- 1Mental Health Center and Psychiatric Laboratory, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Neurobiology, Affiliated Mental Health Center and Hangzhou Seventh People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
Introduction: Insomnia is a major public health problem that determines the quality of life. Among the many causes of insomnia, psychological factors have an important influence on the process, duration of insomnia, help-seeking behavior, and treatment choice. Regarding medical treatment, zolpidem is always chosen to treat acute and transient insomnia due to its few side effects. Although some randomized controlled trials have verified its safety, zolpidem abuse and withdrawal reactions have been reported in recent years.
Case report: A 25-year-old unmarried man with a college degree who worked as a graphic designer was referred and admitted to the inpatient ward for a chief complaint of “alternative episodes of lowering and elevation of mood for 10 years, overdosage use of zolpidem for two years.” He underwent a time-dependent withdrawal reaction after admission. It was characterized by rebound insomnia, anxiety, craving, skin paresthesia, influenza-like symptoms, tonic-clonic-type seizures, and hallucinations. At the 1-year follow-up, he did not exhibit any remaining withdrawal symptoms.
Discussion: The acute cessation of overdosage zolpidem use causes a series of withdrawal symptoms that manifest in chronological order. Additionally, long-term benzodiazepine exposure has potential influences on zolpidem dependence/tolerance. However, patients with a history of abuse or dependence, or mental disorders seem to be at risk of drug abuse. Clinicians should be alert to the potential for zolpidem dependence and addiction. Once the acute cessation of overdosage zolpidem use occurs, the potential of the withdrawal reaction needs to be considered and addressed properly.
Introduction
As a highly prevalent determinant of quality of life, insomnia has become a major public health concern. It was reported that more than half of the people surveyed had experienced insomnia at least once a month (1). Even in surveys using stringent diagnostic criteria, the prevalence of insomnia disorder is reported to be approximately 10% (1–3). The course of insomnia can be chronic and persist for many years (2–4). In addition to being an independent diagnosis, insomnia is also common comorbidity of various psychiatric/neurological disorders, such as mood disorders and anxiety disorders, that require adequate therapy (2, 4–7).
There are many causes of insomnia. Among them, psychological factors have an important influence not only on the process and duration of insomnia but also on help-seeking behavior and treatment choices, including increments or reductions in drug dosage (8). The most frequently reported psycho-social factors that lead to insomnia are stressful events, while the cognitive characteristics of high expectations of sleep duration and excessive worry of sleep loss and sleep distortion may significantly affect the automaticity of the sleep-wake system and drug dosage management (9, 10), which “reminds” people to rely on drugs and increases the dosage used during self-healing and even produces “craving” to maintain addiction (8).
Zolpidem (Stilnox®) is an imidazopyridine that is different from benzodiazepines in chemical structure. It is commonly chosen to treat acute and short-term insomnia due to its rapid onset, short duration of action, and few side effects (11). Zolpidem has been used for decades in the clinic. In 1992, it was approved for use by the U.S. Food and Drug Administration (FDA) (12).
Theoretically, zolpidem has some advantages over benzodiazepines in terms of the receptors it binds to, adverse reactions, and pharmacokinetics (13–15). At the recommended dose, zolpidem acts on the GABAA α1 subunit selectively and has a sedative effect. However, at high doses, it loses selectivity. It also acts on α2, α3, and α5 subunits and causes muscle relaxation, antianxiety and anticonvulsant effects (11, 13). After a single oral dose of 5–10 mg of zolpidem, the peak plasma concentrations (tmax) were found to occur in a range from 0.5 to 2 h (16), and the half-life (t1/2) ranged from 2.5 to 2.6 h (15). Thus, zolpidem acts quickly, have a short half-life, and has nearly no significant residual effect the next day (17).
