- Department of Psychiatry, and National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
Background: Frontotemporal cortex dysfunction has been found to be associated with cognitive impairment in patients with schizophrenia (SCZ). In patients with adolescent-onset SCZ, a more serious type of SCZ with poorer functional outcome, cognitive impairment appeared to occur at an early stage of the disease. However, the characteristics of frontotemporal cortex involvement in adolescent patients with cognitive impairment are still unclear. In the present study, we aimed to illustrate the frontotemporal hemodynamic response during a cognitive task in adolescents with first-episode SCZ.
Methods: Adolescents with first-episode SCZ who were aged 12-17 and demographically matched healthy controls (HCs) were recruited. We used a 48-channel functional near-infrared spectroscopy (fNIRS) system to record the concentration of oxygenated hemoglobin (oxy-Hb) in the participants' frontotemporal area during a verbal fluency task (VFT) and analyzed its correlation with clinical characteristics.
Results: Data from 36 adolescents with SCZ and 38 HCs were included in the analyses. Significant differences were found between patients with SCZ and HCs in 24 channels, mainly covering the dorsolateral prefrontal cortex, superior and middle temporal gyrus and frontopolar area. Adolescents with SCZ showed no increase of oxy-Hb concentration in most channels, while the VFT performance was comparable between the two groups. In SCZ, the intensity of activation was not associated with the severity of symptoms. Finally, receiver operating characteristic analysis indicated that the changes in oxy-Hb concentration could help distinguish the two groups.
Conclusion: Adolescents with first-episode SCZ showed atypical cortical activity in the frontotemporal area during the VFT, and fNIRS features might be more sensitive indicators in cognitive assessment, indicating that the characteristic hemodynamic response pattern might be potential imaging biomarkers for this population.
1. Introduction
Schizophrenia (SCZ) is a major psychiatric disorder that seriously affects the functions and quality of life of patients. Apart from prominent positive and negative symptoms, different types of cognitive impairment also exist in most patients with SCZ. As cognitive impairment tends to be persistent even after the initial symptoms are relieved, it has been found that such impairment is significantly associated with the functional outcome of patients (1), indicating that it may be an important indicator of the prognosis of SCZ. Some researchers proposed that cognitive impairment is a core feature of SCZ (2).
Specifically, SCZ with an onset in adolescence is regarded as a more serious type of SCZ due to the poorer cognitive performance and functional outcomes in these patients (3). While adolescents with SCZ have similar symptoms to adult patients (4), they appeared to have more severe cognitive impairment. Compared with patients with adult-onset SCZ, adolescent patients may exhibit poorer performance in several cognitive domains (5), including executive function, psychomotor processing speed and verbal memory. As adolescence is a critical period for physical and mental development, cognitive impairment in the early stage of the disease (5) may explain the lower academic performance in individuals at a higher risk of psychosis (6). A 3-year follow-up study showed that only 15.8% of adolescents with SCZ were able to catch up with peers academically and up to 36.6% of them were unable to complete their education (7), indicating a heavy disease burden in this population. Studies on the underlying mechanism of cognitive impairment of adolescents with SCZ might help improve the functional outcome of patients as well as improve our understanding of this disorder.
Evidence has shown association between cognitive impairment in adult-onset SCZ and brain dysfunction, especially frontotemporal cortex dysfunction (8–11). Adolescence is a critical period of neurodevelopment, thus, abnormal brain function during this process may also be involved in the mechanism of cognitive impairment (6). However, considering that neurodevelopmental trajectories vary across different populations (12), results from studies on adult-onset SCZ might not be directly extrapolated to adolescent-onset SCZ. While functional magnetic resonance imaging (fMRI) studies of whole-brain resting-state functional connectivity (FC) determined the involvement of frontotemporal cortex in the pathology of adolescent SCZ (13), and abnormal FC in the left superior temporal gyrus was correlated with cognitive impairment in the diseased adolescents (14), few studies have focused on task related brain activity in adolescent SCZ; thus, the activity of the frontotemporal cortex during cognitive tasks is still unclear in adolescents with SCZ. Lately, functional near-infrared spectroscopy (fNIRS) has become a commonly used neuroimaging technology in the study of cerebral function. With its superiority in yielding data with good temporal and spatial resolution, it has been well accepted in studies involving cognitive task related hemodynamics. Moreover, the high safety and tolerance can enable its applicability in specific populations, including children and elderly people (8). Verbal fluency task (VFT) is widely employed in studies on the cognitive function of patients with SCZ (15); with the use of fNIRS, the hemodynamic response can be recorded simultaneously, providing direct cortical activation measurements during the cognitive tasks. Generally, during the VFT, patients with SCZ often show abnormal hemodynamic response patterns (8), decreased prefrontal cortex activity (16), and functional connectivity with lower intensity (17), as compared with healthy individuals. These findings have contributed to the understanding of the mechanism of cognitive impairment of patients with SCZ; however, whether the results can be extrapolated to adolescents still remains unknown.
