
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 12 May 2023
Sec. Addictive Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1083568
This article is part of the Research Topic Benzodiazepine Addiction: From Lab to Street View all 16 articles
 Yumi Aoki1,2
Yumi Aoki1,2 Yoshikazu Takaesu2,3*
Yoshikazu Takaesu2,3* Ken Inada4
Ken Inada4 Hiroki Yamada5
Hiroki Yamada5 Tomohiko Murao6
Tomohiko Murao6 Toshiaki Kikuchi7
Toshiaki Kikuchi7 Masahiro Takeshima8
Masahiro Takeshima8 Masayuki Tani9
Masayuki Tani9 Kazuo Mishima8
Kazuo Mishima8 Tempei Otsubo10
Tempei Otsubo10Aim: We aimed to develop a decision aid (DA) for individuals with anxiety disorders who consider tapering benzodiazepine (BZD) anxiolytics, and if tapering, tapering BZD anxiolytics with or without cognitive behavioral therapy (CBT) for anxiety. We also assessed its acceptability among stakeholders.
Methods: First, we conducted a literature review regarding anxiety disorders to determine treatment options. We cited the results of the systematic review and meta-analysis, which we conducted previously, to describe the related outcomes of two options: tapering BZD anxiolytics with CBT and tapering BZD anxiolytics without CBT. Second, we developed a DA prototype in accordance with the International Patient Decision Aid Standards. We carried out a mixed methods survey to assess the acceptability among stakeholders including those with anxiety disorders and healthcare providers.
Results: Our DA provided information such as explanation of anxiety disorders, options of tapering or not tapering BZD anxiolytics (if tapering, the options of tapering BZD anxiolytics with or without CBT) for anxiety disorder, benefits and risks of each option, and a worksheet for value clarification. For patients (n = 21), the DA appeared to be acceptable language (86%), adequate information (81%), and well-balanced presentation (86%). The developed DA was also acceptable for healthcare providers (n = 10).
Conclusion: We successfully created a DA for individuals with anxiety disorders who consider tapering BZD anxiolytics, which was acceptable for both patients and healthcare providers. Our DA was designed to assist patients and healthcare providers to involve decision-making about whether to taper BZD anxiolytics or not.
Anxiety disorders are common mental disorders characterized by emotional and stress reactions to a threat or anticipation of future concern (1), leading to a significant effect on a person’s physical and social functioning. Previous research revealed that individuals with anxiety disorders are associated with significant impairment to personal life (2) and quality of life (3), suicidal ideation and suicide attempts (4), and high care costs (5). Therefore, continued improvement in the care of people with anxiety disorders is important.
Benzodiazepine (BZD) anxiolytics are one of the treatment choices that are frequently used worldwide for the acute phase of anxiety disorders. However, the long-term BZD anxiolytic use is not recommended because of its disadvantages, including dependence (6), decline in cognitive functions (7), hip fractures associated with falls (8, 9), and impaired driving ability (10). Consequently, most anxiety disorder guidelines recommend that BZD anxiolytics should be used for only a short period (11–15). Moreover, some guidelines do not recommend the use of BZD anxiolytics, even for short-term periods, except in critical situations (16, 17).
Despite the evidence-based recommendations described above, BZD anxiolytics are commonly used worldwide for anxiety disorders (18, 19). Therefore, the safe discontinuation or tapering of BZD anxiolytics for anxiety disorders is essential. Thus, the establishment of treatment strategy against long-term BZD use for anxiety disorders may be warranted in clinical settings.
To address this issue, the evidence that psychological therapy is effective in reducing symptoms for anxiety disorders should be considered (20). Particularly, cognitive behavioral therapy (CBT) is an effective psychological intervention for anxiety disorders (21, 22). Several current guidelines recommend CBT as a first-line therapy because of its effectiveness in improving anxiety symptoms and comparatively fewer risks than BZD anxiolytics (11, 12, 17). Several trials assessing strategies for BZD discontinuation, such as gradual tapering or adding CBT, have reported the effectiveness of adding CBT in the short term (23). On the other hand, CBT has certain disadvantages, such as the lack of a fast-acting effect, longer consultation time, and high cost (24). Therefore, individuals with anxiety disorders deliberating on further non-medication treatment might face the advantages and disadvantages of CBT.
Approaches of treatment decision-making have shifted from the so-called paternalistic approach, where doctors take initiative in the decision-making, to patient-centered communication. In this type of approach, strategies such as “shared decision making” (SDM) have been emphasized, which focus on a patient’s value-based discussion that involves a two-way communication between the patient and their clinician about the positive and negative aspects of each treatment option (25, 26).
In relation to the SDM process, decision aids (DAs) have recently gained attention as patient-centered communication tools that promote two-way conversation between patients and healthcare providers during specific medical or mental conditions that require further treatment planning (27). DAs are intended to support individuals participating in the decision-making process by aiding them to make well-informed, preference-based choices when choosing their treatment options (27). DAs provide related information regarding the available options and aid people to solidify their own preferences, which are associated with different characteristics of each option (27). DAs can promote a patient’s involvement and increase concordance between their choices, preferences, and values during the decision-making process (28).
Various DAs, most of which were for decision-making during treatment initiation, have been developed in many areas including the somatic and psychiatric fields (28). Moreover, we developed several DAs for decision-making about whether the treatment should be continued or discontinued such as DA for depression remission (29) and DA for insomnia remission (30). Ramos-García et al. developed a Spanish version of DA for patients with generalized anxiety disorder (31), based on their needs that patients with GAD preferred an active and collaborative role in decision-making (32). However, to our best knowledge, there is no Japanese version of a DA for patients with anxiety disorders who are receiving BZD anxiolytics and considering further pharmacology treatment.
The aim of this study was to develop a Japanese version of DA for patients with anxiety disorders who are considering whether to discontinue BZD anxiolytics as well as whether to taper them with CBT or without CBT, if discontinuing BZD. The stakeholder’s acceptability of the DA were also examined. We have translated the DA into English so that many more people can utilize it.
