- 1Center of Evidence-Based Health Care, Medizinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
- 2Department of Psychiatry and Psychotherapy, Carl Gustav Carus University Hospital, Technische Universität Dresden, Dresden, Germany
- 3WIG2 Scientific Institute for Health Economics and Health System Research, Leipzig, Germany
Introduction: Flexible and integrated treatment options (FIT) have been established in German psychiatric hospitals to enhance continuous and patient-centered treatment for patients with mental disorders. We hypothesized that patients with experience in FIT treatment showed higher health-related quality of life (HRQoL) and comparable symptom severity compared with patients treated as usual (TAU). Further, we expected that some sub-dimensions of HRQoL determined HRQoL results clearer than others, while certain factors influenced HRQoL and symptom severity stronger in the FIT compared to the TAU group. In addition, we hypothesized that HRQoL is correlated with symptom severity.
Methods: We undertook a controlled, prospective, multicenter cohort study (PsychCare) conducted in 18 psychiatric hospitals in Germany, using the questionnaires Quality of Well Being Self-Administered (QWB-SA) (HRQoL) and Symptom-Checklist-K-9 (SCL-K-9) (symptom severity) at recruitment (measurement I) and 15 months later (measurement II). We assessed overall HRQoL (measured in health utility weights (HUW) and symptom severity score for patients from FIT and TAU treatment. We investigated the QWB-SA dimensions and separated the results by diagnosis. We used beta regressions to estimate the effect of multiple co-variates on both outcomes. To investigate the correlation between HRQoL and symptom severity, we used Pearson correlation.
Results: During measurement I, 1,150 patients were recruited; while 359 patients participated during measurement II. FIT patients reported higher HUWs at measurement I compared to TAU patients (0.530 vs. 0.481, p = 0.003) and comparable HUWs at measurement II (0.581 vs. 0.586, p = 0.584). Symptom severity was comparable between both groups (I: 21.4 vs. 21.1, p = 0.936; II: 18.8 vs. 19.8, p = 0.122). We found lowest HRQoL and highest symptom severity in participants with affective disorders. HRQoL increased and symptom severity decreased over time in both groups. The QWB-SA dimension acute and chronic symptoms was associated with highest detriments in HRQoL. We identified risk/protective factors that were associated with lower quality of life and higher symptom severity in both groups. We confirmed that HRQoL was negatively associated with symptom severity.
Discussion: Health-related quality of life (during hospital treatment) was higher among patients treated in FIT hospitals compared to patients in routine care, while symptom severity was comparable between both groups.
1. Introduction
Mental disorders are associated with decreased health-related quality of life (HRQoL) (1, 2). HRQoL is a comprehensive generic construct and covers many dimensions such as psychological status, functional abilities, subjective wellbeing, social interactions, role performance and physical health (3, 4). Generic instruments that measure HRQoL allow comparing the results across somatic and psychiatric diseases strengthening evidence-based decision-making in health policy and medical practice across diseases and disciplines. HRQoL is reduced among patients with an alcohol use disorder, schizophreniform disorders or affective disorders in comparison to the general population (2, 5–10). In addition, higher symptom severity has been found to be negatively related to HRQoL (11–13). Quality of life is an important concept in mental health care. There is an increasing focus on improving HRQoL, especially in patients with long term impairment and chronic illness (14).
1.1. Flexible and integrated treatment programs (FIT)
Mental health care services in German standard care are very heterogeneous as different cost units and service providers are involved based on different laws with a strong separation between outpatient and inpatient treatment and remuneration (15). The financial sector separation can lead to incentives for hospitals to maximize reimbursement, which then results in less than optional care for patients. The sectoral boundaries are particularly noticeable in the case of mental disorders (16). At the same time, patients with mental disorders in particular need continuous and patient-centered treatment. Therefore, innovative flexible and integrated treatment programs (FIT) for mental health care have been established and tested in several German psychiatric hospitals since 2013. Such FIT programs were established via specific contracts between psychiatric hospitals and health insurance providers. The remuneration of such contracts is based on Global Treatment Budgets (GBT). A GBT is a prospectively fixed budget covering a patient’s treatment in the psychiatric hospital independent from the type or setting of treatment {[inpatient, day care or outpatient as psychiatric outpatient department “Psychiatrische Institutsambulanz” (PIA)]} (17). FIT hospitals receive a fixed remuneration independent from the setting, duration or type of treatment, as long as the number of patients treated is in a specified range. Such remuneration enhances flexible and cross-sectoral care and shifts patients from inpatient to daycare or outpatient treatment. Common FIT concepts are, e.g., case managers, crisis resolution teams, assertive community treatment or treatment groups based on diagnoses not on setting. Patients who are not treated in FIT hospitals receive routine care, i.e., treatment as usual (TAU), with remuneration based on the costs and treatment per setting. As at 2021, 22 of such FIT hospitals have been introduced at German psychiatric hospitals. Previous research has shown that the introduction of FIT programs led to a reduced number of inpatients days and a shift to either increased day care and/or outpatient treatment in the hospital (18–20). However, the influence of such FIT programs on HRQoL is still unclear.
1.2. Hypotheses
We hypothesized that patients with experience in FIT treatment showed higher HRQoL and comparable symptom severity compared with patients treated as usual. Further, we expected that some sub-dimensions of HRQoL determined HRQoL results clearer than others, while certain factors influenced HRQoL and symptom severity stronger in the FIT compared to the TAU group. In addition, we hypothesized that HRQoL is correlated with symptom severity. HRQoL was in the focus of this manuscript, while symptom severity was mainly used to describe the study population and the interrelationship with HRQoL.
2. Materials and methods
2.1. Study design
We present results from the PsychCare study, which is a controlled, prospective, multicenter cohort study conducted in 18 psychiatric hospitals in Germany. For more details on the study design, we published the study protocol elsewhere (21). For the identification of participating FIT hospitals, we ranked all FIT hospitals as of 2017 in a randomized manner stratified by the year of FIT onset (before vs. after 2015). Based on this ranked list, we consecutively asked hospitals for their participation in our study. For each participating FIT hospital, we identified structurally comparable psychiatric hospitals for the TAU group (treatment as usual, more details on hospital selection below in “routine care”) and consecutively asked for their participation in our study.
Participating hospitals recruited all patients who fulfilled all inclusion and none of the exclusion criteria (see below in “inclusion and exclusion criteria”) from February 2018 until September 2019 (measurement I). Consequently, we allocated the participants in either FIT or TAU group on the hospital level. Therefore, if a patient was treated in a FIT or TAU hospital within the recruitment period, the patient was asked to participate in our study and remained in this group (FIT vs. TAU), independent from the length of treatment within the group. We collected data on the same outcomes, including HRQoL and symptom severity, at two measurement time points. Measurement I took place in participating hospitals at recruitment and measurement II 15 months later through written contact (with patients at home). We included all patients from measurement I, independent from whether they participated in the measurement II, and analyzed both time points separately. Our a priori sample size calculation for the QWB-SA (primary outcome) estimated 153 participants for each treatment and diagnosis group (estimated effect size: 0.33, difference: 0.05, SD: 0.16; estimated lost-to-follow-up: 25%; α 5%; power: 80%).
2.2. Inclusion and exclusion criteria
We included patients if they,
– had received in- or outpatient treatment in one of the participating hospitals during recruitment phase,
– were at least 18 years of age,
– had any of the following clinical diagnoses according to the International Classification of Disease, 10th version (ICD-10) (22): mental and behavioral disorders due to use of alcohol (F10, abbreviated to “alcohol use disorders”), schizophrenia, schizotypal disorder, delusional disorders or brief psychotic disorders (F20-23, abbreviated to “schizophreniform disorders”), or affective disorders (F30-39),
– showed sufficient command of German language to take part in the study and,
– provided informed consent.
We excluded patients from the study if they,
– had severe organic brain dysfunction including impairment of cognitive function,
– had severe intellectual disabilities or,
– showed acute suicidality.
The entire PsychCare study population also included younger patients (6–17 years) and other diagnoses (ICD-10: F50, F90-98). However, due to the low number of recruited patients with younger age (n = 58) and other diagnoses (n = 21), we excluded them from the following analyses.
2.3. Routine care
We defined eligible hospitals for the TAU group a priori by selecting those being in the same region, having a psychiatric in- and outpatient unit and not having a FIT-like contract. We matched the structural comparability of the hospitals based on data from structured quality hospital reports and the German spatial sociodemographic and socioeconomic database INKAR (Indicators and Maps for Spatial and Urban Development) (23). We based hospital allocation on the number of cases per diagnosis with a weighting of 50%, structural features of hospitals (e.g., number of beds or number of personnel) with a weighting of 25%, and regional factors (e.g., unemployment rate or household income) with a weighting of 25%. For more details on the selection of control hospitals, see the methods description in Petzold et al. (24) and the PsychCare study protocol (21).
2.4. Outcome measures
We used the results from the German versions of the questionnaires Quality of Well Being Self-Administered (QWB-SA) (25) to examine HRQoL and the Symptom-Checklist-K-9 (SCL-K-9) (26) to examine symptom severity at measurement I and measurement II, and compared them for both groups at both time points.
