
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 14 February 2023
Sec. Public Mental Health
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1014766
Purpose: To evaluate the psychometric properties of a 9-item Concise Health Risk Tracking Self-Report (or CHRT-SR9) to assess suicidal risk in adult primary care outpatients.
Methods: Overall, 369 adults completed the original 14-item version of CHRT-SR at baseline and within 4 months thereafter, from which the CHRT-SR9 was extracted using multigroup confirmatory factor analysis. Measurement invariance (across age and sex) and classical test theory characteristics of the CHRT-SR9 were evaluated. Concurrent validity was assessed by comparing CHRT-SR9 responses to those of the suicide item in the Patient Health Questionnaire (PHQ-9), both cross-sectionally and as a change measure over time.
Results: Confirmatory factor analysis identified the CHRT-SR9 as the optimal solution. Factors included pessimism, helplessness, despair (2 items each) and suicidal thoughts (3 items). Measurement invariance held across sex and age groups, indicating that mean differences among sub-groups were real and not attributable to measurement bias. Classical test theory revealed acceptable item-total correlations overall (0.57–0.79) and internal consistency (Spearman–Brown from 0.76 to 0.90). Concurrent validity analyses revealed that the CHRT-SR9 can measure both improvement and worsening of suicidality over time. A PHQ-9 response of 0, 1, 2, and 3 on the suicide item corresponded to 7.82 (5.53), 16.80 (4.99), 20.71 (5.36), and 25.95 (7.30) (mean and SD) on CHRT-SR9 total score, respectively.
Conclusion: The CHRT-SR9 is a brief self-report evaluating suicidality with excellent psychometric properties that is sensitive to change over time.
Suicide is a significant public health crisis in the United States, with one of the highest rates of suicide among wealthy countries (1). In the US, the annual suicide rate increased 30% between 2000 and 2020, from 10.4 suicides per 100,000 to 13.5/100,000 (2).
A large longitudinal study found that 83% of persons who died by suicide received healthcare services in the year before their death, and 50% received them within the prior month (3). These findings prompted regulatory agencies and healthcare organizations to develop guidelines for physicians to routinely screen patients for depressive symptoms. Screening for risk of suicide, however, was reserved only for those who screened positive for depression or substance abuse (4). There is much debate about whether to extend suicide risk screening to all patients in the primary care setting. Some have pointed out that suicidality can occur even in the absence of major risk factors like depression (5). Sentinel event alert 56 (2016) recommended that physicians in primary care setting screen all patients for suicidal ideation (6). They advised using a brief, standardized, evidence-based screening tool.
There exist a variety of measurement tools that study behaviors related to suicide risk (7). These rating scales typically include the categories of assessment measures such as suicidal ideation and behavior, lethality of suicide attempts, reasoning mechanisms of suicide attempters, etc. This report evaluates a shortened version of the 14-item Concise Health Risk Tracking Self Report (CHRT-SR) (8). The original version included constructs such as pessimism, helplessness, social support, despair, impulsivity, and suicidal thoughts, measured on 5-point likert scales from “0: Strongly Disagree” to “4: Strongly Agree.” Recent work in a representative sample of adolescent outpatients revealed that a 9-item scale was psychometrically the best and most preferred of the various versions (9). These 9 items include items measuring pessimism (items 1 and 2), helplessness (items 3 and 4), despair (items 8 and 9), and current suicidal thought and plans (items 12, 13, and 14) from the 14-item CHRT-SR (Figure 1). This report extends work on the 9-item version of the CHRT-SR (or CHRT-SR9) to evaluate its performance with adult outpatients in primary care. Establishing that the CHRT-SR9 performs satisfactorily in this adult primary care setting would be a significant step in developing evidence of the scale’s reliability and sensitivity to change across a wide age range seen in various settings.
