- 1Department of Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing Brain Hospital, Nanjing, China
- 2Department of Psychiatry, Shenzhen Kangning Hospital, Shenzhen, China
- 3Department of Psychiatry, The Fourth People's Hospital of Chengdu, Chengdu, China
Background: There is limited evidence on the efficacy of electroconvulsive therapy (ECT) in adolescents with mental illness. The present study reported outcomes of adolescents with mental illness treated with ECT aimed at providing evidence for large-scale feasibility.
Objectives: The primary objective of this trial was to examine the differences in demographic and clinical data between responders and non-responders. The secondary objective was to determine whether ECT produced differential readmission rates, the burden of oral medication, and social function in responders and non-responders in the long term.
Methods: Patients aged 14–18 years diagnosed with schizophrenia (SCZ), major depressive disorder (MDD), or bipolar disorder (BD) who received ECT between 2015 and 2020 were included in the study. Demographic and clinical data were compared, and both short-term and long-term outcomes were assessed: response on the Clinical Global Impressions-Improvement scale and readmission at follow-up. The independent-sample t–test was used to compare the continuous variables and the X2 test was used to compare the dichotomous variables with statistical significance at P ≤ 0.05.
Results: Four hundred ten adolescents (aged 14–18 years, 53.90% female) received ECT for SCZ, MDD, and BD. The response rate for SCZ, MDD, and BD were 65.61, 78.57, and 69.95%, respectively. Both SCZ (P = 0.008) and BD (P = 0.008) groups had a significant elder age in responders than in non-responders. Besides that MDD responders had a significantly larger number of ECT sessions than non-responders (P = 0.046), the study failed to find a significant difference in other ECT parameters. A significantly higher proportion of readmission was found in BD non-responders than in responders (P = 0.029), there was no difference in the rate of readmission in other diagnostic groups.
Conclusions: These data suggested that ECT is an effective treatment for adolescents with severe mental illness, and the rate of readmission was low in the long term. The present study supports that large-scale systematic studies are warranted for further investigation of the response rate of ECT for treating adolescents with mental illness.
Introduction
Electroconvulsive therapy (ECT) is a suitable treatment for adults with severe mental disorders. In Asian and African countries, ECT is mainly used for psychotic disorders, in patients with schizophrenia (SCZ) and other psychotic conditions (1). ECT for SCZ has increased over the past years, a national survey demonstrated that ECT treatment of SCZ increased from 4.7 to 7.7% between 2006 and 2012 in China (2). A large body of research in Taiwan showed that ECT could treat SCZ effectively and significantly decrease the rate of re-hospitalization, correspondingly, the total medical expenses increased significantly in the non-ECT patients, but not in the ECT patients (3). ECT also has a positive effect on the clinical response of adults with treatment-resistant SCZ (4). A trial determined the rates of ECT used in adolescents with catatonia in schizophrenia spectrum disorders in America, the results suggested that ECT was used for only 13% of adolescents with catatonia when comorbid schizophrenia spectrum disorders, and a high rate of ECT use was evident for Whites compared with the other race and also was seen in private health insurance beneficiaries (5). These characteristics in the utilization of ECT in adolescents were also found by Trivedi et al. (6). In the United States and European countries, ECT was predominantly used in patients with affective disorders, treated severe depression effectively, and could reduce the risk of suicide (7, 8). A recent meta-analysis demonstrates that the response rates for adults major depressive disorder (MDD) range from 58 to 70% (9). A retrospective cohort study showed the risk of suicide was found to be significantly reduced for adults with MDD who received ECT (10). ECT was also effective for bipolar disorder (BD), a previous study showed that the response rates for bipolar depression, mixed-state, and mania treated by ECT were 68.1, 72.9, and 75%, respectively (11). ECT is relatively well tolerated in adults, with typical side effects being transient cognitive problems, headache, nausea, and muscle soreness (12).
SCZ, BD, MDD, and other severe mental illnesses are also common conditions in adolescence that can impair the social, academic, or daily functioning of affected individuals, quite often predisposing them to a high risk of self-injury or suicide. Rates of self-injury are substantially higher amongst adolescents than in adults, ranging from 7.5–46.5% in adolescents and 4–23% in adults (13). However, there is a lack of available and effective interventions for adolescents in the acute phase of mental illness or for those with tendencies toward suicide and self-injury. Previous literature showed that the first-line antidepressants may not achieve satisfactory effect in 60% of adolescents with MDD (14). Some limitations associated with interventions for acute onset of adolescents with mental disorders include slow effectiveness, lack of well-tolerated and effective pharmaceuticals, and limited pharmaceutical therapeutic options (15, 16). Besides that, there are hidden safety risks in antidepressant medication, such as selective serotonin reuptake inhibitors (SSRIs) could increase the risk of suicidality and self-injury in youth (17, 18). Therefore, ECT could be regarded as an effective treatment.
Although ECT is considered an effective and safe treatment for adults; however, the use of ECT is much less common in adolescents than in adults, for multiple reasons (19). There is a lack of studies regarding the efficacy of ECT in adolescents or children. Due to ethical review, the large sample, randomized control study could not carry out. A previous study estimated the knowledge and attitudes toward the use of ECT in children and adolescents, among child and adolescent psychiatrists, the results showed that about half of the respondents possessed minimal knowledge about the use of ECT in children and adolescents, three-quarters of respondents lack confidence for the efficacy of ECT in children and adolescents, and the majority of respondents regarded ECT as treatment of last resort (20). Similar results were found in the other two studies (21, 22). Moreover, there is specific legislation that inhibits the use of ECT for children under certain ages in some countries and areas, which also limits the research (23). For the above reasons, the proportion of ECT used in adolescents with mental illness only accounts for 1%, which is much lower than in adults (19).