In the present study, we aimed to illustrate the frontotemporal cortical activity patterns during a VFT in adolescents with SCZ. Based on previous studies, our hypothesis is that adolescents with first-episode SCZ show decreased VFT-related frontotemporal cortical activity compared with healthy adolescents. In order to evaluate the characteristics of cognitive impairment in the early stage of this disease, we only included patients with first-episode SCZ and explored whether the characteristics could help effectively distinguish patients from controls.
2. Methods
2.1. Participants
The participants were recruited from March 2021 to September 2022. SCZ patients were recruited from the Second Xiangya Hospital, China, with the inclusion criteria as follows: (1) meeting the diagnostic criteria of schizophrenia according to DSM-5; (2) with first-episode SCZ; (3) aged 12–17 years; (4) with an IQ above 70; and (5) right-handed. The exclusion criteria were (1) having received electroconvulsive therapy in the last 6 months; (2) comorbid with neurological diseases, other mental illnesses, serious physical diseases, cerebral trauma or other organic brain disorders. SCZ patients were diagnosed by child and adolescent psychiatrists with the intermediate professional title or above.
Healthy controls (HC) were recruited from the community with the following criteria: (1) aged 12–17 years; (2) with no mental illness according to the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime (KSADS-PL) interview (18); (3) with no family history of mental illnesses; (4) with an IQ above 70; and (5) right-handed. All the participants and their caregivers provided informed consent after fully informed.
2.2. Demographic statistics and clinical assessment
Demographic data including age, sex, and level of education were collected from all the participants. For patients with SCZ, the Positive and Negative Symptom Scale (PANSS) was used to assess symptoms of SCZ (19). This scale consists of 30 items in three subscales, i.e., positive symptoms, negative symptoms, and global psychopathology, with higher scores indicating higher severity of symptoms. The Chinese version of PANSS has good reliability and validity, with a standardized Cronbach α value of 0.8707 (20).
2.3. Verbal fluency task
All the participants underwent a VFT according to the programmed audio instruction on a computer. The 160-s block-designed task consisted of a 30-s pre-task baseline, a 60-s task, and a 70-s post-task baseline. For the pre- and post-task baseline periods, participants were instructed to repeat the numbers of 1, 2, 3, 4, and 5. During the task period, the participants were instructed to generate as many words including a given Chinese character as they could; referring to previous studies (21, 22), we chose three of the most commonly used Chinese characters for the task, namely “白 (white),” “天 (sky),” and “大 (big),” with each character phase lasting for 20-s. To ensure that the participants truly understood the task, they were allowed to practice with another character before the formal VFT started. During the task, the participants were seated in a comfortable chair and were instructed to avoid any major body movements. The number of valid words were recorded to reflect the task performance.
2.4. fNIRS measurements
Changes in hemoglobin concentration during the VFT were measured using a 48-channel fNIRS system (NirSmart, Danyang Huichuang Medical Equipment Co., Ltd., China) according to the modified Beer-Lambert law (23). Two wavelengths of infrared light (730 nm and 850 nm) were used, with a sampling rate of 11 Hz. Neighboring infrared light sources and detectors were arranged at a distance of 3 cm, and the link between the neighboring source and detector was defined as the “channel.” The 48-channel system consisted of 15 source probes and 16 detector probes covering the prefrontal and bilateral temporal regions, the channels were located according to Brodmann area (24, 25) (Figures 1A, B).
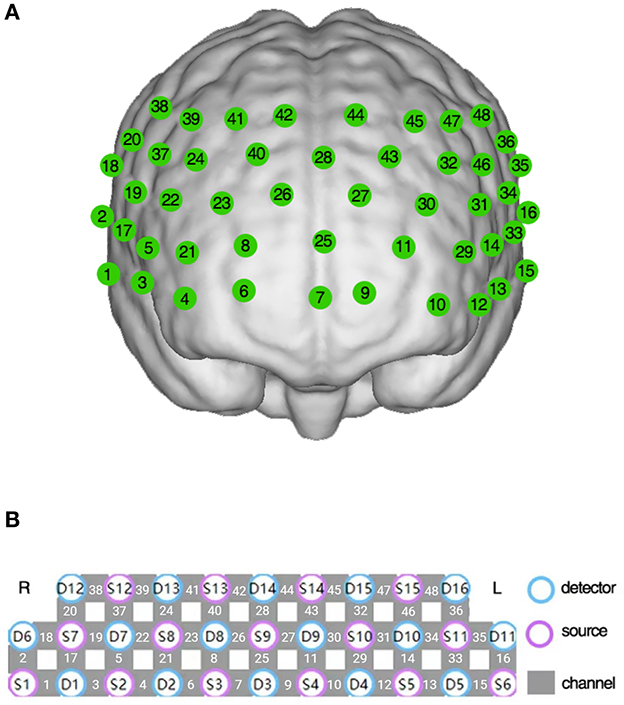
Figure 1. The 48-channel near-infrared spectroscopy system. (A) The location of each channel in the prefrontal and bilateral temporal region. (B) The arrangement of detectors, sources, and channels.