2.4.1. HRQoL
Health-related quality of life is one of two primary outcome measures in the PsychCare study. We used the QWB-SA questionnaire to measure HRQoL as used in other studies (27, 28). The QWB-SA is a preference-based and self-administered instrument to describe HRQoL measured as health utility weights (HUW). The QWB-SA was selected for this study, as it is a short, generic and preference-based instrument to assess the HRQoL. It, therefore, does not overload the study participants together with the other instruments used and can measure the patients’ health utility for health economic evaluations examined in another part of the PsychCare study. The QWB-SA combines three scales of functioning with a measure of symptoms and problems to estimate a point-in-time expression of wellbeing that runs from 0 (equivalent to death) to 1 (equivalent to perfect health) (29). The instrument includes four dimensions. The first dimension describes acute and chronic symptoms (CPX dimension), i.e., the presence or absence of 19 chronic symptoms or problem, followed by 25 acute (or more transient) physical symptoms and 14 mental health symptoms and behaviors (29). The second dimension describes self-care aspects and a person’s mobility (MOB dimension). The third dimension assessed physical activity (PAC dimension). And the fourth dimension describes self-care aspects and usual activity (SAC dimension) including completion of role expectations (29). We calculated the HUW of the QWB-SA by subtracting the maximum weighted item of each dimension for each of the last 3 days from the perfect score 1.0. We then added the daily QWB scores and divided this sum by three to obtain the average self-administered QWB score, the HUW. More information on the scoring can be obtained from the Coding and Scoring Manual (30).
2.4.2. Symptom severity
In addition, we assessed symptom severity using the SCL-K-9 questionnaire. The SCL-K-9 is a short form of the SCL-90-R to define the subjective burden of psychological symptoms. The SCL-90-R was first described by Derogatis (31) and includes 90 items which define the global extent of psychological symptoms on self-rater basis. However, it has been found that 90 items might be too lengthy and time-consuming, especially for (severely) mentally ill patients (26, 32, 33). Therefore, we chose the SCL-K-9 instrument. The SCL-K-9 has been proven to reliably measure the global extent of symptom severity (34, 35). It describes the amount of psychological complaints as well as global distress (36). The SCL-K-9 uses nine items, one item from each of the nine scales of the SCL-90-R, which showed the greatest discriminant power to the average psychological distress level (GSI-90) in a representative German survey (33). The nine items are: uncontrollable emotional outbursts, finding it difficult to start something, feeling that you worry too much, emotional vulnerability, feeling observed or talked about, feeling uptight or agitated, feeling of heaviness in your arms and legs, feeling nervous when left to yourself, feelings of loneliness even in company. The instrument rates each item using a 5-point Likert scale from “not at all” to “very much.” We charged all answers with 1 to 5 each and summed them up to a SCL-K-9 sum score (theoretical range: 9–45). A high sum score indicates high and a low sum score low symptom severity. More information on the instrument and scoring can be obtained elsewhere (26, 37).
2.5. Co-variates
We used the following co-variates in our analyses:
• age (additionally grouped in to the following three categories: 18–39, 40–59, ≥60 years),
• sex (female, male),
• diagnosis at study entry,
• partnership status (married or co-habiting vs. not),
• accommodation (supported living vs. not),
• living situation (living alone vs. not),
• education (lower, intermediate or higher; definition see below),
• occupation (working, not working, incapacitated or unable to work; definition see below),
• time in treatment (at least 5 years of psychiatric treatment vs. less than 5 years) and,
• chronic disease (any chronic disease vs. no; definition see below).
All co-variates were reported at measurement I (recruitment).
We measured education according to the Comparative Analysis of Social Mobility in Industrial Nations (CASMIN) scale (38), translated and adapted to the German context (39). The CASMIN scale is a combination of the attained general education and vocational education based on the reached degree. We used the highest education and occupation reported by the study participants. We grouped the CASMIN into three categories in line with Leopold (40). The category “lower education” comprises study participants holding lower secondary degrees (9 years of schooling) with completed vocational qualification or less (CASMIN 1a–1c). “Intermediate education” ranges from intermediate secondary degrees (at least 10 years of schooling) to higher secondary degrees with vocational qualification (CASMIN 2a–2cvoc). The category “higher education” includes individuals holding tertiary degrees (CASMIN 3a–3b). We omitted participants with missing data from this categorization and separately stated those who were in education (39).
We classified occupation into working (full-time, part-time, other employment), not working (unemployed, pension, housewife/househusband and undergoing training) and incapacitated or unable to work similar to Huber et al. (41). If the study participants indicated more than one mentioned category, we included them in the highest category according to the sequence of the previous sentence.
For the definition of chronic disease, we exclusively considered somatic chronic diseases to avoid correlation with the diagnoses under investigation. Chronic diseases were defined as hypertension (ICD-10: I10-I15), diabetes (E10-E14), heart disease (I05-I09, I30-I52), gastrointestinal diseases (K50, K51), migraine (G43, G44), cancer (C00-C97), thyroid disease (E00-E07), and musculoskeletal disorders (M00-M19, M79.0, M79.1, M80-M82) similar to Domenech et al. (12) and Huber et al. (41).
2.6. Analyses
We assessed overall HUW and symptom severity score at measurement I and measurement II for patients from FIT hospitals contrasting patients from TAU, and by diagnosis at admission. In addition, we visualized the results by diagnosis using box plots to present median, upper and lower quartiles as well as outliers.
The time span between the date when measurement I questionnaire was completed and the date when measurement II questionnaire was completed, varied between study participants (400–825 days, mean = 497 days). If participants completed the measurement II questionnaire later than 850 days after measurement I, we did not consider this questionnaire at all (n = 1). We assessed each time point separately, in contrast to a longitudinal design, as measurement I was conducted already under interventional circumstances, i.e., FIT implementation for at least 2 years at measurement I. Therefore, we expected intervention effects already at measurement I. Results of the follow-up examination are additionally important as the treatment circumstances and disease severity differed between measurement I and measurement II. Recruitment at measurement I took place at either inpatient, day care or outpatient hospital treatment, while measurement II was independent from treatment in a hospital setting.
In order to assess which aspects were associated with the highest detriments in HRQoL and whether there are differences between the included diagnostic groups, we further investigated the role of the four dimensions of the QWB-SA questionnaire and separated the results by diagnosis. We used beta regressions (42) to estimate the effect of multiple co-variates on HUW, as the distribution of the HUW fitted well with the beta distribution. Beta regression has been applied in several studies on HRQoL (43–47). As covariates were present, we used the alternative parameterization with location parameter and scale parameter (47). The mean HUW is represented by μ (alternative parameterization). We did not adjust our regression analysis to the variable “setting” (inpatient, daycare and outpatient) as this variable is only valid during measurement I. Moreover, this variable is a self-explanatory feature (model effect) and, therefore, could distort the regression results due to its correlation with the group (FIT vs. TAU) variable. Therefore, we added a sub-analysis by setting for HUW at measurement I independent from the group (see Supplementary material). Further, as the number of patients, who did not participate in measurement II, was quite high, we additionally reported the HUWs of those who participated in both measurements as part of the sensitivity analyses (see Supplementary material). As the distribution of the symptom severity score fitted well with the normal distribution, we used linear regression to estimate the effect of multiple co-variates on symptom severity. We conducted each regression by each diagnosis group separately and adjusted by each co-variate (see Table 1), excluding diagnosis and setting. To investigate the correlation between HRQoL and symptom severity we used the Pearson correlation coefficient. We applied 5% as the level of significance and conducted all statistical analyses using the statistical software R V.4.0.3 (48).
3. Results
3.1. Characteristics of study participants
Of the 1,150 (FIT: 595; TAU: 555) patients who participated in the PsychCare study during measurement I, 1,084 (FIT: 568; TAU: 516) patients completed the QWB-SA and 1,099 (FIT: 576; TAU: 523) patients the SCL-K-9. Of the 359 (FIT: 217; TAU: 142) patients who participated during measurement II, 339 (FIT: 205; TAU: 134) patients completed the QWB-SA and 348 (FIT: 209; TAU: 139) patients the SCL-K-9. Only 36.5% (FIT) and 25.6% (TAU) of those participating in measurement I also took part in measurement II.
Participants, who completed measurement I, had a mean age of 45.7 (FIT) and 47.5 (TAU) years (Table 1). More than half had an affective disorder diagnosis (FIT: 58.1%; TAU: 59.0%) and had been ill for more than 5 years (FIT: 65.2%; TAU: 64.7%) (Table 1). Participants, who completed measurement I and II, had a mean age of 47.3 (FIT) and 47.6 (TAU), were predominantly diagnosed with affective disorders (FIT: 58.5%; TAU: 64.8%) and had mainly been treated more than 5 years (FIT: 67.7%; TAU: 73.9%).
In sum, the FIT and TAU populations are comparable for further analyses.
3.2. HRQoL and symptom severity
The HUW during measurement I was higher among patients experiencing FIT treatment compared to TAU (0.530 vs. 0.481) (Table 2). This difference was statistically significant (see Table 3, group). A higher HUW among participants from FIT-hospitals could also be found in all diagnosis groups (Figure 1). Participants with F10 had the lowest HUW compared with the other diagnostic groups. During measurement II, however, participants with alcohol use disorders or schizophreniform disorders showed even lower HUW compared to participants from the TAU group. These differences were, however, not statistically significant (Table 3, see variable “group”). In addition, HUW at measurement I were lowest among patients recruited in an inpatient setting, followed by day care and highest for those recruited in an outpatient setting (see Supplementary Table A). Our sensitivity analysis revealed that among those who participated in both measurements, HUW was slightly higher during measurement I in both groups (Supplementary Table B) compared to all who participated in measurement I (Table 2). However, the HUW was again significantly higher in the FIT compared to the TAU group (Supplementary Table B).