In recent years, the literature supporting the use of ECT in adolescents is growing. A recent study (835 samples) reviewed adolescents with schizophrenia from 2007 to 2016 in a single Chinese academic medical center. This study found that the frequency of ECT use was 49.2% and ECT use was independently and positively associated with gender, and high risk for suicide (24). A retrospective study examined the association between baseline futures and clinical outcomes, the results suggested that ECT is safe and efficacy, but the clinical response was not predicted by demographic data (23). However, there are also some limitations in those studies, such as the lack of ECT parameters, long-term social function outcomes, and the rate of readmission. Herein, we compared the demographic and clinical data of adolescents treated with ECT at a single medical center in the People's Republic of China. The objectives of our study were to examine (1) the differences in baseline data between ECT responders and non-responders (2) the future of ECT parameters in ECT responders or non-responders (3) the differences in the rate of readmission, social function, and burden of oral medication between patients with and without response in long-term after ECT treatment.
Methods
Data source
The protocol for the study was approved by the institutional review board at the Affiliated Brain Hospital of Nanjing Medical University. We reviewed the electronic medical records and the electronic prescribing system of SCZ, BD, and MDD patients aged 14–18 years old who received ECT for the first time at the Affiliated Brain Hospital of Nanjing Medical University, from January 2015 through December 2020. Clinical diagnoses of SCZ, MDD, and BD were based on the International Classification of Diseases, Tenth Edition (ICD-10). The exclusion criteria were as follows: diagnosis of other mental disorders; drug or alcohol abuse or dependence in the last 6 months; ECT in the past. Diagnoses at admission, diagnosis changes if patients readmission at follow-up, oral medication, and ECT parameters were collected. We collected demographic data including sex, age, family history of psychiatric disorders, first episode age, duration of illness, and duration of hospitalization.
Adolescents who received ECT were evaluated by at least 2 board-certified psychiatrists, and the medical director of the hospital approved ECT according to the hospital policy. Patients and their legal guardians were provided with information about the diagnosis, treatment options, and the risks and benefits of ECT. Written informed consent from the patients' guardians was obtained prior to starting ECT.
We recorded the number of ECT sessions and collected ECT parameters during the implementation of ECT, including seizure duration time (SDT), seizure energy index, and positive suppression index (PSI). For patients who received more than one ECT treatment series during their adolescence, we analyzed data on the first ECT series only. The course of ECT generally comprised 6–12 sessions for each patient, 3 times per week, between 9 a.m. and 11 a.m. Electroconvulsive therapy was administered using a Thymatron DGx device. Anesthesia was induced with propofol (1–1.5 mg/kg) accompanied by succinylcholine (0.5 mg/kg) and oxygenation. Patients were pre-oxygenated and then manually ventilated using a valve mask and 100% oxygen when adequate muscle relaxation was achieved. A brief pulse wave device with bitemporal electrode placement was used. All patients received hyperventilation before the stimulus was administered.
Outcomes measures
All patients were assigned a Global Clinical Impressions–Improvement scale (CGI-I) score (25). The score of 1 indicated “very much improved” and 2 indicated “much improved” on the CGI-I. All outpatients were assigned a Personal and Social Performance scale (PSP) score (26). All assessments were determined by independent reviews made by 2 primary physicians. If there was a disagreement between 2 physicians, they reviewed the medical record together, thus reaching a consensus.
Statistical analyses
The Statistical Product and Service Solutions (SPSS) version 24.0 was used to analyze the data. Baseline variables included sex (dichotomous), age (continuous), family history of psychiatric disorders (dichotomous), the first episode age (continuous), illness duration (continuous), and days in the hospital (continuous). ECT numbers (continuous), SDT (continuous), SEI (continuous), and PSI (continuous) were also measured. The medication received while on ECT course was also recorded. For outpatients, the types of oral medication were further distinguished, regardless if they were increased or not. To compare the differences in continuous variables between responsive and non-responsive patients and those who were readmitted and not readmitted, the independent-sample t-test was used, and the X2 test was used to compare the dichotomous variables. Statistical significance was set at P < 0.05 (bilateral).
Results
Demographic characteristics and clinical data
A total of 410 adolescents received ECT for mental disorders at the Affiliated Brain Hospital of Nanjing Medical University. 53.90% patients were female, and the mean (SD) age at the start of the treatment series was 16.67 (1.10) years. There were 28.78% patients with a family history of psychiatric disorders. The mean (SD) age at the first episode was 15.07 (1.69) years, and the age range was 10~18 years. The duration of illness ranged from 2 weeks−10 years, and the mean (SD) duration of illness was 22.59 (19.66) months. Patients required a mean (SD) of 34.60 (18.28) days in the hospital. Among patients who were readmitted, the diagnosis was changed in 6 patients. The diagnosis of 1 MDD patient and 1 BD patient were changed to SCZ; the diagnosis of 3 MDD patients and 1 SCZ patient were changed to BD.
SCZ patients
For 157 SCZ patients, the response rate was 65.61%. Among 103 patients who responded to ECT, 30.10% patients were female; the mean (SD) age was 16.87 (1.09) years; 29.13% patients with a family history of psychiatric disorders; the mean (SD) age at the first episode was 15.30 (1.84) years; the mean (SD) duration of illness was 21.41 (19.94) months; the mean (SD) duration of hospitalization was 39.71 (19.47) days. Among 54 non-responders to ECT, 46.30% patients were female; the mean (SD) age was 16.39 (1.02) years; 20.37% patients were with a family history of psychiatric disorders; the mean (SD) age at the first episode was 15.19 (1.55) years; the mean (SD) duration of illness was 18.21 (17.57) months; the mean (SD) duration of hospitalization was 39.63 (32.73) days. There was a significant difference in age between responders and non-responders (t = −2.71, P = 0.008) (Table 1).
