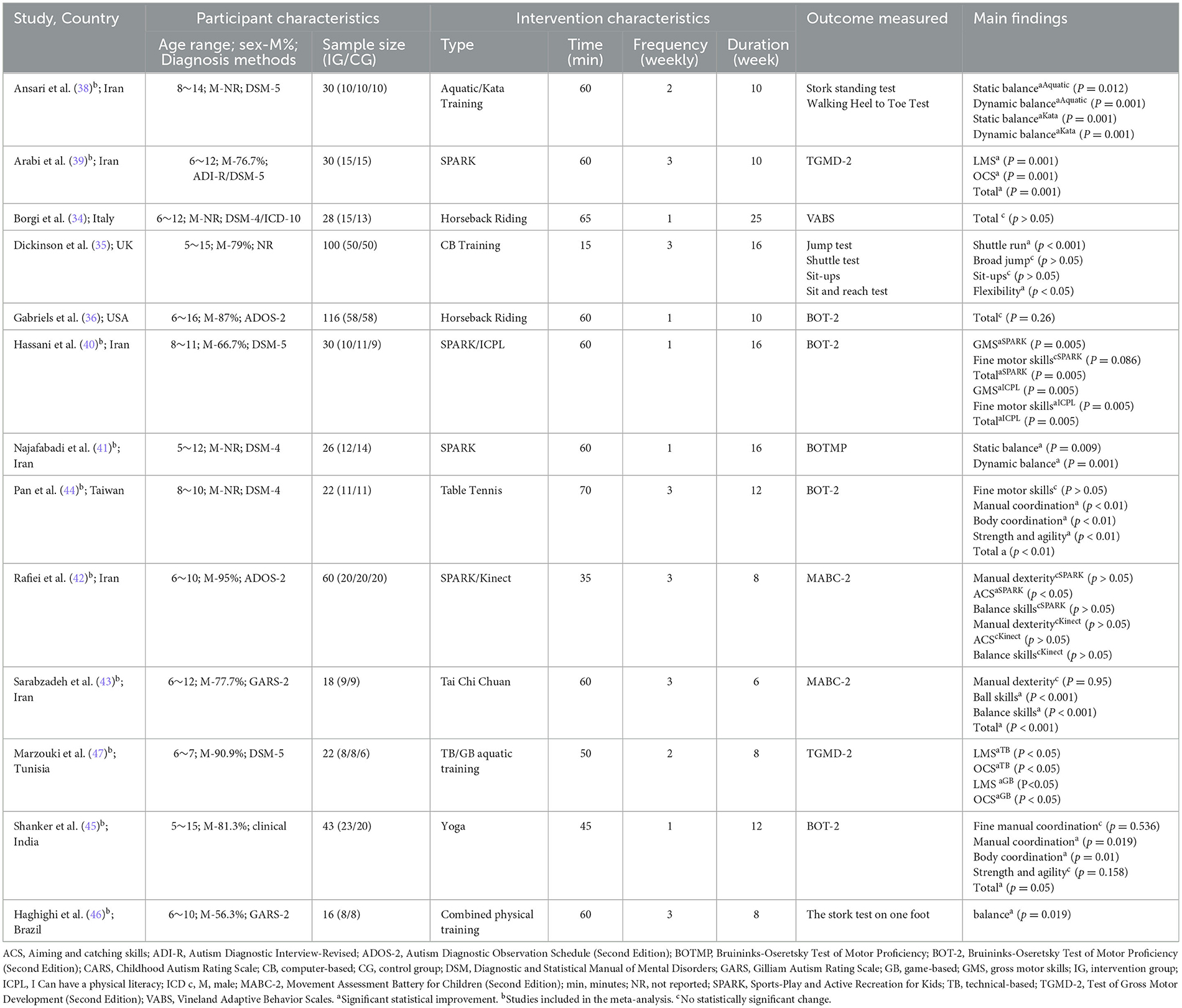
Thirteen included RCTs studies listed in Table 1. In terms of years, ten studies were conducted within the past 5 years (34–36). In terms of the Country and region, seven studies were conducted in Iran (38–43) and Taiwan (44); the remains were from the USA (36), UK (35), Italy (34), India (45), Brazil (46), and Tunisia (47). A total of 541 participants were included, of which 298 were in the exercise intervention group, with a sample size range from 16 to 116. The age of the included sample ranged from 5 to 16, with 84.3% of the sample being male.
Eleven studies reported the ASD diagnosis confirmed using the formal diagnostic criteria. Seven studies (34, 38–41, 44, 47) used the DSM-4 or DSM-5, often the gold standard diagnostic tool for psychologists (33). Additionally, two studies (36, 42) used ADOS-2, two (43, 46) used GARS-2, whereas two studies (35, 45) did not describe the diagnostic process. Moreover, six studies assessed autism severity: four (38, 41, 43, 46) used GARS-2, one (47) used CARS-2, and one (45) used Autism Treatment Evaluation Checklist (ATEC). The other seven studies did not mention autism severity.
The intervention type involved four approaches: game (e.g., Kinect tennis, SPARK), motor skill (e.g., tennis, yoga, tai chi, karate), horseback riding, and aquatic training intervention. The intervention frequency ranged from 1 to 3 times/week, with each session ranging from 15 to 70 min and duration ranging from 6 to 25 weeks. The effects of exercise interventions on FMS were measured by 13 studies using diverse tools, in which six studies assessed LMS, six examined OCS, and seven (38, 41–46) targeted SS. Two studies (39, 47) deployed TGMD-2 to assess LMS and OCS; five studies (36, 40, 41, 44, 45) assessed FMS by BOTMP or BOT-2, and two studies (42, 43) used MABC-2. Moreover, four general measures (Walking Heel to Toe Test, shuttle test, et. al.) were used to assess FMS in children with ASD (34, 35, 38, 46).
3.3. Study quality
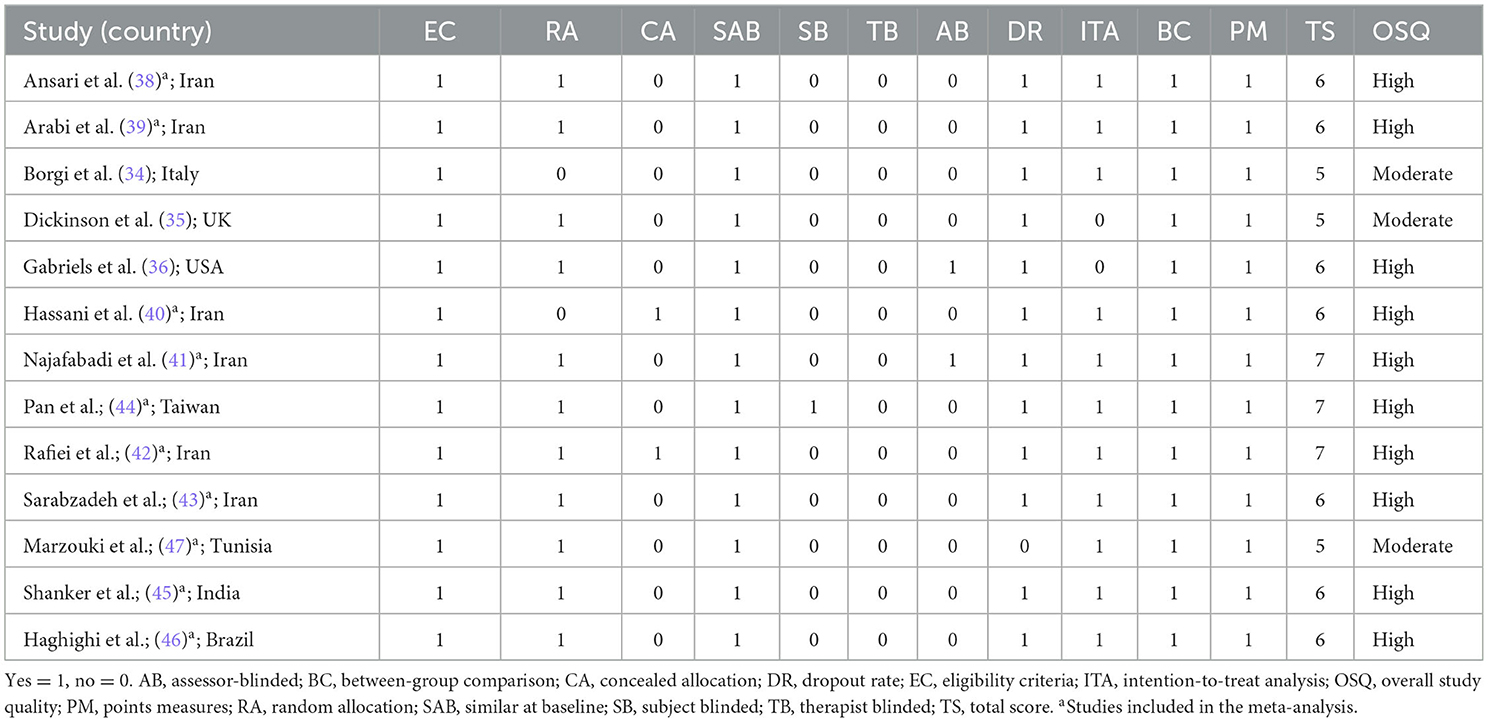
The PEDro scores of included studies ranged from 5 to 7, indicating the study quality from moderate to high (moderate = 23%, high = 77%). All included studies had clear recruitment criteria and were similar at baseline. However, few studies have been conducted on concealed allocation and blinding. The final PEDro scores are presented in Table 2. In addition, sensitivity analysis showed that the combined results changed insignificantly, demonstrating that the meta results were relatively stable, as shown in the Supplementary Figures 1–3.
