
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 06 October 2022
Sec. Social Neuroscience
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.976439
This article is part of the Research Topic Rising Stars in Social Cognition in Psychiatry View all 7 articles
 Liang Qi1
Liang Qi1 Jing Zhao2
Jing Zhao2 PanWen Zhao2
PanWen Zhao2 Hui Zhang2
Hui Zhang2 JianGuo Zhong3
JianGuo Zhong3 PingLei Pan2,3
PingLei Pan2,3 GenDi Wang3
GenDi Wang3 ZhongQuan Yi2*
ZhongQuan Yi2* LiLi Xie3*
LiLi Xie3*Background: Mounting studies have investigated impairments in social cognitive domains (including theory of mind [ToM] and facial emotion recognition [FER] in adult patients with temporal lobe epilepsy (TLE). However, to date, inconsistent findings remain.
Methods: A search of PubMed, Web of Science, and Embase databases was conducted until December 2021. Hedges g effect sizes were computed with a random-effects model. Meta-regressions were used to assess the potential confounding factors of between-study variability in effect sizes.
Results: The meta-analysis included 41 studies, with a combined sample of 1,749 adult patients with TLE and 1,324 healthy controls (HCs). Relative to HCs, adult patients with TLE showed large impairments in ToM (g = −0.92) and cognitive ToM (g = −0.92), followed by medium impairments in affective ToM (g = −0.79) and FER (g = −0.77). Besides, no (statistically) significant differences were observed between the magnitude of social cognition impairment in adult with TLE who underwent and those who did not undergo epilepsy surgery. Meta-regressions exhibited that greater severity of executive functioning was associated with more severe ToM defects, and older age was associated with more severe FER defects.
Conclusions: Results of this meta-analysis suggest that adult patients with TLE show differential impairments in the core aspects of social cognitive domains (including ToM and FER), which may help in planning individualized treatment with appropriate cognitive and behavioral interventions.
Epilepsy is one of the most common brain disorder and affects more than 70 million people worldwide (1, 2). It is characterized by recurrent, chronic and unprovoked seizures (2). In addition to the distress caused by seizures, patients with epilepsy may suffer from cognitive impairment and psychosocial difficulties, which can have serious social consequences, such as poor interpersonal relationships, loss of employment, and reduced social networks (1, 3–6). Although psychosocial function is influenced by many factors, a growing body of evidence shows that social cognitive skills may be an important mediator (7, 8).
Social cognitive skills are the abilities to perceive, encode, process, and interpret social information (9, 10). Social cognition is a multidimensional domain, mainly involving social knowledge, theory of mind (ToM), attribution style, social perception, and emotion recognition. Among them, ToM and facial emotion recognition (FER) are two core domains that have been frequently studied. ToM refers to the ability to attribute mental states of other people [intentions, beliefs, and emotions] (11). It is a complex ability that includes cognitive and affective constructs (12). FER refers to the ability to identify a specific emotional state through the interpretation of another person's facial features (13–15).
According to the International League Against Epilepsy (ILAE) classification (16), epileptic were categorized by seizure onset into focal epilepsy or generalized epilepsy. Temporal lobe epilepsy (TLE), the most common form of focal epilepsy, is characterized by epileptogenic discharges arising from temporal regions, with an incidence of 40% among patients with epilepsy (2, 17, 18). In recent years, there has been an increasing number of studies examining ToM or FER differences between adults with TLE and healthy controls (HCs) (19–24). However, significantly inconsistent results were found in the magnitude of differences between groups. The inconsistent findings may be related to low statistical power, as most of the existing studies had a small sample size. A quantitative meta-analysis may be helpful to improve statistical power and provide the means to draw conclusions from the inconsistent findings of previous studies.
To our knowledge, three meta-analyses have summarized social cognition defects between patients with TLE and HCs (25–27). However, the quantitative results of these meta-analyses between the patients with TLE and social cognition remain inconclusive. Bore et al. (25) observed that patients with TLE underperformed in all six basic emotions recognition (including anger, disgust, fear, happiness, sadness, surprise) compared to HCs. Edwards et al. (27) reported that patients with TLE were only impaired in the recognition of anger, disgust, fear, happy, and sad; however, no group differences were observed for surprise recognition. Furthermore, Bore et al. (25) demonstrated medium impairment in fear recognition (g = 0.70), and small impairment in happy recognition (g = 0.24), whereas Edwards et al. (27) observed a large impairment in fear recognition (g = 1.17), and medium impairment in happy recognition (g = 0.60), compared to HCs. Besides, all previous meta-analyses did not analyze social cognitive performance in adults with TLE as an independent group. In addition, no previous meta-analysis has investigated the differences between cognitive ToM and affective ToM in patients with TLE. Moreover, the above-mentioned meta-analyses only included studies that investigated five specific ToM tasks (faux-pas task [FPT], false belief task [FBT], reading the mind in the eyes task [RMET], strange stories task [SST], and cartoon ToM task [CTT]. It is important to also investigate other individual ToM tasks such as the Moving Triangles and the movie for the assessment of social cognition (MASC).
Recently, in adult patients with TLE, a number of studies assessed the relationship between social cognition defects and general cognitive dysfunction (22, 28–30). Here, general cognitive function includes intelligence ability and non-social cognition (also referred to as neurocognition, mainly including processing speed, learning and memory, executive function [EF], and language fluency, etc.) (31). However, there have been inconsistent findings. For example, some studies have found that there are significant correlations between social cognition defects and intelligence ability (29, 32, 33) or EF (30, 34, 35). In contrast, others found no relationship between general cognitive dysfunction and social cognitive performance (30, 36–40). To date, it has not been determined whether there is a correlation between general cognitive dysfunction and social cognition defects in adults with TLE.
In sum, the present meta-analysis aimed to investigate ToM and FER deficits in adult patients with TLE. Besides, it was investigated whether the magnitude of ToM deficits varied by the type of tasks used to assess ToM. Furthermore, the magnitude of deficits in six individual emotions recognition was investigated. The secondary aim was to investigate the impact of epilepsy surgery on ToM and FER deficits in adults with TLE. The third aims were to determine whether the severity of ToM and FER impairment is affected by demographic factors, epilepsy variables, and treatment factors. The fourth aim was to establish whether ToM or FER is related to general cognitive function in adults with TLE.
Electronic databases including Web of Science, PubMed, and Embase were searched (up to December 13th, 2021). The following terms were used: (“social cognition” or “theory of mind” or “ToM” or “mentalizing” or “mentalising” or “facial expression” or “facial emotion recognition” or “emotion”) AND (“epileps*” or “seizure disorder”). A backward citation search was also undertaken.
Duplicate items were firstly removed. Subsequent primary screening of titles and abstracts were screened to remove ineligibility (i.e., literature reviews, abstracts, animal studies, no mention of epilepsy, or irrelevant measurements; see Figure 1). Finally, full-text screening was performed to exclude unqualified studies.
Studies were included if: (1) were published as a primary peer-reviewed research article in English; (2) had a research design that compared adults with TLE and HCs; (3) included measures to assess at least one domain of ToM or FER performance; (4) presented adequate data to calculate precise group comparison effect sizes between adult patients with TLE and HCs. The authors were contacted if data were insufficient to calculate effect sizes. Studies were excluded if authors did not respond after 4 weeks. Studies were included if they provided data that could be used to calculate effect sizes for group comparisons.
Studies were excluded if: (1) were reviews, single case studies, or editorials; (2) did not include adults with TLE; (3) did not include an HCs group; (4) had a mixed sample: this refers to a sample that group together adults with TLE and other diseases (e.g., frontal lobe epilepsy); (5) did not include comparisons of ToM or FER between adults with TLE and HCs; (6) the sample size was <10 (41).
The Newcastle-Ottawa Scale was used to evaluate the quality of the included studies (42).
Two authors independently completed the article retrieval, screening, and data extraction. Any Discrepancies were resolved with discussion between the two investigators (JZ and PWZ), and further disagreements were arbitrated by the third author (LLX).
The following was extracted:
• Title information, such as name of first author, year of publication, and title.
• Characteristics of the sample, mainly included number of participants in TLE and control groups, gender (female and male), education level, age at testing, age at epilepsy onset, duration of epilepsy, surgery or not, monthly seizure frequency, number of AEDs, general cognitive variables, and the quality assessment score.
• FER task type.
• ToM type. For ToM tasks, tasks were divided into cognitive and affective subcomponents.
• The data used for calculating effect sizes of ToM or FER.
Different individual ToM tasks were used across studies, most common being the FPT (number of studies [k] = 14), SST (k = 5), RMET (k = 4), FBT (k = 2), and CTT (k = 2); other tasks (k = 1, respectively) included MASC, ToM: frith-happé animations, ToM: recognition of irony, ToM: moving triangles, ToM: the comprehension of sarcasm task, ToM: the comprehension of action task, ToM: the animated shapes task, ToM: metaphor and irony. Different FER tasks were used across studies, most commonly the Ekman and Florida Affect Battery.
Cognitive ToM is concerned with understanding another's thoughts, intentions, and beliefs (43–45). It can be evaluated through several tasks such as the SST, FBT, CTT, ToM: recognition of irony, ToM: moving triangles, ToM: the comprehension of sarcasm task, ToM: the comprehension of action task, ToM: the animated shapes task, and ToM: metaphor and irony, as well as the cognitive subcomponents of the MASC, FPT, and ToM: frith-happé animations.
Affective ToM is described as the capacity to infer another person's emotional states (43–45). It can be evaluated through several tasks such as the RMET, as well as the affective subcomponents of the MASC, FPT, and ToM: frith-happé animations.
Data was analyzed using the Stata 15.0 software package with a random-effects model (46). Hedges g and 95% confidence interval (CI) were calculated as the index of effect size between adults with epilepsy and HCs (47). The interpretation of Hedges g was similar to Cohen d: 0.2 indicated a small effect, 0.5 indicated a medium effect, and 0.8 indicated a large effect (48). Negative effect sizes indicated poorer performance for adults with TLE compared to HCs.
For studies that did not provide a total mean score on a particular measure (i.e., ToM, cognitive ToM, affective ToM, and FER), but reported more than one ToM task or individual emotion task, a pooled effect size was aggregated by computing the mean effect size (and standard error) (49). The I2 tests was used to test the heterogeneity of mean weighted effect sizes, and the degree of heterogeneity was deemed low, moderate, or large when I2 was equal to or larger than 0, 50, or 75%, respectively (50).