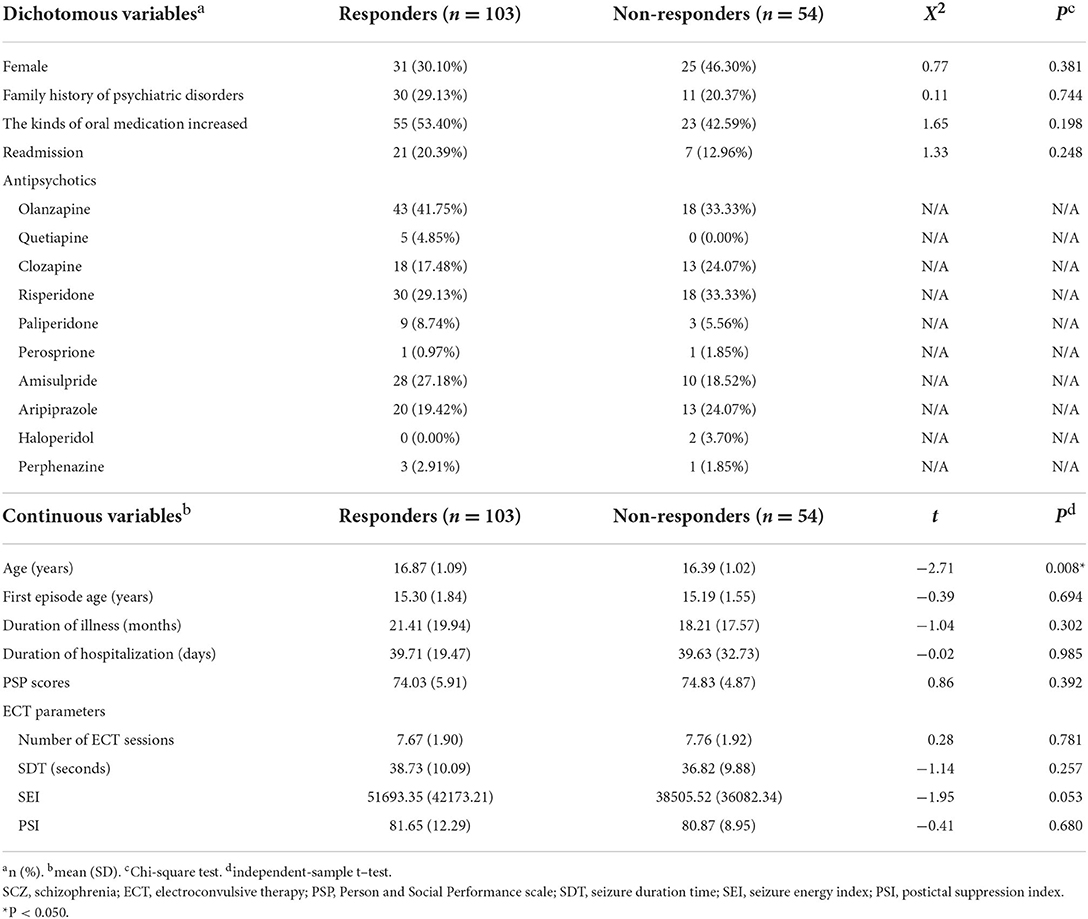
Table 1. Demographic and clinical characteristics of 157 SCZ patients. Comparison between patients with and without response after ECT course.
For antipsychotics that responders received, olanzapine (41.75%) was the most common antipsychotic prescribed to patients undergoing ECT, followed by risperidone (29.13%), amisulpride (27.18%), aripiprazole (19.42%), clozapine (17.48%), paliperidone (8.74%), quetiapine (4.85%), and perphenazine (2.91%). One patient was treated with perosprione. For non-responders, olanzapine (33.33%) and risperidone (33.33%) were both the most common antipsychotic, followed by clozapine (24.07%) and aripiprazole (24.07%), amisulpride (18.52%), paliperidone (5.56%), and haloperidol (3.70%). Two patients were respectively treated with perosprione and perphenazine (Table 1).
MDD patients
For 70 MDD patients, the response rate was 78.57%. Among 55 who responded to ECT, 61.82% patients were female; the mean (SD) age was 16.60 (1.10) years; 34.55% patients with a family history of psychiatric disorders; the mean (SD) age at the first episode was 14.78 (1.59) years; the mean (SD) duration of illness was 25.00 (18.71) months; the mean (SD) duration of hospitalization was 31.04 (10.06) days. Among 15 non-responders to ECT, 60.00% patients were female; the mean (SD) age was 16.33 (1.11) years; 20.00% patients with a family history of psychiatric disorders; the mean (SD) age at the first episode was 15.20 (1.57) years; the mean (SD) duration of illness was 16.72 (17.56) months; the mean (SD) duration of hospitalization was 26.40 (10.03) days. There was no significant difference between responders and non-responders (Table 2).
