
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 18 August 2022
Sec. Public Mental Health
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.976428
Background: Psychiatric disorders have seriously affected human life, one of the risk genes related to psychosis is the methylenetetrahydrofolatereductase (MTHFR) gene. This gene has a potential role in psychiatric disorders. Therefore, a meta-analysis is conducted to investigate the correlations between two prevalent MTHFR single nucleotide polymorphisms (SNPs), MTHFR C677T, A1298C, severe psychological disorders (schizophrenia, major depression, bipolar disorder).
Methods: A total of 81 published studies were screened and selected by a search of electronic databases up to April 2022. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the association between MTHFR polymorphism and psychiatric disorders susceptibility by using random effect models.
Results: We found that MTHFR C677T polymorphism is significantly related to schizophrenia and major depression in the overall population. MTHFR C677T has been linked to an increased risk of bipolar disorder in the recessive model (TT vs. CT + CC). Ethnic subgroup analysis shows that schizophrenia and major depression significantly correlate with MTHFR C677T and A1298C in Asian populations but not Caucasians. Besides, schizophrenia is correlated substantially with MTHFR C677T in the African population. However, the MTHFR A1298C polymorphism is only marginally linked to major depression.
Conclusion: Findings of the current study revealed that MTHFR may contribute to the common pathogenesis of psychiatric diseases and that its variants may be essential in controlling the expression of psychosis-related genes. This study could help the researchers and health specialists in the early diagnosis and treatment of psychiatric disorders.
Mental disorders have seriously affected human life, causing considerable familial and social burden (1). They are among the leading causes of disability globally and have been related to an increase in premature mortality (2). Major psychiatric disorders include schizophrenia (SZ), major depression (MD), bipolar disorder (BPD), and others (3). These mental disorders are more likely to occur in families, suggesting that they are related to genetic factors (4, 5). Many susceptible genes have been found through unbiased genome-wide association studies (GWAS), a kind of analysis comparing allele frequencies of all available polymorphic markers with specific symptoms or disease states (6, 7). GWAS and many other follow-up replication studies have suggested that methylenetetrahydrofolatereductase (MTHFR) polymorphisms are associated with psychiatric disorders.
The MTHFR is a crucial enzyme in the one-carbon metabolism (OCM) process, which involves folate and homocysteine (Hcy) metabolisms. It transforms 5,10-methylenetetrahydrofolate (5,10-methylene THF) to 5-methyltetrahydrofolate (5-methyl THF), and it is involved in folate and homocysteine conversion, which is linked to DNA methylation (8–10). A number of mutations in the MTHFR gene have been found, and the most common mutations are C677T (rs1801133) and A1298C (rs1801131), which are correlated with enzyme deficiency (11–14). In addition, MTHFR polymorphism may significantly decrease MTHFR activity, affect the concentration of Hcy in plasma, and lead to a wide range of mental, neurological, and vascular dysfunction (15).
The human Methylenetetrahydrofolatereductase (MTHFR) gene is located in chromosomal region 1p36.3 (16). The MTHFR gene has 14 common or rare single nucleotide polymorphisms linked with enzyme defects, the most prevalent of which are C677T and A1298C. The C677T gene location is one of the most researched and clinically significant variants in exon 4. The variation in C677T is due to the replacement of cytosine by thymine, which leads to the conversion of valine to alanine at codon 222 (11). The polymorphism of A1298C is due to the adenine substitution by cytosine, leading to the conversion of glutamic acid to alanine at residue 429 (10). The replacement of 677 and 1,298 nucleotides C-T and A-C in the MTHFR gene reduces enzyme activity, and this decrease in MTHFR activity may affect the OCM cycle (17). Abnormal OCM might impair cortical and hippocampal neurogenesis during development and affect brain maturation and function (18–20).
The association between MTHFR polymorphism and mental illnesses has already been explored, but the influence of MTHFR on psychiatric disorders is still disputed, and limited studies have been found (21–23). These inconsistencies might be attributed to limited sample sizes, ethnic heterogeneity, and differences in population substructure. So, in current study these limitations have been overcome and summarized the conflicting data. A meta-analysis is performed to explore the connection of MTHFR C677T and A1298C polymorphisms with major mental disorders (including SZ, MD, and BPD). We also assessed whether ethnicity would affect the results. Therefore, it will provide more powerful evidence of whether MTHFR variants influence psychiatric diseases.
We initially searched PubMed, Embase, Proquest, Web of Science, CNKI (Chinese National Knowledge Infrastructure), VIP (Chinese) database, and Wanfang (Chinese) database for the following terms: MTHFR (methylenetetrahydrofolatereductase), gene (gene or genetic or polymorphism or variants or variation), and psychiatric disorders (psychiatry disorders or mental illness or mental disorders or psychosis). We discovered that most research concentrated on MTHFR C677T and MTHFR A1298C. The researchers investigated the relationships between MTHFR gene variants and susceptibility to mental diseases such as schizophrenia, bipolar disorder, and depression. To guarantee that we missed no studies, we searched these databases again using these gene terms (MTHFR C677T and A1298C) and major mental disorders such as “schizophrenia,” “bipolar disorder,” “depression,” and so on. All of the research was completed and published by April 2022. After that, we selected relevant papers and examined their bibliographies to find additional references.
Selection of articles for analysis purposes was made based on the following criteria: (1) case-control studies; (2) giving comprehensive data of formally diagnosed patients with unrelated healthy control subjects for generating an odds ratio (OR) with a 95% confidence interval (CI); (3) Case status was classified as having a DSM-IV-diagnosed mental condition, with control patients having no history of psychiatric disorders or other neurological abnormalities; (4) the studies used samples that did not overlap with other studies; (5) the use of internationally recognized loci gene polymorphism detection techniques (such as polymerase chain reaction-restriction fragment length polymorphism, real-time quantitative polymerase chain reaction, or amplification block mutation system-polymerase chain reaction); and (6) the demographic characteristics of the control group, such as gender and age, were comparable to those of the case group. In addition, articles were excluded if they (1) not reported the target genotype frequencies, (2) were reviews, letters, or commentaries, or (3) were duplicate reports.
Two reviewers independently extracted the following information from all eligible studies: author, year of publication, country, ethnicity (categorized as Asian, Caucasian, and African populations), and the number of distinct genotypes in cases and controls for C677T or A1298C genotype. In the case of a disagreement, a discussion was held, and if no agreement could be achieved, a third person was consulted for consensus.
We investigated the potential of conducting a meta-analysis of all eligible studies. The odds ratio (OR) and associated 95% confidence intervals (CIs) were used to examine the strength of the connection between MTHFR polymorphism and mental disorders: the allele model (T vs. C, C vs. A), the dominant model (TT + CT vs. CC, CC + AC vs. AA), the homozygote model (TT vs. CC, CC vs. AA) and the recessive model (TT vs. CT + CC, CC vs. AC + AA). The Chi-square test was used to analyze the genotype distribution in the control groups for Hardy Weinberg equilibrium (HWE). The Cochran’s (Q) X2 test and I2 statistic were used to assess the heterogeneity between individual studies (24). Considering the heterogeneity of studies, this meta-analysis adopted a random effect model (25). Subgroup analyses were performed using ethnicity stratification, and sensitivity analyses were undertaken by excluding papers from the meta-analysis that were not in HWE. The funnel plots were displayed and evaluated using Egger’s linear regression test to control publication bias (26). Stata 14.0 was used to conduct all statistical analyses (StataCorp, College Station, TX, United States). A P-value of less than 0.05 was regarded as statistically significant. The article mainly showed the forest plots of T vs. C of MTHFR C677T and C vs. A of MTHFR A1298C; the other results were shown in the tables.
Out of screened articles, 843 unduplicated association studies were found. Figure 1 depicts a flow chart of the research process, the eliminated studies, and the reasons for their exclusion. Following an initial literature search and further screening, 81 (27–106) publications were retrieved. Our meta-analysis comprised 49,775 subjects (20,981 patients and 28,794 controls) with MTHFR C677T genotyping and 16,058 subjects (6,690 patients and 9,368 controls) with MTHFR A1298C genotyping. Detailed information (first author, year of publication, country, ethnicity, case/control, genotype, and PHWE) of included articles are summarized in Tables 1, 2.
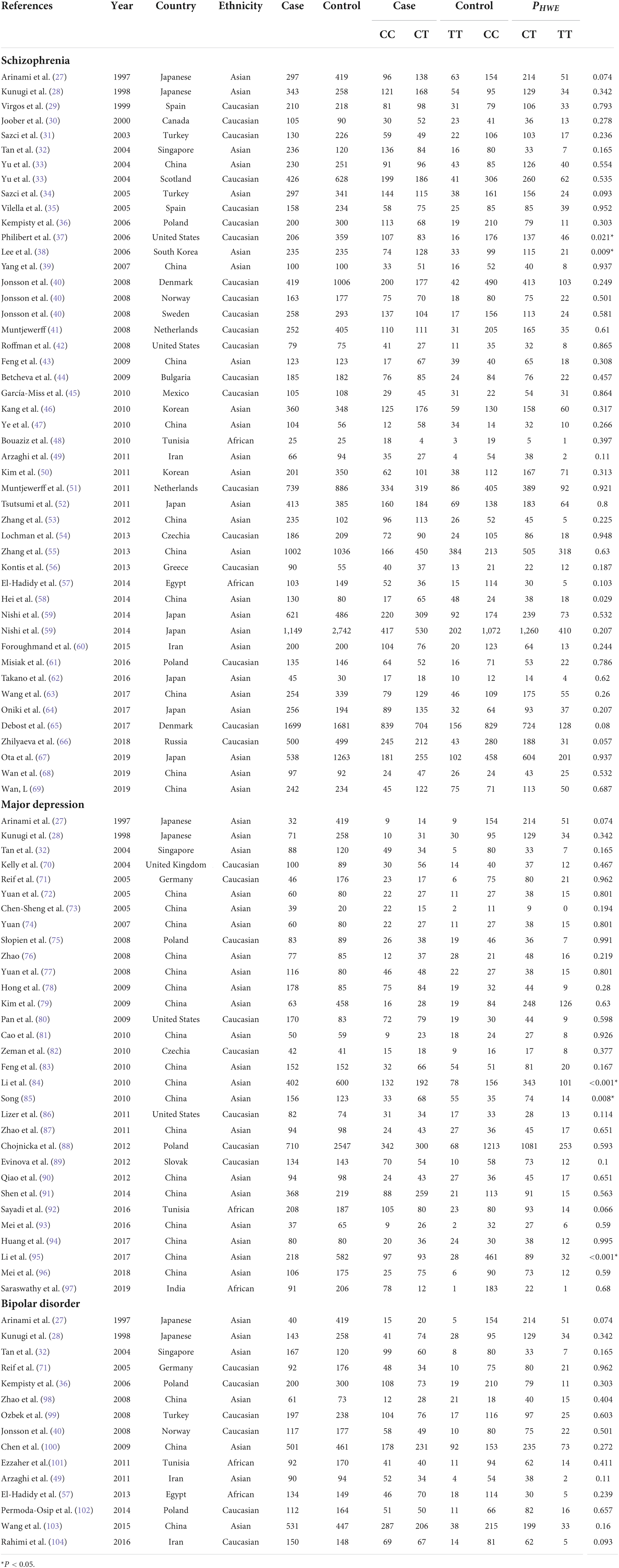
Table 1. Overview of MTHFR C677T genotype distribution of psychosis patients and controls, with information about country, ethnicity, and disease.