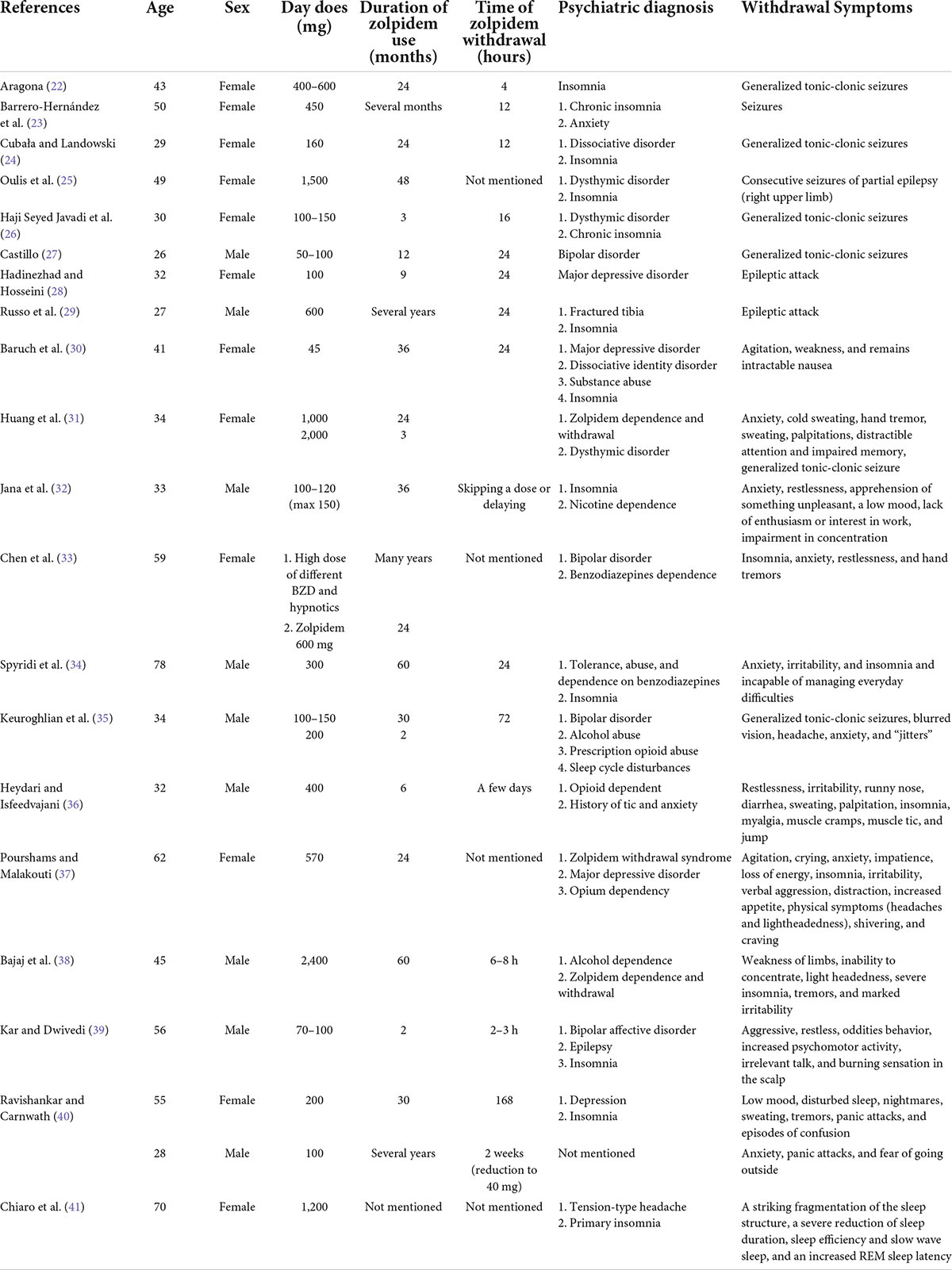
Since zolpidem was marketed, some clinical trials have verified the safety and efficacy of 10 mg of zolpidem for acute and transient insomnia disorder. Studies found no obvious signs of rebound insomnia or withdrawal symptoms after 28 or 180 days of treatment (18–21). Over the past two decades, there have been some case reports regarding the withdrawal symptoms following the cessation of overdose zolpidem use, especially seizures (the summary of reports is presented in Table 1) (22–29). The authors of previous reports focused on one of the serious or typical withdrawal symptoms but did not observe the different kinds of zolpidem withdrawal symptoms in one case, nor the symptom profile over time.
We present a case of chronological symptoms that occurred after overdose zolpidem withdrawal to help clinicians recognize this process early and address it effectively.
Case report
A 25-year-old unmarried man with a college degree who worked as a graphic designer was referred and admitted to the inpatient ward for a chief complaint of “alternative episodes of the lowering and elevation mood for 10 years, overdosage use of zolpidem for two years.”
The patient had a history of psychiatric diseases. Eleven years prior to admission, after experiencing sadness, low energy, hopelessness, and insomnia for half a year, he was diagnosed with depressive disorder in a local hospital. The patient was treated with 20 mg of fluoxetine per day and 10 mg of zolpidem every night. Depression and insomnia were gradually alleviated in 1 year. He reported that he had taken the same drugs and dosage for another 6 years, but symptoms fluctuated during this time. Seven years prior to admission, at the beginning of college, he experienced an episode of elevated mood characterized by the feeling of being in a good condition, increased ability, and high energy levels, and he remained busy for a long time. Five years prior to admission, during the third year of college, when he experienced stress regarding his studies and problems in a closed relationship, he experienced another depressive episode characterized by sadness, loss of interest, low energy, hopelessness, and insomnia for half a year. During this episode, he continued to take the same drugs as those he was first prescribed. When the condition gradually improved, he stopped taking zolpidem and fluoxetine. Four years prior to admission, during the fourth year of college, he experienced a second episode of elevated mood characterized by the feeling of increased energy levels and ability and increased interest and participation in many activities. This period of elevated mood lasted for nearly one and a half years, during which he had driven 20,000 km to travel around the United States. He denied taking drugs during this episode. Two years prior to admission, when he began his first job as a graphic designer, another depressive episode characterized by sadness, loss of interest, low energy, hopelessness, and insomnia started. He reported that he felt similarly to how he felt during the episode 11 years ago. He resumed taking zolpidem 10 mg per night and fluoxetine 20 mg per day. Sometimes he took another 10 mg of zolpidem on his own for a better hypnotic effect. Five months before visiting, his work got busier and his stress levels increased, and he found that taking zolpidem could make him feel relaxed. Thus, the patient took additional zolpidem before bedtime until he relaxed. In total, he took a maximum of 80 mg of zolpidem once or twice weekly, with an average of 30 mg every night. His family members observed that he performed some abnormal and dangerous behaviors after taking an overdose of zolpidem at night, including cheerfulness, talkativeness, frequent movement, excessive eating, and dangerous driving behaviors associated with several traffic accidents. To prevent similar dangerous behaviors and improve sleep and mood, his father brought him to the hospital for help. To improve the possibility of successful drug withdrawal for a patient with comorbid bipolar disorder, insomnia, and long-term zolpidem dependence who repeatedly performed high-risk behaviors and had a higher possibility of severe withdrawal reactions once the drug was stopped, the patient was hospitalized in the psychiatric inpatient ward in the West China Hospital of Sichuan University.