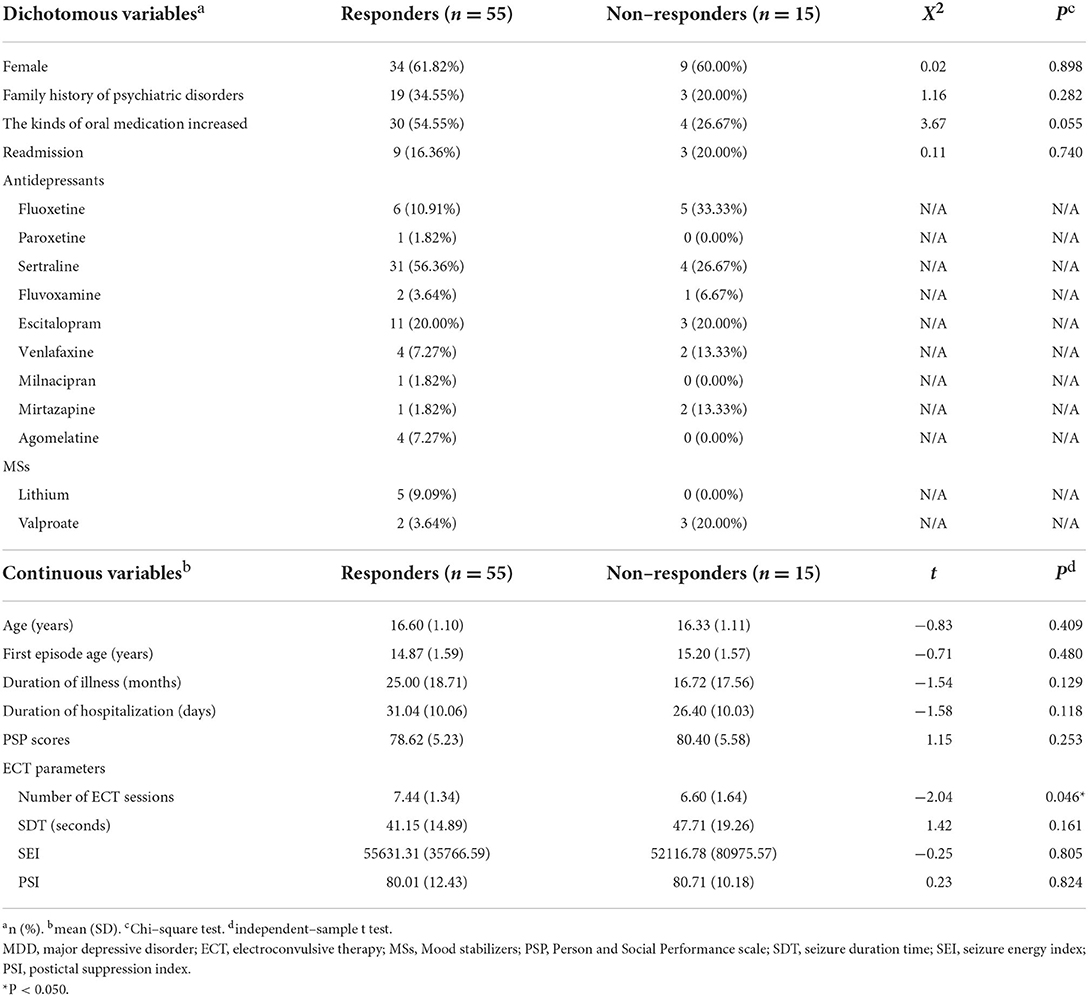
Table 2. Demographic and clinical characteristics of 70 MDD patients. Comparison between patients with and without response after ECT course.
The most common antidepressant which responders received was sertraline (56.36%), followed by escitalopram (20.00%), fluoxetine (10.91%), venlafaxine (7.27%) and agomelatine (7.27%), and fluvoxamine (3.64%). Three patients were treated with paroxetine, milnacipran, and mirtazapine, respectively. Five patients combined with lithium, and two with valproate. For non-responders, the most common antidepressant was fluoxetine (33.33%), followed by sertraline (26.67%), escitalopram (20.00%), venlafaxine (13.33%) and mirtazapine (13.33%). One patient was treated with fluvoxamine. Three patients combined with valproate (Table 2).
BD patients
For 183 BD patients, the response rate was 69.95%. Among 128 responders of ECT, 63.28% patients were female; the mean (SD) age was 16.83 (1.04) years; 27.34% patients with a family history of psychiatric disorders; the mean (SD) age at the first episode was 15.05 (1.68) years; the mean (SD) duration of illness was 25.10 (21.03) months; the mean (SD) duration of hospitalization was 31.31 (11.82) day; the most common diagnosis was bipolar depressive episode (59.38%), followed by mania episode (26.56%), mixed episode (12.50%), and rapid cycling (1.56%). Among 55 non-responders of ECT, 74.55% patients were female; the mean (SD) age was 16.36 (1.16) years; 20 patients (36.36%) with a family history of psychiatric disorders; the mean (SD) age at the first episode was 14.75 (1.68) years; the mean (SD) duration of illness was 22.47 (18.78) months; the mean (SD) duration of hospitalization was 33.55 (13.18) day; the most common diagnosis was bipolar depressive episode (67.27%), followed by mania episode (20.00%), mixed episode (12.73%). There was a significant difference in age between responders and non-responders (t = −2.68, P = 0.008) (Table 3).
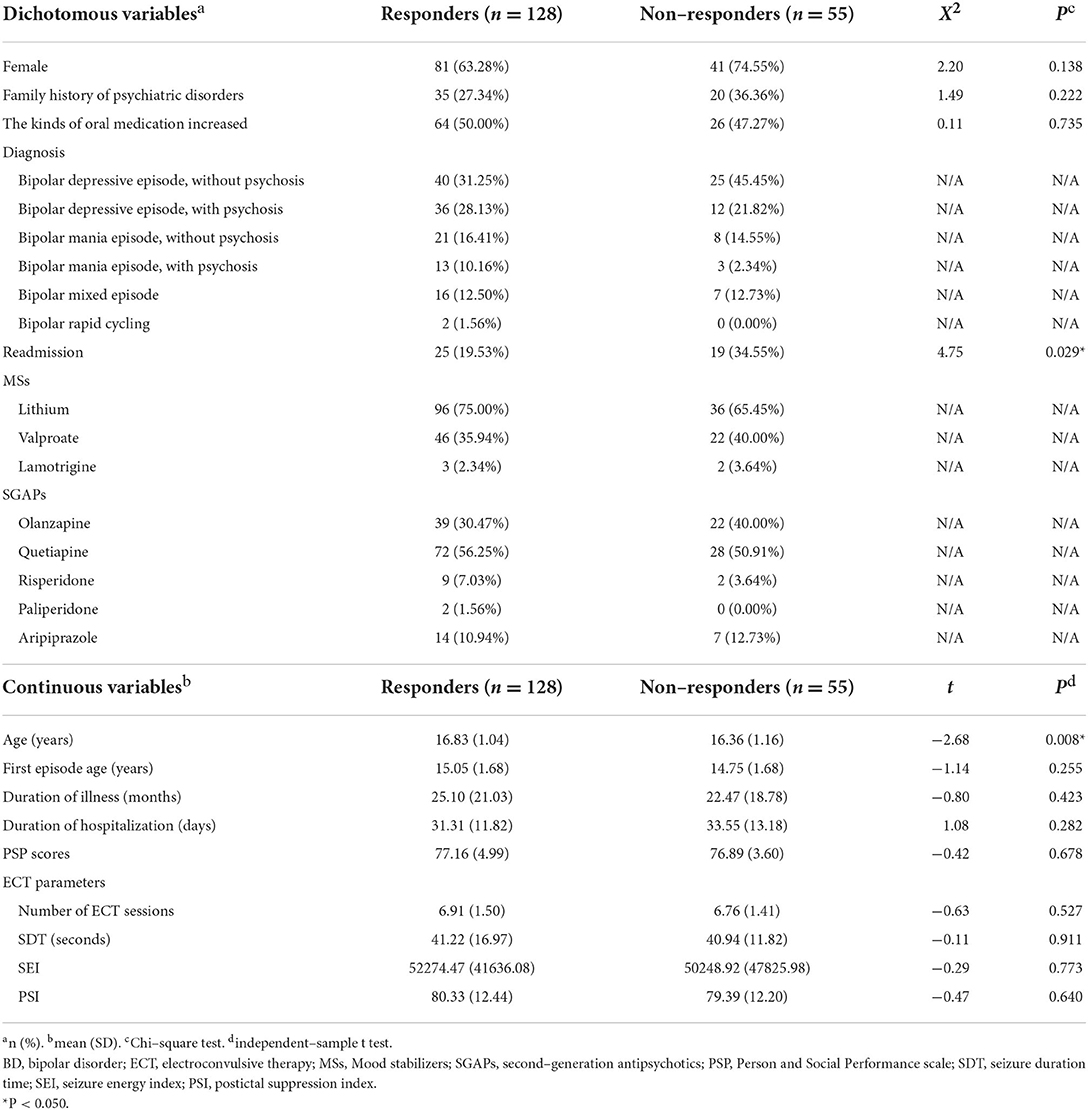
Table 3. Demographic and clinical characteristics of 183 BD patients. Comparison between patients with and without response after ECT course.