2.5. fNIRS data processing
fNIRS data were processed using the NirSpark software package (HuiChuang, China). To remove systemic and physiological noise, we used a bandpass filter with a frequency range of 0.01–0.2 Hz to filter the raw data. The optical density was then translated to hemoglobin concentrations. In this study, we selected the concentration of oxygenated hemoglobin (oxy-Hb) as the target indicator of cortical activity, as the oxy-Hb signals have a higher signal-to-noise ratio (26) and the changes in oxy-Hb concentration is more sensitive for most mental diseases (27). Cortical activity was defined as relative oxy-Hb concentration change between the 60-s task period and the pre- and post-task baseline periods. To focus on task-specific signal changes, we carried out linear fitting on the data between the last 10 s of the pre-task baseline and the 5 s from the 40 to 45-s time points of the post-task baseline. Cortical activity was calculated for each participant during the VFT in all channels.
2.6. Statistical analysis
Shapiro-wilk test was used to test the normality of continuous variables. Independent two-sample t-test or Mann-Whitney U test was used to compare the inter-group difference in continuous variables, and Chi-square test was used to compare the categorical variables. The correlations between oxy-Hb concentration and clinical variables were analyzed using the Spearman correlation analysis. Receiver operating characteristic (ROC) analysis was used to assess the separation performance for oxy-Hb. All statistical analyses were performed using SPSS 22.0 (IBM, Inc., Chicago, Illinois), and figures were generated in R v3.6.3 (R Development Core Team 2020) and Rstudio v1.2.5033 (RStudio, Inc., 2019). P-values were adjusted with the Benjamini-Hochberg false discovery rate (FDR) procedure, with p < 0.05 (two-tailed) indicating that the difference was statistically significant.
3. Results
3.1. Demographic and clinical data