The Ottawa Decision Support Framework (33) and International Patient Decision Aid Standards (IPDAS) were used to systematically develop the DA (34) (Figure 1). The IPDAS is one of the evidence-based frameworks that was established to standardize the development process and elements of DAs (35). The development process is as follows: (1) deciding the target people and assessing their decision-making needs, (2) establishing a steering committee made up by mental health professionals, (3) performing a literature review to decide the treatment options and related evidence-based outcomes, (4) creating a prototype of the DA, (5) assessing the acceptability of the prototype among stakeholders including patients and healthcare providers, (6) correcting the DA using the results of acceptability tests to create a final version of the DA, and (7) testing the developed DA for its effectiveness in clinical environment (35).
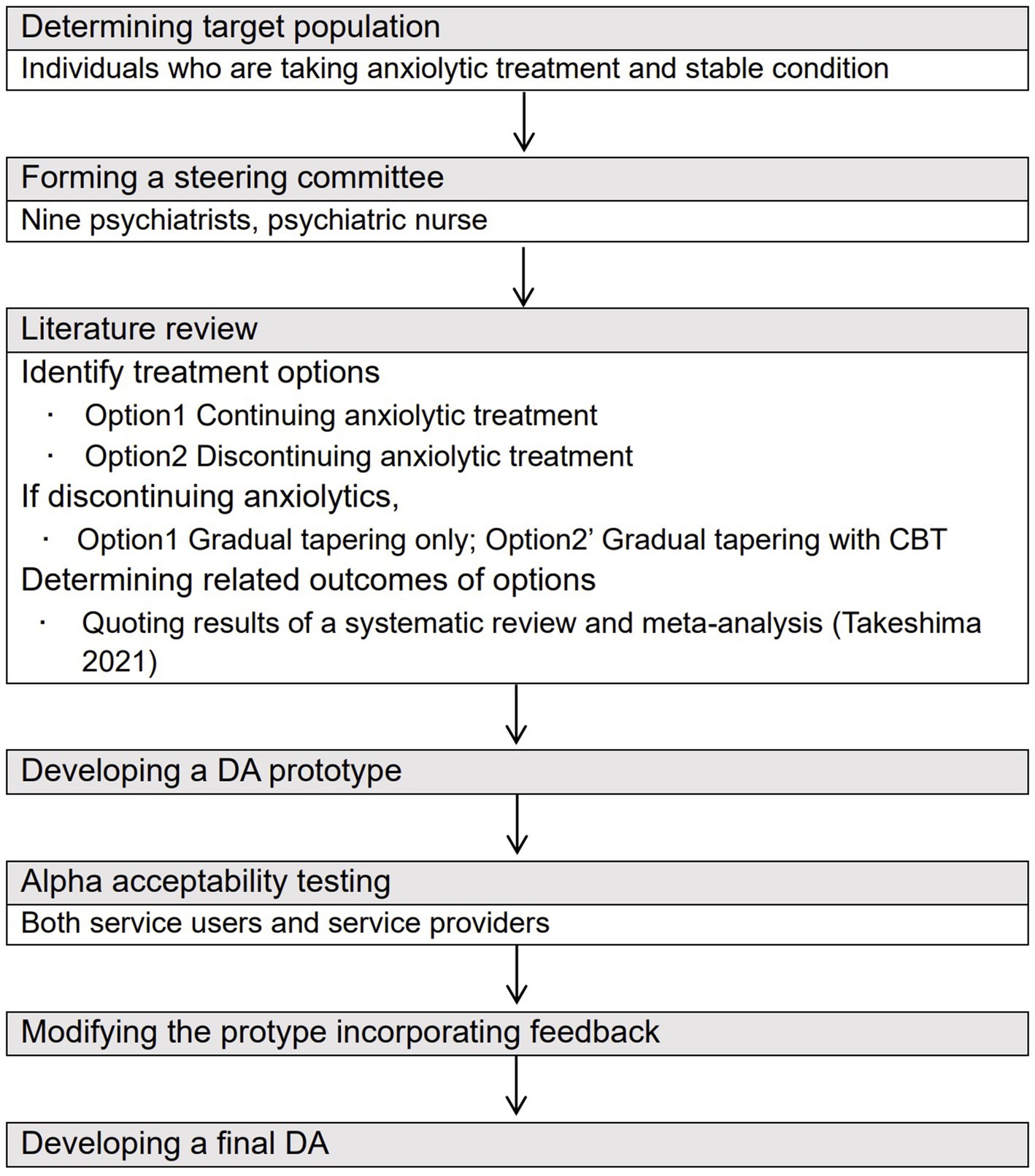
Figure 1. Process of developing a DA for those with anxiety disorder who consider tapering anxiolytics based on the approach of Coulter et al. (2013) (34).
The target people of the DA in this study was those who had been diagnosed with anxiety disorders, such as social anxiety disorder, generalized disorder, and panic disorder, and showed improvements in their symptoms and health conditions following treatment with BZD anxiolytics. Patients who were on medication but still experiencing symptoms were not targeted by the DA. The steering group expect that the DA would be useful in both primary care clinics and psychiatric outpatient clinics.
The authors established a steering committee consisting of mental health professionals on anxiety disorders and DA methodology. The group was consisted of nine psychiatrists who regularly saw people with anxiety disorders and a psychiatric nurse who was familiar with SDM literature in the mental health field (36) and had experience creating DAs for mood disorders (29, 37), insomnia (30), and attention deficit hyperactivity disorder (38).
The steering committee members examined the relevant published articles that explained anxiety disorders as a target disease and explored the advantages and disadvantages of the following treatment options: (1) continuing BZD anxiolytics, (2) tapering BZD anxiolytics, if tapering (3) gradually tapering BZD anxiolytics without CBT, and (4) gradually tapering BZD anxiolytics with CBT.
For the outcomes of the last two options, the committee referred to the results of a systematic review and meta-analysis that the authors had conducted and reported in detail elsewhere earlier (39). The meta-analysis indicated that CBT might be effective for stopping BZD anxiolytics, both in the short term (≤3 months) and long term (12 months) (39). Furthermore, references regarding the lifestyle changes that individuals with anxiety disorders can implement in daily life as self-management were also searched.