For responders, the most common mood stabilizer (MS) was lithium (75.00%), followed by valproate (35.94%), and lamotrigine (2.34%); the most common second-generation antipsychotic (SGAP) was quetiapine (56.25%), followed by olanzapine (30.47%), aripiprazole (10.94%), risperidone (7.03%), and paliperidone (1.56%). For non-responders, the most common MS was lithium (65.45%), followed by valproate (40.00%), and lamotrigine (3.64%); the most common SGAP was quetiapine (50.91%), followed by olanzapine (40.00%), aripiprazole (12.73%), and risperidone (3.64%) (Table 3).
ECT characteristics
SCZ patients
For responders, the mean (SD) number of ECT sessions was 7.67 (1.90); the mean (SD) SDT was 38.73 (10.09) seconds; the mean (SD) SEI was 51693.35 (42173.21), the mean (SD) PSI was 81.65 (12.29). For non-Responders, the mean (SD) number of ECT sessions was 7.76 (1.92); the mean (SD) SDT was 36.82 (9.88) seconds; the mean (SD) SEI was 38505.52 (36082.34), the mean (SD) PSI was 80.87 (8.95). While the study failed to show a significant difference between responders and non-responders for these outcomes specifically, numerical advantage of SEI favoring the responders was observed (Table 1).
MDD patients
For responders, the mean (SD) number of ECT sessions was 7.44 (1.34); the mean (SD) SDT was 41.15 (14.89) sec; the mean (SD) SEI was 55631.31 (35766.59), the mean (SD) PSI was 80.01 (12.43). For non-responders, the mean (SD) number of ECT sessions was 6.60 (1.64); the mean (SD) SDT was 47.71 (19.26) sec; the mean (SD) SEI was 52116.78 (80975.57), the mean (SD) PSI was 80.71 (10.18). There was no significant difference in SDT, SEI and PSI for two groups. But the significant larger number of ECT sessions were received for responders than for non-responders (t = −2.04, P = 0.046) (Table 2).
BD patients
For responders, the mean (SD) number of ECT sessions was 6.91 (1.50); the mean (SD) SDT was 41.22 (16.97) sec; the mean (SD) SEI was 52274.47 (41636.08), the mean (SD) PSI was 80.33 (12.44). For non-responders, the mean (SD) number of ECT sessions was 6.76 (1.41); the mean (SD) SDT was 40.94 (11.82) sec; the mean (SD) SEI was 50248.92 (47825.98), the mean (SD) PSI was 79.39 (12.20). No significant was found in ECT parameters between two groups (Table 3).
Follow-up outcomes
At the end of the follow-up, the readmission rate was 20.49%. There was found a significant higher readmission rate in BD non-responders than in BD responders (34.55% v. 19.53%, X2 = 4.75, P = 0.029) (Table 3). For the other two diagnostic groups, no significant difference was found in readmission rate between responders and non-responders. For outpatients, the mean (SD) PSP scores was 76.35 (5.40), and no significant difference was found respectively in three diagnostic groups between responders and non-responders. While patients who were responded for ECT treatment in three diagnostic groups all had a high proportion of increasing the kinds of oral medication at outpatient setting, no significant difference was found between responders and non-responders at follow-up.
Discussion
In this study, we reviewed the demographic and clinical data of adolescents with certain severe mental disorders treated with ECT at a signal academic medical center. Our research provided evidence to support the efficacy of ECT in adolescents, and also demonstrated the long-term outcome. Our results showed the highest response rate was observed in MDD (78.57%), followed by BD (69.95%), and the lowest response rate was SCZ (65.61%). For SCZ and BD patients, we found that there was a significant difference in age between responders and non-responders. The largest number of ECT sessions was observed in SCZ, which was significantly more than in BD and MDD. At follow-up, we found significant differences in the first episode age, duration of illness, duration of hospitalization, and SEI between patients with or without readmission. The PSP scores showed that the social function recovered well for all three diagnosis groups.
