
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 13 October 2022
Sec. Psychological Therapy and Psychosomatics
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.959171
 Alessandro Gialluisi1,2*‡
Alessandro Gialluisi1,2*‡ Francesca Bracone2‡
Francesca Bracone2‡ Simona Costanzo2
Simona Costanzo2 Federica Santonastaso1†
Federica Santonastaso1† Augusto Di Castelnuovo3
Augusto Di Castelnuovo3 Sabatino Orlandi2
Sabatino Orlandi2 Sara Magnacca3
Sara Magnacca3 Amalia De Curtis2
Amalia De Curtis2 Chiara Cerletti2
Chiara Cerletti2 Maria Benedetta Donati2
Maria Benedetta Donati2 Giovanni de Gaetano2
Giovanni de Gaetano2 Licia Iacoviello1,2 on behalf of the Moli-sani Study Investigators
Licia Iacoviello1,2 on behalf of the Moli-sani Study InvestigatorsBackground: Major depressive disorder is a mental illness associated with chronic conditions like cardiovascular disease (CVD). Circulating inflammation has been proposed as a potential mechanism underlying this link, although the role of specific biomarkers, gender, and symptom domains is not well elucidated.
Methods: We performed multivariable Cox regressions of first hospitalization/all-cause mortality and CVD, ischemic heart (IHD), and cerebrovascular disease (CeVD) causes vs. depression severity in an Italian population cohort (N = 13,191; age ≥ 35 years; 49.3% men; 4,856 hospitalizations and 471 deaths, median follow-up 7.28 and 8.24 years, respectively). In models adjusted for age, sex, and socioeconomic status, we estimated the proportion of association explained by C-reactive protein (CRP), platelet count, granulocyte-to-lymphocyte ratio (GLR), and white blood cell count (WBC). Gender-by-depression interaction and gender-stratified analyses were performed. Associations of polychoric factors tagging somatic and cognitive symptoms with incident clinical risks were also tested, as well as the proportion explained by a composite index of circulating inflammation (INFLA score).
Results: Significant proportions of the influence of depression on clinical risks were explained by CRP (4.8% on IHD hospitalizations), GLR (11% on all-cause mortality), and WBC (24% on IHD/CeVD hospitalizations). Gender-by-depression interaction was significantly associated only with all-cause mortality (p = 0.03), with moderate depression showing a + 60% increased risk in women, but not in men. Stable associations of somatic, but not of cognitive, symptoms with increased hospitalization risk were observed (+ 16% for all causes, + 14% for CVD causes), with INFLA score explaining small but significant proportions of these associations (2.5% for all causes, 8.6% for IHD causes).
Conclusions: These findings highlight the importance of cellular components of inflammation, gender, and somatic depressive symptoms in the link between depression and clinical (especially CVD) risks, pointing to the existence of additional pathways through which depression may play a detrimental effect on the cardiovascular system.
Depression is a common mental illness with a lifelong prevalence of 4.4% worldwide (1). There are several subtypes, with symptoms ranging in terms of their severity (from mild to severe) and duration (from months to years) (2). Among these, major depressive disorder (MDD) represents the most severe form (3, 4). MDD shows diverse signs and symptoms, like low mood, loss of interest or pleasure in daily activities, change in weight or appetite, insomnia/hypersomnia, psychomotor retardation or agitation, loss of energy or fatigue, worthlessness or guilt, impaired concentration or indecisiveness, and thoughts of death or suicidal ideation/attempt (5). MDD is also associated with a significant reduction in life span, in particular due to the remarkable increase in vulnerability to chronic health conditions like cardiovascular disease (CVD) (6), autoimmune disease (7), and renal disease, as well as diabetes (8), and cancer (9). Moreover, MDD is highly comorbid with chronic medical conditions like inflammatory diseases (10), suggesting that inflammation might contribute to depression development and persistence.
Although the pathogenesis of MDD is yet unclear, depression and inflammation seem strictly intertwined and fueling each other (11). The co-occurrence of these two conditions has been hypothesized on the basis of different comorbidities, through a bidirectional link (12). In other words, depression seems to facilitate inflammatory responses, and inflammation promotes depression, with notable health consequences. Indeed, inflammation characterizes a number of disorders and systemic diseases, including CVD, diabetes, metabolic syndrome, rheumatoid arthritis, asthma, multiple sclerosis, chronic pain, and psoriasis, which, in turn, promote an elevated risk for depression (13, 14). In addition, individuals with chronic stress or mood disorders develop negative health behaviors (e.g., a sedentary lifestyle, poor diet, obesity, poor sleep, and smoking) that can lead to uncontrolled inflammation and depression (15).