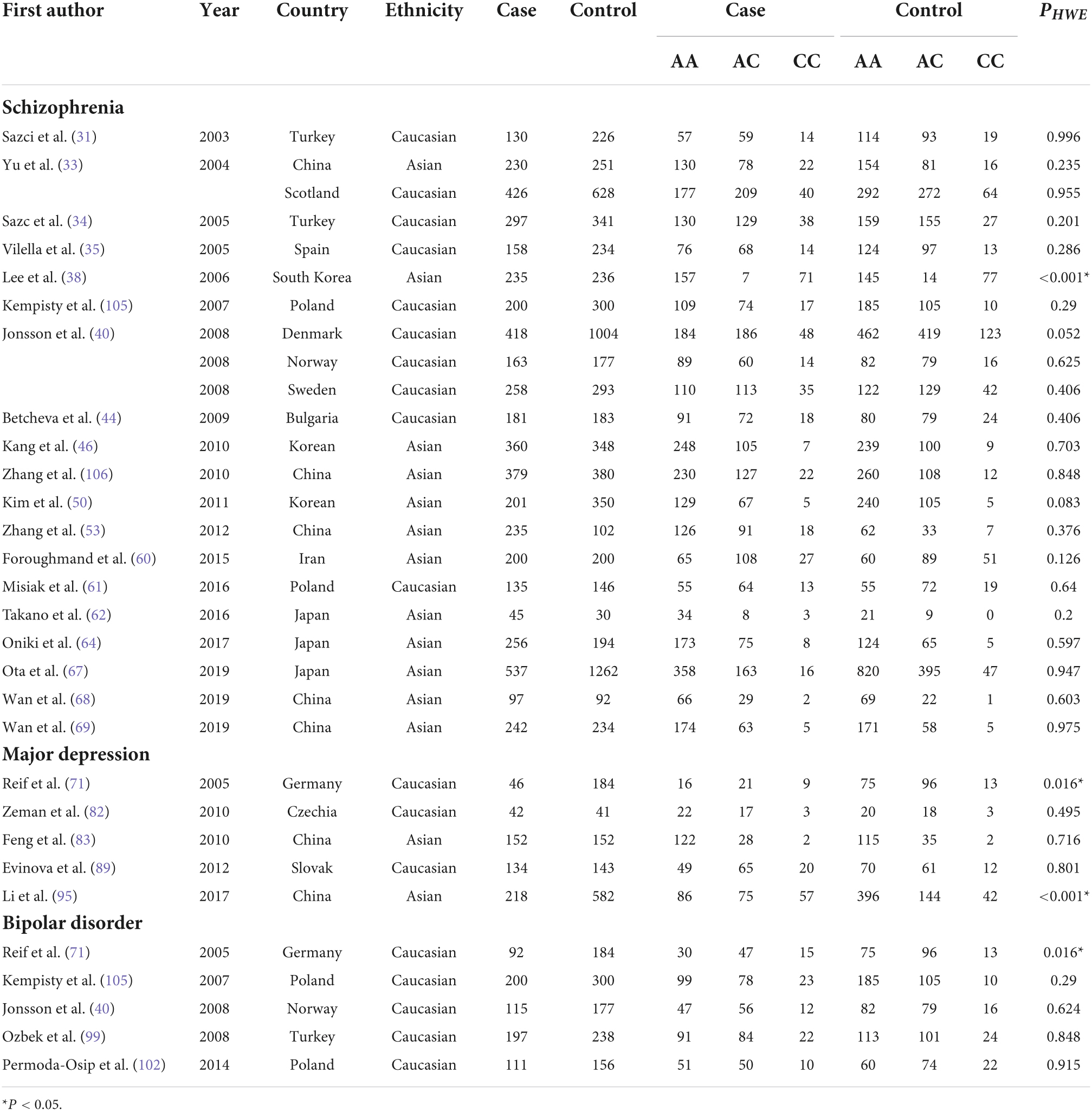
Table 2. Overview of MTHFR A1298C genotype distribution of psychosis patients and controls, with information about country, ethnicity, and disease.
Findings of the association and the heterogeneity test is shown in Table 3. MTHFR C677T polymorphism was shown to be highly associated with an increased risk of developing SZ in all statistical models (for T vs. C, OR = 1.16, 95% CI = 1.10–1.23, P < 0.001; for TT + CT vs. CC: OR = 1.18, 95% CI = 1.10–1.27, P < 0.001; for TT vs. CT + CC: OR = 1.25, 95% CI = 1.13–1.37, P < 0.001; for TT vs. CC: OR = 1.35, 95% CI = 1.19–1.52, P < 0.001) (Figure 2 and Table 3).
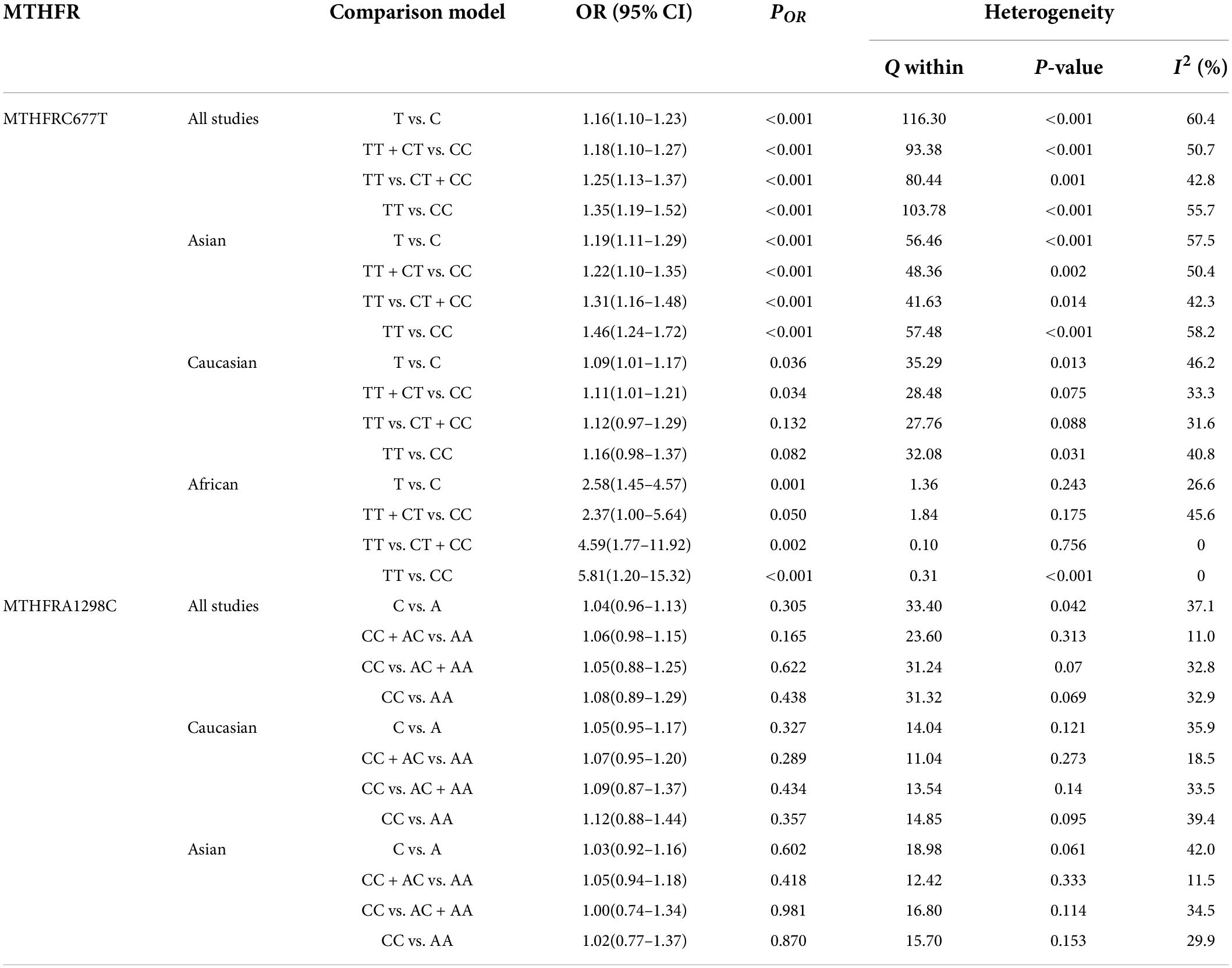
Table 3. Odds ratios and heterogeneity results for the 4 genetic models of the MTHFR C677T and A1298C for SZ.
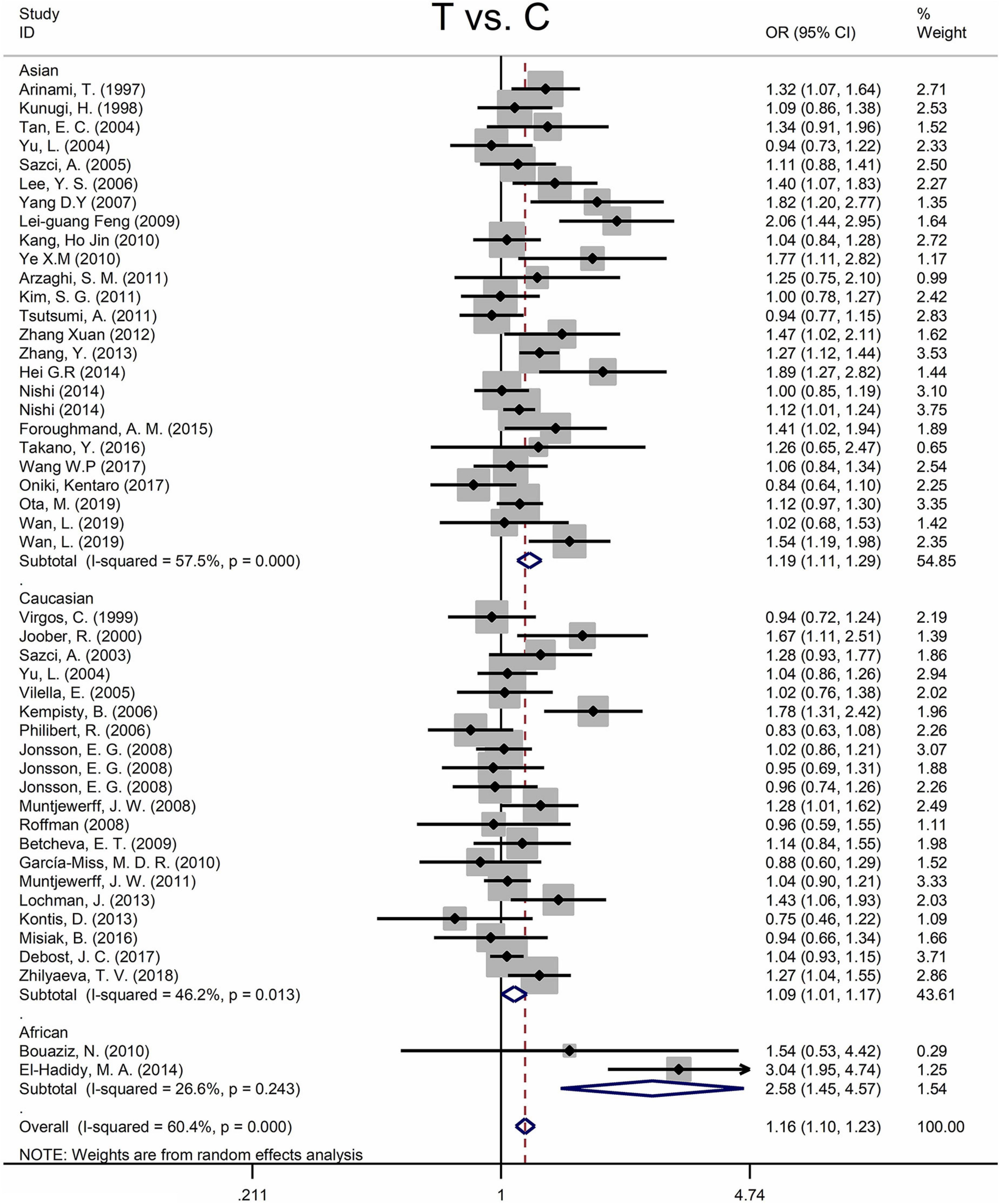
Figure 2. Forest plots for the associations between MTHFR C677T polymorphisms and SZ for the allele model with random effect model.
An ethnic subgroup analysis revealed a substantial association between MTHFR C677T polymorphism and SZ among Asian populations (for T vs. C: OR = 1.19, 95% CI = 1.11–1.29, P < 0.001; for TT + CT vs. CC: OR = 1.22, 95% CI = 1.10–1.35, P < 0.001; for TT vs. CT + CC: OR = 1.31, 95% CI = 1.16–1.48, P < 0.001; for TT vs. CC: OR = 1.46, 95% CI = 1.24–1.72, P < 0.001); in Caucasian populations, a significant association was found with the allele model (for T vs. C: OR = 1.09, 95% Cl = 1.01–1.17, P = 0.036) and the dominant model (for TT + CT vs. CC: OR = 1.11, 95% Cl = 1.01–1.21, P = 0.034); in African populations, there was a significant association with the allele model (for T vs. C: OR = 2.58, 95% Cl = 1.45–4.57, P = 0.001), the recessive model (TT vs. CT + CC: OR = 4.59, 95% CI = 1.77–11.92, P = 0.002) and the homozygote model (for TT vs. CC: OR = 5.81, 95% Cl = 1.20–15.32, P < 0.001). All these findings are summarized in Table 3. Subgroup analysis reveals that the association between MTHFR C677T polymorphism and SZ exists in Asian (all genetic models) and African populations (allele models, recessive models, and homozygous models) but not in Caucasian (only allele models and dominant models).