A total of 40 adolescents with schizophrenia (the SCZ group) and 38 healthy controls (the HC group) provided consent to participate in this study. Data from 36 adolescents with SCZ and 38 HCs were included in the final analyses as 4 patients with SCZ failed to complete the VFT. All the patients were at acute phase, and 17 adolescents with SCZ were medication-naive. No significant inter-group difference was found in age, sex ratio and level of education (Table 1).
3.2. VFT performance and cortical activity
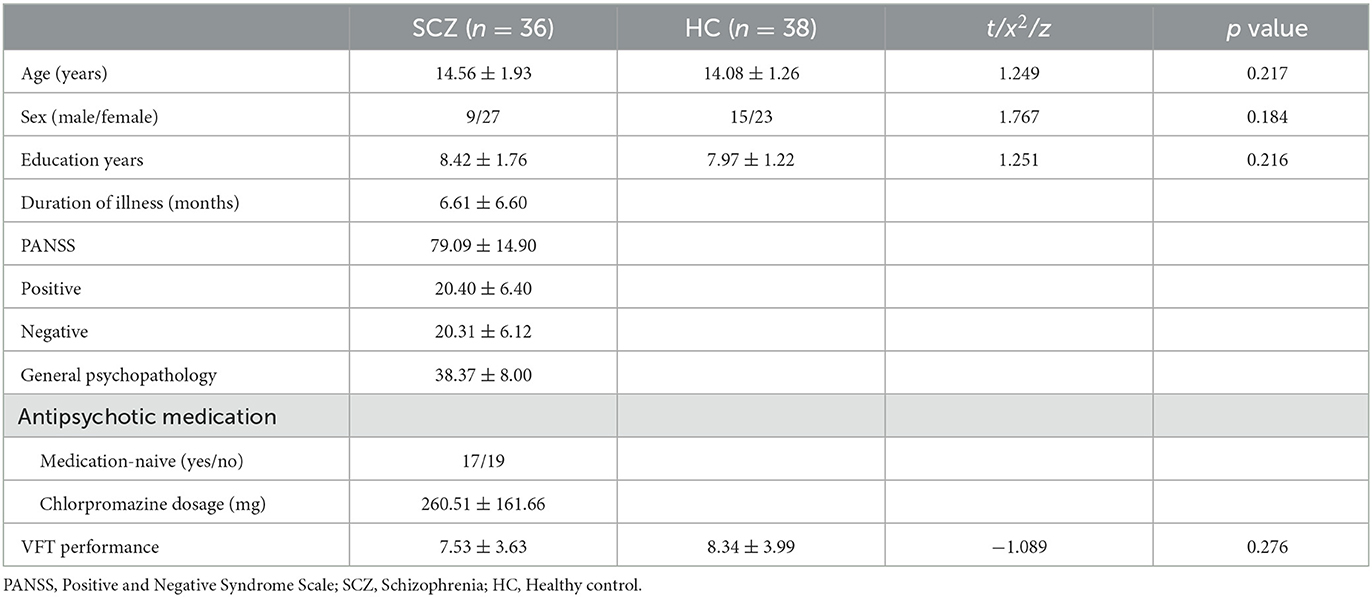
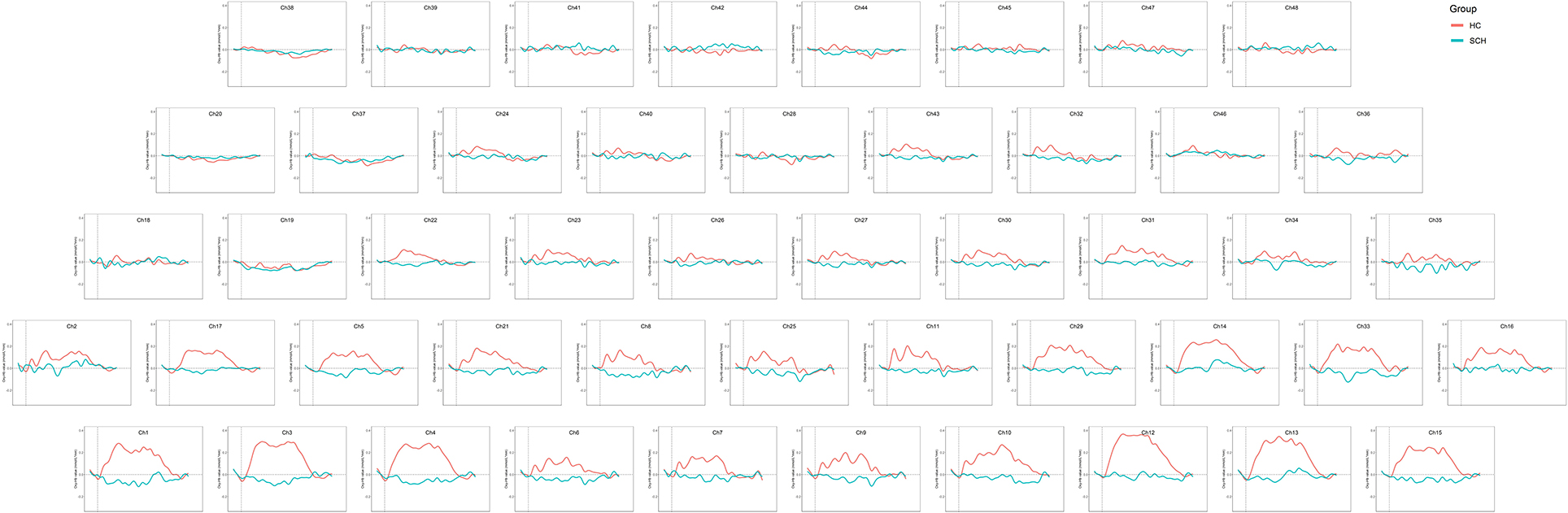
The two groups of adolescents showed no significant difference in the number of words generated during the VFT [(7.53 ± 3.63) for the SCZ group vs. (8.34 ± 3.99) for HCs, p = 0.276]. Higher cortical activation, reflected by a greater increase in oxy-Hb concentration, was found in HCs compared with patients with SCZ in 24 channels: channels 1, 13 and 15 (mainly in the middle temporal gyrus), channels 6–11 (the frontopolar area), channels 16 and 33 (the superior temporal gyrus), channel 3 (the temporopolar area), channel 17 (the subcentral area), channels 5, 12, 14, 21, 29, and 31 (the triangularis Broca's area), and channels 4, 23, 27, 30 and 43 (the dorsolateral prefrontal cortex, DLPFC) (FDR p < 0.05, see Figure 2). Waveforms of oxy-Hb concentration change with time sequence in each channel are presented in Figure 3.
3.3. Correlations between VFT and clinical characteristics in SCZ
In adolescents with SCZ, the VFT performance was significantly correlated with the PANSS negative score (rho = −0.434, p = 0.009). Before FDR correction, positive correlation was found between the VFT performance and oxy-Hb concentration in channel 43 (DLPFC, rho = 0.442, p = 0.008); negative correlation was found between the PANSS total score and oxy-Hb concentration in channel 3 (temporopolar area, rho = −0.374, p = 0.029), between the PANSS positive score and oxy-Hb concentration in channel 4 (DLPFC, rho = −0.398, p = 0.020) and 13 (middle temporal gyrus, rho = −0.346, p = 0.045), between the PANSS general psychopathology score and oxy-Hb concentration in channel 4 (DLPFC, rho = −0.356, p = 0.039), and between the antipsychotic dosage and oxy-Hb concentration in channel 38 (pre-motor and supplementary motor cortex, rho = −0.586, p = 0.0389). However, these results did not pass the FDR correction.