Previous studies showed that – beyond specific illness risk – the link between depression and circulating inflammation has been associated with the risk of deaths (16) and hospitalizations (17) even predicting multiple re-hospitalizations (18). Different studies tested the role of inflammation in the relationship between depression and increased risk of mortality/hospitalizations. Hughes et al. (19) reported an association between depressive symptoms and all-cause mortality in an Irish prospective cohort [2,389 men, 54.7 (2.8) years], which was partly explained by increased C-reactive protein (CRP) levels. Likewise, in independent cohorts, inflammation partly mediated the influence of depressive symptoms on cardiovascular mortality (20) and hospitalization risk (21). Kop et al. found that elevated levels of inflammatory cytokines explained a small proportion (only 6.5%) of the incident CVD mortality associated with depression in a healthy cohort of U.S. elders (N = 907; 71.3 years). In line with this, in a longitudinal Australian cohort (N = 1,692; 55–85 years), the inflammatory markers interleukin-6 (IL-6) and CRP explained 10.9 and 8.1% of the risk of incident CVD hospitalizations associated with depression, respectively. Thus, although inflammation might contribute to the influence of depression on incident CVD, this mechanism accounts for a fairly small part of the total effect (21), supporting the idea that other pathways could be also involved in the depression–mortality link (22). Indeed, in a large longitudinal English cohort of older adults (N = 5,328; 52–89 years), clear sex-specific differences have been observed in the interrelationships between depressive symptoms, inflammation, and mortality (22). The authors observed that older men with both depressive symptoms and high levels of inflammation have an increased risk of CVD and all-cause mortality compared with men with depressive symptoms or inflammation alone; in addition, they reported independent influences of depressive symptoms and inflammation on mortality, finding no evidence of either moderation or mediation (22). In women, neither depressive symptoms nor inflammation predicted CVD or all-cause mortality (22).
More recently, in a longitudinal analysis of a large Italian population cohort, our group reported evidence suggesting not only potential mediation but also additive and interactive effects of circulating inflammation in the relationship between depression and increased hospitalizations/mortality risk, for all and specific (CVD) causes (17). In particular, we tested the role of INFLA score, a composite index based on four circulating markers [C-reactive protein (CRP), platelet count (Plt), white blood cell count (WBC), and granulocyte-to-lymphocyte ratio (GLR)] which tap into different components of the inflammatory process (23), allowing to evaluate combined effects of inflammation biomarkers. Still, we did not investigate effect modifications of gender, which would help clarifying the biological and social background of the relationship between depression, inflammation, and clinical risk. Similarly, the weight of somatic/neurovegetative (e.g., fatigue, psychomotor retardation, and altered appetite) and cognitive/psychological symptoms of depression (e.g., low mood, anhedonia, self-estimate, and suicidality) in this link is largely neglected, as well as the role of inflammation in these specific domains. Clarifying these aspects is of utter importance in light of two main findings: first, the association of somatic symptoms with circulating inflammation markers, consistently supported by independent studies (24–30), and second, a potential explanatory role of circulating inflammation in the relationship between the inflammatory potential of diet and the severity of depressive symptoms, which was prominent for somatic but not for cognitive symptoms, as supported by recent evidence (31). Here, we further explored the influence of depression severity on mortality and hospitalization risk for all and CVD causes by (i) analyzing potential effect modifications of gender, (ii) testing the independent influence of somatic and cognitive depressive symptoms on these clinical outcomes, and iii) examining in detail the potential mediation role of the single component biomarkers of INFLA score. The findings reported here allow to further disentangle the relationship between depression, chronic health conditions, and clinical risk in an Italian population cohort.
Analyses were performed using data from the Moli-sani study, a population-based cohort of 24,325 participants aged ≥ 35 years, randomly recruited among residents in the Molise region (central Italy) between 2005 and 2010. Participants with ongoing pregnancy, disturbances in understanding/willing processes, poly-traumas, or coma were excluded (32). The Moli-sani study was approved by the ethical committee of the Catholic University in Rome, Italy, and all the participants provided written informed consent.
For the present work, we selected participants for whom complete questionnaire data on depressive symptoms were available (N = 13,776). We removed participants with unreliable medical/dietary questionnaire data or blood marker levels (sum of leukocyte fractions < 99% or > 101%) or with signs of acute ongoing inflammation (high-sensitivity serum C-reactive protein levels ≥ 20 mg/L, as in (22). After these filters, 13,191 samples were available for subsequent analyses.
Depressive symptoms were assessed through an alternative validated version of the PHQ-9 (17, 33). This scale (hereafter called PHQ9-6) adapted over eight items, due to the unavailability of data on the feeling of failure, but showed comparable accuracy and robustness in the classification of different depression forms (34). Participants were classified into three different classes based on severity of depressive symptoms, namely, no/minimal depression (PHQ9-6 ≤ 4), mild/moderate depression (4 < PHQ9-6 < 15), and severe depression (PHQ9-6 ≥ 15) (34).