The samples in this study had severe mental illness, but the improvement was significantly well after a course of ECT. The overall response rate in our samples was 69.76%, which is lower than reported in other recent retrospective studies. Two retrospective studies (107 samples and 51 samples, respectively) reviewed the outcomes of adolescents treated with ECT in America, both reporting response rates of 77% (23, 27). In Asia, a cohort study (22 samples) from multi-sites reported a response rate of 77% (28). Our findings revealed a lower response rate compared to those studies, which might be due to a greater proportion of patients with SCZ in the present study compared to American and Indian samples. The present study included 157 SCZ patients (38.29%), which is much higher than the 4.67% of patients with SCZ included in America and 17.65% in India. Moreover, the sample sizes of previous studies were smaller compared to the present study, and there were differences in race and region. Consequently, more large samples, multi-site, and prospective studies are needed to evaluate this issue further.
In our study, although the efficacy of ECT in the treatment of SCZ was lower than affective disorders, the results showed that the majority of adolescents with SCZ had experienced clinically significant improvement. Wang et al. reviewed adolescents with SCZ treated by ECT in China (326 samples), and their results suggested that the response rate was 65%, which was very close to our results (24). A retrospective chart review evaluated the clinical profile of adolescents who had received ECT, the results showed that the rate of remission for SCZ was lower than MDD and mania, which was similar to our results (29). Previous studies have shown that SCZ affects neurodevelopment during the period of adolescence or earlier, the treatment is challenging (30). A meta-analysis in over 18,000 subjects reported that brain loss in SCZ was related to a combination of neurodevelopmental processes reflected in intracranial volume reduction (31). Duan et al. compared the hippocampus volume between patients with adolescent-onset SCZ (AOS) (36 samples) and typically developing controls (TDC) (30 samples), providing evidence on hippocampus volume reduction in AOS, acting as a mediator between hippocampal morphometry and negative symptom (32). The early onset adolescents often have negative symptoms and cognitive deficits, which were predicted poor function outcomes (33). ECT had poor efficacy among early-onset adolescents with negative symptoms, especially first-episode SCZ. In summary, clinicians should pay more attention to the assessment of early-onset SCZ adolescents and formulate a personalized treatment.
For MDD, the response rate was higher than other diagnoses, and the majority were in stable condition at follow-up. Castaneda-Ramirez et al. reviewed 41 studies (601 samples) on the use of ECT for treatment-resistant mood disorders in children and adolescents, and they reported the response rate of ECT ranging from 53–77%, which was consistent with our results (34). ECT could relieve severe depressive symptoms, such as self-injury or suicide, in the short term. A retrospective case series of ECT used in MDD and anorexia nervosa (AN) showed that ECT is safe and well-tolerated in AN with severe comorbid treatment-resistant MDD and/or with increased suicide risk (35). A retrospective chart review found that ECT could decrease suicidal behavior, reduce depressive symptoms, and improve overall function at follow-up after 1 year (36). But fewer literature reported the readmission rate in adolescents with MDD treated by ECT, our study provided evidence for this. The results of the study showed that ECT is a safe and effective treatment for adolescents with MDD.
According to our findings, ECT resulted in an effective treatment for adolescents with BD, the rate of readmission was higher than MDD and SCZ. Pierson et al. reviewed the outcomes of manic patients treated by ECT (16 samples), reporting a response rate of 88% (23). In the other retrospective study (33 samples) of BD patients, the response rate was 93% (37). Medda et al. evaluated the long-term outcome of patients with bipolar depression or mixed state, responsive to ECT (70 samples), they found that 33% of patients showed a depressive relapse and 10% a mixed relapse at the end of the follow-up (38). A meta-analysis reviewed 8 studies on recurrence after the first episode of mania, revealing the recurrence rate of 25.7% at 6 months and 41.0% at 1 year, where a greater risk of recurrence was associated with younger age at onset (39). Based on our outcomes, the readmission rate was high at the follow-up that suggesting a high risk of relapse in the long term, which is consistent with the previous literature on adult patients. For adolescents with BD who received ECT, there is limited evidence of relapse rate. However, ECT is an effective treatment for BD, the factors that influence the rate of relapse after the ECT needs to be further examined.
Our results showed that the low SEI and increase in oral medication were associated with a high risk of readmission. SEI and other parameters have been reported as related to the effectiveness of ECT. They can also be used to assess seizure quality (40). The results suggested that the seizure quality influenced the clinical outcome in the long term. The association between oral medication and readmission suggested that adolescents who could not tolerate medication treatment or lacked treatment compliance had a high risk of relapse. To the best of our knowledge, there are no previous studies on seizure quality and medication treatment that could influence clinical outcomes for adolescents in the long term. Accordingly, this issue should be further examined by future studies.
The PSP score showed that the social function of adolescents recovered well after the ECT course. There have been few studies on the improvement of the social function of adolescents after ECT in long term. A previous study reviewed the long-term improvement of the overall function of adolescents with mood disorders after ECT, the results showed that the score of the Mini-Mental State Examination (MMSE) and school attendance was increased, the self-injurious behaviors and suicidal ideations were decreased at follow-up after 1 year (36). A previous review systematically summarized data on the burden associated with SCZ, MDD, BD, and other major mental disorders, revealing that SCZ and BD had the highest disability rating (41). Green et al. reported that individuals with SCZ exhibit impaired social cognition, which manifests as difficulties identifying emotions, feeling connected to others, inferring people's thoughts, and reacting emotionally to others (42). Currently, available studies on the clinical outcomes of ECT in adolescents failed to focus on social function or cognition after ECT in the long term. Our outcomes showed that the PSP score for SCZ patients was lower than MDD and BD, which also indicated some early-onset SCZ adolescents with cognitive deficits. In the present study, there was no correlation between the PSP score and readmission. Whether ECT could improve the social function of adolescents in the long term needs to be further examined.
Limitations
This retrospective study has a number of limitations. First, we did not record the side effect of ECT and the cognitive impairment of outpatients at follow-up. Previous meta-analyses suggested that cognitive function was impaired immediately following ECT, but returned to at least baseline by two weeks after ECT (43). However, another study reported that autobiographical memory deficits commonly occurred at the completion of the ECT course, and the deficits were long-lasting (44). Second, our results only detected the general increase in the kinds of drugs without recording the exact dose. The changes in dose of oral medication after the ECT course should be assessed by the use of equivalent does. Finally, future independent site validation should be considered, which limits the generalizability of the current study.