The MTHFR A1298C polymorphism was not statistically correlated with SZ in all models (Figure 3 and Table 3). Moreover, subgroup analysis revealed no correlation between the MTHFR A1298C polymorphism and SZ in Asian or Caucasian populations (Figure 3 and Table 3). African populations were not included in the study because of the small number of studies.
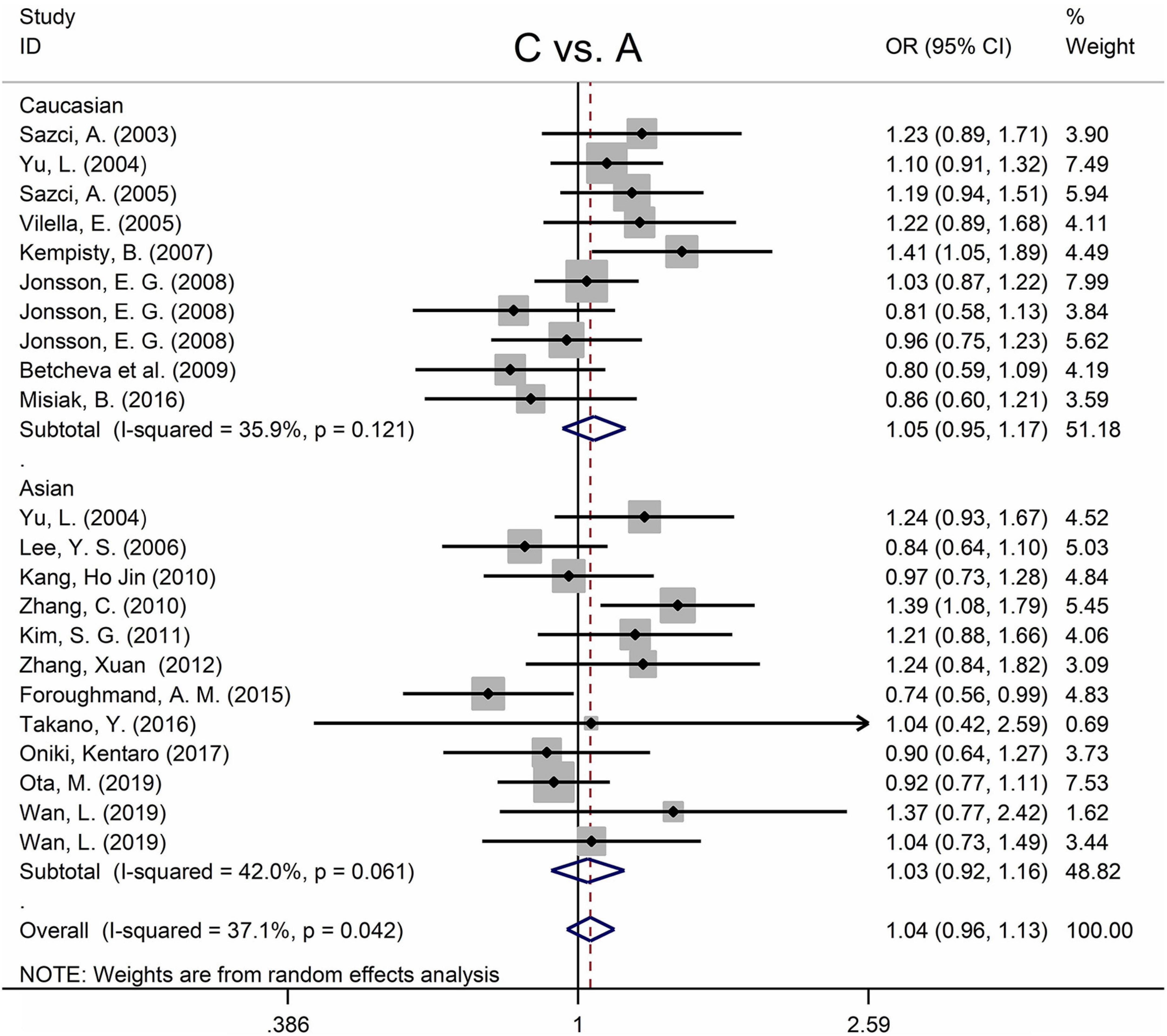
Figure 3. Forest plots for the associations between MTHFR A1298C polymorphisms and SZ for the allele model with random effect model.
There were two articles not in Hardy–Weinberg equilibrium (37, 38) (Tables 1, 2). Sensitivity analysis revealed that the overall association between MTHFR C677T polymorphism and SZ remained unchanged after omitting these two samples from the meta-analysis (for T vs. C: OR = 1.17, 95% CI = 1.10–1.24, P < 0.001, Supplementary Figure 6; for TT + CT vs. CC: OR = 1.18, 95% CI = 1.10–1.28, P < 0.001; for TT vs. CT + CC: OR = 1.25, 95% CI = 1.14–1.38, P < 0.001; for TT vs. CC: OR = 1.35, 95% CI = 1.20–1.53, P < 0.001). Sensitivity analysis for the MTHFR A1298C polymorphism revealed that excluding Lee et al. (38) had no impact on the conclusion of the meta-analysis (Supplementary Figure 7).
Table 4 shows the main results as well as the heterogeneity test. MTHFR C677T polymorphism was shown to be highly associated with an increased risk of developing MD in all statistical models (for T vs. C: OR = 1.33, 95% CI = 1.15–1.55, P < 0.001; for TT + CT vs. CC: OR = 1.35, 95% CI = 1.08–1.70, P = 0.009; for TT vs. CT + CC: OR = 1.58, 95% CI = 1.28–1.95, P < 0.001; for TT vs. CC: OR = 1.66, 95% CI = 1.31–2.11, P < 0.001) (Figure 4 and Table 4).
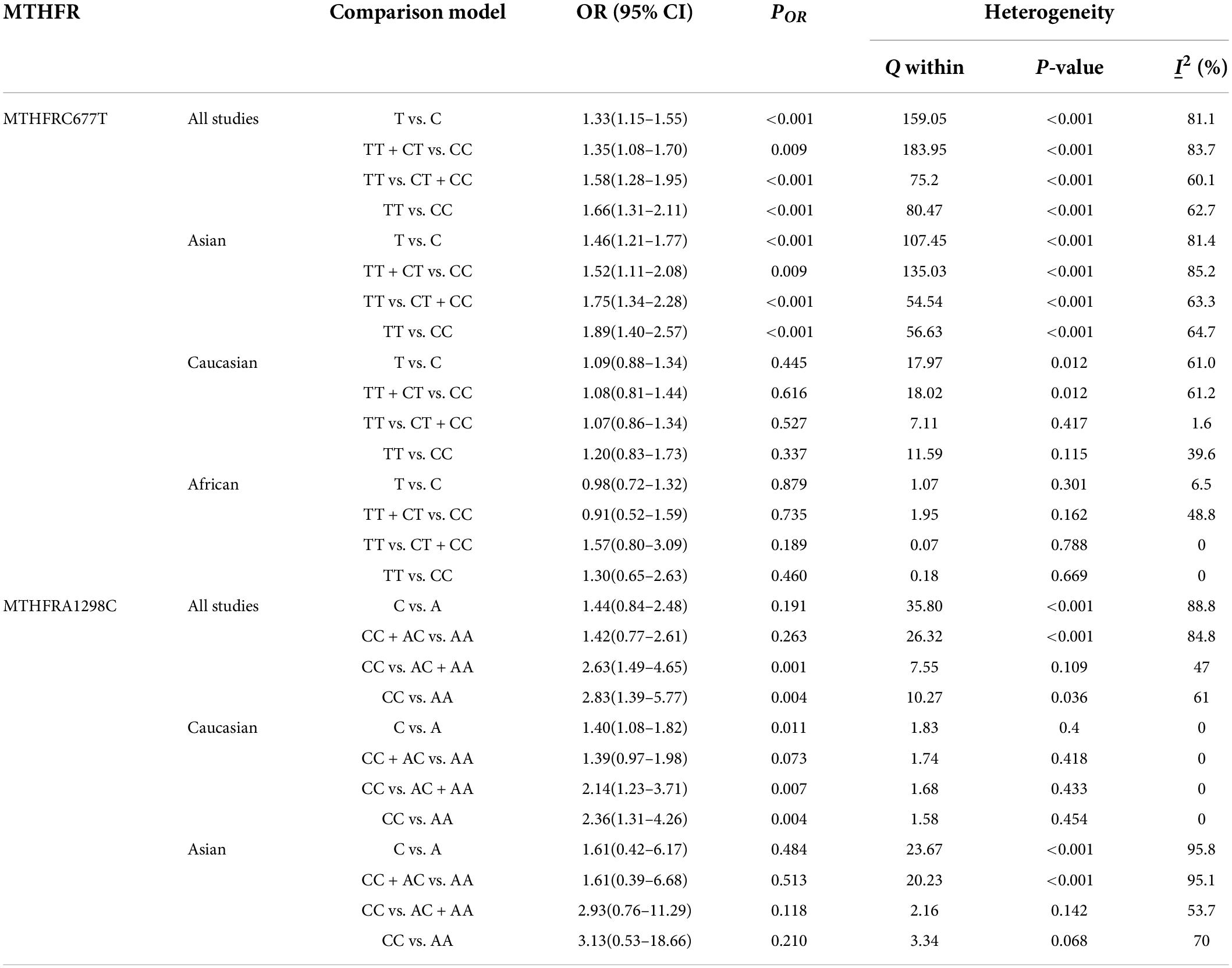
Table 4. Odds ratios and heterogeneity results for the 4 genetic models of the MTHFR C677T and A1298C for MD.
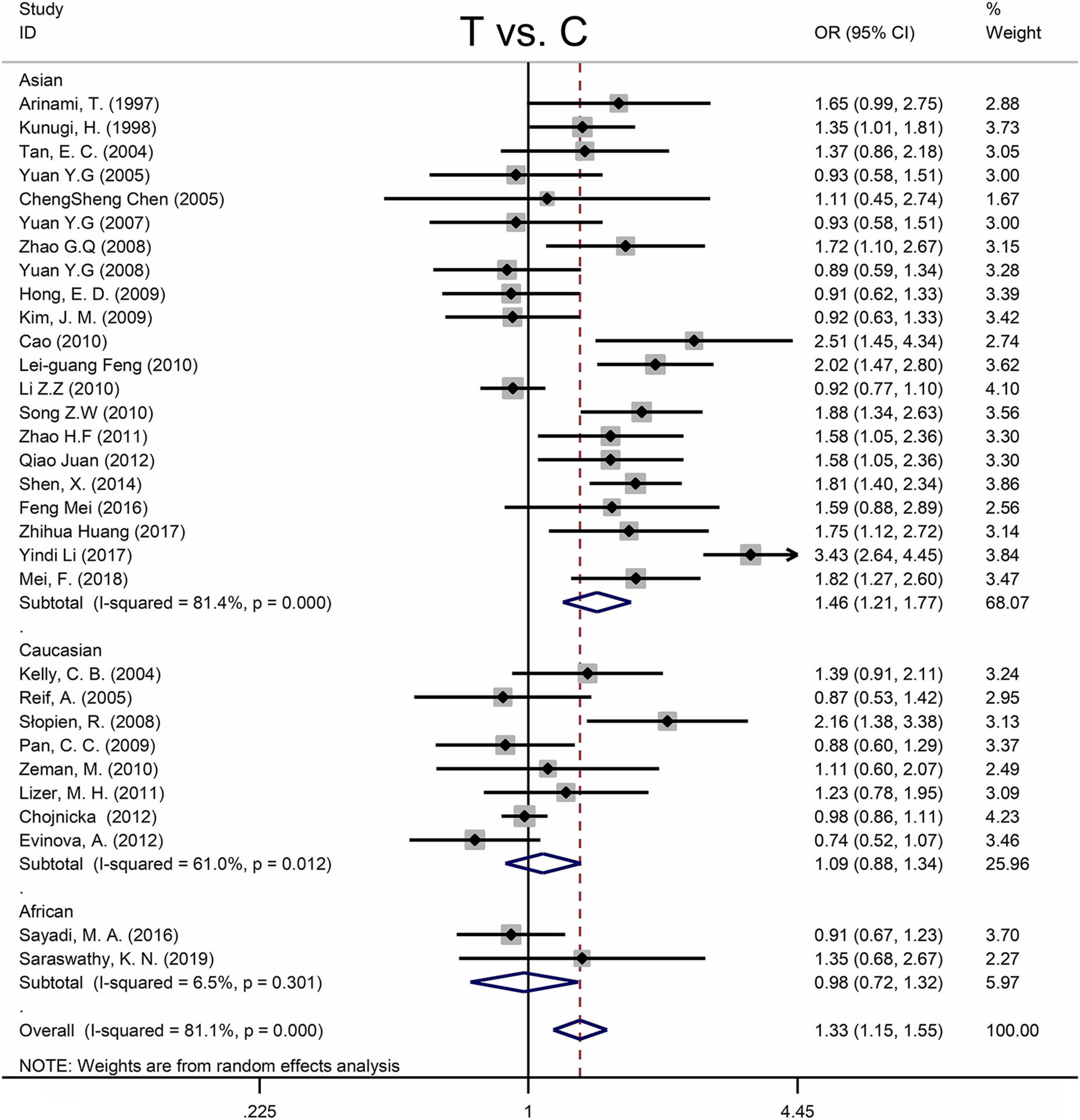
Figure 4. Forest plots for the associations between MTHFR C677T polymorphisms and MD for the allele model with random effect model.