To investigate specific domains of depressive symptoms, we derived latent variables tagging somatic (MR1) and cognitive symptoms (MR2), through a polychoric factor analysis, as described in (31). Briefly, using the psych package in R (see URLs), we first computed a polychoric correlation matrix of the available depression items and then derived two polychoric factors applying Oblimin (oblique) rotation. The derived factors – with a Pearson’s r correlation of 0.72 – showed prominent loadings of neurovegetative/somatic and cognitive/affective symptoms, explaining 35.9 and 17.1% of their shared variance (31). Specifically, MR1 showed moderate to high loadings of impaired movements/speaking and concentration (0.8), altered appetite/eating, tiredness/low energy (0.7), and altered sleeping (0.6), while MR2 showed a high loading of anhedonia (1) and moderate loadings of low mood and suicidal ideation (0.4). These factors were tested like the main exposure (i.e., depression severity) in all downstream analyses (see the following text).
First hospital admissions data were obtained from the Molise regional registry of hospital discharge records, updated to 31 December 2015 (35).
A hospitalization was defined as any stay lasting ≥ 24 h in any hospital, clinic, emergency room, or similar. Primary and secondary diagnoses for hospitalization were coded using major diagnostic categories (MDCs) of the Italian Diagnosis-Related Groups classification (version 24) and the International Classification of Diseases, 9th version (ICD-9; see URLs), and the primary diagnosis was elected as a cause of hospitalization accordingly (35). Here, we focused on hospitalization events for all causes, excluding pregnancy complications, childbirth, rehabilitation, chemotherapy, and/or radiotherapy. Also, we analyzed hospitalizations for specific causes, such as cardiovascular disease (CVD, defined as primary diagnosis MDC: 05, or ICD-9: 390–459 and MDC: 01/05), ischemic heart disease (IHD, primary diagnosis ICD-9: 410–414, or surgical procedure with ICD-9: 360–361), and cerebrovascular events (primary diagnosis ICD-9: 430–434, 436–438, or surgical procedure with ICD-9: 381.2).
Overall and cause-specific mortality data till 31 December 2015 were obtained from the Italian mortality (ReNCaM) registry and with the Moli-sani database through unique identifier codes for each participant. Death events were validated by Italian death certificates (ISTAT form) and coded according to the ICD-9. Cardiovascular mortality was defined when the underlying cause of death included ICD-9 codes 390–459, respectively (36). Within cardiovascular mortality, ICD-9 codes 430–438 were used to define deaths due to cerebrovascular disease (i.e., both hemorrhagic and ischemic stroke), and codes 410–414 and 429 for deaths caused by ischemic heart disease (IHD) (36); then these were collapsed into a single mortality cause due to the paucity of events within each class (37). Non-cardiovascular causes of death were included in death events for all causes.
Following the aforementioned criteria, 13,176 participants were finally involved in the analysis of hospitalizations (4,856 all-cause events; median follow-up 7.28 years) and 13,164 subjects in the analysis of deaths (471 all-cause events; median follow-up 8.24 years).
Statistical analyses were performed by SAS/STAT software, version 9.4 of the SAS System for Windows© 2009. SAS Institute Inc., and SAS is a registered trademark of SAS Institute Inc., Cary, NC, USA. All survival analyses were carried out through case-complete multivariable Cox proportional hazards (PH) regressions, incrementally adjusted for (i) sociodemographic covariates (age, educational attainment, and sex, where applicable), (ii) INFLA score, and (iii) lifestyle factors or their proxies (smoking habits, physical activity, body mass index, adherence to Mediterranean diet, and daily energy intake) and for the main chronic disease conditions (diabetes, history of CVD, and cancer) (17). A detailed description of these variables can be found in the Supplementary Methods.
First, we tested gender-by-depression severity interactive associations (in the total sample) and gender-stratified associations of depression severity with incident hospitalization risk and mortality, for all and specific (CVD) causes. Only significant associations detected both in interaction and in gender-stratified analyses were considered robust evidence of effect modification by gender. Then, we tested specific associations of the two polychoric factors tagging somatic and cognitive symptoms with the same outcomes in the total sample, reciprocally adjusting for the factor other than the exposure, in addition to the aforementioned mentioned covariates.
In our previous study, we tested INFLA score as a potential mediator of the association between depression severity and incident all-cause hospitalizations/mortality risk (17). INFLA score represents a global index capturing both serum and cellular circulating inflammation, based on high-sensitivity C-reactive protein serum (CRP) levels, blood platelet count (Plt), white blood cell count (WBC), and granulocyte-to-lymphocyte ratio (GLR) (23). Indeed, CRP is the most commonly used marker to evaluate systemic inflammation in humans because it is relatively stable and easy to measure (38). Increased Plt and WBC have been frequently studied as cellular inflammation indicators at the epidemiological level (39), as well as the neutrophil-to-lymphocyte ratio (NLR) (40). Here, we used the GLR as a proxy measure of the NLR since neutrophils represent the majority of granulocytes (∼95%), and the NLR showed a much higher missing rate than the GLR in our cohort (41).
To investigate circulating inflammation biomarkers that cause the significant mediation effects observed for INFLA score, we applied the %MEDIATE macro (42) to Cox PH models, predicting the incident risk of hospitalizations and mortality of all and CVD causes, testing each single component of INFLA score as a potential mediator. This analysis was carried out in the total sample, with models adjusted for age, sex, and education level as covariates so as to compare with our previous study (17). These analyses were also repeated using Cox PH regressions testing somatic and cognitive symptom factors as exposures, as well as using gender-stratified analyses.
