
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 02 March 2023
Sec. Sleep Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1033034
Objectives: There is emerging evidence that sleep problems and short sleep duration increase the risk of infection. We aimed to assess whether chronic insomnia disorder, chronic sleep problems, sleep duration and circadian preference based on self-report were associated with risk of infections and antibiotic use among patients visiting their general practitioner (GP).
Methods: We conducted a cross-sectional study of 1,848 unselected patients in Norway visiting their GP during 2020.The patients completed a one-page questionnaire while waiting for the consultation, that included the validated Bergen Insomnia Scale (BIS), questions on self-assessed sleep problem, sleep duration and circadian preference and whether they have had any infections or used antibiotics in the last 3 months. Relative risks (RR) were estimated using modified Poisson regression models.
Results: The risk of infection was 27% (95% CI RR 1.11–1.46) and 44% higher (95% CI 1.12–1.84) in patients sleeping <6 h and >9 h, respectively, compared to those sleeping 7–8 h. The risk was also increased in patients with chronic insomnia disorder or a chronic sleep problem. For antibiotic use, the risk was higher for patients sleeping <6 h, and for those with chronic insomnia disorder or a chronic sleep problem.
Conclusions: Among patients visiting their GP, short sleep duration, chronic insomnia and chronic sleep problem based on self-report were associated with higher prevalence of infection and antibiotic use. These findings support the notion of a strong association between sleep and infection.
Sleep is an important determinant of health and wellbeing (1). Insomnia is the most common sleep problem with a prevalence of 10–20% in the general population (2–4). In Norway, among patients in general practice, the prevalence of insomnia has been found to be as high as 54% (5, 6). The prevalence varies according to factors such as sex, age, socioeconomic status and circadian preference—evening types are reported to be more susceptible (2, 7). There is emerging evidence, both from controlled laboratory and epidemiological observational studies, that sleep disturbances and short sleep duration increase the risk of infection (8–17). The underlying mechanisms behind this association are unclear and is further complicated by the bidirectional relationship between the two (1). Several studies have found an increase in inflammatory markers associated with sleep disturbances, but overall, the results have varied (1, 18). Infections are a common reason for visits to general practice (19, 20), where also a large percentage of antibiotics are prescribed (21). Sleep disturbances are in many instances treatable (3). A causal effect of sleep disturbances on the immune system would therefore imply an opportunity to reduce the risk of infection and the use of antibiotics (22).
Most of the previous observational studies on sleep and risk of infection have been conducted in samples of the adult general population. None have included circadian preference or antibiotic use, and few have used validated instruments to assess sleep disturbances. Thus, in the present study we aimed to assess whether sleep duration, chronic insomnia disorder [based on the Diagnostic and Statistical Manual for Mental disorders (DSM)-version-5], chronic sleep problems and circadian preference based on self-report were associated with risk of infections and antibiotic use among patients visiting their general practitioner (GP).
The study was based on data collected by last year medical students at the University of Bergen who in the spring and fall semester of 2020 were deployed in general practices in Western Norway for 6 weeks. In Norway, the GPs are organized in a list-based system in which all citizens are entitled to a general practitioner (GP). While deployed, the students were asked to collect questionnaire data from 20 consecutive and unselected patients. Patients were recruited in the waiting room of the GP regardless of their reason for the appointment and asked to answer a one-page questionnaire with questions on background factors, infections, and sleep. Of 153 students, 114 collected data for the study.
Information on sleep duration was based on the question “Approximately how long do you sleep per day?” with the answer categories “ < 6 h”, “6–7 h”, “7–8 h” (reference category), “8–9 h” and “>9 h”. Circadian preference (morningness-eveningness) (“Are you a morning (lark) or evening (owl) type?”) was self-reported on a 5-point scale (“definitively a morning type”, “more a morning than an evening type”, “neither a morning nor an evening type”, “more an evening than a morning type” or “definitely an evening type”). To get a larger sample within each group, we created a new variable with three categories with “morning type (lark)” (first two categories), “neither a morning nor an evening type” (middle category) (reference category), and “night type (owl)” (last two categories). Chronic insomnia disorder was measured using the validated Bergen Insomnia Scale (BIS) (23) that was developed according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (24). In the present study the Bergen Insomnia Scale was adapted according to the updated DSM-5 diagnostic criteria (25). The updated scale includes six items that are scored along an eight-point scale indicating the number of days per week during the past 3 months for which a specific insomnia symptom is experienced (0–7 days). Those who reported 3 days or more per week on at least one of the first three items (sleep onset latency >30 min, wake after sleep onset >30 min, early morning awakening >30 min) and 3 days or more per week on at least one of the two latter items (problematic tiredness/sleepiness, dissatisfaction with sleep) were defined as having chronic insomnia disorder (25). Cronbach's α for BIS was 0.88 in the present sample. The participants were also asked for how long they have had a sleep problem with four answer categories (“do not have sleep problems”, “ < 3 months”, “3 months to a year” or “more than a year”). A variable for chronic sleep problem was generated in which those who reported to have had a sleep problem for 3 months or more on this question were coded “yes”.