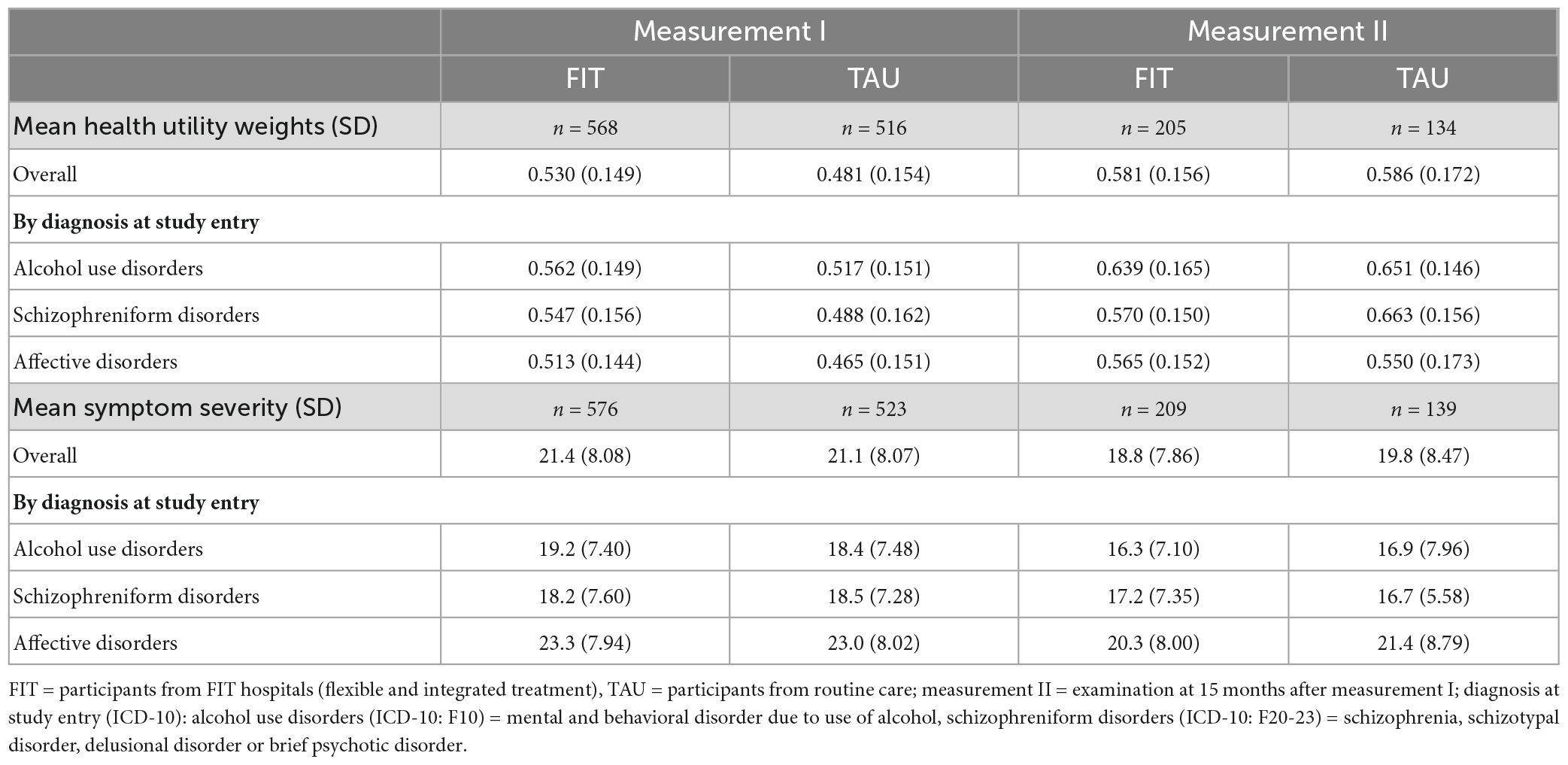
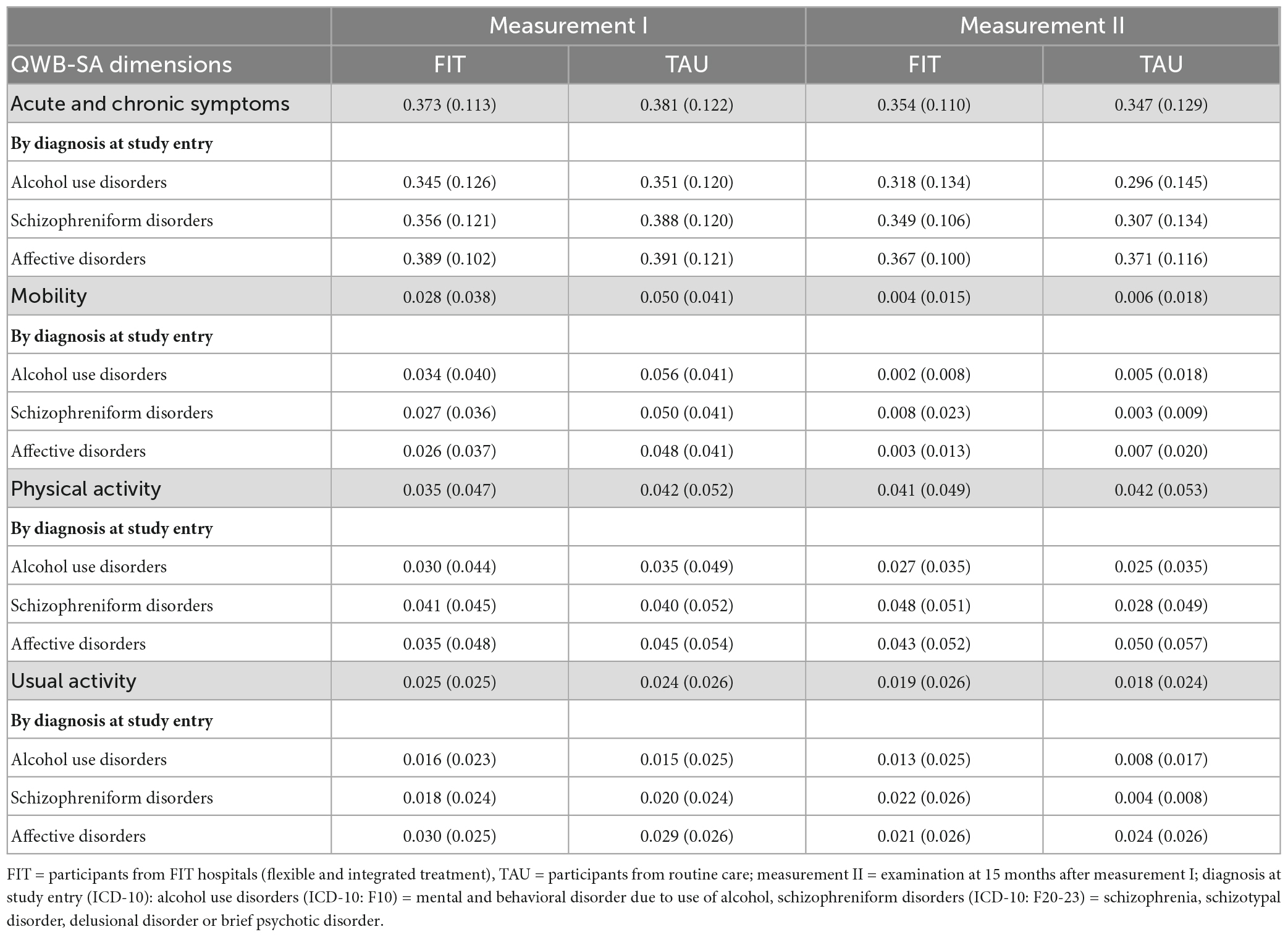
Table 2. Health utility weights and symptom severity scores at measurement I and measurement II, by diagnosis at study entry.
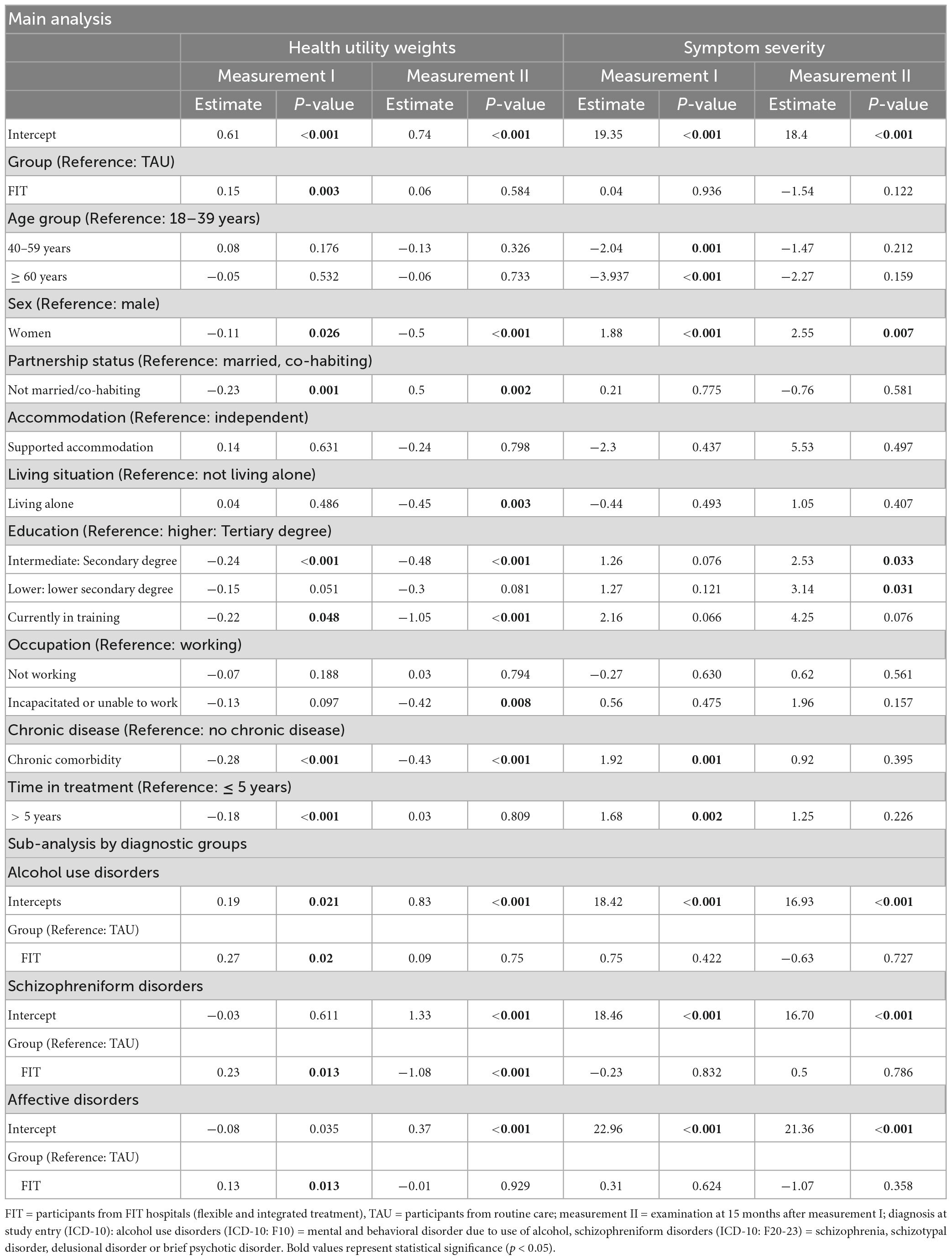
Table 3. Regression analyses, health utility weights and symptom severity, at measurement I and measurement II, by diagnosis at study entry.