A comparison of the population under study (13,191 participants passing) with the removed participants (N = 11,134) revealed a higher frequency of men (49 vs. 47%) and younger age (mean (SD) 53.2 (10.9) vs. 58.9 (12.4) years, p < 0.0001), as well as a generally higher education level (p < 0.0001, Table 1). The analyzed subset showed a lower prevalence of chronic health conditions like CVD, cancer, diabetes, hypertension, and hyperlipidemia (p < 0.0001). This was largely determined by the availability of psychometric assessment data in less than 14,000 participants and is probably due to the fact that people accepting to take self-administered psychological questionnaires are younger and more educated since these topics are not always easily understandable and are possibly considered less important by elders and less educated subjects (41).
Within the analyzed subset, women (N = 6,693) were younger [52.5 (10.7) vs. 53.8 (11.0) years] and less educated (p < 0.0001) and showed a lower prevalence of chronic health conditions like CVD, hypertension, and hyperlipidemia, and a higher prevalence of depression than men (p < 0.0001; Table 1).
We later report associations from the baseline models (model 1), which showed stable effects across all incremental models tested. Gender-by-depression severity interaction was significantly associated with all-cause mortality (p for interaction 0.03), but not with hospitalization risks (Table 2). This evidence was robust across incremental models tested and supported by gender-stratified analyses, where we observed a significant association of moderate depression with death risk in women (HR [CI95%] = 1.60 [1.15; 2.22]), but not in men (1.02 [0.80; 1.31]; Supplementary Tables 1A, B). Conversely, in men, severe depression was associated with mortality more remarkably than in women, although formal tests of interaction were not statistically significant (Table 2).
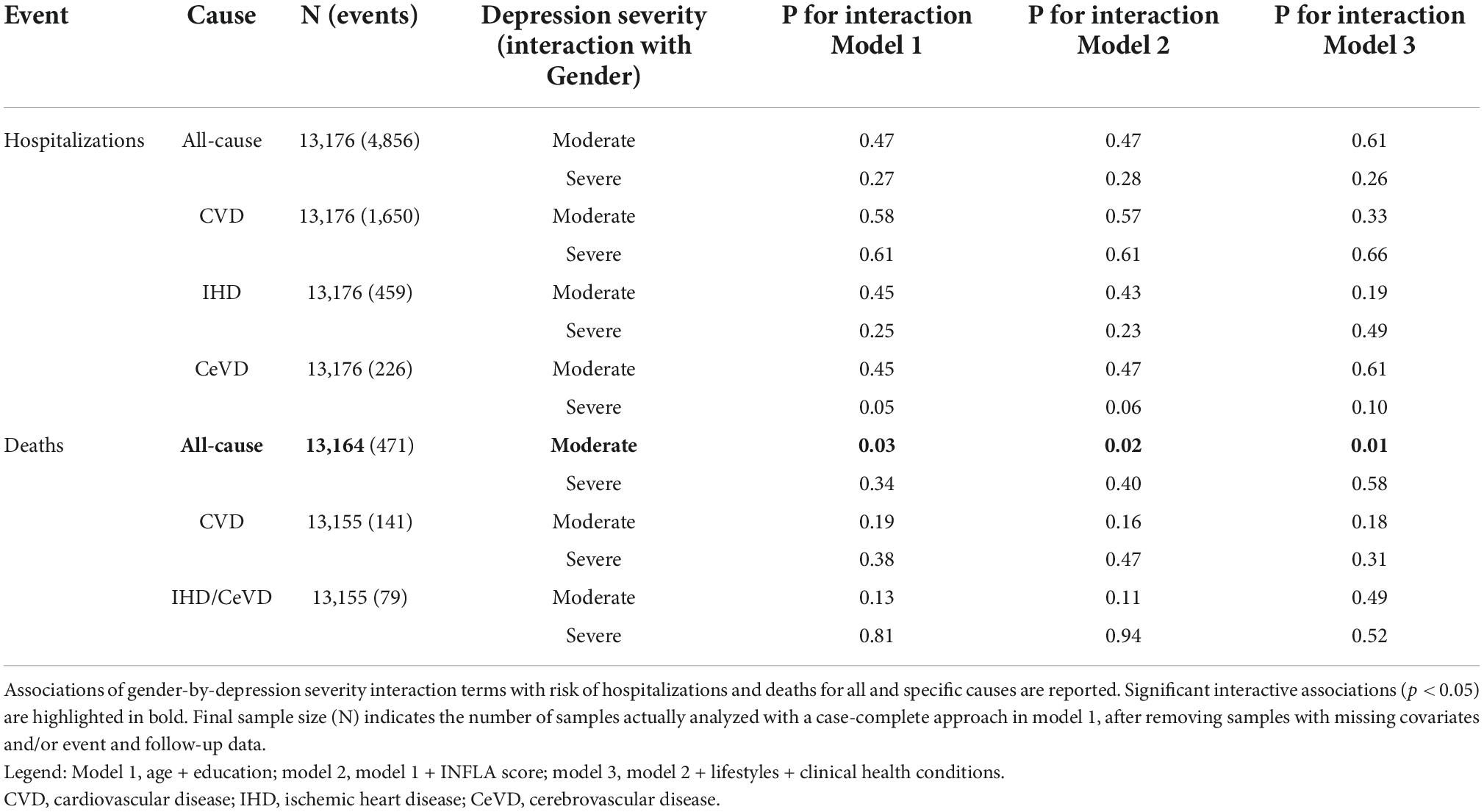
Table 2. Associations of gender-by-depression severity interaction with incident hospitalization risk and mortality.
