- 1Collaborative Innovation Center of Assessment for Basic Education Quality, Beijing Normal University, Beijing, China
- 2Chinese PLA Center for Disease Control and Prevention, Beijing, China
- 3State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, China
- 4College of Physical Education and Sports, Beijing Normal University, Beijing, China
Developmental coordination disorder (DCD) is a motor development disorder that affects an individual’s growth and development, and may persist throughout life. It is not caused by intellectual or physical disability. Studies have suggested DCD often occurs in childhood, resulting in a series of abnormal manifestations that hinder children’s normal development; cohort studies suggest a higher incidence in boys than in girls. Early diagnosis and appropriate interventions can help relieve symptoms. Unfortunately, the relevant research still needs to be further developed. In this paper, we first start from the definition of DCD, systematically investigate the relevant research papers in the past decades and summarize the current research hotspots and research trends in this field. After summarizing, it is found that this research field has attracted more researchers to join, the number of papers published has increased year by year and has become a hot spot in multidisciplinary research, such as education, psychology, sports rehabilitation, neurobiology, and neuroimaging. The continuous development of the correlation between perinatal factors and DCD, various omics studies, and neuroimaging methods also brings new perspectives and working targets to DCD research. DCD-related research will continue to deepen along the research direction of multivariate, multidimensional, and multimodal.
Introduction
Motor development is essential for children’s physical and mental development. Developmental coordination disorder (DCD) is a motor development disorder characterized by inadequate motor performance, which affects individuals’ activities of daily living and academic performance, and cannot be explained by intellectual disability or any congenital/acquired neurological condition. DCD occurs more commonly in children, with detection rates generally ranging from 5% to 20%, and epidemiological findings indicate that boys are usually more likely to be detected than girls, with male to female ratios ranging from 2:1 to 7:1 (1–4). As a limiting condition, DCD seriously affects children’s daily lives (5). These difficulties not only cause problems in children’s motor development, increase the risk of attention, social skills, reading, and spelling difficulties, but also affect children’s self-esteem and motivation development, and they are easily bullied (6), resulting in a series of abnormal manifestations that hinder children’s normal development.
For some children, difficulty in movement is a single symptom; for others, deficits in motor coordination are just one of many problems, and may also involve deficits in speech and language, reading, attention, and/or social and emotional aspects (7–10). The formation of DCD in children may be affected by various factors, such as self-status, maternal pregnancy and childbirth, family, and school environment, etc. (11–13); Although the role of neural factors in the formation of DCD has been increasingly noted with the progress of cognitive neuroscience, the mechanism is still unclear. But it is certain that motor development disorders do not disappear as children age (14, 15); without intervention, these problems can extend from childhood into adulthood and, more seriously, affect an individual’s growth and development. Various interventions, especially those that work at the cognitive level of children, can have a certain effect on children’s motor development (16). Therefore, early detection and intervention of DCD can support the development of children and adolescents as early as possible and avoid long-term adverse effects.
Unity of definition
The field of motor development research initially focused on the difficulties and deficits to movement in children, often without a uniform name. The earliest researchers often used the term “clumsy” to describe children with difficulty with movement or motor acquisition (17, 18).
Subsequently, researchers have called such children “dyspraxia,” “motor learning difficulties,” and “Disorder of Attention, Motor Control, and Perception” (DAMP) from different perspectives, such as neurology. However, the above terms either focus on a specific aspect or only focus on the child’s symptoms and do not refer to the child’s development process (19). Some medical diagnostic criteria, international conferences, and documents have gradually tried to define terminology for them, such as “DCD,” “specific motor skill development disorder,” etc. Until 2013, the latest edition of the Diagnostic and Statistical Manual of Mental Disorders (5th ed.) (DSM-V) included DCD as a neurodevelopmental disorder, cataloging motor disorders under this category and giving four detailed diagnostic criteria:
(A) The acquisition and execution of coordinated motor skills is substantially below that expected given the individual’s chronological age and opportunity for skill learning and use. Difficulties are manifested as clumsiness (e.g., dropping or bumping into objects) as well as slowness and inaccuracy of performance of motor skills (e.g., catching an object, using scissors or cutlery, handwriting, riding a bike, or participating in sports).
(B) The motor skills deficit in Criterion A significantly and persistently interferes with activities of daily living appropriate to chronological age (e.g., self-care and self-maintenance) and impacts academic/school productivity, prevocational and vocational activities, leisure, and play.
(C) Onset of symptoms is in the early developmental period.
(D) The motor skills deficits are not better explained by intellectual disability (intellectual developmental disorder) or visual impairment and are not attributable to a neurological condition affecting movement (e.g., cerebral palsy, muscular dystrophy, and degenerative disorder) (1).
Increasing interest in developing countries
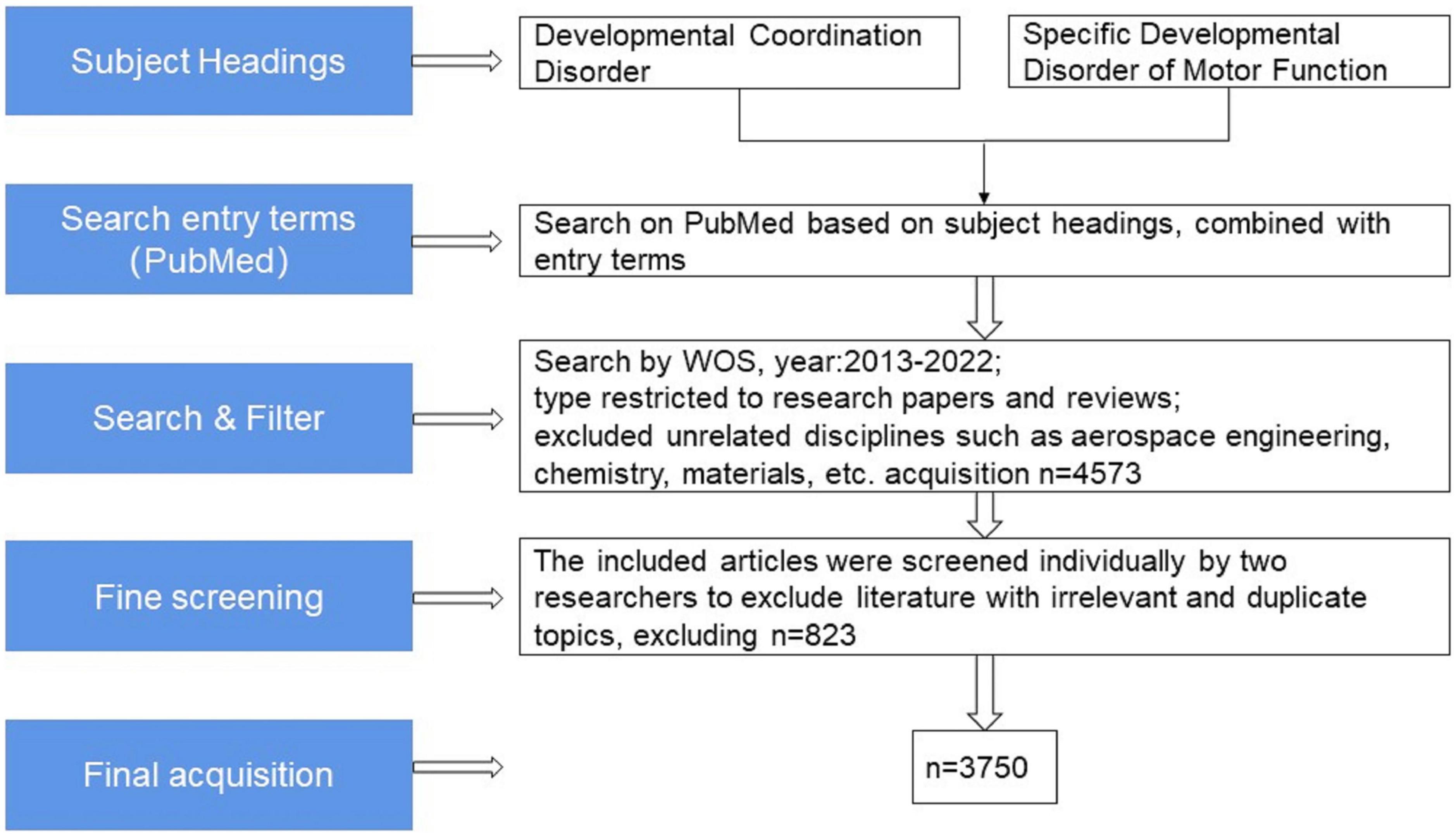
Since then, the field of research on DCD has steadily developed. We grabbed the English literature related to the field of children’s DCD research from January 2013 to November 2022 in the Web of Science (WOS) Core Collection database for bibliometric analysis (20) and removed the Web of Science category and a small number of journals in the fields of aerospace engineering, chemistry, materials, economics, computer and other obvious topics, with a total of 3750 articles meeting the target conditions (see Figure 1). The analysis shows that the annual number of children’s DCD research papers published decreased slightly compared with 2013 in 2014, and the overall trend from 2013 to 2021 showed an upward trend year by year, and the number of papers published in 2021 (640) has reached twice the number published in 2013 (308), and as of 1 November 2022, the number of papers published in 2022 has reached 302 (see Figure 2).