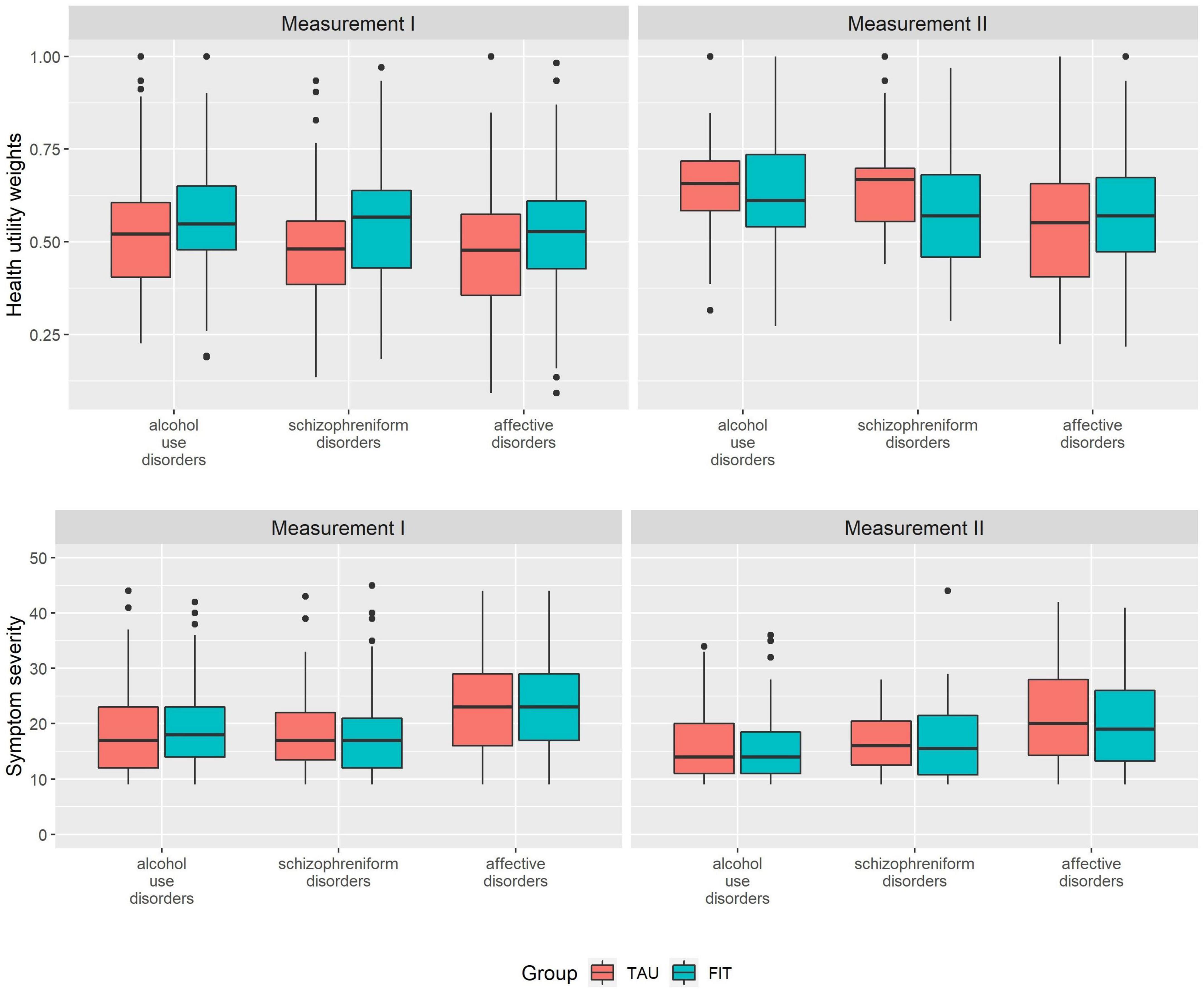
Figure 1. Health utility weights and symptom severity scores at measurement I and measurement II, by diagnosis at study entry. FIT = participants from FIT hospitals (flexible and integrated treatment), TAU = participants from routine care; measurement II = examination at 15 months after measurement I; diagnosis at study entry (ICD-10): Alcohol use disorders (ICD-10: F10) = mental and behavioral disorder due to use of alcohol, schizophreniform disorders (ICD-10: F20-23) = schizophrenia, schizotypal disorder, delusional disorder or brief psychotic disorder.
Symptom severity was comparable between FIT and TAU, both at measurement I and measurement II, and highest among participants with affective disorders (Figure 1 and Table 2). Our sensitivity analysis showed that among those who participated in both measurements, symptom severity was slightly lower among FIT (seen in all diagnostic groups) and higher among TAU patients during measurement I (triggered by participants with affective disorder) (Supplementary Table B) compared to all who participated in measurement I (Table 2). The differences between the FIT and TAU group were again not significant (Supplementary Table B).
3.3. HRQoL by dimensions
The dimension associated with highest detriments was acute and chronic symptoms, independent from the diagnosis at study entry (Table 4). The dimensions mobility, physical activity and usual activity were associated with comparable detriments during measurement I; while during measurement II physical activity detriments were strongest among the three dimensions. During measurement I, all impairments in the dimensions acute and chronic symptoms and mobility among patients treated in FIT hospitals were smaller compared to patients in the TAU group.
Among acute and chronic symptoms, participants with affective disorders showed the highest detriments in both groups and at both time points, followed by patients with schizophreniform disorders and alcohol use disorders. Among the dimension mobility, we observed highest detriments among participants with alcohol use disorders during measurement II, while the impairments between the other two diagnoses during measurement I and among all diagnoses during measurement II were comparable. The absolute detriments during measurement II in this dimension were very small. Among physical activity, the highest impairments among patients in the FIT-group were for patients with schizophreniform disorders, while highest detriments among patients in the TAU-group can be observed among patients with affective disorders. Among the dimension usual activity, highest detriments were among patients with affective disorders, with the exception of the FIT-group during measurement II.
3.4. Influencing factors on and correlation between HRQoL and symptom severity
Our regression analyses (Table 3) show that the HUW during measurement I was significantly higher among patients in FIT hospitals compared to patients treated in the TAU-group. The HUW difference during measurement II and the differences regarding symptom severity were not significant (for descriptive results see Table 2).
Higher age was associated with lower symptom severity during measurement I. Women reported lower HUW and higher symptom severity at both time points compared to men. Not being married or co-habited compared to being married or co-habited decrease HUW during measurement I and increased it during measurement II. Living under supported accommodation situations did not significantly influence HUW nor symptom severity in our study. Those participants who lived alone during measurement II reported lower HUW compared to those who did not live alone. Not having higher education status resulted in lower HUW at both time points, whereas these results were only statistically significant for intermediate education and being currently in training compared to higher education. The comparison of lower education with higher education was, however, not significant. Not having higher education resulted in higher symptom severity compared to higher education during measurement II (currently in training was not significant). Being incapacitated or unable to work was an indicator for lower HUW during measurement II compared to those participants who were working. Having any chronic comorbidity decreased HUW at both time points and increased symptom severity during measurement I. Patients who were under psychiatric treatment for more than 5 years showed lower HUW and higher symptom severity during measurement I.
For each of the three diagnoses, HUW during measurement I was significantly lower among the FIT-group compared to TAU, while during measurement II this was only the case for patients with schizophreniform disorders. We could observe no significant difference between FIT and TAU regarding symptom severity considering each diagnosis group.
3.5. Correlation between HRQoL and symptom severity
Health utility weights und symptom severity were negatively correlated at measurement I and measurement II (Table 5), indicating a higher symptom load was associated with greater detriments in HRQoL. This effect was strong both at measurement I and measurement II according to Cohen’s statistic (49).
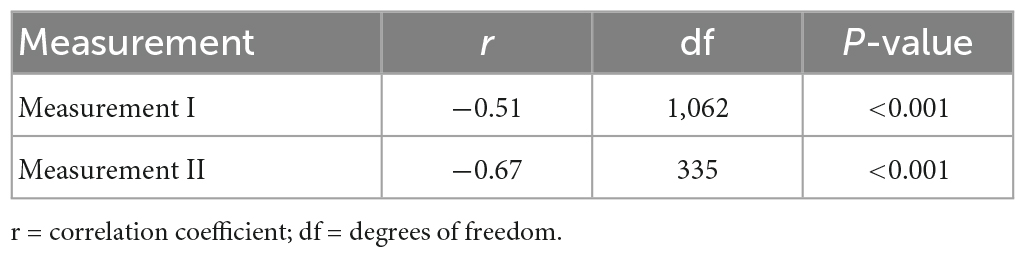
Table 5. Correlation between health utility weights and symptom severity, at measurement I and measurement II.