Subgroup analysis by ethnicity revealed a substantial correlation between the MTHFR C677T polymorphism and MD in Asian populations (for T vs. C: OR = 1.46, 95% CI = 1.21–1.77, P < 0.001; for TT + CT vs. CC: OR = 1.52, 95% CI = 1.11–2.08, P = 0.009; for TT vs. CT + CC: OR = 1.75, 95% CI = 1.34–2.28, P < 0.001; for TT vs. CC: OR = 1.89, 95% CI = 1.40–2.57, P < 0.001), but not in Caucasian and African populations (Figure 4 and Table 4).
The MTHFR A1298C polymorphism was found to be highly associated with MD in the recessive model (for CC vs. AC + AA: OR = 2.63, 95% CI: 1.49–4.65, P = 0.001) and the homozygote model (for CC vs. AA: OR = 2.83, 95% Cl = 1.39–5.77, P = 0.004) (Table 4). Moreover, subgroup analysis demonstrated a positive correlation between the MTHFR A1298C polymorphism and MD in the Caucasian population (for C vs. A: OR = 1.40, 95% CI = 1.08–1.82, P = 0.011; for CC vs. AC + AA: OR = 2.14, 95% CI = 1.23–3.71, P = 0.007; for CC vs. AA: OR = 2.36, 95% CI = 1.31–4.26, P = 0.004) (Figure 5 and Table 4). Nonetheless, there was no statistical correlation between A1298C polymorphism and MD in Asian populations (Figure 5 and Table 4). Subgroup analysis shows that the correlation between MTHFR C677T polymorphism and MD exists in the Asian population (all genetic models) but not in Caucasian and African populations.
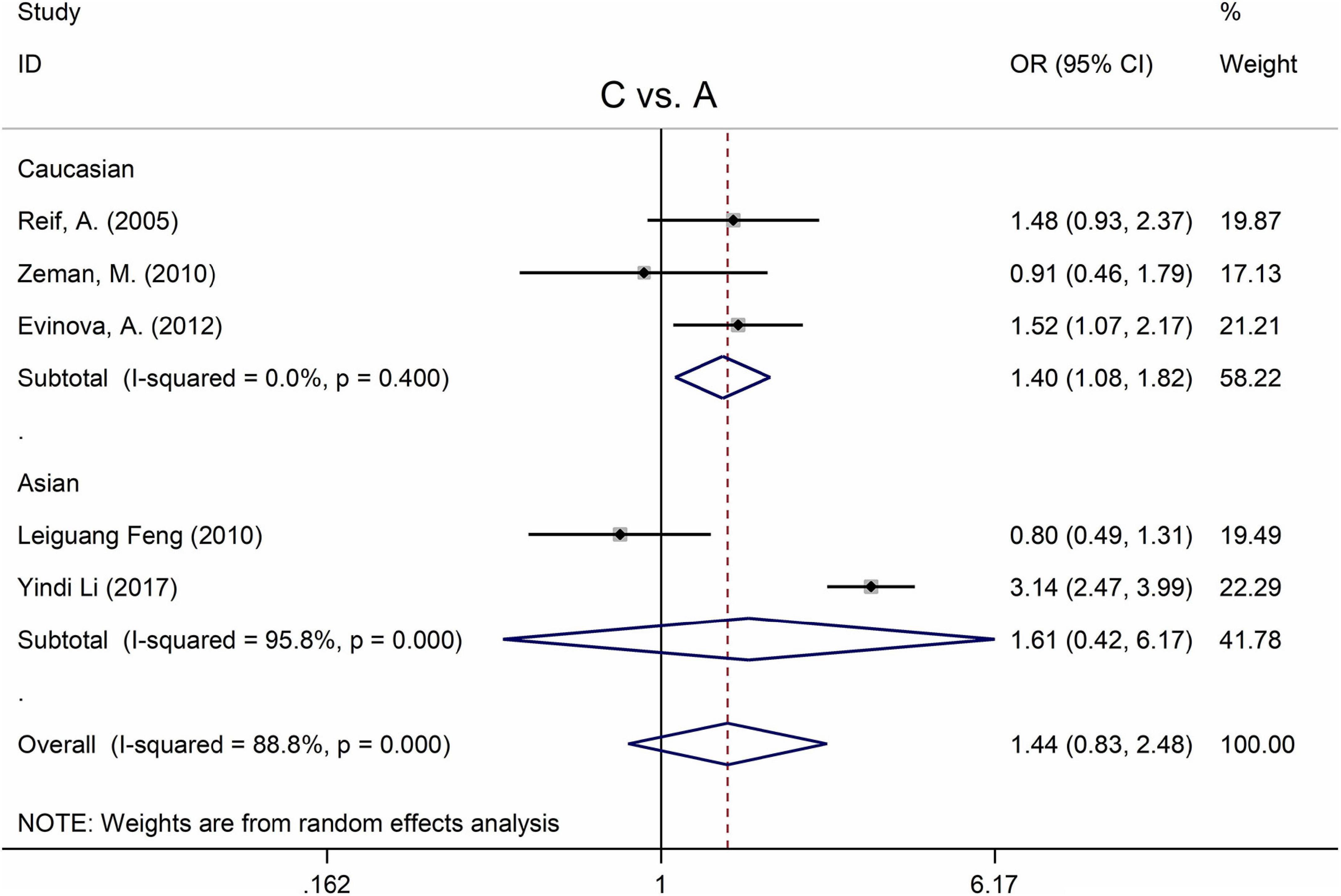
Figure 5. Forest plots for the associations between MTHFR A1298C polymorphisms and MD for the allele model with random effect model.
Four articles were not found in Hardy–Weinberg equilibrium (71, 84, 85, 95) (Tables 1, 2). Sensitivity analysis revealed that the overall correlation between MTHFR C677T polymorphism and MD remained unchanged after eliminating these data from the meta-analysis (Supplementary Figure 8). Sensitivity analyses for MTHFR A1298C polymorphism revealed that excluding Reif A. et al. (71) and Li et al. (95) resulted in a decreasing statistical correlation with MD; nonetheless, all statistical models revealed that MTHFR A1298C polymorphism was not significantly correlated with MD (Supplementary Figure 9).
Table 5 displays the main results and the heterogeneity test. There was a marginal correlation between the MTHFR C677T polymorphism and BPD in the recessive model (for TT vs. CT + CC: OR = 1.31, 95% CI: 1.03–1.67, P = 0.028) and the homozygote model (for TT vs. CC: OR = 1.40, 95% Cl = 1.00–1.94, P = 0.049) (Table 5). Moreover, subgroup analysis indicated no statistical correlation between the MTHFR C677T polymorphism and BPD in Asian, African, or Caucasian populations (Figure 6 and Table 5). Additionally, all models revealed that the MTHFR A1298C polymorphism was not statistically correlated with BPD (Figure 7 and Table 5).
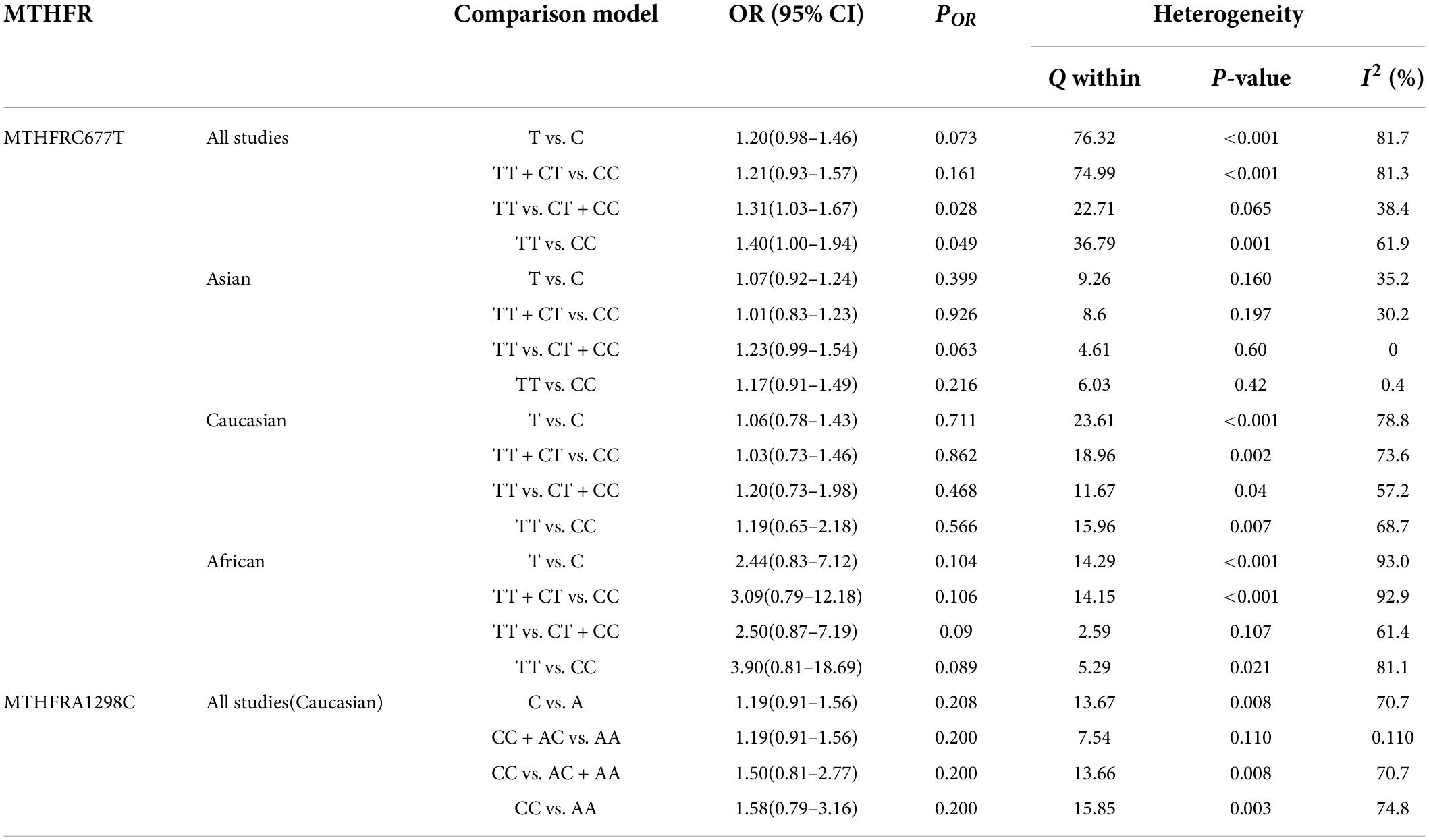
Table 5. Odds ratios and heterogeneity results for the 4 genetic models of the MTHFR C677T and A1298C for BPD.