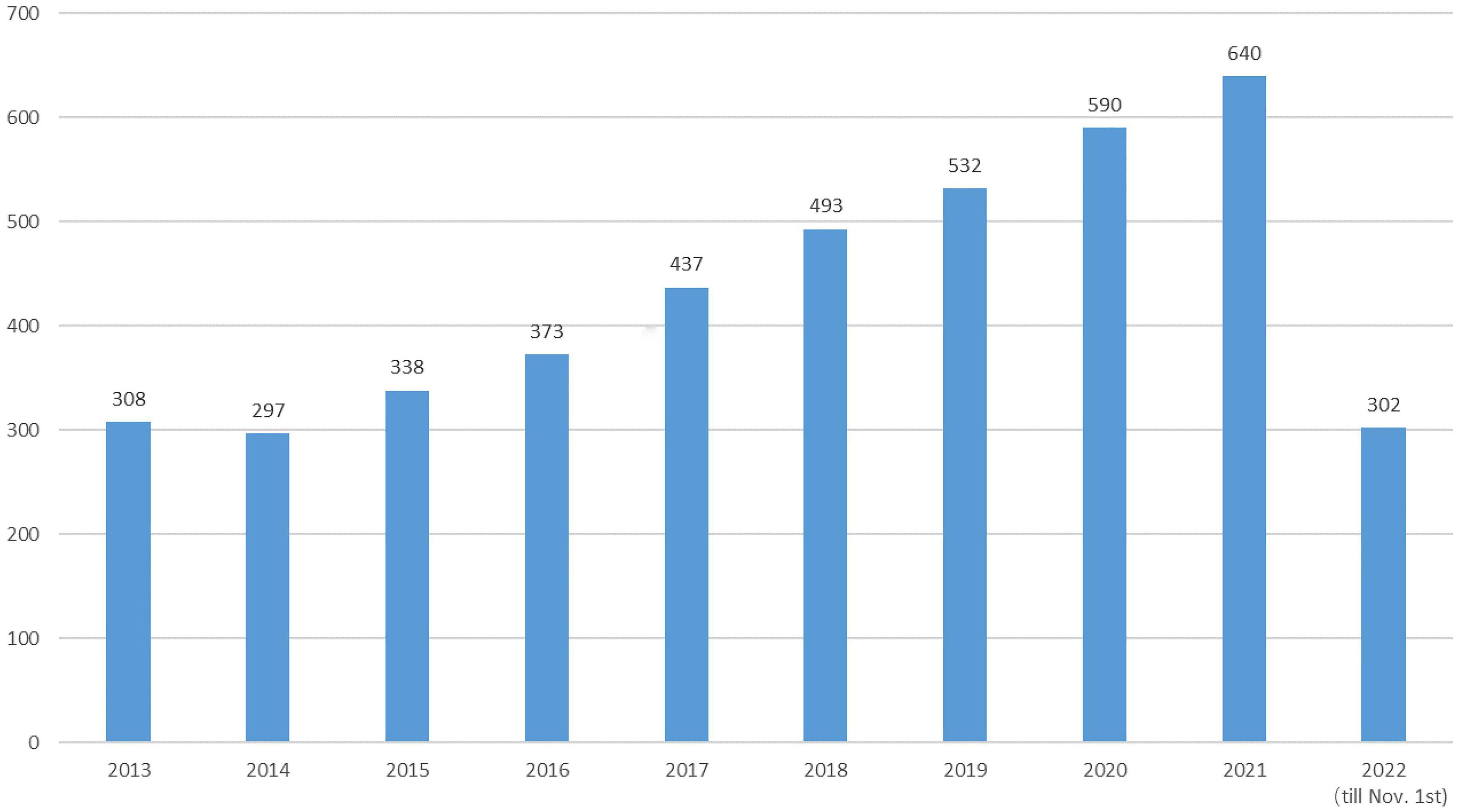
Figure 2. Annual number of articles related to children’s developmental coordination disorder (DCD).
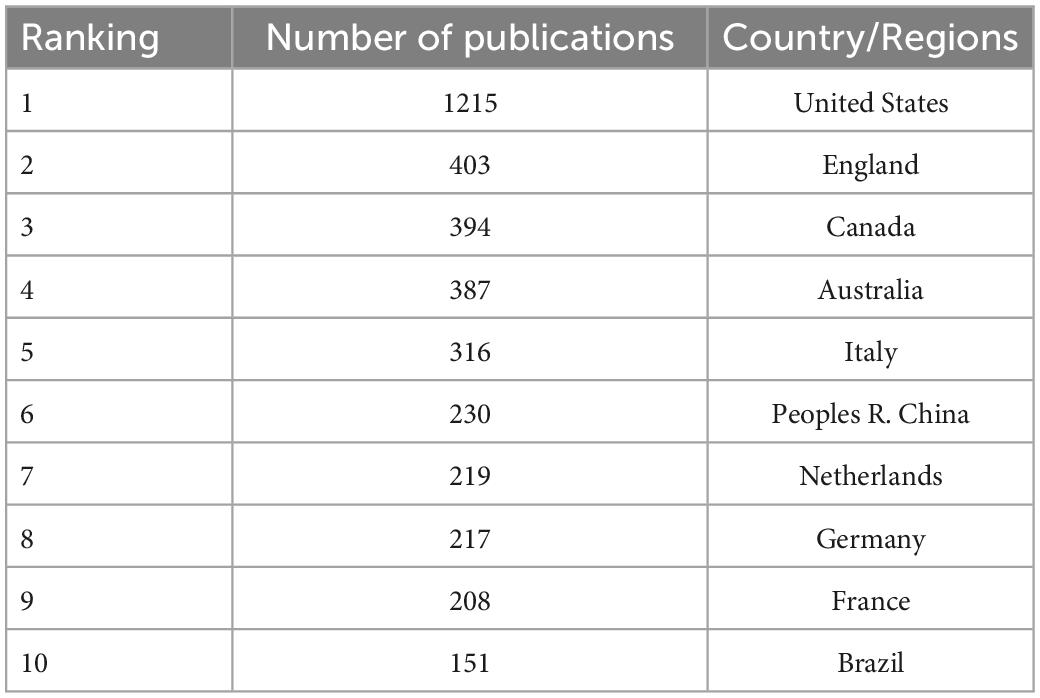
Research on neurodevelopmental disorders, including DCD, is critical to protecting children’s wellbeing and improving the quality of the population. Analysis of the countries or regions where papers were published shows that the United States ranks first in the field of children’s DCD research in terms of the number of papers published; the number of papers published far exceeds that of other countries, and the research contribution is in a central position. Eight of the top 10 countries in terms of number of publications and centrality are developed countries, indicating that the current research on DCD in children is mainly dominated by developed countries (see Figure 3 and Tables 1, 2). The strong economic base of developed countries underpins their focus on child development. In recent years, China and Brazil have gradually emerged in the field of DCD, and China has ranked 6th in the number of articles published so far, about 230. Brazil ranked 10th with 151 publications. China and Brazil also rank in the top 10 research centers, indicating that developing countries are also deepening their understanding of childhood DCD, and their status and role in this research field are becoming more prominent. As populous countries and rapidly emerging economic entities, developing countries are set to play an increasingly important role in the field of neurodevelopmental research in children, including DCD research.