In addition, he had a history of alcohol abuse and cigarette smoking, but he reported that he had given up alcohol and smoking 4 years prior to admission. He admitted that he occasionally used marijuana and nitrous oxide during college, but he denied any use of those drugs for at least 1 year, which was confirmed by his father. He also denied any recent use of alcohol and other sedative-hypnotic drugs, which was confirmed by his father. He had no family history of mental illness.
On admission, the patient was 176 cm tall and 86 kg in weight, and his vital signs were in the normal range. Physical and neurological examinations revealed no notable abnormalities. ECG, electroencephalogram (EEG), chest CT, and brain MRI yielded normal findings. Laboratory tests revealed alanine aminotransferase levels of 62 IU/L, aspartate aminotransferase levels of 30 IU/L, creatinine levels of 65 μmol/L, and an estimated glomerular filtration rate (eGFR) of 128.54 ml/min/1.73 m2. The complete blood count (CBC), electrolyte levels, cortisol levels, adrenotropin levels, and thyroid function were in the normal range. The blood concentration of lithium carbonate was 0.055 mmol/L on the seventh day.
According to the 10th revision of the International Classification of Diseases (ICD-10), he was diagnosed with “Harmful use of sedative or hypnotic drugs; bipolar affective disorder, current episode moderate depressive.” The treatment strategies used for zolpidem withdrawal followed the protocols used in previous case reports (26, 29, 31–34, 37–39, 41, 42). The treatment for comorbid bipolar disorder followed the guidelines for the management of bipolar disorder (43).
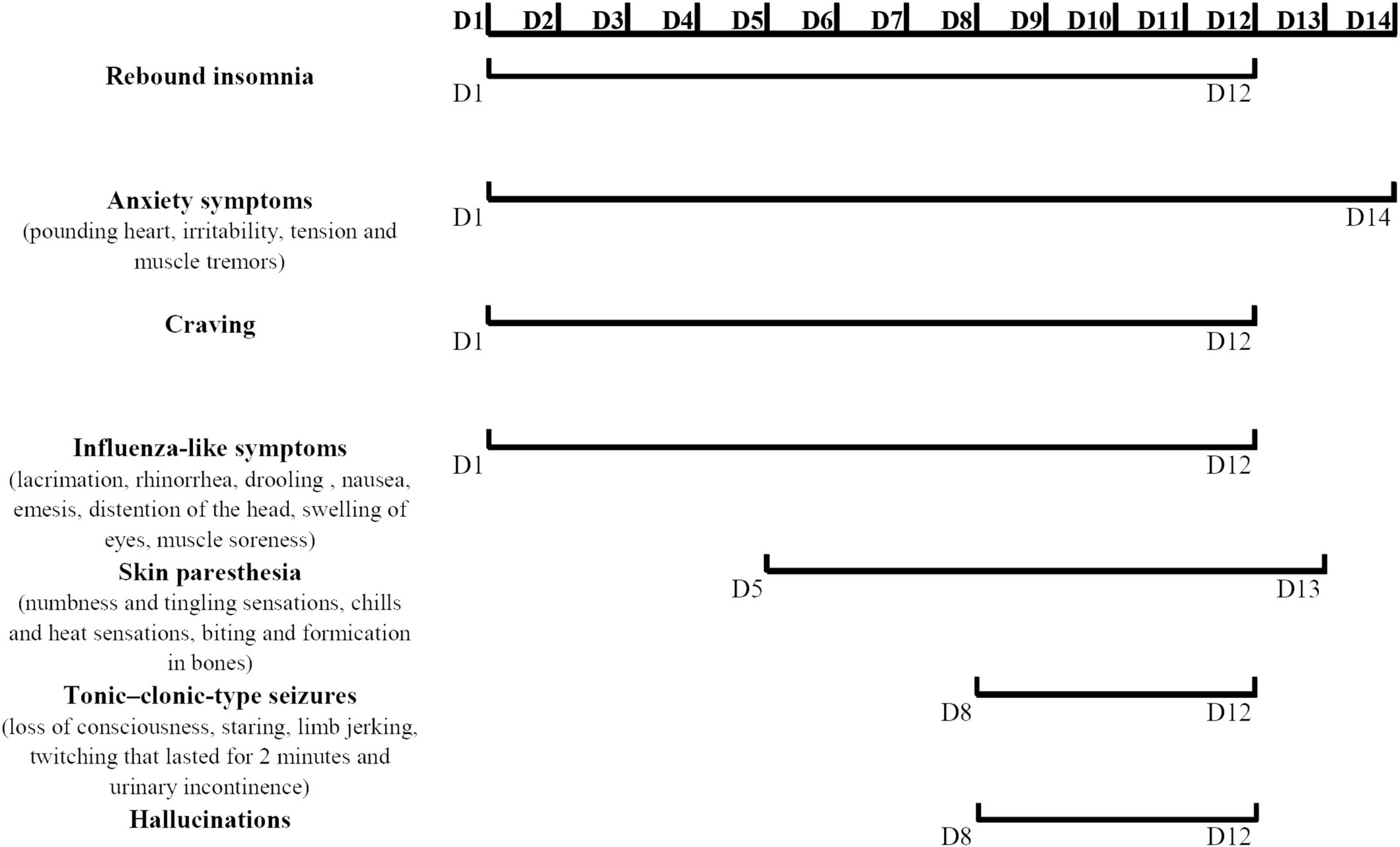
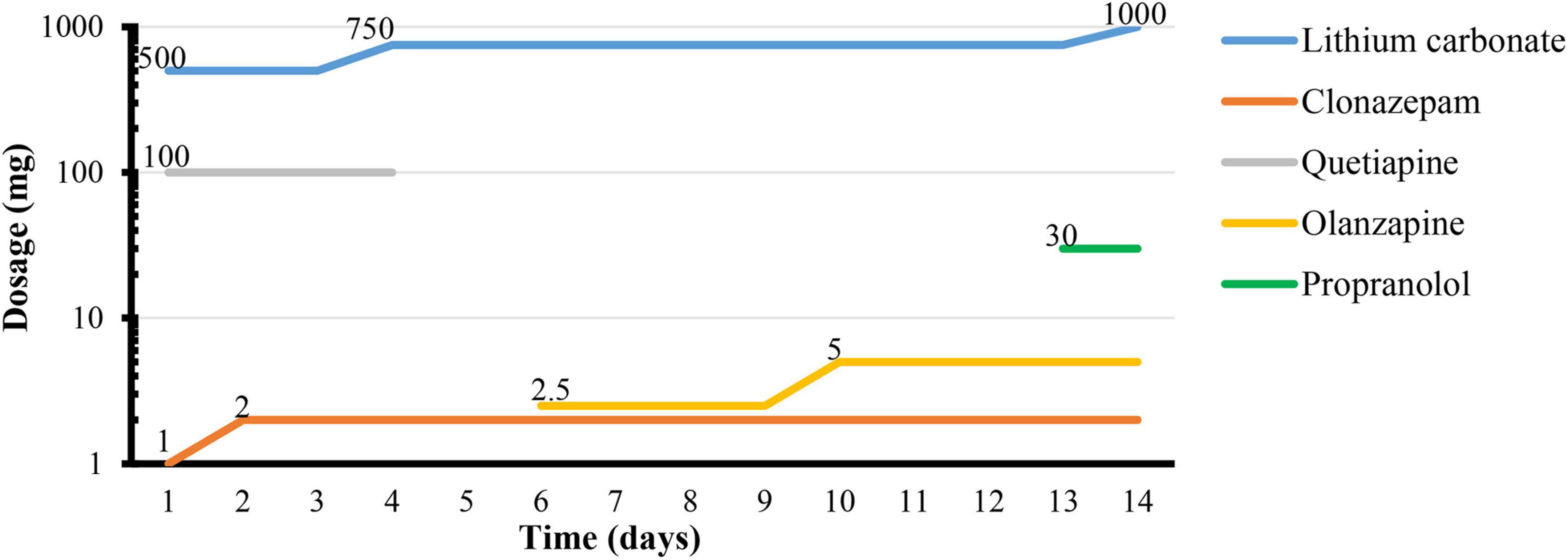
After zolpidem cessation, a series of symptoms appeared gradually during hospitalization. Zolpidem use was terminated the first night, and the patient described obvious rebound insomnia, influenza-like symptoms (manifested as distension of the head, swelling of eyes, and muscle soreness), anxiety symptoms (manifested as muscle tension and muscle tremors) accompanied by strong craving. He was treated with clonazepam (1 mg every night), lithium carbonate (250 mg twice daily), and quetiapine (100 mg every night). On the third day, rebound insomnia became serious, and he could not sleep until 4:00 a.m. Influenza-like symptoms and anxiety symptoms (manifested as pounding heart, irritability, tension, and muscle tremors) persisted during each attack. The attacks lasted for approximately 20 min from onset to symptom relief. The clonazepam dosage was increased to 2 mg per night, and the lithium carbonate dosage was increased to 250 mg three times daily. On the fifth day, skin paresthesia (manifested as numbness and tingling sensations, chills and heat sensations, and biting and formication in bones) appeared and worsened in the following days. The possibility of skin paresthesia that may be caused by quetiapine