
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Psychiatry , 15 March 2023
Sec. Mood Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1128406
This article is part of the Research Topic Perspectives on New Fast-acting Antidepressants View all 5 articles
 Shu-lin Gan1,2†
Shu-lin Gan1,2† Yu-qin Long1,2†
Yu-qin Long1,2† Qin-yun Wang1,2†
Qin-yun Wang1,2† Chang-dong Feng1,2
Chang-dong Feng1,2 Chen-xu Lai1,2
Chen-xu Lai1,2 Chun-tong Liu1,2
Chun-tong Liu1,2 Yun-ying Ding1,2
Yun-ying Ding1,2 Hong Liu3
Hong Liu3 Ke Peng1,2*‡
Ke Peng1,2*‡ Fu-hai Ji1,2*‡
Fu-hai Ji1,2*‡Background: Depressive symptoms are common among patients with lung cancer. We aimed to assess the effects of esketamine on postoperative depressive symptoms after thoracoscopic lung cancer surgery.
Methods: In this randomized, double-blind, placebo-controlled trial, 156 patients undergoing thoracoscopic lung cancer surgery were randomly allocated in a 1:1 ratio to receive intravenous esketamine (intraoperatively and in patient-controlled analgesia until 48 h postoperatively) or normal saline placebo. The primary outcome was the proportion of patients with depressive symptoms at 1 month postoperatively, assessed using the Beck Depression Inventory-II (BDI-II). Secondary outcomes included depressive symptoms at 48 h postoperatively, hospital discharge and 3 months postoperatively, BDI-II scores, anxious symptoms, Beck Anxiety Inventory scores, Quality of Recovery-15 (QoR-15) scores, and 1- and 3-month mortality.
Main results: A total of 151 patients (75 in the esketamine group and 76 in the normal saline group) completed the 1-month follow-up. The esketamine group had a significantly lower incidence of depressive symptoms at 1 month compared to the normal saline group (1.3% vs. 11.8%; risk difference = −10.5, 95%CI = −19.6% to −0.49%; p = 0.018). After excluding patients without lung cancer diagnosis, the incidence of depressive symptoms was also lower in the esketamine group (1.4% vs. 12.2%; risk difference = −10.8, 95%CI = −20.2% to −0.52%; p = 0.018). The secondary outcomes were similar between groups, except that the esketamine group had higher QoR-15 scores at 1 month postoperatively (median difference = 2; 95%CI = 0 to 5; p = 0.048). The independent risk factors for depressive symptoms were hypertension (odds ratio = 6.75, 95%CI = 1.13 to 40.31; p = 0.036) and preoperative anxious symptoms (odds ratio = 23.83, 95%CI = 3.41 to 166.33; p = 0.001).
Conclusion: Perioperative administration of esketamine reduced the incidence of depressive symptoms at 1 month after thoracoscopic lung cancer surgery. History of hypertension and preoperative anxious symptoms were independent risk factors for depressive symptoms.
Clinical trial registration: Chinese Clinical Trial Registry http://www.chictr.org.cn, Identifier (ChiCTR2100046194).
According to the Global Cancer Statistics 2020, lung cancer is the second most common cancer and remains the leading cause of cancer death (1). Patients with lung cancer often suffer from psychiatric problems including depression and anxiety. A study showed that the incidence of anxiety and depression in lung cancer patients was 8 and 12% before surgery, respectively, which changed to 9 and 19% after surgery (2). Another study reported that the prevalence of depression was 8% at 1 month, 5.2% at 3 months, and 4.7% at 1 year after curative resection of non–small-cell lung cancer (3). These symptoms can be caused by chemicals released from lung cancer and treatments such as chemotherapy and corticosteroids (4, 5). Depression and anxiety are associated with poor adherence to treatments, reduced quality of life, prolonged hospital stay, and increased postoperative mortality (6). However, these psychiatric illnesses are often neglected, and there is no report on perioperative interventions to reduce depression and anxiety in patients undergoing thoracoscopic lung cancer surgery.
Ketamine is a racemic mixture of S-ketamine and R-ketamine enantiomers (7). Esketamine is the S-ketamine enantiomer, with more potent analgesic and anesthetic effects. Studies have reported the antidepressant effects of ketamine and esketamine. In patients with treatment-resistant depression, 72% of them responded to the ketamine treatment (8). A multicenter study found that the subanesthetic doses of ketamine vs. normal saline did not prevent depressive symptoms at 3 days (14.7% vs. 17.8%) in elderly patients after major surgery (9). In that study, the proportion of patients with depressive symptoms decreased from 17.5 to 6.9% at 30 days postoperatively, without significant difference after sensitivity analysis. A recent meta-analysis showed that intravenous ketamine was associated with reduced postoperative depressive symptoms within 3 days after surgery (10). Esketamine has been shown to reduce postoperative depression scores from 3 days to 1 month postoperatively in patients with breast cancer (11), while a recent study found that continuous intraoperative esketamine infusion did not reduce depression scores at 3 days after gynecological laparoscopy (12). Therefore, further investigations are still required to ascertain the effects of ketamine or esketamine on depressive symptoms in surgical patients.
The use of ketamine is associated with side effects such as nausea and vomiting, headache, and hallucinations (9, 10). Preclinical studies suggested that those side effects of ketamine may be attributed to S-ketamine, but not R-ketamine (7, 13). However, recent clinical evidence showed that perioperative use of ketamine increased the risk of psychotomimetic adverse events (14), while esketamine did not significantly increase the risk (12, 15).