The funnel plot and Egger's test were used to test the risk of publication bias (51), which is defined by the “dictionary of epidemiology” (52) as “an editorial predilection for publishing particular findings, e.g., positive results, which leads to the failure of authors to submit negative findings for publication” (53). If publication bias was significant (p < 0.05), the trim-and-fill method was applied to provide effect sizes adjusted for publication bias (54).
Meta-regression analyses were performed using a random effects model and the restricted information maximum likelihood method with a significance level set at p < 0.05 to assess whether demographic factors (including gender, age at testing, and education level), epilepsy variables (including age at epilepsy onset, duration of epilepsy, monthly seizure frequency), treatment factors (including AEDs), and general cognitive function (including intelligence ability, severity of processing speed, severity of learning and memory, severity of EF, and severity of verbal fluency) were associated with social cognition in adults with TLE. For each of these analyses, a minimum required of 3 data points was required for each relevant predictor variable and the social cognitive ability under assessment (55). Control measures for ToM tasks, or tasks measuring perceptual processing of facial stimuli were not included.
Figure 1 displays the details of the study selection process. Initially, a total of 3,597 potentially records were identified through three electronic databases searching and 1 records were identified from other sources. After the removal of duplicates, 2,876 records remained, which were then subjected to title and abstract screening. Of these, 63 full text papers seemed to meet inclusion criteria. After further screening, 22 records were excluded: (i) the study did not include an HCs group (k = 7) (56–62); (ii) the study lacked sufficient data to calculate the effect sizes and standard errors of ToM or FER (k = 6) (63–68); (iii) the sample size was under 10 (k = 4) (69–72); (iv) the sample was mixed and included adults with TLE and other diseases (k = 4) (73–76); (v) the sample was mixed and included children or adolescents (k = 1) (77). Eventually, 41records consisting of 1,749 patients with TLE and 1,324 HCs were included in the meta-analysis (Table 1) (19–24, 28–30, 32–40, 78–100).
Table 2 displays the results of the assessment of study quality, with a mean score of 6.80 (SD = 0.81), and 25 of the 41 case-control studies were awarded ≥7 stars and considered of high quality.
Table 3 show the key results from this meta-analysis. Compared to HCs, adult patients with TLE were impaired in ToM and this deficit was large in magnitude (g = −0.92, 95% CI [−1.06, −0.77], k = 19, z = 12.48, p < 0.001, see Figure 2). When considering the different subcomponents of ToM, the findings showed that adult patients with TLE were associated with large impairment for cognitive ToM (g = −0.92, 95% CI [−1.11, −0.73], k = 15, z = 9.68, p < 0.001, see Figure 3) and medium impairment for affective ToM (g = −0.79, 95% CI [−0.92, −0.66], k = 9, z = 11.91, p < 0.001, see Figure 3). For individual ToM tasks (see Figure 4), adult patients with TLE performed significantly worse than HCs with large effect sizes in FPT, SST, CTT, FBT, and medium effect sizes in RMET.
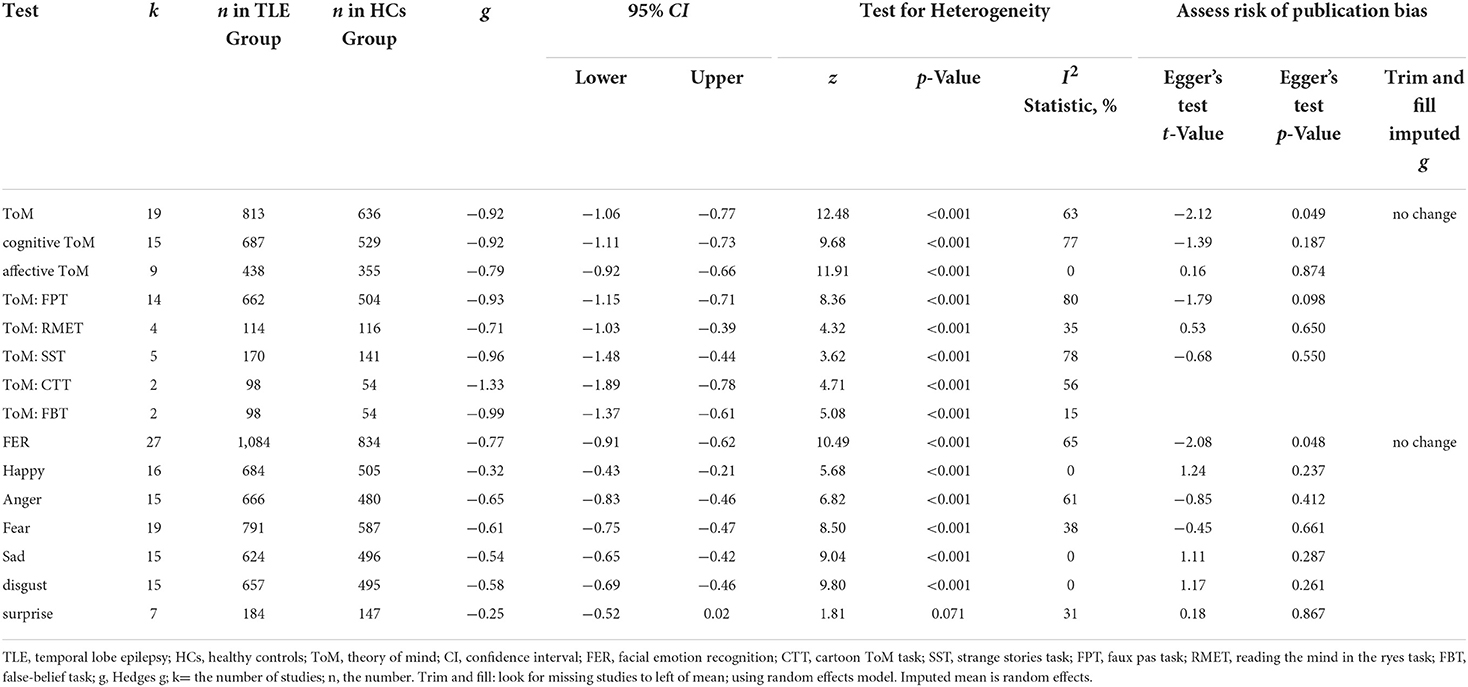
Table 3. Mean effects for ToM and FER subcomponents comparing adult with TLE against healthy controls and tests for publication bias.
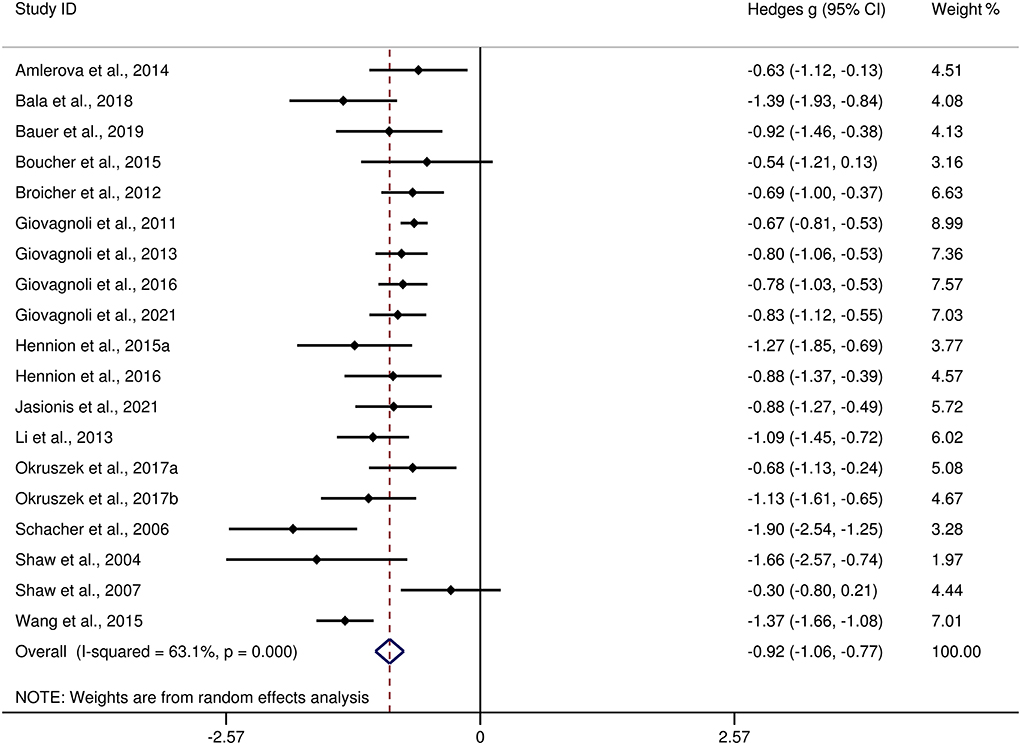
Figure 2. Forest plots showing effect size estimates (Hedges g) for ToM differences between adults with TLE and healthy controls. ToM, theory of mind; CI, confidence interval; TLE, temporal lobe epilepsy.
