- 1Department of Psychiatry, Psychotherapy and Psychosomatics, Bezirkskrankenhaus Augsburg, Medical Faculty, University of Augsburg, Augsburg, Germany
- 2Department of Psychiatry and Psychotherapy, University Hospital, Ludwig Maximilian University of Munich, Munich, Germany
Introduction: The impact of psychiatric medications and their enhancing or impairing effects on physical performance remains inconclusive. Therefore, with this systematic review we provide a comprehensive overview of frequently used psychotropic drugs and their effects on physical performance for the purpose of providing empirical information and deriving prescription and therapy recommendations for clinical practice.
Methods: We systematically searched PubMed, PsycInfo, and Cochrane databases and extracted human studies investigating the effect of psychotropic drugs on parameters associated with the level of physical performance, such as exercise time, oxygen consumption, heart rate, muscle contraction or blood lactate concentration in physically healthy participants. 36 studies - comprising a broad range of psychotropic agents, such as antidepressants, antipsychotics, sedatives, and stimulants - were selected for final analyses.
Results: Most studies (N = 32) were randomized controlled trials (RCT) with a double-blind crossover design. Antidepressants (N = 21) were the most frequently studied drug class, with contradictory results e.g., performance enhancement in warm environment but not in temperate conditions for bupropion or inconsistent findings between studies for other antidepressants. Antipsychotics (N = 3) mainly showed impairing effects on physical performance, while stimulants (N = 4) were often performance-enhancing. Sedatives (N = 9) may cause a hangover effect.
Conclusion: The examined studies with heterogeneous design showed different effects of psychiatric medications on physical performance. Antipsychotics seemed to be performance impairing, while the findings for antidepressants and sedatives were more inconsistent. Stimulants were the only group with consistent performance-enhancing effects. However, most studies were conducted with a small sample size (N < 10), mostly in well-trained subjects rather than in patients with psychiatric disorders, and most studies used single-dose designs. These issues impede the formulation of generalized conclusions for treatment regimes and should therefore be considered in further longitudinal studies for clinically reliable statements. Nevertheless, answering our research question is quite relevant for clinical practice and therapeutic prescription and should be further investigated especially considering the high drop-out rates in drug treatment.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=276103], identifier [CRD42021276103].
Introduction
Psychopharmacological drugs play a pivotal role in the treatment of severe mental illness. Despite indisputable benefits in treatment regimes, the intake of the medication also bears risks and side-effects. Possible side-effects in terms of exercise performance impairments may include, among others, fatigue, muscle stiffness or weakness and these side-effects can negatively impact physical performance (1, 2).
In the past years, physical exercise has become increasingly important in therapeutic regimes of various psychiatric diseases, e.g., aerobic exercise of moderate-vigorous intensity or in combination with resistance training at a frequency of 2–3 times a week, achieving 150 min of moderate-to-vigorous physical activity (3). Physical exercise in this context is also beneficial for the amount of visceral and epicardial adipose tissue and for factors constituting the metabolic syndrome (4). Therefore, side-effects of psychiatric medications with negative impact on physical performance should be strictly avoided.
Previous scientific background for the effects of psychiatric medication on athletic performance was mainly provided in the population of competitive athletes and healthy people. For example one experimental study with N = 6 subjects showed that 70 mg of fluoxetine (selective serotonin reuptake inhibitor = SSRI) reduced the ability to perform prolonged exercise on a bicycle ergometer (5). However, the intake of bupropion (norepinephrine and dopamine reuptake inhibitor = NDRI) increased physical performance in the heat after acute administration (6). Another study examined the intake of tricyclic antidepressants (desipramine 3 mg/kg body weight) in 9 children and 13 adults with no connection to competitive sports. A comparison of treadmill exercise tests before and after a single dose showed no differences in performance, but slight changes in blood pressure and heart rate (7). In summary, the studies were conducted in small sample sizes and results for antidepressive agents were inconclusive. Concerning medication with impact on sleep and anxiety, existing data is also inconsistent. Studies on the so-called Z-substances could not identify impaired athletic performance measured with a 50-m-sprint test after taking two doses of zolpidem 10 mg with N = 8 subjects (8), as well as no increased endurance time after a running time test with two doses of zopiclone 7,5 mg in N = 8 athletes (9). This is in contrast to buspirone with increased exhaustion after a 45 mg single dose in N = 13 athletes after a cycled ergometer test at 80% of maximum oxygen consumption (VO2max) (10). Other studies found negative effects of melatonin 6 mg in N = 23 subjects on psychomotor performance (11).
The described studies mostly referred to healthy adults. Investigations in children/adolescents and elderly people are rare. One study with N = 45 showed that the physical activity of adolescents treated with psychotropic medications was significantly impaired compared to adolescents without medication and to healthy controls (12). Similar results were described among elderly people. A 4-year prospective cohort study conducted at the end of the 1980s with N = 885 older women (over 70 years) showed that the regular use of benzodiazepines had a negative effect on physical performance, such as walking speed or balance (13).
The occurrence of distressing side-effects often lead to the discontinuation of psychotropic medication and should be considered during treatment (14). The impairments of physical performance displayed above are not restricted to athletes, but might be relevant in a larger context to all treated patients. For patients, it might be difficult to differentiate between subjective side-effects, such as tiredness or fatigue, and objectively measurable impairments in physical performance. Therefore, there is a need to systematically investigate the impact of psychotropic mediation on physical performance as which might have been neglected in the scientific literature despite its relevance.
With this systematic review we want to provide the first comprehensive overview of frequently used psychotropic drugs (antidepressants, antipsychotics, sedatives, and stimulants) and their effects on physical performance in order to provide founded information and derive prescription and therapy recommendations for clinical practice.
Objective
To the best of our knowledge, this is the first systematic review summarizing psychopharmacological effects on physical performance parameters. This overview will help to adequately inform patients and clinicians to avoid wrong conclusions regarding the negative impact of psychotropic agents on physical performance.
Methods
Search strategy
This systematic review was registered on PROSPERO (CRD42021276103). We systematically searched PubMed, PsycInfo and Cochrane databases with all combination of the following search terms: psychotropic drugs OR psychiatric medication OR serotonin reuptake inhibitor OR antidepressant drugs OR antipsychotic drugs OR dopamine reuptake inhibitor OR antidepressants OR antipsychotics OR sedatives OR anxiolytics OR hypnotics OR mood stabilizer AND athletic performance OR exercise performance OR physical performance OR physical fitness OR exercise testing OR aerobic capacity OR elite athletes.
The database search was last updated on August 05, 2022. All citations were screened for relevance by title in a first step, by abstract in a second step and by full-text in a last step. In all included records, the citations were screened manually for further relevant studies that may have not been detected by the systematic search. The systematic literature search and selection was performed by AH, the selection was afterward reviewed independently by DS. Both AH and DS retrieved the relevant information, and the results were compared. In case of disagreement, a third author (AR) was consulted. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were considered.
Eligibility criteria
We included studies that contain an evaluation of psychotropic drugs on physical performance, such as exercise time, oxygen consumption, heart rate, muscle contraction or blood lactate concentration in physically healthy adult human participants. No limitations were defined for the type of psychiatric disorder and the type of studies. All studies that were published until December 31, 2021, in English language were considered. We excluded studies without specific data about physical fitness, animal, preclinical and molecular studies as well as expert opinions and position statements.
Quality assessment
Each publication was reviewed using the Scottish Intercollegiate Guidelines Network (SIGN) methodology checklist which assess the internal validity as well as the external validity of our included studies (15). The more the required criteria (e.g., appropriate and clearly focused question, randomization, adequate concealment method, blinding, or percentage of dropout) can be agreed to, the higher the quality is rated. A distinction is made between high quality, acceptable, low quality and unacceptable. We further used the simple Jadad Scale quickly assessing the methodological quality of a clinical trial that included three items (randomization, blinding, and withdrawals/dropouts) and is evaluated with a point system via yes-and-no questions (16).