For the assessment of depressive symptoms, Montgomery-Asberg Depression Rating Scale, 9-item Patient Health Questionnaire, Hamilton Depression Scale and Beck Depression Inventory (BDI) have been used in clinical settings (16–18). Of them, BDI is a validated and commonly used measure of depressive symptoms. In this study, we investigated the effects of esketamine on postoperative psychiatric symptoms and related side effects in patients undergoing lung cancer surgery. We hypothesized that perioperative administration of esketamine would improve postoperative depressive symptoms, anxious symptoms, and recovery after thoracoscopic lung cancer surgery.
This single-center, randomized, double-blind, placebo-controlled trial was conducted at the First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (Approval No. 2020-127). This study was registered on the Chinese Clinical Trial Registry on May 9, 2021 before the enrollment of the first participant. All patients provided their written informed consent. This study was performed following the Declaration of Helsinki and the Consolidated Standards of Reporting Trials guidelines.
Patients aged ≥18 years with American Society of Anesthesiologists (ASA) physical status I–III scheduled for thoracoscopic lung cancer surgery were eligible for inclusion. Preoperative diagnosis of lung cancer was based on computed tomography or pathological results. The exclusion criteria included:
1. non-tumor diseases, such as lung infection, fibrosis, and tuberculosis;
2. preoperative mental illness or use of antipsychotics;
3. serious cardiac, renal and liver diseases (heart failure, myocardial infarction, left ventricular ejection fraction <30%, Child–Pugh grade C, and renal replacement therapy);
4. inability to communicate, read, or write;
5. allergies to study medications; or
6. refusal to participate in this study.
Based on a central randomization system,1 patients were randomly allocated in a 1:1 ratio to either the esketamine group or the normal saline group. The details of allocation were concealed using password protection. A research nurse not involved in the subsequent study prepared the study medications according to the randomization results. Both esketamine and normal saline placebo are clear and colorless fluids, so there is no way to distinguish them. All patients, anesthesiologists, surgeons, perioperative care providers, and postoperative assessors were blinded to the group allocation until the final statistical analysis.
After entering the operating room, patients were monitored with electrocardiography, non-invasive blood pressure, and peripheral oxygen saturation (SpO2). Under local anesthesia with 2% lidocaine, an arterial line was placed via the radial artery for continuous arterial pressure monitoring and blood sampling. Anesthesia depth was assessed using the bispectral index (BIS). Anesthesia was induced with intravenous propofol 1.5–2 mg/kg, sufentanil 0.3–0.5 μg/kg, and cisatracurium 0.2 mg/kg. Patients were intubated with a double-lumen endotracheal tube, and the location was confirmed by capnography, auscultation and fiberoptic bronchoscopy. In the lateral position, single-lung ventilation was started (tidal volume of 4–6 ml/kg, respiratory rate of 12–20 breaths/min, 60–100% oxygen, and positive end-expiratory pressure of 5–10 cm H2O) to maintain end-tidal carbon dioxide within 35–45 mmHg and SpO2 ≥ 90%. Lung recruitment maneuvers were carried out when indicated. Anesthesia was maintained with sevoflurane 1–3% inhalation, titrated to BIS values within 40–60. Intermittent boluses of sufentanil or cisatracurium were given intravenously when needed. Patients received Lactated Ringer’s solution for intraoperative volume repletion. Nasopharyngeal temperature was maintained within 36–37°C using a warming blanket.
Hypotension was defined as mean arterial pressure (MAP) < 65 mmHg and treated with fluid infusion, ephedrine, or phenylephrine. Bradycardia was defined as heart rate (HR) < 50 beats/min and treated with intravenous atropine. Interventions for hypertension (defined as MAP >90 mmHg) and tachycardia (defined as HR > 100 beats/min) included adjustment of anesthesia and administration of medications (nitroglycerine, nicardipine or esmolol). At the end of surgery, all patients received intravenous flurbiprofen axetil 50 mg, ondansetron 4 mg, and wound infiltration with 0.5% ropivacaine 15–20 ml. Patients were transferred to the post-anesthetic care unit and extubated there.
In the esketamine group, esketamine 0.1 mg/kg was administered intravenously at about 3 min before skin incision, followed by a continuous infusion of 0.1 mg/kg/h until the end of surgery. The normal saline group received the same volume of normal saline placebo. Patients received sufentanil-based patient-controlled intravenous analgesia (PCIA) during the first 48 h postoperatively. In the PCIA device, sufentanil 2 μg/kg combined with esketamine 1 mg/kg (the esketamine group) or sufentanil 2 μg/kg only (the normal saline group) was diluted with normal saline to a final volume of 100 ml. The background infusion rate was 1.5 ml/h, the bolus dose was 1.5 ml, and the lockout interval was 10 min. Thus, we used esketamine 0.1 mg/kg bolus +0.1 mg/kg/h intraoperatively, followed by 0.015 mg/kg/h postoperatively for 48 h. This dosing regimen of esketamine was according to our clinical practice and the recent literature in which esketamine was administered as 0.125 to 0.25 mg/kg bolus, with or without 0.015 to 0.125 mg/kg/h for 48 h (11, 19, 20).
The primary outcome was the incidence of depressive symptoms at 1 month postoperatively. An independent investigator assessed the depressive symptoms using a WeChat-based questionnaire of the Beck Depression Inventory-II (BDI-II) (18). The BDI-II is a validated self-report measure of depressive symptoms, consisting of 21 items (3 points for each item and scores ranging from 0–63) (21). A total score of 0–13, 14–19, 20–28, and 29–63 indicates absent, mild, moderate and severe depressive symptoms, respectively (22). Patients completed the questionnaire in their own time to avoid the possibility of observer bias (23).