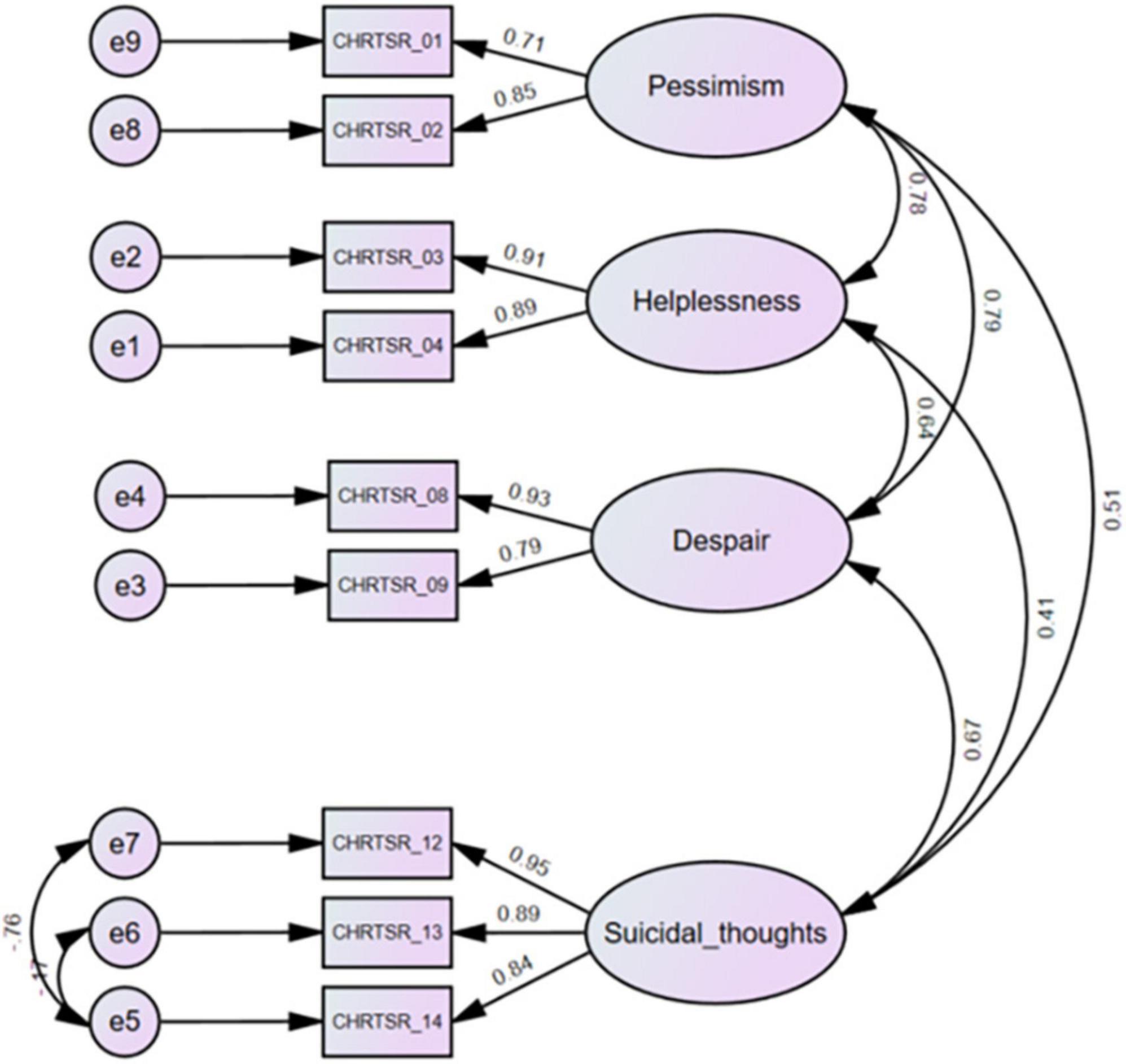
Figure 1. Confirmatory factor analysis of the CHRT-SR9 scale at the first visit (n = 369). CHRTSR_01: I feel as if things are never going to get better; CHRTSR_02: I have no future; CHRTSR_03: It seems as if I can do nothing right; CHRTSR_04: Everything I do turns out wrong; CHRTSR_08: I feel that there is no reason to live; CHRTSR_09: I wish I could just go to sleep and not wake up; CHRTSR_12: I have been having thoughts of killing myself; CHRTSR_13: I have thoughts about how I might kill myself; CHRTSR_14: I have a plan to kill myself.
This report aimed to:
(1) Conduct confirmatory factor analysis of CHRT-SR9 in a representative sample of adults,
(2) Assess the measurement invariance of CHRT-SR9 by sex and age-groups,
(3) Assess the classical test theory (CTT) psychometrics of CHRT-SR9,
(4) Assess its performance against the suicide item of another independent scale (the major depressive disorder module of the Patient Health Questionnaire, PHQ-9) both cross-sectional and as a measure of change over time.
Data used in this report came from a joint quality improvement project of UT Southwestern Medical Center and primary care and specialty care clinics designed to facilitate and enhance access to evidence-based screening and treatment of depression (10). The initiative mandates that participating clinics conduct yearly depression screenings on every patient. Patients fill out a condensed, two-item version of the Patient Health Questionnaire (PHQ-2) on the first screen of the iPad application. If a patient’s PHQ-2 result is positive, indicative of depression, then the application will automatically administer other tests, such as the complete PHQ-9. The application shows these findings to healthcare professionals and helps them choose a diagnosis and a course of treatment. Data collection began in 2014 and is still ongoing. The UT Southwestern Medical Center Institutional Review Board approved this study with a waiver of the need to obtain informed consent from individual patients.