Results
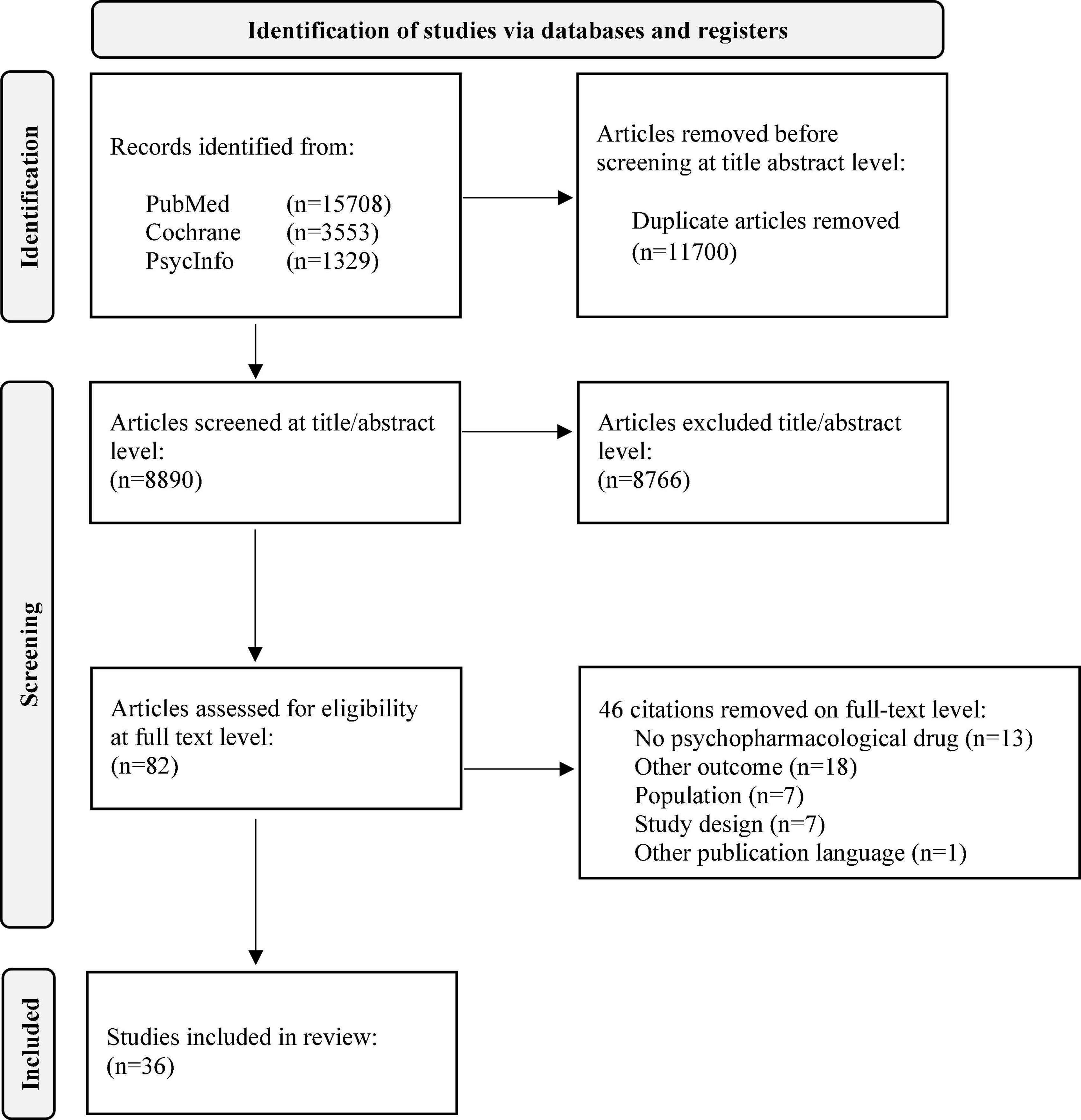
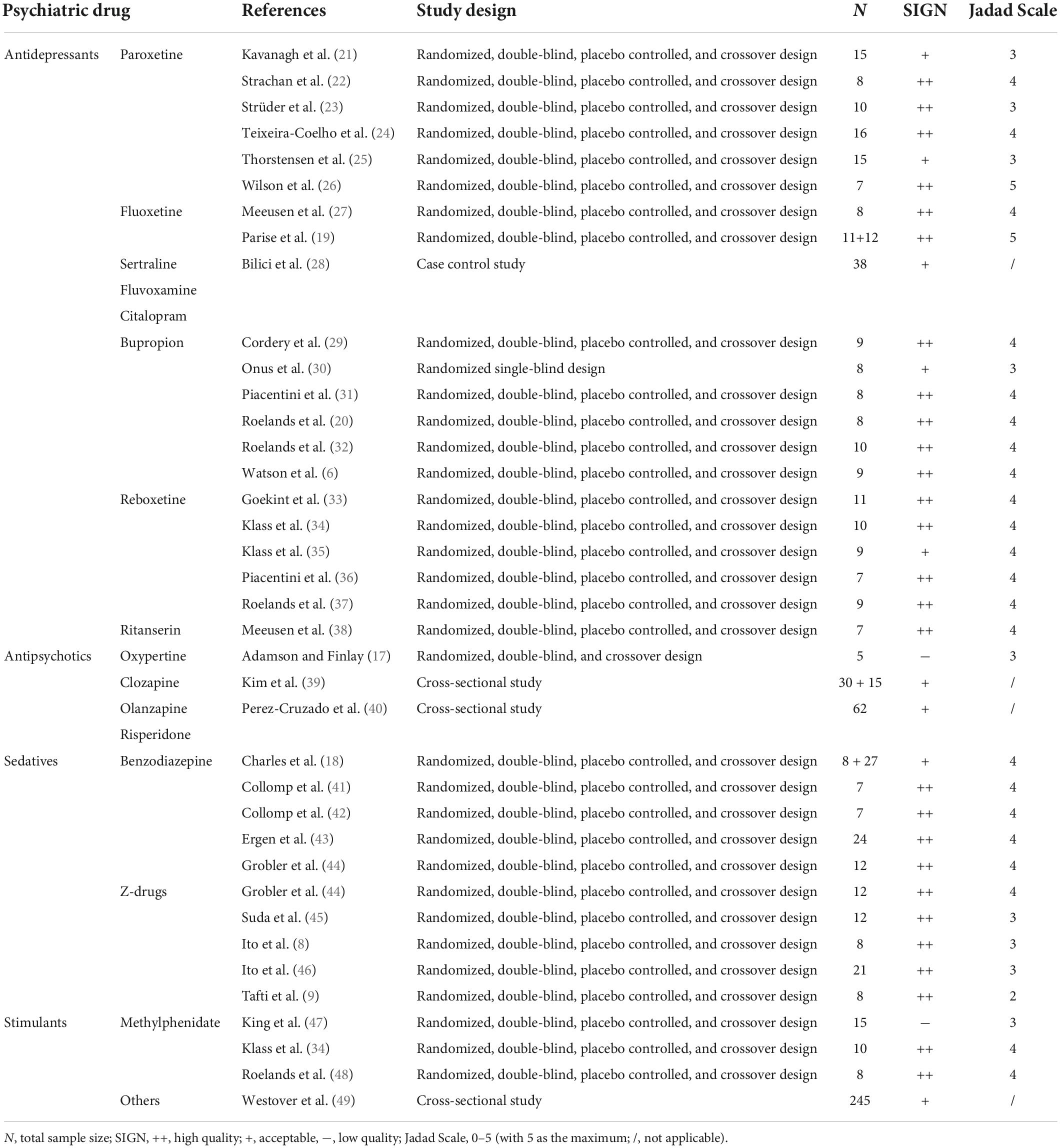
We were able to include 36 studies in the final analysis (see Figure 1). A detailed description of included publications and study types as well as the reviewed quality scores are shown in Table 1.
Study selection
The initial search without further restrictions resulted in 20,590 citations. After elimination of duplicates, 8,890 citations were included for further analysis. The screening on title/abstract level eliminated 8,766 citations (124 remaining). After full-text screening, 36 citations were considered for final analysis. Figure 1 presents the PRISMA chart.
Study characteristics
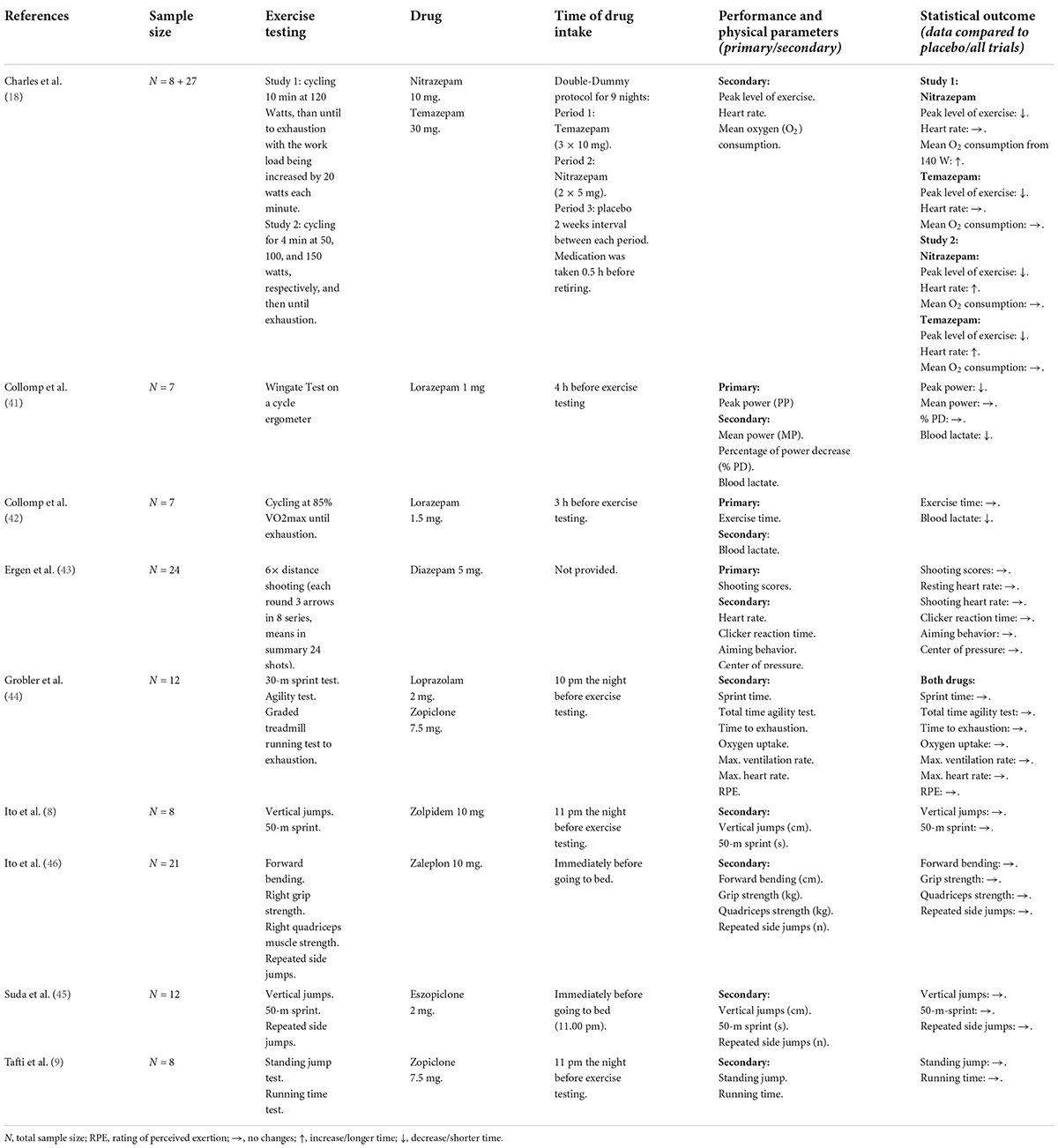
For a better overview, we divided the included studies into four groups: antidepressants (N = 21), antipsychotics (N = 3), sedatives (N = 9), and stimulants (N = 4). The experimental testing consisted of isokinetic measurements of individual muscle (groups) and bicycle cycling at the individual percentage of VO2max or maximum wattage (Wmax) and physical fitness tests (e.g., vertical jumps, standing jumps, and sprints). Across all RCTs, the mean sample size was N < 10 with a minimum of 5 (17) and a maximum of 27 (18). The drugs were mostly administered as a single dose and taken the night before or in the morning of the experiment. Continuous administration was only given in two studies (19, 20).