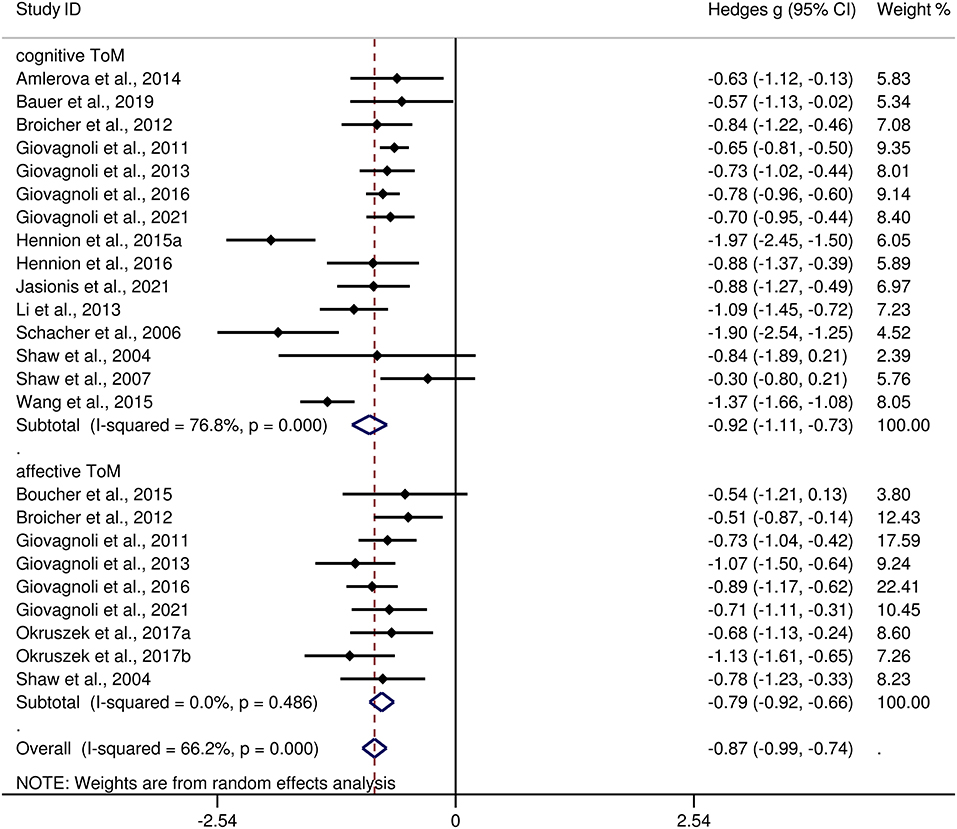
Figure 3. Forest plots showing effect size estimates (Hedges g) for cognitive ToM and affective ToM differences between adults with TLE and healthy controls. ToM, theory of mind; CI, confidence interval; TLE, temporal lobe epilepsy.
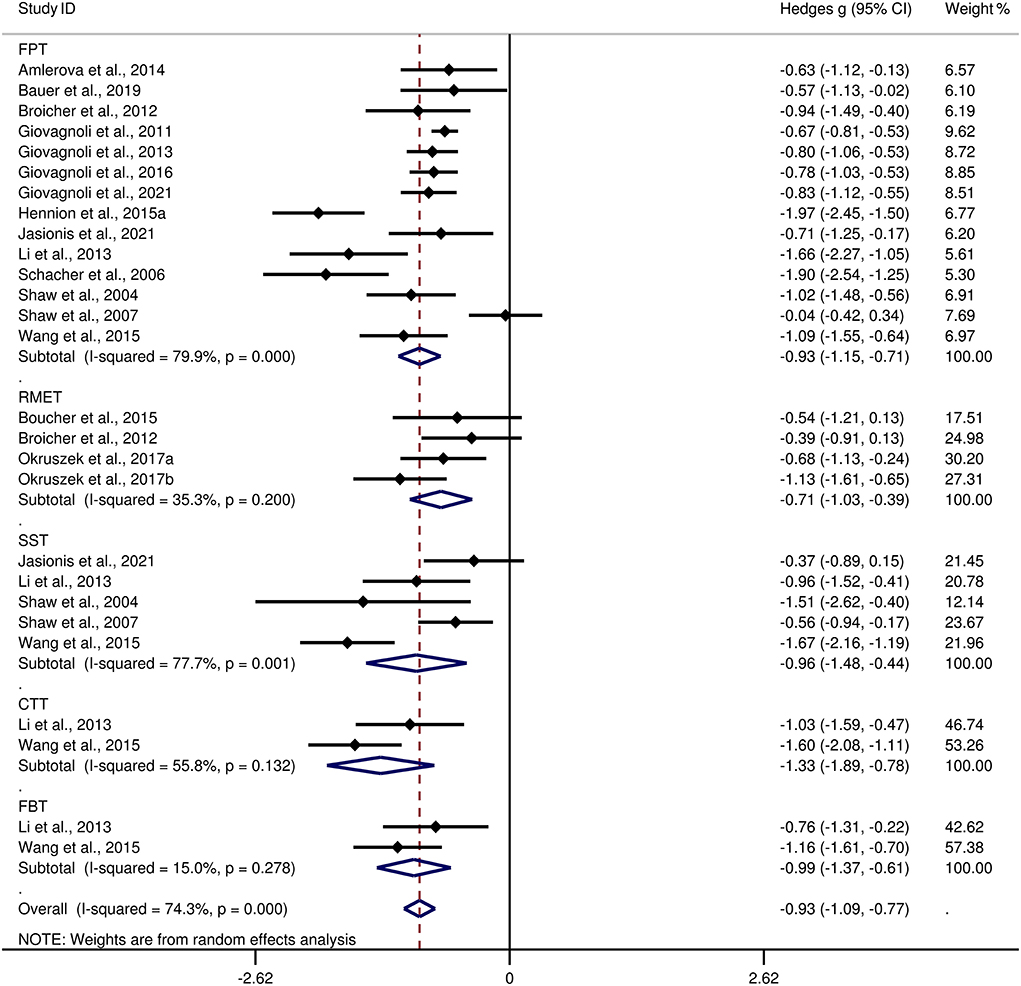
Figure 4. Forest plots showing effect size estimates (Hedges g) for individual ToM tasks differences between adults with TLE and healthy controls. ToM, theory of mind; CI, confidence interval; TLE, temporal lobe epilepsy. CTT, cartoon ToM task; SST, strange stories task; FPT, faux pas task; RMET, reading the mind in the ryes task; FBT, false-belief task.
There was no heterogeneity across studies for affective ToM, small heterogeneity for RMET and FBT (I2 =35% and I2 = 15%, respectively), moderate heterogeneity for ToM and CTT (I2 = 63% and I2 = 56%, respectively), and significant heterogeneity among studies on cognitive ToM, FPT, and SST (I2 = 77%, I2 = 80%, and I2 = 78%, respectively). The funnel plots for ToM, cognitive ToM, affective ToM, FPT, RMET, and SST are displayed in Supplementary Figure 1. The only significant Egger test result was found for ToM. However, a trim-and-fill analysis did not result in the imputation of any further studies, and the effect size remained the same.
Meta-regression analyses found no significant effect of gender (t = 0.84, p = 0.413, k = 19), age at testing (t = 0.52, p = 0.609, k = 19), education level (t = −1.42, p = 0.185, k = 12), age at epilepsy onset (t =1.06, p = 0.309, k = 16), duration of epilepsy (t = 0.08, p = 0.936, k = 15), monthly seizure frequency (t = 0.07, p = 0.947, k = 12), number of AEDs (t = −1.74, p = 0.143, k = 7), intelligence ability (t = 0.63, p = 0.554, k = 8), severity of processing speed (t = 0.32, p = 0.767, k = 5), or severity of verbal fluency (t = 1.22, p = 0.348, k = 4) on the severity of ToM impairment in adult patients with TLE. By contrast, a positive association was noted between ToM defects and severity of EF in adult patients with TLE (t = 3.80, p = 0.019, k = 6; see Figure 5). For further details, see Supplementary Table 1.
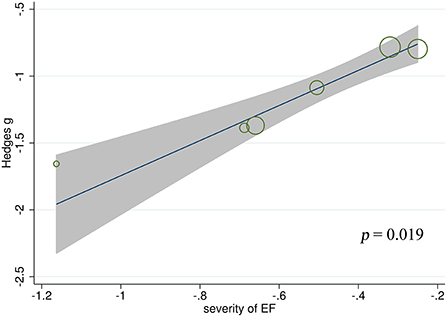
Figure 5. Associations between aggregated effect sizes of the studies investigating ToM performance and severity of EF. ToM, theory of mind; EF, executive functioning.
Meta-regressions were not conducted for the effect of learning and memory on ToM in adult patients with TLE, as less than 3 studies contributed to the data for this subcomponent.
The differences between adult patients with TLE and HCs in FER are presented in Table 3. For FER, adult patients with TLE exhibited a moderate impairment compared to the HCs (g = −0.77, 95% CI [−0.91,−0.62], k = 27, z = 10.49, p < 0.001, see Figure 6). For the analyses of individual emotions recognition (Supplementary Figure 2), adult patients with TLE were associated with medium impairments in anger, fear, sad, and disgust recognition, and small impairments in happy recognition. However, no group differences were evident for surprise recognition.
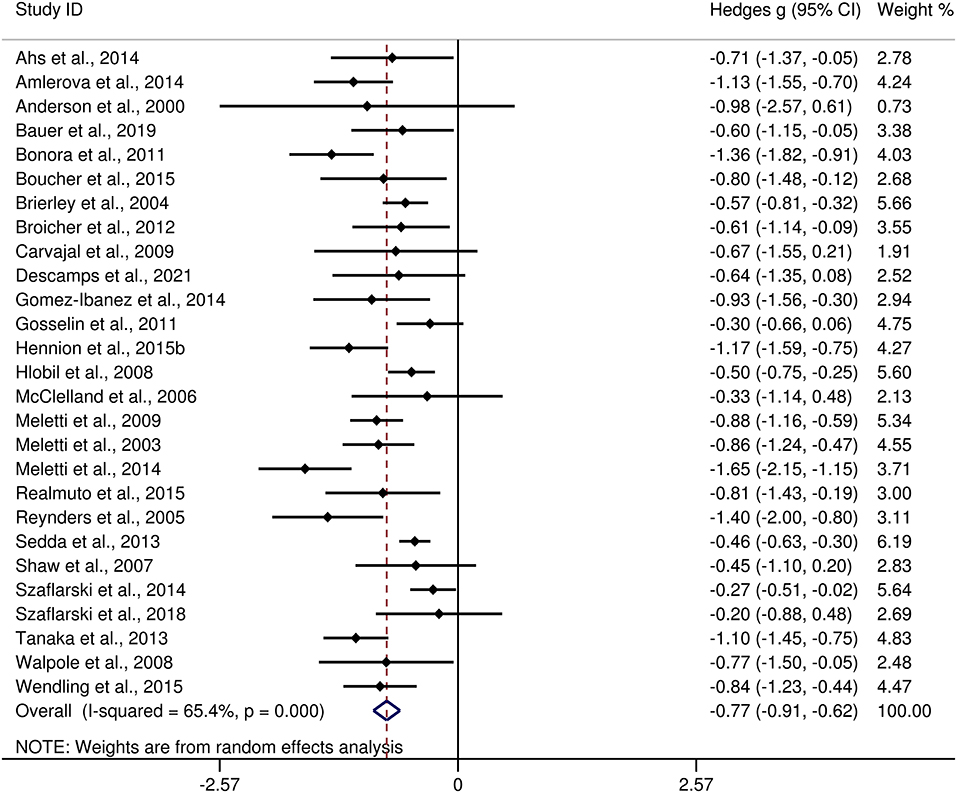
Figure 6. Forest plots showing effect size estimates (Hedges g) for FER differences between adults with TLE and healthy controls. CI, confidence interval; FER, facial emotion recognition; TLE, temporal lobe epilepsy.