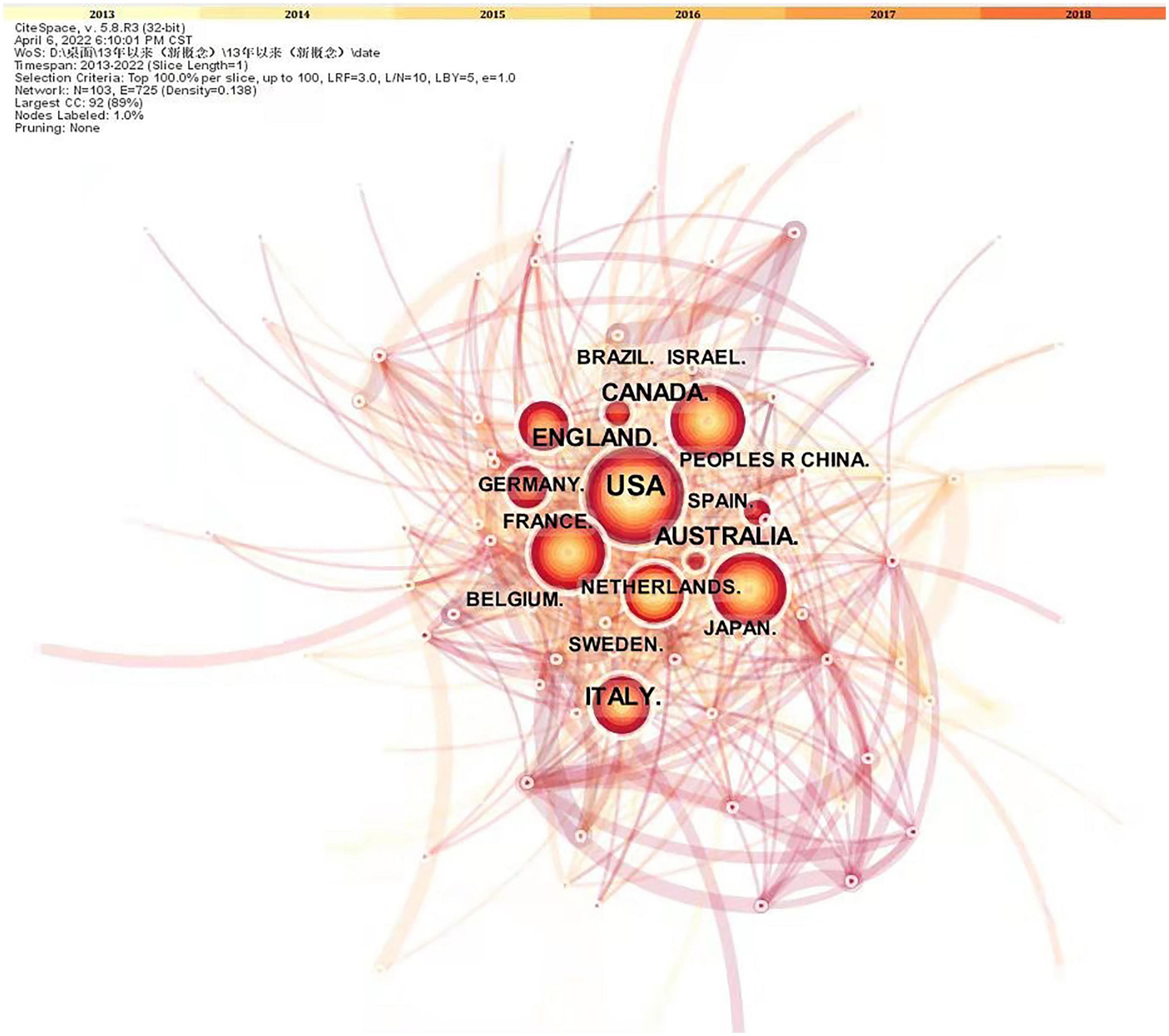
Figure 3. Research papers published on state relations. The nodes in the graph are countries; the larger the node, the higher the volume of articles issued; and the connecting lines indicate the cooperation between countries; the more connecting lines, the better the communication ability of the nodes.
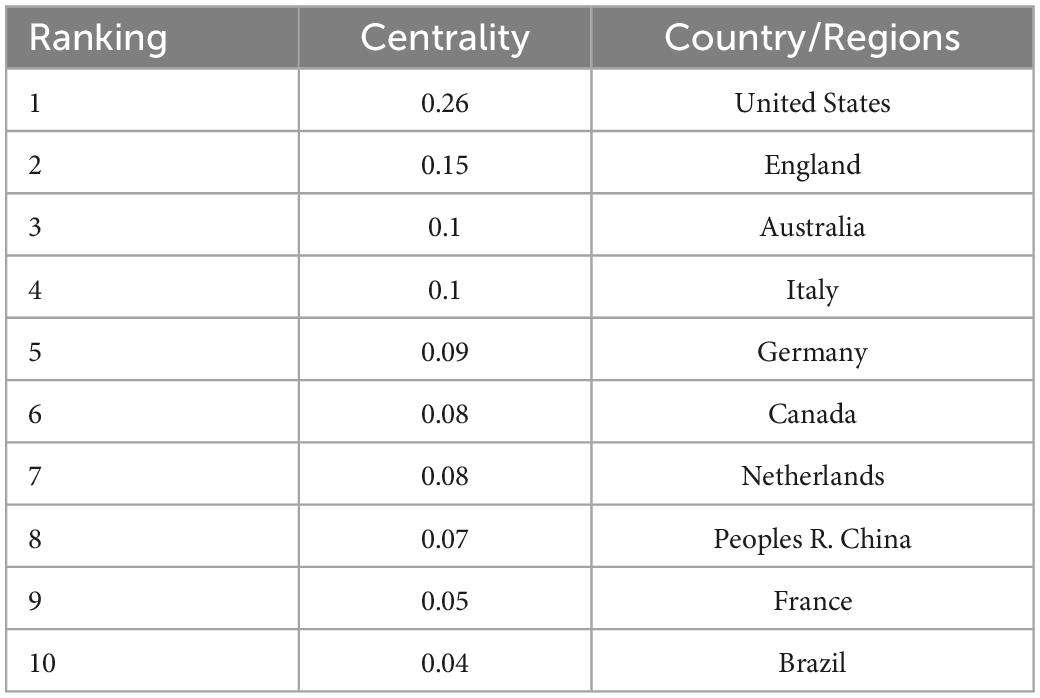
Table 1. The top 10 countries/regions in the country centrality of children’s developmental coordination disorder (DCD) research field.
Perspectives from different research fields
Compared to language development and so on, children’s motor development occurs earlier in early childhood and is easier to observe, meaning there are some intrinsic correlations between motor acquisition and various learning mechanisms in school age. Therefore, research in this field attracts researchers from different disciplinary backgrounds. We conducted keyword cluster analysis on the relevant English literature in the field of childhood DCD research from 2013 to the present and formed a total of 10 clusters: #0 motor skills, #1 executive function, #2 intellectual disability, and #3 movement assessment battery for children-2, #4 motor skill disorders, #5 autism spectrum disorder, #6 motor imagery, #7 language development, #8 autism, #9 early intervention (see Figure 4). The above clusters can be clustered and further divided into four plates: #1/#6 focusing on the cognitive neuroscience underpinnings of children’s motor development; #2/#3/#4/#5/#8/#9 focusing on the assessment, diagnosis, and intervention of specific disorders, as well as comorbid conditions for DCD and other NDDs such as autism; #2/#7 exploring the relationship between children’s motor development and other areas of child development, such as language; #0 focusing more on teaching strategies and learning mastery of motor skills.