The committee members created a DA prototype according to the quality criteria of the IPDAS (33), citing the results of our literature review described above (39). DAs are basically of two types: one DA is for preparation for discussion with healthcare providers (designed to be used by patients at home) and the other DA is for conversation between patients and health care professionals to share decisions during clinical consultations (designed to encourage patients to be actively involved in conversations) (40). Our DA included both of those functions: preparation aid before consultation and conversation aid during consultation. For the preparation aid, the DA prototype provided queries to be selected by putting a check mark (worksheet for value clarification) and a box for any additional comments to be completed at home, which would be shared and discussed with their doctors during consultation. DAs should be understood by people who are unfamiliar with medical knowledge and therefore should be developed using eighth-grade level language (41). Considering this, the committee attempted to use simpler expressions. Moreover, in accordance with previously published evidence-based DAs, we described the outcome probabilities using pictograms, which showed how many people out of 100 would experience an event so that it could be easily understood by people with any literacy level (42).
We conducted acceptability testing of the DA prototype by surveying stakeholders. We adopted a mixed-methods survey.
Following a validated acceptability scoring measurement that assess the comprehensiveness of the DA in terms of its length, amount of information, balance of provided information, and ability to target decisions (43). This is the common DA development process that ensures the quality of the final version of the DA in accordance with stakeholder evaluation.
We recruited patients from the psychiatric outpatient departments of our university hospitals. Outpatients were approached if they fulfilled the following conditions: (i) aged ≥20 years, (ii) using BZD anxiolytics for at least 3 months, and (iii) showing improvements in their symptoms and health condition due to treatment with BZD anxiolytics. Furthermore, health care providers who regularly provided consultation to patients with anxiety disorders from the same department as those used by the outpatients were recruited. Approximately 20 individuals from each group were included in this study. The sample size was determined following the methods used in previous studies on DA development and acceptability testing (29, 30). Both the individuals with anxiety disorder and healthcare professionals were asked to read the DA prototype and participate in the survey. Finally, we modified and improved the DA prototype to create a final version using the results of acceptability testing.
Our DA prototype was a 32-page A5 booklet, which contained a description of the target people, instruction on how to use this tool, and an explanation of anxiety disorders. The prototype next provided the options of continuing (option 1) or tapering BZD anxiolytics (option 2), the advantages and disadvantages of each option, and a worksheet for value clarification. The booklet further prepared a box for those with anxiety disorders to put down any queries or comments to their clinicians, which could be asked in the next consultation on whether to continue or taper BZD anxiolytics. Additionally, for the tapering current anxiolytics option, the DA prototype showed additional options for gradually tapering BZD anxiolytics without CBT (option 1′) or with CBT (option 2′). For each option, the DA prototype recommended gradual tapering which involved reducing the dose by ≤25% over 4–8 weeks to prevent rebound anxiety, based on the current guidelines for BZD (15). Next, our DA described the advantages and disadvantages of these two options, along with a worksheet of value clarification for each option. The outcomes of each option were cited according to the outcomes of the meta-analysis that the authors had previously conducted, which found that gradual tapering with CBT was more effective than gradual tapering without CBT for success of stopping BZD anxiolytics both in the short-term (≤3 months) and long-term (12 months) (39). We described this evidence in the DA prototype using pictorial diagrams consisting of 100 faces, in which the number of colored faces meant the proportion of individuals who were predicted to experience the outcomes (Figure 2). Moreover, the DA prototype had a box for additional comments or queries to their clinicians, which could be asked in the next consultation on whether to taper BZD anxiolytics with CBT or without CBT. Supplementary material S1 showed the detailed information of the DA prototype.
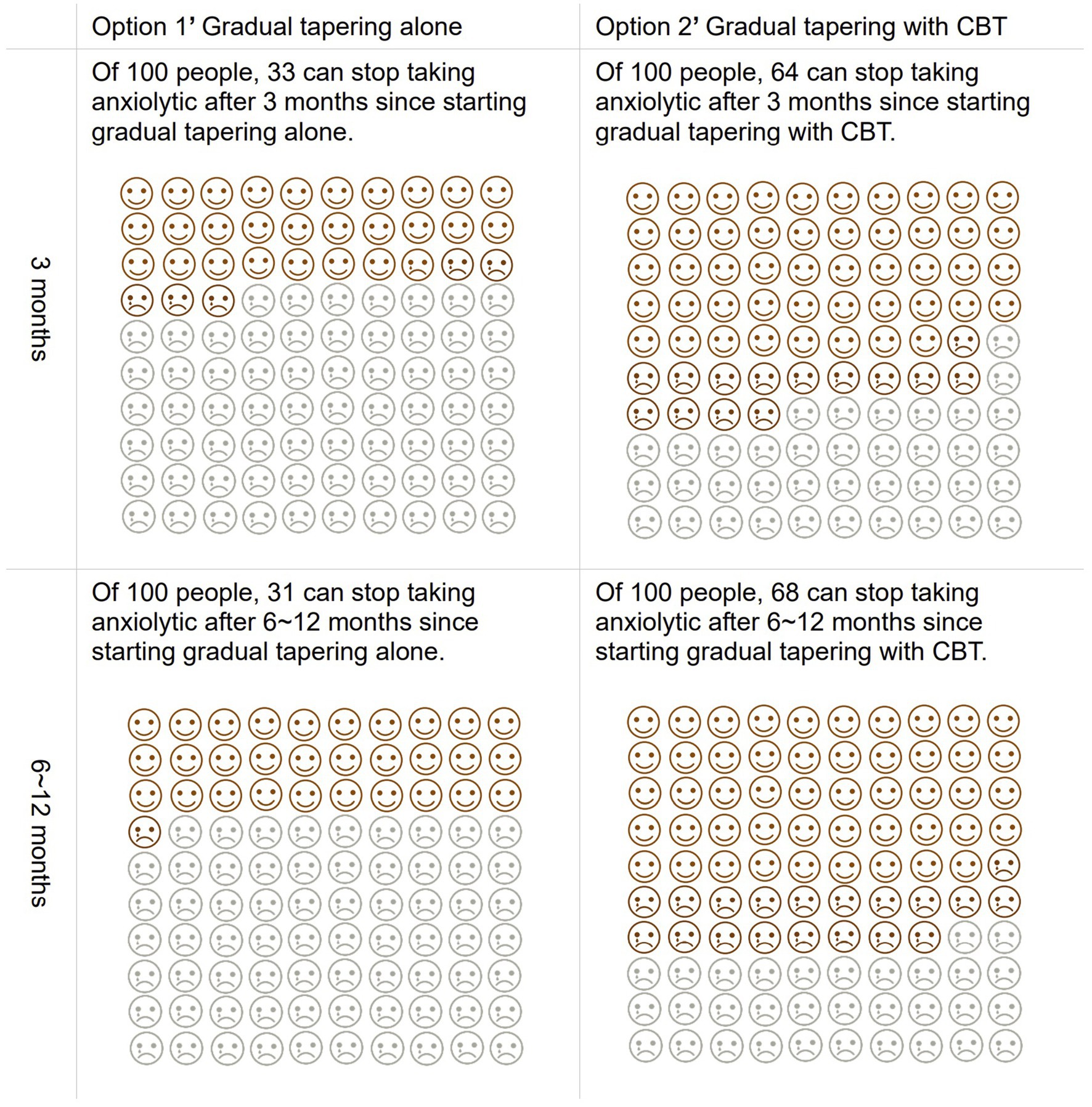
Figure 2. Pictorial diagrams as predicted consequences of tapering anxiolytics with and without CBT described in the DA.