There was no heterogeneity across studies for happy, sad, and disgust recognition, small heterogeneity fear (I2 = 38%) and surprise (I2 = 31%) recognition, and medium heterogeneity for FER (I2 = 65%) and anger recognition (I2 = 61%). The funnel plots for FER and six individual emotions recognition are displayed in Figure 7 and Supplementary Figure 3, respectively. The only significant Egger test result was found for FER (t = −2.08, p = 0.048). However, a trim-and-fill analysis did not result in the imputation of any further studies, and the effect size remained the same.
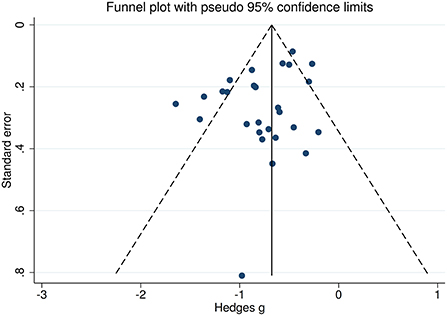
Figure 7. Funnel plots of the actual meta-analyses. The vertical and diagonal dashed lines represent the overall estimated effect size and its 95% confidence limits, respectively, based on the random-effect model.
Meta-regression analyses found no significant effect of gender (t = 1.94, p = 0.064, k = 26), education level (t = 1.55, p = 0.142, k = 17), age at epilepsy onset (t = 0.73, p = 0.473, k = 20), duration of epilepsy (t = −1.27, p = 0.221, k = 18), monthly seizure frequency (t = −2.03, p = 0.179, k = 4), number of AEDs (t = 1.78, p = 0.326, k = 3), intelligence ability (t = 0.99, p = 0.360, k = 8), and severity of EF (t = 0.26, p = 0.835, k = 3) on the severity of FER impairment in adult patients with TLE. By contrast, a negative association was noted between FER defects and age at testing in adult patients with TLE (t = −2.26, p = 0.033, k = 27; Figure 8). For further details, see Supplementary Table 2.
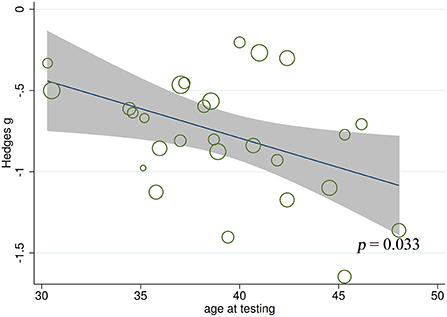
Figure 8. Associations between aggregated effect sizes of the studies investigating FER performance and age at testing. FER, facial emotion recognition.
Meta-regressions were not conducted for the effect of processing speed, learning and memory, or verbal fluency on FER in adult patients with TLE, as <3 studies contributed to the data for this subcomponent.
Table 4 depicts the key results obtained from this meta-analysis. The performance of adult patients with TLE-TL- and adult patients with TLE-TL+ with respect to ToM (g = −0.97 and g = −1.00), cognitive ToM (g = −0.90 and g = −0.78), affective ToM (g = −0.87 and g = −0.73), and FER (g = −0.70 and g = −0.79) was inferior to that of the HCs. Egger's test was not significant except for ToM in adult patients with TLE-TL+ (t = −3.25, p = 0.023). However, a trim-and-fill analysis did not result in imputation of any studies, and the effect size remained the same.

Table 4. Mean effects for ToM and FER subcomponents comparing adults with TLE-TL- and TLE-TL+ against healthy controls and tests for publication bias.
The effect sizes of the TLE-TL- and TLE-TL+ groups were comparable for ToM (Q = 0.02, df = 1, p = 0.896), cognitive ToM (Q = 0.20, df = 1, p = 0.656), affective ToM (Q = 0.42, df = 1, p = 0.515), and FER (Q = 0.27, df = 1, p = 0.603).
The current meta-analysis investigated ToM and FER performance in a large sample of adult patients with TLE in comparison with HCs. It included 41 studies with a total sample size of 1,749 adult patients with TLE and 1,324 HCs. Relative to HCs, adult patients with TLE showed impairments in ToM, ToM subcomponents (cognitive ToM and affective ToM), and FER. For individual ToM tasks, the CTT had the largest effect size. Among individual emotions, adult patients with TLE were more impaired in recognizing negative emotions than positive emotions. In addition, the degree of ToM/FER impairment was not statistically different between adult patients with TLE-TL- and TLE-TL+. Meta-regression analyses indicated that demographic factors, epilepsy variables, treatment factors, and general cognitive function were not related to ToM or FER impairment in adult patients with TLE, except that older age was associated with more severe FER defects, and greater severity of EF was associated with more severe ToM defects
Large effect sizes were observed for ToM (g = −0. 92, k = 19). The results support the findings of Stewart et al. (26) (g = −0.92, k = 9) and Bore et al. (25) (g = −0.86, k = 13, respectively). Regarding the subcomponents of ToM, adult patients with TLE had large impairments in cognitive ToM but moderate impairments in affective ToM. This is consistent with previous consensus that the domains of cognitive ToM and affective ToM appear to have different trajectories (101–104). Specifically, the cognitive ToM is associated with greater activation in the dorsomedial prefrontal cortex, the dorsal anterior cingulate cortex, and the dorsal striatum (105), whereas the affective ToM is associated with greater activation in the ventromedial and orbitofrontal cortices, the ventral anterior cingulate cortex, the ventral striatum, and the amygdala (101, 106). In multiple neuroanatomical reports, gray and white matter pathology in regions implicated in cognitive and affective ToM networks is observed in adult patients with TLE (67, 107–111). Meta-regression analysis showed that more severe EF was associated with more severe ToM defects. This finding is consistent with recent suggestion that ToM is a specific cognitive domain and is associated with EF during neurodevelopment in adulthood (97, 112, 113). At the neural level, it was found that EF and ToM may share certain neural circuits, such as those related to domain general attention (114).
A medium effect size was observed for FER impairment (g = −0.77, k = 27). This was different from the findings of Bora et al. (25) and Edwards et al. (27) (g = −0.87, k = 16 and g = −0.99, k = 14, respectively), which indicate a large-sized impairment. For individual emotions, adult patients with TLE had medium effect sizes in recognition of negative emotions (anger, fear, sad, and disgust), and small effect sizes in recognition of happy; no difference was observed in recognition of surprise. Neuroimaging evidence suggests that higher impairment in negative emotional states in adult patients with TLE may be associated with structural and functional abnormalities in the medial temporal lobe and amygdala (72, 79, 115–117). The relatively intact recognition of positive emotional states (happy and surprise) may be because positive emotions are easier to recognize than negative emotions (118–120). The results of this meta-analysis, in line with previous findings, suggest that different types of neural dysfunction may have different ability to recognize specific emotions (12, 121, 122). The results of meta-regression analyses indicated that the older age appears to be associated with greater FER defects, which are also observed in other types of neural dysfunction, such as multiple sclerosis and Huntington's disease (12, 123).
Notably, the lack of association between epilepsy variables (eg, age at epilepsy onset and duration of epilepsy) and ToM or FER was surprising. It has been reported that cognitive ToM skills develop around 4 to 6 years of age (124–126), affective ToM skills develop from around 8 years of age to late adolescence (124, 125), and FER skills develop gradually from infancy to adolescence (117). Developmental neurology has shown that ToM and FER skills are particularly vulnerable to disruption during periods of development, the so-called critical periods (127–129). Onset of seizures during critical periods may affect the plasticity and maturation of social cognitive neural networks, disrupting the development of ToM and FER skills (26, 117, 130, 131). In addition, the longer duration of epilepsy that begins in critical periods also hinders the continued development of social cognitive abilities (116). Therefore, it can be hypothesized that onset of epilepsy during the critical periods in early childhood may lead to broader social cognitive deficits (25). In this meta-analysis, the mean age at epilepsy onset in adult patients with TLE enrolled in the ToM study was 17.45 years, and the mean age at epilepsy onset in adult patients with TLE enrolled in the FER study was 17.28 years. Their epilepsy began almost in adulthood, not during the critical periods of ToM or FER development. Furthermore, the current meta-analysis only included cross-sectional studies in adult patients with TLE and was unable to investigate the developmental course of ToM and FER deficits. More longitudinal studies are warranted to investigate the developmental trajectories of ToM and FER deficits in patients with TLE.
For adults with TLE, anterior temporal lobectomy (ALT) is the most common type of epilepsy surgery and typically involves resection of the anterior parts of temporal lobe (including the hippocampus, anterior temporal neocortex, and amygdala) (19, 132), which are usually activated in social cognition tasks (133). Therefore, it could be hypothesized that epilepsy surgery may contribute to the risk of a decline in social cognition for adults with TLE (19, 132). However, the current quantitative findings indicated that adults with TLE with and without temporal lobectomy present similar defects in social cognition skills. This may be because this type of surgical treatment is usually performed for patients with drug resistant epilepsy; therefore, most patients undergoing temporal lobectomy have experienced symptoms of epilepsy for many years, and some of them may suffer from epilepsy since birth or early childhood. Such uncontrolled and prolonged seizures may cause alterations in brain tissue that lead to changes in its function (19, 25). In addition, early-onset epilepsy may also trigger early brain reorganization that facilitates a functional compensation after surgical treatment (67). Therefore, temporal lobectomy may not significantly worsen patient's performance in social cognition. However, given methodological heterogeneity between studies and that any individual improvements or decline may be masked by group comparisons, further studies investigating the social cognitive outcomes of epilepsy surgery are warranted (134).