Information on infections during the last 3 months was collected using a table in which the respondents were asked to indicate number of times (0, 1, 2, 3, >3 times) they have had the following infections: common cold, throat, otitis or sinusitis, pneumonia/bronchitis, eye infection, gastrointestinal infection with vomit or diarrhea, urinary tract infection, skin infection or any other infection. The main outcome was any type of infection (“yes”, “no”, including all types of infections). Respiratory (RTI) (common cold, throat, otitis or sinusitis and pneumonia/bronchitis), gastrointestinal (GI) and urinary tract infections (UTI) were looked at specifically as they constituted the three largest infection groups (eye infection (4.3%) and skin infections (7.0 %) were not looked at separately as there were so few who reported these). Information on antibiotics in the last 3 months (“yes”, “no”) was based on a question asking whether they had used antibiotics in the last 3 months (“no”, “yes, one prescription”, “yes, two prescriptions”, “yes, three or more prescriptions”).
The study was approved by The Regional Committee for Medical and Health Research Ethics in Western Norway (REK) (ref. 61165).
Modified Poisson regression models (26) were used to estimate relative risks (RR) with 95% confidence intervals (CI) for the association between the sleep related variables and infection. The analyses were conducted in R statistical package (R Foundation for Statistical Computing) and Stata/SE version 17. In the adjusted analyses we adjusted for sex (“male”, “female”), age (continuous variable, included both age and age squared to account for non-linearity in associations), educational level (“primary or lower secondary education”, “upper secondary education”, “vocational school”, “higher education”), children living at home (“yes”, “no”) and season of data collection [“spring” (February-May), “fall” (September–December)].
