
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 06 April 2023
Sec. Aging Psychiatry
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1017203
 Yuanzhi Zhao1†
Yuanzhi Zhao1† Xiangping Wu1†
Xiangping Wu1† Min Tang2
Min Tang2 Lingli Shi1
Lingli Shi1 Shuang Gong2
Shuang Gong2 Xi Mei1
Xi Mei1 Zheng Zhao1
Zheng Zhao1 Jiayue He1
Jiayue He1 Ling Huang1
Ling Huang1 Wei Cui3*
Wei Cui3*Late-life depression (LLD) is one of the most common mental disorders among the older adults. Population aging, social stress, and the COVID-19 pandemic have significantly affected the emotional health of older adults, resulting in a worldwide prevalence of LLD. The clinical phenotypes between LLD and adult depression differ in terms of symptoms, comorbid physical diseases, and coexisting cognitive impairments. Many pathological factors such as the imbalance of neurotransmitters, a decrease in neurotrophic factors, an increase in β-amyloid production, dysregulation of the hypothalamic-pituitary-adrenal axis, and changes in the gut microbiota, are allegedly associated with the onset of LLD. However, the exact pathogenic mechanism underlying LLD remains unclear. Traditional selective serotonin reuptake inhibitor therapy results in poor responsiveness and side effects during LLD treatment. Neuromodulation therapies and complementary and integrative therapies have been proven safe and effective for the treatment of LLD. Importantly, during the COVID-19 pandemic, modern digital health intervention technologies, including socially assistive robots and app-based interventions, have proven to be advantageous in providing personal services to patients with LLD.
In 2019, more than 9% of the world’s population was over 65 years old, and this proportion is expected to increase to 16% by 2050 (1). The physical and mental health of older adults is important because of the various environmental and social stressors in the modern world. The COVID-19 pandemic has significantly impaired the mental health of older adults (2). Although driven by aging, physical disease, and psychological characteristics, late-life depression (LLD) differs from adult depression in terms of its epidemiology, phenotype, pathogenesis, and clinical treatment (3).
The prevalence of LLD significantly varies worldwide (4). Recent epidemiological meta-findings involving 57,486 older adults showed that the average expected prevalence of LLD is 31.8%. In the subgroup analysis, the pooled prevalence was higher in developing countries (40.78%) than in developed countries (17.05%) (5). The prevalence of LLD ranged from 6.3 to 46.6% in Canada, Brazil, Colombia, and Albania (6). Older adults with depressive symptoms accounted for 19.47% in Western countries (7). The prevalence of depressive symptoms in community-dwelling healthy older populations of Australia and US was 9.8% (8), and in mainland China it ranged from 1.5 to 7.9% (9, 10). The prevalence of LLD might be differ among populations. Latinxs had higher odds of LLD than non-Latinx Whites in the US (11). In Canada, the prevalence of LLD was 19.1, 24.2 and 11.9%, respectively, in home and community care, long-term care and palliative home care (12). Moreover, the comorbid of psychological disorders might also affect the prevalence of LLD (13). The prevalence of comorbid anxiety disorders in LLD was 7.4% for generalized anxiety disorder, 4.7% for panic disorder, 5.3% for agoraphobia, 1.1% for social phobia, 2.1% for obsessive-compulsive disorder and 3.7% for post-traumatic stress disorder, with an overall prevalence of 16.84% (14). In addition, LLD were associated with 20−30% of Alzheimer’s type dementia, 20−50% of Parkinson’s type dementia and 50% of vascular dementia, fronto-temporal dementia, and Lewy body dementia (15). And substance abuse disorder largely increases the prevalence of LLD because of its neurotoxic and neuropathological changes on patients (16).
Late-life depression results in an overall economic burden (17). Health service utilization, including direct and indirect healthcare utilization costs, is the main reason for the excessive economic burden caused by LLD. Compared with non-LLD patients, the total medical expenses for LLD patients including outpatient care and hospitalization have increased by one-third (18). In European countries, the total costs of health-related resources for LLD patients were 3,748 euros, while the total cost for non-LLD patients is 3,090 euros (19).
Age and sex are the main risk factors for LLD (20). Advanced age increased the risk of depression in an inverted J-shaped relationship, with an odd ratio (OR) of approximately 75. Compared with older adults below 70 years of age, the middle-old (70–80 years) population had higher odds of depression, while the oldest-old (> 80 years) population was not at an increased risk of depression (21). Moreover, a national survey in the USA demonstrated that significantly more depressive symptoms were observed among women (26.9%) than men (19.9%) (22). Additionally, marital status and living conditions were associated with the risk of LLD. A large-scale cross-sectional study in Japan emphasized the relation of LLD to “separation/divorce” and “debt,” possibly because loneliness after separation/divorce might cause depression, while debt represents a major adverse event that can increase the likelihood of LLD (23, 24). A study of older adults in rural China found an association between high CESD-10 scores (center for epidemiological studies depression scale-10, indicating symptoms of depression) and low annual personal income, polluted cooking fuel, toilets without seats, and a lack of bathing facilities (25). Consistent with this, a study in Japan showed that socioeconomic status may permanently affect the future incidence of LLD, and that retirement increases the occurrence of LLD (26, 27).
The infection of COVID-19 could lead to psychological consequences, including depression, anxiety, stress and adjustment disorders, poor sleep, and increased substance use (28). Depression was among the most commonly reported symptoms in COVID-19 survivors without pre-existing mental health problems (29). A study in England found that the prevalence of clinically significant depressive symptoms among older adults increased from 12.5% (before the COVID-19 pandemic) to 22.6% (June and July 2020), and further increased to 28.5% (November and December 2020) (30). The Irish Longitudinal Study on Ageing (TILDA) examined trends in depressive symptoms among older adults before and during the COVID-19 pandemic; the prevalence of clinically significant depressive symptoms accounted for 19.8% during the COVID-19 pandemic, which was more than two times higher than before the COVID-19 pandemic (31). A recent study compared trajectories of depressive symptoms before and after contracting COVID-19 between matched long- and short-COVID groups. Depressive symptoms increased immediately after the onset of the infection in both groups. But the long-COVID group showed substantially greater initial increases in depressive symptoms and heightened levels over 22 months of follow-up (28). And during the COVID-19, the number of LLD patients largely increased, especially in patients with other chronic diseases. The prevalence of depression increased from 17 to 33% in diabetic patients during the COVID-19 pandemic (32). The main risk factors associated with LLD that emerged during the COVID-19 pandemic were sex, loneliness, poor sleep quality, and poor motor function (33).
Late-life depression is a significant public health problem owing to its high prevalence and complex risk factors. Population aging and the global COVID-19 pandemic have adversely affected the physical and mental health of older adults (Figure 1). Therefore, it is critical to understand the unique phenotype and pathogenesis of LLD and investigate effective and safe treatments for this disease.