Similarly, a gender-stratified analysis revealed partly differential associations of depression severity with hospitalization risk (Supplementary Tables 2A, B): while in women, moderate and severe depression were associated with an increase in CVD hospitalization risk (HR = 1.36 [1.16; 1.60] and 2.03 [1.39; 3.00], respectively), and in men, only moderate depression was significantly associated with an increased risk (1.32 [1.15; 1.50]). However, severely depressed men showed a notably increased risk of hospitalization for cerebrovascular disease (6.82 [2.97; 15.70]), which was not observed in women (p for interaction 0.05; Table 2).
Of note, INFLA score was associated with a significant additive increase of hospitalization risk both in women (1%) and men (2%), where the increment was more pronounced for CVD (2%), IHD (3%), and CeVD hospitalizations (6%). While this increment was substantial for mortality in men (ranging between 3% for all-cause and 9% for IHD/cerebrovascular deaths), this was not significant in women (Supplementary Tables 3A, B). No significant interactive effects of depression severity and INFLA score on incident risk of hospitalizations and mortality were observed in both genders (Supplementary Tables 4A, B).
The analysis of polychoric factors tagging somatic (MR1) and cognitive depressive symptoms (MR2) in multivariable models reciprocally adjusting for both factors revealed a significant association of the somatic factor with hospitalizations for all (1.16 [1.11; 1.20]) and CVD causes (1.14 [1.07; 1.21]), while associations with IHD and CeVD events were significant but not stable across all incremental models tested (Table 3A). Conversely, we detected no significant association for any of the factors tested with mortality (Table 3B). When modeled jointly with polychoric factors, INFLA score showed small but significant independent increases in the risk of hospitalizations (1% for all, 2% for CVD, 3% for IHD, and 4% for CeVD causes) and of deaths (3% for all, 6% for CVD, and for IHD/CeVD causes) (Supplementary Tables 5A, B). Again, INFLA score did not reveal any significant interactive influence with both polychoric factors on the clinical outcomes analyzed (Supplementary Table 6).
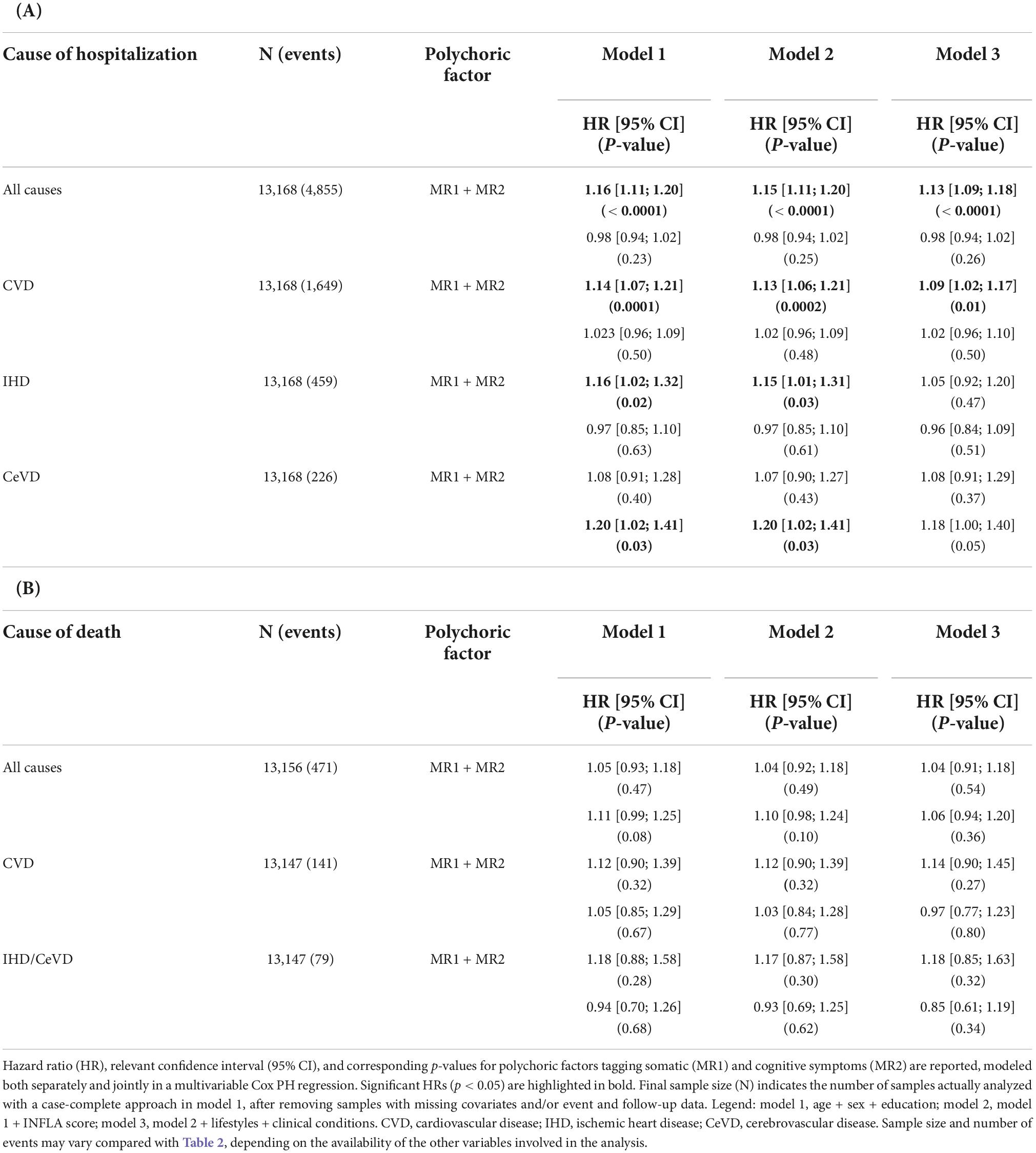
Table 3. Associations of depressive symptom factors with incident (A) hospitalization risk and (B) mortality.
We report here point estimates of the exposure effect on the outcome (PTE) explained by the intermediate variable tested, with relevant p-values, as per %MEDIATE macro applied to minimal models including age, sex, and education as covariates [model 1, as in (17)], unless otherwise stated.