The current study has important clinical implications. In terms of clinical practice recommendations, given the prevalence of ToM and FER impairments in adults with epilepsy (24, 135, 136), we encourage clinicians to be vigilant about indicators of social cognitive impairment in adult patients with TLE, and our results support inclusion of ToM and FER measures in routine neuropsychological testing in adult patients with TLE (137). Additional, given the diversity and complexity of current ToM and FER measures (21, 138, 139) and the fact that few ToM and FER measures have been adjusted and standardized specifically for patients with epilepsy (140), we propose the development of comprehensive, ecologically valid, and economically feasible standardized social cognitive assessment tools for patients with epilepsy. From a therapeutic perspective, interventions targeting social cognition may be an effective approach to address social difficulties in adults with TLE. Currently, a variety of cognitive interventions aimed at ameliorating social cognitive impairment have been developed and validated (135), which are divided into three main categories: targeted interventions aimed at improving a specific social cognitive ability such as ToM or FER; broad interventions aimed at developing interpersonal skills in patients; global interventions aimed at improving a set of social cognitive abilities (135). Although the outcomes of cognitive interventions targeting social cognitive impairment in adult patients with TLE have not been reported, in patients with other neurological or psychiatric disorders such as autism spectrum disorder, schizophrenia, traumatic brain injury, social function and social cognition were significantly improved through social cognitive interventions (141–153). These findings suggest that social cognitive therapy is promising for adults with TLE. Our quantitative results can broaden the understanding of two core domains of social cognition in adults with TLE and may help develop cognitive interventions for this patient population.
There are some limitations to our meta-analysis. First, a field-specific meta-analysis would benefit from a larger number of studies and a larger sample size (154). Our meta-analyses included only English-language peer-reviewed studies that do not represent the likely available evidence in other language areas. Therefore, we conducted an initial search of studies published in other languages, but found no studies that met the inclusion criteria. Second, we only included cross-sectional studies, while more longitudinal studies are required to investigate the dynamic changes in the ToM and FER functions in adult patients with TLE. Third, although 41 studies were included in this meta-analysis, few studies contributed to the mean effect size for some individual ToM tasks (such as CTT, k = 2, FBT, k = 2, and MASC, k = 1). Therefore, further research in this area is warranted in the future.
The results of this meta-analysis suggested that adults with TLE were significantly impaired in ToM and cognitive ToM, and moderately impaired in affective ToM and FER. Additional, temporal resection may have no effect on social cognition performance. Furthermore, in adults with TLE, ToM appeared to be associated with EF, and FER was correlated with patient's age at testing. These quantitative results advance our understanding of the core aspects of social cognitive processing in adult patients with TLE, which may help to further characterize certain epilepsy syndromes and facilitate the development of therapeutic interventions.
Study design: ZQY and GDW. Analysis and interpretation of data: LQ, PWZ, JZ, HZ, JGZ, and PLP. Drafting of the manuscript: LQ and PWZ. Critical revision of the manuscript: ZQY and LLX. Approval of the final version for submission: All authors.
We thank all the authors of the studies included.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.976439/full#supplementary-material
Supplementary Figure 1. Funnel plots of the six actual meta-analyses. The vertical and diagonal dashed lines represent the overall estimated effect size and its 95% confidence limits, respectively, based on the random-effect model. ToM, theory of mind; RMET, reading the mind in the ryes task; FPT, faux pas task; SST, strange stories task. (A) ToM; (B) cognitive ToM; (C) affective ToM; (D) FPT; (E) RMET; (F) SST.
Supplementary Figure 2. Forest plots showing effect size estimates (Hedges g) for individual emotions differences between adults with TLE and healthy controls. CI, confidence interval; TLE, temporal lobe epilepsy.
Supplementary Figure 3. Funnel plots of the six actual meta-analyses. The vertical and diagonal dashed lines represent the overall estimated effect size and its 95% confidence limits, respectively, based on the random-effect model. (A) happy; (B) anger; (C) fear; (D) sad; (E) disgust; (F) surprise.
Supplementary Table 1. Influence of different variables on the effect of ToM in meta-regression analysis.
Supplementary Table 2. Influence of different variables on the effect of FER in meta-regression analysis.
1. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. (2014) 55:475–82. doi: 10.1111/epi.12550
2. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
3. Koponen A, Seppälä U, Eriksson K, Nieminen P, Uutela A, Sillanpää M, et al. Social functioning and psychological well-being of 347 young adults with epilepsy only–population-based, controlled study from Finland. Epilepsia. (2007) 48:907–12. doi: 10.1111/j.1528-1167.2007.01017.x
4. Hermann B, Jacoby A. The psychosocial impact of epilepsy in adults. Epilepsy Behav. (2009) 15:S11–16. doi: 10.1016/j.yebeh.2009.03.029
5. McCagh J, Fisk JE, Baker GA. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. (2009) 86:1–14. doi: 10.1016/j.eplepsyres.2009.04.007
6. Lin PT, Yu HY, Lu YJ, Wang WH, Chou CC, Hsu SPC, et al. Social functioning and health-related quality of life trajectories in people with epilepsy after epilepsy surgery. Epilepsy Behav. (2020) 103:106849. doi: 10.1016/j.yebeh.2019.106849
7. Bora E, Eryavuz A, Kayahan B, Sungu G, Veznedaroglu B. Social functioning, theory of mind and neurocognition in outpatients with schizophrenia; mental state decoding may be a better predictor of social functioning than mental state reasoning. Psychiatry Res. (2006) 145:95–103. doi: 10.1016/j.psychres.2005.11.003
8. Lin X, Zhang X, Liu Q, Zhao P, Zhang H, Wang H, et al. Theory of mind in adults with traumatic brain injury: a meta-analysis. Neurosci Biobehav Rev. (2021) 121:106–18. doi: 10.1016/j.neubiorev.2020.12.010
9. Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. (2008) 34:1211–20. doi: 10.1093/schbul/sbm145
10. Bora E, Walterfang M, Velakoulis D. Theory of mind in Parkinson's disease: a meta-analysis. Behav Brain Res. (2015) 292:515–20. doi: 10.1016/j.bbr.2015.07.012
12. Lin X, Zhang X, Liu Q, Zhao P, Zhong J, Pan P, et al. Social cognition in multiple sclerosis and its subtypes: a meta-analysis. Mult Scler Relat Disord. (2021) 52:102973. doi: 10.1016/j.msard.2021.102973
13. Ekman P, Friesen WV. Unmasking the Face: A Guide to Recognizing Emotions from Facial Clues. Hoboken, NJ: Prentice-Hall (1975).
14. Callahan BL, Ueda K, Sakata D, Plamondon A, Murai T. Liberal bias mediates emotion recognition deficits in frontal traumatic brain injury. Brain Cogn. (2011) 77:412–8. doi: 10.1016/j.bandc.2011.08.017
15. Stewart E, Catroppa C, Gonzalez L, Gill D, Webster R, Lawson J, et al. Facial emotion perception and social competence in children (8 to 16 years old) with genetic generalized epilepsy and temporal lobe epilepsy. Epilepsy Behav. (2019) 100:106301. doi: 10.1016/j.yebeh.2019.04.054
16. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
17. Kaestner E, Reyes A, Chen A, Rao J, Macari AC, Choi JY, et al. Atrophy and cognitive profiles in older adults with temporal lobe epilepsy are similar to mild cognitive impairment. Brain. (2021) 144:236–50. doi: 10.1093/brain/awaa397
18. Johnson GW, Cai LY, Narasimhan S, González HFJ, Wills KE, Morgan VL, et al. Temporal lobe epilepsy lateralisation and surgical outcome prediction using diffusion imaging. J Neurol Neurosurg Psychiatry. (2022) 93:599–608. doi: 10.1136/jnnp-2021-328185
19. Bala A, Okruszek Ł, Piejka A, Głebicka A, Szewczyk E, Bosak K, et al. Social perception in mesial temporal lobe epilepsy: Interpreting social information from moving shapes and biological motion. J Neuropsychiatr Clin Neurosci. (2018) 30:228–35. doi: 10.1176/appi.neuropsych.17080153
20. Szaflarski JP, Allendorfer JB, Nenert R, LaFrance WC, Barkan HI, DeWolfe J, et al. Facial emotion processing in patients with seizure disorders. Epilepsy Behav. (2018) 79:193–204. doi: 10.1016/j.yebeh.2017.12.004
21. Bauer J, Kegel LC, Steiger BK, Jokeit H. Assessment tools for social cognition in epilepsy. Zeitschrift fur Epileptologie. (2019) 32:183–6. doi: 10.1007/s10309-019-0260-z
22. Descamps M, Boucher O, Nguyen DK, Rouleau I. Emotional autobiographical memory associated with insular resection in epileptic patients: a comparison with temporal lobe resection. Brain Sci. (2021) 11:1316. doi: 10.3390/brainsci11101316