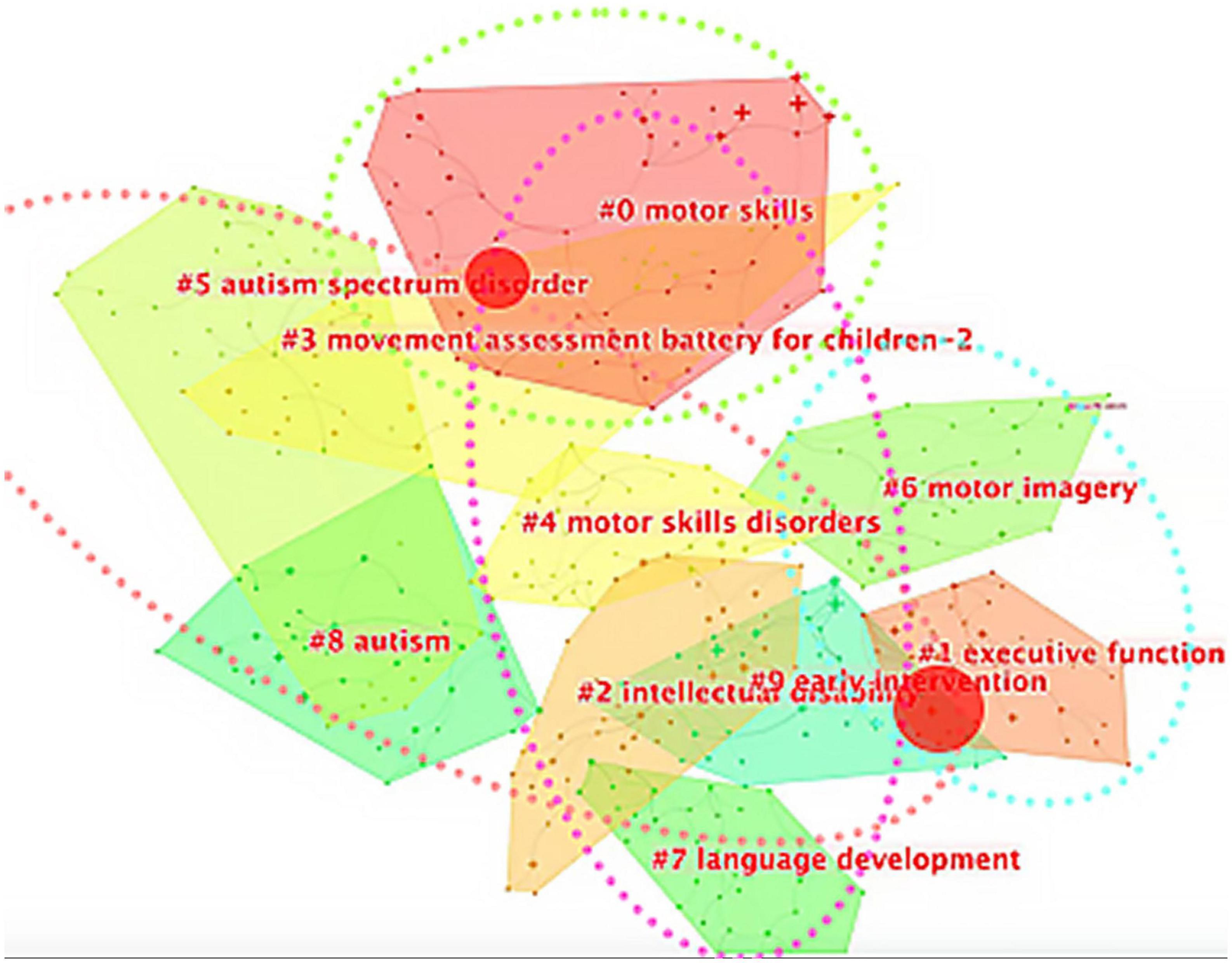
Figure 4. Keywords cluster analysis. The blue circle is plate 1: # 1 and # 6 make up the cognitive neuroscience plate (relationship between cognitive function and process and explicit DCD); the red circle is plate 2: # 5, # 3, # 4, # 8, # 2, and # 9 constitute the medical plate (co-occurrence evaluation intervention of NDDs); the pink circle is plate 4: # 0, # 4, # 2, and # 7 constitute the psychology plate; the green circle is plate 3: # 0 is an education plate.
Among them, the research on the cognitive neural basis of children’s motor development mostly comes from the research perspective of cognitive neuroscience. The evaluation and diagnosis interventions for DCD mostly come from the research perspective of pediatrics, rehabilitation, and other clinical medical disciplines. The research on the developmental status and developmental influencing factors of children with DCD mostly comes from the research perspectives of developmental psychology, experimental psychology, multidisciplinary psychology, and other psychological disciplines. The research on physical activity intervention and physical education psychology mostly comes from the research perspective of special education, exercise science, and other pedagogical disciplines.
The above analysis results reflect the current differences in the focus of different disciplines on the research of children’s motor development disorders. Specifically, physical education pays more attention to the decomposition and training mastery of motor skills, such as Smith et al. (21), who reviewed the differences in gait between children with and without DCD when walking and running; a study conducted in Pakistan showed that trampoline exercise training was associated with improved exercise performance in children with DCD (22). Psychology researchers, on the other hand, focus more on motor development milestones, influencing factors, and developmental outcomes, such as Bolk et al. (23), who revealed that extremely preterm birth is a high-risk factor for DCD and related comorbidities in children; Karras et al. (24), who focused on and studied health-related quality of life in children with DCD.
The discipline of clinical medicine pays more attention to the assessment, diagnosis, and intervention of specific disorders, such as in the journal Archives of Disease in Childhood, Kirby et al. (25) discussed the diagnosis of DCD, including a comprehensive understanding of past history, clinical observation, and the use of standardized assessment tools; In a journal in the pediatric category, research on virtual reality tools for interventions in children with DCD was reviewed (26).
The discipline of cognitive neuroscience pays more attention to the neural mechanisms and patterns of motor development, such as that studied by Rinat et al. (27) using resting-state functional MRI to explore the similarities and differences in brain functional connections between DCD children and normally developing children; Studies have found that children with DCD generally have deficits in language synchronization to auditory and visual routine stimuli compared to normally developing children, and that the stability of audio-verbal synchronization during practice is associated with the thickness of the sensorimotor cortex (28).
In addition, between the above four sections, there are two nodes that reflect the cross-convergence of different research angles in the research focus, namely “Children’s Motor Development Assessment Tool Technology” and “Early Intervention of Children’s Motor Development,” which are the focus of interdisciplinary common concern. These two aspects are also the places where there is much debate between disciplines. For example, there is widespread controversy about the diagnostic criteria and evaluation tools for DCD, one of which is that it is difficult for researchers to determine whether the NDDs are a single barrier or a subtype in terms of detection criteria. There are common comorbidities among all types of NDDs, which some researchers believe may indicate many types (8), but others conversely believe that since difficulties/disorders of motor in children are a universal feature in various types of NDDs and even neurodevelopmental disorders like cerebral palsy (29), it may not even be an independent disorder (30).
Secondly, there is currently no tool that can be used as the “gold standard,” and only the widely used tools with standardized norms should be selected for evaluation. The jury is still out on the advantages and disadvantages of standardized assessment tools and scales. Standardized assessment tools may be interfered with by other factors in assessing athletic performance (31). There is a lack of operability in the diagnostic criteria of “significant interference with academic achievement or activities of daily living” (4, 6), while this type of information is difficult to obtain in standardized assessment tools but can be obtained from scale-based tests (32). However, scale-type tests are currently generally used as an adjunct to detection in clinical samples and are recommended as a screening tool in general population at best, even though the most well-documented scale-type tests such as DCDQ are still less sensitive (31, 32).
The intervention of DCD, from the theoretical basis and method of intervention, is generally classified into three paths: process-oriented, task-oriented, and traditional physical and occupational therapy (33). Interventions in the process-oriented approach focus on the components of the movement itself and the child’s bodily functions. It aims to improve children’s skill performance by paying attention to the details of movement breakdown (34). Sensory integration training is in this category. However, current evidence suggests that process-oriented training is not significant (35, 36). The task-oriented approach is to improve children’s motor performance based on specific tasks that cause motor difficulty in children, considering the fundamentals of motor control/motor learning and ecology (35). Cognitive Orientation to Daily Occupational Performance (CO-OP) falls into this category, and this intervention program is significantly more effective than other interventions (37). The traditional physical and functional therapy approach relies on the basic assumption that motor skills have developmental ladder. The intervention focuses on basic training of gross motor/fine motor, and the development of these basic motor abilities as a prerequisite for motor skill development tends to incorporate some task-oriented approaches (35). But what the active ingredients of its intervention are also controversial. Some researchers believe that the active ingredients of the intervention may be cognition (38–40), sociality (39, 41), or experience/practice (42, 43), and the nature and guidance of activities (13, 43, 44). Among them, the most discussed is the importance of cognition to the intervention effect in children with DCDs. By comparing different intervention methods, Sims et al. (45) believe that the most effective part of the intervention is the process involving cognitive function, that is, the process that facilitates the acquisition, processing, combination, planning, and construction of information in children; In recent years, new cognitive methods of motor imaging training have been arguing that appropriate exercise programs are important for safe and efficient performance of activities of daily living (40). Sugden and Chambers (33) point out that the process-oriented approach and the task-oriented approach have different theoretical starting points but employ some very similar interventions. These measures often point to mental processes such as perception, memory, attention, and planning. Cognitive-directed occupational therapy is generally the acquisition of skills using cognitive strategies (46). With the deepening of the discussion of the mechanism of DCDs, researchers gradually began to map specific intervention tasks to potential cognitive factors and discuss the effectiveness of interventions.
Current hotspots and research trends
In recent years, the research topics related to DCD have also been constantly changing. In general, keywords show the frontiers of research on this topic (47), and we analyze the above collections by year (see Figures 5, 6).