In the association between depression severity and all-cause hospitalizations, a dissection of the significant PTE of INFLA score revealed prominent explanatory effects of the leukocyte components, with WBC and GLR explaining 1.5% (p = 0.02) and 2.0% (p = 3 × 10–3) of the association, respectively. These effects were generally confirmed when we analyzed hospitalizations for CVD causes, and even increased for WBC (Table 4A). The latter explained 2.4% of the influence on all CVD hospitalizations (p = 0.01), increasing up to 5.3% (p = 0.01) and 5.7% (p = 7 × 10–3) for IHD and cerebrovascular events, respectively. Among non-leukocytic components of circulating inflammation, CRP explained 4.6% of the associations between depression severity and IHD events (p = 0.048). As for mortality risk, we detected a significant explanatory role for both WBC (4.8%, p = 0.03) and GLR (11.2%, p = 0.02). Again, PTEs further increased when we analyzed CVD (13.7 and 10.4%) and IHD/CeVD events (23.9 and 16.3%) but remained significant only for WBC (Table 4B).
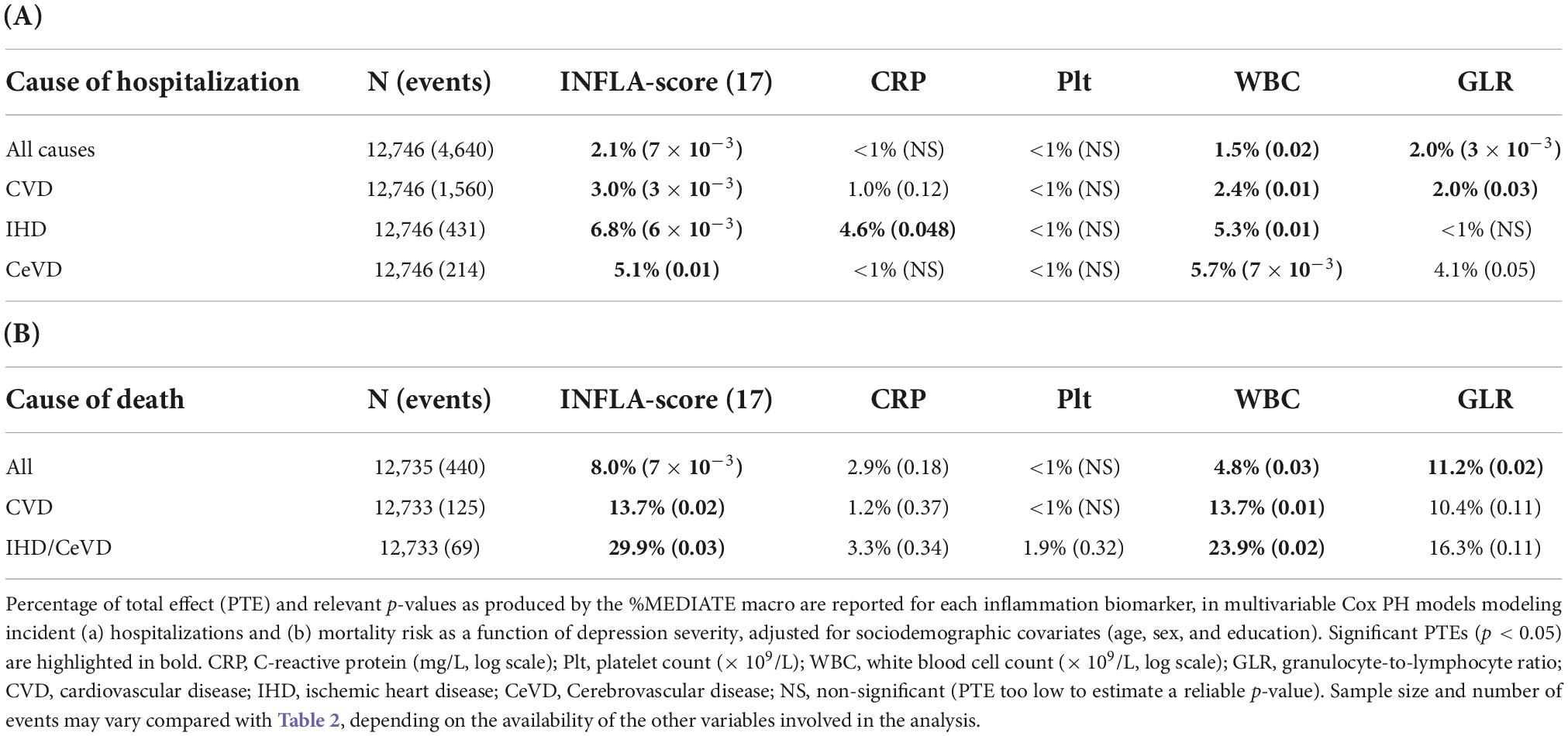
Table 4. Proportion of the association of depression severity with incident (A) hospitalization risk and (B) mortality explained by INFLA score component biomarkers.
Trends similar to those observed in the total sample were observed in the gender-stratified analysis, but only in men, where INFLA score explained 3% of the influence of depression severity on all-cause mortality (p = 0.02), which increased to 5.5% (p = 0.007) for CVD, 11% (p = 0.01) for IHD, and 8.7% (p = 0.006) for CeVD deaths. PTEs were even higher for hospitalizations, namely, 20.5% (p = 0.01) for all-cause mortality and 24.5% (p = 0.01) for CVD events. No significant PTEs were observed for INFLA score in women (Supplementary Tables 7A, B). PTEs of INFLA score in the association of depressive symptom factors with clinical outcomes – adjusting for the other factor not used as exposure – were significant only for the somatic symptom factor (MR1) and for hospitalization risk. INFLA score explained 2.5% (p = 0.006) of the association with all-cause hospitalizations, and 4.1 and 8.6% of the associations with CVD and IHD hospitalizations risk (p < 0.05). Significant PTEs were observed neither for mortality nor for associations with MR2 (Supplementary Tables 8A, B).