The secondary outcomes included BDI-II scores and depressive symptoms incidence at 48 h postoperatively, at hospital discharge, and at 3 months postoperatively, BDI-II scores at 1 month postoperatively, Beck Anxiety Inventory (BAI; 21 items with scores ranging from 0–63) (24) scores and anxious symptoms incidence at 48 h postoperatively and at hospital discharge, Quality of Recovery-15 (QoR-15) (25) scores at 48 h postoperatively, at hospital discharge, and at 1 month postoperatively, and postoperative 1- and 3-month mortality. BAI and QoR-15 questionnaires were also completed via the WeChat application. Studies have validated the Chinese versions of BDI, BAI, and QoR-15 (26–28).
Patients’ demographics and baseline characteristics were collected 1 day before surgery. Patients were assessed for preoperative depressive and anxious symptoms using BDI-II and BAI. Intraoperative data included use of anesthetics and analgesics (propofol, sevoflurane, sufentanil and esketamine), hemodynamic events, blood loss, fluid infusion, urine output, type of surgery, duration of surgery, and duration of anesthesia. Postoperative data included pain scores at rest and on coughing at 24 and 48 h postoperatively, patient-controlled sufentanil consumption within 0–24 h and 0–48 h postoperatively, esketamine consumption during 0–48 h postoperatively, adverse events (postoperative nausea and vomiting [PONV], nightmare, dizziness, blurred vision, dissociation and delirium), and length of postoperative hospital stay. Pain intensity was assessed using the visual analog scale (VAS; ranging from 0–10, higher scores indicate more pain). Delirium was assessed using the Confusion Assessment Method (CAM) or Confusion Assessment Method for Intensive Care Unit (CAM-ICU) (29). Patients were followed up at 1 month and 3 months postoperatively via WeChat questionnaires.
A recent study reported that the incidence of postoperative depressive symptoms was 19% in patients undergoing surgical treatment for lung cancer (30). The effect of esketamine on depressive symptoms after lung cancer surgery is unknown. A recent study showed that a low-dose ketamine compared to normal saline placebo reduced the occurrence of depressive symptoms by 61% (from 17.5 to 6.9%) at 30 days after major surgery in older adults (9). In addition, another study suggested that the postoperative depression scores were lower in the esketamine group than those in the ketamine group for patients with breast cancer (11). Based on these, we hypothesized that use of esketamine could reduce the depressive symptoms by 70% (i.e., from 19 to 5.7%) in our patients. Thus, 70 patients in each group were required with one-sided test at α level of 0.05 and power of 80%. To allow for a 10% dropout rate, we finally enrolled 156 patients with 78 in each group. We calculated the required sample size using the PASS software (NCSS, LCC, Kaysville, UT).
Data are expressed as mean [standard deviation (SD)], median [inter-quartile range (IQR)], or numbers (percentages). Continuous data were checked for normality using the Kolmogorov–Smirnov test and analyzed using the unpaired Student t-test or Mann–Whitney rank–sum test. Categorical data were analyzed with the chi-square test or Fisher exact test. For the primary and secondary outcomes, the between-group differences were analyzed using risk difference or median difference with 95% confidence interval (CI).
The primary analyzes were conducted in the modified intention-to-treat population including patients who underwent randomization and surgery with available primary outcome measurement (1-month depressive symptoms rate). As depressive symptoms were associated with the pathological results, the primary outcome was also analyzed after excluding patients without lung cancer diagnosis.
We analyzed the risk factors for depressive symptoms at 1 month postoperatively in the modified intention-to-treat population. First, we applied the univariate logistic regression analysis for possible predictive factors. We chose the variables with p < 0.25 based on statistical significance and clinical relevance, and we included these variables into the multiple regression analysis. We analyzed the variance inflation factor to assess multicollinearity of the variables in the multiple regression model. The goodness of fit of the model was examined using the Hosmer–Lemeshow test.
No multiple testing corrections were planned for the secondary outcomes, so these results should be regarded exploratory. We did not perform an interim analysis. Missing values were not imputed. In addition, we conducted post hoc subgroup analysis for the 1-month depressive symptoms rate between male and female patients. Except for the subgroup analysis, all analyzes in this study were planned a priori. Statistical analyzes were performed with SPSS software (version 22.0, IBM SPSS, Chicago, IL). A two-sided p < 0.05 was considered statistically significant.
From May 18, 2021 to May 30, 2022, a total of 332 patients scheduled for thoracoscopic lung cancer surgery were screened (Figure 1). Of them, 176 patients were excluded due to not meeting the inclusion criteria (n = 70), refusal for participation (n = 101), and cancelation of surgery (n = 5). Thus, 156 patients were randomized, with 78 in each group. Five patients were lost to 30-day follow-up (3 in the esketamine group and 2 in the normal saline group). The modified intention-to-treat analyzes included 151 patients (75 in the esketamine group and 76 in the normal saline group) who had available primary outcome data. Analyzes were also performed after excluding patients without lung cancer diagnosis (3 in the esketamine group and 2 in the normal saline group). At 90 days postoperatively, 74 patients in the esketamine group and 76 in the normal saline group were assessed for depressive symptoms.