was considered. This drug was replaced with 2.5 mg olanzapine every night. On the sixth day, he experienced typical influenza-like symptoms, such as lacrimation, rhinorrhea, drooling, nausea, and emesis. The duration of each attack increased to more than 30 min from onset to relief. On the eighth to tenth days, the symptoms of some attacks appeared in the following order: first, anxiety symptoms; then, a mixture of skin paresthesias and influenza-like symptoms; then, loss of consciousness, staring, limb jerking, and twitching that lasted approximately 2 min; then, hallucination and urinary incontinence followed the relief of limb twitching and return to consciousness. The patient drew what he saw after symptom relief. The symptoms of loss of consciousness, staring, limb jerking, twitching that lasted for 2 min, and urinary incontinence were considered to be the onset of tonic-clonic-type seizures. However, the EEG was not obtained during the attack. Similar tonic-clonic-type seizures occurred on average once a day during this stage. Craving was also readily reported during this stage. The duration from the onset of anxiety symptoms to the relief of tonic-clonic-type seizures and subsequent urinary incontinence and hallucination lasted not more than 40 min. Tonic-clonic-type seizures were considered one of the withdrawal symptoms, and diazepam was prepared as necessary. He was given 5 mg olanzapine in total to control the hallucinations. On the 12th day, the occurrence of tonic-clonic-type seizures, hallucinations, and cravings decreased. The duration of each symptomatic attack from onset to relief was reduced to approximately 10 min. After 12 days of hospitalization, only the anxiety symptoms (manifested as palpitations and worry) and skin paresthesia (manifested as tingling and formication) appeared occasionally. The duration of each symptomatic attack was reduced to no more than 5 min. Propranolol (10 mg three times daily) was added to the regimen to relieve palpitations. On the 14th day, most of the withdrawal symptoms were relieved except anxiety. The patient was discharged after 15 days. The details of the symptom changes over time are shown in Figure 1, and the drug treatment plan is shown in Figure 2.
After discharge, the patient was followed up in the outpatient department. Three months after discharge, the lithium carbonate dosage was reduced to 250 mg three times daily, the clonazepam dosage was 1 mg every night, and the olanzapine and propranolol dosages were not changed. In the fourth month after discharge, he experienced several anxiety attacks characterized by nervousness, sweating, and trembling that lasted approximately 10 min with spontaneous remission. There were no anxiety attacks reported in the fifth month of follow-up. The olanzapine, propranolol, and clonazepam dosages were reduced gradually. He did not report any skin paresthesia, seizure, or hallucination after discharge. He was sleepless and anxious occasionally, but he could cope with it, and he never took zolpidem again. Finally, 12 months after discharge, he took 250 mg of lithium carbonate twice daily and 0.25 mg of clonazepam every night. He reported that his life and work were normal. This finding confirms that the chronological symptoms observed during hospitalization were most likely caused by zolpidem withdrawal.