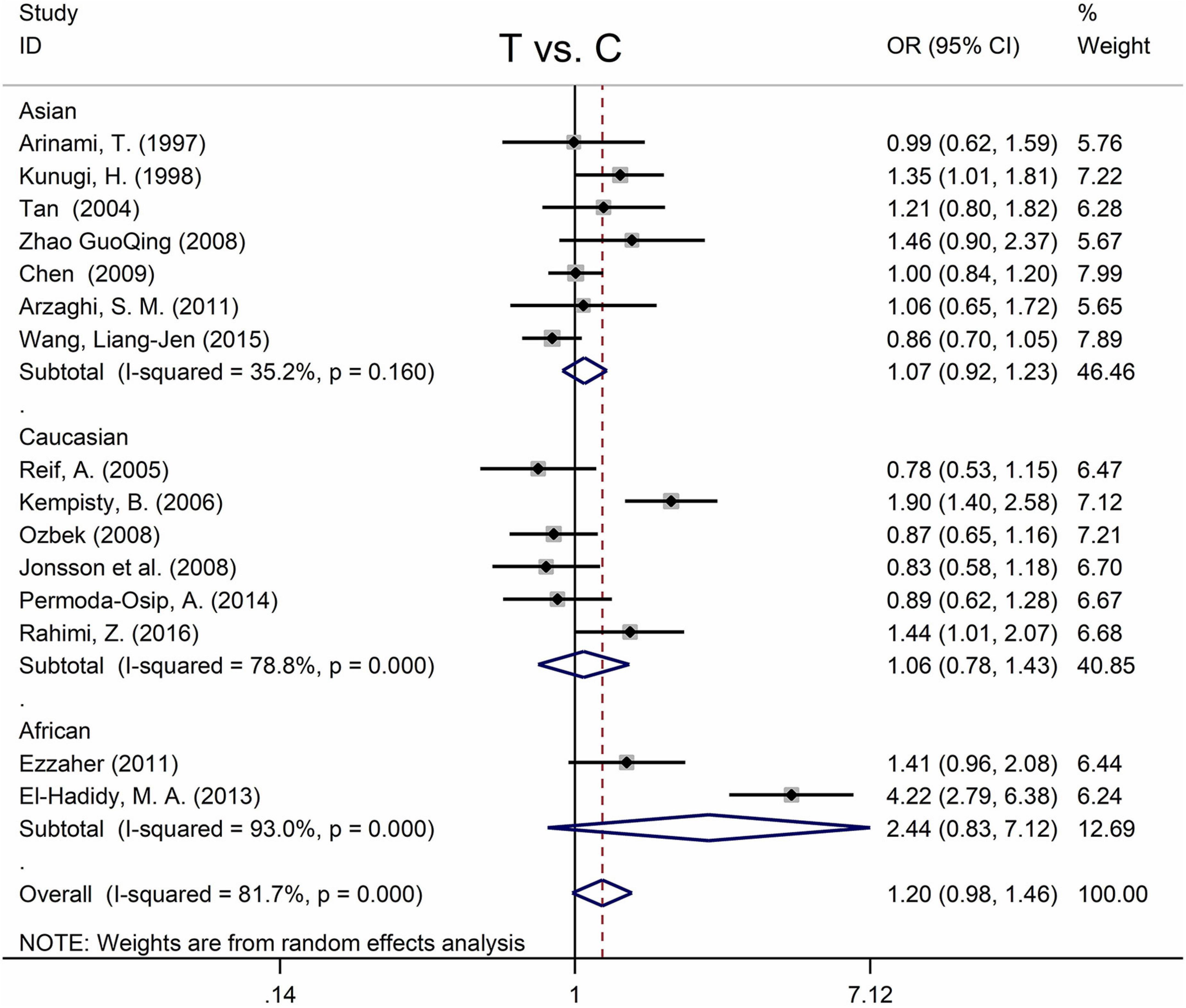
Figure 6. Forest plots for the associations between MTHFR C677T polymorphisms and BPD for the allele model with random effect model.
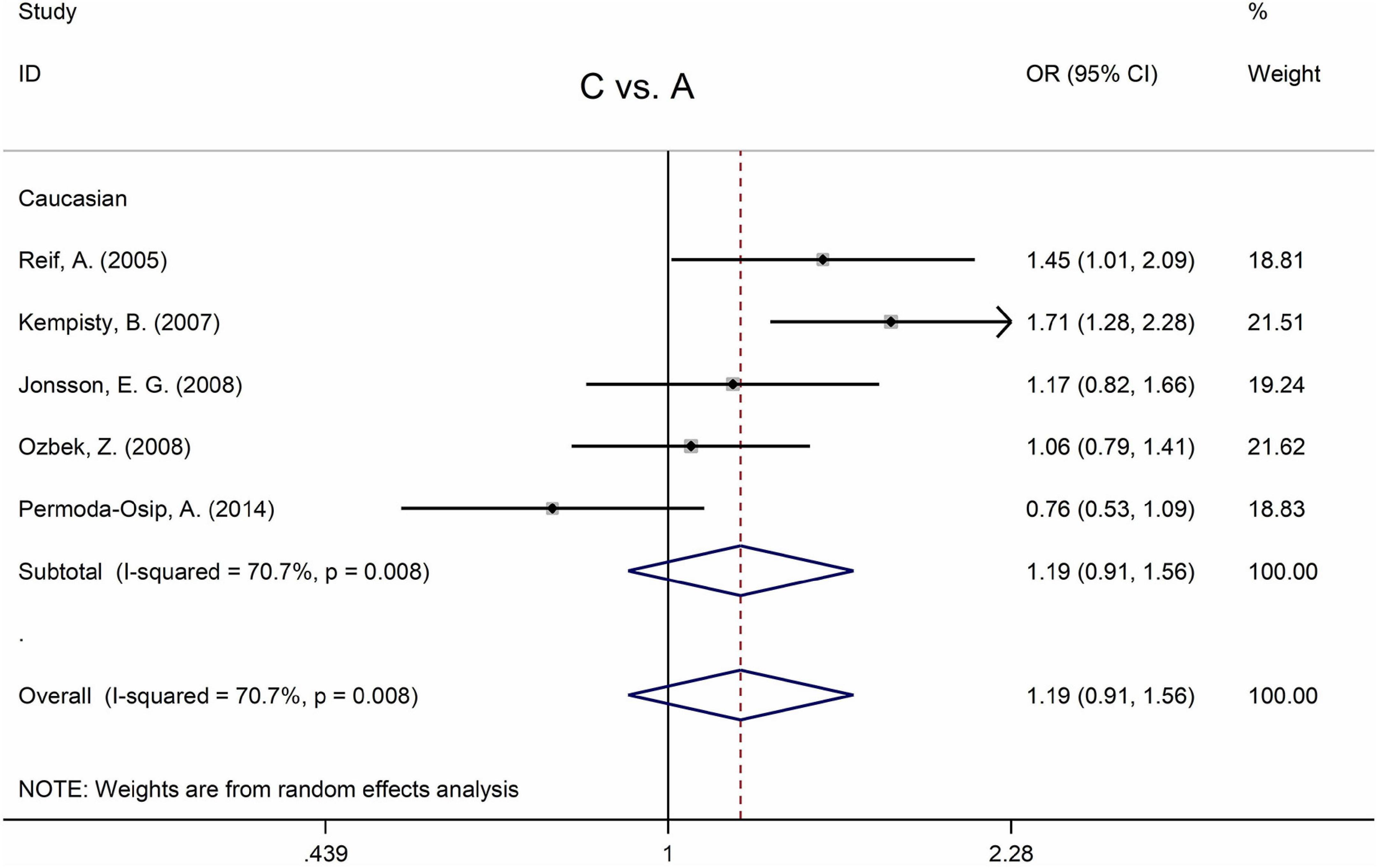
Figure 7. Forest plots for the associations between MTHFR A1298C polymorphisms and BPD for the allele model with random effect model.
Only one study was not in Hardy Weinberg equilibrium (71) (Table 2), and there was no statistical association between A1298C polymorphism and BPD after removing this study (Supplementary Figure 10).
Significant publication biases were found when all diseases were considered (Supplementary Figure 5 and Supplementary Table 2). Therefore, analyses between MTHFR C677T and mental disorders were unsuitable here. However, the main results and the heterogeneity tests between MTHFR C677T and mental disorders were shown in Supplementary Table 1. Furthermore, the forest plots indicated that MTHFR C677T was strongly associated with psychiatric disorders, and sensitivity analysis did not affect the results (Supplementary Figures 1, 2).
Most studies were not in Hardy–Weinberg equilibrium when all diseases were considered. Moreover, analysis between MTHFR A1298C and psychiatric disorders was also unsuitable. Significant correlations were detected between the MTHFR A1298C polymorphism and psychiatric disorders (Supplementary Figure 3). However, sensitivity analysis revealed that excluding did change the conclusion (Supplementary Figure 4).
In order to evaluate publication bias, we used formal statistical methods (Egger’s regression test). Table 6 and Figure 8 presented the funnel plots for the meta-analysis. We observed that for SZ, no publication bias could be observed except in the dominant model (TT + CT vs. CC, PEgger = 0.01). The Egger’s test results for MD were substantial in two genetic models of overall populations (allele model: C vs. A, PEgger = 0.03; homozygote model: CC vs. AA, PEgger = 0.02). And there was no publication bias for BPD. Publication bias may correlate to the editor’s decision for publication. However, it is common that only the positive results are published, and negative findings are unavailable. So, we could not exclude this kind of possibility.
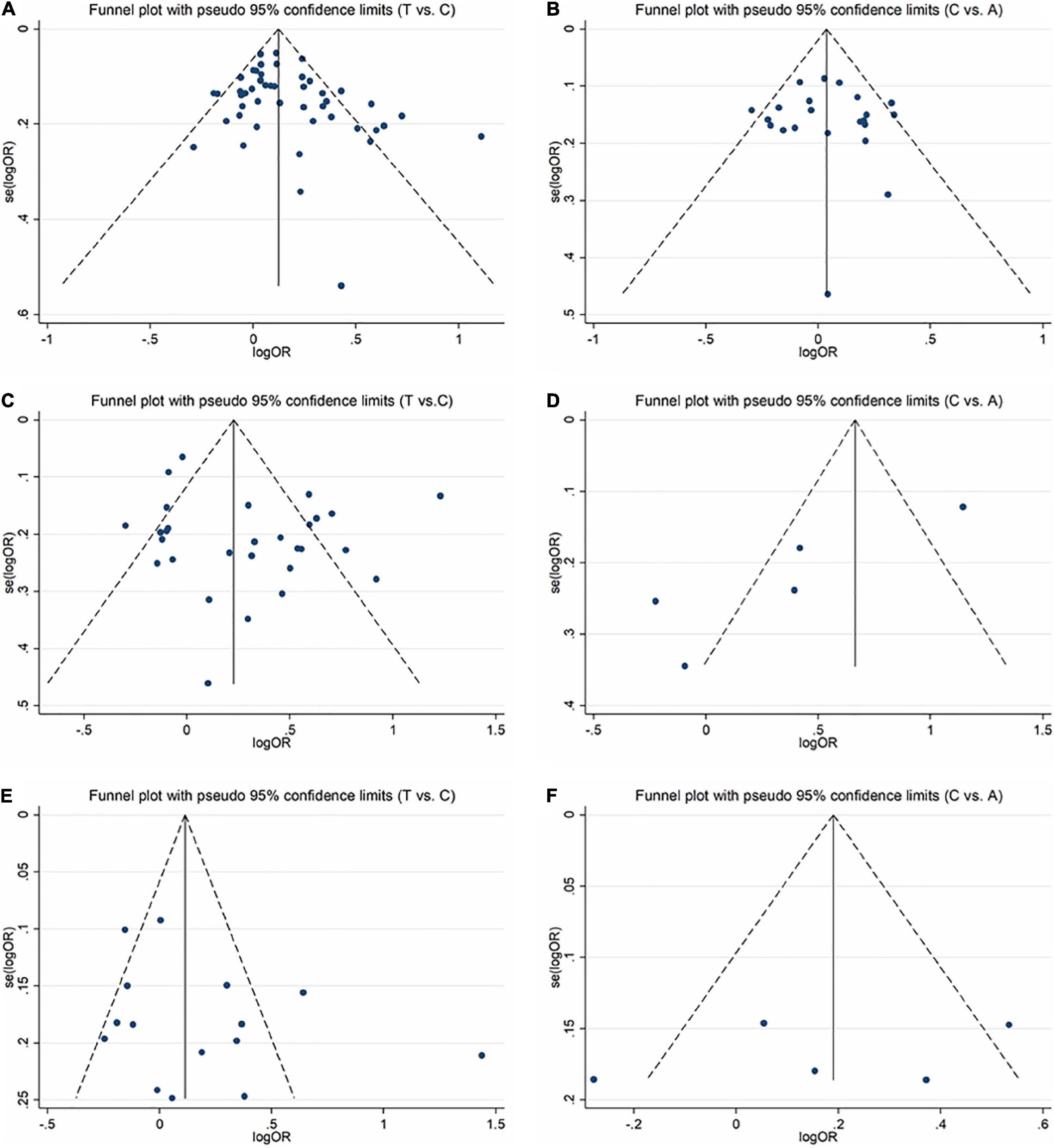
Figure 8. Funnel plots for assessing the publication bias risk in this meta-analysis. (A) Funnel plot for allele contrast (T vs. C) of C677T polymorphism in SZ. (B) Funnel plot for allele contrast (C vs. A) of A1298C polymorphism in SZ. (C) Funnel plot for allele contrast (T vs. C) of C677T polymorphism in MD. (D) Funnel plot for allele contrast (C vs. A) of A1298C polymorphism in MD. (E) Funnel plot for allele contrast (T vs. C) of C677T polymorphism in BPD. (F) Funnel plot for allele contrast (C vs. A) of A1298C polymorphism in BPD.