Results of individual studies
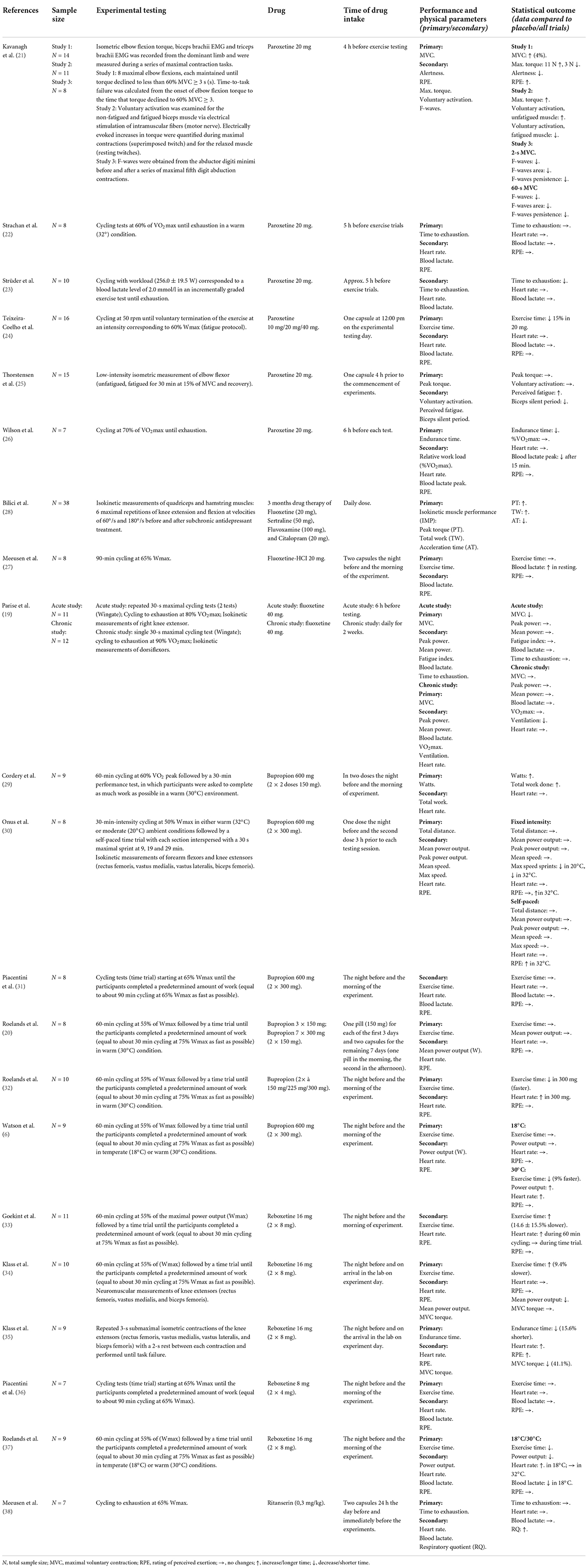
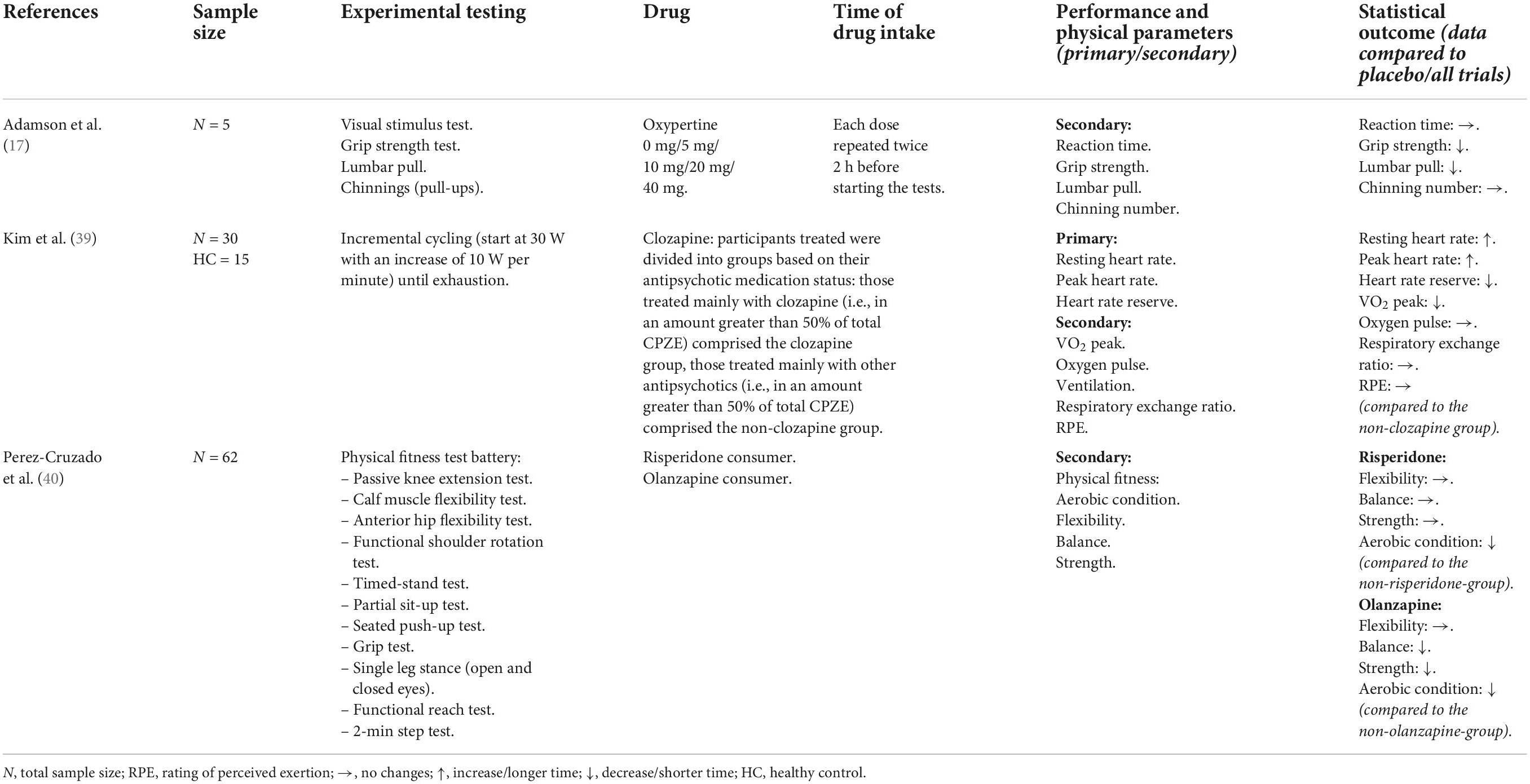
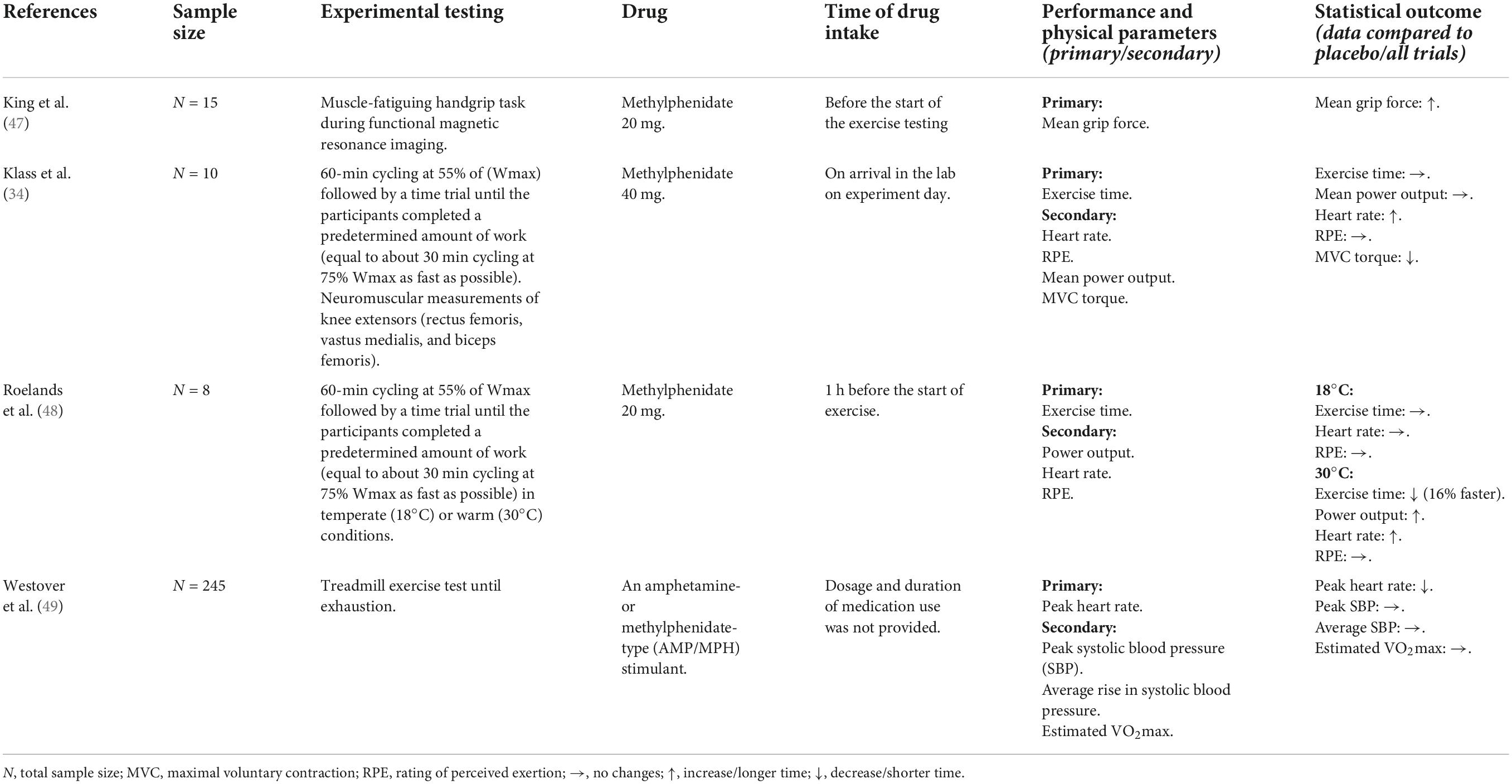
A detailed summary of all publications including study population, experimental testing design, drug, time of drug intake and relevant physical performance outcomes is provided for antidepressants in Table 2, for antipsychotics in Table 3, for sedatives in Table 4, and for stimulants in Table 5. The statistical data are shown in the Supplementary material.