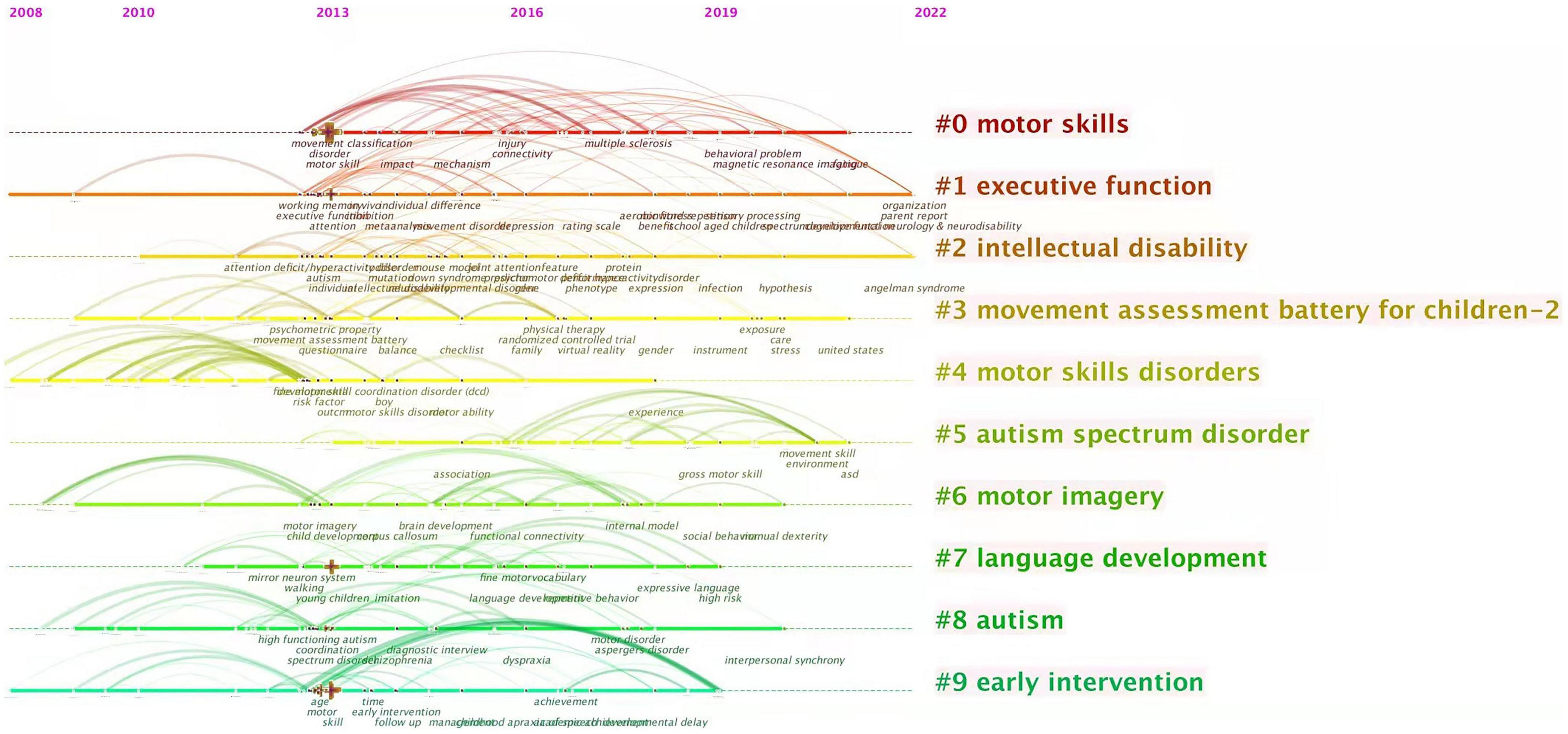
Figure 5. Co-current zone of keywords analysis. The nodes on the ten horizontal lines in the diagram are the keywords and their corresponding occurrence times, the rightmost labels are the keyword clusters, and the keyword clusters from #0 to #9 are arranged in descending order of the number of keywords.
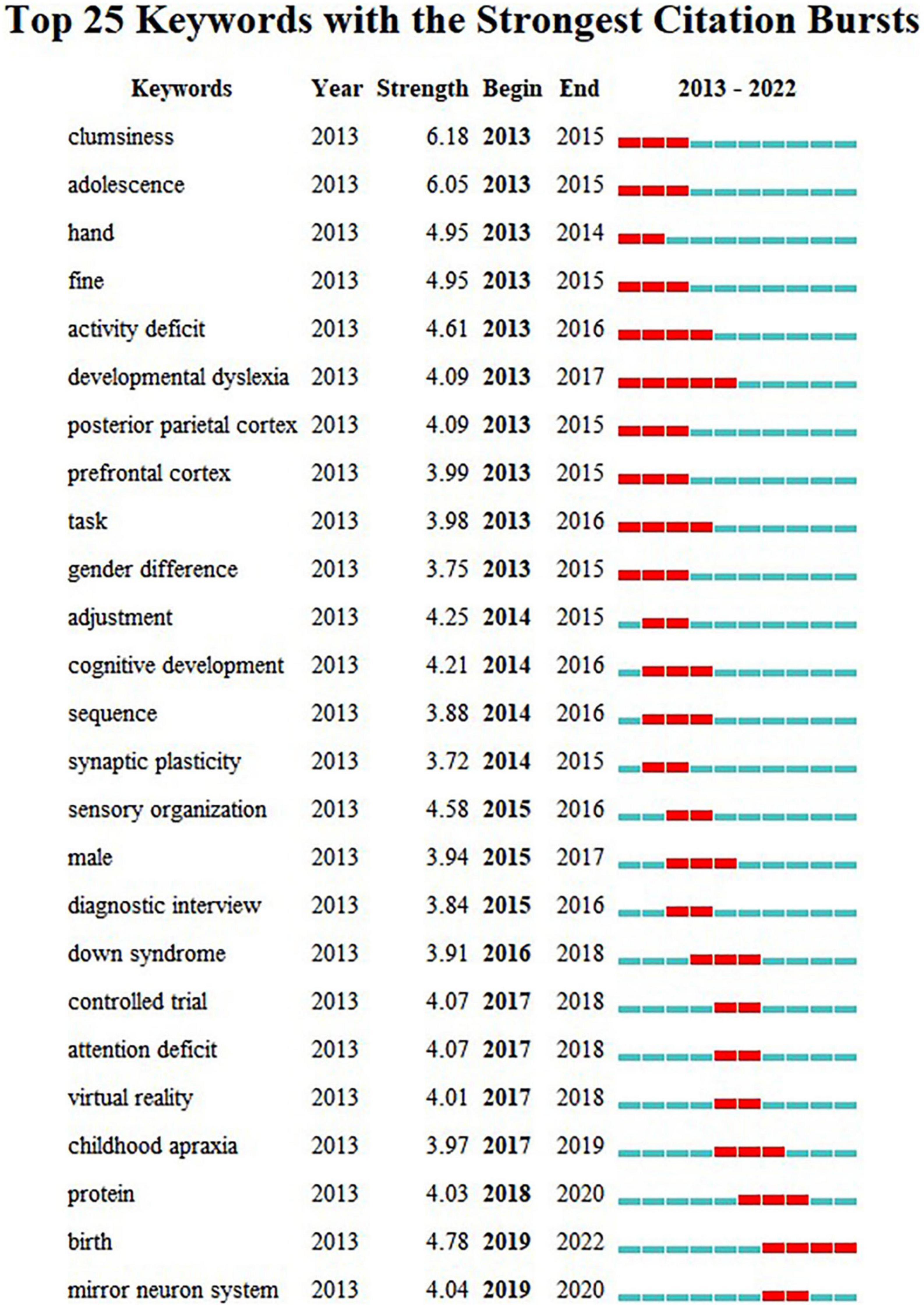
Figure 6. Burst detection of keyword analysis. Each year from 2013 to 2022 is set as a time zone division, with the blue line in the diagram indicating the time interval and the red line indicating the time period when the keyword burst is detected.
According to the analysis of keyword co-current zone, the research hotspots of clinical medicine perspective in recent years are “Comorbid symptoms” and “Behavior disorder.” The research hotspots of psychology perspective are “Parental involvement,” “Intervention in the environment,” “Perinatal intervention,” and “DCD children’s social expression behavior.” The research hotspots of cognitive neuroscience perspective are “Nuclear magnetic resonance (NMR),” “Brain image,” and “Neural mechanism study.” The research hotspots of physical education perspective are “The study of motor skills” and “Improvement of gross motor skills.” New keywords including “neurocognitive,” “proteomics,” “perinatal” have appeared frequently in the past three years. Further according to the analysis of keyword burst detection, the top 25 keywords with the strongest sudden outbreak obviously show a certain time stage. In the past 5 years, the emerging keywords of new outbreaks are: “mirror neuron system,” “protein,” “birth.” The above results reflect the current interest in neurodevelopmental mechanisms, perinatal factors and proteomics in the field of pediatric DCD and reflect the increasing tendency of research to start from behavioral-brain-cellular molecular data when understanding the development process of children.
To study the neurodevelopmental mechanism of DCD, some studies have used brain imaging technology to explore and find that DCD has changes in brain structure and function. A study on brain structure used graph theoretical analysis to reveal differences in global and regional topological attributes of the brain structure network, which showed that children who met the criteria for DCD and ASD showed more widespread topological changes than those with DCD symptoms alone (48).