4. Discussion
4.1. Health related quality of life in general
The range of self-reported HUWs on measurement I and II suggests that the study participants have only about half the value for “self-perceived perfect health.” These values were consistent with and rather in the lower range of values among persons with psychiatric disorders as described in the literature (50–53). We expected that our study population would show slightly lower HRQoL compared to the general population with mental disorders as we recruited patients during treatment in a hospital setting. Based on clinical assessments, Kaplan et al. postulated a 0.03 change in score as a minimum clinically important difference (MCID), which was met here with a difference of 0.049 (54, 55).
We found the lowest HRQoL in participants with affective disorders followed by those with schizophreniform disorders and with alcohol use disorders, regardless of group membership. Other studies also reported diagnosis-specific differences of HRQoL (5, 56–60) and lowest HRQoL among patients with diagnoses in the area of affective disorders (5, 56, 57), whereas other studies observed more severe levels of disability among individuals with schizophrenia compared to those with bipolar affective disorders (59, 60). HRQoL increased over time in both groups. This is probably because recruitment was done during hospital stay or outpatient treatment at the hospital and, therefore, often during a rather acute phase of illness; while measurement II was 15 months after measurement I independent from the stage of illness. Consistent with this observation, symptom severity decreased over time. As hypothesized, HRQoL was negatively related to symptom severity as seen in other studies (11–13). Symptom severity was highest among participants with affective disorders compared to the other two diagnosis groups. As HRQoL and not symptom severity was in the focus of this manuscript, we mainly discuss the results of HRQoL.
4.2. Comparison of study population FIT and TAU
There were no significant differences between FIT and TAU in terms of sociodemographic and clinical characteristics such as age, gender, living conditions, diagnoses, and duration of illness. We only found two differences between the groups at measurement I. First, the proportion not working was slightly smaller among FIT patients, which could hint to a somewhat less severely ill FIT population. However, this was not supported by other sociodemographic factors. Second, fewer patients were recruited during inpatient and more during day care or outpatient treatment among FIT hospitals compared to the TAU group. This difference can be explained by the lower intensity of inpatient and higher intensity of day care and outpatient treatment in FIT hospitals, which can be considered as an interventional effect in itself, introduced by FIT programs, as seen in other studies (18, 19, 61–63). In addition, recruitment across all settings might be facilitated in FIT hospitals as settings are blurred in FIT hospitals due to re-structuring within FIT hospitals, while different staff is often responsible for different settings making recruitment across settings difficult in routine care.
4.3. Comparison of results between FIT and TAU
Even though patient characteristics and symptom severity were comparable between patients experiencing FIT treatment and TAU, HRQoL at measurement I was higher among FIT patients. We observed this difference in all diagnosis groups. Both observations were also valid considering only those patients that completed the questionnaire at both time points. Our data shows lowest HRQoL at measurement I during recruitment at inpatient stay followed by daycare treatment (see Supplementary material). As mentioned above, fewer patients were recruited in an inpatient setting in the FIT group compared to TAU, while the percentage recruited during day care or outpatient treatment was higher in the FIT group. Therefore, the difference in HUW at measurement I can also be associated with the different recruitment in the settings between FIT and TAU. On the other hand, the concept of inpatient, day care vs. outpatient is less structured in FIT hospitals and therefore a less clear classification in comparison to the TAU group.
One of the reasons why the differences in HRQoL between the groups decreased over time could be related to the COVID-19 pandemic. The majority of the measurement II was conducted in March 2020 or later, during the first and second waves of the corona pandemic in Germany (64). The associated restrictions and countermeasures affected all areas of health care. Of particular note, however, were the restrictions and closures in the area of day hospitals (65). Since FIT hospitals reduced the number of beds already before the corona pandemic and partly shifted to the day clinic area, which was structurally anchored, the closure of the day clinics probably had a greater impact on patient care in FIT hospitals than in TAU hospitals. On the other hand, whether FIT hospitals may have been able to act more flexibly in the COVID-19 pandemic because of their more flexible structuring is uncertain. Studies on the effects of the COVID-19 pandemic on the care in FIT vs. TAU hospitals are still pending. Further research is needed in this area.
Another reason might be a ceiling effect of HUW that might have been reached in both groups during measurement II and higher HRQoL cannot be expected in our study population.
4.4. Aspects influencing HRQoL
The dimension that was associated with highest detriments was acute and chronic symptoms, independent from the diagnosis at study entry. The other dimensions (mobility, physical activity and usual activity) only added lower detriments. We expected that the presence of the 19 chronic symptoms or problems (e.g., blindness and speech problems), together with the followed 25 acute (or more transient) physical symptoms (e.g., headache, coughing and pain) as well as the 14 mental health symptoms and behaviors (e.g., sadness, anxiety and irritation) would be associated with the highest detriments in HRQoL, especially in our study population (29). Symptoms are known to be correlated with HRQoL (66–68). In our study, symptom severity was also negatively correlated with HRQoL underlying the importance of this dimension in the QWB-SA instrument.
4.5. Influence of risk factors
In our study, higher age was associated with higher symptom severity during measurement I, while age did not significantly influence HRQoL. In line with our finding, other studies also reported a lack of or only a small association of age on HRQoL (5, 69–72). In contrast, in the German general population, HRQoL was reported to decrease with age (41). This age difference in the general population might diminish focusing on our selected diseases as the detriments of the mental disorders might overweight any age difference or make such differences smaller.
Women in our study consistently reported lower HRQoL and higher symptom severity compared to men. Some studies, however, reported a lack of or only a small association of sex on HRQoL (5, 69–72). Others support our findings that women report lower HRQoL compared to men (73–75), while others found higher HRQoL compared to women (76, 77). In the German general population, HRQoL was slightly lower in women compared to men (41). However, as the proportion of women was lower compared to men among alcohol use disorders (32.6% vs. 67.4%) and the proportion of mood affective disorders was higher (57.8% vs. 42.2%) in our study population, we conducted a sensitivity analysis including the diagnostic group in the regression analysis. The results showed that the lower HRQoL among women at measurement I was no longer significant (p = 0.311). All other variables remained in the same significance cluster (p ≤ 0.05 vs. p > 0.05), including sex at measurement II (data not shown).
Being not married or in co-habiting was associated with a decreased HRQoL during measurement I and with an increased HRQoL during measurement II in our study. An Ethiopian study revealed that being divorced was negatively associated with HRQoL among people with schizophrenia (78). However, due to cultural differences with the German population, these results are only comparable to a limited extent with our study population. Moreover, a study on severely mentally ill patients in Germany could not support those findings (5). One reason why being “not married” or in “co-habiting” was negatively associated during measurement I and positively during measurement II could be the possible different stages of the diseases at both time points. While measurement I was conducted during a clinical setting (inpatient, day care or outpatient) and therefore during a probably more acute phase of disease progression, measurement II was independent from acute treatment. However, further research is necessary in this area.
In our study, living alone was associated with lower HRQoL during measurement II, whereas living in supported accommodation did not reveal significant associations with HRQoL. Living alone and not being married or co-habiting are naturally highly correlated. Therefore, the findings during measurement II were congruent. However, the negative relationship during measurement I indicates that partnership status might add further explanation compared to considering living situation only. Another German study among patients with severely mentally ill patients found highest HRQoL among patients living in an assisted home (5). However, one reason for this difference could be that our study did not only include severely mentally ill patients. Less severely ill patients might be in lower need for supported living.
Lower education was associated with lower HRQoL in our study, which is in line with other studies (41, 79). Further, being incapacitated or unable to work was negatively associated with HRQoL during measurement II. This might reflect the relationship between the severity of the disease and occupational status. However, symptom severity was not associated with occupational status in our study. Other evidence also supports our finding that mentally ill persons with an occupation report better HRQoL (5, 80). Having one’s own income might be associated with financial security and autonomy, but also with having a social network (5, 80).
We found a negative association between HRQoL and chronic comorbidity, both at measurement I and measurement II. This result is supported by other research (41, 81, 82). Longer time in treatment was associated with lower HRQoL in our study during measurement I, whereas time in treatment was not associated with HRQoL during measurement II. In line with that finding, we found that longer time in treatment was also associated with higher symptom severity at measurement I.
4.6. Strengths and limitations
The PsychCare study is the first prospective, controlled, multi-perspective and multi-method evaluation study of FIT programs in Germany. It focusses on the perspectives of patients, which has not yet been considered in other studies evaluating FIT (83, 84). It thus adds important insight on the effects of such programs on patients. Another strength was the establishment of a control group, which was not used in other studies (85, 86). This study included patients from all settings in German psychiatric hospitals (inpatient, daycare and outpatient) and involved hospitals throughout Germany including hospitals with many years of FIT experience and those with less than 3 years of FIT experience by the time of patient recruitment onset.
Nonetheless, we also need to acknowledge some limitations. The COVID-19 pandemic, which led to significant inferences in mental health services, started during measurement II and probably distorted the results, as mentioned above. Further, some hospitals had difficulties to recruit study participants in all hospital settings. One reason for this was, as mentioned above, that the recruiting personnel was often only available for either inpatient, day care or outpatient treatment. This separation of the settings, especially in routine care, within German hospitals made it sometimes difficult to reach all patients, particularly those only experiencing outpatient treatment (15).