The demographic and baseline characteristics were comparable between the two groups (Table 1). The mean (SD) age was 54 (12) years for both groups. 69.2% of patients in the esketamine group and 76.9% in the normal saline group were female sex. At 1 day before surgery, patients were assessed for depressive and anxious symptoms. Two patients in the normal saline group showed preoperative depressive symptoms; however, they had no history of previous depressive episodes, nor they were on antidepressants. Three patients in the esketamine group and 5 patients in the normal saline group exhibited anxious symptoms. The median (IQR) time between suspected diagnosis and surgery was 2.5 (0.6, 7.3) and 2 (0.5, 9.3) months in the esketamine group and the normal saline group, respectively.
Table 2 shows the intraoperative and postoperative data. The two groups had similar intraoperative propofol, sevoflurane, and sufentanil consumption. The mean (SD) total dose of intraoperative esketamine was 21.6 (7.0) mg. The two groups were similar in terms of hemodynamic events, blood loss, fluid infusion, urine output, type of surgery, duration of surgery, or duration of anesthesia. For the postoperative data, the two groups had similar VAS pain scores and required similar amount of patient-controlled sufentanil. The mean (SD) total dose of postoperative esketamine was 45.1 (8.8) mg. Adverse events were comparable between groups. 43.6% of patients in both groups experienced PONV; most episodes were mild, and 3 patients required additional antiemetics. No patients experienced dissociation or delirium. The median (IQR) length of postoperative hospital stay was 4 (3.0, 6.3) days in the esketamine group and 5 (3, 6) days in the normal saline group.
The primary outcome of depressive symptoms at 1 month postoperatively was assessed in two study populations, respectively, (Table 3). In the modified intention-to-treat population, patients receiving esketamine had a significantly lower incidence of depressive symptoms at 1 month postoperatively than those receiving normal saline placebo (1.3% vs. 11.8%; risk difference = −10.5, 95%CI = −19.6% to −0.49%; p = 0.018). Postoperative pathological diagnosis confirmed non-cancerous lesions but not lung cancer in 5 patients (3 in the esketamine group and 2 in the normal saline group). After excluding these patients, the incidence of depressive symptoms was also lower in the esketamine group (1.4% vs. 12.2%; risk difference = −10.8, 95%CI = −20.2% to −0.52%; p = 0.018). The post hoc subgroup analysis showed that the 1-month depressive symptoms rate did not differ significantly between males and females (Figure 2).
The details of patients with depressive symptoms are presented in Supplementary Table S1. These patients aged between 34 and 70 years, and most of them (9 out of 10) were female sex. At 1 month postoperatively, the depressive symptoms were mild in 7 patients, experienced moderate in 2 patients, and severe in 1 patient. Two patients with preoperative depressive symptoms (case 6 and case 9) still had symptoms at 1 month postoperatively. Three patients (cases 1, 3, and 6) needed postoperative psychological counseling and received antidepression medications.
The secondary outcomes are shown in Table 3. The two study groups were similar in terms of the BDI-II scores and incidence of depressive symptoms at 48 h postoperatively and at the time of hospital discharge. Of note, at 1 month postoperatively, the median (IQR) BDI-II score was 5 (4, 7) in the esketamine group, which was similar to 5 (4, 8) in the normal saline group (p = 0.664). At 3 months postoperatively, 1 patient in the normal saline group still had depressive symptoms. Two patients in the normal saline group showed anxious symptoms at 48 h postoperatively and at hospital discharge. Regarding the quality of recovery after surgery, the two groups had similar QoR-15 scores at 48 h postoperatively and at hospital discharge; however, the esketamine group had higher median QoR-15 scores at 1 month postoperatively compared to the normal saline group (median difference = 2, 95%CI = 0 to 5; p = 0.048). One patient in the esketamine group died at postoperative day 45 due to respiratory failure.
Based on the univariate logistic regression analysis, female sex, in paid employment, history of hypertension, and preoperative anxious symptoms were associated with increased risk for depressive symptoms at 1 month postoperatively, while use of esketamine was associated with a lower depressive symptoms rate (Table 4). Thus, these 5 factors were included into the multiple regression model and analyzed as categorical variables.