4. Meta-analysis results
Among the 13 studies, 10 RCTs investigated exercise intervention effects on FMS in children with ASD and were included in the meta-analysis. Due to the different FMS measurement tools, the content included in the total FMS score is inconsistent. Moreover, the overall improvement effect on the FMS of children with ASD is hard to calculate. Therefore, we analyzed the effect on the three lower categories of the FMS (LMS/OCS/SS). In addition, four studies included two intervention groups, which were distinguished in the meta-analysis using a and b.
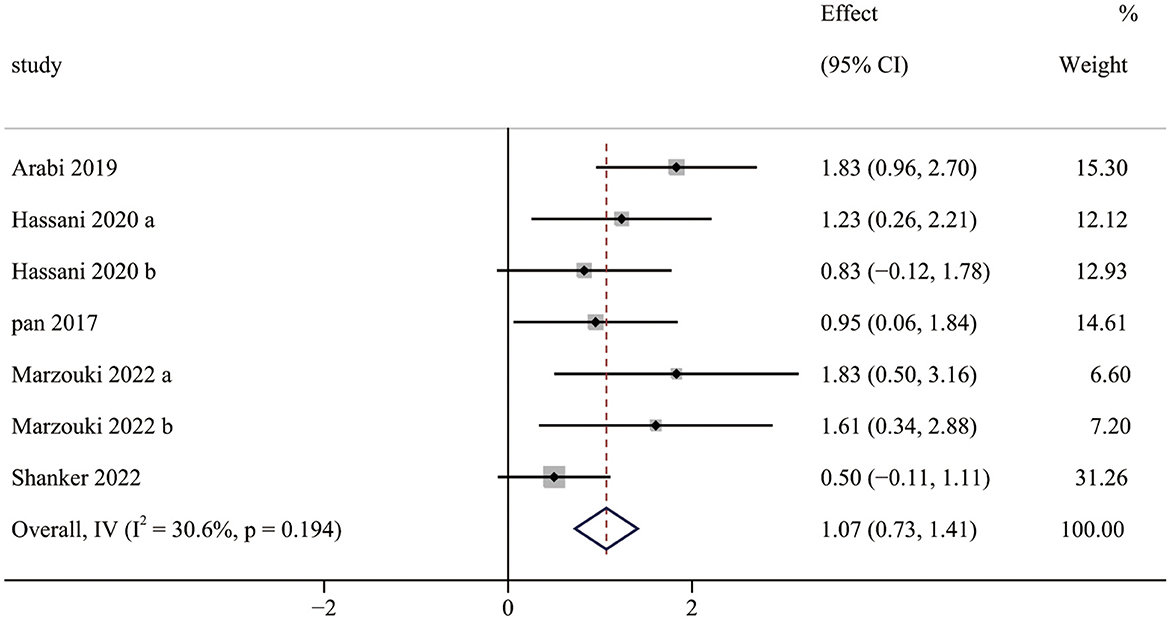
Five studies (seven pairwise comparisons) investigated the effect of exercise interventions on LMS. The combined results showed that exercise interventions significantly improved the LMS of children with ASD (SMD = 1.07; 95% CI 0.73 to 1.41; p < 0.001) (Figure 2). The SMDs of exercise intervention were considered high ESs.
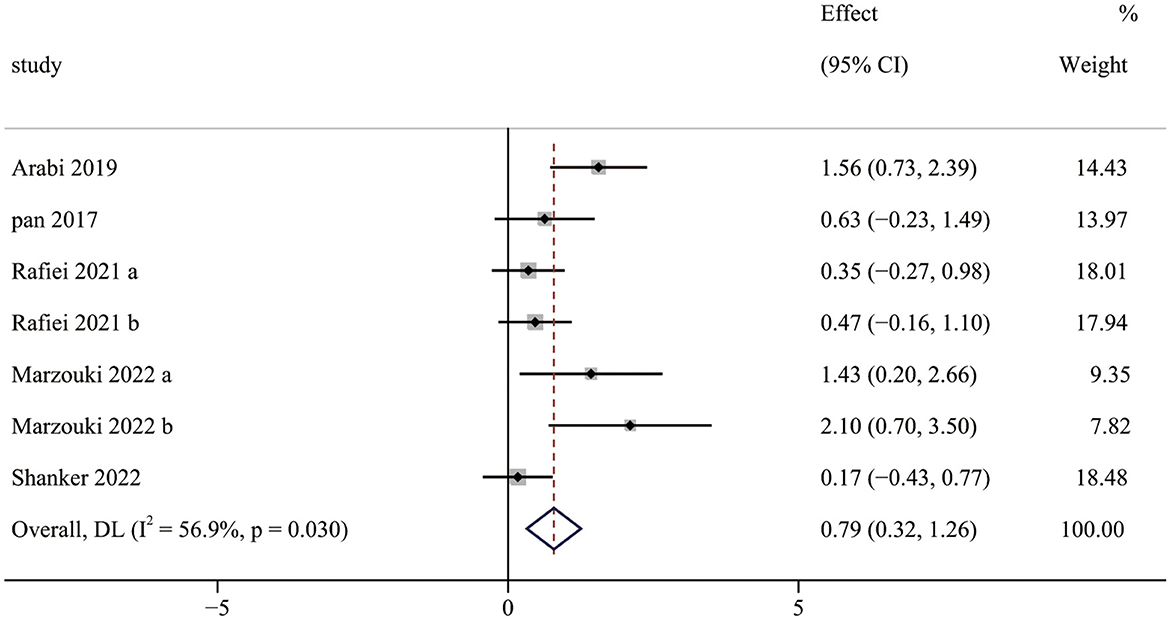
Five studies (7 pairwise comparisons) investigated the effect of exercise interventions on OCS. The combined results showed that exercise intervention significantly improved the OCS of children with ASD (SMD = 0.79; 95% CI 0.32 to 1.26; p = 0.001) (Figure 3). The SMDs of exercise intervention were considered moderate ESs.