In this study, we untangled the potential role of circulating inflammation in the link of depression with mortality and hospitalization risk for all and specific causes, which we demonstrated in a previous study (17). In that study, we reported significant proportions of the influence of depression on clinical risks – ∼30% of coronary heart disease/stroke mortality and ∼3–8% of CVD hospitalizations to be explainable by INFLA score, a composite marker of low-grade inflammation. In the current study, we observed that leukocyte-related components like total counts and granulocyte-to-lymphocyte ratio explained a small but significant proportion of effect on mortality and hospitalization risks, which was especially pronounced for CVD causes. These proportions ranged from 11% (for GLR on all-cause mortality) to 24% (for WBC on IHD/CeVD mortality) and are comparable to (and sometimes higher than) those observed for INFLA score in our previous work (17). Overall, this evidence suggests that cellular components of inflammation may represent a more direct mediation pathway between mental health and clinical risks, potentially explaining at least part of the bidirectional link between mental and cardiovascular health (6).
White blood cell count has been reported to fully mediate the relationship between depressive symptoms and all-cause mortality in a recent Chinese study (N = 4,053; ≥ 60 years) (44), although contrasting evidence links WBC with depression risk. In a patient cohort from the United States [N = 2,400; 54.1 (16.8) years], those with low WBC levels had a greater risk of subsequent hospitalization with depression (45), while in a large study including patients with bipolar disorder from two clinical trials in the United States (N = 765; 18–62 years), those with both high and low WBC levels showed increased symptom severity and specific clusters of symptoms, which differed depending on gender but were most pronounced among men (46).
The neutrophil-to-lymphocyte ratio (NLR), representing the innate immunity component of inflammation (47) and tagged here by GLR, has also been proposed as a useful biomarker to predict cardiovascular risk (47, 48), poor outcomes in cardiovascular diseases, and all-cause mortality (47, 49, 50). Recent studies showed that the NLR is a good indicator of inflammation state and is higher in patients with MDD (51, 52), a finding further corroborated by evidence that the NLR is positively correlated with severity of depression (53). Suicidal depressive patients also have higher NLRs than both non-suicidal patients and healthy controls, suggesting that inflammation may also play a central role in the pathogenesis of suicidal behavior in MDD (54).