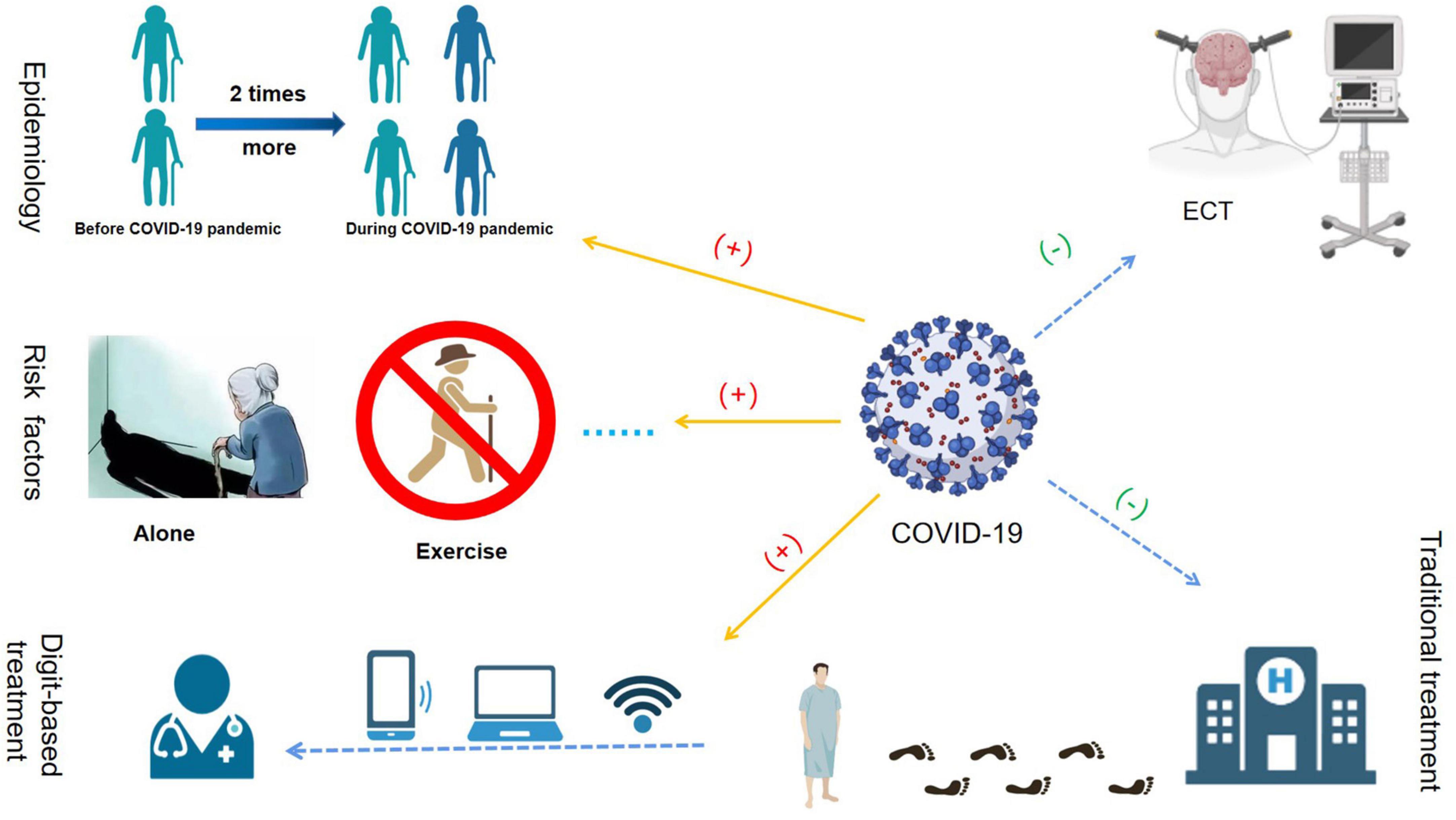
Figure 1. The effects of the COVID-19 pandemic on late-life depression (LLD). The COVID-19 pandemic increased the prevalence of LLD significantly. The prevalence of clinically significant depressive symptoms during the COVID-19 pandemic, which was more than two times higher than before the COVID-19 pandemic. The main risk factors associated with LLD during the COVID-19 pandemic were female sex, loneliness, poor sleep quality, and poor motor function. During the COVID-19 pandemic, owing to the possibility of virus transmission via bag-mask ventilation in the electroconvulsive therapy (ECT) procedure, many ECT units in hospitals worldwide were closed. During the COVID-19 pandemic, the conventional medical and healthcare service mode was “devastating,” while modern digital health intervention technology developed rapidly.
Although the diagnostic criteria for LLD and adult depression are similar, the clinical presentations between LLD and adult depression tend to vary widely.
Central depressive symptom networks differed between the LLD and adult groups. Loss of interest and depressed mood were central to the depressive symptom network structure in both older and younger adults. Additionally, wishes for death and pessimism were the central depressive symptoms in the network of older adults, while fatigue and appetite changes were consistently found among the depressive symptoms in young adults, but not in older adults (34). Moreover, compared with adult depression, LLD is mainly characterized by lower mood and more physical discomfort symptoms. Loss of appetite and weight, insomnia, and difficulty falling asleep are also common among older adults (35). Other unique features of LLD are indifference, weakened interest in all aspects of life, and guilt (36).
Compared to adults with depression, patients with LLD have a higher rate of concomitant drug use. A systematic review and meta-analysis of 16 observational studies that followed nearly 500,000 people showed that the OR for depression was high among individuals with diabetes (37). Due to their physical illness, LLD patients are also likely to be exposed to multiple drugs, which may increase their symptoms of depression. Regarding patients with cerebrovascular disease, the use of calcium channel blockers and nitrate esters is positively associated with a higher risk of depression (38). In addition, complex polypharmacy easily results in drug interactions and multiple adverse effects (39). Therefore, extreme caution should be exercised when prescribing antidepressants to patients with LLD and heart, liver, or kidney disease.
Coexisting cognitive impairments are common in patients with LLD. Approximately 30% of LLD patients exhibit abnormal performance in verbal fluency, response inhibition, novel problem-solving, cognitive flexibility, working memory, and ideomotor planning tests (40). Moreover, a large cohort study of the older adult population in the UK showed that LLD was associated with an 85, 65, and 152% increased risk of all-cause dementia, Alzheimer’s disease (AD), and vascular dementia, respectively, (41). Cumulative exposure to long-term depressive symptoms among older adults could contribute to predicting accelerated subsequent cognitive decline in a dose-response pattern (42).
Late-life depression is typically diagnosed according to the diagnostic criteria for adult depression. However, some clinical phenotypes of LLD and adult depression, including a unique central depressive symptom network, high rate of concomitant drug use, and co-existing cognitive impairments in LLD, differed. These phenotypes are associated with the pathogenesis of LLD.
The pathogenesis of LLD is complex. Multiple etiological hypotheses have been proposed to explain the underlying biological mechanisms of LLD (Figure 2).
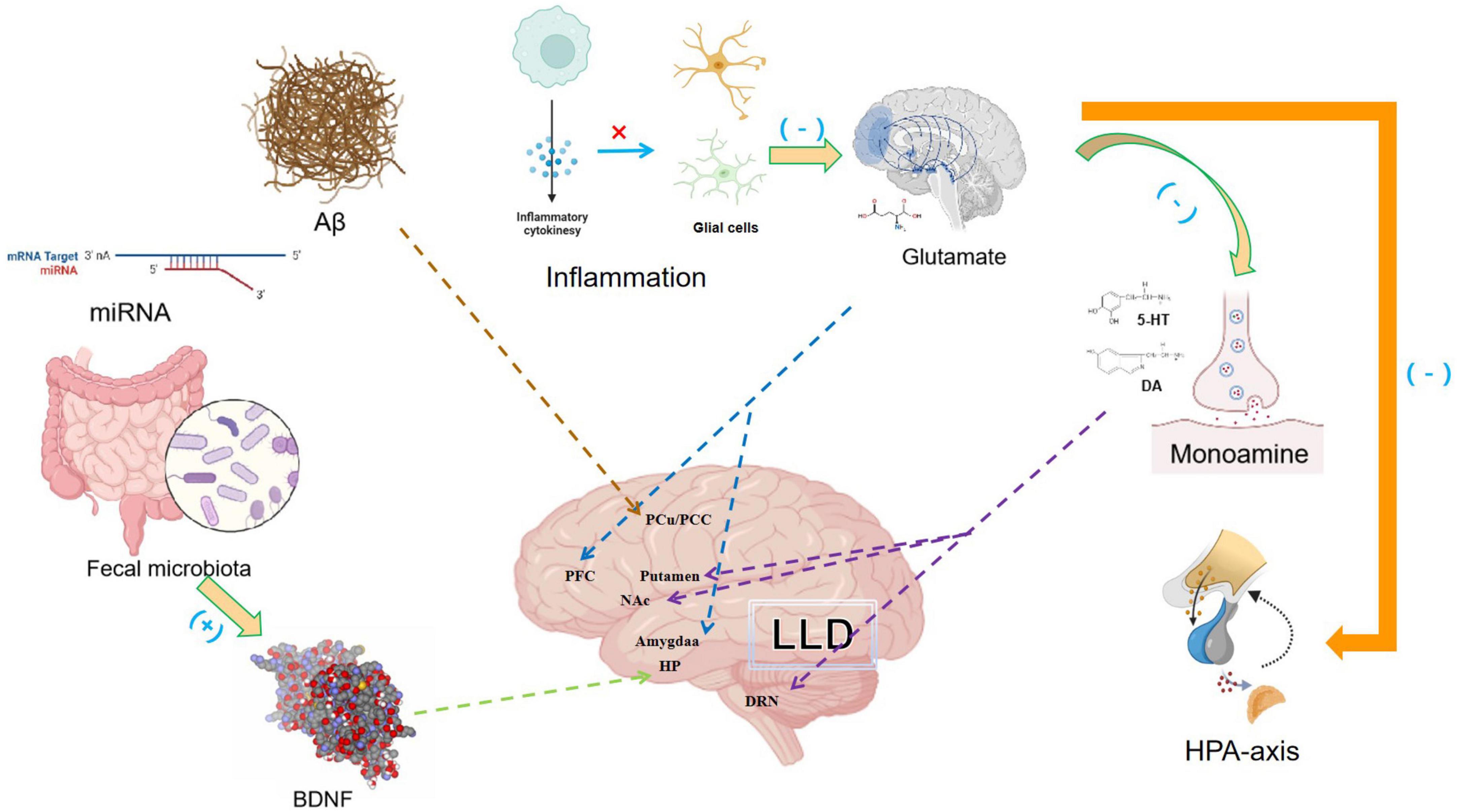
Figure 2. The multiple pathogenesis of late-life depression (LLD) include insufficient monoamine neurotransmission, increased inflammation, abnormal glutamate input, decreased neurotrophic factor production, the dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis, age-related Aβ deposition and low diversity of the gut microbiome. The interplay between the different pathogenesis of LLD and the partial localization of some of the mechanisms in the brain was also shown. Pro-inflammatory cytokines can dysregulate the glutamate system, reduces the synthesis of serotonin and affect HPA axis.