3.4. Receiver operating characteristic (ROC) analysis
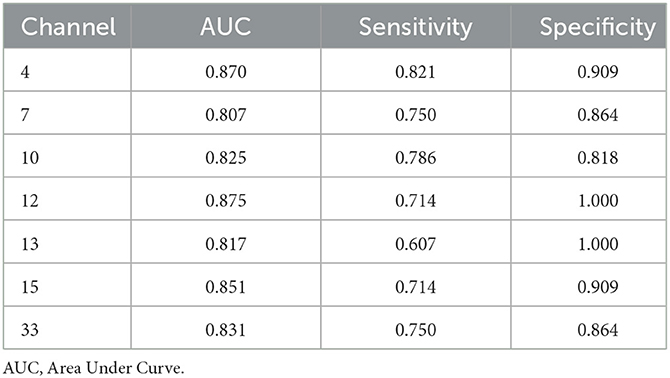
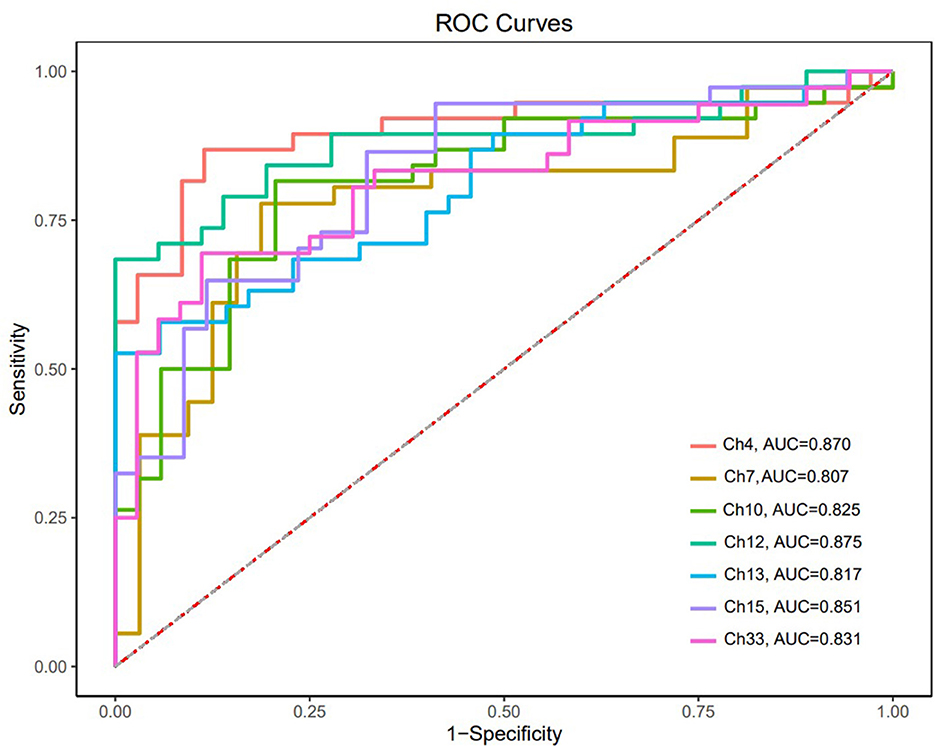
The ROC curves were generated based on oxy-Hb changes in each channel. We calculated the areas under the curve (AUC) to assess their capability to distinguish between patients with SCZ and HCs. When the AUC was >0.8, the changes in oxy-Hb concentration in channel 4 (DLPFC), channels 7 and 10 (the frontopolar area), channel 12 (the triangularis Broca's area), channels 13 and 15 (the middle temporal gyrus), and channel 33 (the superior temporal gyrus) could help distinguish between the two groups effectively. The sensitivity and specificity of these channels are presented in Table 2, and the ROC curves are presented in Figure 4.
4. Discussion
The present study found significant differences in VFT related frontotemporal cortical activity between adolescents with first-episode SCZ and their healthy counterparts, while the VFT performance was comparable between the two groups. We also found that the intensity of activation was not associated with the severity of symptoms, and that the impairment was similar across all patients with SCZ. To our knowledge, this is the first study on the pattern of frontotemporal cortical activity during the VFT in adolescents with first-episode SCZ.
4.1. Atypical cortical activity in SCZ
Adolescents with SCZ exhibited atypical cortical activity in 24 channels during VFT, covering half of the targeted areas. The greatest inter-group differences were found in channels 3, 4, 12, 13, 15, 33, involving the DLPFC, superior and middle temporal gyrus, and triangularis Broca's area. Channels 6–11 covers most of the frontopolar area where cortical activity also differed significantly between the two groups, suggesting abnormal frontotemporal function in the early stage of adolescent SCZ. A study revealed the association between lower activation in the frontopolar area and functional impairment in SCZ (28), indicating that the frontopolar function might be an important marker of functional outcome. The present study also found frontopolar abnormality in adolescents with SCZ, but whether this feature is associated with the functional outcome of adolescents with SCZ is worth further exploration.
Our findings are partly consistent with a former fMRI study showing lower activation in the middle frontal cortices in patients with acute psychosis and in remission (15). But interestingly, they had no remarkable finding in lateral prefrontal activation in patients with acute psychosis, which was inconsistent with the result of DLPFC dysfunction in the present study; their finding suggested that additional prefrontal activation may be involved in SCZ patients as neural compensation to maintain task performance. The VFT can reflect the executive function (29) and retrieval of information (30), considering the role of DLPFC in this process (9), the inconsistency in DLPFC dysfunction involvement might be associated with the diversity of neural compensation of DLPFC (31) in different SCZ population. The present study indicated that neural compensation might be uncommon among adolescents with first-episode SCZ.