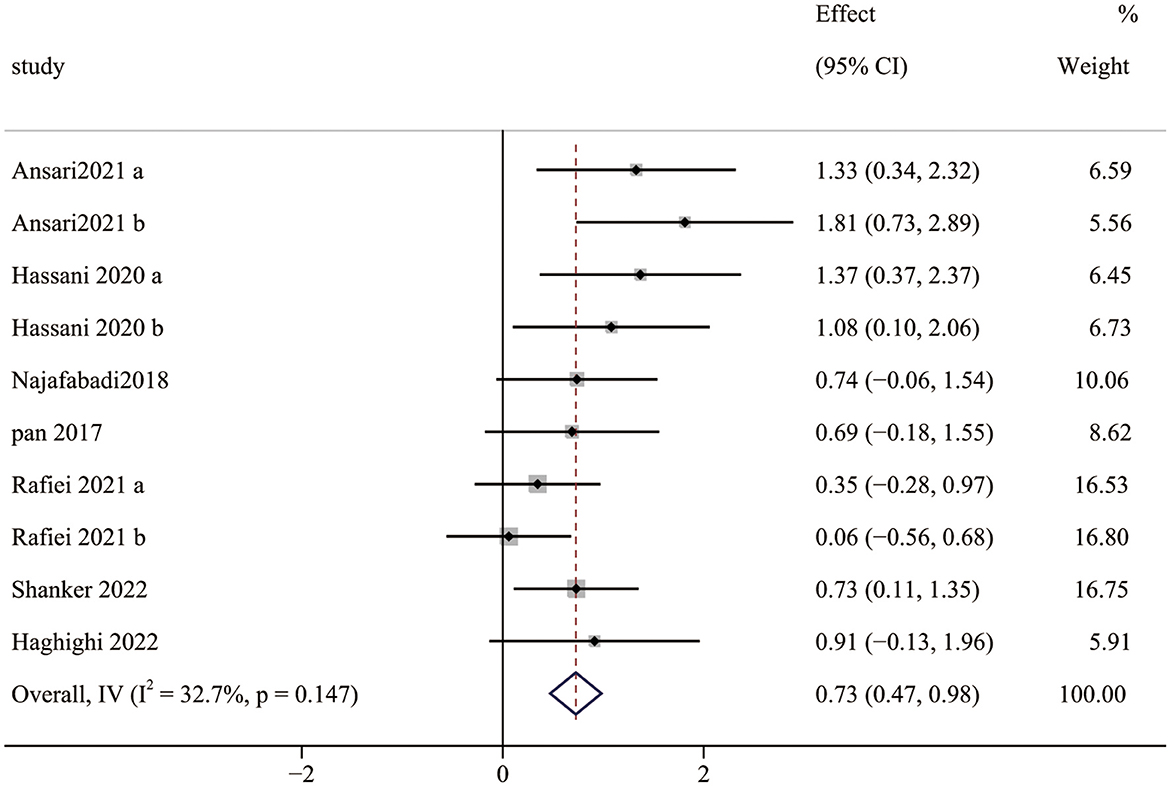
Seven studies (10 pairwise comparisons) examined the effect of exercise interventions on SS. The combined results showed that exercise intervention significantly improved the SS of children with ASD (SMD = 0.73; 95% CI 0.47 to 0.98; p < 0.0001) (Figure 4). The SMDs of exercise intervention were considered moderate ESs.
4.1. GRADE quality evaluation
According to the criteria of GRADE, this study evaluated the certainty of the evidence regarding the significant improvement of exercise interventions on the categories (LMS, OCS, and SS) of FMS in children with ASD. Specifically, exercise interventions exhibited moderate-quality evidence for OCS and low-quality evidence for LMS and SS, as shown in the Supplementary Table 2.
4.2. Subgroup analysis
For exercise intervention, the variables of exercise intervention (type, duration, and frequency) and outcome measures are likely to influence children with ASD in their LMS, OCS, and SS. A moderator analysis was conducted using the corresponding model to investigate potential sources of variance.
4.2.1. Locomotor skills
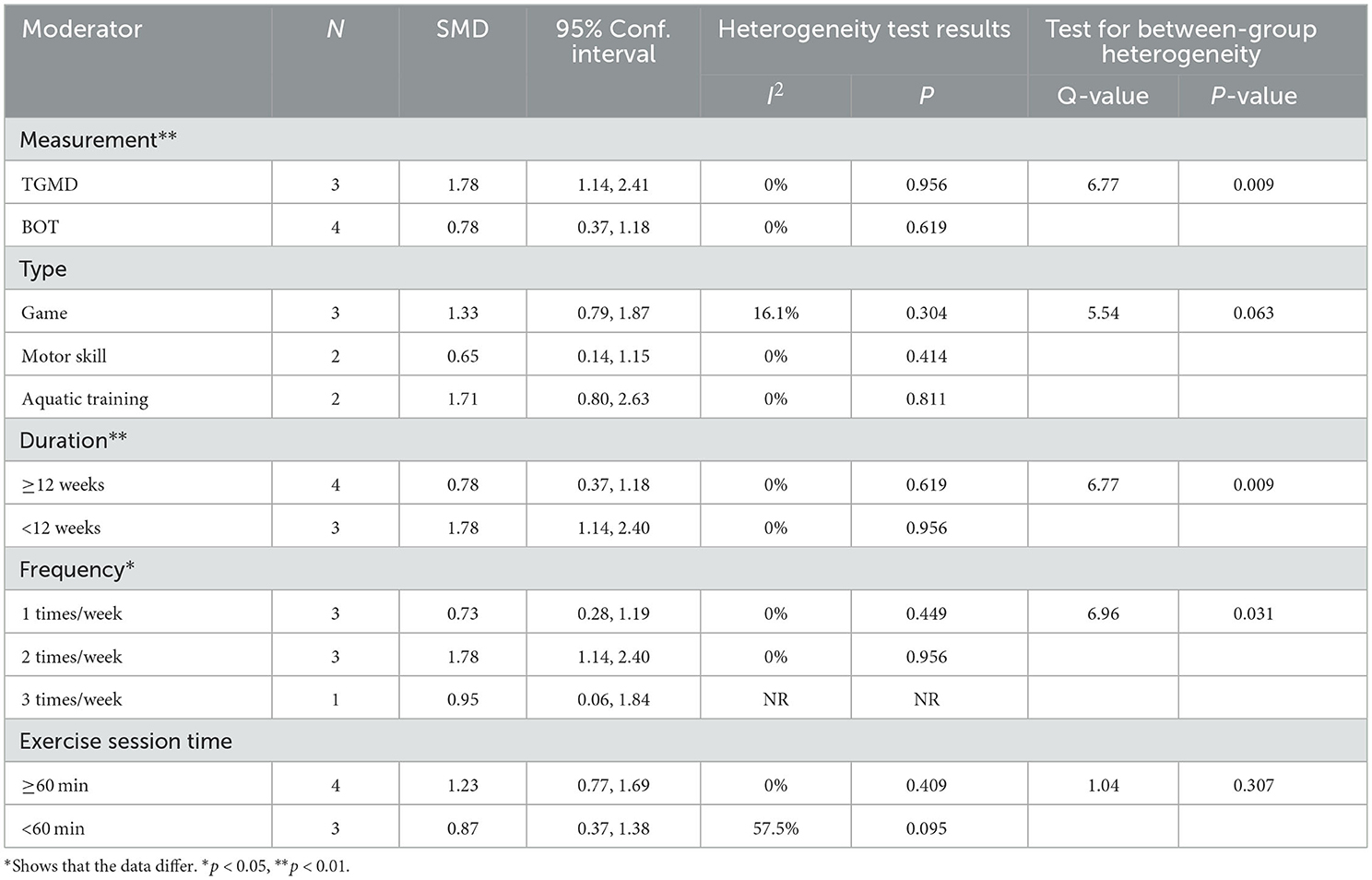
The subgroup analysis showed that measurement tools significantly moderated the effect of exercise interventions on LMS (Q = 6.77, p = 0.009). The ESs of the TGMD results (SMD = 1.78; 95% CI 1.14 to 2.40; p < 0.001) were significantly higher than that of the BOT results (SMD = 0.78; 95% CI 0.37 to 1.18; p < 0.001). Furthermore, the exercise intervention groups with 1–3 times/weeks were included in our current meta-analysis, indicating a statistically significant difference in the ESs (Q = 6.96; p = 0.031). The 2 times/week intervention had significant improvement in LMS (SMD = 1.78; 95% CI 1.14 to 2.40; p < 0.001) compared with 1 time/week (SMD = 0.73; 95% CI 0.28 to 1.19; p = 0.002) and 3 times/weeks (SMD = 0.95; 95% CI 0.06 to 1.84; p = 0.036). Similarly, the duration of intervention produced a statistically significant difference between the two subgroups (Q = 6.77, p = 0.009). The ESs for duration <12 weeks (SMD = 1.78; 95% CI 1.14 to 2.40; p < 0.001) was larger than for duration ≥ 12 weeks (SMD = 0.78; 95% CI 0.37 to 1.18; p < 0.001). Moreover, there were no statistical differences in the type (Q = 5.54, p = 0.063), and session time (Q = 1.04, p = 0.307) (Table 3).