The monoamine hypothesis of depression attributes the symptoms of major depressive disorders (MDDs) to imbalances in monoamine neurotransmitters including serotonin, dopamine, and norepinephrine (43). Low levels of serotonin transporters have been found in the frontal, temporal, and parietal cortical regions in patients with LLD and depressive symptoms (44). Moreover, the dysfunction of 5-HT1A receptor activity in the brainstem region of the dorsal raphe nucleus has been observed in LLD (45). Dysfunction of the dopamine system causes alterations in the cognitive, positive valence, and sensorimotor systems, resulting in depressive symptoms. Low dopamine transporter binding potentials have also been found in the nucleus accumbens and putamen of patients with LLD compared to healthy controls. Thus, it is suggested that symptom of anhedonia found in LLD is associated with reduced dopamine neurotransmission in the reward system (46).
Furthermore, a positive correlation was observed between inflammation and LLD. Inflammatory cytokines may participate in the pathogenesis of LLD through multiple mechanisms. Inflammatory cytokines act directly on astrocytes and microglia, dysregulate the glutamate system, promote excitotoxicity, and activate indoleamine 2,3-dioxygenase, which reduces serotonin synthesis and increases kynurenine production. Increased kynurenine levels can result in oxidative stress, which consequently damages glial cells in the prefrontal cortex and amygdala. Moreover, inflammatory cytokines can affect the hypothalamus-pituitary-adrenal (HPA) axis, thereby destroying the inhibitory effects of glucocorticoids on inflammatory cytokines. Continuous activation of microglia can result in inefficient clearance of neurotoxic molecules, neuronal loss, and decreased neurogenesis (40, 47–49). A longitudinal study conducted over 2 years found that compared to non-depressive patients, the levels of IL-1β, IL-6, and IL-8 were higher in patients with LLD (50). IL-6 not only reduces the concentration of serotonin, but also damages the plasticity of synaptic nerves, which consequently results in cognitive impairment in LLD (51). Moreover, complaints and anhedonia, two important symptoms of depression, were positively correlated with C-reactive protein levels in patients with LLD, further supporting the link between inflammation and LLD (52).
Glutamate, a major excitatory neurotransmitter in the central nervous system, was found to have significantly higher glutamine-to-glutamate ratios at baseline in subjects with LLD. This ratio decreased in individuals with LLD over a 3 year follow-up, which correlated with a decrease in the severity of depression, suggesting that abnormalities in the glutamine–glutamate cycle may contribute to the pathophysiology of LLD (53). Glutamatergic synapses are excitatory synapses closely related to stress. Abnormal levels of N-methyl-D-aspartate (NMDA) receptors are also present in the brain of patients (54, 55). Ketamine, a non-competitive NMDA receptors agonist, has been used clinically to treat refractory depression. The safety and effectiveness of ketamine as an antidepressant for LLD have been proven in clinical trials, further supporting the involvement of NMDA receptors in the pathogenesis of LLD (56).
Neurotrophic factors are growth factors that regulate synaptic plasticity and neurotransmission, which can also play a role in LLD. Brain-derived neurotrophic factors (BDNF) can reverse defects in synaptic plasticity caused by stress, thereby enhancing flexibility against depression. BDNF levels in patients with LLD are significantly lower than those in healthy individuals (57, 58). And when compared to a control group, DNA methylation of BDNF in the LLD group was significantly higher (59). Higher BDNF methylation is independently associated with the prevalence and incidence of depression and major depressive symptoms (60). Longitudinal studies have reported 1−2% annual hippocampal atrophy among adults older than 55 years without dementia, and such age-related hippocampal deterioration and memory impairment may exacerbate depression in LLD patients (61).
The HPA axis is an important neuroendocrine system involved in controlling stress responses (62). Patients with LLD displayed significantly higher levels of basal cortisol during all phases of the diurnal cycle and higher levels of post-dexamethasone cortisol than healthy controls, suggesting that patients with LLD have HPA axis dysregulation (63). Moreover, patients with LLD have higher cortisol levels during morning awakening and a lower response to dynamic awakening, indicating that LLD responds to awakening stress. Older participants with depression showed a high degree of HPA axis activity dysregulation compared to younger adults, possibly because LLD lacks a buffering mechanism and displays higher HPA axis activity than its younger counterparts (63, 64).
The pathophysiological mechanisms of LLD and AD overlap. β-amyloid (Aβ) accumulation, a pathological hallmark of AD, has also been observed among patients with LLD. Aβ deposition results in a series of neurobiological processes that can damage neural networks related to depression (65). A community follow-up study of 270 older adults with un-impaired cognition showed that Aβ deposition was positively associated with an increase in anxiety-depressive symptoms (66). Moreover, older adults with depressive symptoms had more significant Aβ deposition in the anterior/posterior cingulate cortex than older adults without depressive symptoms (67). Furthermore, subjects with mild cognitive impairment and depression had severe Aβ deposits in the frontal cortex on both sides (68).
Age-related neurological conditions in LLD may also be a result of low-diversity gut microbiome (69). This is a common feature of biological aging, and age-dependent changes in the gut microbiota can manipulate neuroimmune responses (70). Gray matter volume (GMV) deficiency in marginal regions is strongly associated with LLD. And the great brain volume and increased gut diversity measures appear was positively associated with health, further informing the involvement of the brain-gut axis in LLD (71). In fact, rodents that receive fecal microbiome transplantation (FMT) from depressed humans can exhibit a heightened state of inflammation and increased anhedonia-like and anxiety-like behavior compared to those who receive FMT from healthy volunteers (72, 73). The baseline enrichment of Faecalibacterium, Agathobacter and Roseburia was positively associated with the treatment outcome of remission, indicating that the information from fecal microbiota might predict the response of antidepressants to LLD (74). Moreover, a randomized double-blind placebo-controlled multicenter trial found that probiotics containing Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI administered for 12 weeks significantly improved mental flexibility and alleviated stress among healthy older adults, supporting the regulation of LLD by affecting the gut microbiota (75).
The classic pathogenesis of LLD includes insufficient monoamine neurotransmission, increased inflammation, abnormal glutamate input, decreased neurotrophic factor production, and dysregulation of the HPA axis. Moreover, age-related Aβ deposition and low diversity of the gut microbiome might result in LLD with cognitive impairments. Therefore, further treatments should target LLD pathogenesis.
Selective serotonin reuptake inhibitors (SSRI) antidepressants are typically used to treat LLD. A study of 6,373 patients with LLD receiving SSRI antidepressants found that 50.7% of the patients achieved a reduction of at least 50% on the Hamilton depression scale (HAMD). This finding suggests that approximately 50% of older adults with MDD treated with SSRI antidepressants could exhibit symptom improvement when treated (76). According to the 2016 Canadian Network for Mood and Anxiety Treatments (CANMAT) guideline, first-line SSRI antidepressants recommendations for depression in older adults were duloxetine, mirtazapine, nortriptyline (level of evidence: level 1), bupropion, citalopram/escitalopram, desvenlafaxine, duloxetine, sertraline, venlafaxine, and vortioxetine (level of evidence: level 2) (54). Previously, there was a concern that paroxetine might cause adverse outcomes in the geriatric population owing to its anticholinergic properties. However, a recent study reported no increase in mortality, dementia risk, or cognitive measures in patients with paroxetine-treated LLD (77).
Although initial treatment, continuous treatment, and medication adjustment plans for treatment-resistant depression with LLD are similar to those for adult depression, several concerns should be considered when treating LLD with SSRI antidepressants (78). Aging affects pharmacokinetics, pharmacodynamics, and interactions between antidepressants. Moreover, liver function and kidney creatinine clearance rates continue to decline, and the pharmacodynamic sensitivity of older adults increase with age, resulting in reduced therapeutic effects, non-compliance to treatment, side effects, and poor tolerance of antidepressants in LLD (77). SSRI antidepressants can cause hyponatremia in older adults. Moreover, the incidence of these adverse effects can be greater than 10% when SSRI treatment is initiated for LLD (39). Additionally, the use of SSRI antidepressants was associated with a higher risk of hip fracture in older adults. In a large case-control study in Taiwan, the current use of SSRI antidepressants was associated with > two fold increase in the risk of hip fractures (79). Similarly, in a Danish nationwide population study, the prevalence of SSRI use among patients with hip fractures was approximately double that in the general population (80). In addition, patients with LLD and cognitive deficits do not respond well to antidepressants; the response of patients with LLD with executive function complaints to escitalopram was slower than that of patients with LLD without executive function complaints (81).