Antidepressants
Our search resulted in 21 studies that met our inclusion criteria. The majority examined the effects of antidepressants (fluoxetine, paroxetine, bupropion, and reboxetine) on physical/athletic performance, which were measured through ergometric cycling or isokinetic muscle measurements. There were 17/21 studies for antidepressants, that only recruited male participants with similar age (approximately between 19 and 27 years old). There is only one study with female and just 2/21 studies with mixed participants.
Effects of paroxetine
Our search resulted in six publications examining paroxetine (21–26). All drug administration of paroxetine were given as a single-dose of 20 mg in physically active men (21–26) and women (21, 25). In addition, one study further compared 10 and 40 mg of paroxetine but they did not affect physical performance (24). Another study showed that paroxetine did not influence exercise duration of cycling as well (22). Three other studies found that paroxetine decreased physical performance in cycling (23, 24, 26). Paroxetine increased activation of unfatigued muscle but exacerbated central fatigue during prolonged sustained contractions (21). During a sustained submaximal contraction, paroxetine had no influence on motor performance or on the development of central fatigue (25). Heart rate and lactate blood concentration increased during exercise but did not differ between trials (22–24, 26).
Effects of fluoxetine
Exercise performance during cycling was not affected by fluoxetine (20 mg) in well-trained young men (27). Another RCT examined that there were no significant effects of fluoxetine on strength or high-intensity exercise performance in young male athletes and no parallel change in muscle strength of knee extensors was measured after acute fluoxetine administration (40 mg, 6 h before testing) (19). There was no suppression of maximal voluntary contraction strength with continuous fluoxetine administration (40 mg, every day for 2 weeks) (19). Blood lactate concentration during exercise testing was only measured in one study (27). Lactate increased throughout exercise but was not influenced by fluoxetine. Another study examined further SSRI and showed that the isokinetic muscle performance of depressed middle-aged patients (m/w) was improved after a 3-month treatment with different SSRI (fluoxetine, sertraline, fluvoxamine, or citalopram) (28).
Effects of bupropion
Six publications examined the effects of bupropion (6, 20, 29–32). One clinical trial showed that the acute administration of a single dose of bupropion (2 × 300 mg) during prolonged exercise cycling in a temperate conditions had no effect on performance in well-trained male cyclists (31). But in warm conditions (30°), acute bupropion administration enabled endurance-trained males to 9% faster completed time trials (cycling) in the bupropion condition compared to placebo (6). Two further trials supported this finding, both in physically active women (29) and in well-trained male cyclists (32). Continuous administration of bupropion (10 days of medication) caused no differences in cycling performance in trained males (20). Neuromuscular data showed that maximal voluntary contraction and voluntary activation were unaffected by the administration of bupropion (2 × 300 mg) during cycling in temperature (20°) and warm (32°) conditions in physically active males (30). Heart rates increased over time during exercise cycling (6, 20, 31, 32) and exercise intensity (30), but were not influenced by drug administration. The results of Cordery et al. (29) showed significant changes in heart rate, but only at the end of the exercise test. Blood lactate concentration was not influenced by bupropion (31). The subjects of all six studies were of similar age (mid-late 20s).
Effects of reboxetine
We identified five randomized controlled trials with reboxetine (33–37). All drug administration of reboxetine were given as a single-dose, examined in young physically active males. One study showed no differences in cycling performance between reboxetine (8 mg) intake and placebo (36). Nearly all other studies showed decreases in exercise performance after reboxetine (16 mg) (33–35, 37). Lactate blood concentration (36) and heart rate (33, 36) changed but were not influenced by reboxetine intake. But in one study, heart rate was significantly higher in the reboxetine condition compared to placebo in cooler environment (18°) (37). This drug-induced effect could not be replicated in the heat (30°) (37). Blood pressure was not influenced by drug. At the end of the time trials and after recovery in reboxetine (18°), the blood lactate concentrations were significantly lower than during placebo (18°) (37).
Effects of a further antidepressant drug
The results of one study showed that a specific centrally acting 5-HT2A/2C antagonist (5-Hydroxytryptamin/Serotonin, single-dose Ritanserin, 0,3 mg/kg) did not influenced the time to exhaustion in cycling in trained males (38). Respiratory quotient at the end of exercise was significantly higher in Ritanserin group, while the increase during exercise was comparable in all groups (38).
Antipsychotics
Our search resulted in three publications using the antipsychotic drugs oxypertine (17) (rarely used in clinical practice), clozapine (39), olanzapine and risperidone (40). There are 2/3 studies that recruited mixed participant and 1/3 study was only with male participants. In all studies the subjects were approximately between 20 and 30 years old. Oxypertine was examined in different single-doses from 0 to 40 mg. Physical performance in several exercise tests (muscular force, muscular endurance) in trained athletes (gender unknown) declined progressively as the dosage was increased (17). Negative effects were also found with olanzapine and risperidone (no single-dose) in several physical exercise tests (flexibility, balance, strength, and aerobic condition) examined in a cross-sectional study with middle-aged patients (m/w) (40). Clozapine treatment (no single-dose) was associated with reduced cycling performance in middle-aged patients (m/w) with schizophrenia or schizoaffective disorder compared with non-clozapine antipsychotics (39).
Sedatives
Our search resulted in nine studies using benzodiazepines (18, 41–44) and z-drugs (8, 9, 44–46). For sedatives there are 4/7 studies that recruited mixed participants and 3/7 were only with males. In all studies the subjects were approximately between 18 and 26 years old.
Benzodiazepine
One study applied temazepam 30 mg, nitrazepam 10 mg and placebo for 9 days in young university students (m/w) (18). On day 2, maximal attained exercise levels in cycling with temazepam 30 mg and nitrazepam 10 mg were comparable to placebo, on day 9, physical performance with nitrazepam was lower than with temazepam and placebo (18). One study showed that 4 h after drug intake, a single-dose of lorazepam 1 mg impaired anaerobic peak power and induced a significant decrease in blood lactate concentration during a Wingate test in healthy volunteers (m/w) (41), while another study found that 3 h after a single-dose of lorazepam 1.5 mg time of cycling was not significantly changed in young male triathletes (42). Diazepam 5 mg (exact time of intake was not provided) was shown to not significantly change shooting performance in Turkish archers (m/w) (43). Ten hours after night-time administration, a further study showed no significant impairment of physical performance in sprint, agility and running after a single-dose of loprazolam 2 mg, but drug intake resulted in a greater hangover effect physically active volunteers (m/w) (44).
Z-drugs
Five further studies examined the effects of z-drugs on physical performance with no significant adverse effects on performance. Two RCTs could not identify impaired athletic performance measured with a vertical jump and a 50-m sprint after taking zolpidem 10 mg (8), as well as no increased endurance time after a running test with zopiclone 7,5 mg (9), both with two doses over two nights in young male athletes. As well, 10 h after night-time administration of single-dose zopiclone 7.5 mg physical performance in sprint, agility and running was not significantly impaired in physically active volunteers (m/w) (44). A recent study assessed the residual effects of a single-dose eszopiclone 2 mg on physical performance in male athletes and resulted in no significant differences in vertical jump, 50-m sprint and repeated side jumps (45), as well as a single-dose of zaleplon 10 mg that did not affect physical performances in forward bending, grip strength, quadriceps strength and side jumps (46).