4.2.2. Object control skills
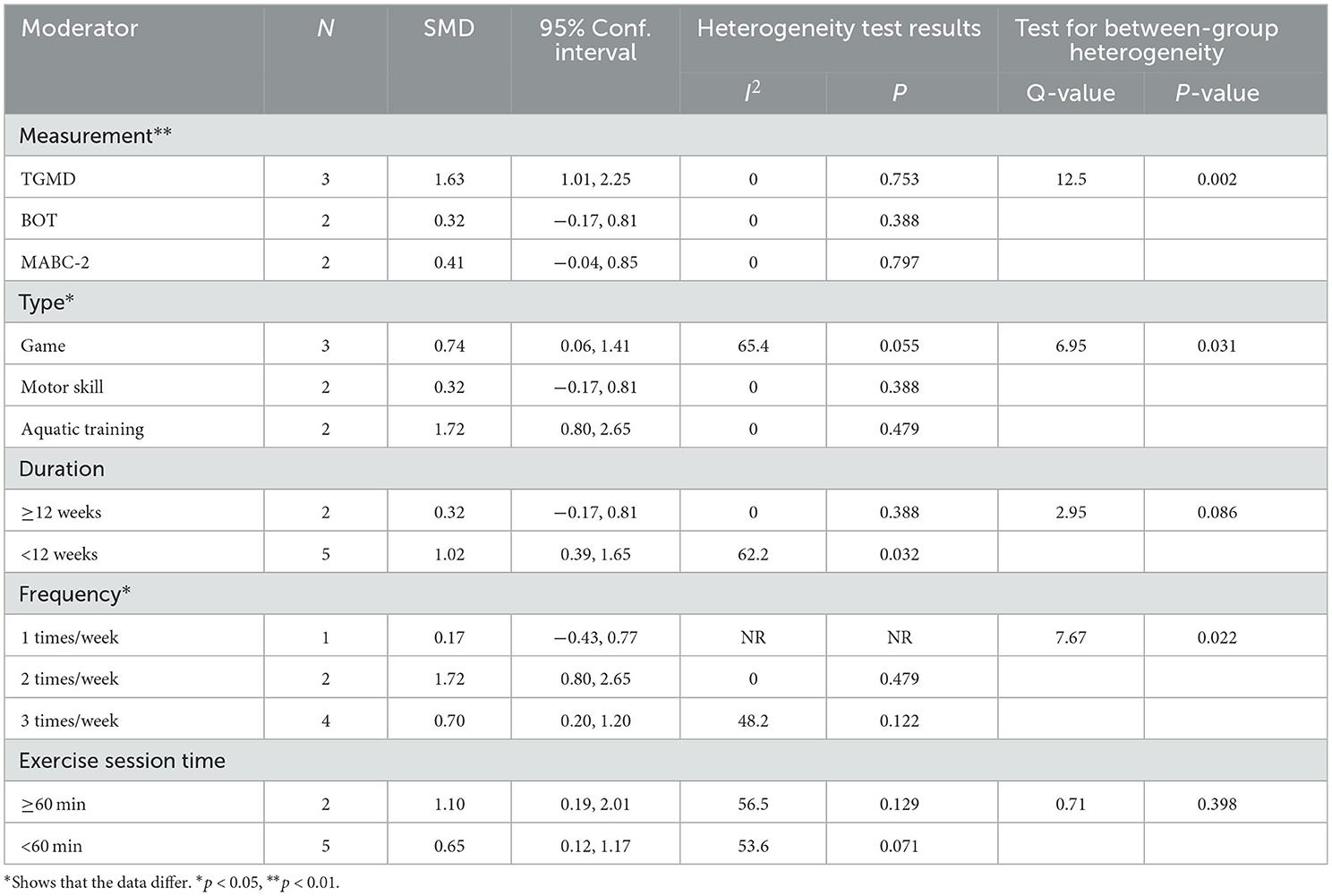
The subgroup analysis showed that measurement tools significantly moderated the effect of exercise interventions on OCS (Q = 12.5, p = 0.002). The ESs of the TGMD results (SMD = 1.63; 95% CI 1.01 to 2.25; p < 0.001) was higher than that of the BOT (SMD = 0.32; 95% CI−0.17 to 0.81; p = 0.202) and MABC-2 results (SMD = 0.41; 95% CI−0.04 to 0.85; p = 0.007). In terms of the type, a statistically significant ESs was found (Q = 6.95, p = 0.031) in the games (SMD = 0.74; 95% CI 0.06 to 1.41, p = 0.032), motor skill (SMD = 0.32; 95% CI−0.17 to 0.81; p = 0.20) and aquatic training (SMD = 1.72; 95% CI 0.80 to 2.65; p < 0.001). Notably, frequency significantly moderated the effect of exercise interventions on OCS (Q = 7.67, p = 0.022). Results revealed that the ESs for children with ASD engaged in a moderate-frequency (2 times/weeks) (SMD = 1.72; 95% CI 0.80 to 2.65; p < 0.001) was greater than those of in low-frequency (1 time/weeks) (SMD = 0.17; 95% CI−0.43 to 0.77; p = 0.581) or high-frequency (3 times/weeks) (SMD = 0.70; 95% CI 0.20 to 1.20; p = 0.006). By contrast, there were no statistical differences in duration (Q = 2.95, p = 0.086) and session time (Q = 0.71, p = 0.398) (Table 4).