A mental disorder is a neurological disease with complicated etiology, which may be closely related to genetic factors. A great number of research on the susceptibility to mental illnesses (including SZ, MD, and BPD) have been undertaken using MTHFR gene polymorphism. Some studies supported the susceptibility variation of MTHFR in mental diseases (27, 31, 70, 96, 105, 69), whereas other studies showed a negative correlation (28, 29, 33, 46, 102, 46). These variations might be due to the type of disease, ethnicity, or sample size. Our meta-analysis incorporates all previous research and provides more reliable evidence for the association between mental illness and MTHFR SNPs.
For Sz, our meta-analysis found a substantial association between MTHFR C677T polymorphism and higher incidence of SZ, which is consistent with research by Hu et al. (22) and Peerbooms et al. (23). In addition, we found that MTHFR A1298C polymorphism was not correlated with increased SZ risk, which is consistent with Peerbooms et al. (23). However, Hu et al. (22) discovered a marginal correlation between the MTHFR A1298C polymorphism and SZ. The inconsistency may be mainly owing to the limited sample size in the previous meta-analyses. For MTHFR C677T and A1298C, the sensitivity analysis has no substantial change to the results. As a result, the study’s findings are relatively consistent.
For MD, our meta-analysis’s results showed a significant correlation between MTHFR C677T polymorphism and increased risk of MD. The meta-analysis of Wu et al. (107) supports our view, whereas Gaysina et al. (108) and Peerbooms et al. (23) discovered no association between the C677T and MD. These discrepancies might be due to ethnicity, sample size, and other factors. Sensitivity analysis showed no change in the overall correlation between C677T polymorphism and MD. Also, we found a correlation between the A1298C polymorphism and MD (in recessive models and homozygous models). However, after excluding two studies not in Hardy–Weinberg equilibrium (38, 95), we discovered that A1298C polymorphism was not correlated with depression. We suspect that the reason for this is the insufficient number of studies included.
For BPD, meta-analysis reveals that MTHFR C677T polymorphism is weakly related to the occurrence of diseases (in recessive models and homozygous models). The meta-analysis of Hu et al. (22) found a marginal connection of C677T with an elevated risk of BPD (the recessive model), but some studies (21, 109, 110) found no associations. Different numbers of studies included may cause the inconsistency. Our meta-analysis included all current research, providing more reliable evidence for the association between MTHFR C677T polymorphism and BPD. As for A1298C, we only found studies in Caucasian people, and we did not find any association in these studies. The sensitivity analysis has no change to the results. Therefore, the results of this study are generally robust.
Many researchers have discovered that MTHFR is closely related to cognitive function, such as verbal fluency, visual-motor coordination, attention selectivity, and distribution (111–113). MTHFR polymorphism may also cause central nerve injury and microvascular injury, affect the synthesis of central neurotransmitters and the methylation of central neural system amines and phospholipids, and eventually lead to various mental diseases (114). All these impairments are not specific to one disease; therefore, we guess MTHFR may work on the common pathogenesis of these psychiatric disorders.
Some limitations of this meta-analysis should be considered when interpreting the findings. Firstly, we can only search for English and Chinese articles with some language limitations. Second, publication bias cannot be ignored in the current study since Egger test findings are substantial in several SZ and MD genetic models. It may correlate to the editor’s decision for publication and so on. However, it is common that only the positive results are published, and negative findings are unavailable. And we could not exclude this kind of possibility. Furthermore, the number of articles on A1298C polymorphism with MD are insufficient to provide conclusive evidence. More original research is required to validate our results. Despite some limitations, our current research also has some value. First of all, our meta-analysis includes a large sample size, which can reduce errors. Secondly, we fully considered and analyzed the impact of race on the disease.
Our meta-analysis findings demonstrate that MTHFR C677T polymorphism increases the risk of schizophrenia and severe depression in the general population, and a marginal correlation of MTHFR C677T with a higher risk of bipolar disorder has also been reported for the recessive model. More original research and a bigger sample size are required to validate our results. Nevertheless, the findings of our meta-analysis imply that MTHFR may play a significant role in the common pathogenesis of mental illness and that its variation may be involved in controlling the expression of genes associated with it. It would help in the early diagnosis and treatment of related mental disorders. Moreover, studies on risk factor analysis could be performed on psychiatric disorders to better prevent these mental health problems.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Y-XZ: conceptualization, software, data curation, and writing – original draft preparation. DH: conceptualization, methodology, and funding acquisition. L-PY: data curation and validation. CG: visualization and investigation. C-CC: software and validation. Z-YG: writing – reviewing and editing. H-MS: project administration and supervision. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant No. 81803857) and Autonomous Subject of Beijing University of Chinese Medicine (Grant No. 2018-JYBZZ-XJSJJ002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.976428/full#supplementary-material
1. Barnett R. Depression. Lancet (London, England). (2019) 393:2113. doi: 10.1016/s0140-6736(19)31151-1
2. Adorjan K, Falkai P. Premature mortality, causes of death, and mental disorders. Lancet (London, England). (2019) 394:1784–6. doi: 10.1016/s0140-6736(19)32521-8
3. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. (2003) 54:515–28. doi: 10.1016/s0006-3223(03)00171-9
4. Malhi GS, Mann JJ. Depression. Lancet (London, England). (2018) 392:2299–312. doi: 10.1016/s0140-6736(18)31948-2
5. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet (London, England). (2016) 388:86–97. doi: 10.1016/s0140-6736(15)01121-6
6. Dennison CA, Legge SE, Pardinas AF, Walters JTR. Genome-wide association studies in schizophrenia: Recent advances, challenges, and future perspective. Schizophr Res. (2019) 217:4–12. doi: 10.1016/j.schres.2019.10.048
7. Schwab SG, Wildenauer DB. Genetics of psychiatric disorders in the GWAS era: An update on schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2013) 263(Suppl. 2):S147–54. doi: 10.1007/s00406-013-0450-z
8. Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci U S A. (1998) 95:13217–20. doi: 10.1073/pnas.95.22.13217
9. Födinger M, Hörl WH, Sunder-Plassmann G. Molecular biology of 5,10-methylenetetrahydrofolate reductase. J Nephrol. (2000) 13:20–33.
10. Wan L, Li Y, Zhang Z, Sun Z, He Y, Li R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. (2018) 8:242. doi: 10.1038/s41398-018-0276-6
11. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. (1995) 10:111–3. doi: 10.1038/ng0595-111
12. Lewis SJ, Lawlor DA, Smith GD, Araya R, Timpson N, Day INM, et al. The thermolabile variant of Mthfr is associated with depression in the British Women’s heart and health study and a meta-analysis. Mol Psychiatry. (2006) 11:352–60. doi: 10.1038/sj.mp4001790
13. Lewis SJ, Zammit S, Gunnell D, Smith GD. A meta-analysis of the MTHFR C677t polymorphism and schizophrenia risk. Am J Med Genet Part B, Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2005) 135b:2–4. doi: 10.1002/ajmg.b.30170
14. van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects? Am J Hum Genet. (1998) 62:1044–51. doi: 10.1086/301825
15. Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism: Epidemiology, metabolism and the associated diseases. Eur J Med Genet. (2015) 58:1–10. doi: 10.1016/j.ejmg.2014.10.004
16. Goyette P, Pai A, Milos R, Frosst P, Tran P, Chen Z, et al. Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome Off J Int Mamm Genome Soc. (1998) 9:652–6. doi: 10.1007/s003359900838
17. Krebs MO, Bellon A, Mainguy G, Jay TM, Frieling H. One-carbon metabolism and schizophrenia: Current challenges and future directions. Trends Mol Med. (2009) 15:562–70. doi: 10.1016/j.molmed.2009.10.001
18. Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: Current knowledge and possible mechanisms. Nutr Rev. (2008) 66:250–5. doi: 10.1111/j.1753-4887.2008.00031.x
19. Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. (2005) 10:40–68; image 5. doi: 10.1038/sj.mp.4001558
20. Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, Velakoulis D, et al. Brain surface contraction mapped in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Mol Psychiatry. (2009) 14:976–86. doi: 10.1038/mp.2008.34
21. Cohen-Woods S, Craig I, Gaysina D, Gray J, Gunasinghe C, Craddock N, et al. The bipolar association case-control study (BACCS) and meta-analysis: No association with the 5,10-methylenetetrahydrofolate reductase gene and bipolar disorder. Am J Med Genet Part B Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2010) 153b:1298–304. doi: 10.1002/ajmg.b.31101
22. Hu CY, Qian ZZ, Gong FF, Lu SS, Feng F, Wu YL, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: An updated meta-analysis. J Neural Transmission (Vienna, Austria 1996). (2015) 122:307–20. doi: 10.1007/s00702-014-1261-8
23. Peerbooms OL, van Os J, Drukker M, Kenis G, Hoogveld L, MTHFR in Psychiatry Group, et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: Evidence for a common genetic vulnerability? Brain Behav Immun. (2011) 25:1530–43. doi: 10.1016/j.bbi.2010.12.006
24. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. (2014) 20:123–9. doi: 10.1111/1469-0691.12494
25. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clin Trials. (2015) 45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002
26. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, Graphical Test. BMJ (Clin Res Ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
27. Arinami T, Yamada N, Yamakawa-Kobayashi K, Hamaguchi H, Toru M. Methylenetetrahydrofolate reductase variant and schizophrenia/depression. Am J Med Genet. (1997) 74:526–8. doi: 10.1002/(sici)1096-8628(19970919)74:53.0.co;2-e
28. Kunugi H, Fukuda R, Hattori M, Kato T, Tatsumi M, Sakai T, et al. C677t polymorphism in methylenetetrahydrofolate reductase gene and psychoses. Mol Psychiatry. (1998) 3:435–7. doi: 10.1038/sj.mp.4000390
29. Virgos C, Martorell L, Simó JM, Valero J, Figuera L, Joven J, et al. Plasma homocysteine and the methylenetetrahydrofolate reductase C677t gene variant: Lack of association with schizophrenia. Neuroreport. (1999) 10:2035–8. doi: 10.1097/00001756-199907130-00008
30. Joober R, Benkelfat C, Lal S, Bloom D, Labelle A, Lalonde P, et al. Association between the Methylenetetrahydrofolate reductase 677c–>T missense mutation and schizophrenia. Mol Psychiatry. (2000) 5:323–6. doi: 10.1038/sj.mp.4000724
31. Sazci A, Ergül E, Güzelhan Y, Kaya G, Kara I. Methylenetetrahydrofolate reductase gene polymorphisms in patients with schizophrenia. Brain Res Mol Brain Res. (2003) 117:104–7. doi: 10.1016/s0169-328x(03)00327-9
32. Tan EC, Chong SA, Lim LC, Chan AO, Teo YY, Tan CH, et al. Genetic analysis of the thermolabilemethylenetetrahydrofolate reductase variant in schizophrenia and mood disorders. Psychiatric Genet. (2004) 14:227–31. doi: 10.1097/00041444-200412000-00012
33. Yu L, Li T, Robertson Z, Dean J, Gu NF, Feng GY, et al. No association between polymorphisms of methylenetetrahydrofolate reductase gene and schizophrenia in both chinese and scottish populations. Mol Psychiatry. (2004) 9:1063–5. doi: 10.1038/sj.mp.4001566
34. Sazci A, Ergul E, Kucukali I, Kara I, Kaya G. Association of the C677t and A1298c polymorphisms of methylenetetrahydrofolate reductase gene with schizophrenia: Association is significant in men but not in women. Progr Neuro Psychopharmacol Biol Psychiatry. (2005) 29:1113–23. doi: 10.1016/j.pnpbp.2005.06.022
35. Vilella E, Virgos C, Murphy M, Martorell L, Valero J, Simó JM, et al. Further evidence that hyperhomocysteinemia and methylenetetrahydrofolate reductase C677t and A1289c polymorphisms are not risk factors for schizophrenia. Progr Neuro Psychopharmacol Biol Psychiatry. (2005) 29:1169–74. doi: 10.1016/j.pnpbp.2005.07.001
36. Kempisty B, Mostowska A, Gorska I, Luczak M, Czerski P, Szczepankiewicz A, et al. Association of 677c>T polymorphism of methylenetetrahydrofolate reductase (Mthfr) gene with bipolar disorder and schizophrenia. Neurosci Lett. (2006) 400:267–71. doi: 10.1016/j.neulet.2006.02.055
37. Philibert R, Gunter T, Hollenbeck N, Adams WJ, Bohle P, Packer H, et al. No association of the C677t methylenetetrahydrofolate reductase polymorphism with schizophrenia. Psychiatric Genet. (2006) 16:221–3. doi: 10.1097/01.ypg.0000242192.28526.fa
38. Lee YS, Han DH, Jeon CM, Lyoo IK, Na C, Chae SL, et al. Serum Homocysteine, folate level and methylenetetrahydrofolate reductase 677, 1298 gene polymorphism in Korean schizophrenic patients. Neuroreport. (2006) 17:743–6. doi: 10.1097/01.wnr.0000215777.99473.52
39. Yang D-Y, Lu X-B, Li Y-H, Tong Z-S, Fu Y, Yang M-X, et al. The association of methylenetetrahydrofolate reductase gene polymorphism, plasma homocysteine level and first-episode schizophrenia [in Chinese]. Chin Hehavioral Med Sci. (2007) 16:901–2. doi: 10.3760/cma.j.issn.1674-6554.2007.10.013
40. Jonsson EG, Larsson K, Vares M, Hansen T, Wang AG, Djurovic S, et al. Two methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms, schizophrenia and bipolar disorder: An association study. Am J Med Genet Part B Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2008) 147B:976–82. doi: 10.1002/ajmg.b.30671
41. Muntjewerff J-W. Meta-analysis of plasma homocysteine and methylenetetrahydrofolate reductase (MTHFR) polymorphisms in schizophrenia. Biol Psychiatry. (2008) 63:134S–S.
42. Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, et al. MTHFR 677c –> T genotype disrupts prefrontal function in schizophrenia through an interaction with Comt 158val –> Met. Proc Natl Acad Sci U S A. (2008) 105:17573–8. doi: 10.1073/pnas.0803727105
43. Feng LG, Song ZW, Xin F, Hu J. Association of plasma homocysteine and methylenetetrahydrofolate reductase C677t gene variant with schizophrenia: A chinese han population-based case-control study. Psychiatry Res. (2009) 168:205–8. doi: 10.1016/j.psychres.2008.05.009
44. Betcheva ET, Mushiroda T, Takahashi A, Kubo M, Karachanak SK, Zaharieva IT, et al. Case-control association study of 59 candidate genes reveals the DRD2 SNP Rs6277 (C957t) as the only susceptibility factor for schizophrenia in the bulgarian population. J Hum Genet. (2009) 54:98–107. doi: 10.1038/jhg.2008.14
45. García-Miss MR, Pérez-Mutul J, López-Canul B, Solís-Rodríguez F, Puga-Machado L, Oxté-Cabrera A, et al. Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677t polymorphism, are risk factors for schizophrenia. J Psychiatric Res. (2010) 44:441–6. doi: 10.1016/j.jpsychires.2009.10.011
46. Kang HJ, Choe BM, Kim SH, Son SR, Lee KM, Kim BG, et al. No association between functional polymorphisms in COMT and MTHFR and schizophrenia risk in Korean population. Epidemiol Health. (2010) 32:e2010011. doi: 10.4178/epih/e2010011
47. Ye X, Zhang X, Wang H. Study association of MTHFR C677t polymorphism and schizophrenia [in Chinese]. Heilongjiang Med J. (2010) 34:641–5.
48. Bouaziz N, Ayedi I, Sidhom O, Kallel A, Rafrafi R, Jomaa R, et al. Plasma homocysteine in schizophrenia: Determinants and clinical correlations in tunisian patients free from antipsychotics. Psychiatry Res. (2010) 179:24–9. doi: 10.1016/j.psychres.2010.04.008
49. Arzaghi SM, Hossein-Nezhad A, Shariat SV, Ghodsipour A, Shams J, Larijani B. C677t methylenetetrahydrofolate reductase (MTHFR) gene polymorphism in schizophrenia and bipolar disorder: An association study in Iranian population. Iranian J Psychiatry. (2011) 6:1–6.
50. Kim SG, Song JY, Joo EJ, Jeong SH, Kim SH, Lee KY, et al. No Association of functional polymorphisms in methlylenetetrahydrofolate reductase and the risk and minor physical anomalies of schizophrenia in Korean population. J Korean Med Sci. (2011) 26:1356–63. doi: 10.3346/jkms.2011.26.10.1356
51. Muntjewerff JW, Ophoff RA, Buizer-Voskamp JE, Strengman E, den Heijer M, Consortium G. Effects of season of birth and a common MTHFR gene variant on the risk of schizophrenia. Eur Neuropsychopharmacol J Eur College Neuropsychopharmacol. (2011) 21:300–5. doi: 10.1016/j.euroneuro.2010.10.001
52. Tsutsumi A, Glatt SJ, Kanazawa T, Kawashige S, Uenishi H, Hokyo A, et al. The genetic validation of heterogeneity in schizophrenia. Behav Brain Funct BBF. (2011) 7:43. doi: 10.1186/1744-9081-7-43
53. Zhang X, Liu T, Yang Y, Deng X, Yan X, Yang K, et al. Association analysis of methylenetetrahydrofolate reductase (MTHFR) gene polymorphism and treatment-resistant schizophrenia [in Chinese]. J Clin Psychiatry. (2012) 22:293–7.
54. Lochman J, Plesník J, Janout V, Povová J, Míšek I, Dvořáková D, et al. Interactive effect of MTHFR and Adra2a gene polymorphisms on pathogenesis of schizophrenia. Neuro Endocrinol Lett. (2013) 34:792–7.
55. Zhang Y, Yan H, Tian L, Wang F, Lu T, Wang L, et al. Association of MTHFR C677t polymorphism with schizophrenia and its effect on episodic memory and gray matter density in patients. Behav Brain Res. (2013) 243:146–52. doi: 10.1016/j.bbr.2012.12.061
56. Kontis D, Theochari E, Fryssira H, Kleisas S, Sofocleous C, Andreopoulou A, et al. Comt and MTHFR polymorphisms interaction on cognition in schizophrenia: An exploratory study. Neurosci Lett. (2013) 537:17–22. doi: 10.1016/j.neulet.2013.01.012
57. El-Hadidy MA, Abdeen HM, Abd El-Aziz SM, Al-Harrass M. MTHFR gene polymorphism and age of onset of schizophrenia and bipolar disorder. BioMed Res Int. (2014) 2014:318483. doi: 10.1155/2014/318483
58. Hei G, Pang L, Chen X, Zhang W, Zhu Q, Lü L, et al. [Association of serum folic acid and homocysteine levels and 5, 10-methylenetetrahydrofolate reductase gene polymorphism with schizophrenia]. Zhonghuayixuezazhi. (2014) 94:2897–901.
59. Nishi A, Numata S, Tajima A, Kinoshita M, Kikuchi K, Shimodera S, et al. Meta-analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677t polymorphism in schizophrenia. Schizophrenia Bull. (2014) 40:1154–63. doi: 10.1093/schbul/sbt154
60. Foroughmand AM, Galehdari H, Pooryasin A, Ajam T, Kazemi-Nezhad SR. Additive effect of MTHFR and GRIN1 genetic polymorphisms on the risk of schizophrenia. Mol Biol Res Commun. (2015) 4:33–42.
61. Misiak B, Laczmanski L, Sloka NK, Szmida E, Piotrowski P, Loska O, et al. Metabolic dysregulation in first-episode schizophrenia patients with respect to genetic variation in one-carbon metabolism. Psychiatry Res. (2016) 238:60–7. doi: 10.1016/j.psychres.2016.01.077
62. Takano Y, Ozeki Y, Sekine M, Fujii K, Watanabe T, Okayasu H, et al. Multi-regression analysis revealed a relationship between l-serine and methionine, a component of one-carbon metabolism, in the normal control but not in the schizophrenia. Ann Gen Psychiatry. (2016) 15:23. doi: 10.1186/s12991-016-0113-3
63. Wang W-P, Fan W, Shi B, Tong C, Wang X, Cai J, et al. Effect of MTHFR gene on the schizophrenia and its cognitive function [in Chinese]. Chin J Med Genet. (2017) 34:905–8.
64. Oniki K, Ishioka M, Osaki N, Sakamoto Y, Yoshimori Y, Tomita T, et al. Association between oxidative stress-related genes polymorphisms and metabolic abnormalities among schizophrenia patients. Clin Neuropsychopharmacol Ther. (2017) 8:25–37. doi: 10.5234/cnpt.8.25
65. Debost JC, Debost M, Grove J, Mors O, Hougaard DM, Borglum AD, et al. Comt Val158met and MTHFR C677t moderate risk of schizophrenia in response to childhood adversity. ActapsychiatricaScandinavica. (2017) 136:85–95. doi: 10.1111/acps.12761
66. Zhilyaeva TV, Sergeeva AV, Blagonravova AS, Kasimova LN, Kuznetsov KV, Golovanova VI, et al. Association study of methylenetetrahydrofolate reductase genetic polymorphism 677c>T with schizophrenia in hospitalized patients in population of European Russia. Asian J Psychiatry. (2018) 32:29–33. doi: 10.1016/j.ajp.2017.11.027
67. Ota M, Sato N, Yoshida F, Hattori K, Hidese S, Teraishi T, et al. A polymorphism of the methylenetetrahydrofolate reductase gene confers susceptibility to schizophrenia and related brain changes. Schizophr Res. (2019) 208:462–4. doi: 10.1016/j.schres.2019.03.002
68. Wan L, Li Y, Zhou Y, Li R, Zheng Y. Age matters: An atypical association between polymorphism of MTHFR and clinical phenotypes in children with schizophrenia. J Mol Neurosci MN. (2019) 69:485–93. doi: 10.1007/s12031-019-01382-0
69. Wan L, Zhang G, Liu M, Wang C, Li Y, Li R. Sex-specific effects of methylenetetrahydrofolate reductase polymorphisms on schizophrenia with methylation changes. Compr Psychiatry. (2019) 94:152121. doi: 10.1016/j.comppsych.2019.152121
70. Kelly CB, McDonnell AP, Johnston TG, Mulholland C, Cooper SJ, McMaster D, et al. The MTHFR C677t polymorphism is associated with depressive episodes in patients from northern Ireland. J Psychopharmacol (Oxford, England). (2004) 18:567–71. doi: 10.1177/0269881104047285
71. Reif A, Pfuhlmann B, Lesch KP. Homocysteinemia as well as methylenetetrahydrofolate reductase polymorphism are associated with affective psychoses. Progr Neuro Psychopharmacol Biol Psychiatry. (2005) 29:1162–8. doi: 10.1016/j.pnpbp.2005.06.027
72. Yuan Y-G, Li H, Wu R. The association of plasma homocysteine level, methylene tetrahydrofolate reductase gene polymorphism and depression in the elderly [in Chinese]. Chin J Psychiatry. (2005) 38:150.