Notably, no obvious increased frontotemporal activity was observed in adolescents with SCZ. This is inconsistent with a former VFT study on adult patients with first-episode SCZ (32); they found that although oxy-Hb concentration increase in the patient group was lower than in HC, the patients showed significantly increased frontotemporal activities during the VFT in most channels. Similar frontotemporal activation pattern was also found in adult patients with chronic SCZ (22, 33, 34). However, in the present study, the patient group showed no obvious increase in oxy-Hb concentration after the 60-s task period started, and even a decrease of oxy-Hb concentration was found in some channels (Figure 3). The broad inhibition of hemodynamic response in the frontotemporal area in adolescents with SCZ demonstrated an atypical cortical activity pattern which might be specific to adolescent patients. Existing evidence suggested abnormal neurodevelopment in adolescents with SCZ. In children and adolescents with SCZ, decreased gray matter volume was found mainly in the frontotemporal cortex (35). In a resting-state fMRI study, the researchers attributed the abnormal anatomical FC pattern in DLPFC to functional overpruning in the neurodevelopment process (13). The present study might provide support for the neurodevelopmental disorder theory of SCZ and indicated that adolescent-onset SCZ might be a more serious type of SCZ.
In addition, we found no significant correlation between the intensity of activation and the PANSS score or other clinical characteristics, indicating that the change in oxy-Hb concentration during VFT was not sensitive to SCZ symptoms.
4.2. VFT performance
We found that the adolescents with SCZ performed equally well in generating words compared with HC. Concerning the VFT performance is associated with the difficulty of task (36), the phonological fluency task used might be equally difficult for the adolescents with first-episode SCZ and HCs. Some researchers proposed that the category fluency task (CFT) might be superior for the evaluation of Chinese-speaking patients with psychiatric disorders (34). Thus, further studies on potential inter-task effects in adolescents are still needed. Meanwhile, although there was no significant difference in the VFT performance between the two groups, the patients' atypical cortical activity pattern suggested that the functional changes in the frontotemporal area occurred prior to cognitive impairment and that fNIRS features might be more sensitive in cognitive assessment. Moreover, in SCZ group, no significant correlation was found between the VFT performance and oxy-Hb concentration, indicating that, decreased frontotemporal cortical activity was common among adolescents with first-episode SCZ and may be associated with neural inefficiency or abnormalities in neurovascular coupling rather than lack of motivation for cognitive task (27).
In adolescents with SCZ, the VFT performance was negatively correlated with the PANSS negative score, i.e., patients with more severe negative symptoms came up with fewer words in the VFT, indicating an association between negative symptoms and cognitive impairment (37, 38). According to prior studies, the brain regions associated with negative symptoms and those associated with cognitive impairments overlapped mainly in the frontotemporal cortex (9, 39).
4.3. ROC analysis
The ROC analysis found that the change in oxy-Hb concentration in 7 channels had a good sensitivity and specificity for distinguishing adolescents with SCZ from their healthy counterparts, with a high sensitivity of 0.821 and specificity of 0.909 obtained in channel 4. In addition to previous findings that neurological features based on the frontotemporal cortex are potential biomarkers for SCZ (40), our study suggested that oxy-Hb changes during the VFT might be a promising imaging biomarker for adolescents with SCZ; however, large-scale studies are needed to verify our findings.
5. Limitations
There are still some limitations to this study. First, some of the participants were on antipsychotic medications. Although we found no significant correlation between antipsychotic dosage and oxy-Hb changes, the potential influence of medications might need to be investigated in further studies involving all medication-naive adolescents with SCZ. Second, the results of ROC analysis have not been extrapolated to external samples, thus, the oxy-Hb change during VFT in distinguishing patients with SCZ from HCs needs to be further verified. Last, we focused on adolescents with first-episode SCZ in this study, future studies involving adolescents in the chronic phase of SCZ, or a longer duration of illness are warranted.
6. Conclusion
Adolescents with SCZ showed atypical cortical activity in the frontotemporal area during the VFT, and the characteristic hemodynamic response pattern may be a potential imaging biomarker for this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Second Xiangya Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
XL and KZ were responsible for the study design. KZ, XJ, and YH were responsible for recruiting the participants. KZ, SW, and XJ were involved in statistical analysis. KZ, XC, and YH were involved in manuscript preparation and drafting the paper. CH and XG were involved in editing and revising the manuscript. XL was responsible for the critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (No. 2017YFC1309904), Development Program of Hunan Province (No. 2019SK2081), National Natural Science Foundation of China (No. 82201703), and the research sailing program supported by Second Xiangya Hospital of Central South University.