Discussion
To our knowledge, this report represents the first case of chronological symptoms after overdose zolpidem withdrawal. From the data collected, the lowest dosage that induced withdrawal symptoms was 45 mg (Table 1). Symptoms may appear within 2 h after withdrawal, but the duration from symptom onset to relief is not mentioned. It is well known that zolpidem can lead to withdrawal symptoms if overdosed. The details of symptoms were not complete in previous case reports, but they were described in chronological order in our case report. It could be assumed that the withdrawal symptoms did not appear randomly. This inference was related to the timing of the cessation of drug intake. The knowledge provided by this study may help to clinically diagnose and treat zolpidem withdrawal in different stages.
The patient with a long history of fluoxetine use with zolpidem may have had an influence on the symptoms that appeared during zolpidem withdrawal due to drug-drug interactive effects and the acute cessation of fluoxetine use. A case report indicated that the combination of zolpidem and fluoxetine might cause hallucinations (44). Studies of the interaction between zolpidem and fluoxetine show that the time of zolpidem onset may be shortened in the presence of fluoxetine (45–47). To some extent, fluoxetine may prolong the duration of zolpidem-associated hallucinations (47). Both zolpidem and fluoxetine are highly protein bound. The interaction between them may occur through competitive binding and increase the serum levels of zolpidem (48). However, some symptoms that occur during zolpidem withdrawal as shown in case reports and clinical trials, such as nervousness, insomnia, dizziness, headache, drowsiness, tremor, and sweating, have also been reported as fluoxetine-related adverse effects (49–51), but those adverse symptoms tended to occur in the early stage of fluoxetine use and infrequently occur during long-term treatment (51). The abrupt interruption of fluoxetine use may cause dizziness and sleep disturbances, but the severity of the discontinuation syndrome is slight, and the score of discontinuation emergent signs and symptoms (DESS) is lower than that of other selective serotonin reuptake inhibitors (SSRIs) (52). This might be because fluoxetine and norfluoxetine have metabolites with longer half-lives, and the blood concentration does not drop quickly. Thus, most symptoms that occurred during zolpidem withdrawal, in this case, were not attributable to the long-term use or abrupt interruption of fluoxetine treatment.