Twenty-one patients with anxiety disorders, such as general anxiety disorder (GAD) with sleep disorder (n = 6), GAD (n = 2), panic disorder (PD) (n = 2), PD with sleep disorder (n = 1), depression with GAD and sleep disorder (n = 1), depression with PD and sleep disorder (n = 1), PD with social anxiety disorder and sleep disorder (n = 1), and unknown (n = 7) participated in the DA acceptability testing. Ten patients (48%) were taking antidepressants as well as benzodiazepine anxiolytics, 4 (19%) were not, and 7 (33%) were unknown. Ten patients were taking hypnotics besides benzodiazepine anxiolytics, 4 (19%) were not taking them, and 7 (33%) were unknown whether to take them. Among the 21 patients, 14 (67%) have no CBT experience, while 7 (33%) were unknown. The mean age of the participants was 48.0 (±9.2) years, among which 14 (67%) were women, 5 (24%) were men, and 2 (10%) were unknown. Nine participants (43%) had a high school degree or lower level of education, 4 (19%) had vocational college level education, and 8 (38%) were university graduates.
Table 1 shows that the results of the patients’ feedback. The length of explanation or instruction was reported to be “just right” in 18 of 21 participants (86%). The amount of provided information was judged as “just right” in 17 of 21 participants (81%). The presentation of both options was rated as not biased but well balanced in 20 of 21 participants (95%). The DA was considered to be useful for decision-making about whether to taper anxiolytic drug or not in 17 of 20 patients (85%). A total of 14 of 20 patients (70%) thought that they could foresee their chance of successful stopping of current anxiolytics using the DA. Finally, 17 of 19 participants (89%) reported that the DA enabled easy decision making, while 18 of 21 participants (86%) thought that the DA had enough information to support to decide whether to continue or taper anxiolytics.
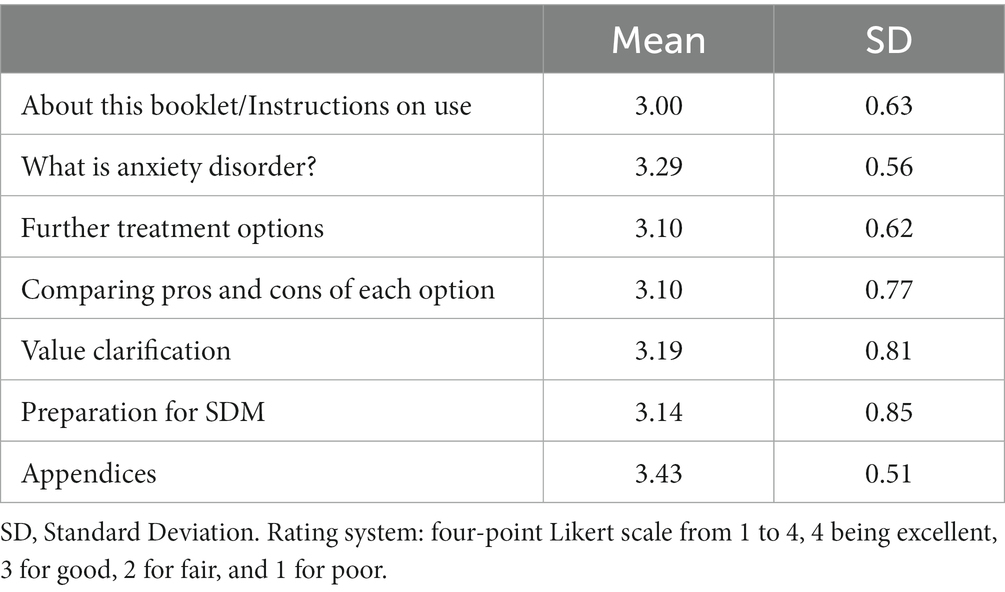
Table 1. Patient assessment on the way information is presented in each section of the prototype (n = 21).
In the comments from the participants, overall positive feedback on the DA prototype were observed. Some quotations are shown below.
“I thought it was a good way to discuss and decide together.” (Patient 8).
“This is a good opportunity to fully think about anxiety disorder and my current treatment.” (Patient 10).
“I liked that it was explained in a way that made it easy for my family members who do not have a good knowledge about anxiety disorder to be able to read and understand it.” (Patient 11).
“This is good because I had felt that my doctors had not given me much detailed information about my treatment so far.” (Patient 17).
“I could understand my current condition. This booklet gave me an indication of what stage of treatment I was at.” (Patient 19).
“I thought it was good to be able to organize my thoughts and concerns in advance for the consultation.” (Patient 20).
Furthermore, suggestions were provided to include additional explanations of some terms.
Ten clinicians participated in the DA acceptability testing. The mean age of the clinicians was 37.3 (±10.1) years, and they included 2 (20%) women and 8 (80%) men.
The overall reaction of the DA prototype was preferable (Table 2). The comments from the clinicians contained several positive aspects of the DA prototype, including the concept of shared decision-making, visualization and friendly illustration, simple wording, and presentation of not biased either option.