Conclusions
Overall, we provided retrospective evidence suggesting that ECT is an effective treatment for adolescents with severe mental disorders. The benefits of ECT include reducing the risk of relapse and the burden of oral medications. The data presented in this study showed that patients aged 14–18 years with SCZ, BD, and MDD responded well to ECT in the short term, also obtaining long-term benefits. However, whether patients younger than 14 years respond similarly to adolescents and adults remains unclear.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board at the Affiliated Brain Hospital of Nanjing Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
YS and YL designed the study. XZ and JL conducted the literature searches and analysis. QS and CC performed the statistical analysis. FR and GX managed the assessment of the risk bias. QS wrote the manuscript. YL contributed to the final review of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Scientific Research Foundation of Nanjing Medical University (201715048 and ZKX17030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. (2012) 2:283–344. doi: 10.1002/brb3.37
2. Li Q, Su YA, Xiang YT, Shu L, Yu X, Ungvari G, et al. Electroconvulsive therapy in schizophrenia in China: A National Survey. J ECT. (2017) 33:138–42. doi: 10.1097/YCT.0000000000000361
3. Lin HT, Liu SK, Hsieh MH, Chien YL, Chen IM, Liao S, et al. Impacts of electroconvulsive therapy on 1-year outcomes in patients with schizophrenia: a controlled, population-based mirror-image study. Schizophr Bull. (2018) 44:798–806. doi: 10.1093/schbul/sbx136
4. Sinclair DJM, Zhao S, Qi F, Nyakyoma K, Kwong JSW. Electroconvulsive therapy for treatment-resistant schizophrenia. Schizophr Bull. (2019) 45:730–2. doi: 10.1093/schbul/sbz037
5. Patel RS, Hobart K, Wadhawan A, Chalia A. Electroconvulsive treatment utilization for inpatient management of catatonia in adolescents with schizophrenia spectrum disorders. J ECT. (2022). doi: 10.1097/YCT.0000000000000858. [Epub ahead of print].
6. Trivedi C, Motiwala F, Mainali P, Mansuri Z. Trends for electroconvulsive therapy utilization in children and adolescents in the United States from 2002 to 2017: a nationwide inpatient sample analysis. J ECT. (2021) 37:100–6. doi: 10.1097/YCT.0000000000000750
7. Kellner CH, Fink M, Knapp R, Petrides G, Husain M, Rummans T, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. (2005) 162:977–82. doi: 10.1176/appi.ajp.162.5.977
8. Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ECT. (2004) 20:13–20. doi: 10.1097/00124509-200403000-00004
9. Haq AU, Sitzmann AF, Goldman ML, Maixner DF. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry. (2015) 76:1374–84. doi: 10.4088/JCP.14r09528
10. Kaster TS, Vigod SN, Gomes T, Sutradhar R, Wijeysundera DN. Risk of serious medical events in patients with depression treated with electroconvulsive therapy: a propensity score-matched, retrospective cohort study. Lancet Psychiatry. (2021) 8:686–95. doi: 10.1016/S2215-0366(21)00168-1
11. Perugi G, Medda P, Toni C, Mariani MG, Socci C. The Role of Electroconvulsive Therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. (2017) 15:359–71. doi: 10.2174/1570159X14666161017233642
13. Cipriano A, Cella S. Nonsuicidal self-injury: a systematic review. Front Psychol. (2017) 8:1946. doi: 10.3389/fpsyg.2017.01946
14. Miller L. Depression in adolescents. New Eng J Med. (2021) 385:445–9. doi: 10.1056/NEJMra2033475
15. Edgcomb JB. Medication adherence among children and adolescents with severe mental illness: a systematic review and meta-analysis. J Child Adolesc Psychopharmacol. (2018) 28:508–20. doi: 10.1089/cap.2018.0040
16. Mead L, Ayres A, Blake JA. Monitoring of metabolic side-effects in children and adolescents prescribed antipsychotic medication: a systematic review. Aust N Z J Psychiatry. (2021) 55:763–71. doi: 10.1177/00048674211009620
17. Isacsson G. Antidepressants and the risk of suicide in young persons–prescription trends and toxicological analyses. Acta Psychiatr Scand. (2014) 129:296–302. doi: 10.1111/acps.12160
18. Sørensen J, Rasmussen A, Roesbjerg T, Verhulst FC. Suicidality and self-injury with selective serotonin reuptake inhibitors in youth: Occurrence, predictors and timing. Acta Psychiatr Scand. (2022) 145:209–22. doi: 10.1111/acps.13360
19. Rey JM. Half a century of ECT use in young people. Am J Psychiatry. (1997) 154:595–602. doi: 10.1176/ajp.154.5.595
20. Ghaziuddin N, Kaza M, Ghazi N, King C, Walter G. Electroconvulsive therapy for minors: experiences and attitudes of child psychiatrists and psychologists. J ECT. (2001) 17:109–17. doi: 10.1097/00124509-200106000-00005
21. Bilginer Ç. Knowledge, attitudes, and experience of child and adolescentpsychiatrists in Turkey concerning pediatric electroconvulsive therapy. Asian J Clin Psychiatry. (2019) 46:74–8. doi: 10.1016/j.ajp.2019.09.035
22. de Meulenaere M, de Meulenaere J. Experience, knowledge, and attitudes of child and adolescent psychiatrists in belgium toward pediatric electroconvulsive therapy. J ECT. (2018) 34:47–252. doi: 10.1097/YCT.0000000000000489
23. Pierson MD, Mickey BJ, Gilley LB, Weeks HR III. Outcomes of youth treated with electroconvulsive therapy: a retrospective cohort study. J Clin Psychiatry. (2021) 82:19m13164. doi: 10.4088/JCP.19m13164
24. Wang S, Yang C, Jia J, Zhou Y. Use of electroconvulsive therapy in adolescents with schizophrenia in China. Child Adolesc Psychiatry Ment Health. (2018) 12:49. doi: 10.1186/s13034-018-0254-z
25. W G. Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology Revised, Rockville (Maryland), U.S. Dept of Health, Education and Welfare, Public Health Service: Alcohol, Drug Abuse and Mental Health (1967).