Acknowledgments
We would like to show our gratitude to Junli Dong for her help in data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carruthers SP, Gurvich CT, Rossell SL. The muscarinic system, cognition and schizophrenia. Neurosci Biobehav Rev. (2015) 55:393–402. doi: 10.1016/j.neubiorev.2015.05.011
2. Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA psychiatry. (2013) 70:1107–12. doi: 10.1001/jamapsychiatry.2013.155
3. Rhinewine JP, Lencz T, Thaden EP, Cervellione KL, Burdick KE, Henderson I, et al. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biol Psychiatry. (2005) 58:705–12. doi: 10.1016/j.biopsych.2005.04.031
4. Madaan V, Dvir Y, Wilson DR. Child and adolescent schizophrenia: pharmacological approaches. Expert Opin Pharmacother. (2008) 9:2053–68. doi: 10.1517/14656566.9.12.2053
5. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. The British journal of psychiatry: the journal of mental science. (2009) 195:286–93. doi: 10.1192/bjp.bp.108.060723
6. Ramsay H, Barnett JH, Murray GK, Miettunen J, Mäki P, Järvelin MR, et al. Cognition, psychosis risk and metabolic measures in two adolescent birth cohorts. Psychol Med. (2018) 48:2609–23. doi: 10.1017/S0033291718001794
7. Kang C, Zhou H, Yang J, Yang R, Sun N, Wang S, et al. Course, outcome and diagnosis stability of early-onset schizophrenia in Yunnan Province, China-a three years follow-up study. Psychiatry Res. (2019) 271:144–9. doi: 10.1016/j.psychres.2018.11.013
8. Ehlis AC, Schneider S, Dresler T, Fallgatter AJ. Application of functional near-infrared spectroscopy in psychiatry. NeuroImage. (2014) 85 Pt 1:478–88. doi: 10.1016/j.neuroimage.2013.03.067
9. Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. (2016) 61:108–20. doi: 10.1016/j.neubiorev.2015.12.007
10. Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. (2006) 20:497–510. doi: 10.1037/0894-4105.20.5.497
11. Wang X, Xia M, Lai Y, Dai Z, Cao Q, Cheng Z, et al. Disrupted resting-state functional connectivity in minimally treated chronic schizophrenia. Schizophr Res. (2014) 156:150–6. doi: 10.1016/j.schres.2014.03.033
12. Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. Local brain connectivity and associations with gender and age. Dev Cogn Neurosci. (2011) 1:187–97. doi: 10.1016/j.dcn.2010.10.001
13. Alexander-Bloch AF, Vértes PE, Stidd R, Lalonde F, Clasen L, Rapoport J, et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cerebral cortex. (2013) 23:127–38. doi: 10.1093/cercor/bhr388
14. Wang S, Zhan Y, Zhang Y, Lyu L, Lyu H, Wang G, et al. Abnormal long- and short-range functional connectivity in adolescent-onset schizophrenia patients: A resting-state fMRI study. Progr Neuro-Psychopharmacol Biologic Psychiatr. (2018) 81:445–51. doi: 10.1016/j.pnpbp.2017.08.012
15. Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK. Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. Am J Psychiatry. (2005) 162:485–94. doi: 10.1176/appi.ajp.162.3.485
16. Xia D, Quan W, Wu T. Optimizing functional near-infrared spectroscopy (fNIRS) channels for schizophrenic identification during a verbal fluency task using metaheuristic algorithms. Front Psychiatry. (2022) 13:939411. doi: 10.3389/fpsyt.2022.939411
17. Ji X, Quan W, Yang L, Chen J, Wang J, Wu T. Classification of schizophrenia by seed-based functional connectivity using prefronto-temporal functional near infrared spectroscopy. J Neurosci Methods. (2020) 344:108874. doi: 10.1016/j.jneumeth.2020.108874
18. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. (1997) 36:980–8. doi: 10.1097/00004583-199707000-00021
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
20. Si T, Yang J, Shu L, Wang X, Kong Q, Zhou M, et al. The reliability and validity of positive and negative symptom scale (PANSS) Chinese version. Chinese Mental Health J. (2004) 18:45–7.
21. Quan W, Wu T, Li Z, Wang Y, Dong W, Lv B. Reduced prefrontal activation during a verbal fluency task in Chinese-speaking patients with schizophrenia as measured by near-infrared spectroscopy. Prog Neuropsychopharmacol Biol Psychiatr. (2015) 58:51–8. doi: 10.1016/j.pnpbp.2014.12.005
22. Fu X, Quan W, Liu L, Li T, Dong W, Wang J, et al. Similarities and differences in brain activation between patients with schizophrenia and obsessive-compulsive disorder: a near-infrared spectroscopy study. Front Psychiatr. (2022) 13:853428. doi: 10.3389/fpsyt.2022.853428
23. Obrig H, Villringer A. Beyond the visible–imaging the human brain with light. J Cerebr Blood Flow Metabol Offic J Int Soc Cerebr Blood Flow Metabol. (2003) 23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29
24. McKendrick R, Harwood A. Cognitive workload and workload transitions elicit curvilinear hemodynamics during spatial working memory. Front Hum Neurosci. (2019) 13:405. doi: 10.3389/fnhum.2019.00405