The project included a depression screening using the first 2 items of the PHQ-9. Patients who screened positive were given the full PHQ-9, the 14-item CHRT-SR, and a variety of other ratings scales measuring factors associated with depression (10). For this report, we focused on adults (≥18 years of age) who completed the CHRT-SR on two successive clinic visits, second visit being within 4 months of the first to approximate the time for a treatment trial and to minimize time differences between the two visits. Our analyzable sample included 369 adult patients in mostly primary care clinics (18 primary care and two specialty, cancer and obstetrics and gynecology).
Demographic factors included sex (male/female), race (White, Black, Other) and age, based on self-report.
Concise Health Risk Tracking Scale Self-Report (CHRT-SR14): The 14-item CHRT-SR was designed to assess psychosocial and behavioral factors associated with increased risk of suicidal thoughts and behaviors. The items were designed as self-referent statements which respondents rated on 5-point scales starting at strongly disagree (0) to strongly agree (4), with higher scores indicative of greater severity of suicidality.
PHQ-9 is a nine-item, self-report inventory including all nine criterion symptoms that define a major depressive episode (11). Item 9 (“Thoughts that you would be better off dead, or of hurting yourself”) relates to suicidality/ideation. Each item is rated on a 4-point scale starting with “Not at all” (0) to “Nearly every day” (3), with higher scores indicating greater depression severity. Studies comprising eight primary care and seven obstetrical clinics demonstrated the diagnostic validity of the nine-item PHQ-9. Major Depressive Disorder was detected with 88% sensitivity and 88% specificity using PHQ-9 values greater than 10. The tool’s reliability and validity have shown that it possesses excellent psychometric features (11).
Confirmatory Factor Analysis (CFA) was conducted using AMOS 28 (12) to determine whether the 9-item CHRT-SR9 fits well to the CHRT-SR data in adults. We used maximum likelihood estimation to estimate model parameters and standard errors. Model-fit indices such as chi-square test, Comparative Fit Index (CFI), Tucker Lewis index (TLI), and Root-Mean-Square Error of Approximation (RMSEA) were investigated to assess model fit. Good fit thresholds for these indices are CFI > 0.95, TLI > 0.95, and RMSEA < 0.05 (13). Bollen–Stine bootstrap p was used as an indicator of model fit, since it operates without normality assumptions and p > 0.05 indicates excellent fit (14).
To assess whether CHRT-SR9 measured the same constructs across all respondents and demographic sub-groups, we evaluated measurement invariance by sex (male and female) and age-groups (young: 18–35, middle aged: 36–55, and older: >55 years). Measurement invariance can be categorized into three hierarchical levels, namely, configural (where the factor structure is the same across groups), metric (where factor loadings are similar across groups) and scalar (where values/means are also equivalent across groups) (15). We first tested whether the CFA fit for each sub-group separately (16). Thereafter, we tested for evidence of configural, metric and scalar invariance.
We calculated the Spearman–Brown coefficient to examine the internal consistency of the CHRT-SR9 using data from both the first and second visits. While Cronbach’s alpha is a popular measure of internal consistency, for a two-item scale, it usually underestimates the true reliability. On the other hand, the Spearman–Brown coefficient is less biased on average, especially if the correlation between the items is relatively strong (17).
We assessed its sensitivity to change over time by testing whether total and subscale means were different between first and second visits using paired sample t-tests.
We assessed its performance against Item 9 (the suicidal item) of the PHQ-9 as an anchor by testing whether means for each item, total score, and all subscale scores varied across response levels (0–3) to Item 9 of the PHQ-9 at the first visit.
Finally, we assessed whether CHRT-SR9 changed over time when individuals experienced a change in suicidality by looking at the mean change in response for each CHRT-SR9 item (as well as mean of total and subscale scores) against change in the PHQ-9 Item 9 over time (between second and first visits).
Table 1 summarizes the sample characteristics. The majority were Whites (68.42%) and female (74.53%). The mean total score was 15.69 ± 8.14 for the CHRT-SR9 and 15.65 ± 6.77 for the PHQ-9.
We fit CHRT-SR9 to the data (Figure 1). The Bollen–Stine bootstrap p = 0.16 indicated good model fit. Model fit statistics such as CMIN/DF = 1.54, CFI = 0.995, TLI = 0.991, and RMSEA = 0.038 also indicated excellent model fit to the data. Means and SDs for the subfactor and total scores can be found for the overall sample at first and second visits in Tables 1, 2.
We checked model fit for males and females (Supplementary Figure 1); both fit well on all metrics. Configural invariance (CMIN/DF = 1.44, CFI = 0.992, TLI = 0.985, and RMSEA = 0.035), metric invariance ( p-value = 0.29) and scalar invariance ( p-value = 0.30) were upheld, suggesting that full scalar invariance held.
Model fit for the three age-groups: Young, middle aged, and older (Supplementary Figure 1) was excellent. Configural invariance (CMIN/DF = 1.36, CFI = 0.99, TLI = 0.98, and RMSEA = 0.03), metric invariance ( p-value = 0.51) and scalar invariance ( p-value = 0.08) were upheld.