Stimulants
For stimulants there are 2/4 studies that recruited mixed participants and 2/4 were only with males. In all studies the subjects were approximately between 26 and 42 years old. In contrast to sedatives, stimulants could possibly enhance physical performance. This hypothesis was supported by the results of two studies with acute administration of methylphenidate 20 mg after a handgrip task (in young healthy volunteers m/w) and cycling (in young well-trained males) (47, 48), while one study revealed no significant changes in cycling performance after single-dose methylphenidate 40 mg in well-trained males (34). A cross-sectional study examined stimulant medication use and maximal exercise running test outcomes in a large community sample (49). Stimulant medication use was not associated with increases in peak systolic blood pressure (SBP) and average SBP rise, nor did it impact cardiorespiratory fitness (VO2max). Dosage and duration of use of medications were not available (49). Heart rate significantly increased by methylphenidate in warm condition (48) and was higher at the end of the time trial for methylphenidate than for the placebo (34). But it was also shown, that stimulant medication users had a significantly lower peak heart rate (49).
Discussion
With 33 included studies, this is the first systematic review examining the effects of psychiatric medication on physical performance in physically healthy adult humans. Differentiated by their medical compound, dose and period of use, psychotropic drugs can have performance-limiting or performance-enhancing effects. These findings can help to provide further information about the medication to the patients and thereby lead to reduced discontinuation rates. In the following, we discuss four medication groups separately (antidepressants, antipsychotics, sedatives, and stimulants).
Antidepressants
Within the antidepressants, nine studies have been conducted examining the effects of SSRI on exercise performance using paroxetine (21–26) and fluoxetine (19, 27). Three studies (22, 23, 26) with comparable dosages and application times of paroxetine led to contradictory conclusions. Two studies (23, 26) showed reduced total exercise time, one did not observe any difference after the intake of paroxetine (22). In contrast, another study (22) using the same dose and same time of administration did not observe any difference in total exercise time. The major difference in the study protocols and therefore a possible explanation for the different findings was the manipulation of the ambient temperature (30°C) (22) at which the exercise was performed compared to that of the previous studies (23, 26). It has been suggested that serotonin may play a role in thermal regulation and elevated body temperature is a major factor in the development of fatigue during prolonged exercise (50). The results suggest that oral administration of 20 mg paroxetine did not significantly influence the thermoregulatory factors that may limit exercise capacity (22). Based on these results, the authors of a later published study (24) hypothesized that the dose of 20 mg paroxetine might be insufficient to consistently influence the serotonergic system and consequently physical performance. In their study, they tested doses ranging from 10 to 40 mg of paroxetine. Surprisingly, the higher dose of 40 mg did not affect physical performance. Physical performance only decreased after administration of 20 mg of paroxetine (24). The authors explained this difference based on the pharmacological profile of this agent. Paroxetine increases the extracellular concentration of brain 5-HT, which can promote the release of dopamine (51). A higher dose of 40 mg paroxetine may result in a higher stimulation of 5-HT3 receptors compared to 20 mg of paroxetine, thus increasing dopamine release into the synaptic cleft (24). Furthermore, the authors assumed that 5-HT receptor responses could be influenced by an individual’s aerobic capacity level. They suggest that individuals with higher aerobic capacity would be less affected by psychiatric medication than those with a low aerobic capacity level. However, their results revealed decreased physical performance in the high aerobic capacity group which suggested that individuals with higher aerobic capacity might be more responsive to pharmacological activation of the serotonergic system during exercise (24). The authors discussed various explanations for this observed phenomenon, such as higher body fat content in volunteers with lower aerobic capacity, differences in thermoregulation or changes in carbohydrate availability or acid-basic balance between the groups, but they could not postulate a ‘one-size-fits-all’ hypothesis (24).
Two studies observed no impact of fluoxetine on physical performance, neither after acute (19, 27) nor after continuous drug administration (19). The authors of another study grappled with the central fatigue hypothesis, which states that an increased concentration of brain 5-HT as a result of a long-term exercise may contribute to the perception of central fatigue. However, their results showed that subjects were not hindered by the central fatigue hypothesis during the 80 and 90% VO2max trials (19) also not in the previously mentioned study during 65% Wmax (27). This can possibly be attributed to peripheral mechanisms that contribute to fatigue during short duration exercise training (52). A previously published study showed that, at a lower intensity, there is an earlier onset of fatigue during 70% VO2max cycle ergometer test, after the administration of a SSRI (paroxetine) (26). Similarly, subjects expressed a greater perceived exertion during a cycle ergometer test after receiving fluoxetine (5). Both of the cited studies indicated that SSRI administration may play a role in fatigue during endurance exercise. Interestingly, the results of the chronic study (cycling at 90% VO2max) showed reduced ventilation (7.5%) during the fluoxetine trial with no alteration in performance (19). The authors discussed that this may have been caused by inhibitory and excitatory 5-HT receptors that have been identified in the respiratory system of the cat (53). The role of the 5-HT metabolism during exercise is very complex which was also discussed in Kavanagh et al. (21) and Thorstensen et al. (25). The studies showed that with increased 5-HT availability voluntary muscle activation and torque generation increased during unfatigued maximal contraction and in contrast, the ability to generate maximal torque was compromised under fatigue conditions (21). The authors suggested that serotonergic drive, that occurred with prolonged maximal contractions, provided a spinal mechanism by which higher concentrations of 5-HT may contribute to central fatigue (21). Interestingly, during sustained submaximal contractions, enhanced availability of 5-HT did not directly influence motor performance (25). Only strong fatiguing contractions causing strong serotonergic drive may cause 5-HT-associated reductions in motor output. It should be further studied to understand its mechanisms of action during exercise and especially its role in the onset of fatigue. However, the serotonergic system (could potentially) impact fatigue during exercise, but the mechanisms underlying this relationship remain elusive. Of the mentioned studies investigating how 5-HT concentration affects exercise performance, most of them used well-trained/physically active subjects (19, 22–24, 26, 27). In fact, only one publication included patients in their study. Its results showed that isokinetic muscle performance in patients with major depressive disorder was reduced compared to healthy controls. After 3 months of antidepressive treatment (comprising a trial of fluoxetine), muscle performance improved and the patients approached levels similar to the healthy control group. The authors suggested that this may be related to an increasing metabolism in brain structures, such as prefrontal cortex and basal ganglia (28). Although two other studies identified SSRI-mediated changes in central fatigue during sustained isometric contraction, their participants had no training history (21, 25), SSRI responses may be associated with the type of exercise as well as the individual’s fitness level which was discussed earlier (24). In conclusion, it can be stated that the here presented studies have mostly shown limitations in physical performance. The explanations for the mentioned impairments may relate to the modification of central fatigue, which has not been adequately investigated so far. Moreover, there is no data on the nowadays commonly used preparations citalopram, escitalopram and sertraline.
With its norepinephrine dopamine reuptake inhibitor function, bupropion does not typically cause weight gain or sedation (54). The included studies suggest that there may be performance enhancement in heat (6, 29) but not in temperate conditions (31). Similar performance and thermoregulatory effects did not occur after continuous administration of bupropion over several days, which might be due to an adaptation of central neurotransmitter homeostasis (20). Further examinations (32) investigated a dose-response relationship. The study demonstrated that an ergogenic effect occurred when the highest dose (600 mg) of bupropion was taken, which is consistent to prior findings (6). Bupropion was shown to cause a suppressed sensation of heat enabling humans to push themselves to higher temperature without perceiving greater effort (6). This may potentially increase the risk of developing heat illness (6). Because of its ergogenic effect, which can lead to enhanced performance, it is included in the Monitoring Program of WADA (World Anti-Doping Agency) competition but is not prohibited in competition (54).
Similar examinations have been conducted with the norepinephrine reuptake inhibitor reboxetine. One study (36) found no effect of reboxetine 8 mg on performance (36). In contrast, two other studies showed that this medication may limit performance (33, 37). To identify the mechanisms that contributed to the decrease in performance after reboxetine 16 mg (37), other researchers analyzed the potential link between neural impairments and changes in brain neurotransmission (34, 35). Their results suggest that noradrenaline reuptake inhibition could contribute to the development of supraspinal fatigue observed after a prolonged cycling exercise (34) and after a fatiguing task involving intermittent submaximal contractions (35). Since corticospinal excitability was not modified after both exercising tests, norepinephrine appears to more specifically affect supraspinal centers located prior to the motor cortex.
In conclusion, the studies of antidepressants on physical performance must be considered heterogeneous and thus the observed effects inconsistent. Possible reasons for this include differences in study protocols (doses and acute versus continuous intake of the medication) and limitations (e.g., usually very small sample sizes). Only bupropion 600 mg resulted in performance enhancement in heat (6, 29, 32) but not in temperate conditions (31) with risk of contracting heat illnesses. Other antidepressants (paroxetine, fluoxetine, and reboxetine) led to impairments or showed no medication-induced changes during exercise. The mentioned drugs are usually not related to sedation. Therefore, further research is needed to elucidate the effects of psychotropic drugs that can cause greater sedation (e.g., mirtazapine or tricyclic antidepressants).