A total of 2201 questionnaires were collected, of which 1,875 were answered (response rate 85.2%). The analytic sample included 1848 patients after excluding those who were under 18 years. Of the total sample, 60.6% were female, the mean age was 52 years (standard deviation (SD) 18), 38.0% had higher education and 33.3% children living at home (Table 1). A total of 21.0% reported a sleep duration of < 6 h, and 2.0% >9 h. Chronic insomnia disorder based on the DSM-5 criteria was present in 48.3% of the participants, while 46.9% fulfilled the criteria for chronic sleep problem. The prevalence of self-report of any type of infection, RTI, GI and UTI in the last 3 months were 53.9, 35.9, 11.0, and 9.8%, respectively, and 16.0% reported to have used antibiotics at least once during this period.
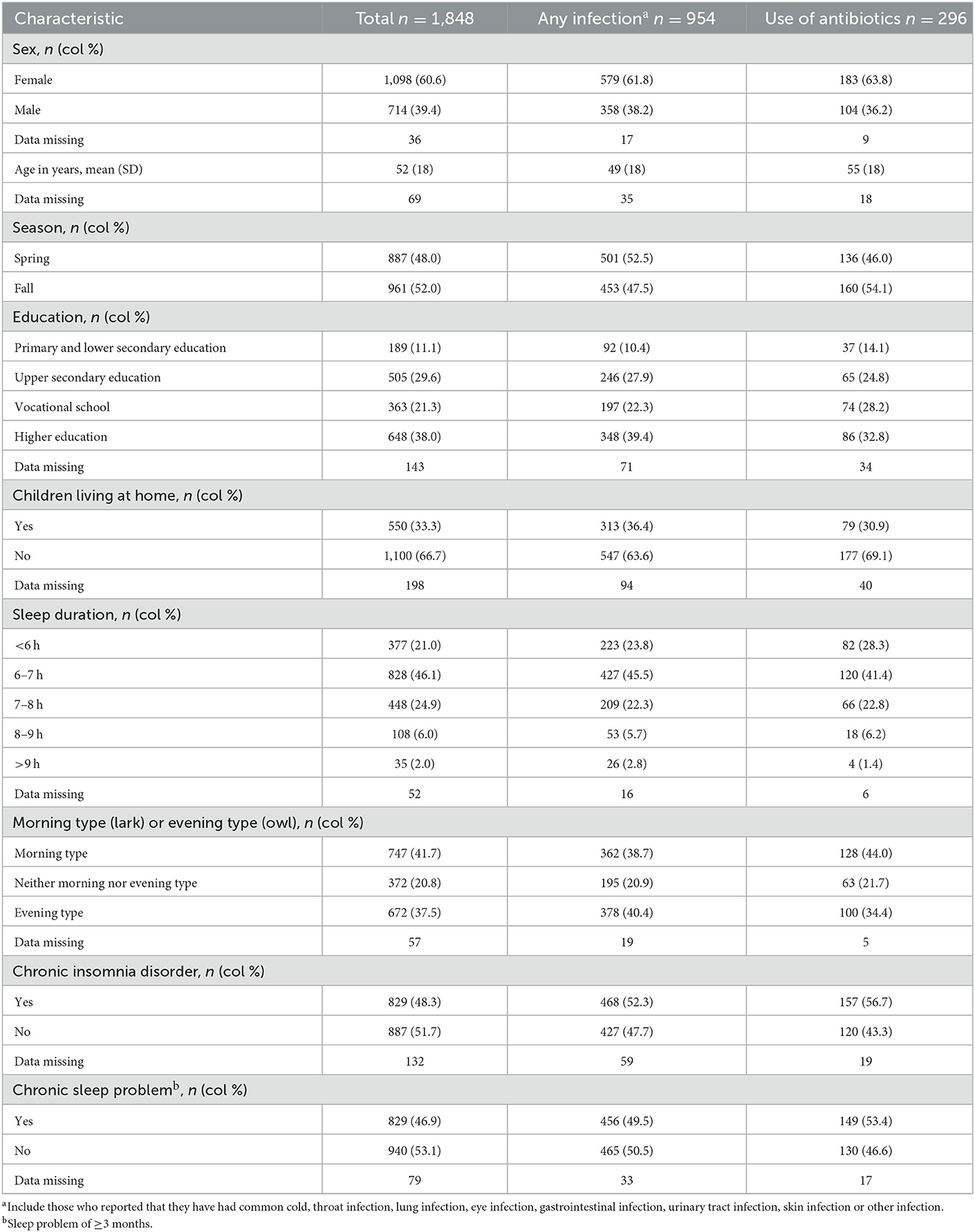
Table 1. Patient characteristics of 1,848 patients in Norway visiting their general practitioner during the spring and fall of 2020, total and for any type of infection and use of antibiotics.
The absolute risk of any type of infection and antibiotic use varied within most of the explanatory variables (Table 2). Patients who reported a sleep duration of < 6 h had an increased risk of infection (adjusted RR (aRR) 1.27, 1.11–1.46) and antibiotic use (aRR 1.57, 95% CI 1.13–2.18) compared to those with sleep duration of 7–8 h (Figure 1, Supplementary Table 1). The risk of infection was also increased in those with >9 h (aRR 1.44, 95% CI 1.12–1.84). Patients with chronic insomnia disorder and with a chronic sleep problem had an increased risk of infection (aRR 1.15, 95% CI 1.05–1.27, aRR 1.13, 95% CI 1.03–1.24) and antibiotic use (aRR 1.47, 95% CI 1.16–1.87, aRR 1.33, 95% CI 1.05–1.69).
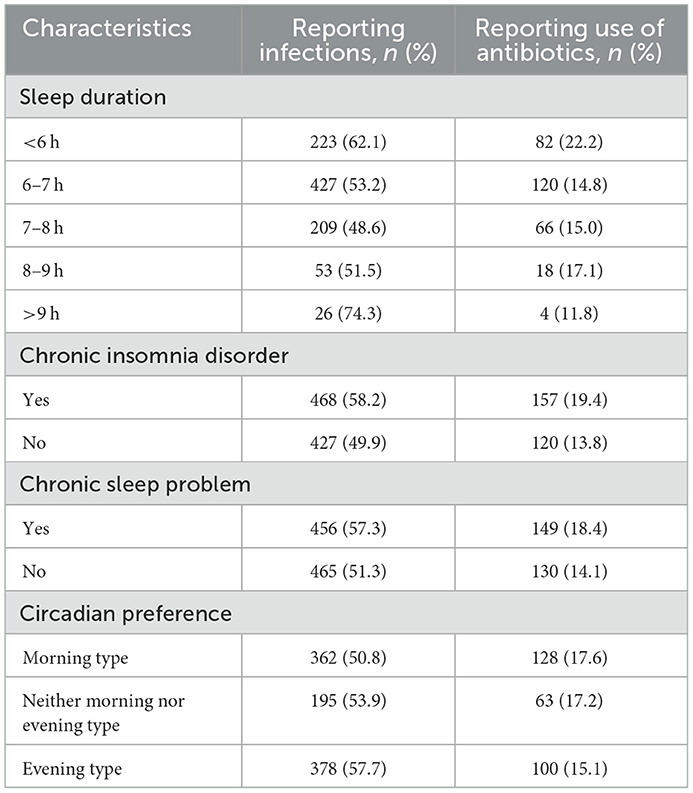
Table 2. Absolute risk of infection (any type) and antibiotic use among 1,848 patients visiting their GPs in the spring and fall of 2020.