The Spearman–Brown coefficient was calculated as a measure of internal consistency for each subscale and the total score. These reliability coefficients indicated excellent reliability for the total score and all subfactors at both first (0.76–0.90) and second visit (0.81–0.92) (Table 2). Additionally, we calculated the corrected item-total correlation for each item at the first visit and these varied between 0.57 and 0.79 (Table 3).
In total, 369 adults completed both first and second visit CHRT-SR9 measurements. Although the length of time between visits varied by individual, all second visits occurred within 4 months of the first (mean time to second visit = 40.7 days, median = 30 days, max = 120 days). Table 2 shows that CHRT-SR9 scores were sensitive to change following the first visit, with the average subfactor and total scores decreasing significantly by the second visit.
The cross-sectional and change analyses allowed us to estimate the relative current risk, as measured by the CHRT-SR9, against the single suicide item in PHQ-9. All participants completed the PHQ-9 questionnaires at both visits. At the first visit, 31.3% (n = 115) adults indicated “Not at all” to Item 9, 39.0% (n = 143) “several days,” 18.8% (n = 69) “More than half the days,” and 10.9% (n = 40) “Nearly every day.” Table 4 shows the means/SDs for each item, as well as for the subscale scores, across the response categories of Item 9. The CHRT-SR9 means were significantly higher with greater levels of suicidality as reflected in responses to Item 9. Taking the total score in CHRT-SR9 as the overall measure of suicidality and comparing it to the levels of Item 9, we found that no risk (0 on Item 9) corresponded to a mean total score on CHRT-SR9 of 7.82 (SD = 5.53), mild (1 on Item 9) was 16.80 (SD = 4.99) on CHRT-SR9, moderate was 20.71 (SD = 5.36) and severe was 25.95 (SD = 7.30).
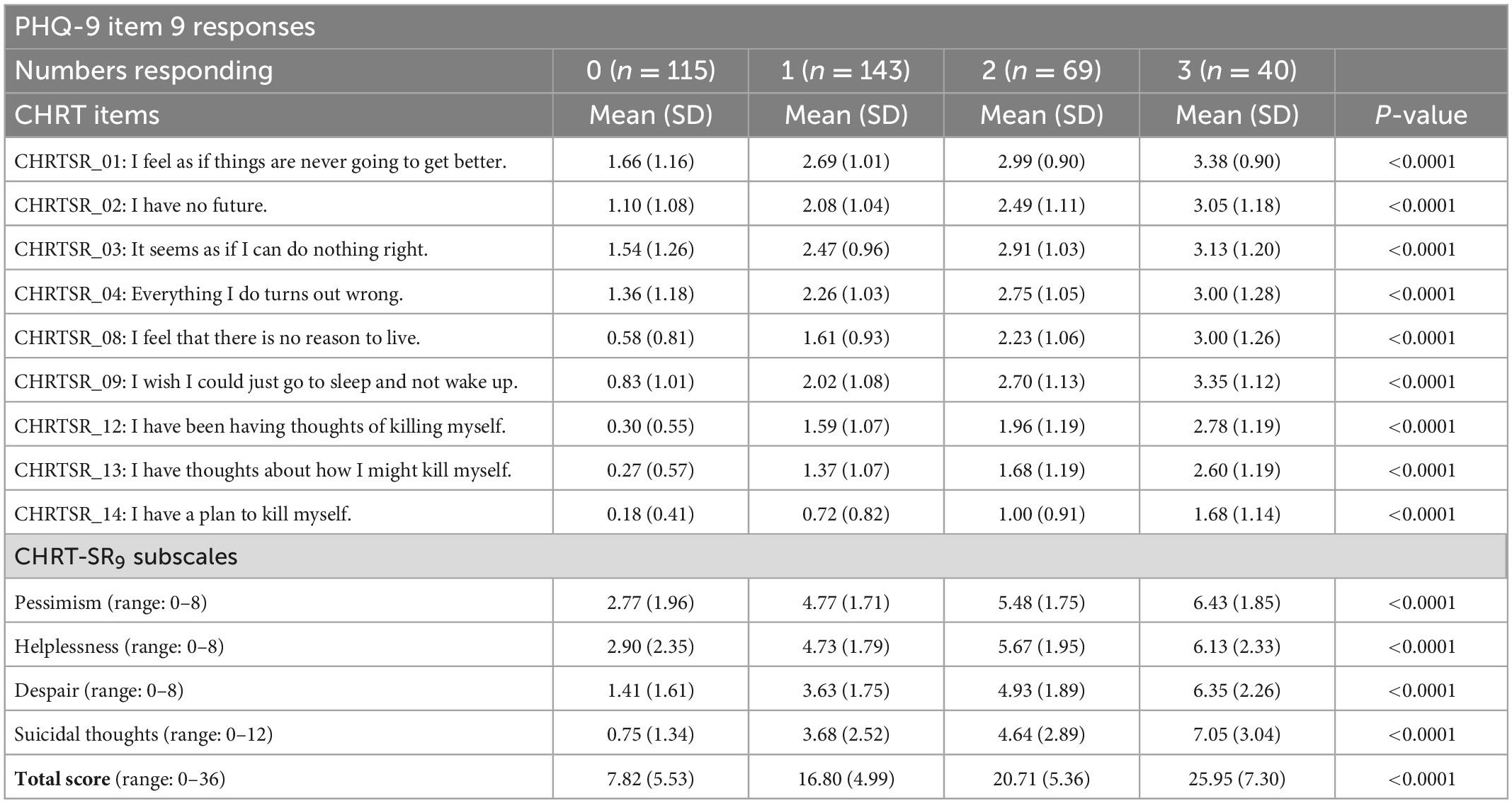
Table 4. Means of CHRT item/subscale scores for each response to the PHQ-9 suicide item at 1st visit.