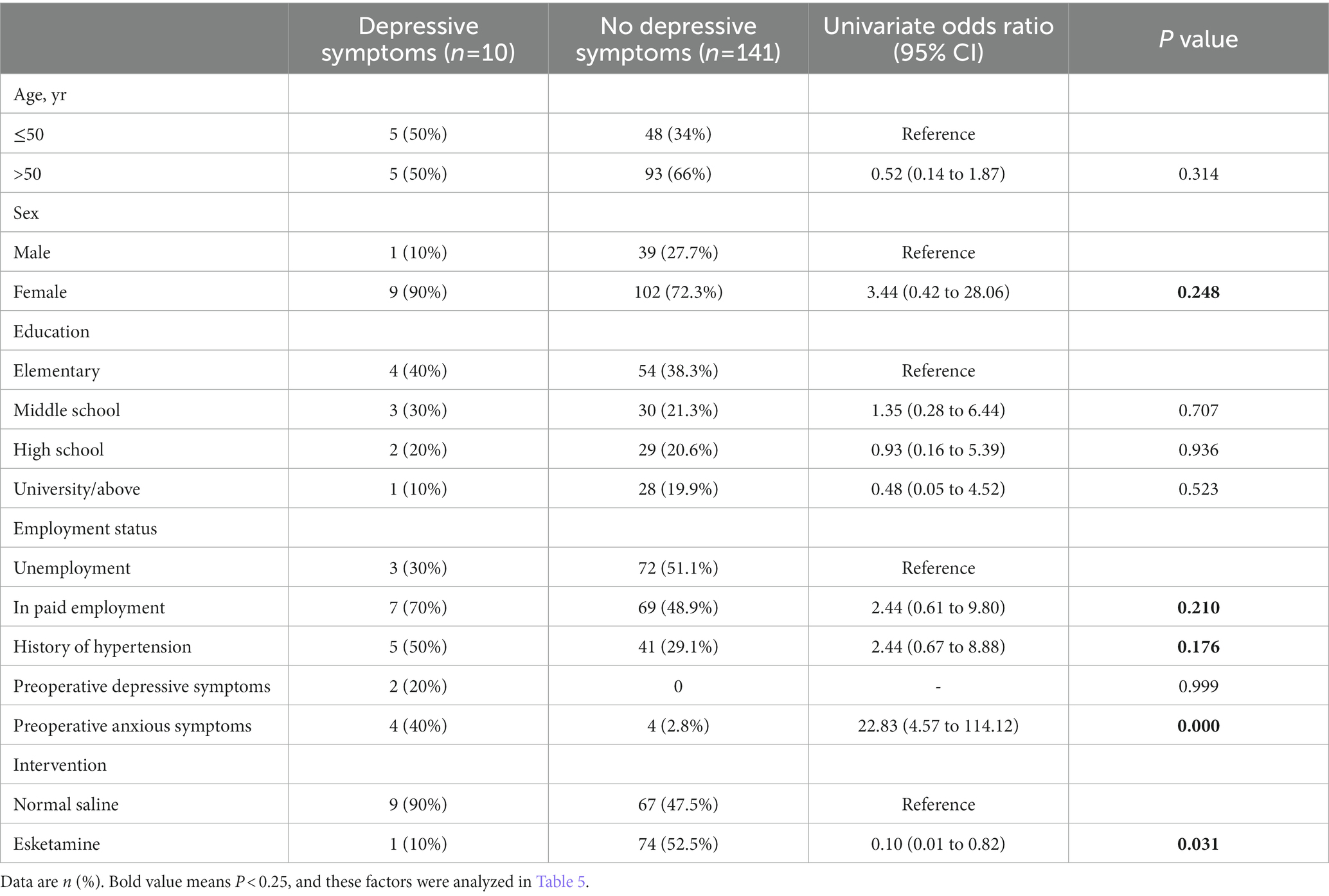
Table 4. Univariate logistic regression analysis of possible predictive factors for depressive symptoms at 1 month postoperatively in the modified intention-to-treat population.
In this multiple logistic regression analysis, the values of variance inflation factor were < 2 for all factors (1.058 for female sex, 1.126 for in paid employment, 1.167 for history of hypertension, 1.017 for preoperative anxious symptoms, and 1.012 for use of esketamine), which indicates minimal collinearity among these predictors. The Hosmer–Lemeshow test showed that p = 0.698, suggesting a high goodness of fit of our model. The independent risk factors for depressive symptoms at 1 month postoperatively were hypertension (multivariate odds ratio = 6.75, 95%CI = 1.13 to 40.31; p = 0.036) and preoperative anxious symptoms (multivariate odds ratio = 23.83, 95%CI = 3.41 to 166.33; p = 0.001). The use of esketamine was a protective factor against 1-month depressive symptoms postoperatively (multivariate odds ratio = 0.09, 95%CI = 0.01 to 0.91; p = 0.042; Table 5).
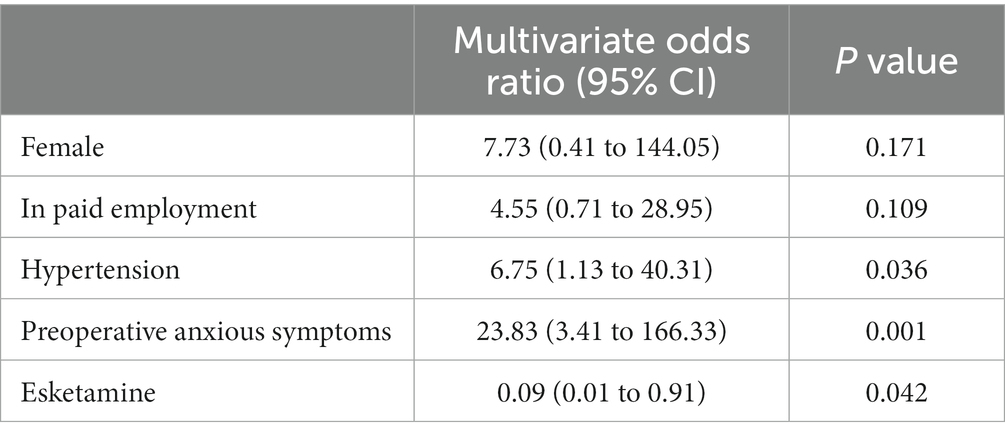
Table 5. Multiple logistic regression analysis of potential risk factors for depressive symptoms at 1 month postoperatively in the modified intention-to-treat population.
This study showed that the perioperative infusion of esketamine prevented the occurrence of depressive symptoms at 1 month after thoracoscopic lung cancer surgery. This finding was evident in the modified intention-to-treat patient population, as well as after excluding patients without lung cancer diagnosis. Moreover, the QoR-15 scores at 1 month were slightly higher in patients receiving esketamine, suggesting that the improvement in depressive symptoms contributed to better postoperative recovery. Our results did not show between-group differences in anxious symptoms, and the use of esketamine did not induce concerning adverse events in our patients. The regression analyzes suggested that history of hypertension and preoperative anxious symptoms were independent risk factors for depressive symptoms and that the use of esketamine was a protective factor.