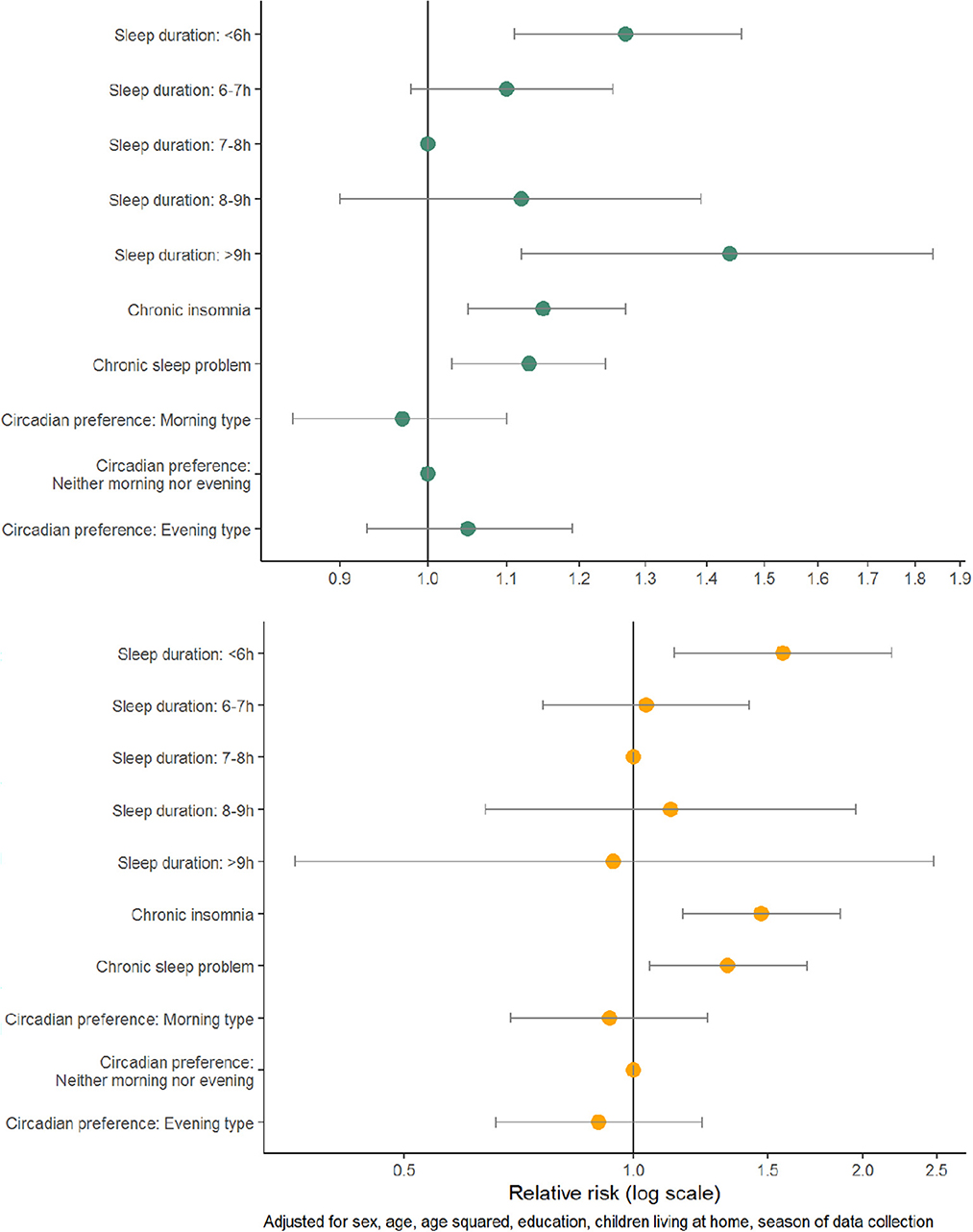
Figure 1. Adjusted relative risks with 95% confidence interval of any type of infection (top panel) and antibiotic use (bottom panel) among 1,848 patients visiting their GPs in the spring and fall of 2020.
In analyses of specific infections, there was some indication of an association between sleep duration and risk of RTI, but the association was not statistically significant (Table 3). For patients with a sleep duration of < 6 h or >9 h, and for those with a chronic sleep problem, the risk of GI was increased (Table 3). For UTI, the risk was higher for patients with chronic insomnia disorder.