A meaningful change in risk can be estimated by the degree of change in PHQ-9 Item 9 responses. Table 5 lists the mean change in each item, as well as total and subscale scores of the CHRT-SR9, against change in Item 9, from the first visit to the second visit. Lower change scores implied improvement at second visit compared to first visit while higher values implied worsening. As the PHQ-9 Item 9 progressed from improvement to worsening, CHRT-SR9 items moved similarly from improvement to worsening. For example, on average, (i) a 3-point improvement in Item 9 (from 3 at first visit to 0 at second visit) corresponded to 13 points (SD = 7.48) drop in CHRT-SR9 total score, (ii) a 2-point improvement in Item 9 (e.g., 3–1 or 2–0) corresponded to 7.23 points (SD = 8.16) drop in CHRT-SR9 total score, etc. (Table 5).
In a large sample of adults seen in primary care practices, the brief, 9-item version of the CHRT-SR was identified and evaluated. These nine known items were identical to those identified by similar methods in a representative sample of adolescent outpatients (9). In addition, the performance of the total scale and the subscales were highly comparable to results found in the adolescent population.
The four factors or subscales (pessimism, helplessness, despair, and suicidal thoughts) have clinical face validity, as each construct has been associated with suicidal risk in many studies over the years. For example, pessimism (“I feel as if things are never going to get better” and “I have no future”) is well known to be associated with suicidal risk (18). The helplessness subscale includes responses to “It seems as if I can do nothing right” and “Everything I do turns out wrong,” which certainly reflects a sense of inefficacy, which also is associated with suicidal risk (19). The third subscale we call despair, as “I feel that there is no reason to live” and “I wish I could just go to sleep and not wake up” reflects a resignation to fate and a sense that struggling does not matter, which also is often found in suicidal notes (19). Finally, the 3-item suicidal thinking/planning subscale (“I have been having thoughts of killing myself;” “I have thoughts about how I might kill myself;” “I have a plan to kill myself”) would be expected to relate to the propensity to end things. In addition, the three 2-item subscales on the CHRT-SR9 (pessimism, helplessness, and despair) are distinct and have clinical face validity.
Of note, is the lower corrected item-total correlation for item 14 (“I have a plan to kill myself”). This is likely because this is a general population not seeking care for suicidal thoughts, and not restricted to depressed patients (although there were depressed adults included in the sample). Thus, the prevalence of patients with a suicide plan would be low and thereby reduce the item-total correlation.
Establishment of measurement invariance by gender and age groups indicated that mean differences among sub-groups were real and not attributable to measurement bias, making comparisons of CHRT-SR9 total and subscale scores between males and females or among different age groups valid. A granular look at suicidality by gender and age, through the lens of CHRT-SR9, is of significant interest and deserves further study.
Overall, the CTT and the concurrent validity analyses revealed a coherent, internally consistent instrument that can detect both the improvement and worsening over time in this population. The cross-sectional and change analyses allow us to estimate the relative current risk as measured by the CHRT-SR9 against the single PHQ-9 suicide Item 9. It gives us estimates of CHRT-SR9 total scores that correspond to no, mild, moderate, and severe risk on Item 9. It also gives us estimates of changes in CHRT-SR9 total score corresponding to changes in Item 9 score between visits.
Prior reports on the psychometric properties of the CHRT-SR have largely been in adult samples acquired in the conduct of clinical trials, with the exception of our report in adolescents who were enrolled in a tertiary care suicide prevention program (9). There have been attempts to explore a variety of versions of the CHRT-SR to facilitate implementation in practice and research studies, with different versions allowing for assessment of specific symptoms (i.e., impulsivity and irritability), as well as shortening of the scale. These versions include the 16, 14, 12, and the 7-item CHRT-SR (8, 20–27). These versions of the CHRT-SR have been found to have acceptable psychometric properties. It would, therefore, not be surprising that a limited number (9) of items in adults have similar desirable properties.