For patients who underwent lung cancer surgery, the incidence of postoperative depressive symptoms at 2 weeks after discharge was reported to be 19% (30). In our study, 11.8% of patients in the normal saline group developed depressive symptoms, whereas the esketamine treatment reduced the depressive symptoms rate to 1.3%. For patients who had breast cancer and preoperative mild/moderate depressive symptoms, the use of esketamine led to lower postoperative depression scores when compared to saline or racemic ketamine (11). Another recent study showed that esketamine alleviated short-term depressive symptoms after laparoscopic modified radical hysterectomy (19). Furthermore, several studies also suggested that the use of esketamine prevented postpartum depressive symptoms after cesarean section (31, 32). A recent study showed that intraoperative esketamine infusion improved postoperative sleep disturbance, but not depression symptoms, after gynecological laparoscopy (12). However, there is lack of evidence regarding the effects of esketamine on depression after cancer surgery. Our study is the first to demonstrate the impact of esketamine on depressive symptoms in patients who underwent lung cancer surgery.
Studies have suggested that perioperative use of esketamine was associated with reduced pain intensity and opioid requirements after surgery (12, 15, 20). However, our study did not show such a relationship. The two groups had similar pain scores and sufentanil consumption. Both groups reported low VAS pain scores, which can be attributable to the multimodal analgesia strategy used in all our patients (flurbiprofen axetil, wound infiltration with ropivacaine, and sufentanil-based PCIA). In terms of adverse effects, recent studies found that esketamine did not significantly increase the risk for nausea, vomiting, and psychotomimetic adverse events (12, 15). On the other hand, a recent meta-analysis showed that perioperative use of ketamine reduced postoperative depression scores, but increased the risk of adverse effects including nausea and vomiting, headache, dizziness, and hallucination (14). In our study, the most commonly reported adverse events were mild PONV and dizziness, and the rates were similar between groups. Events of nightmare and blurred vision were uncommon, and no patients had dissociation or delirium.
Recent experimental studies have explored the mechanisms underlying the antidepressant role of ketamine or its two enantiomers. Yu and colleagues found that ketamine produced antidepressant effects which was mediated by Neurocan in the prelimbic cortex of adolescent rats (33). Another study showed that the antidepressant-dose ketamine reversed the depressive behaviors in mice by restoring lost spines and rescuing coordinated ensemble activity (34). Rawat et al. revealed that the activation of immature granule neurons in the dentate gyrus of hippocampus was necessary and sufficient for the antidepressant effect of ketamine (35). In lipopolysaccharide-induced depression mice models, esketamine conveyed antidepressant effects by inhibiting autophagy via the mTOR- brain-derived neurotrophic factor (BDNF) pathway (36). For mice subjected to abdominal surgery, esketamine alleviated postoperative depression-like behaviors by inhibiting inflammatory effects in the prefrontal cortex (37). Preclinical studies showed that R-ketamine has greater potency and longer-lasting antidepressant effects than esketamine in animal models of depression. BDNF is the key to the antidepressant-like effects of racemic ketamine and its two enantiomers (38). R-ketamine enhanced BDNF transcription by activation of ERK-NRBP1-CREB signaling in microglia in chronic social defeat stress susceptible mice (38). In addition, another study reported that TGF-β1 signaling pathway plays a role in the antidepressant effect of R-ketamine (39).
The strengths of our study included the randomized, double-blind, controlled design, 3 months of follow-up, and the use of WeChat-based questionnaires. Questionnaires were completed by our patients via WeChat, which avoided the observer bias. In addition, we utilized regression models to determine the risk factors for depressive symptoms at 1 month postoperatively in the modified intention-to-treat population, and our results suggested that preoperative hypertension and anxious symptoms were independent risk factors and esketamine treatment was a protective factor. Last, we conducted post hoc subgroup analysis to explore the effects of esketamine between male and female patients.
Our study also has some limitations. First, although the number of included patients was consistent with the sample size calculation, including more patients may give the trial more power to test between-group differences. Second, this study was conducted at a single center situated in eastern China. Whether the results could be generalizable to other institutions needs further investigation. Third, the usage of esketamine in this study was based on the recent literature and our clinical practice, but it may not be definitive. In addition, we did not collect the pharmacokinetic data in our patients. Last, the follow-up period was limited to 3 months. Further studies are needed to ascertain the generalizability of our results and longer-term effects of esketamine in thoracoscopic lung cancer surgery.
In conclusion, the perioperative administration of esketamine reduced the incidence of depressive symptoms at 1 month postoperatively for patients undergoing thoracoscopic lung cancer surgery, without incurring significant adverse events. History of hypertension and preoperative anxious symptoms were independent risk factors for depressive symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study involving human participants was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (Approval No. 2020-127). All patients provided written informed consent.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the Beijing Medical Award Foundation Project (YXJL-2021-0170-0256), National Natural Science Foundation of China (82072130 and 81873925), 333 High-level Talent Training Project in Jiangsu Province (BRA2020089), and Six Talent Peaks Project in Jiangsu Province (WSN-022). The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the manuscript.