In addition, there were high rejection rates during the request for study participation among clinics in standard care (n = 14), which illustrates the difficulty to implement research in hospitals with TAU character (neither FIT nor university hospital). The general lack of personnel, especially in patient care, is a major limitation (87). FIT and university hospitals often have other personnel options (e.g., case managers) and structures (e.g., research coordination) supporting a successful recruitment. The number of patients included also fell short of expectations in some hospitals. Through harmonized training, follow-up training and close monitoring in the study, the targeted total number of cases at measurement I could, nevertheless, be achieved. In addition, the high loss-to-follow-up (63.5% and 74.4% vs. 25% estimated) is a limitation in this study. Furthermore, in various clinics, not all settings could be involved, particularly for some hospitals in the outpatient setting where recruitment was very low. This could influence the comparability between FIT and TAU. However, characteristics at measurement I and symptom severity hint to comparable groups in our analyses. Unfortunately, fewer patients than planned were reached during measurement II. One reason might be the severity of the illness at the time of study inclusion, which makes longitudinal research with (severely) mentally ill persons very difficult. Another reason might be that the recruitment process during measurement I was during hospital stay and measurement II was conducted via written questionnaires by the study centers. Patients might have higher motivation to participate being invited by the treating staff, which they know in person, instead of a non-related research team, which they have never seen.
Furthermore, measurement I was done in a clinical setting (inpatient, day care or outpatient), while measurement II was independent from the setting. We also did not control for phase of treatment (e.g., first treatment vs. longer duration of treatment). We expect the clinical pathways after study inclusion to be quite diverse. Due to the low number of patients participating in measurement II, outliers could distort our results.
In addition, we used self-reported data, which can lead to self-report bias, e.g., participants might feel obliges to provide socially desirable answers. However, on the one hand, this bias would occur in both groups (non-differential). On the other hand, we judge the outcomes HRQoL and symptom severity to be less prone to such a bias in contrast to, for example, satisfaction with care.
We purposely used a generic preference-based instrument to measure HRQoL. This allows us to compare the results across different diseases and use it for health-economic evaluations enriching evidence-based health policy decision-making. We are, however, aware that such generic instruments might not perfectly fit the targeted population. On the other hand, the QWB-SA has been found to be a valid instrument for our population under study (27, 53, 88). In addition, we could only include patients with sufficient command of the German language, as all study documents used were only in German. This might limit the generalizability of our study results to non-German speaking patients. However, we expect this limitation to be minor and present to the same extent in both FIT and TAU group.
The selection of potential co-variates were limited to the variables that we assessed in the context of the PsychCare study. Other factors such as self-esteem (5), self-stigma (89), self-efficacy (90), illness insight (91), or pharmaceutic side effects (91) etc., which are important factors influencing HRQoL could not be considered. Some severely ill patients could not be included; therefore, the HRQoL and symptom severity is likely to be underestimated. However, this was true for both the FIT and TAU group and should not infer with the group comparison.
5. Conclusion
In sum, HRQoL in our study was, at the time of recruitment during hospital treatment, higher among patients treated in FIT hospitals compared to patients in routine care, while symptom severity was comparable between both groups. HRQoL increased and symptom severity decreased from the time of recruitment to 15 months later. However, the difference between FIT and TAU observable during time of recruitment diminished 15 months later. Symptom severity remained comparable between both groups 15 months after recruitment. The dimension acute and chronic symptoms was associated with the highest detriments in HRQoL in both groups. We identified risk/protective factors associated with lower quality of life and higher symptom severity in both groups. We confirmed that HRQoL was negatively associated with symptom severity.
While HRQoL alone should not define the effect of an intervention, HRQoL is an important patient-centered outcome alongside with other outcomes, such as treatment satisfaction or recovery. They are necessary outcomes for a patient-centered evaluation of an intervention effect, such as the introduction of FIT with a GBT seen in Germany. The question asked in German politics is whether FIT hospitals provide better results to overcome the fragmented system compared to routine care. If they do so, ways to integrate aspects of FIT into routine care are politically discussed. Effects visible in other studies on FIT, e.g., the shift from inpatient to day care or outpatient treatment, needs to be strengthened by patient-centered outcomes, such as HRQoL. This is the first study providing evidence of FIT treatment on the patient-centered outcome HRQoL compared to routine care and shows evidence whether to integrate FIT into routine care.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB00001473 and IORG0001076) of the Medical Faculty of the Technische Universität Dresden and at each site where a separate approval was mandatory. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AN drafted the manuscript. AN and BS coordinated the study. FB analyzed the data and created the figure. AN, BS, RK, IW, JS, and AP contributed to the study design. AP and JS were principle investigators. AN, BS, RK, IW, JS, and FB contributed to the data interpretation. All authors read and commented on the manuscript and approved the final version of the manuscript.
Funding
This study was funded by the Innovation Fund of the Federal Joint Committee (G-BA) in Germany (grant reference no. 01VSF16053). The funder had no role in the design of the study and was not involved in its execution, data analysis, and dissemination of results.
Acknowledgments
We acknowledge the important contribution of the participating study hospitals and the study participants. We further acknowledge the contribution of the whole PsychCare team to the study design and implementation of the entire PsychCare study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1068087/full#supplementary-material
References
1. Mack S, Jacobi F, Beesdo-Baum K, Gerschler A, Strehle J, Hofler M, et al. Functional disability and quality of life decrements in mental disorders: results from the mental health module of the German health interview and examination survey for adults (DEGS1-MH). Eur Psychiatry. (2015) 30:793–800. doi: 10.1016/j.eurpsy.2015.06.003
2. Evans S, Banerjee S, Leese M, Huxley P. The impact of mental illness on quality of life: a comparison of severe mental illness, common mental disorder and healthy population samples. Qual Life Res. (2007) 16:17–29. doi: 10.1007/s11136-006-9002-6
3. Woo J, Jeon H, Noh E, Kim H, Lee S, Lee K, et al. Importance of remission and residual somatic symptoms in health-related quality of life among outpatients with major depressive disorder: a cross-sectional study. Health Qual Life Outcomes. (2014) 12:188. doi: 10.1186/s12955-014-0188-y
4. Cramer J, Rosenheck R, Xu W, Thomas J, Henderson W, Charney D. Quality of life in schizophrenia: a comparison of instruments. Department of veterans affairs cooperative study group on clozapine in refractory schizophrenia. Schizophr Bull. (2000) 26:659–66. doi: 10.1093/oxfordjournals.schbul.a033484
5. Berghofer A, Martin L, Hense S, Weinmann S, Roll S. Quality of life in patients with severe mental illness: a cross-sectional survey in an integrated outpatient health care model. Qual Life Res. (2020) 29:2073–87. doi: 10.1007/s11136-020-02470-0
6. IsHak W, Brown K, Aye S, Kahloon M, Mobaraki S, Hanna R. Health-related quality of life in bipolar disorder. Bipolar Disord. (2012) 14:6–18. doi: 10.1111/j.1399-5618.2011.00969.x
7. Pascual-Sanchez A, Jenaro C, Montes-Rodriguez J. Quality of life in euthymic bipolar patients: a systematic review and meta-analysis. J Affect Disord. (2019) 255:105–15. doi: 10.1016/j.jad.2019.05.032
8. Lahmek P, Berlin I, Michel L, Berghout C, Meunier N, Aubin H. Determinants of improvement in quality of life of alcohol-dependent patients during an inpatient withdrawal programme. Int J Med Sci. (2009) 6:160–7. doi: 10.7150/ijms.6.160
9. Morgan M, Landron F, Lehert P, New European Alcoholism Treatment Study Group. Improvement in quality of life after treatment for alcohol dependence with acamprosate and psychosocial support. Alcohol Clin Exp Res. (2004) 28:64–77. doi: 10.1097/01.ALC.0000108652.73143.4B
10. Dong M, Lu L, Zhang L, Zhang Y, Ng C, Ungvari G, et al. Quality of life in schizophrenia: a meta-analysis of comparative studies. Psychiatr Q. (2019) 90:519–32. doi: 10.1007/s11126-019-09633-4
11. Klompstra L, Ekdahl A, Krevers B, Milberg A, Eckerblad J. Factors related to health-related quality of life in older people with multimorbidity and high health care consumption over a two-year period. BMC Geriatr. (2019) 19:187. doi: 10.1186/s12877-019-1194-z
12. Domenech C, Pastore A, Altamura A, Bernasconi C, Corral R, Elkis H, et al. Correlation of health-related quality of life in clinically stable outpatients with schizophrenia. Neuropsychiatr Dis Treat. (2019) 15:3475–86. doi: 10.2147/NDT.S218578
13. Velure G, Muller B, Hauken M. Symptom burden, psychological distress, and health-related quality of life in cancer survivors with pelvic late radiation tissue injuries. Support Care Cancer. (2022) 30:2477–86. doi: 10.1007/s00520-021-06684-x
14. Chaudhury S, Rani Das P, Murthy P, Diwan C, Patil A, Jagtap B. Quality of life in psychiatric disorders. J Tre Bio Res. (2018) 1:2–4. doi: 10.15761/JTBR.1000103
15. Brieger P. [The German community mental health system-a review]. Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz. (2019) 62:121–7. doi: 10.1007/s00103-018-2861-5
16. Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen. Koordinierte Versorgung von Menschen mit psychischen Erkrankungen [Coordinated Care for People With Mental Illness]. Berlin: Medizinisch Wissenschaftlicher Verlagsgesellschaft (2018).