Antipsychotics
The effects of antipsychotics are more consistent and point to limited physical performance, even though only three studies could be included in this review (17, 39, 40). One study showed dose-dependent effects, with a gradual impairment as the dose level of oxypertine was increased from 5 to 40 mg (17). Another study showed that olanzapine had negative effects on muscle strength, balance and aerobic endurance and risperidone had negative effects on the amount of physical activity (40). The authors assumed that patients with severe psychiatric symptoms have a decrease in their activity levels and physical fitness in general. These facts may also be explained by side-effects such as increased fatigue or sedation, both of which might affect physical performance (40). These data confirm previous findings from a study that was not included in our review (different population), but showed that adults with severe mental illness (SMI) who were taking antipsychotic medication were less physically active and had lower body balance as well as muscular strength compared with unmedicated patients (12). Another study from our review showed that cardiovascular fitness was impaired during clozapine treatment which might be explained by the higher antagonistic activity at alpha-adrenergic receptors. This antagonism could lead to limited blood flow in the working skeletal muscle and compromise cardiovascular stability during exercise (39). We found no articles on the use of antipsychotic medication in athletes but based on the assumptions from the cited studies (12, 40), this collective might expect less limitations compared to those previously mentioned in patients. Due to a favorable side-effect profile, aripiprazole or lurasidone are recommended for this special collective (2). In addition, an important difference to the results from the antidepressants should be emphasized. The presented studies using antipsychotics have mostly included patients with a mental illness who have been taking the medication for a longer time period (39, 40). This may explain the consistent deterioration in physical performance with antipsychotic treatment. For antidepressants, mostly acute administration and well-trained subjects were investigated.
Sedatives
Sedatives are mostly used in clinical practice to treat insomnia and other sleep disturbances (55). They are a common issue for athletes after jet lag or as isolated symptoms in stressful situations. Commonly used sleep aids among the general population include benzodiazepines and z-drugs.
It would seem obvious that the intake of sedating medication at night may result in residual morning drowsiness, which may impair the participant’s motivation and may increase perceived exertion. However, these assumptions were not confirmed by the identified studies in our review. The intake of loprazolam (2 mg) and zopiclone (7.5 mg) at 10 pm the night before the exercise test resulted in unchanged physical performance (44). The authors suggested that zopiclone may have a weaker (self-reported) hangover effect compared to loprazolam, but both drugs did not significantly impair objectively measured physical performance (44). A greater number of subjects reported feeling alert after the ingestion of zopiclone without having any residual drowsiness the morning following the night-time administration. This can possibly be attributed to the different half-lives of zopiclone [4–5 h, (56)] and loprazolam [6–12 h, (57)]. Previously published studies that were not included in our review (missing data about physical fitness) supported that finding and stated that due to the relatively short half-life of zopiclone, the hangover effect the morning after night-time administration was minimal (56) and a 7.5 mg dose of zopiclone has been shown to have the optimum sedative properties with negligible side-effects (58). Similar effects are shown with three further ‘z’ agents that were used in double-doses but showed no adverse effect on physical performance (8, 9, 46). This can possibly be explained by their short plasma half-life and limited duration of action (59). Zaleplon is characterized by an ultrashort half-life (approximately 1 h). Zolpidem and zopiclone have longer half-lives (1.5–2.4 h and 4–5 h) (59). These properties, together with the low risk of residual effect, may explain the limited negative influences on physical performance (59). Instead, the authors concluded that zopiclone and zolpidem have useful hypnotic activity without disturbing physical performance on the following day (8, 9), suggesting that zolpidem may be used in healthy humans to adjust sleep disturbances (8).
Similar results were shown by another study evaluating psychomotor and physical performances on the following day after taking eszopiclone (half-life 5–6 h) at bedtime (45). The authors did not identify any impairment. Another study examined the longer acting benzodiazepines temazepam [half-life of 5.3 h (60)] and nitrazepam [half-life of 30 h (61)] and their effects on exercise performance using two separate study designs (study 1: drug for two nights, study 2: drugs for nine nights) with night-time administration (18). In study 1, at all work-loads both drugs showed an increase in ventilation, gas exchange and heart rate when compared to placebo suggesting that the subjects were working harder at all work loads and this might have reduced the maximum load they could achieve. In study 2, a similar trend in the maximal exercise load was displayed with a significant difference in the nitrazepam group on day 9 compared to temazepam and placebo. The authors attributed this finding to a probable hangover effect of nitrazepam (18).
Two further studies investigated the effects of benzodiazepines on physical performance (41, 42). Subjects who had been treated with lorazepam (1.5 mg) 4 h before cycling at 85% of their VO2max (submaximal exercise) showed no difference when compared to placebo but exhibited significantly lower values in selected hormones and lactate (42). On the other hand, subjects treated with lorazepam (1.0 mg) 3 h before exercising a Wingate Test (supramaximal exercise) showed significantly lower values in peak power and maximal lactate when compared to placebo (41). Authors in both studies suggested that the lower lactate values may be due to either a decrease in muscle glycogenolysis or a change in lactate removal (41, 42) but in Collomp et al. they did not find a clear association between drug effects and impairment in peak power (41). Melatonin is one of the most studied sleep aids among athletes (55), but no study met our inclusion criteria due to an inappropriate study design. However, one study indicated no impairment of performance the following day after intake (62).
In summary, due to the heterogeneous study designs (drug, dosage, and exercise testing) of the studies included in our review, it is difficult to derive consistent findings about sedatives and their effects on physical performance. Sedatives may result in residual morning drowsiness, which may impair the participant’s motivation but not directly physical performance which may be explained by different half-lives. Especially with regard to their use in sleep disorders or stage fright, sedatives should be further investigated, since reduced muscle strength and poorer running times in the endurance sport have already been reported (63).
Stimulants
Stimulants are known to be performance-enhancing and they are banned (only) in competition (64). This was first reported in the Journal of the American Medical Association (JAMA) (65). Since then, further studies have reported similar effects with performance enhancements in anaerobic capacity, strength, time to exhaustion, acceleration, and maximum heart rate (66). Some researchers suggest that athletes taking stimulants (e.g., methylphenidate) may be able to exercise to higher core temperature without any change in the perception of effort or thermal stress. The ergogenic effect was confirmed by one of our included studies (48). Subjects showed improved performance in the heat but not in temperate conditions without any greater perception of effort or thermal stress. Concerning this matter, stimulants might not only be ergogenic but also harmful in the heat as athletes may be unaware of increasing thermal/heat stress promoting heat illness (6, 48).
In another experiment, subjects demonstrated enhanced force and changes in brain connectivity throughout a muscle-fatiguing handgrip test using acute ingestion of 20 mg methylphenidate at room temperature (47). The authors discussed, that given agents may affect dopamine transmission and consequently cause an increase in fatigue and a decrease in motivation (67), methylphenidates influence on neurotransmission make subjects more willing to exert forces closer to their maximum (68). The ergogenic mechanism of methylphenidate remained elusive in this study, further experiments were proposed to detect the neurochemical underpinnings of motivation and muscle fatigue.
One RCT tested the effects of acute noradrenaline (reboxetine 8 mg) and acute dopamine (methylphenidate 40 mg) reuptake inhibitors on prolonged cycling exercise and supraspinal fatigue in a temperate environment (34). Compared to reboxetine, methylphenidate did not affect exercise performance and did not contribute to the development of supraspinal fatigue. It can be speculated that compared to dopamine, norepinephrine has an inhibitory effect on performance. The effects of reboxetine have already been discussed in the paragraph regarding antidepressants (34).
We were further able to identify a cross-sectional study examining stimulant medication use and maximal exercise test outcomes in a large community sample (49). In contrast to previous studies that have shown an increase in heart rate associated with acute application of methylphenidate (34, 48), this study showed a lower peak heart rate in stimulant medication users compared to non-users (49). The reasons were unclear, however, authors suggested that unmeasured confounding of stimulant use or confounding by contraindication and survivor bias caused lower peak heart rates.
Conclusion
This is the first systematic review evaluating the effects of psychiatric medication on physical performance. We identified studies with antidepressants, antipsychotics, sedatives and stimulants. Antipsychotics seemed to be performance impairing, while the findings for antidepressants and sedatives were more inconsistent. Stimulants were the only group with consistent performance-enhancing effects.
Several issues impede the formulation of generalized conclusions for treatment regimes and should therefore be further considered in future studies. Most studies were conducted in populations of athletes, limiting general transferability of the results. Although 27 of our included studies have powered their sample size calculation to the primary sport outcome, most of them were conducted in small sample sizes (N < 10). To get an impression of sample sizes for future studies, we made some assumption using G*Power (69). Assuming a two-sided independent t-test for a respective analysis, a power of 0.8 and an α-prolity of 0.05, a sample size of 34 subjects per group would be valid to identify a moderate effect size d = 0.5 and 19 subjects per group to identify a large effect size of d = 0.7 between two groups in a conservative between-group design. A within-group design using pared sample t-tests would results in slightly smaller sample sizes. Of our included studies five out of 36 included >26 subjects. Thus, most studies may have been underpowered and we recommend larger sample sizes for future studies. Moreover, most study protocols used single dose medication, which does not reflect real-world settings in clinical practice. Within the different medication groups, there was a bias toward specific drugs (e.g., six studies with paroxetine and none for the often-used venlafaxine). In addition, there were 17/21 studies for antidepressants, that only recruited male participants. This shows good comparability among these studies, since the age of the participants was almost identical, but there is only one study with female and just 2/21 studies with mixed participants. For antipsychotics, there are 2/3 studies, for sedatives there are 4/7 studies and for stimulants there are 2/4 studies that recruited mixed participants. However, future studies especially with antidepressants in female and mixed participants are necessary to be able to make sex-related conclusions and to state more details for the treatment reality.