23. Giovagnoli AR, Parente A, Ciuffini R, Tallarita GM, Turner K, Maialetti A, et al. Diversified social cognition in temporal lobe epilepsy. Acta Neurol Scand. (2021) 143:396–406. doi: 10.1111/ane.13386
24. Jasionis A, Puteikis K, Mameniškiene R. The impact of social cognition on the real-life of people with epilepsy. Brain Sci. (2021) 11:877. doi: 10.3390/brainsci11070877
25. Bora E, Meletti S. Social cognition in temporal lobe epilepsy: a systematic review and meta-analysis. Epilepsy Behav. (2016) 60:50–7. doi: 10.1016/j.yebeh.2016.04.024
26. Stewart E, Catroppa C, Lah S. Theory of Mind in Patients with Epilepsy: a Systematic Review and Meta-analysis. Neuropsychol Rev. (2016) 26:3–24. doi: 10.1007/s11065-015-9313-x
27. Edwards M, Stewart E, Palermo R, Lah S. Facial emotion perception in patients with epilepsy: a systematic review with meta-analysis. Neurosci Biobehav Rev. (2017) 83:212–25. doi: 10.1016/j.neubiorev.2017.10.013
28. Giovagnoli AR, Parente A, Villani F, Franceschetti S, Spreafico R. Theory of mind and epilepsy: What clinical implications? Epilepsia. (2013) 54:1639–46. doi: 10.1111/epi.12255
29. Amlerova J, Cavanna AE, Bradac O, Javurkova A, Raudenska J, Marusic P, et al. Emotion recognition and social cognition in temporal lobe epilepsy and the effect of epilepsy surgery. Epilepsy Behav. (2014) 36:86–9. doi: 10.1016/j.yebeh.2014.05.001
30. Realmuto S, Zummo L, Cerami C, Agrò L, Dodich A, Canessa N, et al. Social cognition dysfunctions in patients with epilepsy: Evidence from patients with temporal lobe and idiopathic generalized epilepsies. Epilepsy Behav. (2015) 47:98–103. doi: 10.1016/j.yebeh.2015.04.048
31. Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. (2019) 18:146–61. doi: 10.1002/wps.20624
32. Broicher SD, Kuchukhidze G, Grunwald T, Krämer G, Kurthen M, Jokeit H, et al. “Tell me how do I feel”–emotion recognition and theory of mind in symptomatic mesial temporal lobe epilepsy. Neuropsychologia. (2012) 50:118–28. doi: 10.1016/j.neuropsychologia.2011.11.005
33. Boucher O, Rouleau I, Lassonde M, Lepore F, Bouthillier A, Nguyen DK, et al. Social information processing following resection of the insular cortex. Neuropsychologia. (2015) 71:1–10. doi: 10.1016/j.neuropsychologia.2015.03.008
34. Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS, et al. The impact of early and late damage to the human amygdala on 'theory of mind' reasoning. Brain. (2004) 127:1535–48. doi: 10.1093/brain/awh168
35. Giovagnoli AR, Franceschetti S, Reati F, Parente A, MacCagnano C, Villani F, et al. Theory of mind in frontal and temporal lobe epilepsy: cognitive and neural aspects. Epilepsia. (2011) 52:1995–2002. doi: 10.1111/j.1528-1167.2011.03215.x
36. Reynders HJ, Broks P, Dickson JM, Lee CE, Turpin G. Investigation of social and emotion information processing in temporal lobe epilepsy with ictal fear. Epilepsy Behav. (2005) 7:419–29. doi: 10.1016/j.yebeh.2005.07.013
37. Schacher M, Winkler R, Grunwald T, Kraemer G, Kurthen M, Reed V, et al. Mesial temporal lobe epilepsy impairs advanced social cognition. Epilepsia. (2006) 47:2141–6. doi: 10.1111/j.1528-1167.2006.00857.x
38. Bonora A, Benuzzi F, Monti G, Mirandola L, Pugnaghi M, Nichelli P, et al. Recognition of emotions from faces and voices in medial temporal lobe epilepsy. Epilepsy Behav. (2011) 20:648–54. doi: 10.1016/j.yebeh.2011.01.027
39. Li YH, Chiu MJ, Yeh ZT, Liou HH, Cheng TW, Hua MS, et al. Theory of mind in patients with temporal lobe epilepsy. J Int Neuropsychol Soc. (2013) 19:594–600. doi: 10.1017/S1355617713000143
40. Gomez-Ibañez A, Urrestarazu E, Viteri C. Recognition of facial emotions and identity in patients with mesial temporal lobe and idiopathic generalized epilepsy: An eye-tracking study. Seizure. (2014) 23:892–8. doi: 10.1016/j.seizure.2014.08.012
41. Leppanen J, Sedgewick F, Treasure J, Tchanturia K. Differences in the Theory of Mind profiles of patients with anorexia nervosa and individuals on the autism spectrum: a meta-analytic review. Neurosci Biobehav Rev. (2018) 90:146–63. doi: 10.1016/j.neubiorev.2018.04.009
42. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
43. Kalbe E, Grabenhorst F, Brand M, Kessler J, Hilker R, Markowitsch HJ, et al. Elevated emotional reactivity in affective but not cognitive components of theory of mind: a psychophysiological study. 1. (2007) 27–38. doi: 10.1348/174866407X180792
44. Ayribas B, Ayhan G, Topcuoglu V, Kose S, Sayar K. Theory of Mind Abilities and Insight Dimension in Patients with Obsessive-Compulsive Disorder and Schizophrenia. Psychiatr Clin Psychopharmacol. (2020) 30:434. doi: 10.5455/PCP.20201221104133
45. Hillmann K, Neukel C, Krauch M, Spohn A, Schnell K, Herpertz SC, et al. Cognitive and Affective Theory of Mind in Female Patients With Borderline Personality Disorder. J Pers Disord. (2021) 35:672–90. doi: 10.1521/pedi.2021.35.5.672
46. Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ, et al. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. (2015) 20:440–6. doi: 10.1038/mp.2014.59
47. Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat. (1981) 6:107–28. doi: 10.3102/10769986006002107
48. Cohen J, Cohen J, Cohen JW, Cohen J, Cohen J, Cohen J, et al. Statistical power analysis for the behavioral science. Technometrics. (1988) 31:499–500. doi: 10.1063/1.866910
49. Scammacca N, Roberts G, Stuebing KK. Meta-analysis with complex research designs: dealing with dependence from multiple measures and multiple group comparisons. Rev Educ Res. (2014) 84:328–64. doi: 10.3102/0034654313500826
50. Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
51. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
52. Ferreira Antunes JL. A dictionary of epidemiology. J Epidemiol Community Health. (2009) 63:337. doi: 10.1136/jech.2008.082511
53. Page MJ, Sterne JAC, Higgins JPT, Egger M. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: a review. Res Synth Methods. (2021) 12:248–59. doi: 10.1002/jrsm.1468
54. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2015) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
55. Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to Meta-Analysis || When does it Make Sense to Perform a Meta-Analysis? Hoboken, NJ: John Wiley and Sons (2009) 357–64.
56. Sanz-Martin A, Guevara MA, Corsi-Cabrera M, Ondarza-Rovira R, Ramos-Loyo J. Differential effect of left and right temporal lobectomy on emotional recognition and experience in patients with epilepsy. Rev Neurol. (2006) 42:391–8. doi: 10.33588/rn.4207.2004572
57. Brand JG, Burton LA, Schaffer SG, Alper KR, Devinsky O, Barr WB, et al. Emotional recognition in depressed epilepsy patients. Epilepsy Behav. (2009) 15:333–8. doi: 10.1016/j.yebeh.2009.04.021
58. Lomlomdjian C, Múnera CP, Low DM, Terpiluk V, Solís P, Abusamra V, et al. The right hemisphere's contribution to discourse processing: a study in temporal lobe epilepsy. Brain Lang. (2017) 171:31–41. doi: 10.1016/j.bandl.2017.04.001
59. Sung C, Connor A. Social-cognitive predictors of vocational outcomes in transition youth with epilepsy: Application of social cognitive career theory. Rehabil Psychol. (2017) 62:276–89. doi: 10.1037/rep0000161
60. Abraira L, Sanabria A, Ortega G, Quintana M, Santamarina E, Salas-Puig J, et al. [Social cognition and cognitive functions in patients with epilepsy treated with eslicarbazepine acetate]. Rev Neurol. (2018) 66:361–7.
61. Inanç L, Ünal Y, Semiz ÜB, Kutlu G. Do mentalization skills affect the perception of stigma in patients with epilepsy? Epilepsy Behav. (2018) 88:49–53. doi: 10.1016/j.yebeh.2018.08.022
62. Keni RR, Radhakrishnan A. Using McGurk effect to detect speech-perceptional abnormalities in refractory epilepsy. Epilepsy Behav. (2021) 114:107600. doi: 10.1016/j.yebeh.2020.107600
63. Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. (2001) 15:396–404. doi: 10.1037/0894-4105.15.3.396
64. Benuzzi F, Meletti S, Zamboni G, Calandra-Buonaura G, Serafini M, Lui F, et al. Impaired fear processing in right mesial temporal sclerosis: a fMRI study. Brain Res Bull. (2004) 63:269–81. doi: 10.1016/j.brainresbull.2004.03.005
65. Fowler HL, Baker GA, Tipples J, Hare DJ, Keller S, Chadwick DW, et al. Recognition of emotion with temporal lobe epilepsy and asymmetrical amygdala damage. Epilepsy Behav. (2006) 9:164–72. doi: 10.1016/j.yebeh.2006.04.013
66. Giovagnoli AR, Canafoglia L, Reati F, Raviglione F, Franceschetti S. The neuropsychological pattern of Unverricht-Lundborg disease. Epilepsy Res. (2009) 84:217–23. doi: 10.1016/j.eplepsyres.2009.02.004
67. Cohn M, St-Laurent M, Barnett A, McAndrews MP. Social inference deficits in temporal lobe epilepsy and lobectomy: risk factors and neural substrates. Soc Cogn Affect Neurosci. (2015) 10:636–44. doi: 10.1093/scan/nsu101
68. Gilliam F. Social cognition and epilepsy: Understanding the neurobiology of empathy and emotion. Epilepsy Curr. (2015) 15:118–9. doi: 10.5698/1535-7597-15.3.118
69. Ammerlaan EJ, Hendriks MP, Colon AJ, Kessels RP. Emotion perception and interpersonal behavior in epilepsy patients after unilateral amygdalohippocampectomy. Acta Neurobiol Exp (Wars). (2008) 68:214–8.