17. Konig H, Heinrich S, Heider D, Deister A, Zeichner D, Birker T, et al. [The regional psychiatry budget (RPB): a model for a new payment system of hospital based mental health care services]. Psychiatr Prax. (2010) 37:34–42. doi: 10.1055/s-0029-1223418
18. Neumann A, Baum F, Seifert M, Schoffer O, Kliemt R, March S, et al. [Reduction of days in inpatient care in psychiatric hospitals with flexible and integrated treatment for patient-centered care with a global budget – results with three-year follow-up from the evaluation study EVA64]. Psychiatr Prax. (2021) 48:127–34. doi: 10.1055/a-1274-3731
19. Baum F, Schoffer O, Neumann A, Seifert M, Kliemt R, March S, et al. Effectiveness of global treatment budgets for patients with mental disorders—claims data based meta-analysis of 13 controlled studies from Germany. Front Psychiatry. (2020) 11:131. doi: 10.3389/fpsyt.2020.00131
20. Baum F, Schmitt J, Seifert M, Kliemt R, Kubat D, March S, et al. Lengths of inpatient stay and sick leave of patients with mental diseases: disorder-specific effects of flexible and integrated treatment programs in Germany. Transl Psychiatry. (2022) 12:370. doi: 10.1038/s41398-022-02131-5
21. Soltmann B, Neumann A, March S, Weinhold I, Hackl D, Kliemt R, et al. Multiperspective and multimethod evaluation of flexible and integrative psychiatric care models in Germany: study protocol of a prospective, controlled multicenter observational study (Psychcare). Front Psychiatry. (2021) 12:659773. doi: 10.3389/fpsyt.2021.659773
22. WHO. Internationale Klassifikation Psychischer Störungen: ICD-10, Kapitel V (F): Diagnostische Kriterien für Forschung und Praxis. Berlin: Hogrefe AG (2000).
23. Bundesinstitut für Bau- S-uRIfRoB, Urban Affairs and Spatial Development (BBSR)]. INKAR – Indikatoren und Karten zur Raum- und Stadtentwicklung Bonn. (2022). Available online at: http://inkar.de/ (accessed February 10, 2022).
24. Petzold T, Neumann A, Seifert M, Kuster D, Pfennig A, Weiss J, et al. [Identification of control hospitals for the implementation of the nationwide and standardized evaluation of model projects according to section sign 64b SGB V: analysis of data from structured quality reports]. Gesundheitswesen. (2019) 81:63–71. doi: 10.1055/s-0042-116436
25. Frosch D, Porzsolt F, Heicappell R, Kleinschmidt K, Schatz M, Weinknecht S, et al. Comparison of German language versions of the QWB-SA and SF-36 evaluating outcomes for patients with prostate disease. Qual Life Res. (2001) 10:165–73. doi: 10.1023/A:1016771205405
26. Wilkening A, Zeschky M, Ziegenbein M, Pfefferer-Wolf H, Harms E, Lubbe G, et al. [Evaluation of therapy outcome on a psychiatric admission ward. Background, methods and first results of a project on quality management]. Wiener Klin Wochenschr. (2007) 119:654–62. doi: 10.1007/s00508-007-0895-z
27. Pyne J, Sullivan G, Kaplan R, Williams D. Comparing the sensitivity of generic effectiveness measures with symptom improvement in persons with schizophrenia. Med Care. (2003) 41:208–17. doi: 10.1097/01.MLR.0000044900.72470.D4
28. Thwin S, Hermes E, Lew R, Barnett P, Liang M, Valley D, et al. Assessment of the minimum clinically important difference in quality of life in schizophrenia measured by the quality of well-being scale and disease-specific measures. Psychiatry Res. (2013) 209:291–6. doi: 10.1016/j.psychres.2013.01.016
29. Sieber W, Groessl E, David K, Ganiats T, Kaplan R. Quality of Well Being. Self-Administered (QWB-SA) Scale. User’s Manual. San Diego, CA: University of California (2008).
30. Kaplan R, Ganiats T, Sieber W. Coding & Scoring the Self-Administered QWB Form 1.04 (QWB-SA). San Diego, CA: University of California (1997).
31. Derogatis L. SCL-90-R: Administration, Scoring & Procedures Manual-II, for the R (Revised) Version and Other Instruments of the Psychopathology Rating Scale Series. Towson: Clinical Psychometric Research, Inc (1992).
32. Wetterling T, Junghanns K, Mussigbrodt H, Freyberger H, Dilling H. [Assessment of therapy outcome within the scope of quality assurance in a psychiatric clinic. A report of experiences]. Nervenarzt. (1997) 68:742–51. doi: 10.1007/s001150050189
33. Petrowski K, Schmalbach B, Kliem S, Hinz A, Brahler E. Symptom-checklist-K-9: norm values and factorial structure in a representative German sample. PLoS One. (2019) 14:e0213490. doi: 10.1371/journal.pone.0213490
34. Prinz U, Nutzinger D, Schulz H, Petermann F, Braukhaus C, Andreas S. Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry. (2013) 13:104. doi: 10.1186/1471-244X-13-104
35. Müller J, Postert C, Beyer T, Furniss T, Achtergarde S. Comparison of eleven short versions of the symptom checklist 90-revised (SCL-90-R) for use in the assessment of general psychopathology. J Psychopathol Behav Assess. (2010) 32:246–54. doi: 10.1007/s10862-009-9141-5
36. Berth H, Petrowski K, Balck F. The Amsterdam preoperative anxiety and information scale (APAIS) – the first trial of a German version. Psychosoc Med. (2007) 4:Doc01.
37. Klaghofer R, Brähler E. Konstruktion und teststatistische Prüfung einer kurzform der SCL-90-R [construction and test-statistical examination of a short form of the SCL-90-R]. Z Klin Psychol Psychiat Psychother. (2001) 49:115–24.
38. König W, Lüttinger P, Müller W. A Comparative Analysis of the Development and Structure of Educational Systems. Methodological Foundations and the Construction of a Comparative Education Scale. Mannheim: University Mannheim (1988).
39. Lechert Y, Schroedter J, Lüttinger P. Die Umsetzung der Bildungsklassifikation CASMIN für die Volkszählung 1970, die Mikrozensus-Zusatzerhebun 1971 und die Mikrozensen 1976-2004. Mannheim: ZUMA (2006).
40. Leopold L. Health measurement and health inequality over the life course: a comparison of self-rated health, SF-12, and grip strength. Demography. (2019) 56:763–84. doi: 10.1007/s13524-019-00761-x
41. Huber M, Felix J, Vogelmann M, Leidl R. Health-related quality of life of the general german population in 2015: results from the EQ-5D-5L. Int J Environ Res Public Health. (2017) 14:426. doi: 10.3390/ijerph14040426
42. Ferrari S, Cribari-Neto F. Beta regression for modelling rates and proportions. J Appl Stat. (2004) 31:799–815. doi: 10.1080/0266476042000214501
43. Neumann A, Schoffer O, Norstrom F, Norberg M, Klug S, Lindholm L. Health-related quality of life for pre-diabetic states and type 2 diabetes mellitus: a cross-sectional study in Vasterbotten Sweden. Health Qual Life Outcomes. (2014) 12:150. doi: 10.1186/s12955-014-0150-z
44. Bilcke J, Hens N, Beutels P. Quality-of-life: a many-splendored thing? Belgian population norms and 34 potential determinants explored by beta regression. Qual Life Res. (2017) 26:2011–23. doi: 10.1007/s11136-017-1556-y
45. Basu A, Manca A. Regression estimators for generic health-related quality of life and quality-adjusted life years. Med Decis Making. (2012) 32:56–69. doi: 10.1177/0272989X11416988
46. Hunger M, Doring A, Holle R. Longitudinal beta regression models for analyzing health-related quality of life scores over time. BMC Med Res Methodol. (2012) 12:144. doi: 10.1186/1471-2288-12-144
47. Smithson M, Verkuilen J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods. (2006) 11:54–71. doi: 10.1037/1082-989X.11.1.54
48. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2012).
49. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates (1988).