The examples of comments from clinicians are provided below.
“I found the explanations with illustrations on how to taper off medication easy to understand.” (Clinician 1).
“I wanted to use it immediately in my clinic.” (Clinician 4).
“I did not know that I could make use of this kind of booklet before, so it’s a novelty.” (Clinician 5).
“It is nice that patients can gain basic knowledge about anxiety disorders and its treatment, which would help them to develop their own preferences and take the initiative in discontinuation decision-making.” (Clinician 6).
“I like that it describes alternative methods, such as breathing and relaxation techniques, along with medicines.” (Clinician 9).
“A detailed explanation of how this is used would be helpful.” (Clinician 10).
The committee assembled and shared the results of the stakeholder’s acceptability test described above. We fully discussed and deliberated the results to utilize them to modify the DA prototype.
Our final DA was developed (Supplementary material S2) to ensure a high-quality decision support tool (Table 3). The final DA fulfilled all the IPDAS qualifying criteria (six of six), which were required for consideration as a DA (35), as well as all the IPDAS certification criteria (six of six), which judged the DA to contain a low risk of harmful bias (35). Moreover, the DA covered most IPDAS quality criteria (19 of 23), which added strength to the DA but whose lack did not mean a high risk of harmful bias (35). The status of the IPDAS criteria fulfilled by the final DA was considered higher than other Ottawa DAs that target other healthcare treatments or health screenings (44).
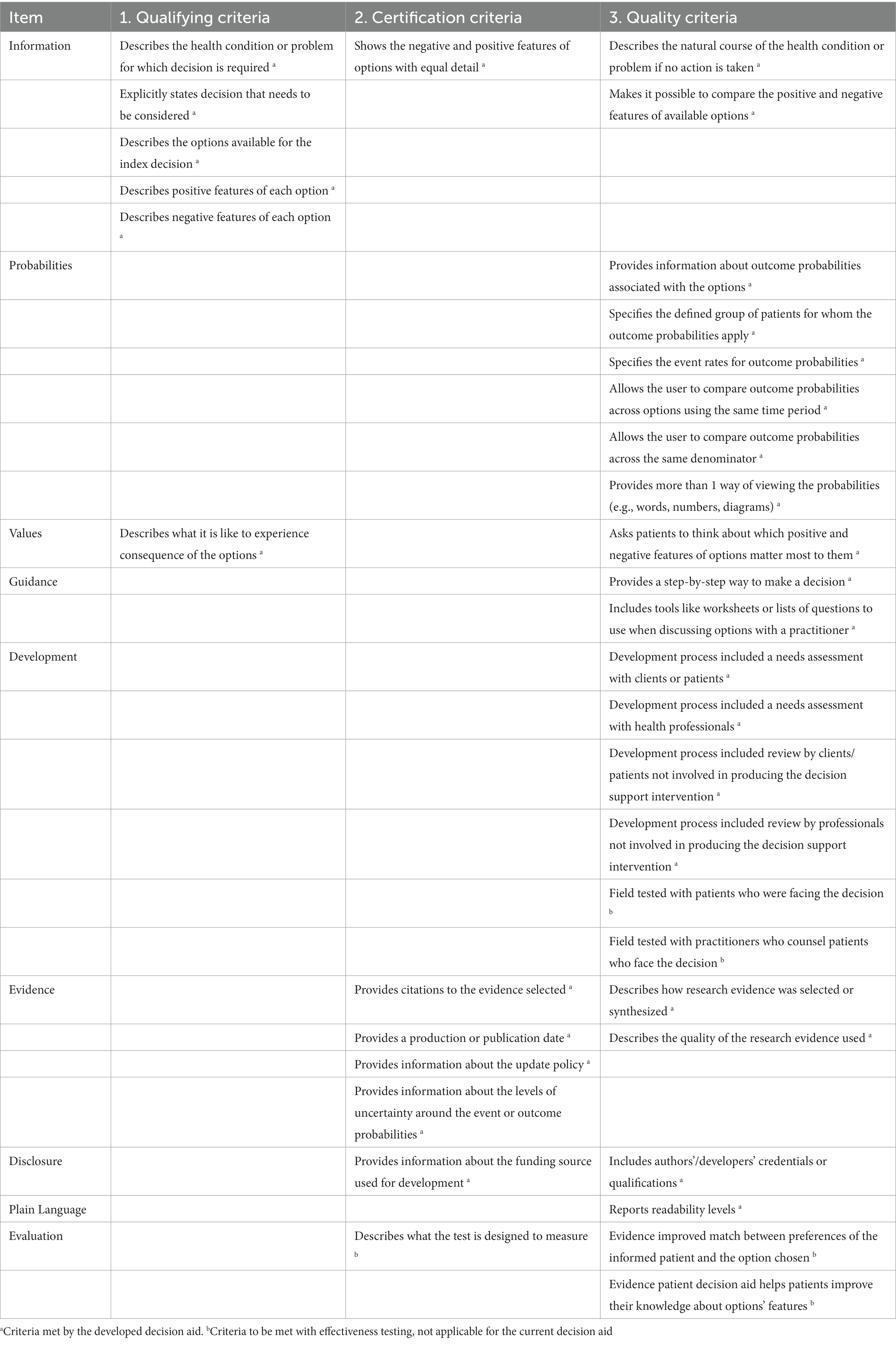
Table 3. International patient decision aid standards criteria met by current decision aid (30).
Additionally, the healthcare professionals who will be utilizing this DA will be required to be familiar with this tool. Therefore, the committee also created a DA manual for healthcare professionals that presented a detailed explanation of how to use the DA during decision-making in the clinical setting (Supplementary material S3).
This is the first study to develop and assess the acceptability of a Japanese/English version of the DA for individuals with anxiety disorders for considering whether to continue BZD anxiolytics and whether CBT for anxiety should be added, if BZD is being discontinued.
The acceptability testing results suggested that the DA was well acceptable and favored by both patients and clinicians. This indicates that the DA was confirmed by stakeholders who were expected to use our DA. The strong point of the DA is that the committee systematically developed this tool using evidence-based criteria, in which both patients and clinicians, who were not involved in the development process, confirmed the DA. This implies that DA can be used in clinical settings. Ramos-García et al. also reported that their Spanish DA for patients with generalized anxiety disorder was easy to use, virtually appealing, and accepted by patients and clinical experts (31). These studies supported the suitability of DAs for anxiety-related disorders. Given that most people are highly motivated in contributing to the decision-making about their own treatment (32), these novel DAs could address the needs of patients with anxiety disorders.