4.2.3. Stability skills
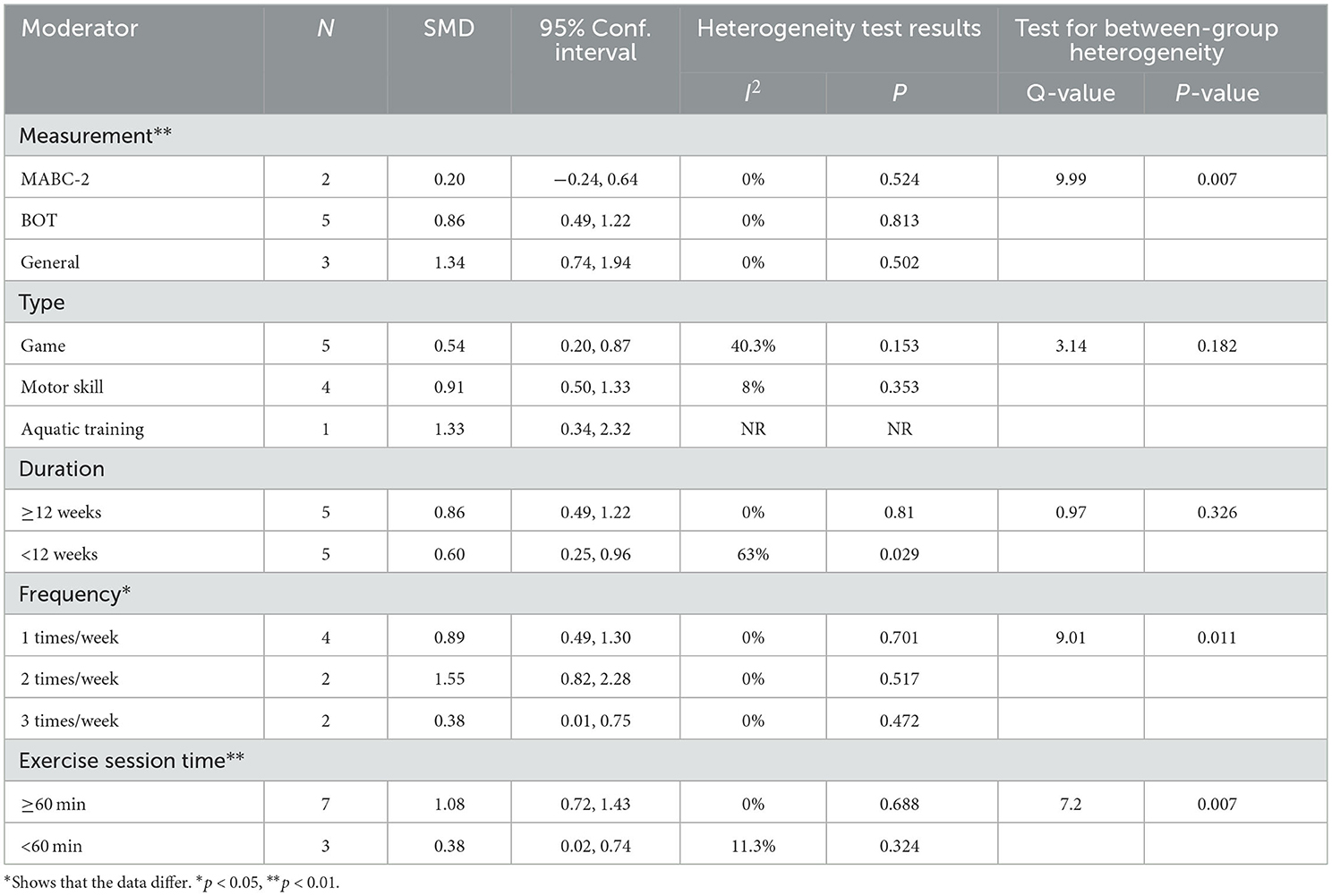
The subgroup analysis showed that measurement tools significantly moderated the effect of exercise intervention on SS (Q = 9.99, p = 0.007). The ESs of the general test results (SMD = 1.34; 95% CI 0.74 to 1.94; p < 0.001) was higher than that of the BOT (SMD = 0.86; 95% CI 0.49 to 1.22; p < 0.001) and TGMD results (SMD = 0.20; 95% CI−0.24 to 0.64; p = 0.366). For duration, the long-term (≥12 weeks) (SMD = 0.86; 95% CI 0.49 to 1.22; p < 0.001) had a significant improvement in SS compared with the short-term (<12 weeks) (SMD = 0.60; 95% CI 0.25 to 0.96; p = 0.001). Moreover, frequency significantly moderated the effect of exercise on OCS (Q = 9.01, p = 0.011). Results revealed that the ESs for children with ASD engaged in a moderate-frequency (2 times/weeks) (SMD = 1.55; 95% CI 0.82 to 2.28; p < 0.001) was greater than those of in low-frequency (1 time/weeks) (SMD = 0.89; 95% CI 0.49 to 1.30; p < 0.001) or high-frequency (3 times/weeks) (SMD = 0.38; 95% CI 0.01 to 0.75; p = 0.044). In addition, there were no statistical differences in type (Q = 3.14, p = 0.182) and duration (Q = 0.97, p = 0.326) (Table 5).
4.3. Meta-regression analysis
In order to investigate the exercise intervention effects on LMS, OCS, and SS, a meta-regression analysis was conducted to identify variables that might influence the effect of the intervention. Results indicated that measurement (β = −0.9962322; p = 0.048; CI−1.98 to−0.01) and duration (β = −0.9962322; p = 0.048; CI 0.01 to 1.98) significantly influenced LMS (Supplementary Table 3). Regarding the exercise intervention effects on OCS, all variables did not influence the ESs (Supplementary Table 4). In addition, results revealed that measurement (β = 0.581979; p = 0.014; CI 0.16 to 1.01) and exercise session time (β = −0.6958256; p = 0.028; CI−1.29 to−0.10) had a significant influence on SS, with higher session time leading to a more remarkable improvement in SS (Supplementary Table 5).
5. Discussion
To the best of our knowledge, the present study is the first systematic review and meta-analysis examining the effects of exercise interventions on FMS in children with ASD. The results demonstrated that exercise intervention has a moderate to high effect on three categories of FMS. Available evidence suggests that exercise intervention can significantly improve LMS (SMD = 1.07), OCS (SMD = 0.79), and SS (SMD = 0.73) compared with controls receiving no intervention.
5.1. Measurement
This study found a statistically significant difference between different FMS measurement tools. Every measurement tool has dominated function. For example, the TGMD-2 mainly assesses LMS (running, galloping, hopping, etc.) and OCS (two-hand strike, two-hand catch, kick, etc.) (48). The BOT-2 includes eight subtests (balance, upper limb coordination, strength, running speed and agility, etc.) that measure the LMS, OCS, and SS (49). In the included studies, FMS measurement in children with ASD involved various standardized tools, including TGMD-2, BOT-2, and MABC-2. By comparing their differences, the reasons for heterogeneity can be identified.
The present study found that TGMD-2 is better than BOT-2 and MABC-2. There are two general types of FMS measurement tools: product-oriented and process-oriented. Product-oriented tools measure the outcome of a motor skill performance, such as speed, distance, or time, also known as quantitative evaluation (50). In contrast, process-oriented tools mainly measure specific motor skill performance and completion process, also known as qualitative evaluation (50). Studies show that process- and product-oriented tools assess different aspects and do not equally evaluate intervention efficacy (51). In this study, the TGMD-2 is a process-oriented tool, while the MABC-2 and the BOT-2 are product-oriented tools, which explains the significant differences between the effects of separate measurement tools.
5.2. Locomotor skills
The meta-analysis demonstrates that exercise intervention has a large positive effect on LMS (SMD = 1.07), which is similar to previous results (9, 52). LMS is the ability of an individual to move within a spatial location, which was measured primarily through the TGMD and BOT. The possible reason is that specifically-designed and structured exercise interventions provide sufficient opportunities for children to perform the correct movement patterns to improve FMS (53). Foulkes et al. (54) implemented a 6-week active play (AP) intervention program in 162 preschool children aged 3–5 years and found no significant improvement in LMS and OCS. Moghaddaszadeh et al. (55) used a 7-week guided AP intervention program in 52 school-aged children aged 5–7 years, and found that LMS improved more significantly compared to the AP intervention group. It indicates that structured, guided exercise interventions improve the LMS of children with ASD rather than just giving children the opportunity to play freely.
Furthermore, our meta-analysis found moderate and significant heterogeneity between studies (I2 = 30.6%). The subgroup analysis revealed that a duration of <12 weeks had significantly higher effects than those of ≥12 weeks. This finding is in contrast to previous studies (11). Case et al. investigated the effect of different intervention approaches on gross motor outcomes of children with ASD and found that a total intervention time of ≥16 h was significantly higher than of <16 h. The possible reason is that the outcome measurement tools used for the studies (<12 weeks) were both TGMD, whereas the studies (≥12 weeks) were all evaluated by BOT, in which TGMD is a qualitative measurement tool, while BOT is a quantitative measurement tool. It hardly compared the results between the two types of measurement tools. Furthermore, the effects of interventions measured by TGMD were significantly higher than those measured by BOT. Notably, the effects of intervention with a moderate frequency (2 times/week) were significantly higher than those with low frequency (1 time/week) or high frequency (3 times/week). The reason for this finding is unclear due to limited relevant research. In addition, meta-regression demonstrated that measurement tools and duration were moderators of the effect of exercise interventions on LMS. It implied that the effect of exercise interventions on LMS was influenced by measurement tools and duration, which is consistent with the results of the subgroup analysis.