Antipsychotics (such as aripiprazole), mood stabilizers (such as lithium salt), and dopamine agonists (such as methylphenidate) are commonly used as synergists for adjuvant treatment of refractory LLD (82). Aripiprazole has been reported to improve the achievement of remission among LLD patients treated with venlafaxine, possibly via the partial activation of dopamine (83). Moreover, methylphenidate has been reported to potentiate the anti-depressive effects of citalopram, such as alleviation of depression severity, increased treatment response, and improved cognitive performance in patients with LLD (84). Additionally, 33.3% of patients with refractory LLD achieved remission using lithium salt synergistic therapy, and citalopram combined with lithium could be used to treat imipramine resistant LLD (85, 86).
Therefore, several novel antidepressant drugs have been developed for this purpose. The most studied novel antidepressants are the NMDA receptors antagonists, ketamine and memantine (40). Memantine has been used as an adjunct agent to citalopram in the treatment of LLD. The remission rate was higher in the escitalopram + memantine group than in the escitalopram-alone group. Moreover, a combination of memantine and escitalopram is well tolerated by patients with LLD (87). Ketamine is a racemic mixture of equal amounts of S-ketamine- and R-ketamine, which has a high affinity for the NMDA receptors esketamine (S-ketamine) and generates rapid and sustained antidepressant effects in patients with depression without notable side effects. A double-blind pilot study among older patients with treatment-resistant depression showed that ketamine was more effective than midazolam (88). Moreover, benzoate is a d-amino acid oxidase (DAAO) inhibitor and an indirect NMDAR enhancer. A recent randomized clinical trial found that benzoate decreases perceived stress, improves cognitive function, and enhances treatment adherence in patients with LLD (89).
Classical antidepressants are represented by SSRI antidepressants as the first-line treatment for LLD. However, these classical antidepressants are associated with various difficulties in LLD therapy, such as poor responsiveness to cognitive deficits, drug-drug interactions, and side effects among older adults. Although novel antidepressants with different mechanisms of action have been developed and are used as synergists for the adjuvant treatment of LLD, it is still necessary to investigate non-pharmacological treatments for this disorder.
Non-pharmacological treatments, which are safe and operable, have been widely used to treat LLD. Neuromodulation therapies use physical methods to implant treatment facilities in the body or outside the skin, to further adjust the function of the central nervous system and improve disease symptoms (90). The most common forms of neuro-modulatory therapy currently used for LLD include electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), and light treatment (Table 1) (91–94).
Electroconvulsive therapy stimulates the brain with a short and appropriate current, causing the patient’s brain cells to discharge synchronously, resulting in severe epileptic seizures. ECT is one of the most effective interventions for refractory depression. A randomized controlled trial on LLD, comparing the antidepressant effects of 6 weeks of ECT with those of 12 weeks of drug therapy found that patients who underwent ECT experienced faster symptom relief than the drug-treated patients (95). After the trial, the response rates in the two groups were 63.8 and 33.3%, respectively. Old age is also associated with a rapid response to ECT (96). ECT was more effective among older than younger patients. Moreover, no serious adverse events were observed in either group (92). Furthermore, most patients with LLD who underwent ECT had mild cognitive impairment within 1 week. However, 2 weeks after the end of ECT treatment, most cognitive functions remained unchanged compared to baseline functions (97). Mini-mental state examination (MMSE) scores tended to improve significantly 6 months after ECT in the event of baseline cognitive impairment with LLD (93). However, ECT requires an oxygen mask for spontaneous ventilation after anesthesia. During the COVID-19 pandemic, because of the possibility of virus transmission via bag-mask ventilation during the ECT procedure, many ECT units in hospitals worldwide were closed (Figure 1) (98).
Transcranial magnetic stimulation is an electrophysiological technique that uses a time-varying magnetic field to induce a time-varying electric field in the skull, resulting in enhanced brain metabolism and electrical nerve activity. TMS has proven to be effective for LLD treatment, with a response rate of approximately 20–50% (95). Moreover, a systematic review suggested that TMS is a safe and well-tolerated option for older patients with LLD with a relatively low percentage of side effects (12.4% in total) (99). Intermittent theta pulse stimulation (iTBS), a specialized form of TBS is effective in increasing cortical excitability and has been proven to be advantageous for patients with LLD and cognitive impairments (100). iTBS can reverse depression and executive function deficits in older patients (91). Moreover, a clinical case report suggested that iTBS can be used to treat LLD with comorbid mild cognitive impairments (101). Additionally, deep TMS can stimulate connections between subcortical structures and deep brain fibers. The remission rate of LLD patients in the deep TMS group was higher than in the sham TMS group, indicating the usefulness of deep TMS in the treatment of LLD (102).
Light therapies use sunlight at specific wavelengths. Light therapies can reduce symptoms of depression and sleep disruption in older adults residing in long-term care facilities. Bright light treatment can improve mood, enhance sleep efficiency, and increase the upslope melatonin level gradient of non-seasonal MDD among older adults with non-seasonal MDD (94, 103).
Psychotherapy has a moderate-to-strong effect on the improvement of depressive symptoms in LLD. Moreover, the therapeutic effects can last for at least 6 months (104). Cognitive behavioral therapy (CBT), problem-solving therapy (PST), interpersonal relationship therapy (IPT), and life review therapy are the main evidence-based psychotherapy methods for LLD treatment (40). In clinical practice, LLD patients in the context of hopelessness and age-related stressors have high suicide risk and great treatment challenges. Therefore, it is an urgent need to find alternative managements for treating such patients. The combination of psychotropic medications and the joy journal, a CBT-informed therapeutic intervention, could improve depressive symptoms of LLD associated with hopelessness during the COVID-19 pandemic (105).
Lifestyle behaviors may be an effective, low-cost intervention for improving the overall wellbeing of older adults. An active lifestyle, such as physical exercise or a balanced diet, could be a valid low-cost preventive strategy to counteract LLD (106). A study in South Korea suggested that it is important to encourage older individuals to exercise regularly to relieve depressive symptoms and that hand-grip strength may increase the effect of regular exercise on depressive symptoms among individuals 65 years and older (107).
Non-pharmacological treatments such as neuromodulation therapies and psychotherapy have been rapidly developed for the treatment of psychological disorders. ECT, TMS and phototherapy are among the most effective and safe non-pharmacological treatments for LLD. Importantly, neuromodulation therapy has proven to be advantageous for patients with LLD and cognitive impairments.
Many complementary and alternative medicine interventions, including Qigong, acupuncture, and traditional Chinese medicine were proved to be benefit for LLD patients, especially during the COVID-19 pandemic (108).
Qigong has gained worldwide popularity and was suitable for LLD patients who cannot go out during the COVID-19 pandemic. 10-week Tai Chi intervention decreased the depressive symptoms of participants aged 60−78 years during the COVID-19 pandemic (109). Moreover, there was significant improvement of Sahaj Samadhi meditation on the Hamilton rating scale for depression in old patients during the COVID-19 period (110). Traditional Chinese herb formulations, such as Chaihu Shugan San, Xiaoyao San, and Sini San were proved to be effective to reduce depressive symptoms in patients infected with COVID-19 virus (111). Moreover, acupuncture was proofed not only to relief primary and secondary depressive symptoms, but also to reduce the side effects of the medical treatments (112). And the safety and efficacy of acupuncture in the treatment of depression was also proved by a previous study (113).
Social distancing, quarantine, and limitations in outdoor activities have resulted in sarcopenia, which has been closely associated with LLD during the COVID-19 pandemic. Dietary essential amino acids (EAAs) could be a good alternative for counteracting against loss of muscle mass and function (114). Moreover, deficiencies in the EAAs isoleucine, leucine, and histidine could be used to predict depression in older women (115). The Mediterranean diet, characterized by an abundance of whole grains, plant foods, and olive oil, and a moderate intake of fish and wine, has been associated with a variety of disease outcomes. A longitudinal study of older residents in Chicago found that greater adherence to a Mediterranean diet was positively associated with a reduced number of newly occurring depressive symptoms (116).
Following technological progress, modern digital health interventions have become mainstream in medical and healthcare services. During the COVID-19 pandemic, the conventional medical and healthcare service mode (hospital outpatient service and routine healthcare projects) has been “devastating,” whereas modern digital health intervention technologies have developed rapidly (Table 2 and Figure 1) (117–122).
A socially assistive robot is an artificial intelligence system designed to interact with humans by following social behaviors and rules. Social robots are being used increasingly to provide personal support to older adults living in long-term care facilities. Moreover, they can help alleviate LLD when used in group activities (123).
An 8 weeks mobile app-based intervention with remote therapist support was found to decrease depressive and anxiety symptoms among community-dwelling middle-aged and older adults. Moreover, 45% of participants showed clinically significant improvements in either depressive or anxiety symptoms (120).