Clinicians should not ignore the potential for zolpidem dependence and addiction because it has high receptor selectivity. Although the number of pharmacological studies on the dependence/tolerance of zolpidem is very limited, it is likely appropriate to interpret its potential mechanisms based on studies of benzodiazepine tolerance solely because the GABA receptor is the major targeted receptor for both zolpidem and benzodiazepines. There are five potential mechanisms underlying the influences of long-term benzodiazepine exposure (53, 54). First, a change in GABAA receptor gene expression, which might be related to the exposure time (55), might result in the development of tolerance, although most reports suggested that the number of GABAA receptors might not change because of the maximal binding capacity (Bmax) of [3H] flunitrazepam (a non-selective benzodiazepine) did not change significantly during the studied periods (54). Second, the changes in the subunit composition of GABAA receptors might influence the binding of drugs to receptors, especially any changes in the expression of γ submit or α submit (53, 54). Third, uncoupling of the GABA and benzodiazepine binding sites may reduce benzodiazepine activity (53, 54). Vlainić et al. documented that the number of functional interactions occurring at GABAA receptors in the zolpidem group was diminished by approximately 40% of the control values (54). Fourth, the phosphorylation of the GABAA submits might regulate the components of GABAA receptors and the effects of allosteric modulators and consequently influences the function of GABAA receptors (53, 54). Fifth, the internalization of GABAA receptors, which might be controlled by phosphorylation or by the mediation of uncoupling, may decrease the number of receptors at the cell surface (53, 54), although it probably does not influence the total number of GABAA receptors. More pharmacological studies are needed to confirm these potential mechanisms.
Patients with a history of abuse or dependence or mental disorders are potentially at risk for drug abuse (56). It is important to manage the use of benzodiazepines in those patients (57). As shown in this report and others, in addition to insomnia, these patients can exhibit comorbid mental disorders, such as dysthymic disorder, bipolar disorder, alcohol abuse, and so on (Table 1). Patients reported that zolpidem use not only helped with sleep but also relieved anxiety and promoted relaxation (32, 38). Clinicians should be more vigilant about drug abuse and addiction.
This case report had certain limitations. First, there is the possibility that some symptoms of this patient might be caused by substance-related exogenous psychosis (58). It was difficult to rule out that the symptoms were not caused by the history of substance use or the clinical deterioration of bipolar episodes. Second, the urine test which may help distinguish the withdrawal reaction of drugs from intoxication for a patient who had a history of drugs and alcohol abuse, was not complete when the patient was admitted. It is noteworthy that not only the patient but also his father denied that he had recently used alcohol or any other sedative-hypnotic drugs. Third, drug abuse or addiction is a chronic and recurring process, and a systemic follow-up plan of patient monitoring will help evaluate the therapeutic effect and progression of the disease. The time interval of follow-up also needs to be considered.
Conclusion
The present case report describes the chronological symptom profile of overdose zolpidem withdrawal. It emphasizes the potential of zolpidem abuse and the variability of withdrawal symptoms that change over time. Clinicians should be able to recognize zolpidem early and accurately during the process of zolpidem withdrawal.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Z-XM wrote the first draft. W-JG and H-YW supervised the patient and provided the treatment. W-JG and XY edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by grants from the “1.3.5” project for disciplines of excellence, West China Hospital, Sichuan University (grant no. 2019HXFH026).
Acknowledgments
We thank the assistant from the library of Sichuan University and the patient for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aernout E, Benradia I, Hazo JB, Sy A, Askevis-Leherpeux F, Sebbane D, et al. International study of the prevalence and factors associated with insomnia in the general population. Sleep Med. (2021) 82:186–92. doi: 10.1016/j.sleep.2021.03.028
2. Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. (2022) 17:173–91. doi: 10.1016/j.jsmc.2022.03.003
3. Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, et al. Insomnia disorder. Nat Rev Dis Primers. (2015) 1:15026. doi: 10.1038/nrdp.2015.26
4. Dopheide JA. Insomnia overview: epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am J Manag Care. (2020) 26:S76–84. doi: 10.37765/ajmc.2020.42769
5. Malhotra RK. Neurodegenerative disorders and sleep. Sleep Med Clin. (2022) 17:307–14. doi: 10.1016/j.jsmc.2022.02.009
6. Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. (2010) 14:35–46. doi: 10.1016/j.smrv.2009.09.003
7. Freeman D, Sheaves B, Waite F, Harvey AG, Harrison PJ. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. (2020) 7:628–37. doi: 10.1016/S2215-0366(20)30136-X
8. Espie CA, Broomfield NM, MacMahon KM, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. (2006) 10:215–45. doi: 10.1016/j.smrv.2006.03.002
9. Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. (2012) 138:77–101. doi: 10.1037/a0025730
10. Fernandez-Mendoza J, Calhoun SL, Bixler EO, Karataraki M, Liao D, Vela-Bueno A, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. (2011) 73:88–97. doi: 10.1097/PSY.0b013e3181fe365a
11. Kim H, Shin C, Ko YH, Han C. Comorbid zolpidem dependence and over-the-counter compound analgesic abuse. Clin Psychopharmacol Neurosci. (2019) 17:323–5. doi: 10.9758/cpn.2019.17.2.323
12. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. (1995) 29:142–53. doi: 10.2165/00003088-199529030-00002
13. Liappas IA, Malitas PN, Dimopoulos NP, Gitsa OE, Liappas AI, Nikolaou CHK, et al. Zolpidem dependence case series: possible neurobiological mechanisms and clinical management. J Psychopharmacol. (2003) 17:131–5. doi: 10.1177/0269881103017001723
14. Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. (2017) 13:307–49. doi: 10.5664/jcsm.6470
15. Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs. (2000) 59:865–89. doi: 10.2165/00003495-200059040-00014
16. Dong-shan Y, Mao-hong G, Hai-ling S. Handbook of Rational Use of Drugs in Psychiatry. 3rd ed. Nanjing: Phoenix Science Press Ltd (2016). p. 340.