5.3. Object control skills
The meta-analysis demonstrates that exercise intervention has a moderate positive effect on OCS (SMD = 0.79), which is similar to previous results (9). OCS are complex motor skills that reflect coordination, attention, and information integration by all body systems. It is an essential basis for developing special motor skills. MacDonald et al. found that levels of OCS in children with ASD significantly predicted the Severity of autism. Children with lower OCS were more likely to have deficits in social communicative skills (56). The mountain of motor development theory states that early (1–7 years) and middle childhood (7–12 years) are critical periods for the development of FMS. An earlier FMS acquisition can positively affect subsequent growth and life (57). It proved that the development of FMS occurred unnaturally (58), and appropriate instruction and practice were necessary (59). Bo et al. (60) suggest that the poorer development of FMS in children with ASD may be due to a lack of physical activity participation and an optimal environment for them to practice. Exercise interventions create an appropriate environment for motor development and opportunities to practice, allowing for partial compensation for otherwise deficient motor skills. Therefore, suitable environments and practice opportunities provide targeted instruction and guide repetitive practice for children with ASD. It is essential to their FMS improvement and reinforcements (59).
Furthermore, our meta-analysis found moderate and significant heterogeneity between studies (I2 = 56.9%). The subgroup analysis indicated that aquatic training interventions could improve the ESs (1.72) on OCS and achieve a more significant effect than motor skill and game interventions. Previous studies have demonstrated that aquatic training intervention improves a variety of gross motor outcomes in children with ASD (61–63). The reason for this finding is that aquatic training interventions provide a variety of sensory stimuli through water temperature, weight reduction, and vestibular input. Meanwhile, the properties of water provide postural support and promote relaxation of spastic muscles, allowing a variety of FMS to be performed depending on the individual's skill level (38). A 12-week aquatic training intervention was conducted with three ASD children aged 11–15 years and showed significant improvements in LMS and OCS (16). The increase in OCS was attributed to aquatic training intervention improving the link between perceptual ability, visual-motor integration, and motor skills. In addition, several studies have confirmed the effect of game on the improvement of OCS. It implied that appropriate types of exercise interventions are vital to improving OCS.
5.4. Stability skills
The meta-analysis demonstrates that exercise intervention has a moderate positive effect on SS (SMD = 0.73), which is similar to previous results (64). Balance is the ability of an individual to maintain a specific state of the body under dynamic, static, or kinesthetic conditions (65). Balance control involves a complex interplay between information processing, motor planning, and timing and sequencing of muscle movements (66). Therefore, the reasons for SS improvement in children with ASD can be summarized as follows. First, ankle and knee joints endured adequately exercised during exercise interventions. It effectively improves the integration of associated efferent neuromuscular and sensorimotor inputs, which benefits static postural and dynamic postural control (67). Second, the complex exercise environment constantly stimulated their sensory functions, encouraged positive behavioral and strategic choices, and reduced weight shifts (68). Since SS is the fundament for gross motor skills (running, jumping etc.), especially in the earlier school age (7–10 years) (69), it is critical to improving the SS of children with ASD.
Furthermore, our meta-analysis found moderate and significant heterogeneity between studies (I2 = 32.7%). The subgroup analysis indicated that a session time of ≥60 min could improve the ESs (1.08) on SS and achieve a more significant effect than that of <60 min. Notably, only one in included studies (≥60 min) adopted a session time of 70 min, while others were 60 min. Because most participants had repeated behaviors and hardly focused on themselves (70), too long a session time made them feel bored and neglected to engage in the intervention experiment. Similarly, there is a significant difference among the interventions with different frequencies (one to three times). Although there were no significant differences in intervention duration, some studies suggest that longer exercise intervention duration has a better effect. Zolghadr et al. (71) found that 12 weeks of balance training was better than 6 weeks for children and adolescents with intellectual disabilities. With the extension of the exercise intervention duration, the neuromuscular system of children with ASD adapts to environmental changes due to repeated stimulation, leading to motor ability development and balance skills improvement. Finally, meta-regression showed that measurement tools and exercise session time were moderators of the effect of exercise interventions on SS, implying that the effect of exercise interventions on SS improved with time and was influenced by measurement tools, which is consistent with the results of the subgroup analysis.
5.5. Study limitations
There are several limitations to the present review. Firstly, strict inclusion criteria limited the number of included studies and sample size, which may influence the accuracy. Moreover, some effective exercise interventions may be omitted, for only RCTs were included. Secondly, the wide age span makes it hard to achieve the essential moderating variable of age for the optimal exercise intervention period. Thirdly, various measurement tools were adopted to assess FMS, making it hard to calculate the total ESs and leading to difficulties in synthesizing the results. Fourthly, only a few studies provide scientific monitoring of exercise intensity, which limits the interpretation of heterogeneity. Finally, most studies had no long-term follow-up, and whether exercise intervention has a long-term benefit for FMS remains unclear.
6. Conclusions
This systematic review and meta-analysis demonstrate that exercise interventions may have moderate to large beneficial effects on FMS in children with ASD, including LMS, OCS, and SS. To better understand the exercise intervention effects on children with ASD, well-designed studies are necessary. Specifically, In terms of LMS, TGMD is most appropriate for LMS measurements, and the effective intervention frequency is 2 times/week. In terms of OCS, TGMD is also most effective for OCS measurements, the intervention frequency of 2 times/week is most efficient, and aquatic training is the optimal type of exercise intervention to enhance OCS. In terms of SS, standardized measurement tools (e.g., Borg scale, Stork test) are most effective for SS measurements, the intervention frequency of 2 times/week is most efficient, and exercise session time ≥60 min is more effective. In addition, the effects of children's age and intervention intensity on the effects of exercise interventions need to be further explored.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Y-QJ and HT: acquisition and analysis of data for the study, drafting the paper, and interpretation of data for the study. Y-QJ, Z-YY, and Z-YZ: design and acquisition of data for the study. QY: drafting the paper, revising the paper for important intellectual content, and interpretation of data for the study. All authors contributed to the article and approved the submitted version.
Funding
This work received funding by the Major Project of Natural Science of Jiangsu Provincial Department of Education (17KJA33001).