Further research with longitudinal studies and therapeutic doses is recommended to focus on precisely these issues to be able to derive even more well-founded data and clinically reliable recommendations for therapy. We would suggest adding physical performance parameters such as e.g., exercise time, oxygen consumption, heart rate, muscle contraction or blood lactate concentration to the safety assessments in future clinical and approval studies for psychopharmacological compounds. On the one hand, one could assume that with an approval in psychopathology also physical performance parameters may improve. However, the here evaluated outcomes from mainly single-dose studies imply that a worsening of physical performance parameters need to be defined and evaluated as compound-specific side-effects. Moreover, with a deeper understanding of these effects of psychopharmacological compounds on physical performance parameters, medication prescriptions could become more tailored to individual requirements and addressed by specific side-management interventions. Less side effects due to impairments of physical performance (which might be misinterpreted as sedation) could lead to reduced discontinuation rates of treatment and therefore more successful treatments in terms of less discontinuation-based relapses.
Author contributions
AHi, DL, and AR conceived the study and performed the qualitative analyses. AHi wrote the first draft of this manuscript. AR and AlH supervised the project. EW provided methodological advice. All authors were involved in reviewing the manuscript and approved the final version of the manuscript.
Funding
This work was funded by an institutional grant of Medical Faculty (University of Augsburg) to AR (Sport study Move = Motivation for Exercise).
Conflict of interest
Within the last 5 years, AlH has received paid speakerships from Janssen, Otsuka, Recordati, and Lundbeck. He was member of Rovi, Recordati, Otsuka, Lundbeck, and Janssen advisory boards. He is the editor of the German AWMF S3 and the WFSBP schizophrenia guidelines.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.985983/full#supplementary-material
References
1. Johnston A, McAllister-Williams RH. Psychotropic drug prescribing. In: Currie A, Owen B editors. Sports Psychiatry (Oxford Psychiatry Library). (Oxford: Oxford University Press) (2016). p. 133–43. doi: 10.1093/med/9780198734628.003.0010
2. Reardon CL, Creado S. Psychiatric medication preferences of sports psychiatrists. Phys Sportsmed. (2016) 44:397–402. doi: 10.1080/00913847.2016.1216719
3. Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and position statement from the European psychiatric association (EPA), supported by the international organization of physical therapists in mental health (IOPTMH). Eur Psychiatry. (2018) 54:124–44. doi: 10.1016/j.eurpsy.2018.07.004
4. Kahl KG, Kerling A, Tegtbur U, Gützlaff E, Herrmann J, Borchert L, et al. Effects of additional exercise training on epicardial, intra-abdominal and subcutaneous adipose tissue in major depressive disorder: a randomized pilot study. J Affect Disord. (2016) 192:91–7. doi: 10.1016/j.jad.2015.12.015
5. Davis JM, Bailey SP, Jackson DA, Strasner AB, Morehouse SL. 438 Effects of a serotonin (5-H) agonist during prolonged exercise to fatigue in humans. Med Sci Sports Exerc. (1993) 25:78. doi: 10.1249/00005768-199305001-00440
6. Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol. (2005) 565:873–83. doi: 10.1113/jphysiol.2004.079202
7. Waslick BD, Walsh BT, Greenhill LL, Giardina EG, Sloan RP, Bigger JT, et al. Cardiovascular effects of desipramine in children and adults during exercise testing. J Am Acad Child Adolesc Psychiatry. (1999) 38:179–86. doi: 10.1097/00004583-199902000-00017
8. Ito SU, Kanbayashi T, Takemura T, Kondo H, Inomata S, Szilagyi G, et al. Acute effects of zolpidem on daytime alertness, psychomotor and physical performance. Neurosci Res. (2007) 59:309–13. doi: 10.1016/j.neures.2007.07.009
9. Tafti M, Besset A, Billiard M. Effects of zopiclone on subjective evaluation of sleep and daytime alertness and on psychomotor and physical performance tests in athletes. Prog Neuropsychopharmacol Biol Psychiatry. (1992) 16:55–63. doi: 10.1016/0278-5846(92)90008-3
10. Marvin G, Sharma A, Aston W, Field C, Kendall MJ, Jones DA. The effects of buspirone on perceived exertion and time to fatigue in man. Exp Physiol. (1997) 82:1057–60. doi: 10.1113/expphysiol.1997.sp004080
11. Paul MA, Gray G, Kenny G, Pigeau RA. Impact of melatonin, zaleplon, zopiclone, and temazepam on psychomotor performance. Aviat Space Environ Med. (2003) 74:1263–70.
12. Vancampfort D, Probst M, Daenen A, Damme TV, De Hert M, Rosenbaum S, et al. Impact of antipsychotic medication on physical activity and physical fitness in adolescents: an exploratory study. Psychiatry Res. (2016) 242:192–7. doi: 10.1016/j.psychres.2016.05.042
13. Gray SL, Penninx BW, Blough DK, Artz MB, Guralnik JM, Wallace RB, et al. Benzodiazepine use and physical performance in community-dwelling older women. J Am Geriatr Soc. (2003) 51:1563–70. doi: 10.1046/j.1532-5415.2003.51502.x
14. Undurraga J, Baldessarini RJ. Direct comparison of tricyclic and serotonin-reuptake inhibitor antidepressants in randomized head-to-head trials in acute major depression: systematic review and meta-analysis. J Psychopharmacol. (2017) 31:1184–9. doi: 10.1177/0269881117711709
15. Scottish, Intercollegiate, Guidelines, Network [SIGN]. SIGN 50: A Guideline Developer’s Handbook. (2021). Available online at: https://www.sign.ac.uk/what-we-do/methodology/checklists/ (accessed April 24, 2022).
16. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
17. Adamson GT, Finlay SE. A comparison of the effects of varying dose levels of oxypertine on mood and physical performance of trained athletes. Br J Psychiatry. (1966) 112:1177–80. doi: 10.1192/bjp.112.492.1177
18. Charles RB, Kirkham AJ, Guyatt AR, Parker SP. Psychomotor, pulmonary and exercise responses to sleep medication. Br J Clin Pharmacol. (1987) 24:191–7. doi: 10.1111/j.1365-2125.1987.tb03161.x
19. Parise G, Bosman MJ, Boecker DR, Barry MJ, Tarnopolsky MA. Selective serotonin reuptake inhibitors: their effect on high-intensity exercise performance. Arch Phys Med Rehabil. (2001) 82:867–71. doi: 10.1053/apmr.2001.23275
20. Roelands B, Hasegawa H, Watson P, Piacentini MF, Buyse L, De Schutter G, et al. Performance and thermoregulatory effects of chronic bupropion administration in the heat. Eur J Appl Physiol. (2009) 105:493–8. doi: 10.1007/s00421-008-0929-x
21. Kavanagh JJ, McFarland AJ, Taylor JL. Enhanced availability of serotonin increases activation of unfatigued muscle but exacerbates central fatigue during prolonged sustained contractions. J Physiol. (2019) 597:319–32. doi: 10.1113/JP277148
22. Strachan AT, Leiper JB, Maughan RJ. Paroxetine administration failed [corrected] to influence human exercise capacity, perceived effort or hormone responses during prolonged exercise in a warm environment. Exp Physiol. (2004) 89:657–64. doi: 10.1113/expphysiol.2004.027839
23. Strüder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K. Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Horm Metab Res. (1998) 30:188–94. doi: 10.1055/s-2007-978864
24. Teixeira-Coelho F, Uendeles-Pinto JP, Serafim AC, Wanner SP, de Matos Coelho M, Soares DD. The paroxetine effect on exercise performance depends on the aerobic capacity of exercising individuals. J Sports Sci Med. (2014) 13:232–43.