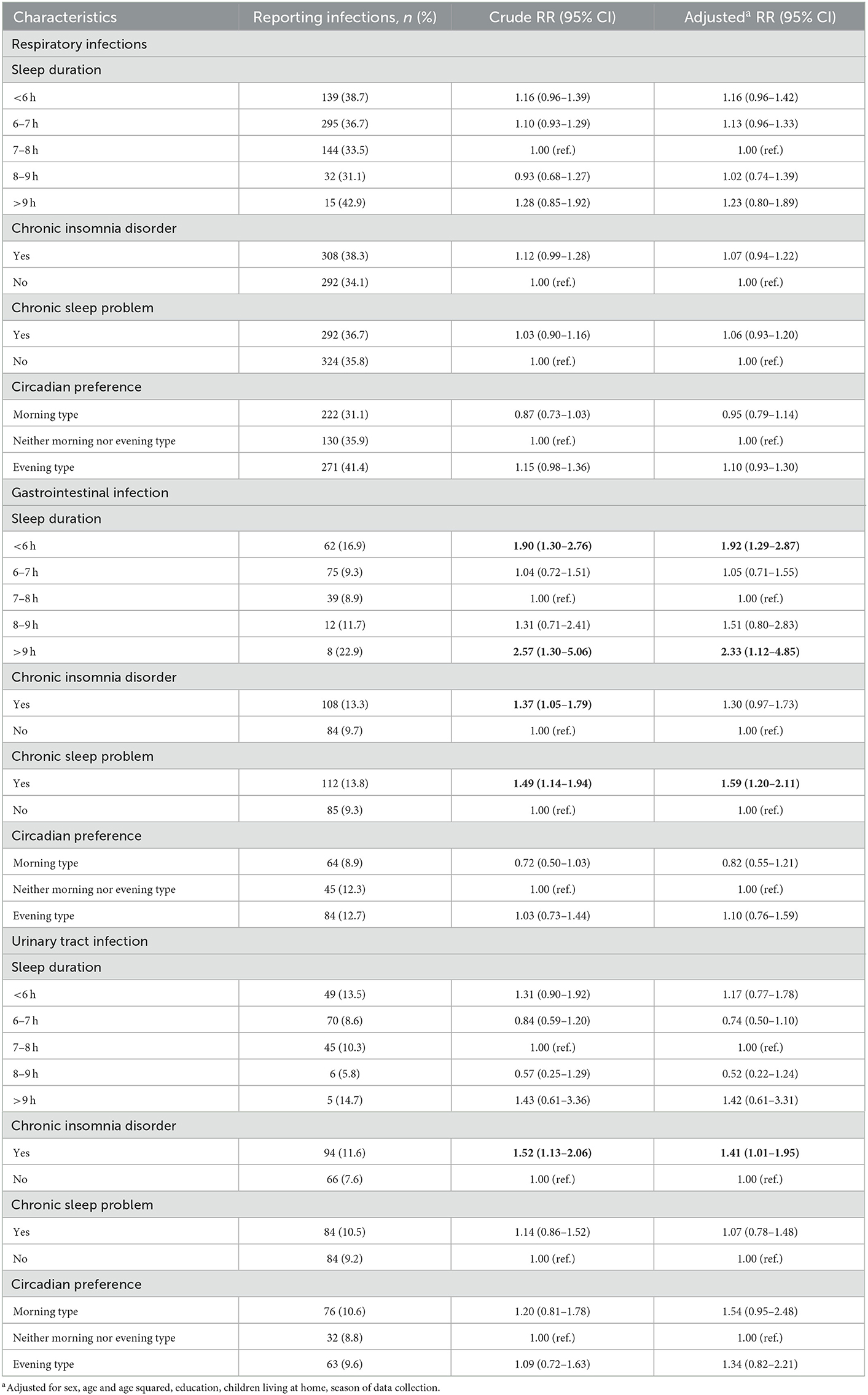
Table 3. Absolute risk, crude and adjusted relative risk (RR) with 95% confidence interval (CI) for respiratory, gastrointestinal and urinary tract infection among 1,848 patients visiting their GPs in the spring and fall of 2020, statistically significant results are indicated in bold.
In this cohort of 1,848 unselected patients in general practice with self-reported data, we found that both short and long sleep duration, chronic insomnia disorder and having a chronic sleep problem were associated with increased risk of reporting any type of infection in the last 3 months. Short sleep duration, chronic insomnia disorder and chronic sleep problem were also associated with increased risk of antibiotic use. The risk of gastrointestinal infections was increased in patients who reported short (< 6 h) and long sleep (≥9 h), and for those who reported a chronic sleep problem. For urinary tract infections, the risk was increased in patients with chronic insomnia disorder.
Strengths of the study include a high response rate (85.2%) and the fact that we included an unselected sample of patients visiting their GPs. Therefore, the results are likely generalizable to patients in general practice. A validated instrument—the Bergen Insomnia Scale (BIS)—was used to assess insomnia based on the DSM-5 criteria (validated based on DSM-IV) (23). The measure used to assess circadian preference has not been validated against other circadian measures but has been used in several previous publications (6, 7, 27).
We do not know why the patient visited their GP and our study did not include any clinical assessment of sleep problems, chronotype, nor infection. At the same time, we were able to assess clinically relevant measures using a questionnaire. Some of the results were imprecise with broad confidence intervals due to a small sample in some of the sub-groups of exposure-outcome combinations. In addition, our measure of RTI included very broad and crude categories of infections. There could be a problem with recall bias in which patients with a self-assessed sleep problem are more or less likely to recall episodes of infection or antibiotic use. If so, this could result in bias.
The data were collected during the 1st year of the COVID-19 pandemic. The pandemic may have made patients more aware of symptoms of respiratory infections. At the same time, it probably also resulted in a lower prevalence of common RTIs due to infection control measures. It is unlikely that this have affected the observed associations in the present study. However, an increase in virtual online consultations (28) may have affected the representatives of our sample. We found a higher proportion of older patients in the present study compared to a similar Norwegian study from 2014 with the same design and source population (5). This was less pronounced for the spring semester (February–May 2020) that also included data collected before the first COVID cases were detected in Norway. All adjusted analyses included semester as a covariate, and this had little effect on the estimates.