The discontinuation of BZD anxiolytics has several advantages and disadvantages. The advantages include avoidance of adverse events, such as falls, drowsiness, and cognitive decline, whereas the disadvantages include worsening of anxiety and possible withdrawal symptoms. Thus, even if the patients desire to discontinue their medication, they may face conflicts between the advantages and disadvantages. Our DA might possibly reduce this conflict, since this tool successfully provides the evidenced-based characteristics of each option and asks the patients to clarify their own preferences. Using our DA with healthcare providers might also help patients to deliberate on further treatment courses with less conflict.
Several studies have been conducted to develop and assess psychosocial interventions for dealing with the risks of BZD use thus far (23). Heather et al. (45) reported that individuals with insomnia who received a letter warning about the harms of long-term use of BZD hypnotics showed larger reductions in BZD consumption than those who did not receive such a letter (23, 43). Thus, the presentation of not only the advantages but also the disadvantages of anxiolytic use to patients might lead to successful medication reduction. Our DA included both advantages and disadvantages of anxiolytics in a well-balanced manner. Moreover, our DA succeeded in supplying daily activities and relaxation techniques to reduce anxiety, which individuals with anxiety disorder could adopt in their everyday lives. In these regards, our DA contributes to the current literature, which suggests useful psychosocial interventions focusing on the prevention of the adverse aspects of long-term anxiolytic use. Furthermore, the uniqueness of our DA is that we have created a framework that allows patients to discuss and decide their options together with their clinicians, rather than unilaterally providing them with related information.
This study has some limitations. First, although our DA fulfilled most IPDAS quality criteria (35), some items should be covered in the future to improve the quality. Those items include field-testing and providing evidence of the intervention. To address this issue, the steering committee plans to conduct beta field-testing during the decision-making process of whether to discontinue BZD anxiolytics in a clinical setting. Second, there may be differences in the level of acceptance and appreciation among the patients who were shown their diagnosed disorder through the DA. Therefore, we plan to examine the differences between the diagnoses in beta field-testing. Third, patients with anxiety disorders often take antidepressant and BZD including some participants in this study. Therefore, there may be differences in the difficulties of discontinuing BZD if an antidepressant was also taken. We then plan to examine the differences between those on antidepressants and those who were not on antidepressants, in the beta field test. Forth, CBT for anxiety disorders include different elements and unique skills are required for each anxiety disorder. Our DA provided only non-specific general information of CBT for anxiety disorders, which is a limitation of this study. Additionally, the intervention effects of this DA need to be verified in a clinical setting.
This study described the development process and acceptability of a DA for the tapering BZD anxiolytics for anxiety disorders. The developed DA was acceptable to all stakeholders. The results could help in the treatment decisions of both individuals with anxiety disorder and their clinicians who are deliberating on the discontinuation of anxiolytic therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Board of Kyorin University. The patients/participants provided their written informed consent to participate in this study.
YA: study design, drafting and revising the DA prototype, data analysis and interpretation, revising the DA, and drafting the manuscript. KI, MTak, and TO: study design, revising the DA prototype, data collection, data analysis and interpretation, revising the DA, drafting, and editing the manuscript. HY, TM, TK, and MTan: study design, revising the DA prototype, data interpretation, revising the DA, and editing the manuscript. YT and KM: study design, revising the DA prototype, data collection and interpretation, revising the DA, editing the manuscript, and funding acquisition. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
This study was supported by research grants from the Ministry of Health, Labor and Welfare of Japan (19GC1012 and 21GC1016).
The authors sincerely thank the patients for participating in this study.
YA received speaker’s honoraria from Sumitomo Pharma, Meiji Seika Pharma, Viatris Pharmaceuticals Japan. YT received a lecture sponsorship from Takeda Pharmaceutical, Sumitomo Pharma, Otsuka Pharmaceutical, Meiji Seika Pharma, Kyowa Pharmaceutical, Eisai, MSD, and Yoshitomi and re-search funding from Otsuka Pharmaceutical, Meiji Seika Pharma, MSD, and Eisai. KI has received personal fees/grant support from Eisai, Eli Lilly, Janssen, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Mochida, MSD, Novartis, Otsuka, Shionogi, Sumitomo Pharma, and Yoshitomiyakuhin in the last three years. HY received lecture fees from Takeda Pharmaceutical, Lundbeck Japan, Sumitomo Pharma, Otsuka Pharmaceutical, Meiji Seika Pharma, Janssen Pharma, Kyowa Pharmaceutical, Eisai, MSD, Yoshitomiyakuhin, Mochida Pharmaceutical and Viatris in the last three years. TM declares no interest of conflict. TK has received speaker’s honoraria from Takeda Pharmaceutical, Sumitomo Pharma, Viatris Pharmaceuticals Japan, MSD, Eisai, Ltd., and Yoshitomi Pharmaceutical. MTak has received speaker’s honoraria from Takeda Pharmaceutical, Otsuka Pharmaceutical, Daiichi Sankyo Company, Sumitomo Pharma, Meiji Seika Pharma, Viatris Pharmaceuticals Japan, MSD, Eisai, Ltd., and Yoshitomi Pharmaceutical, and research grants from Otsuka Pharmaceutical, Eisai, Shionogi and the Japanese Ministry of Health, Labour and Welfare (R3-21GC1016) outside the submitted work. MTan declares no interest of conflict. KM received speaker’s honoraria from EISAI Co., Ltd., Nobelpharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., MSD Inc. and research grants from Eisai Co., Ltd., Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., AMED (JP21dk0307103KM), the Japanese Ministry of Health, Labour and Welfare (19GC1012, 21GC0801). TO has received speaker’s honoraria from IQVIA, Takeda Pharmaceutical, Otsuka Pharmaceutical, Daiichi Sankyo Company, Sumitomo Pharma, Meiji Seika Pharma, Viatris Pharmaceuticals Japan, MSD, Eisai, Ltd., Kyowa Pharma, Lundbeck Japan, Lily, and Yoshitomi Pharmaceutical.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1083568/full#supplementary-material
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition: DSM-5. Arlington, VA, Washington, DC: American Psychiatric Association (2013) 189–195.