A briefer tool with acceptable psychometric features, which is demonstrated to be applicable to both adolescents and adults, makes identification of suicidality in a busy primary care practices less demanding and more feasible, especially if a self-report is used. Secondly, nine items are few enough to be adaptable to digital administration via smartphones or otherwise, thus offering a therapeutic opportunity for early and perhaps targeted intervention. Thirdly, this 9-item scale with four clinically relevant subscales, can help clinicians identify and focus their discussion on factors that are particularly contributing to the risk of suicidality. This report is an initial attempt to establish the degree of suicidal risk benchmarking the total score and subscale scores against the four possible responses to the PHQ-9 suicide Item 9.
This report has several limitations. The generalizability of findings to other primary care practices as well as public and private sector psychiatric practices is unknown. Concurrent validity based on other accepted measures of suicidality such as the Columbia-Suicide Severity Rating Scale (CSSRS), (28) and the Sheehan Suicidality Tracking Scale (Sheehan-STS) (29) is unknown. The degree to which the CHRT-SR9 predicts actual suicidal attempts or efforts to prevent such attempts in adolescents, as well as adults, is not known. Finally, its clinical utility as compared to clinical impression alone has not been assessed.
In conclusion, the CHRT-SR9 is a brief self-report with excellent psychometric properties in both adolescents (based on our prior report) and adults (based on this report) that can estimate the degree of suicidality and whether a clinically important degree of improvement or worsening in suicidality has occurred. Its subscales provide clinical clues about psychological factors contributing to the risk.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the UT Southwestern Medical Center Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KN, AR, and MT conceptualized the study. KN and AR reviewed the literature, interpreted the results from the data analysis, and drafted the manuscript. KN analyzed the data. KN, AR, TC, TM, and MT revised the manuscript for important intellectual content. AR and MT provided project oversight. All authors approved the final version of the manuscript and take responsibility for the content herein.
This study was funded by the Center for Depression Research and Clinical Care (CDRC) at UT Southwestern.
We thank the patients, clinics, and staff and colleagues who made this project possible. We also thank Ms. Kathryn Forbes for her administrative support on this manuscript.
Licensing and distribution of the CHRT-SR is managed by Mapi Research Trust on behalf of the copyright holder, University of Texas Southwestern Medical Center. At the time of publishing, the CHRT-SR is available without charge to non-commercial users. Fees may apply to commercial users, IT companies, funded academic users or healthcare organizations. Requests for information and licensing of the CHRT-SR should be submitted through Mapi Research Trust’s ePROVIDE platform (https://eprovide.mapi-trust.org/).
MT has served as an advisor or consultant for Abbott Laboratories, Abdi Ibrahim, AcademyHealth, ACI Clinical, Akili Interactive, Akzo (Organon Pharmaceuticals), Alkermes, Allergan Pharmaceuticals, Alto Neuroscience, American Psychiatric Association, American Society of Clinical Psychopharmacology, Arcadia Pharmaceuticals, AstraZeneca, Avanir Pharmaceuticals Axon Advisors, Axsome Therapeutics, Biogen MA Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Cephalon, Cerebral Inc., Cerecor, Circular Genomics Inc., CME Institute of Physicians, Compass Pathfinder Limited, Concert Pharmaceuticals, Eli Lilly, Evotec, Fabre Kramer Pharmaceuticals, Forest Pharmaceuticals, GH Research Limited, GlaxoSmithKline, Janssen Global Services, GreenLight VitalSign6 Inc., Heading Health Inc., Janssen Pharmaceutical Products, Jazz Pharmaceutical, Johnson and Johnson Pharmaceutical Research and Development, Legion Health Inc., Libby, Lundbeck, Meade Johnson, MedAvante, Medscape, Medtronic, Merck Sharp and Dohme Corp., Mind Medicine (MindMed) Inc., Mitsubishi Tanabe Pharma Development America, Naki Health, Ltd., Naurex, Navitor, Neurocrine Biosciences Inc., Neuronetics, Noema Pharma AG, Orexo US Inc., Otsuka American Pharmaceutical Inc., Otsuka Canada Pharmaceutical Inc., Otsuka Pharmaceutical Development and Commercialization Inc., Pamlab, Parke-Davis Pharmaceuticals, Perception Neuroscience Holdings, Pfizer, PgxHealth, Pharmerit International, Phoenix Marketing Solutions, Research USA, Praxis Precision Medicines Inc., Relmada Therapeutics, Inc., Rexahn Pharmaceuticals, Ridge Di-agnostics, Roche Products, SAGE Therapeutics, Sepracor, Shire Development, Sierra, SK Life and Science, Signant Health, Sparian Biosciences Inc., Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, WebMD, and WyethAyerst Laboratories and has received grants or research support from the Agency for Healthcare Research and Quality, American