Most of the previous observational studies on the association between sleep parameters and risk of infection have looked at respiratory infections and have generally reported a higher risk in short sleepers or in those with a self-reported sleep problem (13–15, 17, 29). The lack of statistically significant associations for risk of RTI in the present study was therefore surprising. We have identified two previous studies within patient populations and one study with a sample from the general population including records from hospitals and primary care. In a Taiwanese registry-based study, including a random sample of 8,061 patients with an insomnia diagnosis and 16 112 without such a diagnosis, the risk of pneumonia was more than doubled in those with an insomnia diagnosis (13). In that study, the diagnoses of both pneumonia and insomnia were made by a clinician and not self-reported, hence it is likely that only the most severe cases were included. In a Japanese study including 39 524 adults without any serious medical conditions who underwent a medical health checkup, those who slept 5 h or less had higher odds of self-reported predisposition to the common cold compared to those who slept 7 h (14). More recently, a large longitudinal registry-based study found that a prior diagnosis of insomnia was associated with an increased risk of later being diagnosed with upper respiratory infections (17). This study included data from two population-based cohorts—the UK Biobank and FinnGen—with up to 23 years of follow-up data from primary care and/or hospitals. In the present study, we found an increased risk of self-reported antibiotic use for patients who reported sleep duration of < 6 h, chronic insomnia disorder or a chronic sleep problem. This may indicate that sleep disturbances are more strongly associated with more severe types of RTI (such as pneumonia). If our study had included more patients and if the included categories of RTI had been more specific, we might have detected effects of the sleep variables on the specific types of RTI.
We have not identified any previous studies that have specifically reported on the association between sleep parameters and the risk of gastroenteritis or UTI in an adult or patient population. Sleep disturbances are common in patients with gastrointestinal disease (30), and there is suggestive evidence from clinical trials that treatment of sleep disorders may improve gastrointestinal symptoms in patients with irritable bowel syndrome (31, 32). The questionnaire used in the present study asked the respondents whether they have had any “gastrointestinal infection with vomit or diarrhea”. This may be interpreted in different ways, but we think that most patients only reported acute gastrointestinal infections. Our results indicate an association between chronic insomnia disorder and the risk of UTI. Like for all infections explored in the present study, we were not able to assess the direction of this effect due to the cross-sectional design.
We found no clear associations between circadian preference and risk of infection or antibiotic use. We have not identified any previous studies on how circadian preference might affect the risk of infections. This question deserves further exploration.
The results from the present study are in line with previous experimental studies in humans that have found increased risk of infection with sleep deprivation or insomnia. In two studies in which healthy adult individuals were infected with rhinovirus, those who had short sleep duration prior to the exposure of the virus were more likely to develop a clinical cold (8, 10). Similarly, previous studies have found a reduction in number of virus-specific antibody titers for influenza, hepatitis A, hepatitis B, and H1N1 (swine flu) in individuals with poor sleep before and after vaccination (33–36). Poor sleep can affect various immune parameters which in turn could reduce the body's ability to fight an infection (1, 18). A systematic review and meta-analysis of cohort and experimental studies on sleep disturbances, sleep duration and inflammation, reported an increase in the inflammatory markers C-reactive protein (CRP) and Interleukin 6 (IL-6) with the presence of sleep disturbance defined by use of validated questionnaires (18). The same study reported an association between long sleep duration (>8 h) and an increase in CRP and IL-6, while no evidence of increase in markers of inflammation were found for short sleep. There has been less focus on the effect of sleep disorders, such as insomnia, on immune response. Insomnia is very common among patients in general practice (5, 6), but under-recognized among GPs (37, 38). Cognitive behavioral therapy for insomnia (CBT-I) has been found to be highly effective in a primary care setting (39), and there is also suggestive evidence that such treatment can reduce the level of CRP in the blood (40). Recently, a more direct link between insomnia and risk of infection was demonstrated in a Mendelian randomization study with genetic data from a large Finnish cohort (17). By using genetic variants strongly associated with insomnia, the authors were able to assess the causal effect of insomnia on respiratory infections. They found that insomnia increased the risk of upper respiratory infection, and COVID-19 hospitalization and severity. Hence, interventions aimed at treating individuals with insomnia could potentially reduce the risk of infections.
Unlike in experimental studies, we were not able to exclude the role of potential unobserved confounding. An underlying health problem could affect both the risk of sleep problems and infections. For example, long sleep duration is associated with cardiovascular disease, diabetes and obesity (41), and has also been linked to depression, low education, low physical activity levels, and high drinking and smoking rates (42). Of these, we only had information on educational level. We view the results on chronic insomnia disorder as more robust than the results on sleep duration as insomnia is a long-term condition that is regarded as independent of other conditions (43). Although an underlying health problem could be a potential unobserved causal factor of both sleep problems and infection risk in the present study, it is likely that better sleep nevertheless could serve as a moderator reducing the risk of infection. More longitudinal studies in the general population and among patients in general practice, as well as clinical studies on the effect of treatment of insomnia on risk of infection, are needed. Data on different groups of infections and their potential differences in associations with sleep could give us important clues about potential underlying mechanisms.