17. Roth T, Soubrane C, Titeux L, Walsh JK, Zoladult Study Group. Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia. Sleep Med. (2006) 7:397–406. doi: 10.1016/j.sleep.2006.04.008
18. Schlich D, L’Heritier C, Coquelin JP, Attali P, Kryrein HJ. Long-term treatment of insomnia with zolpidem: a multicentre general practitioner study of 107 patients. J Int Med Res. (1991) 19:271–9. doi: 10.1177/030006059101900313
19. Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T, Zolong Study Group. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. (2008) 31:79–90. doi: 10.1093/sleep/31.1.79
20. Herrmann WM, Kubicki ST, Boden S, Eich FX, Attali P, Coquelin JP. Pilot controlled double-blind study of the hypnotic effects of zolpidem in patients with chronic ‘learned’ insomnia: psychometric and polysomnographic evaluation. J Int Med Res. (1993) 21:306–22. doi: 10.1177/030006059302100602
21. Xiang T, Cai Y, Hong Z, Pan J. Efficacy and safety of zolpidem in the treatment of insomnia disorder for one month: a meta-analysis of a randomized controlled trial. Sleep Med. (2021) 87:250–6. doi: 10.1016/j.sleep.2021.09.005
22. Aragona M. Abuse, dependence, and epileptic seizures after zolpidem withdrawal: review and case report. Clin Neuropharmacol. (2000) 23:281–3. doi: 10.1097/00002826-200009000-00008
23. Barrero-Hernández FJ, Ruiz-Veguilla M, López-López MI, Casado-Torres A. Crisis epil pticas como manifestaci n en la abstinencia al consumo cr nico de zolpidem [Epileptic seizures as a sign of abstinence from chronic consumption of zolpidem]. Rev Neurol. (2002) 34:253–6.
24. Cubała WJ, Landowski J. Seizure following sudden zolpidem withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. (2007) 31:539–40. doi: 10.1016/j.pnpbp.2006.07.009
25. Oulis P, Nakkas G, Masdrakis VG. Pregabalin in zolpidem dependence and withdrawal. Clin Neuropharmacol. (2011) 34:90–1. doi: 10.1097/WNF.0b013e31820a3b5a
26. Haji Seyed Javadi SA, Hajiali F, Nassiri-Asl M. Zolpidem dependency and withdrawal seizure: a case report study. Iran Red Crescent Med J. (2014) 16:e19926. doi: 10.5812/ircmj.19926
27. Castillo JL. Crisis epilépticas post suspensión de zolpidem [Epileptic seizures after zolpidem withdrawal]. Rev Med Chil. (2020) 148:1695. doi: 10.4067/S0034-98872020001101695
28. Hadinezhad P, Hosseini SH. Zolpidem withdrawal seizure in an Iranian young woman: a case presentation. Caspian J Intern Med. (2021) 12:S376–8. doi: 10.22088/cjim.12.0.376
29. Russo AD, Hodgman M, Calleo V. Seizures secondary to zolpidem withdrawal. Clin Toxicol (Phila). (2021) 59:174–5. doi: 10.1080/15563650.2020.1778718
30. Baruch E, Vernon LF, Hasbun RJ. Intractable nausea caused by zolpidem withdrawal: a case report. J Addict Med. (2007) 1:48–50. doi: 10.1097/ADM.0b013e31804259cb
31. Huang MC, Lin HY, Chen CH. Dependence on zolpidem. Psychiatry Clin Neurosci. (2007) 61:207–8. doi: 10.1111/j.1440-1819.2007.01644.x
32. Jana AK, Arora M, Khess CR, Praharaj SK. A case of zolpidem dependence successfully detoxified with clonazepam. Am J Addict. (2008) 17:343–4. doi: 10.1080/10550490802139168
33. Chen CY, Shiah IS, Lee WK, Kuo SC, Huang CC, Wang TY. Dependence on quetiapine in combination with zolpidem and clonazepam in bipolar depression. Psychiatry Clin Neurosci. (2009) 63:427–8. doi: 10.1111/j.1440-1819.2009.01953.x
34. Spyridi S, Diakogiannis I, Nimatoudis J, Iacovides A, Kaprinis G. Zolpidem dependence in a geriatric patient: a case report. J Am Geriatr Soc. (2009) 57:1962–3.
35. Keuroghlian AS, Barry AS, Weiss RD. Circadian dysregulation, zolpidem dependence, and withdrawal seizure in a resident physician performing shift work. Am J Addict. (2012) 21:576–7. doi: 10.1111/j.1521-0391.2012.00273.x
36. Heydari M, Isfeedvajani MS. Zolpidem dependence, abuse and withdrawal: a case report. J Res Med Sci. (2013) 18:1006–7.