On resting functional MRI, Rinat et al. (27) found that children with DCD showed altered functional connectivity between the sensorimotor network and the posterior cingulate cortex (PCC), precuneus, and posterior middle temporal gyrus (pMTG). Fuelscher et al. (49) used activation likelihood estimation (ALE) to perform a meta-analysis of seven studies using fMRI and found that, compared to the control group, children with DCD had reduced in activation in middle frontal gyrus, superior frontal gyrus, cerebellum, supramarginal gyrus, and inferior parietal lobule when performing manual dexterity tasks.
On diffusion tensor imaging (DTI), Langevin et al. (50) found white matter parameters changed in children with ADHD and DCD. Further researchers found that neurodevelopmental disorders such as DCD may be related to white matter development delay and/or structural disorders, and the DCD children are more prone to abnormal white matter development than ordinary children (51–53), correspondingly with relatively higher detection rates of various types of NDDs. Moreover, there are differences in the pattern of white matter abnormalities in children with different NDDs such as DCD and ADHD. In studies of preterm very low birth weight (VLBW) children, abnormalities in the white matter structure of children with DCD were mostly present in white matter in areas related to motor function, including corticospinal tracts, cerebellar tracts, and cerebellum (51). White matter retardation in ADHD is mainly manifested in the frontostriatal and superior longitudinal fasciculus (54).
Other studies have shown that perinatal factors such as preterm birth and VLBW greatly increase the risk of children developing various neurological injuries, such as cerebral palsy, and also increase the risk of NDDs in K-12 children. Powls et al. (55) reported that VLBW children had more significant motor impairments at 6 and 8 years of age than controls; a review by Williams et al. (56) showed that the combined estimate of mild to moderate motor impairment in preterm infants was 40.5/100, and the composite estimate for moderate sports impairment was 19.0/100. An Italian study showed that preterm children scored significantly lower on DCDQ-Italian than control children, and 30% of them were at risk for DCD (57). In a study exploring brain structure and neurobehavioral function in preterm infants, it was discussed that changes in white matter parameters may also be associated with white matter development and myelination (58). Whether this is related to protein expression requires further research.
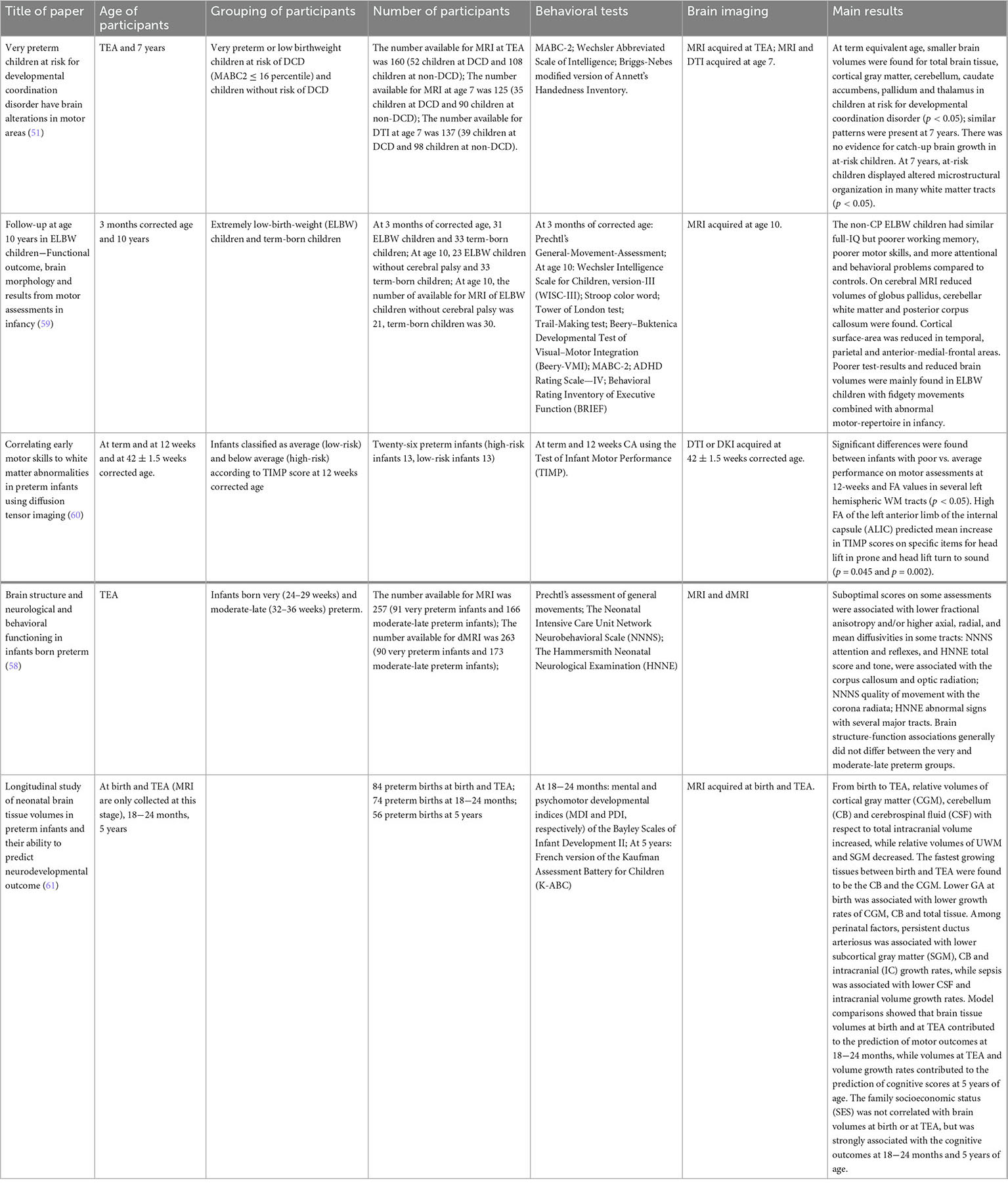
Studies are beginning to try to combine perinatal and brain imaging. For example, Grunewaldt et al. (59) reported that early ELBW children without CP with abnormal motor skills had significantly smaller white matter volume and cortical surface-area at age 10; Coker-Bolt et al. (60) conducted exercise assessment (at term and at 12 weeks corrected age) and DTI and DKI (mean corrected gestational age 42.2 ± 1.5 weeks) and there were significant differences in FA values in many regions of the left hemisphere between infants with poor and average performance on exercise assessment; while Kelly et al. (58) measured brain structure and neurobehavioral function in preterm infants at term-equivalent age (TEA), showing that there was no overall difference in brain structure-function relationship between very preterm and moderate-late preterm infants; Gui et al. (61) did two MRI scans of preterm infants at birth and TEA, providing information on brain development from birth to TEA, and models showing brain tissue capacity at birth and TEA helped predict motor outcomes at 18−24 months. We briefly summarize the factors and results of the above studies considering brain imaging and perinatal factors in the Table 3.
Among the studies mentioned above, there are no study in the age group of 3 to 6 years old, and most studies have a sample size of less than 100 people. From the brain imaging database, brain imaging data of children aged 3−6 is rarely collected, and even few NDDs samples are available. Due to differences in research methods, ages, and research tools, there is no consistent conclusion from the perspective of protective factors and risk factors. Early identification and treatment facilitate the prognosis of DCD and other NDDs. Meanwhile, the systematic evaluation of perinatal factors, DCD detection rate, fMRI, motor development assessment, and other factors is crucial for the healthy development of children with neurodevelopmental disorders under perinatal factors.
Some large cohort study databases are increasingly incorporating motor development data, perinatal and neurodevelopment factors, and brain imaging data from children, such as GUSTO (62) and the FinnBrain Birth Cohort Study (63), which track participants for several years from prenatal and monitoring their brain, cognitive, and behavioral development. Such studies are helpful for understanding the relationship between perinatal factors, motor development, and brain development. However, these cohorts are not publicly available, and it is not clear whether they have assessed motor development and other NDD risk factors, Limiting the possibility of other researchers to use them for more extensive exploration. Some other public child development cohorts did not start to monitor brain development with imaging technology until children were around year 10 years old, including the ABCD study (64) and NCANDA (65), which cannot fully cover the high-risk period (3−6 years) of NDDs. Considering the complexity of human brain development and the high incidence of NDD, it is necessary to conduct larger sample and more targeted cohort studies.
PeriCBD’s outlook for the field
In view of above, a group of Chinese experts in the fields of psychology, pediatrics, cognitive neuroscience, and neuroimaging jointly launched the project “Multicenter Database on Perinatal Factors in Child Brain-Mind Development” (PeriCBD). We hope to explore the significance of the PeriCBD database in predicting NDDs based on brain imaging data, motor development data, and DCD detection rate. By further verifying the relationship between abnormal development of children’s neural network and various types of NDDs, through the comparison between the experimental group and control group, we hope to establish an early diagnosis model and a multimodal rapid assessment model for different types of NDDs, taking the level of motor development, perinatal adverse factors, and other sensitive indicators as core indications, in order to realize the early rapid screening and diagnosis of children’s NDDs. By the understanding and mastering the brain and cognitive mechanism of NDDs, attempts should be made to intervene for children with NDDs.