Internet-based cognitive behavioral therapy (iCBT) has also been used to treat patients with LLD (122). The potential efficacy and cost-effectiveness of iCBT in the treatment of LLD were examined in randomized interventions with telephone-delivered CBT or telephone-delivered non-directive supportive therapy (NST) for patients with LLD. After 4 months, both treatments resulted in a reduction in clinical symptoms. Moreover, telephone-delivered CBT yielded significantly greater improvements in depressive symptoms than NST (118, 121). An iCBT and mindfulness techniques were found to be effective in improving distress, depression and loneliness in older adults in Israel during the COVID-19 pandemic (124). MoodTech is a pilot study of internet-based interventions for LLD that provides a medium for empathy, encouragement, appreciation, affirmation, and collaborative problem-solving. Such internet-based interventions have proven to be viable and potentially cost-effective options for disseminating online treatments for LLD (119). Another study conducted a short-term intervention with internet-based cognitive-behavioral and mindfulness techniques for community-dwelling older adults in Israel during the COVID-19 pandemic. The study concluded that these interventions were effective in improving distress, depression, and loneliness in older adults and that different psychological techniques seemed to have different effects on these symptoms.
Digit-based treatments are rapidly being developed. In particular, following the devastating conventional medical supply during the COVID-19 pandemic, digit-based medical supply proved advantageous in providing personal services to patients with LLD and was proven to be effective and safe for LLD therapy.
This article reviews the research progress on LLD in epidemiology, clinical phenotype, pathogenesis, and treatment, focusing on the comparison of the differences between LLD and adult depression. Population aging, the COVID-19 pandemic, and societal stress significantly affect the emotional health of older adults, resulting in a high prevalence of LLD worldwide. The clinical phenotypes of LLD and adult depression differ. However, pathological mechanisms underlying LLD remain unclear. Traditional SSRI therapy yields poor response and side effects in the treatment of LLD. Although several forms of neuromodulation therapies, complementary and integrative therapies and digit-based treatments have been proven to be safe and effective in treating LLD, there is still a need to investigate the detailed pathogenesis of LLD and develop novel anti-LLD treatments.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This research was funded by the Natural Science Foundation of Ningbo (2021J276 and 2021J274) and the NINGBO Medical & Health Leading Academic Discipline Project (2022-F28).
We sincerely appreciate the academic supports by each teacher from the Ningbo 3 units. We thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LLD, late-life depression; OR, odd ratio; AD: Alzheimer’s disease; HPA, hypothalamus-pituitary-adrenal; NMDA, N-methyl -D-aspartate; DRN, dorsal raphe nucleus; NAc, nucleus accumbens; PFC, prefrontal cortex; ACC/PCC, anterior/posterior cingulate cortex; HP, hippocampal; BDNF, brain-derived neurotrophic factors; A β, β -amyloid; GMV, gray matter volume; FMT, fecal microbiome transplantation; HAMD, Hamilton depression scale; CANMAT, Canadian Network for Mood and Anxiety Treatments; DAAO, d-amino acid oxidase; ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation; MMSE, mini-mental state examination; iTBS, intermittent theta pulse stimulation; MDD, major depressive disorder; CBT, cognitive behavioral therapy; PST, problem-solving therapy; IPT, interpersonal relationship therapy; EAAs, essential amino acids; iCBT, inter-net-based cognitive behavioral therapy; NST, non-directive supportive therapy; SSRI, Selective serotonin reuptake inhibitors; RAVLT, Rey Auditory Verbal Learning Test.
1. Available online at: https://www.un.org/en/desa/world-population-prospects-2019-highlights
2. Manca R, De Marco M, Venneri A. The impact of Covid-19 Infection and enforced prolonged social isolation on neuropsychiatric symptoms in older adults with and without dementia: a review. Front Psychiatry. (2020) 11:585540. doi: 10.3389/fpsyt.2020.585540
3. Haigh E, Bogucki O, Sigmon S, Blazer D. Depression among older adults: a 20-year update on five common myths and misconceptions. Am J Geriatr Psychiatry. (2018) 26:107–22. doi: 10.1016/j.jagp.2017.06.011
4. Andreas S, Schulz H, Volkert J, Dehoust M, Sehner S, Suling A, et al. Prevalence of mental disorders in elderly people: the european mentdis_Icf65+ study. Br J Psychiatry. (2017) 210:125–31. doi: 10.1192/bjp.bp.115.180463
5. Zenebe Y, Akele B, Necho M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann Gen Psychiatry. (2021) 20:55. doi: 10.1186/s12991-021-00375-x
6. Ylli A, Miszkurka M, Phillips S, Guralnik J, Deshpande N, Zunzunegui M. Clinically relevant depression in old age: an international study with populations from Canada, Latin America and eastern Europe. Psychiatry Res. (2016) 241:236–41. doi: 10.1016/j.psychres.2016.04.096
7. Volkert J, Schulz H, Harter M, Wlodarczyk O, Andreas S. The prevalence of mental disorders in older people in western countries - a meta-analysis. Ageing Res Rev. (2013) 12:339–53. doi: 10.1016/j.arr.2012.09.004
8. Mohebbi M, Agustini B, Woods R, McNeil J, Nelson M, Shah R, et al. Prevalence of depressive symptoms and its associated factors among healthy community-dwelling older adults living in Australia and the united states. Int J Geriatr Psychiatry. (2019) 34:1208–16. doi: 10.1002/gps.5119
9. Available online at: https://govt.chinadaily.com.cn/s/202006/29/WS5ef995fd498ed1e2f34075ef/china-statistical-yearbook-2010-2019.html
10. Tang T, Jiang J, Tang X. Prevalence of depressive symptoms among older adults in mainland china: a systematic review and meta-analysis. J Affect Disord. (2021) 293:379–90. doi: 10.1016/j.jad.2021.06.050
11. Jimenez A, Cruz-Gonzalez M, Calhoun T, Cohen L, Alegria M. Late life anxiety and depression symptoms, and suicidal behaviors in racial/ethnic minority older adults in community-based organizations and community clinics in the United Status. Cult Div Ethnic Minor Psychol. (2022) 2022:524. doi: 10.1037/cdp0000524
12. Neufeld E, Freeman S, Spirgiene L, Horwath UA. Cross-sectoral comparison of prevalence and predictors of symptoms of depression over time among older adults in Ontario, Canada. J Geriatr Psychiatry Neurol. (2021) 34:11–20. doi: 10.1177/0891988720901790
13. Zhang C, Chang Y, Yun Q, Lu J, Zheng X, Xue Y, et al. The impact of chronic diseases on depressive symptoms among the older adults: the role of sleep quality and empty nest status. J Affect Disord. (2022) 302:94–100. doi: 10.1016/j.jad.2022.01.090
14. Suradom C, Wongpakaran N, Wongpakaran T, Lerttrakarnnon P, Jiraniramai S, Taemeeyapradit U, et al. Prevalence and Associated factors of comorbid anxiety disorders in late-life depression: findings from geriatric tertiary outpatient settings. Neuropsychiatr Dis Treat. (2019) 15:199–204. doi: 10.2147/NDT.S184585
15. Invernizzi S, Simoes Loureiro I, Kandana Arachchige K, Lefebvre L. Late-life depression, cognitive impairment, and relationship with Alzheimer’s disease. Dement Geriatr Cogn Disord. (2021) 50:414–24. doi: 10.1159/000519453
16. Joshi P, Duong K, Trevisan L, Wilkins K. Evaluation and Management of alcohol use disorder among older adults. Curr Geriatr Rep. (2021) 10:82–90. doi: 10.1007/s13670-021-00359-5
17. Avasthi A, Grover S. Clinical practice guidelines for management of depression in elderly. Indian J Psychiatry. (2018) 60:S341–62. doi: 10.4103/0019-5545.224474
18. Luppa M, Sikorski C, Motzek T, Konnopka A, Konig H, Riedel-Heller S. Health service utilization and costs of depressive symptoms in late life - a systematic review. Curr Pharm Des. (2012) 18(36):5936–57. doi: 10.2174/138161212803523572
19. Bock J, Hajek A, Weyerer S, Werle J, Wagner M, Maier W, et al. The impact of depressive symptoms on healthcare costs in late life: longitudinal findings from the agemoode study. Am J Geriatr Psychiatry. (2017) 25:131–41. doi: 10.1016/j.jagp.2016.10.011