37. Pourshams M, Malakouti SK. Zolpidem abuse and dependency in an elderly patient with major depressive disorder: a case report. Daru. (2014) 22:54. doi: 10.1186/2008-2231-22-54
38. Bajaj V, Kalra I, Bajaj A, Sharma D, Kumar R. A case of zolpidem dependence with extremely high daily doses. Asia Pac Psychiatry. (2019) 11:e12356. doi: 10.1111/appy.12356
39. Kar SK, Dwivedi S. Zolpidem dependence in an adult with bipolar affective disorder and epilepsy: a case report. Gen Psychiatr. (2019) 32:e100102. doi: 10.1136/gpsych-2019-100102
40. Ravishankar A, Carnwath T. Zolpidem tolerance and dependence–two case reports. J Psychopharmacol. (1998) 12:103–4. doi: 10.1177/026988119801200114
41. Chiaro G, Castelnovo A, Bianco G, Maffei P, Manconi M. Severe chronic abuse of zolpidem in refractory insomnia. J Clin Sleep Med. (2018) 14:1257–9. doi: 10.5664/jcsm.7240
42. Rappa LR, Larose-Pierre M, Payne DR, Eraikhuemen NE, Lanes DM, Kearson ML. Detoxification from high-dose zolpidem using diazepam. Ann Pharmacother. (2004) 38:590–4. doi: 10.1345/aph.1D339
43. Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. (2018) 20:97–170. doi: 10.1111/bdi.12609
44. Coleman DE, Ota K. Hallucinations with zolpidem and fluoxetine in an impaired driver. J Forensic Sci. (2004) 49:392–3.
45. Piergies AA, Sweet J, Johnson M, Roth-Schechter BF, Allard S. The effect of co-administration of zolpidem with fluoxetine: pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther. (1996) 34:178–83.
46. Allard S, Sainati S, Roth-Schechter B, MacIntyre J. Minimal interaction between fluoxetine and multiple-dose zolpidem in healthy women. Drug Metab Dispos. (1998) 26:617–22.
47. Mandrioli R, Forti GC, Raggi MA. Fluoxetine metabolism and pharmacological interactions: the role of cytochrome p450. Curr Drug Metab. (2006) 7:127–33. doi: 10.2174/138920006775541561
48. Skourides D, Samartzis L. Initiation of illusions after combination of zolpidem and paroxetine in a young woman: a case report. Prim Care Companion CNS Disord. (2012) 14:PCC.12l01361. doi: 10.4088/PCC.12l01361
49. Benfield P, Heel RC, Lewis SP. Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs. (1986) 32:481–508. doi: 10.2165/00003495-198632060-00002
50. Stokes PE, Holtz A. Fluoxetine tenth anniversary update: the progress continues. Clin Ther. (1997) 19:1135–250. doi: 10.1016/s0149-2918(97)80066-5
51. Wernicke JF. Safety and side effect profile of fluoxetine. Expert Opin Drug Saf. (2004) 3:495–504. doi: 10.1517/14740338.3.5.495
52. Renoir T. Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. Front Pharmacol. (2013) 4:45. doi: 10.3389/fphar.2013.00045
53. Gravielle MC. Activation-induced regulation of GABAA receptors: is there a link with the molecular basis of benzodiazepine tolerance? Pharmacol Res. (2016) 109:92–100. doi: 10.1016/j.phrs.2015.12.030
54. Vlainić J, Švob Štrac D, Jazvinšćak Jembrek M, Vlainić T, Perièić D. The effects of zolpidem treatment on GABA(A) receptors in cultured cerebellar granule cells: changes in functional coupling. Life Sci. (2012) 90:889–94. doi: 10.1016/j.lfs.2012.04.021
55. Holt RA, Bateson AN, Martin IL. Chronic zolpidem treatment alters GABA(A) receptor mRNA levels in the rat cortex. Eur J Pharmacol. (1997) 329:129–32. doi: 10.1016/s0014-2999(97)00168-4
56. Roehrs TA, Randall S, Harris E, Maan R, Roth T. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. (2012) 26:1088–95. doi: 10.1177/0269881111424455
57. Dell’Osso B, Albert U, Atti AR, Carmassi C, Carrà G, Cosci F, et al. Bridging the gap between education and appropriate use of benzodiazepines in psychiatric clinical practice. Neuropsychiatr Dis Treat. (2015) 11:1885–909. doi: 10.2147/NDT.S83130
Keywords: zolpidem, withdrawal symptoms, overdose, chronological feature, bipolar disorder
Citation: Mao Z-x, Yang X, Wang H-y and Guo W-j (2022) Case report: Chronological symptom profile after cessation of overdose zolpidem in a patient with comorbid bipolar disorder—from anxiety, craving, paresthesia and influenza-like symptoms to seizures and hallucinations. Front. Psychiatry 13:962836. doi: 10.3389/fpsyt.2022.962836
Received: 06 June 2022; Accepted: 25 July 2022;
Published: 17 August 2022.
Edited by:
Giovanni Martinotti, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Josipa Vlainic, Rudjer Boskovic Institute, CroatiaDebora Luciani, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2022 Mao, Yang, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan-jun Guo, Z3Vvd2pjbkAxNjMuY29t
 Zi-xin Mao
Zi-xin Mao Xia Yang1
Xia Yang1 Hui-yao Wang
Hui-yao Wang Wan-jun Guo
Wan-jun Guo