70. Banks SJ, Bellerose J, Douglas D, Jones-Gotman M. The insular cortex: relationship to skin conductance responses to facial expression of emotion in temporal lobe epilepsy. Appl Psychophysiol Biofeedback. (2014) 39:1–8. doi: 10.1007/s10484-013-9236-3
71. Benuzzi F, Zamboni G, Meletti S, Serafini M, Lui F, Baraldi P, et al. Recovery from emotion recognition impairment after temporal lobectomy. Front Neurol. (2014) 5:92. doi: 10.3389/fneur.2014.00092
72. Wang S, Yu RJ, Tyszka JM, Zhen SS, Kovach C, Sun S, et al. The human amygdala parametrically encodes the intensity of specific facial emotions and their categorical ambiguity. Nat Commun. (2017) 8:14821. doi: 10.1038/ncomms14821
73. Shaw P, Bramham J, Lawrence EJ, Morris R, Baron-Cohen S, David AS, et al. Differential effects of lesions of the amygdala and prefrontal cortex on recognizing facial expressions of complex emotions. J Cogn Neurosci. (2005) 17:1410–9. doi: 10.1162/0898929054985491
74. Bujarski KA, Flashman L, Li Z, Tosteson TD, Jobst BC, Thadani VM, et al. Investigating social cognition in epilepsy using a naturalistic task. Epilepsia. (2016) 57:1515–20. doi: 10.1111/epi.13477
75. Morou N, Papaliagkas V, Markouli E, Karagianni M, Nazlidou E, Spilioti M, et al. Theory of Mind impairment in focal vs. generalized epilepsy. Epilepsy Behav. (2018) 88:244–50. doi: 10.1016/j.yebeh.2018.09.026
76. Hatlestad-Hall C, Bruña R, Erichsen A, Andersson V, Syvertsen MR, Skogan AH, et al. The organization of functional neurocognitive networks in focal epilepsy correlates with domain-specific cognitive performance. J Neurosci Res. (2021) 99:2669–87. doi: 10.1002/jnr.24896
77. Pinabiaux C, Bulteau C, Fohlen M, Dorfmüller G, Chiron C, Hertz-Pannier L, et al. Impaired emotional memory recognition after early temporal lobe epilepsy surgery: the fearful face exception? Cortex. (2013) 49:1386–93. doi: 10.1016/j.cortex.2012.06.008
78. Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. (2000) 14:526–36. doi: 10.1037/0894-4105.14.4.526
79. Meletti S, Benuzzi F, Rubboli G, Cantalupo G, Stanzani Maserati M, Nichelli P, et al. Impaired facial emotion recognition in early-onset right mesial temporal lobe epilepsy. Neurology. (2003) 60:426–31. doi: 10.1212/WNL.60.3.426
80. Brierley B, Medford N, Shaw P, David AS. Emotional memory and perception in temporal lobectomy patients with amygdala damage. J Neurol Neurosurg Psychiatry. (2004) 75:593–9. doi: 10.1136/jnnp.2002.006403
81. McClelland S 3rd, Garcia RE, Peraza DM, Shih TT, Hirsch LJ, Hirsch J, et al. Facial emotion recognition after curative nondominant temporal lobectomy in patients with mesial temporal sclerosis. Epilepsia. (2006) 47:1337–42. doi: 10.1111/j.1528-1167.2006.00557.x
82. Shaw P, Lawrence E, Bramham J, Brierley B, Radbourne C, David AS, et al. A prospective study of the effects of anterior temporal lobectomy on emotion recognition and theory of mind. Neuropsychologia. (2007) 45:2783–90. doi: 10.1016/j.neuropsychologia.2007.04.020
83. Hlobil U, Rathore C, Alexander A, Sarma S, Radhakrishnan K. Impaired facial emotion recognition in patients with mesial temporal lobe epilepsy associated with hippocampal sclerosis (MTLE-HS): side and age at onset matters. Epilepsy Res. (2008) 80:150–7. doi: 10.1016/j.eplepsyres.2008.03.018
84. Walpole P, Isaac CL, Reynders HJ. A comparison of emotional and cognitive intelligence in people with and without temporal lobe epilepsy. Epilepsia. (2008) 49:1470–4. doi: 10.1111/j.1528-1167.2008.01655.x
85. Carvajal F, Rubio S, Martin P, Serrano JM, Garcia-Sola R. Perception and recall of faces and facial expressions following temporal lobectomy. Epilepsy Behav. (2009) 14:60–5. doi: 10.1016/j.yebeh.2008.08.016
86. Meletti S, Benuzzi F, Cantalupo G, Rubboli G, Tassinari CA, Nichelli P, et al. Facial emotion recognition impairment in chronic temporal lobe epilepsy. Epilepsia. (2009) 50:1547–59. doi: 10.1111/j.1528-1167.2008.01978.x
87. Gosselin N, Peretz I, Hasboun D, Baulac M, Samson S. Impaired recognition of musical emotions and facial expressions following anteromedial temporal lobe excision. Cortex. (2011) 47:1116–25. doi: 10.1016/j.cortex.2011.05.012
88. Sedda A, Rivolta D, Scarpa P, Burt M, Frigerio E, Zanardi G, et al. Ambiguous emotion recognition in temporal lobe epilepsy: the role of expression intensity. Cogn Affect Behav Neurosci. (2013) 13:452–63. doi: 10.3758/s13415-013-0153-y
89. Tanaka A, Akamatsu N, Yamano M, Nakagawa M, Kawamura M, Tsuji S, et al. A more realistic approach, using dynamic stimuli, to test facial emotion recognition impairment in temporal lobe epilepsy. Epilepsy Behav. (2013) 28:12–6. doi: 10.1016/j.yebeh.2013.03.022
90. Ahs F, Engman J, Persson J, Larsson EM, Wikström J, Kumlien E, et al. Medial temporal lobe resection attenuates superior temporal sulcus response to faces. Neuropsychologia. (2014) 61:291–8. doi: 10.1016/j.neuropsychologia.2014.06.030
91. Meletti S, Picardi A, De Risi M, Monti G, Esposito V, Grammaldo LG, et al. The affective value of faces in patients achieving long-term seizure freedom after temporal lobectomy. Epilepsy Behav. (2014) 36:97–101. doi: 10.1016/j.yebeh.2014.05.002
92. Szaflarski JP, Allendorfer JB, Heyse H, Mendoza L, Szaflarski BA, Cohen N, et al. Functional MRI of facial emotion processing in left temporal lobe epilepsy. Epilepsy Behav. (2014) 32:92–9. doi: 10.1016/j.yebeh.2014.01.012
93. Hennion S, Delbeuck X, Duhamel A, Lopes R, Semah F, Tyvaert L, et al. Characterization and prediction of theory of mind disorders in temporal lobe epilepsy. Neuropsychology. (2015) 29:485–92. doi: 10.1037/neu0000126
94. Hennion S, Szurhaj W, Duhamel A, Lopes R, Tyvaert L, Derambure P, et al. Characterization and prediction of the recognition of emotional faces and emotional bursts in temporal lobe epilepsy. J Clin Exp Neuropsychol. (2015) 37:931–45. doi: 10.1080/13803395.2015.1068280
95. Wang WH, Shih YH, Yu HY, Yen DJ, Lin YY, Kwan SY, et al. Theory of mind and social functioning in patients with temporal lobe epilepsy. Epilepsia. (2015) 56:1117–23. doi: 10.1111/epi.13023
96. Wendling AS, Steinhoff BJ, Bodin F, Staack AM, Zentner J, Scholly J, et al. Selective amygdalohippocampectomy vs. standard temporal lobectomy in patients with mesiotemporal lobe epilepsy and unilateral hippocampal sclerosis: Post-operative facial emotion recognition abilities. Epilepsy Res. (2015) 111:26–32. doi: 10.1016/j.eplepsyres.2015.01.002
97. Giovagnoli AR, Parente A, Didato G, Deleo F, Villani F. Expanding the spectrum of cognitive outcomes after temporal lobe epilepsy surgery: a prospective study of theory of mind. Epilepsia. (2016) 57:920–30. doi: 10.1111/epi.13384
98. Hennion S, Delbeuck X, Koelkebeck K, Brion M, Tyvaert L, Plomhause L, et al. A functional magnetic resonance imaging investigation of theory of mind impairments in patients with temporal lobe epilepsy. Neuropsychologia. (2016) 93:271–9. doi: 10.1016/j.neuropsychologia.2016.11.007
99. Okruszek Ł, Bala A, Dziekan M, Szantroch M, Rysz A, Marchel A, et al. Gaze matters! The effect of gaze direction on emotional enhancement of memory for faces in patients with mesial temporal lobe epilepsy. Epilepsy Behav. (2017) 72:35–8. doi: 10.1016/j.yebeh.2017.04.016
100. Okruszek Ł, Bala A, Wordecha M, Jarkiewicz M, Wysokiński A, Szczepocka E, et al. Social cognition in neuropsychiatric populations: a comparison of theory of mind in schizophrenia and mesial temporal lobe epilepsy. Sci Rep. (2017) 7:484. doi: 10.1038/s41598-017-00565-2
101. Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. (2007) 45:3054–67. doi: 10.1016/j.neuropsychologia.2007.05.021
102. Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. (2011) 49:2971–84. doi: 10.1016/j.neuropsychologia.2011.07.012
103. Sebastian CL, Fontaine NM, Bird G, Blakemore SJ, Brito SA, McCrory EJ, et al. Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Soc Cogn Affect Neurosci. (2012) 7:53–63. doi: 10.1093/scan/nsr023
104. Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci Biobehav Rev. (2016) 65:276–91. doi: 10.1016/j.neubiorev.2016.03.020
105. Kalbe E, Schlegel M, Sack AT, Nowak DA, Dafotakis M, Bangard C, et al. Dissociating cognitive from affective theory of mind: a TMS study. Cortex. (2010) 46:769–80. doi: 10.1016/j.cortex.2009.07.010
106. Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. (2005) 18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99
107. Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. (2008) 49:741–57. doi: 10.1111/j.1528-1167.2007.01485.x
108. Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. (2009) 30:2313–35. doi: 10.1002/hbm.20671
109. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. (2009) 21:489–510. doi: 10.1162/jocn.2008.21029
110. Park CH, Ohn SH. A challenge of predicting seizure frequency in temporal lobe epilepsy using neuroanatomical features. Neurosci Lett. (2019) 692:115–21. doi: 10.1016/j.neulet.2018.11.005
111. Rivera Bonet CN, Hermann B, Cook CJ, Hwang G, Dabbs K, Nair V, et al. Neuroanatomical correlates of personality traits in temporal lobe epilepsy: findings from the epilepsy connectome project. Epilepsy Behav 98(Pt A). (2019) 220–7. doi: 10.1016/j.yebeh.2019.07.025
112. Phillips LH, Bull R, Allen R, Insch P, Burr K, Ogg W, et al. Lifespan aging and belief reasoning: Influences of executive function and social cue decoding. Cognition. (2011) 120:236–47. doi: 10.1016/j.cognition.2011.05.003
113. Giovagnoli AR. Theory of mind across lifespan from ages 16 to 81 years. Epilepsy Behav. (2019) 100:106349. doi: 10.1016/j.yebeh.2019.05.044