Acknowledgments
The authors thank all the research staff for their team collaboration work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1132074/full#supplementary-material
References
1. Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: a systematic review update. Autism Res. (2022) 15:778–90. doi: 10.1002/aur.2696
2. American Psychiatric Association A, Association AP. Diagnostic and Statistical Manual of Mental Disorders: Dsm-5: Washington, DC: American psychiatric association (2013). doi: 10.1176/appi.books.9780890425596
3. Colombo-Dougovito AM, Block ME. Fundamental motor skill interventions for children and adolescents on the autism spectrum: a literature review. Rev J Autism Dev Disord. (2019) 6:159–71. doi: 10.1007/s40489-019-00161-2
4. Calhoun SL, Pearl AM, Fernandez-Mendoza J, Durica KC, Mayes SD, Murray MJ. Sleep disturbances increase the impact of working memory deficits on learning problems in adolescents with high-functioning autism spectrum disorder. J Autism Dev Disord. (2020) 50:1701–13. doi: 10.1007/s10803-019-03928-y
5. Curtin C, Jojic M, Bandini LG. Obesity in children with autism spectrum disorder. Harv Rev Psychiatry. (2014) 22:93–103. doi: 10.1097/HRP.0000000000000031
6. Craig F, Margari F, Legrottaglie AR, Palumbi R, de Giambattista C, Margari L, et al. Review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. (2016) 12:1191–202. doi: 10.2147/NDT.S104620
7. Liang X, Li R, Wong SHS, Sum RKW, Sit CHP. Accelerometer-measured physical activity levels in children and adolescents with autism spectrum disorder: a systematic review. Prev Med Rep. (2020) 19:101147. doi: 10.1016/j.pmedr.2020.101147
8. MacDonald M, Lord C, Ulrich DA. Motor skills and calibrated autism severity in young children with autism spectrum disorder. Adapt Phys Activ Q. (2014) 31:95–105. doi: 10.1123/apaq.2013-0068
9. Healy S, Nacario A, Braithwaite RE, Hopper C. The effect of physical activity interventions on youth with autism spectrum disorder: a meta-analysis. Autism Res. (2018) 11:818–33. doi: 10.1002/aur.1955
10. Petrus C, Adamson SR, Block L, Einarson SJ, Sharifnejad M, Harris SR. Effects of exercise interventions on stereotypic behaviours in children with autism spectrum disorder. Physiother Can. (2008) 60:134–45. doi: 10.3138/physio.60.2.134
11. Case L, Yun J. The effect of different intervention approaches on gross motor outcomes of children with autism spectrum disorder: a meta-analysis. Adapt Phys Activ Q. (2019) 36:501–26. doi: 10.1123/apaq.2018-0174
12. Barnett LM, Stodden D, Cohen KE, Smith JJ, Lubans DR, Lenoir M, et al. Fundamental movement skills: an important focus. J Teach Phys Educ. (2016) 35:219–25. doi: 10.1123/jtpe.2014-0209
13. Lubans DR, Morgan PJ, Cliff DP, Barnett LM, Okely AD. Fundamental movement skills in children and adolescents. Sports Med. (2010) 40:1019–35. doi: 10.2165/11536850-000000000-00000
14. Cattuzzo MT, dos Santos Henrique R, Ré AHN, de Oliveira IS, Melo BM, de Sousa Moura M, et al. Motor competence and health related physical fitness in youth: a systematic review. J Sci Med Sport. (2016) 19:123–9. doi: 10.1016/j.jsams.2014.12.004
15. Leonard HC, Hill EL. Review: the impact of motor development on typical and atypical social cognition and language: a systematic review. Child Adolesc Ment Health. (2014) 19:163–70. doi: 10.1111/camh.12055
16. Battaglia G, Agrò G, Cataldo P, Palma A, Alesi M. Influence of a Specific aquatic program on social and gross motor skills in adolescents with autism spectrum disorders: three case reports. J Funct Morphol Kinesiol. (2019) 4:27. doi: 10.3390/jfmk4020027
17. Holfelder B, Schott N. Relationship of fundamental movement skills and physical activity in children and adolescents: a systematic review. Psychol Sport Exerc. (2014) 15:382–91. doi: 10.1016/j.psychsport.2014.03.005
18. Logan SW, Kipling Webster E, Getchell N, Pfeiffer KA, Robinson LE. Relationship between fundamental motor skill competence and physical activity during childhood and adolescence: a systematic review. Kinesiol Rev. (2015) 4:416–26. doi: 10.1123/kr.2013-0012
19. Liu T, Breslin CM. Fine and gross motor performance of the Mabc-2 by CHILDREN with autism spectrum disorder and typically developing children. Res Autism Spectr Disord. (2013) 7:1244–9. doi: 10.1016/j.rasd.2013.07.002
20. Whyatt CP, Craig CM. Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. J Autism Dev Disord. (2012) 42:1799–809. doi: 10.1007/s10803-011-1421-8
21. Pan C-Y, Tsai C-L, Chu C-H. Fundamental movement skills in children diagnosed with autism spectrum disorders and attention deficit hyperactivity disorder. J Autism Dev Disord. (2009) 39:1694. doi: 10.1007/s10803-009-0813-5
22. Ruggeri A, Dancel A, Johnson R, Sargent B. The effect of motor and physical activity intervention on motor outcomes of children with autism spectrum disorder: a systematic review. Autism. (2019) 24:544–68. doi: 10.1177/1362361319885215
23. Healy S, Obrusnikova I, Getchell N. Fundamental motor skill interventions in children with autism spectrum disorder: a systematic review of the literature including a methodological quality assessment. Res Autism Spectr Disord. (2021) 81:101717. doi: 10.1016/j.rasd.2020.101717
24. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (Prisma-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
25. Sherrington C, Herbert RD, Maher CG, Moseley AM. Pedro. a database of randomized trials and systematic reviews in physiotherapy. Man Ther. (2000) 5:223–6. doi: 10.1054/math.2000.0372
26. Liang X, Li R, Wong SHS, Sum RKW, Wang P, Yang B, et al. The effects of exercise interventions on executive functions in children and adolescents with autism spectrum disorder: a systematic review and meta-analysis. Sports Med. (2022) 52:75–88. doi: 10.1007/s40279-021-01545-3
27. Sherrington C, Moseley AM, Herbert RD, Elkins MR, Maher CG. Ten years of evidence to guide physiotherapy interventions: physiotherapy evidence database (Pedro). Br J Sports Med. (2010) 44:836. doi: 10.1136/bjsm.2009.066357
28. Liu S, Yu Q, Li Z, Cunha PM, Zhang Y, Kong Z, et al. Effects of acute and chronic exercises on executive function in children and adolescents: a systemic review and meta-analysis. Front Psychol. (2020) 11:554915. doi: 10.3389/fpsyg.2020.554915
29. Brinsley J, Schuch F, Lederman O, Girard D, Smout M, Immink MA, et al. Effects of yoga on depressive symptoms in people with mental disorders: a systematic review and meta-analysis. Br J Sports Med. (2021) 55:992. doi: 10.1136/bjsports-2019-101242
30. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for T-Tests and anovas. Front Psychol. (2013) 4:863. doi: 10.3389/fpsyg.2013.00863
31. Hedges LV, Olkin I. Estimation of a single effect size: Parametric and nonparametric methods. In: Statistical Methods for Meta-Analysis. Academic Press (1985). p. 75–106. doi: 10.1016/B978-0-08-057065-5.50010-5
32. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
33. Zhang Y, Alonso-Coello P, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, et al. Grade Guidelines: 19. assessing the certainty of evidence in the importance of outcomes or values and preferences—risk of bias and indirectness. J Clin Epidemiol. (2019) 111:94–104. doi: 10.1016/j.jclinepi.2018.01.013
34. Borgi M, Loliva D, Cerino S, Chiarotti F, Venerosi A, Bramini M, et al. Effectiveness of a standardized equine-assisted therapy program for children with autism spectrum disorder. J Autism Dev Disord. (2016) 46:1–9. doi: 10.1007/s10803-015-2530-6
35. Dickinson K, Place M. A randomised control trial of the impact of a computer-based activity programme upon the fitness of children with autism. Autism Res Treat. (2014) 2014:419653. doi: 10.1155/2014/419653
36. Gabriels RL, Pan Z, Dechant B, Agnew JA, Brim N, Mesibov G. Randomized controlled trial of therapeutic horseback riding in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. (2015) 54:541–9. doi: 10.1016/j.jaac.2015.04.007
37. Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. (2022) 18:e1230. doi: 10.1002/cl2.1230