Foundation for Suicide Prevention, Blue Cross Blue Shield of Texas, Cancer Prevention and Research Institute of Texas, Cyberonics, Janssen Research and Development, Johnson and Johnson, NARSAD, the National Institute of Drug Abuse, the National Institute of Mental Health, and Patient-Centered Outcomes Research Institute, and has received editorial compensation from The American Psychiatric Association, Engage Health Media, Healthcare Global Village, and Oxford University Press. He owns stock in Alto Neuroscience Inc., Cerebral Inc., Circular Genomics Inc., GreenLight VitalSign6 Inc., and Legion Health Inc. AR has received consulting fees from Compass Inc., Curbstone Consultant LLC., Emmes Corp., Evecxia Therapeutics, Inc., Holmusk Technologies, Inc., ICON, Johnson and Johnson (Janssen), Liva-Nova, Mind Street Inc., Neurocrine Biosciences Inc., Otsuka-US; speaking fees from Johnson and Johnson (Janssen), and Liva-Nova, and royalties from Wolters Kluwer Health, Guilford Press and the University of Texas Southwestern Medical Center, Dallas, TX (for the Inventory of Depressive Symptoms and its derivatives) and AR is also named co-inventor on two patents: U.S. patent no. 7,795,033: Methods to Predict the Outcome of Treatment with Antidepressant Medication, Inventors: McMahon F. J., Laje G., Manji H., AR, Paddock S., Wilson A. S., and U.S. patent no. 7,906,283: Methods to Identify Patients at Risk of Developing Adverse Events During Treatment with Antidepressant Medication, Inventors: McMahon F. J., Laje G., Manji H., AR, Paddock S. TC has served as a consultant for Alkermes, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the various funding organizations.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1014766/full#supplementary-material
CHRT-SR, concise health risk tracking self report; CTT, classical test theory; PHQ-9, patient health questionnaire; CFA, confirmatory factor analysis; CFI, comparative fit index; TLI, tucker lewis index; RMSEA, root-mean-square error of approximation; CMIN\DF, chi-square fit statistics/degree of freedom; SD, standard deviation; CSSRS, Columbia-suicide severity rating scale; Sheehan-STS, Sheehan suicidality tracking scale.
1. The Commonwealthfund. New International Report on Health Care: U.S. Suicide Rate Highest Among Wealthy Nations. New York, NY: Commonwealth Fund (2020).
2. Centers for Disease Control and Prevention. Products - Data Briefs - Number 431 - January 2022. Atlanta: CDC (2022).
3. Ahmedani B, Simon G, Stewart C, Beck A, Waitzfelder B, Rossom R, et al. Health care contacts in the year before suicide death. J Gen Intern Med. (2014) 29:870–7. doi: 10.1007/s11606-014-2767-3
4. O’Connor E, Gaynes B, Burda B, Soh C, Whitlock E. Screening for and treatment of suicide risk relevant to primary care: a systematic review for the U.S. preventive services task force. Ann Intern Med. (2013) 158:741–54. doi: 10.7326/0003-4819-158-10-201305210-00642
5. Raue PJ, Ghesquiere AR, Bruce ML. Suicide risk in primary care: identification and management in older adults. Curr Psychiatry Rep. (2014) 16:466. doi: 10.1007/s11920-014-0466-8
6. The Joint Commission. Sentinel Event Alert 56 Detecting and Treating Suicide Ideation in all Settings. Oakbrook Terrace, IL: The Joint Commission (2016).
7. Ghasemi P, Shaghaghi A, Allahverdipour H. Measurement scales of suicidal ideation and attitudes: a systematic review article. Health Promotion Perspect. (2015) 5:156–68. doi: 10.15171/hpp.2015.019
8. Trivedi MH, Wisniewski SR, Morris DW, Fava M, Gollan JK, Nierenberg AA, et al. Concise health risk tracking scale: a brief self-report and clinician rating of suicidal risk. J Clin Psychiatry. (2011) 72:757–64. doi: 10.4088/JCP.11m06837
9. Nandy K, Rush A, Carmody T, Kulikova A, Mayes T, Emslie G, et al. The concise health risk tracking - self-report (CHRT-SR)-a measure of suicidal risk: performance in adolescent outpatients. Int J Methods Psychiatr Res. (2022) 10:e1944. doi: 10.1002/mpr.1944
10. Trivedi M, Jha M, Kahalnik F, Pipes R, Levinson S, Lawson T, et al. VitalSign6: a primary care first (PCP-First) model for universal screening and measurement-based care for depression. Pharmaceuticals (Basel). (2019) 12:71. doi: 10.3390/ph12020071
11. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J General Int Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
13. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equat Model. (1999) 6:1–55. doi: 10.1080/10705519909540118
14. Bollen KA, Stine RA. Bootstrapping goodness-of-fit measures in structural equation models. Soc. Methods Res. (1992) 21:205–29. doi: 10.1177/0049124192021002004
15. Widaman K, Reise S. Exploring the measurement invariance of psychological instruments: applications in the substance use domain. In: Bryant K, Windle M, West S editors. The Science of Prevention: Methodological Advances from Alcohol and Substance Abuse Research. Washington, DC: American Psychological Association (1997). p. 281–324. doi: 10.1037/10222-009
16. Byrne BM. Structural Equation Modeling with AMOS: Basic Concepts, Applications, and Programming. New York, NY: Routledge (2010).