2. Pine, DS, Helfinstein, SM, Bar-Haim, Y, Nelson, E, and Fox, NA. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology. (2009) 34:213–28. doi: 10.1038/npp.2008.113
3. Olatunji, BO, Cisler, JM, and Tolin, DF. Quality of life in the anxiety disorders: a meta-analytic review. Clin Psychol Rev. (2007) 27:572–81. doi: 10.1016/j.cpr.2007.01.015
4. Sareen, J, Cox, BJ, Afifi, TO, Ron de, G, Asmundson, GJG, ten Have, M, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. (2005) 62:1249–57. doi: 10.1001/archpsyc.62.11.1249
5. Simon, G, Ormel, J, VonKorff, M, and Barlow, W. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. (1995) 152:352–7. doi: 10.1176/ajp.152.3.352
6. Rickels, K, Case, WG, Downing, RW, and Winokur, A. Long-term diazepam therapy and clinical outcome. JAMA. (1983) 250:767–71. doi: 10.1001/jama.1983.03340060045024
7. Picton, JD, Marino, AB, and Nealy, KL. Benzodiazepine use and cognitive decline in the elderly. Am J Health Syst Pharm. (2018) 75:e6–e12. doi: 10.2146/ajhp160381
8. Woolcott, JC, Richardson, KJ, Wiens, MO, Patel, B, Marin, J, Khan, KM, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. (2009) 169:1952–60. doi: 10.1001/archinternmed.2009.357
9. Xing, D, Ma, XL, Ma, JX, Wang, J, Yang, Y, and Chen, Y. Association between use of benzodiazepines and risk of fractures: a meta-analysis. Osteoporos Int. (2014) 25:105–20. doi: 10.1007/s00198-013-2446-y
10. Smink, BE, Egberts, AC, Lusthof, KJ, Uges, DR, and de Gier, JJ. The relationship between benzodiazepine use and traffic accidents: a systematic literature review. CNS Drugs. (2010) 24:639–53. doi: 10.2165/11533170-000000000-00000
11. Baldwin, DS, Anderson, IM, Nutt, DJ, Allgulander, C, Bandelow, B, den Boer, JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. (2014) 28:403–39. doi: 10.1177/0269881114525674
12. Bandelow, B, Sher, L, Bunevicius, R, Hollander, E, Kasper, S, Zohar, J, et al. WFSBP task force on mental disorders in primary care, WFSBP task force on anxiety disorders, OCD and PTSD guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. (2012) 16:77–84. doi: 10.3109/13651501.2012.667114
13. American Psychiatric Association. Practice guidelines for the treatment of patients with panic disorder (2009). Available at: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf (Accessed August 17, 2022).
14. Katzman, MA, Bleau, P, Blier, P, Chokka, P, Kjernisted, K, Van Ameringen, M, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. (2014) 14:S1. doi: 10.1186/1471-244X-14-S1-S1
15. Kumano, H, and Kuboki, T. Handbook of panic disorder: treatment guidelines and practice. Tokyo, Japan: Igakusyoin (2008) (in Japanese).
16. NICE. National Institute for health and care excellence. Generalised anxiety disorder and panic disorder in adults: Management (2011). Available at: https://www.nice.org.uk/guidance/cg113/resources/generalised-anxiety-disorder-and-panic-disorder-in-adults-management-pdf-35109387756997 (Accessed August 17, 2022).
17. Bandelow, B, Lichte, T, Rudolf, S, Wiltink, J, and Beutel, ME. The German guidelines for the treatment of anxiety disorders. Eur Arch Psychiatry Clin Neurosci. (2015) 265:363–73. doi: 10.1007/s00406-014-0563-z
18. Tanguay Bernard, MM, Luc, M, Carrier, JD, Fournier, L, Duhoux, A, Côté, E, et al. Patterns of benzodiazepines use in primary care adults with anxiety disorders. Heliyon. (2018) 4:e00688. doi: 10.1016/j.heliyon.2018.e00688
19. Vasile, RG, Bruce, SE, Goisman, RM, Pagano, M, and Keller, MB. Results of a naturalistic longitudinal study of benzodiazepine and SSRI use in the treatment of generalized anxiety disorder and social phobia. Depress Anxiety. (2005) 22:59–67. doi: 10.1002/da.20089
20. Hunot, V, Churchill, R, Silva de Lima, M, and Teixeira, V. Psychological therapies for generalised anxiety disorder. Cochrane Database Syst Rev. (2007) 1:CD001848. doi: 10.1002/14651858.CD001848.pub4
21. Hoffman, SG, and Smits, JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. (2008) 69:621–32. doi: 10.4088/JCP.v69n0415
22. Ninomiya, A, Sado, M, Park, S, Fujisawa, D, Kosugi, T, Nakagawa, A, et al. Effectiveness of mindfulness-based cognitive therapy in patients with anxiety disorders in secondary-care settings: a randomized controlled trial. Psychiatry Clin Neurosci. (2020) 74:132–9. doi: 10.1111/pcn.12960
23. Darker, CD, Sweeney, BP, Barry, JM, Farrell, MF, and Donnelly-Swift, E. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Sys Rev. (2015) 5:CD009652. doi: 10.1002/14651858.CD009652.pub2
24. Takaesu, Y, Utsumi, T, Okajima, I, Shimura, A, Kotorii, N, Kuriyama, K, et al. Psychosocial intervention for discontinuing benzodiazepine hypnotics in patients with chronic insomnia: a systematic review and meta-analysis. Sleep Med Rev. (2019) 48:101214. doi: 10.1016/j.smrv.2019.101214
25. Légaré, F, Adekpedjou, R, Stacey, D, Turcotte, S, Kryworuchko, J, Graham, ID, et al. Cochrane effective practice and organisation of care group interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. (2018) 2018:CD006732. doi: 10.1002/14651858.CD006732.pub4
26. Elwyn, G, Frosch, D, Thomson, R, Joseph-Williams, N, Lloyd, A, Kinnersley, P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. (2012) 27:1361–7. doi: 10.1007/s11606-012-2077-6