26. Morosini PL, Magliano L, Brambilla L, Ugolini S. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. (2000) 101:323–9. doi: 10.1111/j.1600-0447.2000.tb10933.x
27. Puffer CC, Wall CA, Huxsahl JE. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. (2016) 26:632–6. doi: 10.1089/cap.2015.0139
28. Jacob P, Gogi PK, Srinath S, Thirthalli J, Girimaji S, Seshadri S, et al. Review of electroconvulsive therapy practice from a tertiary Child and Adolescent Psychiatry Centre. Asian J Psychiatr. (2014) 12:95–9. doi: 10.1016/j.ajp.2014.06.023
29. Grover S, Raju V, Chakrabarti S, Sharma A, Shah R, Avasthi A. Use of electroconvulsive therapy in adolescents: a retrospective study. Indian J Psychol Med. (2021) 43:119–24. doi: 10.1177/0253717620956730
30. Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. (2012) 61:397–406. doi: 10.1016/j.neuroimage.2011.11.080
31. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. (2013) 39:1129–38. doi: 10.1093/schbul/sbs118
32. Duan X, He C, Ou J, Wang R, Xiao J, Li L, et al. Reduced hippocampal volume and its relationship with verbal memory and negative symptoms in treatment-naive first-episode adolescent-onset schizophrenia. Schizophr Bull. (2021) 47:64–74. doi: 10.1093/schbul/sbaa092
33. Mørch-Johnsen L, Smelror RE, Andreou D, Barth C, Johannessen C, Wedervang-Resell K, et al. Negative symptom domains are associated with verbal learning in adolescents with early onset psychosis. Front Psychiatry. (2021) 12:825681. doi: 10.3389/fpsyt.2021.825681
34. Castaneda-Ramirez S, Becker TD, Bruges-Boude A, Kellner C, Rice TR. Systematic review: Electroconvulsive therapy for treatment-resistant mood disorders in children and adolescents. Eur Child Adolesc Psychiatry. (2022). doi: 10.1007/s00787-022-01942-7. [Epub ahead of print].
35. Shilton T, Enoch-Levy A, Giron Y, Yaroslavsky A, Amiaz R, Gothelf D, et al. A retrospective case series of electroconvulsive therapy in the management of comorbid depression and anorexia nervosa. Int J Eating Disorders. (2020) 53:210–8. doi: 10.1002/eat.23181
36. Ghaziuddin N, Shamseddeen W, Gettys G. Electroconvulsive therapy for the treatment of severe mood disorders during adolescence: a retrospective chart review. J Child Adolesc Psychopharmacol. (2020) 30:235–43. doi: 10.1089/cap.2019.0054
37. Karayagmurlu A, Coşkun M, Elboga G, Ghaziuddin N, Karayagmurlu E, Gökçen C, et al. Efficacy and Safety of electroconvulsive therapy in adolescents: a retrospective chart review study from Turkey. J ECT. (2020) 36:54–9. doi: 10.1097/YCT.0000000000000602
38. Medda P, Barbuti M, Novi M, Boccolini A, Tripodi B, Simone D, et al. Naturalistic follow-up in bipolar patients after successful electroconvulsive therapy. J Affective Disorders. (2020) 271:152–9. doi: 10.1016/j.jad.2020.03.079
39. Gignac A, McGirr A, Lam RW. Recovery and recurrence following a first episode of mania: a systematic review and meta-analysis of prospectively characterized cohorts. J Clin Psychiatr. (2015) 76:1241–8. doi: 10.4088/JCP.14r09245
40. Kranaster L, Jennen-Steinmetz C. A novel seizure quality index based on ictal parameters for optimizing clinical decision-making in electroconvulsive therapy. part 2: validation. Eur Arch Psychiatry Clin Neurosci. (2019) 269:859–65. doi: 10.1007/s00406-018-0962-7
41. Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D. The burden of mental disorders. Epidemiologic Rev. (2008) 30:1–14. doi: 10.1093/epirev/mxn011
42. Green MF, Horan WP. Social cognition in schizophrenia. Nat Rev: Neurosci. (2015) 16:620–31. doi: 10.1038/nrn4005
43. Porter RJ, Baune BT, Morris G, Hamilton A, Bassett D, Boyce P, et al. Cognitive side-effects of electroconvulsive therapy: what are they, how to monitor them and what to tell patients. BJPsych Open. (2020) 6:e40. doi: 10.1192/bjo.2020.17
44. Rajkumar AP, Petit CP, Rachana A, Deinde F, Shyamsundar G, Thangadurai P, et al. Correlates of self-reported, autobiographical, and mini-mental status examination defined memory deficits following electroconvulsive therapy in South India. Asian J Psychiatr. (2018) 34:47–53. doi: 10.1016/j.ajp.2018.04.016
Keywords: adolescent, mental illness, electroconvulsive therapy, retrospective study, efficacy
Citation: Si Q, Zhang X, Lei J, Chen C, Ren F, Xu G, Li Y and Sui Y (2022) Electroconvulsive therapy efficacy in adolescents with mental illness: A retrospective comparison. Front. Psychiatry 13:990660. doi: 10.3389/fpsyt.2022.990660
Received: 11 July 2022; Accepted: 18 August 2022;
Published: 09 September 2022.
Edited by:
Murat Ilhan Atagün, Çanakkale Onsekiz Mart University, TurkeyReviewed by:
Shile Qi, Nanjing University of Aeronautics and Astronautics, ChinaRikinkumar S. Patel, Duke University Medical Center, United States
Copyright © 2022 Si, Zhang, Lei, Chen, Ren, Xu, Li and Sui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxiu Sui, c3VpeXV4aXVAYWxpeXVuLmNvbQ==; Yuan Li, TGl5dWFuOTd5MzVAMTYzLmNvbQ==
 Qi Si1
Qi Si1 Congxin Chen
Congxin Chen Yuxiu Sui
Yuxiu Sui