Through a series of preparations, the PeriCBD project carried out data collection in 2021 with two county-level hospitals in China. We selected children aged 3−10 years old who had experienced preterm birth (<37 weeks gestation) and low birth weight (<2500 g) as the experimental group of adverse perinatal factors, and recruited ordinary children with the same age and demographic indicators as control. Data of children’s brain magnetic resonance imaging, demographic factors, cognitive ability, motor development, intelligence, detection rate of multiple NDDs disorders, behavioral index, family parenting style, maternal risk factors, maternal mood and others were collected through self-assessment, other assessments, standardized assessments and other methods. Relevant data have been preprocessed and are under further analysis.
In general, with the unity of the definition of DCD, research in this field has steadily increased in the past 10 years, especially in populous and developing countries. This trend is increasingly evident given the importance of motor development in child development. At present, research in this field pays more and more attention to the combination of multidisciplinary, multimodal data such as brain imaging data, perinatal factors, biomarkers, and conventional behavioral data. It is certain that, with the support of mass comprehensive data, the subtype classification and mechanism of DCD can be discussed, and the practical activities such as monitoring, diagnosis, prevention, and intervention of DCD, can be well guided in the near future.
Author contributions
YW, LK, and XS contributed to the discussion and design of the study. ZD and SH performed the statistical analysis. SY wrote the first draft of the manuscript. LK and XS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Dsm-V). 5th ed. Arlington, VA: American Psychiatric Publishing (2013).
2. Zoia S, Barnett A, Wilson P, Hill E. Developmental coordination disorder: current issues. Child Care Health Dev. (2006) 32:613–8. doi: 10.1111/j.1365-2214.2006.00697.x
3. Tan S, Parker H, Larkin D. Concurrent validity of motor tests used to identify children with motor impairment. Adapt Phys Act Q. (2001) 18:168–82. doi: 10.1123/apaq.18.2.168
4. Ke L. Developmental Coordination Disorders of Urban Children in China: Assessment, Influencing Factors and Interventions. Ph.D. thesis. Beijing: Beijing Normal University (2019).
5. Barnhart R, Davenport M, Epps S, Nordquist V. Developmental coordination disorder. Phys Ther. (2003) 83:722–31. doi: 10.1093/ptj/83.8.722
6. Henderson S, Barnett A. The classification of specific motor coordination disorders in children: some problems to be solved (reprinted from perspectives on the classification of specific developmental disorders). Hum Mov Sci. (1998) 17:449–69. doi: 10.1016/s0167-9457(98)00009-8
7. Dewey D, Cantell M, Crawford S. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc. (2007) 13:246–56. doi: 10.1017/s1355617707070270
8. Gillberg C. Deficits in attention, motor control, and perception: a brief review. Arch Dis Child. (2003) 88:904–10. doi: 10.1136/adc.88.10.904
9. Henderson S, Henderson L. Toward an understanding of developmental coordination disorder: terminological and diagnostic issues. Neural Plast. (2003) 10:1–13. doi: 10.1155/np.2003.1
10. Lingam R, Golding J, Jongmans M, Hunt L, Ellis M, Emond A. The association between developmental coordination disorder and other developmental traits. Pediatrics. (2010) 126:E1109–18. doi: 10.1542/peds.2009-2789
11. Davis N, Ford G, Anderson P, Doyle L. Victorian infant collaborative S. Developmental coordination disorder at 8 years of age in a regional cohort of extremely-low-birthweight or very preterm infants. Dev Med Child Neurol. (2007) 49:325–30. doi: 10.1111/j.1469-8749.2007.00325.x
12. Harris S, Mickelson E, Zwicker J. Diagnosis and management of developmental coordination disorder. Can Med Assoc J. (2015) 187:659–65. doi: 10.1503/cmaj.140994
13. Venetsanou F, Kambas A. Environmental factors affecting preschoolers’ motor development. Early Child Educ J. (2010) 37:319–27. doi: 10.1007/s10643-009-0350-z
14. Cousins M, Smyth M. Developmental coordination impairments in adulthood. Hum Mov Sci. (2003) 22:433–59. doi: 10.1016/j.humov.2003.09.003
15. Hellgren L, Gillberg I, Bagenholm A, Gillberg C. Children with deficits in attention, motor control and perception (damp) almost grown up – psychiatric and personality-disorders at age 16 years. J Child Psychol Psychiatry Allied Discip. (1994) 35:1255–71. doi: 10.1111/j.1469-7610.1994.tb01233.x
16. Withers R, Tsang Y, Zwicker J. Intervention and management of developmental coordination disorder: are we providing evidence-based services? Can J Occup Ther. (2017) 84:158–67. doi: 10.1177/0008417417712285
17. Gubbay S, Ellis E, Walton J, Court S. Clumsy children a study of apraxic and agnosic defects in 21 children. Brain. (1965) 88:295–312. doi: 10.1093/brain/88.2.295
18. Sigmundsson H. Perceptual deficits in clumsy children: inter- and intra-modal matching approach–a window into clumsy behavior. Neural Plast. (2003) 10:27–38. doi: 10.1155/np.2003.27
19. Henderson S, Sugden D, Barnett A. Movement Assessment Battery for Children-2 (Mabc-2). Examiner’s Manual. 2nd ed. London: Pearson Assessment (2007).
20. Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. (2019) 14:25. doi: 10.1371/journal.pone.0223994
21. Smith M, Ward E, Williams C, Banwell H. Differences in walking and running gait in children with and without developmental coordination disorder: a systematic review and meta-analysis. Gait Posture. (2021) 83:177–84. doi: 10.1016/j.gaitpost.2020.10.013
22. Sulaiman M, Baig M, Memon S, Ashraf N, Butt P, Afzal K, et al. Effects of trampoline exercises in children with developmental coordination disorder: a randomized controlled trial. Phys Med Rehabilitationsmed Kurort. (2022) 32:279–84. doi: 10.1055/a-1755-8655
23. Bolk J, Farooqi A, Hafstrom M, Aden U, Serenius F. Developmental coordination disorder and its association with developmental comorbidities at 6.5 years in apparently healthy children born extremely preterm. JAMA Pediatr. (2018) 172:765–74. doi: 10.1001/jamapediatrics.2018.1394
24. Karras H, Morin D, Gill K, Izadi-Najafabadi S, Zwicker J. Health-related quality of life of children with developmental coordination disorder. Res Dev Disabil. (2019) 84:85–95. doi: 10.1016/j.ridd.2018.05.012
25. Kirby A, Sugden D, Purcell C. Diagnosing developmental coordination disorders. Arch Dis Child. (2014) 99:292–6. doi: 10.1136/archdischild-2012-303569
26. Lino F, Arcangeli V, Chieffo D. The virtual challenge: virtual reality tools for intervention in children with developmental coordination disorder. Children Basel. (2021) 8:10. doi: 10.3390/children8040270
27. Rinat S, Izadi-Najafabadi S, Zwicker J. Children with developmental coordination disorder show altered functional connectivity compared to peers. Neuroimage Clin. (2020) 27:12. doi: 10.1016/j.nicl.2020.102309
28. Le M, Blais M, Jucla M, Chauveau N, Maziero S, Biotteau M, et al. Procedural learning and retention of audio-verbal temporal sequence is altered in children with developmental coordination disorder but cortical thickness matters. Dev Sci. (2021) 24:14. doi: 10.1111/desc.13009
29. Zielinski I, Steenbergen B, Baas C, Aarts P, Jongsma M. Event-related potentials during target-response tasks to study cognitive processes of upper limb use in children with unilateral cerebral palsy. J Vis Exp. (2016) 107:e53420. doi: 10.3791/53420
30. Skranes J. Is developmental coordination disorder in preterm children the motor phenotype of more widespread brain pathology? Acta Paediatr. (2019) 108:1559–61. doi: 10.1111/apa.14825
31. Blank R, Barnett A, Cairney J, Green D, Kirby A, Polatajko H, et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev Med Child Neurol. (2019) 61:242–85. doi: 10.1111/dmcn.14132
32. Barnett A. Motor assessment in developmental coordination disorder: from identification to intervention. Int J Disabil Dev Educ. (2008) 55:113–29. doi: 10.1080/10349120802033436
33. Sugden D, Chambers M. Intervention approaches and children with developmental coordination disorder. Pediatr Rehabil. (1998) 2:139–47.