73. Cheng-Sheng C, Tsai J-C, Hin-Yeung T, Yu-Ting K. Homocysteine levels, MTHFR C677t genotype, and mrihyperintensities in late-onset major depressive disorder. Am J Geriatric Psychiatry. (2005) 13:869–75. doi: 10.1176/appi.ajgp.13.10.869
74. Yuan YG. Plasma homocysteine levels and MTHFR polymorphisms in senile depression and mild Alzheimer’s disease [in Chinese]. Chin J Geriatr. (2007) 26:767–69.
75. Slopien R, Jasniewicz K, Meczekalski B, Warenik-Szymankiewicz A, Lianeri M, Jagodzinski PP. Polymorphic variants of genes encoding MTHFR, MTR, and MTHFD1 and the risk of depression in postmenopausal women in Poland. Maturitas. (2008) 61:252–5. doi: 10.1016/j.maturitas.2008.08.002
76. Zhao G-Q. Association of polymorphism of homocysteine and MTHFR gene C677t with major depression [in Chinese]. Chin J Nerv Ment Dis. (2008) 34:378–80.
77. Yuan YG, Zhang ZJ, Li JJ. Plasma homocysteine but not MTHFR gene polymorphism is associated with geriatric depression in the Chinese Population. Actaneuropsychiatrica. (2008) 20:251–5. doi: 10.1111/j.1601-5215.2008.00290.x
78. Hong ED, Taylor WD, McQuoid DR, Potter GG, Payne ME, Ashley-Koch A, et al. Influence of the MTHFR C677t polymorphism on magnetic resonance imaging hyperintensity volume and cognition in geriatric depression. Am J Geriatric Psychiatry Off J Am Assoc Geriatric Psychiatry. (2009) 17:847–55. doi: 10.1097/JGP.0b013e3181aad5b2
79. Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Modification by two genes of associations between general somatic health and incident depressive syndrome in older people. Psychosomatic Med. (2009) 71:286–91. doi: 10.1097/PSY.0b013e3181990fff
80. Pan CC, McQuoid DR, Taylor WD, Payne ME, Ashley-Koch A, Steffens DC. Association analysis of the COMT/MTHFR genes and geriatric depression: An MRI study of the putamen. Int J Geriatric Psychiatry. (2009) 24:847–55. doi: 10.1002/gps.2206
81. Cao L, Liu Z, Zhao L. Correlation analysis of plasma homocysteine and MTHFR gene polymorphism with post-stroke depression [in Chinese]. Guangdong Med J. (2010) 31:2946–9.
82. Zeman M, Jachymova M, Jirak R, Vecka M, Tvrzicka E, Stankova B, et al. Polymorphisms of genes for brain-derived neurotrophic factor, methylenetetrahydrofolate reductase, tyrosine hydroxylase, and endothelial nitric oxide synthase in depression and metabolic syndrome. Folia Biol. (2010) 56:19–26.
83. Feng L-G, Shao C, Liu Y, Tao Y, Hao X. The detection of genepolymorphisms of MTHFR C677t and A1298c in severe depression patients [in Chinese]. Basic Clin Med. (2010) 30:811–4.
84. Li ZZ, Yu SY, Zhang C, Yuan CM, Hong W, Wang Y, et al. Association of MTHFR gene C677t polymorphisms and depression in han populations. J Shanghai Jiaotong Univ (Med Sci). (2010) 30:624–7.
85. Song Z-W. Association of Methylenetetrahydrofolate reductase gene polymorphism and plasma homocysteine with major depression [in Chinese]. J Med Postgraduates. (2010) 23:934–7.
86. Lizer MH, Bogdan RL, Kidd RS. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism in depressed versus nondepressed patients. J Psychiatric Pract. (2011) 17:404–9. doi: 10.1097/01.pra.0000407963.26981.a6
87. Zhao H-F, Qiao J, Zhu X-H. The association study of MTHFR gene polymorphism with depression [in Chinese]. J Psychiatry. (2011) 24:435–7. doi: 10.3969/j.issn.1009-7201.2011.06.011
88. Chojnicka I, Sobczyk-Kopciol A, Fudalej M, Fudalej S, Wojnar M, Waskiewicz A, et al. No association between MTHFR C677t polymorphism and completed suicide. Gene. (2012) 511:118–21. doi: 10.1016/j.gene.2012.09.019
89. Evinova A, Babusikova E, Straka S, Ondrejka I, Lehotsky J. Analysis of genetic polymorphisms of brain-derived neurotrophic factor and methylenetetrahydrofolate reductase in depressed patients in a slovak (Caucasian) population. Gen Physiol Biophys. (2012) 31:415–22. doi: 10.4149/gpb_2012_049
90. Qiao J, Zhao H-F, Zhu X-H, Geng D-Q. The study of MTHFR Gene Polymorphism in Depression [in Chinese]. J Clin Psychiatry. (2012) 22:92–4.
91. Shen X, Wu Y, Guan T, Wang X, Qian M, Lin M, et al. Association analysis of COMT/MTHFR polymorphisms and major depressive disorder in Chinese Han population. J Affect Disord. (2014) 161:73–8. doi: 10.1016/j.jad.2014.03.008
92. Sayadi MA, Achour O, Ezzaher A, Hellara I, Omezzine A, Douki W, et al. Ct Genotype of 5,10-methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism is protector factor of major depressive disorder in the Tunisian Population: A case control study. Ann Gen Psychiatry. (2016) 15:18. doi: 10.1186/s12991-016-0103-5
93. Mei F, Wu Y, Ding G. Association analysis of the MTHFR polymorphism and post-stroke depression in the elderly [in Chinese]. Geriatrics Health Care. (2016) 22:363–5+9.
94. Huang Z-H, Zhou H-Y, Iv W-C, Cheng X-Y, Chen F. The correlation between MTHFR gene, Erαgene Polymorphism and Postpartum Depression Susceptibility [in Chinese]. J Med Theory Pract. (2017) 30:1108–9+22.
95. Li Y, Yue H, Li H. Effect of folic acid intake during pregnancy and MTHFR polymorphism on postpartum depression [in Chinese]. Chin J Woman Child Health Res. (2017) 28:632–5. doi: 10.1038/ejcn.2011.136
96. Mei F, Wu Y, Ding G, Pan F, Chen L, Wu J. Association of methylenetetrahydrofolate reductase Gene 677c>T polymorphism with post-stroke depression risk and antidepressant treatment response in Han Chinese. JPMA J Pakistan Med Assoc. (2018) 68:888–92.
97. Saraswathy KN, Ansari SN, Kaur G, Joshi PC, Chandel S. Association of vitamin B12 mediated hyperhomocysteinemia with depression and anxiety disorder: A cross-sectional study among bhil indigenous population of India. ClinNutr ESPEN. (2019) 30:199–203. doi: 10.1016/j.clnesp.2019.01.009
98. Zhao G-Q, Jiao Z-A, Wang S-M, Liu Z-X, Shi H. An association analysis of methylenetetrahydrofolate dehydrogenase polymorphism, plasma homocysteine levels and bipolar affective disorder [in Chinese]. J Psychiatry. (2008) 21:130–2.
99. Ozbek Z, Kucukali CI, Ozkok E, Orhan N, Aydin M, Kilic G, et al. Effect of the methylenetetrahydrofolate reductase gene polymorphisms on homocysteine, folate and vitamin B12 in patients with bipolar disorder and relatives. Progr Neuro Psychopharmacol Biol Psychiatry. (2008) 32:1331–7. doi: 10.1016/j.pnpbp.2008.04.016
100. Chen Z, Liu Y, Zhang D, Liu Z, Wang P, Zhou D, et al. C677t methylenetetrahydrofolate reductase gene polymorphisms in bipolar disorder: An association study in the Chinese population and a meta-analysis of genetic association studies. Neurosci Lett. (2009) 449:48–51. doi: 10.1016/j.neulet.2008.10.077
101. Ezzaher A, Mouhamed DH, Mechri A, Omezzine A, Neffati F, Douki W, et al. Hyperhomocysteinemia in Tunisian Bipolar I patients. Psychiatry Clin Neurosci. (2011) 65:664–71. doi: 10.1111/j.1440-1819.2011.02284.x
102. Permoda-Osip A, Dmitrzak-Weglarz M, Hauser J, Rybakowski JK. Are genes connected with homocysteine metabolism associated with bipolar disorder? Neuropsychobiology. (2014) 69:107–11. doi: 10.1159/000358091
103. Wang LJ, Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, et al. A potential interaction between COMT and MTHFR genetic variants in Han Chinese patients with Bipolar II disorder. Sci Rep. (2015) 5:8813. doi: 10.1038/srep08813
104. Rahimi Z, Kakabaraee K, Garavand A, Rahimi Z. The T allele of MTHFR C.C677t and Its synergism with G (Val 158) allele of COMT C.G472a polymorphism are associated with the risk of Bipolar I disorder. Genet Test Mol Biomarkers. (2016) 20:510–5. doi: 10.1089/gtmb.2016.0061
105. Kempisty B, Bober A, Luczak M, Czerski P, Szczepankiewicz A, Hauser J, et al. Distribution of 1298a>C polymorphism of methylenetetrahydrofolate reductase gene in patients with bipolar disorder and schizophrenia. Eur Psychiatry J Assoc Eur Psychiatrists. (2007) 22:39–43. doi: 10.1016/j.eurpsy.2006.11.003
106. Zhang C, Xie B, Du Y, Cheng W, Fang Y, Yu S. Further evidence that methylenetetrahydrofolate reductase A1298c polymorphism is a risk factor for schizophrenia. J Neural Transm (Vienna). (2010) 117:1115–7. doi: 10.1007/s00702-010-0442-3
107. Wu YL, Ding XX, Sun YH, Yang HY, Chen J, Zhao X, et al. Association between MTHFR C677t polymorphism and depression: An updated meta-analysis of 26 Studies. Progr Neuro-Psychopharmacol Biol Psychiatry. (2013) 46:78–85. doi: 10.1016/j.pnpbp.2013.06.015
108. Gaysina D, Cohen S, Craddock N, Farmer A, Hoda F, Korszun A, et al. No Association with the 5,10-methylenetetrahydrofolate reductase gene and major depressive disorder: Results of the Depression Case Control (DECC) study and a meta-analysis. Am J Med Genet Part B Neuropsychiatric Genet Off Publ Int Soc Psychiatric Genet. (2008) 147B:699–706. doi: 10.1002/ajmg.b.30665
109. Rai V. Evaluation of methylenetetrahydrofolate reductase gene variant (C677t) as risk factor for bipolar disorder. Cell Mol Biol (Noisy-le-Grand, France). (2011) 57:OL1558–66.
110. Zintzaras E. C677t and A1298c methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: A meta-analysis of genetic association studies. Psychiatric Genet. (2006) 16:105–15. doi: 10.1097/01.ypg.0000199444.77291.e2
111. Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Purcell S, et al. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677t polymorphism on executive function in schizophrenia. Schizophr Res. (2007) 92:181–8. doi: 10.1016/j.schres.2007.01.003
112. Wang X, Wang Z, Wu Y, Yuan Y, Hou Z, Hou G. Association analysis of the Catechol-O-Methyltransferase/methylenetetrahydrofolate reductase genes and cognition in late-onset depression. Psychiatry Clin Neurosci. (2014) 68:344–52. doi: 10.1111/pcn.12133
113. Zhilyaeva TV, Sergeyeva AV, Kasimova LN, Blagonravova AS. Cognitive function dynamics during folate augmented therapy in patients with schizophrenia carrying MTHFR677c>T gene polymorphism: A pilot study. Sovremennyetehnologii Med. (2015) 7:147–53. doi: 10.17691/stm2015.7.4.20
Keywords: MTHFR C677T, MTHFR A1298C, disorders, meta-analysis, gene variants
Citation: Zhang Y-X, Yang L-P, Gai C, Cheng C-C, Guo Z-y, Sun H-M and Hu D (2022) Association between variants of MTHFR genes and psychiatric disorders: A meta-analysis. Front. Psychiatry 13:976428. doi: 10.3389/fpsyt.2022.976428
Received: 23 June 2022; Accepted: 28 July 2022;
Published: 18 August 2022.
Edited by:
Muhammad Muddassir Ali, University of Veterinary & Animal Sciences, PakistanReviewed by:
Furqan Awan, University of Veterinary & Animal Sciences, PakistanCopyright © 2022 Zhang, Yang, Gai, Cheng, Guo, Sun and Hu. This is an open-access article distributed under the tersms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Die Hu, aHVkaWUwNjEwQGJ1Y20uZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.