20. Maier A, Riedel-Heller S, Pabst A, Luppa M. Risk factors and protective factors of depression in older people 65+. a systematic review. PLoS One. (2021) 16:e0251326. doi: 10.1371/journal.pone.0251326
21. Barrenetxea J, Pan A, Feng Q, Koh W. Factors associated with depression across age groups of older adults: the singapore chinese health study. Int J Geriatr Psychiatry. (2022) 37:5666. doi: 10.1002/gps.5666
22. Cheung E, Mui A. Gender variation and late-life depression: findings from a national survey in the United Status. Ageing Int. (2021) 2021:1–18. doi: 10.1007/s12126-021-09471-5
23. Kaji T, Mishima K, Kitamura S, Enomoto M, Nagase Y, Li L, et al. Relationship between late-life depression and life stressors: large-scale cross-sectional study of a representative sample of the Japanese general population. Psychiatry Clin Neurosci. (2010) 64:426–34. doi: 10.1111/j.1440-1819.2010.02097.x
24. Vyas C, Okereke O. Late-life depression: a narrative review on risk factors and prevention. Harv Rev Psychiatry. (2020) 28:72–99. doi: 10.1097/HRP.0000000000000240
25. Fang M, Mirutse G, Guo L, Ma X. Role of socioeconomic status and housing conditions in geriatric depression in rural china: a cross-sectional study. BMJ Open. (2019) 9:e024046. doi: 10.1136/bmjopen-2018-024046
26. Shiba K, Kondo N, Kondo K, Kawachi I. Retirement and mental health: dose social participation mitigate the association? A fixed-effects longitudinal analysis. BMC Public Health. (2017) 17:526. doi: 10.1186/s12889-017-4427-0
27. Tani Y, Fujiwara T, Kondo N, Noma H, Sasaki Y, Kondo K. Childhood socioeconomic status and onset of depression among Japanese older adults: the Jages prospective cohort study. Am J Geriatr Psychiatry. (2016) 24:717–26. doi: 10.1016/j.jagp.2016.06.001
28. Fancourt D, Steptoe A, Bu F. Psychological consequences of long Covid: comparing trajectories of depressive and anxiety symptoms before and after contracting sars-cov-2 between matched long- and short-Covid groups. Br J Psychiatry. (2023) 222:74–81. doi: 10.1192/bjp.2022.155
29. Efstathiou V, Stefanou M, Demetriou M, Siafakas N, Katsantoni E, Makris M, et al. New-onset neuropsychiatric sequelae and “long-covid” syndrome (review). Exp Ther Med. (2022) 24:705. doi: 10.3892/etm.2022.11641
30. Zaninotto P, Iob E, Demakakos P, Steptoe A. Immediate and longer-term changes in the mental health and well-being of older adults in England during the Covid-19 pandemic. JAMA Psychiatry. (2022) 79:151–9. doi: 10.1001/jamapsychiatry.2021.3749
31. Briggs R, McDowell C, De Looze C, Kenny R, Ward M. Depressive Symptoms among older adults pre- and post-covid-19 pandemic. J Am Med Dir Assoc. (2021) 22(11):2251–7. doi: 10.1016/j.jamda.2021.09.003
32. Garcia-Lara R, Suleiman-Martos N, Membrive-Jimenez M, Garcia-Morales V, Quesada-Caballero M, Guisado-Requena I, et al. Prevalence of depression and related factors among patients with chronic disease during the covid-19 pandemic: a systematic review and meta-analysis. Diagnostics. (2022) 12:3094. doi: 10.3390/diagnostics12123094
33. Ciuffreda G, Cabanillas-Barea S, Carrasco-Uribarren A, Albarova-Corral M, Arguello-Espinosa M, Marcen-Roman Y. Factors associated with depression and anxiety in adults >/=60 years old during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. (2021) 18:22. doi: 10.3390/ijerph182211859
34. Belvederi Murri M, Amore M, Respino M, Alexopoulos G. The symptom network structure of depressive symptoms in late-life: results from a european population study. Mol Psychiatry. (2020) 25:1447–56. doi: 10.1038/s41380-018-0232-0
35. Wilkowska-Chmielewska J, Szelenberger W, Wojnar M. Age-dependent symptomatology of depression in hospitalized patients and its implications for dsm-5. J Affect Disord. (2013) 150:142–5. doi: 10.1016/j.jad.2012.12.012
36. Schaakxs R, Comijs H, Lamers F, Beekman A, Penninx B. Age-related variability in the presentation of symptoms of major depressive disorder. Psychol Med. (2017) 47:543–52. doi: 10.1017/S0033291716002579
37. Almeida O, McCaul K, Hankey G, Yeap B, Golledge J, Norman P, et al. Duration of diabetes and its association with depression in later life: the health in men study (hims). Maturitas. (2016) 86:3–9. doi: 10.1016/j.maturitas.2016.01.003
38. Zhang L, Bao Y, Tao S, Zhao Y, Liu M. The association between cardiovascular drugs and depression/anxiety in patients with cardiovascular disease: a meta-analysis. Pharmacol Res. (2022) 175:106024. doi: 10.1016/j.phrs.2021.106024
39. Brender R, Mulsant B, blumberger d. an update on antidepressant pharmacotherapy in late-life depression. Expert Opin Pharmacother. (2021) 22(14):1909–17. doi: 10.1080/14656566.2021.1921736
40. Alexopoulos G. Mechanisms and treatment of late-life depression. Trans Psychiatry. (2019) 9:188. doi: 10.1038/s41398-019-0514-6
41. Richard E, Reitz C, Honig L, Schupf N, Tang M, Manly J, et al. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. (2013) 70:374–82. doi: 10.1001/jamaneurol.2013.603
42. Zheng F, Zhong B, Song X, Xie W. Persistent depressive symptoms and cognitive decline in older adults. Br J Psychiatry. (2018) 213:638–44. doi: 10.1192/bjp.2018.155
43. Edinoff A, Fort J, Woo J, Causey C, Burroughs C, Cornett E, et al. Selective serotonin reuptake inhibitors and clozapine: clinically relevant interactions and considerations. Neurol Int. (2021) 13:445–63. doi: 10.3390/neurolint13030044
44. Smith G, Workman C, Protas H, Su Y, Savonenko A, Kuwabara H, et al. Positron emission tomography imaging of serotonin degeneration and beta-amyloid deposition in late-life depression evaluated with multi-modal partial least squares. Trans Psychiatry. (2021) 11:473. doi: 10.1038/s41398-021-01539-9
45. Meltzer C, Price J, Mathis C, Butters M, Ziolko S, Moses-Kolko E, et al. Serotonin 1a receptor binding and treatment response in late-life depression. Neuropsychopharmacology. (2004) 29:2258–65. doi: 10.1038/sj.npp.1300556
46. Moriya H, Tiger M, Tateno A, Sakayori T, Masuoka T, Kim W, et al. Low dopamine transporter binding in the nucleus accumbens in geriatric patients with severe depression. Psychiatry Clin Neurosci.(2020) 74:424–30. doi: 10.1111/pcn.13020
47. Forbes M, O’Neil A, Lane M, Agustini B, Myles N, Berk M. Major depressive disorder in older patients as an inflammatory disorder: implications for the pharmacological management of geriatric depression. Drugs Aging. (2021) 38:451–67. doi: 10.1007/s40266-021-00858-2
48. Miller A. Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry. (2020) 19:108–9. doi: 10.1002/wps.20723
49. Treadway M, Cooper J, Miller A. Can’t or won’t? Immunometabolic constraints on dopaminergic drive. Trends Cogn Sci. (2019) 23:435–48. doi: 10.1016/j.tics.2019.03.003
50. Kim J, Stewart R, Kim J, Kang H, Bae K, Kim S, et al. Changes in pro-inflammatory cytokine levels and late-life depression: a two year population based longitudinal study. Psychoneuroendocrinology. (2018) 90:85–91. doi: 10.1016/j.psyneuen.2018.02.006
51. Charlton R, Lamar M, Zhang A, Ren X, Ajilore O, Pandey G, et al. Associations between pro-inflammatory cytokines, learning, and memory in late-life depression and healthy aging. Int J Geriatr Psychiatry. (2018) 33:104–12. doi: 10.1002/gps.4686
52. Straka K, Tran M, Millwood S, Swanson J, Kuhlman K. Aging as a context for the role of inflammation in depressive symptoms. Front Psychiatry. (2020) 11:605347. doi: 10.3389/fpsyt.2020.605347
53. Hashimoto K, Bruno D, Nierenberg J, Marmar C, Zetterberg H, Blennow K, et al. Abnormality in glutamine-glutamate cycle in the cerebrospinal fluid of cognitively intact elderly individuals with major depressive disorder: a 3-year follow-up study. Trans Psychiatry. (2016) 6:e744. doi: 10.1038/tp.2016.8
54. Kuo C, Lin C, Lane H. Molecular basis of late-life depression. Int J Mol Sci. (2021) 22:14. doi: 10.3390/ijms22147421
55. Niu M, Yang X, Li Y, Sun Y, Wang L, Ha J, et al. Progresses in Glun2a-containing nmda receptors and their selective regulators. Cell Mol Neurobiol. (2022) 2022:1185. doi: 10.1007/s10571-021-01185-1