34. Sugden D. Current approaches to intervention in children with developmental coordination disorder. Dev Med Child Neurol. (2007) 49:467–71. doi: 10.1111/j.1469-8749.2007.00467.x
35. Smits-Engelsman B, Blank R, Van der Kaay A, Mosterd-Van der Meijs R, Vlugt-Van den Brand E, Polatajko H, et al. Efficacy of interventions to improve motor performance in children with developmental coordination disorder: a combined systematic review and meta-analysis. Dev Med Child Neurol. (2013) 55:229–37. doi: 10.1111/dmcn.12008
36. Wilson P. Practitioner review: approaches to assessment and treatment of children with DCD: an evaluative review. J Child Psychol Psychiatry. (2005) 46:806–23. doi: 10.1111/j.1469-7610.2005.01409.x
37. Miller L, Polatajko H, Missiuna C, Mandich A, Macnab JJA. Pilot trial of a cognitive treatment for children with developmental coordination disorder. Hum Mov Sci. (2001) 20:183–210. doi: 10.1016/s0167-9457(01)00034-3
38. EbrahimiSani S, Sohrabi M, Taheri H, Agdasi M, Amiri S. Effects of virtual reality training intervention on predictive motor control of children with DCD – a randomized controlled trial. Res Dev Disabil. (2020) 107:15. doi: 10.1016/j.ridd.2020.103768
39. Missiuna C, Moll S, Law M, King S, King G. Mysteries and mazes: parents’ experiences of children with developmental coordination disorder. Can J Occup Ther. (2006) 73:7–17. doi: 10.2182/cjot.05.0010
40. Steenbergen B, Krajenbrink H, Lust J, Wilson P. Motor imagery and action observation for predictive control in developmental coordination disorder. Dev Med Child Neurol. (2020) 62:1352–5. doi: 10.1111/dmcn.14612
41. Gagnon-Roy M, Jasmin E, Camden C. Social participation of teenagers and young adults with developmental co-ordination disorder and strategies that could help them: results from a scoping review. Child Care Health Dev. (2016) 42:840–51. doi: 10.1111/cch.12389
42. Loftesnes J, Ingvaldsen R, Sigmundsson H. Children with developmental coordination disorder: can underlying perceptual disability be remediated through specific training? Psychol Rep. (2017) 120:242–54. doi: 10.1177/0033294116687761
43. Sit C, Yu J, Wong S, Capio C, Masters RA. School-based physical activity intervention for children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil. (2019) 89:1–9. doi: 10.1016/j.ridd.2019.03.004
44. Zachopoulou E, Tsapakidou A, Derri V. The effects of a developmentally appropriate music and movement program on motor performance. Early Childhood Res Q. (2004) 19:631–42. doi: 10.1016/j.ecresq.2004.10.005
45. Sims K, Henderson S, Morton J, Hulme C. The remediation of clumsiness .2. Is kinaesthesis the answer? Dev Med Child Neurol. (1996) 38:988–97. doi: 10.1111/j.1469-8749.1996.tb15059.x
46. Schwartz S, Northrup S, Izadi-Najafabadi S, Zwicker J. Co-Op for children with DCD: goals addressed and strategies used. Can J Occup Ther. (2020) 87:278–86. doi: 10.1177/0008417420941980
47. Sun H. Authour keyword co-occurrence network analysis: an empirical research. J Intell. (2012) 31:63–7.
48. Caeyenberghs K, Taymans T, Wilson P, Vanderstraeten G, Hosseini H, van Waelvelde H. Neural signature of developmental coordination disorder in the structural connectome independent of comorbid autism. Dev Sci. (2016) 19:599–612. doi: 10.1111/desc.12424
49. Fuelscher I, Caeyenberghs K, Enticott P, Williams J, Lum J, Hyde C. Differential activation of brain areas in children with developmental coordination disorder during tasks of manual dexterity: an ale meta analysis. Neurosci Biobehav Rev. (2018) 86:77–84. doi: 10.1016/j.neubiorev.2018.01.002
50. Langevin L, MacMaster F, Crawford S, Lebel C, Dewey D. Common white matter microstructure alterations in pediatric motor and attention disorders. J Pediatr. (2014) 164:1157–64.e1. doi: 10.1016/j.jpeds.2014.01.018
51. Dewey D, Thompson D, Kelly C, Spittle A, Cheong J, Doyle L, et al. Very preterm children at risk for developmental coordination disorder have brain alterations in motor areas. Acta Paediatr. (2019) 108:1649–60. doi: 10.1111/apa.14786
52. Skranes J, Vangberg T, Kulseng S, Indredavik M, Evensen K, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. (2007) 130:654–66. doi: 10.1093/brain/awm001
53. Zhang Y, Inder T, Neil J, Dierker D, Alexopoulos D, Anderson P, et al. Cortical structural abnormalities in very preterm children at 7 years of age. Neuroimage. (2015) 109:469–79. doi: 10.1016/j.neuroimage.2015.01.005
54. Bouziane C, Caan M, Tamminga H, Schrantee A, Bottelier M, de Ruiter M, et al. ADHD and maturation of brain white matter: a DTI study in medication naive children and adults. Neuroimage Clin. (2018) 17:53–9. doi: 10.1016/j.nicl.2017.09.026
55. Powls A, Botting N, Cooke R, Marlow N. Motor impairment in children 12 to 13 years old with a birth-weight of less-than 1250 G. Arch Dis Child Fetal Neonatal Ed. (1995) 73:F62–6. doi: 10.1136/fn.73.2.F62
56. Williams J, Lee K, Anderson P. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. (2010) 52:232–7. doi: 10.1111/j.1469-8749.2009.03544.x
57. Caravale B, Herich L, Zoia S, Capone L, Voller F, Carrozzi M, et al. Risk of developmental coordination disorder in Italian very preterm children at school age compared to general population controls. Eur J Paediatr Neurol. (2019) 23:296–303. doi: 10.1016/j.ejpn.2019.01.002
58. Kelly C, Thompson D, Cheong J, Chen J, Olsen J, Eeles A, et al. Brain structure and neurological and behavioural functioning in infants born preterm. Dev Med Child Neurol. (2019) 61:820–31. doi: 10.1111/dmcn.14084
59. Grunewaldt K, Fjortoft T, Bjuland K, Brubakk A, Eikenes L, Haberg A, et al. Follow-up at age 10 years in elbw children – Functional outcome, brain morphology and results from motor assessments in infancy. Early Hum Dev. (2014) 90:571–8. doi: 10.1016/j.earlhumdev.2014.07.005
60. Coker-Bolt P, Barbour A, Moss H, Tillman J, Humphries E, Ward E, et al. Correlating early motor skills to white matter abnormalities in preterm infants using diffusion tensor imaging. J Pediatr Rehabil Med. (2016) 9:185–93. doi: 10.3233/prm-160380
61. Gui L, Loukas S, Lazeyras F, Huppi P, Meskaldji D, Tolsa C. Longitudinal study of neonatal brain tissue volumes in preterm infants and their ability to predict neurodevelopmental outcome. Neuroimage. (2019) 185:728–41. doi: 10.1016/j.neuroimage.2018.06.034
62. Soh S, Tint M, Gluckman P, Godfrey K, Rifkin-Graboi A, Chan Y, et al. Cohort profile: growing up in Singapore towards healthy outcomes (gusto) birth cohort study. Int J Epidemiol. (2014) 43:1401–9. doi: 10.1093/ije/dyt125
63. Karlsson L, Tolvanen M, Scheinin N, Uusitupa H, Korja R, Ekholm E, et al. Cohort profile: the finnbrain birth cohort study (finnbrain). Int J Epidemiol. (2018) 47:15j–6j. doi: 10.1093/ije/dyx173
64. Casey B, Cannonier T, Conley M, Cohen A, Barch D, Heitzeg M, et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. (2018) 32:43–54. doi: 10.1016/j.dcn.2018.03.001
Keywords: developmental coordination disorders, bibliometrics, visual analysis, cluster analysis, neuroimaging
Citation: Ke L, Su X, Yang S, Du Z, Huang S and Wang Y (2023) New trends in developmental coordination disorder: Multivariate, multidimensional and multimodal. Front. Psychiatry 14:1116369. doi: 10.3389/fpsyt.2023.1116369
Received: 05 December 2022; Accepted: 16 January 2023;
Published: 27 January 2023.
Edited by:
Qinghua He, Southwest University, ChinaReviewed by:
Liang Zhang, Institute of Psychology (CAS), ChinaLeilei Mei, South China Normal University, China
Copyright © 2023 Ke, Su, Yang, Du, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Wang,  d2FuZ3l1bkBibnUuZWR1LmNu
d2FuZ3l1bkBibnUuZWR1LmNu
†These authors share first authorship
 Li Ke
Li Ke Xueting Su
Xueting Su Sijia Yang
Sijia Yang Zhihao Du
Zhihao Du Shunsen Huang
Shunsen Huang Yun Wang
Yun Wang