In conclusion, we found a higher risk of infection in patients that reported short or long sleep duration, chronic insomnia, or a chronic sleep problem. The risk of antibiotic use was also increased in patients who reported short sleep duration, chronic insomnia disorder or a chronic sleep problem. Sleep could be a potential target when developing measures to prevent infections and reduce the use of antibiotics.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Regional Committee for Medical and Health Research Ethics in Western Norway (REK) (ref. 61165). The patients/participants provided their written informed consent to participate in this study.
IF performed the data analysis and drafted the manuscript. All authors contributed to the interpretation of the data, reviewed, critically revised and approved the manuscript, take full responsibility for the work, the conduct of the study, controlled the decision to publish, conceived the idea for the present study, and contributed to the data collection.
We thank medical students Sunniva Torsvik and Mats Robin Blindheim for help with coding the questionnaires.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1033034/full#supplementary-material
1. Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. (2019) 99:1325–80. doi: 10.1152/physrev.00010.2018
2. Pallesen S, Sivertsen B, Nordhus IH, Bjorvatn B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. (2014) 15:173–9. doi: 10.1016/j.sleep.2013.10.009
3. Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. (2017) 26:675–700. doi: 10.1111/jsr.12594
4. Bjorvatn B, Waage S, Pallesen S. The association between insomnia and bedroom habits and bedroom characteristics: an exploratory cross-sectional study of a representative sample of adults. Sleep Health. (2018) 4:188–93. doi: 10.1016/j.sleh.2017.12.002
5. Bjorvatn B, Meland E, Flo E, Mildestvedt T. High prevalence of insomnia and hypnotic use in patients visiting their general practitioner. Fam Pract. (2017) 34:20–4. doi: 10.1093/fampra/cmw107
6. Torsvik S, Bjorvatn B, Eliassen KE, Forthun I. Prevalence of insomnia and hypnotic use in Norwegian patients visiting their general practitioner. Fam Pract. (2022). doi: 10.1093/fampra/cmac103
7. Merikanto I, Kortesoja L, Benedict C, Chung F, Cedernaes J, Espie CA, et al. Evening-types show highest increase of sleep and mental health problems during the COVID-19 pandemic-multinational study on 19,267 adults. Sleep. (2022) 45:zsab216. doi: 10.1093/sleep/zsab216
8. Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. (2015) 38:1353–9. doi: 10.5665/sleep.4968
9. Prather AA, Janicki-Deverts D, Adler NE, Hall M, Cohen S. Sleep habits and susceptibility to upper respiratory illness: the moderating role of subjective socioeconomic status. Ann Behav Med. (2016) 51:137–46. doi: 10.1007/s12160-016-9835-3
10. Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. (2009) 169:62–7. doi: 10.1001/archinternmed.2008.505
11. Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW, et al. prospective study of sleep duration and pneumonia risk in women. Sleep. (2012) 35:97–101. doi: 10.5665/sleep.1594
12. Ghilotti F, Pesonen AS, Raposo SE, Winell H, Nyrén O, Trolle Lagerros Y, et al. Physical activity, sleep and risk of respiratory infections: a Swedish cohort study. PLoS ONE. (2018) 13:e0190270. doi: 10.1371/journal.pone.0190270
13. Lin C-L, Liu T-C, Chung C-H, Chien W-C. Risk of pneumonia in patients with insomnia: a nationwide population-based retrospective cohort study. J Infect Public Health. (2018) 11:270–4. doi: 10.1016/j.jiph.2017.08.002
14. Shibata M, Iwane T, Higuchi R, Suwa K, Nakajima K. Potential common factors associated with predisposition to common cold in middle-aged and elderly Japanese: a community-based cross-sectional study. Medicine. (2018) 97:e10729. doi: 10.1097/MD.0000000000010729
15. Nieters A, Blagitko-Dorfs N, Peter H-H, Weber S. Psychophysiological insomnia and respiratory tract infections: results of an infection-diary-based cohort study. Sleep. (2019) 42:zsz098. doi: 10.1093/sleep/zsz098
16. Orzech KM, Acebo C, Seifer R, Barker D, Carskadon MA. Sleep patterns are associated with common illness in adolescents. J Sleep Res. (2014) 23:133–42. doi: 10.1111/jsr.12096
17. Jones SE, Maisha FI, Strausz SJ, Cade BE, Tervi AM, Helaakoski V, et al. The public health impact of poor sleep on severe COVID-19, influenza and upper respiratory infections. medRxiv. (2022). doi: 10.1101/2022.02.16.22271055
18. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014
19. Grimsmo A, Hagman E, Faikø E, Matthiessen L, Njálsson T. Patients, diagnoses and processes in general practice in the Nordic countries. An attempt to make data from computerised medical records available for comparable statistics. Scand J Prim Health Care. (2001) 19:76–82. doi: 10.1080/028134301750235277
20. Wändell P, Carlsson AC, Wettermark B, Lord G, Cars T, Ljunggren G. Most common diseases diagnosed in primary care in Stockholm, Sweden, in 2011. Fam Pract. (2013) 30:506–13. doi: 10.1093/fampra/cmt033