25. Chen Y, Wan A, Mao M, Sun W, Song Q, Mao D. Tai Chi practice enables prefrontal cortex bilateral activation and gait performance prioritization during dual-task negotiating obstacle in older adults. Front Aging Neurosci. (2022) 14:1000427. doi: 10.3389/fnagi.2022.1000427
26. Pinti P, Tachtsidis I, Hamilton A, Hirsch J, Aichelburg C, Gilbert S, et al. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann N Y Acad Sci. (2020) 1464:5–29. doi: 10.1111/nyas.13948
27. Yeung MK, Lin J. Probing depression, schizophrenia, and other psychiatric disorders using fNIRS and the verbal fluency test: a systematic review and meta-analysis. J Psychiatr Res. (2021) 140:416–35. doi: 10.1016/j.jpsychires.2021.06.015
28. Takizawa R, Kasai K, Kawakubo Y, Marumo K, Kawasaki S, Yamasue H, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res. (2008) 99:250–62. doi: 10.1016/j.schres.2007.10.025
29. Liu X, Cheng F, Hu S, Wang B, Hu C, Zhu Z, et al. Cortical activation and functional connectivity during the verbal fluency task for adolescent-onset depression: a multi-channel NIRS study. J Psychiatr Res. (2022) 147:254–61. doi: 10.1016/j.jpsychires.2022.01.040
30. Berberian AA, Moraes GV, Gadelha A, Brietzke E, Fonseca AO, Scarpato BS, et al. Is semantic verbal fluency impairment explained by executive function deficits in schizophrenia? Revista brasileira de psiquiatria. (2016) 38:121–6. doi: 10.1590/1516-4446-2015-1663
31. Dienel SJ, Enwright JF. 3rd, Hoftman GD, Lewis DA. Markers of glutamate and GABA neurotransmission in the prefrontal cortex of schizophrenia subjects: disease effects differ across anatomical levels of resolution. Schizophrenia Res. (2020) 217:86–94. doi: 10.1016/j.schres.2019.06.003
32. Chou PH, Lin WH, Lin CC, Hou PH Li WR, Hung CC, et al. Duration of untreated psychosis and brain function during verbal fluency testing in first-episode schizophrenia: a near-infrared spectroscopy study. Sci Rep. (2015) 5:18069. doi: 10.1038/srep18069
33. Xiang Y, Li Y, Shu C, Liu Z, Wang H, Wang G. Prefrontal cortex activation during verbal fluency task and tower of london task in schizophrenia and major depressive disorder. Front Psychiatr. (2021) 12:709875. doi: 10.3389/fpsyt.2021.709875
34. Li J, Mu J, Shen C, Yao G, Feng K, Zhang X, et al. Abnormal cortical activation patterns among chinese-speaking schizophrenia patients during category and letter verbal fluency tasks revealed by multi-channel functional near-infrared spectroscopy. Front Psychiatr. (2021) 12:790732. doi: 10.3389/fpsyt.2021.790732
35. Liu J, Wen F, Yan J, Yu L, Wang F, Wang D, et al. Gray matter alterations in pediatric schizophrenia and obsessive-compulsive disorder: a systematic review and meta-analysis of voxel-based morphometry studies. Front Psychiatr. (2022) 13:785547. doi: 10.3389/fpsyt.2022.785547
36. Kinou M, Takizawa R, Marumo K, Kawasaki S, Kawakubo Y, Fukuda M, et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophr Res. (2013) 150:459–67. doi: 10.1016/j.schres.2013.08.026
37. Moore DJ, Savla GN, Woods SP, Jeste DV, Palmer BW. Verbal fluency impairments among middle-aged and older outpatients with schizophrenia are characterized by deficient switching. Schizophr Res. (2006) 87:254–60. doi: 10.1016/j.schres.2006.06.005
38. Khalil AH, El-Meguid MA, Bastawy M, Rabei S, Ali R, Abd Elmoneam MHE. Correlating cognitive functions to symptom domains and insight in Egyptian patients with schizophrenia. Int J Soc Psychiatry. (2020) 66:240–8. doi: 10.1177/0020764019897697
39. van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry. (2018) 84:644–54. doi: 10.1016/j.biopsych.2018.04.023
Keywords: adolescent-onset schizophrenia, first-episode, functional near infrared spectroscopy (fNIRS), frontotemporal dysfunction, cognitive impairment
Citation: Zhang K, Jin X, He Y, Wu S, Cui X, Gao X, Huang C and Luo X (2023) Atypical frontotemporal cortical activity in first-episode adolescent-onset schizophrenia during verbal fluency task: A functional near-infrared spectroscopy study. Front. Psychiatry 14:1126131. doi: 10.3389/fpsyt.2023.1126131
Received: 29 December 2022; Accepted: 17 February 2023;
Published: 08 March 2023.
Edited by:
Chao Yan, East China Normal University, ChinaReviewed by:
Po-Han Chou, China Medical University, TaiwanZhi Li, Lieber Institute for Brain Development, United States
Copyright © 2023 Zhang, Jin, He, Wu, Cui, Gao, Huang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuerong Luo, bHVveHVlcm9uZyYjeDAwMDQwO2NzdS5lZHUuY24=
 Kun Zhang
Kun Zhang Xingyue Jin
Xingyue Jin Yuqiong He
Yuqiong He Xilong Cui
Xilong Cui Xueping Gao
Xueping Gao Chunxiang Huang
Chunxiang Huang Xuerong Luo
Xuerong Luo