56. Lijffijt M, Murphy N, Iqbal S, Green C, Iqbal T, Chang L, et al. Identification of an optimal dose of intravenous ketamine for late-life treatment-resistant depression: a bayesian adaptive randomization trial. Neuropsychopharmacology. (2022) 47:1088–95. doi: 10.1038/s41386-021-01242-9
57. Dimitriadis M, van den Brink R, Comijs H, Oude Voshaar R. Prognostic effect of serum bdnf levels in late-life depression: moderated by childhood trauma and ssri usage? Psychoneuroendocrinology. (2019) 103:276–83. doi: 10.1016/j.psyneuen.2019.02.003
58. Tsang R, Mather K, Sachdev P, Reppermund S. Systematic review and meta-analysis of genetic studies of late-life depression. Neurosci Biobehav Rev. (2017) 75:129–39. doi: 10.1016/j.neubiorev.2017.01.028
59. Januar V, Ancelin M, Ritchie K, Saffery R, Ryan J. Bdnf promoter methylation and genetic variation in late-life depression. Trans Psychiatry. (2015) 5:e619. doi: 10.1038/tp.2015.114
60. Kang H, Kim J, Bae K, Kim S, Shin I, Kim H, et al. Longitudinal Associations between bdnf promoter methylation and late-life depression. Neurobiol Aging. (2015) 36:e1–7. doi: 10.1016/j.neurobiolaging.2014.12.035
61. Erickson K, Miller D, Roecklein K. The aging hippocampus: interactions between exercise, depression, and bdnf. Neuroscientist. (2012) 18:82–97. doi: 10.1177/1073858410397054
62. Menke A. Is the hpa axis as target for depression outdated, or is there a new hope? Front Psychiatry. (2019) 10:101. doi: 10.3389/fpsyt.2019.00101
63. Humphreys K, Moore S, Davis E, MacIsaac J, Lin D, Kobor M, et al. DNA methylation of hpa-axis genes and the onset of major depressive disorder in adolescent girls: a prospective analysis. Trans Psychiatry. (2019) 9:245. doi: 10.1038/s41398-019-0582-7
64. Rhebergen D, Korten N, Penninx B, Stek M, van der Mast R, Oude Voshaar R, et al. Hypothalamic-pituitary-adrenal axis activity in older persons with and without a depressive disorder. Psychoneuroendocrinology. (2015) 51:341–50. doi: 10.1016/j.psyneuen.2014.10.005
65. Mahgoub N, Alexopoulos G. Amyloid hypothesis: is there a role for antiamyloid treatment in late-life depression? Am J Geriatr Psychiatry. (2016) 24:239–47. doi: 10.1016/j.jagp.2015.12.003
66. Donovan N, Locascio J, Marshall G, Gatchel J, Hanseeuw B, Rentz D, et al. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry. (2018) 175:530–7. doi: 10.1176/appi.ajp.2017.17040442
67. Yasuno F, Kazui H, Morita N, Kajimoto K, Ihara M, Taguchi A, et al. High amyloid-beta deposition related to depressive symptoms in older individuals with normal cognition: a pilot study. Int J Geriatr Psychiatry. (2016) 31:920–8. doi: 10.1002/gps.4409
68. Chung J, Plitman E, Nakajima S, Chow T, Chakravarty M, Caravaggio F, et al. Lifetime history of depression predicts increased amyloid-beta accumulation in patients with mild cognitive impairment. J Alzheimers Dis. (2016) 49:1189–90. doi: 10.3233/JAD-159007
69. Shiels P, Buchanan S, Selman C, Stenvinkel P. Allostatic load and ageing: linking the microbiome and nutrition with age-related health. Biochem Soc Trans. (2019) 47:1165–72. doi: 10.1042/BST20190110
70. Dinan T, Cryan J. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. (2017) 595:489–503. doi: 10.1113/JP273106
71. Lee S, Milillo M, Krause-Sorio B, Siddarth P, Kilpatrick L, Narr K, et al. Gut microbiome diversity and abundance correlate with gray matter volume (gmv) in older adults with depression. Int J Environ Res Public Health. (2022) 19:405. doi: 10.3390/ijerph19042405
72. Kelly J, Borre Y, Brien C, Patterson E, Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
73. Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. (2016) 21:786–96. doi: 10.1038/mp.2016.44
74. Lee S, Dong T, Krause-Sorio B, Siddarth P, Milillo M, Lagishetty V, et al. The intestinal microbiota as a predictor for antidepressant treatment outcome in geriatric depression: a prospective pilot study. Int Psychogeriatr. (2022) 34:33–45. doi: 10.1017/S1041610221000120
75. Kim C, Cha L, Sim M, Jung S, Chun W, Baik H, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. (2021) 76:32–40. doi: 10.1093/gerona/glaa090
76. Gutsmiedl K, Krause M, Bighelli I, Schneider-Thoma J, Leucht S. How well do elderly patients with major depressive disorder respond to antidepressants: a systematic review and single-group meta-analysis. BMC Psychiatry. (2020) 20:102. doi: 10.1186/s12888-020-02514-2
77. Beyer J, Johnson K. Advances in pharmacotherapy of late-life depression. Curr Psychiatry Rep. (2018) 20:34. doi: 10.1007/s11920-018-0899-6
78. Borges S, Chen Y, Laughren T, Temple R, Patel H, David P, et al. Review of maintenance trials for major depressive disorder: a 25-year perspective from the us food and drug administration. J Clin Psychiatry. (2014) 75:205–14. doi: 10.4088/JCP.13r08722
79. Hung S, Lin C, Hung H, Lin C, Lai S. Use of selective serotonin reuptake inhibitors and risk of hip fracture in the elderly: a case-control study in taiwan. J Am Med Dir Assoc. (2017) 18:350–4. doi: 10.1016/j.jamda.2016.12.003
80. Bruun S, Petersen I, Kristensen N, Cronin-Fenton D, Pedersen A. Selective serotonin reuptake inhibitor use in hip fracture patients: a danish nationwide prevalence study. Acta Orthop. (2019) 90:33–9. doi: 10.1080/17453674.2018.1543842
81. Manning K, Alexopoulos G, Banerjee S, Morimoto S, Seirup J, Klimstra S, et al. Executive functioning complaints and escitalopram treatment response in late-life depression. Am J Geriatr Psychiatry. (2015) 23:440–5. doi: 10.1016/j.jagp.2013.11.005
82. Aguera-Ortiz L, Claver-Martin M, Franco-Fernandez M, Lopez-Alvarez J, Martin-Carrasco M, Ramos-Garcia M, et al. Depression in the elderly. consensus statement of the spanish psychogeriatric association. Front Psychiatry (2020) 11:380. doi: 10.3389/fpsyt.2020.00380
83. Lenze E, Mulsant B, Blumberger D, Karp J, Newcomer J, Anderson S, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet (2015) 386:2404–12. doi: 10.1016/S0140-6736(15)00308-6
84. Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli L, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. (2015) 172:561–9. doi: 10.1176/appi.ajp.2014.14070889
85. Kok R, Vink D, Heeren T, Nolen W. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. (2007) 68:1177–85. doi: 10.4088/jcp.v68n0803
86. Navarro V, Boulahfa I, Obach A, Jerez D, Diaz-Ricart M, Gasto C, et al. Lithium augmentation versus citalopram combination in imipramine-resistant major depression: a 10-week randomized open-label study. J Clin Psychopharmacol. (2019) 39:254–7. doi: 10.1097/JCP.0000000000001024
87. Lavretsky H, Laird K, Krause-Sorio B, Heimberg B, Yeargin J, Grzenda A, et al. A randomized double-blind placebo-controlled trial of combined escitalopram and memantine for older adults with major depression and subjective memory complaints. Am J Geriatr Psychiatry. (2020) 28:178–90. doi: 10.1016/j.jagp.2019.08.011
88. Ochs-Ross R, Daly E, Zhang Y, Lane R, Lim P, Morrison R, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-transform-3. Am J Geriatr Psychiatry. (2020) 28:121–41. doi: 10.1016/j.jagp.2019.10.008
89. Lin C, Wang S, Lane H. Effects of sodium benzoate, a d-amino acid oxidase inhibitor, on perceived stress and cognitive function among patients with late-life depression: a randomized, double-blind, sertraline- and placebo-controlled trial. Int J Neuropsychopharmacol. (2022) 2022:6. doi: 10.1093/ijnp/pyac006
90. Elias G, Boutet A, Parmar R, Wong E, Germann J, Loh A, et al. Neuromodulatory treatments for psychiatric disease: a comprehensive survey of the clinical trial landscape. Brain Stimul. (2021) 14:1393–403. doi: 10.1016/j.brs.2021.08.021
91. Cristancho P, Kamel L, Araque M, Berger J, Blumberger D, Miller J, et al. Itbs to relieve depression and executive dysfunction in older adults: an open label study. Am J Geriatr Psychiatry. (2020) 28(11):1195–9. doi: 10.1016/j.jagp.2020.03.001
92. Dominiak M, Antosik-Wojcinska A, Wojnar M, Mierzejewski P. Electroconvulsive therapy and age: effectiveness, safety and tolerability in the treatment of major depression among patients under and over 65 years of age. Pharmaceuticals. (2021) 14:582. doi: 10.3390/ph14060582