17. Eisinga R, Grotenhuis MT, Pelzer B. The reliability of a two-item scale: pearson, cronbach, or spearman-brown? Int J Public Health. (2013) 58:637–42. doi: 10.1007/s00038-012-0416-3
18. Minkoff K, Bergman E, Beck AT, Beck R. Hopelessness, depression, and attempted suicide. Am J Psychiatry. (1973) 130:455–9. doi: 10.1176/ajp.130.4.455
19. Beck AT, Kovacs M, Weissman A. Hopelessness and suicidal behavior: an overview. JAMA. (1975) 234:1146–9. doi: 10.1001/jama.234.11.1146
20. Zisook S, Lesser I, Lebowitz B, Rush A, Kallenberg G, Wisniewski S, et al. Effect of antidepressant medication treatment on suicidal ideation and behavior in a randomized trial: an exploratory report from the combining medications to enhance depression outcomes study. J Clin Psychiatry. (2011) 72:1322–32. doi: 10.4088/JCP.10m06724
21. Ostacher M, Nierenberg A, Rabideau D, Reilly-Harrington N, Sylvia L, Gold A, et al. clinical measure of suicidal ideation, suicidal behavior, and associated symptoms in bipolar disorder: psychometric properties of the concise health risk tracking self-report (CHRT-SR). J Psychiatr Res. (2015) 71:126–33. doi: 10.1016/j.jpsychires.2015.10.004
22. Reilly-Harrington N, Shelton R, Kamali M, Rabideau D, Shesler L, Trivedi M, et al. tool to predict suicidal ideation and behavior in bipolar disorder: the concise health risk tracking self-report. J Affect Disord. (2016) 192:212–8. doi: 10.1016/j.jad.2015.12.036
23. Villegas A, DuBois C, Celano C, Beale E, Mastromauro C, Stewart J, et al. longitudinal investigation of the CONCISE HEALTH RISK TRACKING SELF-REPORT (CHRT-SR) in suicidal patients during and after hospitalization. Psychiatry Res. (2018) 262:558–65. doi: 10.1016/j.psychres.2017.09.044
24. Sanchez K, Killian M, Mayes T, Greer T, Trombello J, Lindblad R, et al. psychometric evaluation of the concise health risk tracking self-report (CHRT-SR)- a measure of suicidality-in patients with stimulant use disorder. J Psychiatr Res. (2018) 102:65–71. doi: 10.1016/j.jpsychires.2018.03.012
25. Mayes T, Kennard B, Killian M, Carmody T, Grannemann B, Rush A, et al. Psychometric properties of the concise health risk tracking (CHRT) in adolescents with suicidality. J Affect Disord. (2018) 235:45–51. doi: 10.1016/j.jad.2018.03.007
26. Trombello J, Killian M, Grannemann B, Rush A, Mayes T, Parsey R, et al. The concise health risk tracking-self report: psychometrics within a placebo-controlled antidepressant trial among depressed outpatients. J Psychopharmacol. (2019) 33:185–93. doi: 10.1177/0269881118817156
27. De La Garza N, Rush A, Killian M, Grannemann B, Carmody T, Trivedi M. The concise health risk tracking self-report (CHRT-SR) assessment of suicidality in depressed outpatients: a psychometric evaluation. Depress Anxiety. (2019) 36:313–20. doi: 10.1002/da.22855
28. Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry (2011) 168:1266–77. doi: 10.1176/appi.ajp.2011.10111704
Keywords: psychometrics, concise health risk tracking scale self-report (CHRT-SR), adults, depression, suicidal risk, suicidality
Citation: Nandy K, Rush AJ, Carmody TJ, Mayes TL and Trivedi MH (2023) The 9-item Concise Health Risk Tracking – Self-Report (CHRT-SR9) measure of suicidal risk: Performance in adult primary care patients. Front. Psychiatry 14:1014766. doi: 10.3389/fpsyt.2023.1014766
Received: 08 August 2022; Accepted: 23 January 2023;
Published: 14 February 2023.
Edited by:
Yuan Yuan Wang, De Montfort University, United KingdomReviewed by:
Ricardo Gusmão, University of Porto, PortugalCopyright © 2023 Nandy, Rush, Carmody, Mayes and Trivedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhukar H. Trivedi,  bWFkaHVrYXIudHJpdmVkaUB1dHNvdXRod2VzdGVybi5lZHU=
bWFkaHVrYXIudHJpdmVkaUB1dHNvdXRod2VzdGVybi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.