27. O'Connor, AM, Wennberg, JE, Legare, F, Llewellyn-Thomas, HA, Moulton, BW, Sepucha, KR, et al. Toward the 'tipping point': decision aids and informed patient choice. Health Aff (Millwood). (2007) 26:716–25. doi: 10.1377/hlthaff.26.3.716
28. Stacey, D, Légaré, F, Lewis, K, Barry, MJ, Bennett, CL, Eden, KB, et al. Cochrane consumers and communication group: decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2017) 2017:CD001431. doi: 10.1002/14651858.CD001431.pub5
29. Aoki, Y, Takaesu, Y, Baba, H, Iga, JI, Hori, H, Inoue, T, et al. Development and acceptability of a decision aid for major depressive disorder considering discontinuation of antidepressant treatment after remission. Neuropsychopharmacol Rep. (2022) 42:306–14. doi: 10.1002/npr2.12269
30. Aoki, Y, Takaesu, Y, Suzuki, M, Okajima, I, Takeshima, M, Shimura, A, et al. Development and acceptability of a decision aid for chronic insomnia considering discontinuation of benzodiazepine hypnotics. Neuropsychopharmacol Rep. (2022) 42:10–20. doi: 10.1002/npr2.12219
31. Ramos-García, V, Perestelo-Pérez, L, Rivero-Santana, A, Peñate-Castro, W, Duarte-Díaz, A, Álvarez-Pérez, Y, et al. Decision aids linked to the recommendations in clinical practice guidelines: results of the acceptability of a decision aid for patients with generalized anxiety disorder. BMC Med Inform Decis Mak. (2022) 22:171. doi: 10.1186/s12911-022-01899-2
32. Ramos-García, V, Rivero-Santana, A, Duarte-Díaz, A, Perestelo-Pérez, L, Peñate-Castro, W, Álvarez-Pérez, Y, et al. Shared decision-making and information needs among people with generalized anxiety disorder. Eur J Investig Health Psychol Educ. (2021) 11:423–35. doi: 10.3390/ejihpe11020031
33. Hoefel, L, O'Connor, AM, Lewis, KB, Boland, L, Sikora, L, Hu, J, et al. 20th anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Mak. (2020) 40:555–81. doi: 10.1177/0272989X20936209
34. Coulter, A, Stilwell, D, Kryworuchko, J, Mullen, PD, Ng, CJ, and van der Weijden, T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. (2013):13. doi: 10.1186/1472-6947-13-S2-S2
35. Joseph-Williams, N, Newcombe, R, Politi, M, Durand, MA, Sivell, S, Stacey, D, et al. Toward minimum standards for certifying patient decision aids: a modified delphi consensus process. Med Decis Mak. (2014) 34:699–710. doi: 10.1177/0272989X13501721
36. Aoki, Y. Shared decision making for adults with severe mental illness: a concept analysis. Jpn J Nurs Sci. (2020) 17:e12365. doi: 10.1111/jjns.12365
37. Aoki, Y, Takaesu, Y, Inoue, M, Furuno, T, Kobayashi, Y, Chiba, H, et al. Seven-day shared decision making for outpatients with first episode of mood disorders among university students: a randomized controlled trial. Psychiatry Res. (2019) 281:112531. doi: 10.1016/j.psychres.2019.112531
38. Aoki, Y, Tsuboi, T, Takaesu, Y, Watanabe, K, Nakayama, K, Kinoshita, Y, et al. Development and field testing of a decision aid to facilitate shared decision making for adults newly diagnosed with attention-deficit hyperactivity disorder. Health Expect. (2022) 25:366–73. doi: 10.1111/hex.13393
39. Takeshima, M, Otsubo, T, Funada, D, Murakami, M, Usami, T, Maeda, Y, et al. Does cognitive behavioral therapy for anxiety disorders assist the discontinuation of benzodiazepines among patients with anxiety disorders? A systematic review and meta-analysis. Psychiatry Clin Neurosci. (2021) 75:119–27. doi: 10.1111/pcn.13195
40. Lovell, K, Bee, P, Brooks, H, Cahoon, P, Callaghan, P, Carter, LA, et al. Embedding shared decision-making in the care of patients with severe and enduring mental health problems: the EQUIP pragmatic cluster randomised trial. PLoS One. (2018) 13:e0201533. doi: 10.1371/journal.pone.0201533
41. Oshima Lee, E, and Emanuel, EJ. Shared decision making to improve care and reduce costs. N Engl J Med. (2013) 368:6–8. doi: 10.1056/NEJMp1209500
42. Sletvold, H, Sagmo, LAB, and Torheim, EA. Impact of pictograms on medication adherence: a systematic literature review. Patient Educ Couns. (2020) 103:1095–103. doi: 10.1016/j.pec.2019.12.018
43. O’Conner, AM, and Cranney, A. User manual – acceptability. Ottawa: Ottawa Hospital Research Institute (1996). Available at: https://www.ohri.ca/decisionaid/ (Accessed August 17, 2022).
44. Ottawa Hospital Research Institute. Patient Decision Aids. Available at: https://decisionaid.ohri.ca/AZlist.html (Accessed August 17, 2022).
Keywords: anxiolytic, anxiety disorder, benzodiazepine, decision aid, shared decision making
Citation: Aoki Y, Takaesu Y, Inada K, Yamada H, Murao T, Kikuchi T, Takeshima M, Tani M, Mishima K and Otsubo T (2023) Development and acceptability of a decision aid for anxiety disorder considering discontinuation of benzodiazepine anxiolytic. Front. Psychiatry. 14:1083568. doi: 10.3389/fpsyt.2023.1083568
Received: 29 October 2022; Accepted: 24 April 2023;
Published: 12 May 2023.
Edited by:
Vitor Tardelli, University of Toronto, CanadaReviewed by:
Riccardo Maccioni, The Scripps Research Institute, United StatesCopyright © 2023 Aoki, Takaesu, Inada, Yamada, Murao, Kikuchi, Takeshima, Tani, Mishima and Otsubo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshikazu Takaesu, dGFrYWVzdXlAbWVkLnUtcnl1a3l1LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.