93. Obbels J, Vansteelandt K, Verwijk E, Dols A, Bouckaert F, Oudega M, et al. Mmse changes during and after ect in late-life depression: a prospective study. Am J Geriatr Psychiatry. (2019) 27:934–44. doi: 10.1016/j.jagp.2019.04.006
94. Wu M, Sung H, Lee W, Smith G. The effects of light therapy on depression and sleep disruption in older adults in a long-term care facility. Int J Nurs Pract. (2015) 21:653–9. doi: 10.1111/ijn.12307
95. Galvez V, Ho K, Alonzo A, Martin D, George D, Loo C. Neuromodulation therapies for geriatric depression. Curr Psychiatry Rep. (2015) 17:59. doi: 10.1007/s11920-015-0592-y
96. Rhebergen D, Huisman A, Bouckaert F, Kho K, Kok R, Sienaert P, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. (2015) 23:274–82. doi: 10.1016/j.jagp.2014.05.002
97. Dybedal G, Tanum L, Sundet K, Gaarden T, Bjolseth T. Cognitive side-effects of electroconvulsive therapy in elderly depressed patients. Clin Neuropsychol. (2014) 28:1071–90. doi: 10.1080/13854046.2014.958536
98. Lapid M, Seiner S, Heintz H, Hermida A, Nykamp L, Sanghani S, et al. Electroconvulsive therapy practice changes in older individuals due to covid-19: expert consensus statement. Am J Geriatr Psychiatry. (2020) 28(11):1133–45. doi: 10.1016/j.jagp.2020.08.001
99. Cappon D, den Boer T, Jordan C, Yu W, Metzger E, Pascual-Leone A. Transcranial magnetic stimulation (tms) for geriatric depression. Ageing Res Rev. (2022) 74:101531. doi: 10.1016/j.arr.2021.101531
100. Konstantinou G, Downar J, Daskalakis Z, Blumberger D. Accelerated intermittent theta burst stimulation in late-life depression: a possible option for older depressed adults in need of ect during the covid-19 pandemic. Am J Geriatr Psychiatry. (2020) 28(10):1025–9. doi: 10.1016/j.jagp.2020.07.007
101. Hodzic-Santor B, Meltzer J, Verhoeff N, Blumberger D, Mah L. Intermittent theta burst stimulation using the h1-coil for treatment of late-life depression with comorbid mild cognitive impairment. Am J Geriatr Psychiatry. (2021) 29:409–10. doi: 10.1016/j.jagp.2020.08.016
102. Kaster T, Daskalakis Z, Noda Y, Knyahnytska Y, Downar J, Rajji T, et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology. (2018) 43(11):2231–8. doi: 10.1038/s41386-018-0121-x
103. Kim H, Lee S, Lee S, Hong S, Kang H, Kim N. Depression prediction by using ecological momentary assessment, actiwatch data, and machine learning: observational study on older adults living alone. JMIR Mhealth Uhealth (2019) 7(10):e14149. doi: 10.2196/14149
104. Cuijpers P, Karyotaki E, Eckshtain D, Ng M, Corteselli K, Noma H, et al. Psychotherapy for depression across different age groups: a systematic review and meta-analysis. JAMA Psychiatry. (2020) 77:694–702. doi: 10.1001/jamapsychiatry.2020.0164
105. Taylor R, Bodoukhin N, Botros M, Luca L. Joy journal: a behavioral activation technique used in the treatment of late-life depression associated with hopelessness during the covid-19 pandemic. Prim Care Companion CNS Disord. (2021) 23:2817. doi: 10.4088/PCC.20l02817
106. Farioli Vecchioli S, Sacchetti S, Nicolis di Robilant V, Cutuli D. The role of physical exercise and omega-3 fatty acids in depressive illness in the elderly. Curr Neuropharmacol. (2018) 16:308–26. doi: 10.2174/1570159X15666170912113852
107. Kim Y, Cho S. Regular exercise and depressive symptoms in korean older adults. Int J Environ Res Public Health. (2021) 18:10303. doi: 10.3390/ijerph18010303
108. Badakhsh M, Dastras M, Sarchahi Z, Doostkami M, Mir A, Bouya S. Complementary and alternative medicine therapies and covid-19: a systematic review. Rev Environ Health. (2021) 36:443–50. doi: 10.1515/reveh-2021-0012
109. Solianik R, Mickeviciene D, Zlibinaite L, Cekanauskaite A. Tai chi improves psychoemotional state, cognition, and motor learning in older adults during the covid-19 pandemic. Exp Gerontol. (2021) 150:111363. doi: 10.1016/j.exger.2021.111363
110. Ionson E, Limbachia J, Rej S, Puka K, Newman R, Wetmore S, et al. Effects of sahaj samadhi meditation on heart rate variability and depressive symptoms in patients with late-life depression. Br J Psychiatry. (2019) 214:218–24. doi: 10.1192/bjp.2018.265
111. Da X, Yue L, Li X, Chen J, Yuan N, Chen J. Potential therapeutic effect and methods of traditional chinese medicine on covid-19-induced depression: a review. Anat Rec. (2021) 304(11):2566–78. doi: 10.1002/ar.24758
112. Yang N, Lin L, Li Y, Li H, Cao Y, Tan C, et al. Potential mechanisms and clinical effectiveness of acupuncture in depression. Curr Neuropharmacol. (2022) 20:738–50. doi: 10.2174/1570159X19666210609162809
113. Feng J, Wang W, Zhong Y, Xing C, Guo T. Acupuncture for perimenopausal depressive disorder: a systematic review and meta-analysis protocol. Medicine (2019) 98:e14574. doi: 10.1097/MD.0000000000014574
114. Park S, Chang Y, Wolfe R, Kim I. Prevention of loss of muscle mass and function in older adults during covid-19 lockdown: potential role of dietary essential amino acids. Int J Environ Res Public Health. (2022) 19:8090. doi: 10.3390/ijerph19138090
115. Solis-Ortiz S, Arriaga-Avila V, Trejo-Bahena A, Guevara-Guzman R. Deficiency in the essential amino acids l-isoleucine, l-leucine and l-histidine and clinical measures as predictors of moderate depression in elderly women: a discriminant analysis study. Nutrients. (2021) 13:11. doi: 10.3390/nu13113875
116. Skarupski K, Tangney C, Li H, Evans D, Morris M. Mediterranean diet and depressive symptoms among older adults over time. J Nutr Health Aging (2013) 17:441–5. doi: 10.1007/s12603-012-0437-x
117. Moya C, Soares F, Lima R, de Barros M, Bezerra J. Depressive symptoms in older adults: the role of physical activity and social support. Trends Psychiatry Psychother. (2021) 2020:93. doi: 10.47626/2237-6089-2020-0093
118. Brenes G, Danhauer S, Lyles M, Hogan P, Miller M. Telephone-delivered cognitive behavioral therapy and telephone-delivered nondirective supportive therapy for rural older adults with generalized anxiety disorder: a randomized clinical trial. JAMA Psychiatry (2015) 72(10):1012–20. doi: 10.1001/jamapsychiatry.2015.1154
119. Chen A, Slattery K, Tomasino K, Rubanovich C, Bardsley L, Mohr D. Challenges and benefits of an internet-based intervention with a peer support component for older adults with depression: qualitative analysis of textual data. J Med Internet Res. (2020) 22:e17586. doi: 10.2196/17586
120. Gould C, Carlson C, Ma F, Forman-Hoffman V, Ranta K, Kuhn E. Effects of mobile app-based intervention for depression in middle-aged and older adults: mixed methods feasibility study. JMIR Form Res. (2021) 5:e25808. doi: 10.2196/25808
121. Titov N, Dear B, Ali S, Zou J, Lorian C, Johnston L, et al. Clinical and cost-effectiveness of therapist-guided internet-delivered cognitive behavior therapy for older adults with symptoms of depression: a randomized controlled trial. Behav Ther. (2015) 46:193–205. doi: 10.1016/j.beth.2014.09.008
122. Xiang X, Kayser J, Sun Y, Himle J. Internet-based psychotherapy intervention for depression among older adults receiving home care: qualitative study of participants’. Exp JMIR Aging. (2021) 4:e27630. doi: 10.2196/27630
123. Chen S, Jones C, Moyle W. Social robots for depression in older adults: a systematic review. J Nurs Scholarsh. (2018) 50:612–22. doi: 10.1111/jnu.12423
Keywords: late-life depression (LLD), epidemiology, phenotype, pathogenesis, treatment, COVID-19 pandemic
Citation: Zhao Y, Wu X, Tang M, Shi L, Gong S, Mei X, Zhao Z, He J, Huang L and Cui W (2023) Late-life depression: Epidemiology, phenotype, pathogenesis and treatment before and during the COVID-19 pandemic. Front. Psychiatry 14:1017203. doi: 10.3389/fpsyt.2023.1017203
Received: 11 August 2022; Accepted: 15 March 2023;
Published: 06 April 2023.
Edited by:
Manoj Prithviraj, All India Institute of Medical Sciences Gorakhpur, IndiaReviewed by:
Helen Lavretsky, David Geffen School of Medicine, The University of California, Los Angeles, United StatesCopyright © 2023 Zhao, Wu, Tang, Shi, Gong, Mei, Zhao, He, Huang and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Cui, Y3Vpd2VpQG5idS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.