We gratefully acknowledge the nurses and surgeons for their assistant at the Department of Anesthesiology and Department of Thoracic surgery, First Affiliated Hospital of Soochow University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1128406/full#supplementary-material
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Park, S, Kang, CH, Hwang, Y, Seong, YW, Lee, HJ, Park, IK, et al. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancerdagger. Eur J Cardiothorac Surg. (2016) 49:e16–21. doi: 10.1093/ejcts/ezv336
3. Uchitomi, Y, Mikami, I, Nagai, K, Nishiwaki, Y, Akechi, T, and Okamura, H. Depression and psychological distress in patients during the year after curative resection of non-small-cell lung cancer. J Clin Oncol. (2003) 21:69–77. doi: 10.1200/JCO.2003.12.139
4. Pitman, A, Suleman, S, Hyde, N, and Hodgkiss, A. Depression and anxiety in patients with cancer. BMJ. (2018) 361:k1415. doi: 10.1136/bmj.k1415
5. Chen, HM, Tsai, CM, Wu, YC, Lin, KC, and Lin, CC. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer. (2015) 112:438–45. doi: 10.1038/bjc.2014.612
6. Sullivan, DR, Forsberg, CW, Ganzini, L, Au, DH, Gould, MK, Provenzale, D, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a National Cohort Assessment. J Clin Oncol. (2016) 34:3984–91. doi: 10.1200/JCO.2016.66.8459
7. Bonaventura, J, Lam, S, Carlton, M, Boehm, MA, Gomez, JL, Solis, O, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. (2021) 26:6704–22. doi: 10.1038/s41380-021-01093-2
8. Tiger, M, Veldman, ER, Ekman, CJ, Halldin, C, Svenningsson, P, and Lundberg, J. A randomized placebo-controlled PET study of ketamine s effect on serotonin(1B) receptor binding in patients with SSRI-resistant depression. Transl Psychiatry. (2020) 10:159. doi: 10.1038/s41398-020-0844-4
9. Mashour, GA, Ben Abdallah, A, Pryor, KO, El-Gabalawy, R, Vlisides, PE, Jacobsohn, E, et al. Intraoperative ketamine for prevention of depressive symptoms after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Br J Anaesth. (2018) 121:1075–83. doi: 10.1016/j.bja.2018.03.030
10. Wang, J, Sun, Y, Ai, P, Cui, V, Shi, H, An, D, et al. The effect of intravenous ketamine on depressive symptoms after surgery: a systematic review. J Clin Anesth. (2022) 77:110631. doi: 10.1016/j.jclinane.2021.110631
11. Liu, P, Li, P, Li, Q, Yan, H, Shi, X, Liu, C, et al. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J Investig Surg. (2021) 34:883–8. doi: 10.1080/08941939.2019.1710626
12. Qiu, D, Wang, XM, Yang, JJ, Chen, S, Yue, CB, Hashimoto, K, et al. Effect of intraoperative Esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2244514. doi: 10.1001/jamanetworkopen.2022.44514
13. Hashimoto, K, Kakiuchi, T, Ohba, H, Nishiyama, S, and Tsukada, H. Reduction of dopamine D(2/3) receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci. (2017) 267:173–6. doi: 10.1007/s00406-016-0692-7
14. Guo, J, Qiu, D, Gu, HW, Wang, XM, Hashimoto, K, Zhang, GF, et al. Efficacy and safety of perioperative application of ketamine on postoperative depression: a meta-analysis of randomized controlled studies. Mol Psychiatry. (2023). 1–11 doi: 10.1038/s41380-023-01945-z
15. Wang, X, Lin, C, Lan, L, and Liu, J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: a systematic review and meta-analysis. J Clin Anesth. (2021) 68:110071. doi: 10.1016/j.jclinane.2020.110071
16. Hudgens, S, Floden, L, Blackowicz, M, Jamieson, C, Popova, V, Fedgchin, M, et al. Meaningful change in depression symptoms assessed with the patient health questionnaire (PHQ-9) and Montgomery-Asberg depression rating scale (MADRS) among patients with treatment resistant depression in two, randomized, double-blind, active-controlled trials of Esketamine nasal spray combined with a new Oral antidepressant. J Affect Disord. (2021) 281:767–75. doi: 10.1016/j.jad.2020.11.066
17. Carney, CE, Edinger, JD, Kuchibhatla, M, Lachowski, AM, Bogouslavsky, O, Krystal, AD, et al. Cognitive behavioral insomnia therapy for those with insomnia and depression: a randomized controlled clinical trial. Sleep. (2017) 40:zsx019. doi: 10.1093/sleep/zsx019
18. Beck, AT, Steer, RA, Ball, R, and Ranieri, W. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. (1996) 67:588–97. doi: 10.1207/s15327752jpa6703_13
19. Wang, J, Wang, Y, Xu, X, Peng, S, Xu, F, and Liu, P. Use of various doses of S-ketamine in treatment of depression and pain in cervical carcinoma patients with mild/moderate depression after laparoscopic Total hysterectomy. Med Sci Monit. (2020) 26:e922028. doi: 10.12659/MSM.922028
20. Bornemann-Cimenti, H, Wejbora, M, Michaeli, K, Edler, A, and Sandner-Kiesling, A. The effects of minimal-dose versus low-dose S-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: a triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva Anestesiol. (2016) 82:1069–76.