38. Ansari S, Hosseinkhanzadeh AA, AdibSaber F, Shojaei M, Daneshfar A. The effects of aquatic versus kata techniques training on static and dynamic balance in children with autism spectrum disorder. J Autism Dev Disord. (2021) 51:3180–6. doi: 10.1007/s10803-020-04785-w
39. Arabi M, Saberi Kakhki A, Sohrabi M, Soltani Kouhbanani S, Jabbari Nooghabi M. Is visuomotor training an effective intervention for children with autism spectrum disorders? Neuropsychiatr Dis Treat. (2019) 15:3089–102. doi: 10.2147/NDT.S214991
40. Hassani F, Shahrbanian S, Shahidi SH, Sheikh M. Playing games can improve physical performance in children with autism. Int J Dev Disabil. (2020) 68:219–26. doi: 10.1080/20473869.2020.1752995
41. Najafabadi MG, Sheikh M, Hemayattalab R, Memari A-H, Aderyani MR, Hafizi S. The effect of spark on social and motor skills of children with autism. Pediatr Neonatol. (2018) 59:481–7. doi: 10.1016/j.pedneo.2017.12.005
42. Rafiei Milajerdi H, Sheikh M, Najafabadi MG, Saghaei B, Naghdi N, Dewey D. The effects of physical activity and exergaming on motor skills and executive functions in children with autism spectrum disorder. Games Health J. (2021) 10:33–42. doi: 10.1089/g4h.2019.0180
43. Sarabzadeh M, Azari BB, Helalizadeh M. The effect of six weeks of tai chi chuan training on the motor skills of children with autism spectrum disorder. J Bodyw Mov Ther. (2019) 23:284–90. doi: 10.1016/j.jbmt.2019.01.007
44. Pan C-Y, Chu C-H, Tsai C-L, Sung M-C, Huang C-Y, Ma W-Y. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. (2017) 21:190–202. doi: 10.1177/1362361316633562
45. Shanker S, Pradhan B. Effect of yoga on the motor proficiency of children with autism spectrum disorder and the feasibility of its inclusion in special school environments. Adapt Phys Activ Q. (2022) 39:247–67. doi: 10.1123/apaq.2021-0108
46. Haghighi AH, Broughani S, Askari R, Shahrabadi H, Souza D, Gentil P. Combined physical training strategies improve physical fitness, behavior, and social skills of autistic children. J Autism Dev Disord. (2022). doi: 10.1007/s10803-022-05731-8
47. Marzouki H, Soussi B, Selmi O, Hajji Y, Marsigliante S, Bouhlel E, et al. Effects of aquatic training in children with autism spectrum disorder. Biology. (2022) 11:657. doi: 10.3390/biology11050657
48. Houwen S, Hartman E, Jonker L, Visscher C. Reliability and validity of the Tgmd-2 in primary-school-age children with visual impairments. Adapt Phys Activ Q. (2010) 27:143–59. doi: 10.1123/apaq.27.2.143
49. Piek JP, Hands B, Licari MK. Assessment of motor functioning in the preschool period. Neuropsychol Rev. (2012) 22:402–13. doi: 10.1007/s11065-012-9211-4
50. Khodaverdi Z, Bahram A, Khalaji H, Kazemnejad A, Ghadiri F, Lopes VP. Performance assessments on three different motor competence testing batteries in girls aged 7–10. Sport Sci Health. (2020) 16:747–53. doi: 10.1007/s11332-020-00653-3
51. Palmer KK, Stodden DF, Ulrich DA, Robinson LE. Using process- and product-oriented measures to evaluate changes in motor skills across an intervention. Meas Phys Educ Exerc Sci. (2021) 25:273–82. doi: 10.1080/1091367X.2021.1876069
52. Sam K-L, Chow B-C, Tong K-K. Effectiveness of exercise-based interventions for children with autism: a systematic review and meta-analysis. Int J Learn Teach. (2015) 1:98–103. doi: 10.18178/ijlt.1.2.98-103
53. Edwards J, Jeffrey S, May T, Rinehart NJ, Barnett LM. Does playing a sports active video game improve object control skills of children with autism spectrum disorder? J Sport Health Sci. (2017) 6:17–24. doi: 10.1016/j.jshs.2016.09.004
54. Foulkes JD, Knowles Z, Fairclough SJ, Stratton G, O'Dwyer M, Ridgers ND, et al. Effect of a 6-week active play intervention on fundamental movement skill competence of preschool children: a cluster randomized controlled trial. Percept Mot Skills. (2017) 124:393–412. doi: 10.1177/0031512516685200
55. Moghaddaszadeh A, Belcastro AN. Guided active play promotes physical activity and improves fundamental motor skills for school-aged children. J Sports Sci Med. (2021) 20:86–93. doi: 10.52082/jssm.2021.86
56. MacDonald M, Lord C, Ulrich DA. The relationship of motor skills and social communicative skills in school-aged children with autism spectrum disorder. Adapt Phys Activ Q. (2013) 30:271–82. doi: 10.1123/apaq.30.3.271
57. Clark JE, Metcalfe JS. The mountain of motor development: A metaphor. In: Clark JE, Humphrey J, editors. Motor Development: Research and Reviews. Reston, VA: NASPE Publications (2002). p. 163–90.
58. Clark JE. From the beginning: a developmental perspective on movement and mobility. Quest. (2005) 57:37–45. doi: 10.1080/00336297.2005.10491841
59. Goodway JD, Branta CF. Influence of a motor skill intervention on fundamental motor skill development of disadvantaged preschool children. Res Q Exerc Sport. (2003) 74:36–46. doi: 10.1080/02701367.2003.10609062
60. Bo J, Pang Y, Dong L, Xing Y, Xiang Y, Zhang M, et al. Brief report: does social functioning moderate the motor outcomes of a physical activity program for children with autism spectrum disorders-a pilot study. J Autism Dev Disord. (2019) 49:415–21. doi: 10.1007/s10803-018-3717-4
61. Caputo G, Ippolito G, Mazzotta M, Sentenza L, Muzio MR, Salzano S, et al. Effectiveness of a multisystem aquatic therapy for children with autism spectrum disorders. J Autism Dev Disord. (2018) 48:1945–56. doi: 10.1007/s10803-017-3456-y
62. Pan C-Y. Effects of water exercise swimming program on aquatic skills and social behaviors in children with autism spectrum disorders. Autism. (2010) 14:9–28. doi: 10.1177/1362361309339496
63. Pan C-Y. The efficacy of an aquatic program on physical fitness and aquatic skills in children with and without autism spectrum disorders. Res Autism Spectr Disord. (2011) 5:657–65. doi: 10.1016/j.rasd.2010.08.001
64. Djordjević M, Memisevic H, Potic S, Djuric U. Exercise-based interventions aimed at improving balance in children with autism spectrum disorder: a meta-analysis. Percept Mot Skills. (2022) 129:90–119. doi: 10.1177/00315125211060231
65. Majnemer A, Shikako-Thomas K, Schmitz N, Shevell M, Lach L. Stability of leisure participation from school-age to adolescence in individuals with cerebral palsy. Res Dev Disabil. (2015) 47:73–9. doi: 10.1016/j.ridd.2015.08.009
66. Stins JF, Emck C. Balance performance in autism: a brief overview. Front Psychol. (2018) 9:901. doi: 10.3389/fpsyg.2018.00901
67. Fotiadou EG, Neofotistou KH, Giagazoglou PF, Tsimaras VK. The effect of a psychomotor education program on the static balance of children with intellectual disability. J Strength Cond Res. (2017) 31:1702–8. doi: 10.1519/JSC.0000000000001612
68. Golubović Š, Maksimović J, Golubović B, Glumbić N. Effects of exercise on physical fitness in children with intellectual disability. Res Dev Disabil. (2012) 33:608–14. doi: 10.1016/j.ridd.2011.11.003
69. Mickle KJ, Munro BJ, Steele JR. Gender and age affect balance performance in primary school-aged children. J Sci Med Sport. (2011) 14:243–8. doi: 10.1016/j.jsams.2010.11.002
70. Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder:symptom or syndrome? J Atten Disord. (2009) 13:117–26. doi: 10.1177/1087054708326261
Keywords: autism spectrum disorder, children, exercise, fundamental motor skills, meta-analysis
Citation: Ji Y-Q, Tian H, Zheng Z-Y, Ye Z-Y and Ye Q (2023) Effectiveness of exercise intervention on improving fundamental motor skills in children with autism spectrum disorder: a systematic review and meta-analysis. Front. Psychiatry 14:1132074. doi: 10.3389/fpsyt.2023.1132074
Received: 26 December 2022; Accepted: 23 May 2023;
Published: 12 June 2023.
Edited by:
Asma Bouden, Tunis El Manar University, TunisiaReviewed by:
Riccardo Bravi, Università degli Studi di Firenze, ItalyLiu Yueying, Affiliated Hospital of Jiangnan University, China
Copyright © 2023 Ji, Tian, Zheng, Ye and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Ye, eWVxaWFuZ0Buc2kuZWR1LmNu
 Yu-Qin Ji
Yu-Qin Ji Hao Tian
Hao Tian Ze-Yu Zheng
Ze-Yu Zheng Zhuo-Yan Ye4
Zhuo-Yan Ye4 Qiang Ye
Qiang Ye