114. Batista S, Freitas S, Afonso A, Macário C, Sousa L, Cunha L, et al. Theory of mind and executive functions are dissociated in multiple sclerosis. Arch Clin Neuropsychol. (2018) 33:541–51. doi: 10.1093/arclin/acx101
115. Ives-Deliperi VL, Jokeit H. Impaired social cognition in epilepsy: a review of what we have learnt from neuroimaging studies. Front Neurol. (2019) 10:940. doi: 10.3389/fneur.2019.00940
116. Stewart E, Lah S, Smith ML. Patterns of impaired social cognition in children and adolescents with epilepsy: The borders between different epilepsy phenotypes. Epilepsy Behav. (2019) 100:106146. doi: 10.1016/j.yebeh.2019.01.031
117. Pastorino GMG, Operto FF, Padovano C, Vivenzio V, Scuoppo C, Pastorino N, et al. Social cognition in neurodevelopmental disorders and epilepsy. Front Neurol. (2021) 12:658823. doi: 10.3389/fneur.2021.658823
118. Rosenberg H, McDonald S, Dethier M, Kessels RP, Westbrook RF. Facial emotion recognition deficits following moderate-severe Traumatic Brain Injury (TBI): re-examining the valence effect and the role of emotion intensity. J Int Neuropsychol Soc. (2014) 20:994–1003. doi: 10.1017/S1355617714000940
119. Garrido MV, Prada M. KDEF-PT: valence, emotional intensity, familiarity and attractiveness ratings of angry, neutral, and happy faces. Front Psychol. (2017) 8:2181. doi: 10.3389/fpsyg.2017.02181
120. Operto FF, Pastorino GMG, Mazza R, Di Bonaventura C, Marotta R, Pastorino N, et al. Social cognition and executive functions in children and adolescents with focal epilepsy. Eur J Paediatric Neurol. (2020) 28:167–75. doi: 10.1016/j.ejpn.2020.06.019
121. Henry JD, Phillips LH, Beatty WW, McDonald S, Longley WA, Joscelyne A, et al. Evidence for deficits in facial affect recognition and theory of mind in multiple sclerosis. J Int Neuropsychol Soc. (2009) 15:277–85. doi: 10.1017/S1355617709090195
122. Gray HM, Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in Parkinson's disease. Neuropsychology. (2010) 24:176–91. doi: 10.1037/a0018104
123. Bora E, Velakoulis D, Walterfang M. Social cognition in Huntington's disease: a meta-analysis. Behav Brain Res. (2016) 297:131–40. doi: 10.1016/j.bbr.2015.10.001
124. Pons F, Harris PL, Rosnay MD. Emotion comprehension between 3 and 11 years: Developmental periods and hierarchical organization. Eur J Dev Psychol. (2004) 1:127–52. doi: 10.1080/17405620344000022
125. Vetter NC, Weigelt S, Döhnel K, Smolka MN, Kliegel M. Ongoing neural development of affective theory of mind in adolescence. Soc Cogn Affect Neurosci. (2014) 9:1022–9. doi: 10.1093/scan/nst081
126. Stewart E, Catroppa C, Gill D, Webster R, Lawson J, Mandalis A, et al. Theory of mind and social competence in children and adolescents with genetic generalised epilepsy (GGE): relationships to epilepsy severity and anti-epileptic drugs. Seizure. (2018) 60:96–104. doi: 10.1016/j.seizure.2018.06.015
127. Anderson V, Moore C. Age at injury as a predictor of outcome following pediatric head injury: a longitudinal perspective. Child Neuropsychol. (2007) 1:187–202. doi: 10.1080/09297049508400224
128. Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld JV. Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics. (2009) 124:e1064–1071. doi: 10.1542/peds.2009-0365
129. Anderson V, Brown S, Newitt H, Hoile H. Long-term outcome from childhood traumatic brain injury: intellectual ability, personality, and quality of life. Neuropsychology. (2011) 25:176–84. doi: 10.1037/a0021217
130. Giovagnoli AR. The importance of theory of mind in epilepsy. Epilepsy Behav. (2014) 39:145–53. doi: 10.1016/j.yebeh.2014.05.021
131. Stewart E, Catroppa C, Gonzalez L, Gill D, Webster R, Lawson J, et al. Theory of mind and social competence in children and adolescents with temporal lobe epilepsy. Neuropsychology. (2019) 33:986–95. doi: 10.1037/neu0000543
132. Kuang Y, Yang T, Gu J, Kong B, Cheng L. Comparison of therapeutic effects between selective amygdalohippocampectomy and anterior temporal lobectomy for the treatment of temporal lobe epilepsy: a meta-analysis. Br J Neurosurg. (2014) 28:374–7. doi: 10.3109/02688697.2013.841854
133. Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci. (2013) 8:123–33. doi: 10.1093/scan/nss119
134. Mikula B, Lencsés A, Borbély C, Demeter G. Emotion recognition and theory of mind after temporal lobe epilepsy surgery: a systematic review. Seizure. (2021) 93:63–74. doi: 10.1016/j.seizure.2021.10.005
135. Mirabel H, Guinet V, Voltzenlogel V, Pradier S, Hennion S. Social cognition in epilepsy: state of the art and perspectives. Rev Neurol (Paris). (2020) 176:468–79. doi: 10.1016/j.neurol.2020.02.010
136. Ziaei M, Arnold C, Thompson K, Reutens DC. Social cognition in temporal and frontal lobe epilepsy: systematic review, meta-analysis, and clinical recommendations. J Int Neuropsychol Soc. (2022) 1–25. doi: 10.1017/S1355617722000066
137. Cotter J, Granger K, Backx R, Hobbs M, Barnett JH. Social cognitive dysfunction as a clinical marker: a systematic review of meta-analyses across 30 clinical conditions. Neurosci Biobehav Rev. (2018) 84:92–9. doi: 10.1016/j.neubiorev.2017.11.014
138. Henry JD, von Hippel W, Molenberghs P, Lee T, Sachdev PS. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. (2016) 12:28–39. doi: 10.1038/nrneurol.2015.229
139. Eicher M, Jokeit H. Toward social neuropsychology of epilepsy: a meta-analysis on social cognition in epilepsy phenotypes and a critical narrative review on assessment methods. Acta Epileptologica. (2022) 4:1–17. doi: 10.1186/s42494-022-00093-1
140. Brissart H, Planton M, Bilger M, Bulteau C, Forthoffer N, Guinet V, et al. French neuropsychological procedure consensus in epilepsy surgery. Epilepsy Behav. (2019) 100:106522. doi: 10.1016/j.yebeh.2019.106522
141. Guercio JM, Podolska-Schroeder H, Rehfeldt RA. Using stimulus equivalence technology to teach emotion recognition to adults with acquired brain injury. Brain Inj. (2004) 18:593–601. doi: 10.1080/02699050310001646116
142. Bornhofen C, McDonald S. Comparing strategies for treating emotion perception deficits in traumatic brain injury. J Head Trauma Rehabil. (2008) 23:103–15. doi: 10.1097/01.HTR.0000314529.22777.43
143. Hadwin JA, Kovshoff H. A review of theory of mind interventions for children and adolescents with autism spectrum conditions. Rfo. (2013) 137:605–8. doi: 10.1093/acprof:oso/9780199692972.003.0023
144. Neumann D, Babbage DR, Zupan B, Willer B. A randomized controlled trial of emotion recognition training after traumatic brain injury. J Head Trauma Rehabil. (2015) 30:E12–23. doi: 10.1097/HTR.0000000000000054
145. Hofmann SG, Doan SN, Sprung M, Wilson A, Ebesutani C, Andrews LA, et al. Training children's theory-of-mind: a meta-analysis of controlled studies. Cognition. (2016) 150:200–12. doi: 10.1016/j.cognition.2016.01.006
146. Grant N, Lawrence M, Preti A, Wykes T, Cella M. Social cognition interventions for people with schizophrenia: a systematic review focussing on methodological quality and intervention modality. Clin Psychol Rev. (2017) 56:55–64. doi: 10.1016/j.cpr.2017.06.001
147. Westerhof-Evers HJ, Visser-Keizer AC, Fasotti L, Schönherr MC, Vink M, van der Naalt J, et al. Effectiveness of a Treatment for Impairments in Social Cognition and Emotion Regulation (T-ScEmo) after traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil. (2017) 32:296–307. doi: 10.1097/HTR.0000000000000332
148. Corbett BA, Ioannou S, Key AP, Coke C, Muscatello R, Vandekar S, et al. Treatment effects in social cognition and behavior following a theater-based intervention for youth with autism. Dev Neuropsychol. (2019) 44:481–94. doi: 10.1080/87565641.2019.1676244
149. Tseng A, Biagianti B, Francis SM, Conelea CA, Jacob S. Social Cognitive interventions for adolescents with autism spectrum disorders: a systematic review. J Affect Disord. (2020) 274:199–204. doi: 10.1016/j.jad.2020.05.134
150. Cheung PPP, Brown T, Yu ML, Siu AMH. The effectiveness of a school-based social cognitive intervention on the social participation of Chinese children with autism. J Autism Dev Disord. (2021) 51:1894–908. doi: 10.1007/s10803-020-04683-1
151. Darling SJ, Goods M, Ryan NP, Chisholm AK, Haebich K, Payne JM, et al. Behavioral intervention for social challenges in children and adolescents: a systematic review and meta-analysis. JAMA Pediatr. (2021) 175:e213982. doi: 10.1001/jamapediatrics.2021.3982
152. Lahera G, Reboreda A, Vallespí A, Vidal C, López V, Aznar A, et al. Social Cognition and Interaction Training (SCIT) vs. Training in Affect Recognition (TAR) in patients with schizophrenia: a randomized controlled trial. J Psychiatr Res. (2021) 142:101–9. doi: 10.1016/j.jpsychires.2021.07.029
153. Nahum M, Lee H, Fisher M, Green MF, Hooker CI, Ventura J, et al. Online social cognition training in schizophrenia: a double-blind, randomized, controlled multi-site clinical trial. Schizophr Bull. (2021) 47:108–17. doi: 10.1093/schbul/sbaa085
Keywords: temporal lobe epilepsy, theory of mind, facial emotion recognition, meta-analysis, cognitive, affective
Citation: Qi L, Zhao J, Zhao PW, Zhang H, Zhong JG, Pan PL, Wang GD, Yi ZQ and Xie LL (2022) Theory of mind and facial emotion recognition in adults with temporal lobe epilepsy: A meta-analysis. Front. Psychiatry 13:976439. doi: 10.3389/fpsyt.2022.976439
Received: 23 June 2022; Accepted: 16 September 2022;
Published: 06 October 2022.
Edited by:
Jan B. Engelmann, University of Amsterdam, NetherlandsReviewed by:
Gaelle Eve Doucet, Boys Town National Research Hospital, United StatesCopyright © 2022 Qi, Zhao, Zhao, Zhang, Zhong, Pan, Wang, Yi and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ZhongQuan Yi, eWl6aG9uZ3F1YW5AMTYzLmNvbQ==; LiLi Xie, TGlsaWx1Y2t5MzMzQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.