21. NORM/NORM-VET 2020. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo (2021).
22. Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol. (2021) 4:1304. doi: 10.1038/s42003-021-02825-4
23. Pallesen S, Bjorvatn B, Nordhus IH, Sivertsen B, Hjørnevik M, Morin CM, et al. New scale for measuring insomnia: the bergen insomnia scale. Percept Mot Skills. (2008) 107:691–706. doi: 10.2466/pms.107.3.691-706
24. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th edn. Washington, DC: American Psychiatric Association (1994).
25. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th edn. Washington, DC: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
26. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. (2004) 159:702–6. doi: 10.1093/aje/kwh090
27. Bjorvatn B, Waage S, Saxvig IW. Do people use methods or tricks to fall asleep? A comparison between people with and without chronic insomnia. J Sleep Res. (2022) 31:e13763. doi: 10.1111/jsr.13763
28. Helsedirektoratet. E-konsultasjoner hos fastleger. Use of virtual online consultations among GPs. (2021). Available online at: https://www.helsedirektoratet.no/statistikk/statistikk-om-allmennlegetjenester/e-konsultasjoner-hos-fastleger (accessed May 5, 2022).
29. Prather AA, Leung CW. Association of insufficient sleep with respiratory infection among adults in the United States. JAMA Int Med. (2016) 176:850–2. doi: 10.1001/jamainternmed.2016.0787
30. Khanijow V, Prakash P, Emsellem HA, Borum ML, Doman DB. Sleep dysfunction and gastrointestinal diseases. Gastroenterol Hepatol. (2015) 11:817–25.
31. Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. (2005) 22:927–34. doi: 10.1111/j.1365-2036.2005.02673.x
32. Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. (2005) 54:1402–7. doi: 10.1136/gut.2004.062034
33. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. (2002) 288:1471–2. doi: 10.1001/jama.288.12.1471-a
34. Prather AA, Hall M, Fury JM, Ross DC, Muldoon MF, Cohen S, et al. Sleep and antibody response to hepatitis B vaccination. Sleep. (2012) 35:1063–9. doi: 10.5665/sleep.1990
35. Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. (2003) 65:831–5. doi: 10.1097/01.PSY.0000091382.61178.F1
36. Benedict C, Brytting M, Markström A, Broman JE, Schiöth HB. Acute sleep deprivation has no lasting effects on the human antibody titer response following a novel influenza A H1N1 virus vaccination. BMC Immunol. (2012) 13:1. doi: 10.1186/1471-2172-13-1
37. Ogeil RP, Chakraborty SP, Young AC, Lubman DI. Clinician and patient barriers to the recognition of insomnia in family practice: a narrative summary of reported literature analysed using the theoretical domains framework. BMC Fam Pract. (2020) 21:1. doi: 10.1186/s12875-019-1070-0
38. Sivertsen B, Nordhus IH, Bjorvatn B, Pallesen S. Sleep problems in general practice: a national survey of assessment and treatment routines of general practitioners in Norway. J Sleep Res. (2010) 19:36–41. doi: 10.1111/j.1365-2869.2009.00769.x
39. Davidson JR, Dickson C, Han H. Cognitive behavioural treatment for insomnia in primary care: a systematic review of sleep outcomes. Br J Gen Pract. (2019) 69:e657–e64. doi: 10.3399/bjgp19X705065
40. Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. (2014) 37:1543–52. doi: 10.5665/sleep.4008
41. Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. (2018) 39:25–36. doi: 10.1016/j.smrv.2017.06.011
42. Liu T-Z, Xu C, Rota M, Cai H, Zhang C, Shi M-J, et al. Sleep duration and risk of all-cause mortality: a flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. (2017) 32:28–36. doi: 10.1016/j.smrv.2016.02.005
Keywords: sleep initiation and maintenance disorders, infection, antibiotics, primary care, insomnia
Citation: Forthun I, Eliassen KER, Emberland KE and Bjorvatn B (2023) The association between self-reported sleep problems, infection, and antibiotic use in patients in general practice. Front. Psychiatry 14:1033034. doi: 10.3389/fpsyt.2023.1033034
Received: 03 October 2022; Accepted: 30 January 2023;
Published: 02 March 2023.
Edited by:
Seockhoon Chung, University of Ulsan College of Meidicine, Republic of KoreaReviewed by:
Dhirendra Paudel, Transcultural Psychosocial Organization Nepal, NepalCopyright © 2023 Forthun, Eliassen, Emberland and Bjorvatn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ingeborg Forthun, SW5nZWJvcmcuRm9ydGh1bkB1aWIubm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.