21. Beck, AT, Ward, CH, Mendelson, M, Mock, J, and Erbaugh, J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
22. Huang, CC, Wu, PW, Fu, CH, Huang, CC, Chang, PH, and Lee, TJ. Impact of psychologic burden on surgical outcome in empty nose syndrome. Laryngoscope. (2021) 131:E694–701. doi: 10.1002/lary.28845
23. Lewis, G, Duffy, L, Ades, A, Amos, R, Araya, R, Brabyn, S, et al. The clinical effectiveness of sertraline in primary care and the role of depression severity and duration (PANDA): a pragmatic, double-blind, placebo-controlled randomised trial. Lancet Psychiatry. (2019) 6:903–14. doi: 10.1016/S2215-0366(19)30366-9
24. Beck, AT, Epstein, N, Brown, G, and Steer, RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. (1988) 56:893–7. doi: 10.1037/0022-006X.56.6.893
25. Stark, PA, Myles, PS, and Burke, JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. (2013) 118:1332–40. doi: 10.1097/ALN.0b013e318289b84b
26. Zhao, P, Wu, Z, Li, C, Yang, G, Ding, J, Wang, K, et al. Postoperative analgesia using dezocine alleviates depressive symptoms after colorectal cancer surgery: a randomized, controlled, double-blind trial. PLoS One. (2020) 15:e0233412. doi: 10.1371/journal.pone.0233412
27. Liang, Y, Wang, L, and Zhu, J. Factor structure and psychometric properties of Chinese version of Beck anxiety inventory in Chinese doctors. J Health Psychol. (2018) 23:657–66. doi: 10.1177/1359105316658971
28. Bu, XS, Zhang, J, and Zuo, YX. Validation of the Chinese version of the quality of Recovery-15 score and its comparison with the post-operative quality recovery scale. Patient. (2016) 9:251–9. doi: 10.1007/s40271-015-0148-6
29. Subramaniam, B, Shankar, P, Shaefi, S, Mueller, A, O'Gara, B, Banner-Goodspeed, V, et al. Effect of intravenous acetaminophen vs placebo combined with Propofol or Dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. (2019) 321:686–96. doi: 10.1001/jama.2019.0234
30. Signorelli, MS, Surace, T, Migliore, M, and Aguglia, E. Mood disorders and outcomes in lung cancer patients undergoing surgery: a brief summery. Future Oncol. (2020) 16:41–4. doi: 10.2217/fon-2018-0835
31. Wang, W, Xu, H, Ling, B, Chen, Q, Lv, J, and Yu, W. Effects of esketamine on analgesia and postpartum depression after cesarean section: a randomized, double-blinded controlled trial. Medicine (Baltimore). (2022) 101:e32010. doi: 10.1097/MD.0000000000032010
32. Han, Y, Li, P, Miao, M, Tao, Y, Kang, X, and Zhang, J. S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: a randomized controlled trial. BMC Anesthesiol. (2022) 22:49. doi: 10.1186/s12871-022-01588-7
33. Yu, Z, Han, Y, Hu, D, Chen, N, Zhang, Z, Chen, W, et al. Neurocan regulates vulnerability to stress and the anti-depressant effect of ketamine in adolescent rats. Mol Psychiatry. (2022) 27:2522–32. doi: 10.1038/s41380-022-01495-w
34. Moda-Sava, RN, Murdock, MH, Parekh, PK, Fetcho, RN, Huang, BS, Huynh, TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. (2019) 364. doi: 10.1126/science.aat8078
35. Rawat, R, Tunc-Ozcan, E, McGuire, TL, Peng, CY, and Kessler, JA. Ketamine activates adult-born immature granule neurons to rapidly alleviate depression-like behaviors in mice. Nat Commun. (2022) 13:2650. doi: 10.1038/s41467-022-30386-5
36. Jiang, G, Wang, Y, Liu, Q, Gu, T, Liu, S, Yin, A, et al. Autophagy: a new mechanism for Esketamine as a depression therapeutic. Neuroscience. (2022) 498:214–23. doi: 10.1016/j.neuroscience.2022.05.014
37. Wang, T, Weng, H, Zhou, H, Yang, Z, Tian, Z, Xi, B, et al. Esketamine alleviates postoperative depression-like behavior through anti-inflammatory actions in mouse prefrontal cortex. J Affect Disord. (2022) 307:97–107. doi: 10.1016/j.jad.2022.03.072
38. Yao, W, Cao, Q, Luo, S, He, L, Yang, C, Chen, J, et al. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol Psychiatry. (2022) 27:1618–29. doi: 10.1038/s41380-021-01377-7
Keywords: anxiety, depression, esketamine, lung cancer, thoracoscopic surgery
Citation: Gan S-l, Long Y-q, Wang Q-y, Feng C-d, Lai C-x, Liu C-t, Ding Y-y, Liu H, Peng K and Ji F-h (2023) Effect of esketamine on postoperative depressive symptoms in patients undergoing thoracoscopic lung cancer surgery: A randomized controlled trial. Front. Psychiatry. 14:1128406. doi: 10.3389/fpsyt.2023.1128406
Received: 17 January 2023; Accepted: 23 February 2023;
Published: 15 March 2023.
Edited by:
Rodrigo Simonini Delfino, Federal University of São Paulo, BrazilReviewed by:
Alina Wilkowska, Medical University of Gdansk, PolandCopyright © 2023 Gan, Long, Wang, Feng, Lai, Liu, Ding, Liu, Peng and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Peng, cGVuZ2tlMDQyMkAxNjMuY29t; Fu-hai Ji, amlmdWhhaXN1ZGFAMTYzLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Ke Peng https://orcid.org/0000-0003-2879-1759
Fu-hai Ji https://orcid.org/0000-0001-6649-665X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.