25. Thorstensen JR, Taylor JL, Tucker MG, Kavanagh JJ. Enhanced serotonin availability amplifies fatigue perception and modulates the TMS-induced silent period during sustained low-intensity elbow flexions. J Physiol. (2020) 598:2685–701. doi: 10.1113/JP279347
26. Wilson WM, Maughan RJ. Evidence for a possible role of 5-hydroxytryptamine in the genesis of fatigue in man: administration of paroxetine, a 5-HT re-uptake inhibitor, reduces the capacity to perform prolonged exercise. Exp Physiol. (1992) 77:921–4. doi: 10.1113/expphysiol.1992.sp003660
27. Meeusen R, Piacentini MF, Van Den Eynde S, Magnus L, De Meirleir K. Exercise performance is not influenced by a 5-HT reuptake inhibitor. Int J Sports Med. (2001) 22:329–36. doi: 10.1055/s-2001-15648
28. Bilici M, Cakirbay H, Koroglu MA, Guler M, Tosun M, Aydin T, et al. Isokinetic muscle performance in major depressive disorder: alterations by antidepressant therapy. Int J Neurosci. (2001) 110:9–23. doi: 10.3109/00207450108994218
29. Cordery P, Peirce N, Maughan RJ, Watson P. Dopamine/noradrenaline reuptake inhibition in women improves endurance exercise performance in the heat. Scand J Med Sci Sports. (2017) 27:1221–30. doi: 10.1111/sms.12753
30. Onus K, Cannon J, Liberts L, Marino FE. Acute effects of a dopamine/norepinephrine reuptake inhibitor on neuromuscular performance following self-paced exercise in cool and hot environments. J Therm Biol. (2016) 60:60–9. doi: 10.1016/j.jtherbio.2016.06.003
31. Piacentini MF, Meeusen R, Buyse L, De Schutter G, De Meirleir K. Hormonal responses during prolonged exercise are influenced by a selective DA/NA reuptake inhibitor. Br J Sports Med. (2004) 38:129–33. doi: 10.1136/bjsm.2002.000760
32. Roelands B, Watson P, Cordery P, Decoster S, Debaste E, Maughan R, et al. A dopamine/noradrenaline reuptake inhibitor improves performance in the heat, but only at the maximum therapeutic dose. Scand J Med Sci Sports. (2012) 22:e93–8. doi: 10.1111/j.1600-0838.2012.01502.x
33. Goekint M, Heyman E, Roelands B, Njemini R, Bautmans I, Mets T, et al. No influence of noradrenaline manipulation on acute exercise-induced increase of brain-derived neurotrophic factor. Med Sci Sports Exerc. (2008) 40:1990–6. doi: 10.1249/MSS.0b013e31817eee85
34. Klass M, Roelands B, Lévénez M, Fontenelle V, Pattyn N, Meeusen R, et al. Effects of noradrenaline and dopamine on supraspinal fatigue in well-trained men. Med Sci Sports Exerc. (2012) 44:2299–308. doi: 10.1249/MSS.0b013e318265f356
35. Klass M, Duchateau J, Rabec S, Meeusen R, Roelands B. Noradrenaline reuptake inhibition impairs cortical output and limits endurance time. Med Sci Sports Exerc. (2016) 48:1014–23. doi: 10.1249/MSS.0000000000000879
36. Piacentini MF, Meeusen R, Buyse L, De Schutter G, Kempenaers F, Van Nijvel J, et al. No effect of a noradrenergic reuptake inhibitor on performance in trained cyclists. Med Sci Sports Exerc. (2002) 34:1189–93. doi: 10.1097/00005768-200207000-00021
37. Roelands B, Goekint M, Heyman E, Piacentini MF, Watson P, Hasegawa H, et al. Acute norepinephrine reuptake inhibition decreases performance in normal and high ambient temperature. J Appl Physiol (1985) 105:206–12. doi: 10.1152/japplphysiol.90509.2008
38. Meeusen R, Roeykens J, Magnus L, Keizer H, De Meirleir K. Endurance performance in humans: the effect of a dopamine precursor or a specific serotonin (5-HT2A/2C) antagonist. Int J Sports Med. (1997) 18:571–7. doi: 10.1055/s-2007-972683
39. Kim DD, Lang DJ, Procyshyn RM, Woodward ML, Kaufman K, White RF, et al. Reduced cardiovascular fitness associated with exposure to clozapine in individuals with chronic schizophrenia. Psychiatry Res. (2018) 262:28–33. doi: 10.1016/j.psychres.2018.01.029
40. Perez-Cruzado D, Cuesta-Vargas A, Vera-Garcia E, Mayoral-Cleries F. Medication and physical activity and physical fitness in severe mental illness. Psychiatry Res. (2018) 267:19–24. doi: 10.1016/j.psychres.2018.05.055
41. Collomp KR, Ahmaidi SB, Caillaud CF, Audran MA, Chanal JL, Préfaut CG. Effects of benzodiazepine during a Wingate test: interaction with caffeine. Med Sci Sports Exerc. (1993) 25:1375–80. doi: 10.1249/00005768-199312000-00010
42. Collomp K, Fortier M, Cooper S, Long A, Ahmaidi S, Prefaut C, et al. Performance and metabolic effects of benzodiazepine during submaximal exercise. J Appl Physiol. (1985) 77:828–33. doi: 10.1152/jappl.1994.77.2.828
43. Ergen E, Açikada C, Hazir T, Güner R, Çilli M, Ergün Acar Y. Effects of benzodiazepine on neuromuscular activity performance in archers. J Sports Med Phys Fitness. (2015) 55:995–1003.
44. Grobler LA, Schwellnus MP, Trichard C, Calder S, Noakes TD, Derman WE. Comparative effects of zopiclone and loprazolam on psychomotor and physical performance in active individuals. Clin J Sport Med. (2000) 10:123–8. doi: 10.1097/00042752-200004000-00007
45. Suda H, Kanbayashi T, Ito SU, Sagawa Y, Imanishi A, Tsutsui K, et al. Residual effects of eszopiclone on daytime alertness, psychomotor, physical performance and subjective evaluations. Sleep Biol Rhythms. (2017) 15:311–6. doi: 10.1007/s41105-017-0112-z
46. Ito W, Kanbayashi T, Shimizu K, Ito SU, Wakasa M, Inoue Y, et al. Acute effects of zaleplon on daytime functions on the following day: psychomotor and physical performances, arousal levels and mood. Gazzetta Med Italiana Arch LE Sci Med. (2017) 176:257−64. doi: 10.23736/S0393-3660.16.03353-2
47. King M, Rauch LHG, Brooks SJ, Stein DJ, Lutz K. Methylphenidate enhances grip force and alters brain connectivity. Med Sci Sports Exerc. (2017) 49:1443–51. doi: 10.1249/MSS.0000000000001252
48. Roelands B, Hasegawa H, Watson P, Piacentini MF, Buyse L, De Schutter G, et al. The effects of acute dopamine reuptake inhibition on performance. Med Sci Sports Exerc. (2008) 40:879–85. doi: 10.1249/MSS.0b013e3181659c4d
49. Westover AN, Nakonezny PA, Barlow CE, Vongpatanasin W, Adinoff B, Brown ES, et al. Exercise outcomes in prevalent users of stimulant medications. J Psychiatr Res. (2015) 64:32–9. doi: 10.1016/j.jpsychires.2015.03.011
50. González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. (1985) 86:1032–9. doi: 10.1152/jappl.1999.86.3.1032
51. Nakayama K. Effect of paroxetine on extracellular serotonin and dopamine levels in the prefrontal cortex. Naunyn Schmiedebergs Arch Pharmacol. (2002) 365:102–5. doi: 10.1007/s00210-001-0497-7
52. Sahlin K. Metabolic factors in fatigue. Sports Med. (1992) 13:99–107. doi: 10.2165/00007256-199213020-00005
53. Lalley PM. The excitability and rhythm of medullary respiratory neurons in the cat are altered by the serotonin receptor agonist 5-methoxy-N,N, dimethyltryptamine. Brain Res. (1994) 648:87–98. doi: 10.1016/0006-8993(94)91909-7
54. Berg X, Colla M, Stefan V, Erich S, Christian C. Psychopharmacological Treatment in Athletes. Sports Exerc Med Open J. (2020) 68.
55. Reardon CL, Factor RM. Sport psychiatry: a systematic review of diagnosis and medical treatment of mental illness in athletes. Sports Med. (2010) 40:961–80. doi: 10.2165/11536580-000000000-00000
56. Billiard M, Besset A, de Lustrac C, Brissaud L. Dose-response effects of zopiclone on night sleep and on nighttime and daytime functioning. Sleep. (1987) 10(Suppl. 1):27–34. doi: 10.1093/sleep/10.suppl_1.27
57. Ashton H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs. (1994) 48:25–40. doi: 10.2165/00003495-199448010-00004
58. Nicholson AN, Stone BM. Zopiclone: sleep and performance studies in healthy man. Pharmacology. (1983) 27(Suppl. 2):92–7. doi: 10.1159/000137915
59. Terzano MG, Rossi M, Palomba V, Smerieri A, Parrino L. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem and zaleplon. Drug Saf. (2003) 26:261–82. doi: 10.2165/00002018-200326040-00004
60. Fuccella LM, Bolcioni G, Tamassia V, Ferrario L, Tognoni G. Human pharmacokinetics and bioavailability of temazepam administered in soft gelatin capsules. Eur J Clin Pharmacol. (1977) 12:383–6. doi: 10.1007/BF00562455
61. Breimer DD, Bracht H, De Boer AG. Plasma level profile of nitrazepam (Mogadon§) following oral administration. Br J Clin Pharmacol. (1977) 4:709–11. doi: 10.1111/j.1365-2125.1977.tb00444.x
62. Atkinson G, Drust B, Reilly T, Waterhouse J. The relevance of melatonin to sports medicine and science. Sports Med. (2003) 33:809–31. doi: 10.2165/00007256-200333110-00003
63. Wilmore JH. Exercise testing, training, and beta-adrenergic blockade. Phys Sportsmedicine. (1988) 16:46–51. doi: 10.1080/00913847.1988.11709662
64. WADA. World Anti-Doping Agency Prohibited List 2022. (2022). Available online at: https://www.usada.org/substances/prohibited-list/ (accessed June 14, 2022).
65. Smith GM, Beecher HK. Amphetamine sulfate and athletic performance. I. Objective effects. J Am Med Assoc. (1959) 170:542–57. doi: 10.1001/jama.1959.63010050001008
66. Chandler JV, Blair SN. The effect of amphetamines on selected physiological components related to athletic success. Med Sci Sports Exerc. (1980) 12:65–9. doi: 10.1249/00005768-198021000-00013
67. Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. (2012) 69:1044–53. doi: 10.1001/archgenpsychiatry.2011.2094
68. Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. (2012) 32:6170–6. doi: 10.1523/JNEUROSCI.6459-11.2012
Keywords: psychiatric medication, psychotropic drugs, physical performance, athletic performance, sport, exercise, fitness, systematic review
Citation: Hirschbeck A, Leao DS, Wagner E, Hasan A and Roeh A (2022) Psychiatric medication and physical performance parameters – Are there implications for treatment? Front. Psychiatry 13:985983. doi: 10.3389/fpsyt.2022.985983
Received: 04 July 2022; Accepted: 16 August 2022;
Published: 06 September 2022.
Edited by:
Huixuan Zhou, Beijing Sport University, ChinaReviewed by:
Chong Chen, Yamaguchi University Graduate School of Medicine, JapanKai G. Kahl, Hannover Medical School, Germany
Copyright © 2022 Hirschbeck, Leao, Wagner, Hasan and Roeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Hirschbeck, YW5uYS5oaXJzY2hiZWNrQGJraC1hdWdzYnVyZy5kZQ==
 Anna Hirschbeck
Anna Hirschbeck Douglas Silva Leao1
Douglas Silva Leao1 Astrid Roeh
Astrid Roeh