50. Patterson T, Kaplan R, Grant I, Semple S, Moscona S, Koch W, et al. Quality of well-being in late-life psychosis. Psychiatry Res. (1996) 63:169–81. doi: 10.1016/0165-1781(96)02797-7
51. Pyne J, Patterson T, Kaplan R, Gillin J, Koch W, Grant I. Assessment of the quality of life of patients with major depression. Psychiatr Serv. (1997) 48:224–30. doi: 10.1176/ps.48.2.224
52. Kasckow J, Twamley E, Mulchahey J, Carroll B, Sabai M, Strakowski S, et al. Health-related quality of well-being in chronically hospitalized patients with schizophrenia: comparison with matched outpatients. Psychiatry Res. (2001) 103:69–78. doi: 10.1016/S0165-1781(01)00260-8
53. Pyne J, French M, McCollister K, Tripathi S, Rapp R, Booth B. Preference-weighted health-related quality of life measures and substance use disorder severity. Addiction. (2008) 103:1320–9; discussion 30–2. doi: 10.1111/j.1360-0443.2008.02153.x
54. Kaplan R. The minimally clinically important difference in generic utility-based measures. COPD. (2005) 2:91–7. doi: 10.1081/COPD-200052090
55. Le Q, Doctor J, Zoellner L, Feeny N. Minimal clinically important differences for the EQ-5D and QWB-SA in post-traumatic stress disorder (PTSD): results from a doubly randomized preference trial (DRPT). Health Qual Life Outcomes. (2013) 11:59. doi: 10.1186/1477-7525-11-59
56. Spitzer R, Kroenke K, Linzer M, Hahn S, Williams J, deGruy F III et al. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 Study. JAMA. (1995) 274:1511–7. doi: 10.1001/jama.274.19.1511
57. Rapaport M, Clary C, Fayyad R, Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry. (2005) 162:1171–8. doi: 10.1176/appi.ajp.162.6.1171
58. Candilis P, McLean R, Otto M, Manfro G, Worthington J III, Penava S, et al. Quality of life in patients with panic disorder. J Nerv Ment Dis. (1999) 187:429–34. doi: 10.1097/00005053-199907000-00006
59. Adegbaju D, Olagunju A, Uwakwe R. A comparative analysis of disability in individuals with bipolar affective disorder and schizophrenia in a sub-Saharan African mental health hospital: towards evidence-guided rehabilitation intervention. Soc Psychiatry Psychiatr Epidemiol. (2013) 48:1405–15. doi: 10.1007/s00127-013-0654-6
60. Waghorn G, Chant D, Jaeger J. Employment functioning and disability among community residents with bipolar affective disorder: results from an Australian community survey. Bipolar Disord. (2007) 9:166–82. doi: 10.1111/j.1399-5618.2007.00417.x
61. Ziguras S, Stuart GW. A meta-analysis of the effectiveness of mental health case management over 20 years. Psychiatr Serv. (2000) 51:1410–21. doi: 10.1176/appi.ps.51.11.1410
62. Lambert M, Bock T, Schottle D, Golks D, Meister K, Rietschel L, et al. Assertive community treatment as part of integrated care versus standard care: a 12-month trial in patients with first- and multiple-episode schizophrenia spectrum disorders treated with quetiapine immediate release (ACCESS trial). J Clin Psychiatry. (2010) 71:1313–23. doi: 10.4088/JCP.09m05113yel
63. Ignatyev Y, Mundt A, von Peter S, Heinze M. Hospital length of stay among older people treated with flexible and integrative psychiatric service models in Germany. Int J Geriatr Psychiatry. (2019) 34:1557–64. doi: 10.1002/gps.5165
64. Schilling J, Tolksdorf K, Marquis A, Faber M, Pfoch T, Buda S, et al. [The different periods of COVID-19 in Germany: a descriptive analysis from January 2020 to February 2021]. Bundesgesundheitsblatt Gesundheitsforsch Gesundheitssch. (2021) 64:1093–106. doi: 10.1007/s00103-021-03394-x
65. Adorjan K, Pogarell O, Probstl L, Rub M, Wiegand H, Tuscher O, et al. [Impact of the COVID-19 pandemic on the care situation in psychiatric hospitals in Germany]. Nervenarzt. (2021) 92:562–70. doi: 10.1007/s00115-021-01129-6
66. Galuppi A, Turola M, Nanni M, Mazzoni P, Grassi L. Schizophrenia and quality of life: how important are symptoms and functioning? Int J Ment Health Syst. (2010) 4:31. doi: 10.1186/1752-4458-4-31
67. Fitzgerald P, Williams C, Corteling N, Filia S, Brewer K, Adams A, et al. Subject and observer-rated quality of life in schizophrenia. Acta Psychiatr Scand. (2001) 103:387–92. doi: 10.1034/j.1600-0447.2001.00254.x
68. Norman R, Malla A, McLean T, Voruganti L, Cortese L, McIntosh E, et al. The relationship of symptoms and level of functioning in schizophrenia to general wellbeing and the quality of life scale. Acta Psychiatr Scand. (2000) 102:303–9. doi: 10.1034/j.1600-0447.2000.102004303.x
69. Mercier C, Peladeau N, Tempier R. Age, gender and quality of life. Commun Ment Health J. (1998) 34:487–500. doi: 10.1023/A:1018790429573
70. Vatne S, Bjorkly S. Empirical evidence for using subjective quality of life as an outcome variable in clinical studies. A meta-analysis of correlates and predictors in persons with a major mental disorder living in the community. Clin Psychol Rev. (2008) 28:869–89. doi: 10.1016/j.cpr.2008.01.001
71. Caron J, Lecomte Y, Stip E, Renaud S. Predictors of quality of life in schizophrenia. Commun Ment Health J. (2005) 41:399–417. doi: 10.1007/s10597-005-5077-8
72. Bengtsson-Tops A, Hansson L. Subjective quality of life in schizophrenic patients living in the community. Relationship to clinical and social characteristics. Eur Psychiatry. (1999) 14:256–63. doi: 10.1016/S0924-9338(99)00173-X
73. Dubreucq M, Plasse J, Gabayet F, Blanc O, Chereau I, Cervello S, et al. Sex Differences in recovery-related outcomes and needs for psychiatric rehabilitation in people with schizophrenia spectrum disorder. J Clin Psychiatry. (2021) 82:20m13732. doi: 10.4088/JCP.20m13732
74. Chan S, Yu IW. Quality of life of clients with schizophrenia. J Adv Nurs. (2004) 45:72–83. doi: 10.1046/j.1365-2648.2003.02863.x
75. Kujur N, Kumar R, Verma A. Differences in levels of disability and quality of life between genders in schizophrenia remission. Ind Psychiatry J. (2010) 19:50–4. doi: 10.4103/0972-6748.77638
76. Cardoso C, Caiaffa W, Bandeira M, Siqueira A, Abreu M, Fonseca J. Factors associated with low quality of life in schizophrenia. Cad Saude Publica. (2005) 21:1338–40. doi: 10.1590/S0102-311X2005000500005
77. Salokangas R, Honkonen T, Stengard E, Koivisto A. To be or not to be married–that is the question of quality of life in men with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. (2001) 36:381–90. doi: 10.1007/s001270170028
78. Desalegn D, Girma S, Abdeta T. Quality of life and its association with psychiatric symptoms and socio-demographic characteristics among people with schizophrenia: a hospital-based cross-sectional study. PLoS One. (2020) 15:e0229514. doi: 10.1371/journal.pone.0229514
79. Kim J, Park E. Impact of socioeconomic status and subjective social class on overall and health-related quality of life. BMC Public Health. (2015) 15:783. doi: 10.1186/s12889-015-2014-9
80. Ruesch P, Graf J, Meyer P, Rossler W, Hell D. Occupation, social support and quality of life in persons with schizophrenic or affective disorders. Soc Psychiatry Psychiatr Epidemiol. (2004) 39:686–94. doi: 10.1007/s00127-004-0812-y
81. Lee H, Shin J, Lim Y, Kim K, Kim S, Kim J, et al. Health-related quality of life in coronary heart disease in Korea: the Korea national health and nutrition examination survey 2007 to 2011. Angiology. (2015) 66:326–32. doi: 10.1177/0003319714533182
82. Golicki D, Dudzinska M, Zwolak A, Tarach J. Quality of life in patients with type 2 diabetes in Poland – comparison with the general population using the EQ-5D questionnaire. Adv Clin Exp Med. (2015) 24:139–46. doi: 10.17219/acem/38137
83. Neumann A, Swart E, Häckl D, Kliemt R, March S, Kuster D, et al. The influence of cross-sectoral treatment models on patients with mental disorders in Germany: study protocol of a nationwide long-term evaluation study (EVA64). BMC Psychiatry. (2018) 18:139. doi: 10.1186/s12888-018-1721-z
84. Neumann A, Hense H, Baum F, Kliemt R, Seifert M, Harst L, et al. Evaluation of a flexible and integrative psychiatric care model in a department of child and adolescent psychiatry in Tubingen, Germany: study protocol (EVA_TIBAS). BMC Health Serv Res. (2021) 21:1262. doi: 10.1186/s12913-021-07226-1
85. Deister A, Zeichner D, Witt T, Forster H. [Changes in mental health care by a regional budget: results of a pilot project in Schleswig-Holstein (Germany)]. Psychiatr Prax. (2010) 37:335–42. doi: 10.1055/s-0030-1248438
86. Johne J, von Peter S, Schwarz J, Timm J, Heinze M, Ignatyev Y. Evaluation of new flexible and integrative psychiatric treatment models in Germany-assessment and preliminary validation of specific program components. BMC Psychiatry. (2018) 18:278. doi: 10.1186/s12888-018-1861-1
87. Hillienhof A. [Psychiatrische Kliniken: bundeskammer fordert mehr therapeutisches Personal]. Deutsch Ärzteblatt. (2019) Heft 7:293.
88. Pyne J, Tripathi S, French M, McCollister K, Rapp R, Booth B. Longitudinal association of preference-weighted health-related quality of life measures and substance use disorder outcomes. Addiction. (2011) 106:507–15. doi: 10.1111/j.1360-0443.2010.03299.x
89. Corrigan P, Sokol K, Rusch N. The impact of self-stigma and mutual help programs on the quality of life of people with serious mental illnesses. Commun Ment Health J. (2013) 49:1–6. doi: 10.1007/s10597-011-9445-2
90. Hansson L. Determinants of quality of life in people with severe mental illness. Acta Psychiatr Scand Suppl. (2006) 113:46–50. doi: 10.1111/j.1600-0447.2005.00717.x
Keywords: health-related quality of life, mental health care, psychiatric care models, affective disorders, schizophreniform disorders, alcohol use disorders, flexible and integrative care, symptom severity
Citation: Neumann A, Soltmann B, Kliemt R, Weinhold I, Schmitt J, Pfennig A and Baum F (2023) Health-related quality of life among patients with treated alcohol use disorders, schizophreniform disorders or affective disorders and the influence of flexible and integrative psychiatric care models in Germany (PsychCare). Front. Psychiatry 14:1068087. doi: 10.3389/fpsyt.2023.1068087
Received: 12 October 2022; Accepted: 06 March 2023;
Published: 31 March 2023.
Edited by:
Andrew T. Olagunju, McMaster University, CanadaReviewed by:
Mauro Ceccanti, Sapienza University of Rome, ItalyBabatunde Fadipe, Lagos University Teaching Hospital, Nigeria
Copyright © 2023 Neumann, Soltmann, Kliemt, Weinhold, Schmitt, Pfennig and Baum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne Neumann, QW5uZS5OZXVtYW5uQHVuaWtsaW5pa3VtLWRyZXNkZW4uZGU=
 Anne Neumann
Anne Neumann Bettina Soltmann2
Bettina Soltmann2 Ines Weinhold
Ines Weinhold Jochen Schmitt
Jochen Schmitt