Of interest, CRP explained a non-negligible proportion (4.6%) of the influence of depression severity only on IHD hospitalization risk, a finding not that surprising, in light of contrasting results on its involvement in the link between depressive symptoms and all-cause or CVD mortality and hospitalization risk. Indeed, some previous studies detected a small but significant mediating role of cytokine-related inflammatory biomarkers (i.e., CRP and IL-6) in the link between depressive symptoms and all-cause or CVD mortality (19, 20), which was slightly higher for CVD hospitalization outcomes (21, 55). However, Lawes et al. observed no significant mediation effect of inflammation between depression and all-cause/CVD mortality in both genders (22), while Davidson et al. observed that depressive symptoms were associated with an increased risk of incident coronary heart disease (CHD) events, in a way independent of both traditional risk factors and inflammatory biomarkers, particularly CRP levels (56). Overall, these data suggest that classical inflammatory biomarkers only partially explain the association between depression and clinical (in particular cardiovascular) risks, at best, and warrant further research on other inflammatory markers and alternative mediation pathways, which may help filling the gap.
Significant gender-specific differences were observed in the relationship between depression severity and mortality, but not hospitalization risk. Moderately depressed women showed increased all-cause death incidence, while in men, severe depression was associated with mortality of all and specific (IHD/CeVD) causes. This evidence seems concordant with the scarce literature on the topic (22, 57), and may be due to the trend in men not to report milder symptoms or seek treatment until depressive symptoms are more severe (58), which may result in lower power in the comparison between non- and mildly depressed subjects. Similarly, the role that INFLA score played in these associations was more pronounced for men, both as an additive term and as a potential mediator.
The present study also showed that mostly somatic depressive symptoms were associated with hospitalizations for all and CVD causes, in line with previous reports of these symptoms better predicting health status and cardiovascular prognosis in patients with cardiovascular disease (59, 60), although another study showed comparable associations with incident cardiac events between symptom domains (61). Moreover, we observed that a small but significant proportion of the association between the somatic symptom factor and hospitalization risks was explained by INFLA score, while associations with the cognitive factor were not, in line with recent evidence in another large British population cohort (62). This suggests that inflammation may play a minor role in mediating the effects of depression on cardiac events, especially when considering cognitive affective symptoms (63). Again, further research is warranted to clarify the differential associations between somatic and cognitive symptoms, especially in population settings, and substantiate the role of inflammation in these links.
This study presents some strengths. First, to our knowledge, no study so far has investigated cellular inflammatory components of inflammation as possible mediators in the link between depression and all-cause/CVD mortality and hospitalization. Second, the advantage of a large homogeneous population in the targeted analysis on each single component biomarker of INFLA score allowed detecting novel explanatory effects of the link between depression and clinical risks, which were never observed before in the field. Third, the analysis of two factors tagging different depressive domains, such as somatic and cognitive symptoms, and of interactions with gender allowed to further untangle this association, identifying potential gender-specific mechanisms. Although independent studies previously used factor analysis applied to depressive symptoms to analyze their relationship with inflammatory markers and arterial stiffness (e.g., 24, 25, 64), we are not aware of any study using this approach to analyze the link among depression, inflammation, later risk of hospitalization, and death. Therefore, further studies are warranted to substantiate this approach and the reported findings.
Limitations of our study should also be noted. The simultaneous assessment of depressive symptoms and inflammatory markers did not allow us to establish clear directionality in the mediation analysis. The lack of an item in the PHQ-9 assessment represents a further limitation in defining depression severity classes, although this subscale already showed comparable performance (34) and the polychoric factor analysis of depressive symptoms should have minimized the resulting bias. Moreover, owing to the observational design, we cannot fully rule out the potential of residual confounding by unmeasured factors. Data on depressive symptoms are self-reported, and this could lead to misclassification of exposures. In addition, the lack of precise information on medical treatments for some health conditions (e.g., CVD and cancer) makes it difficult to estimate the effect these may have on our findings. Finally, data were measured at baseline only; thus, potential changes occurred over life course might have modified the strength of the findings.
In conclusion, this study suggests a prominent explanatory role of cellular components of inflammation, in particular granulocytes and lymphocytes, in the relationship between depression severity and mortality/hospitalization risk for all and CVD causes. Moreover, it sheds light on the depression–inflammation–cardiovascular disease pathway, highlighting the importance of gender and of somatic depressive symptoms in this link (Figure 1). However, much remains to be done to clarify the relationship between immunity, depression, and its comorbidities like cardiovascular disorders in detail, especially to enlighten their shared genetic and epigenetic underpinnings and the effect of medications for chronic health conditions on this complex interplay. Future studies should be aimed to clarify these aspects and identify gender- and symptom-specific mechanisms.
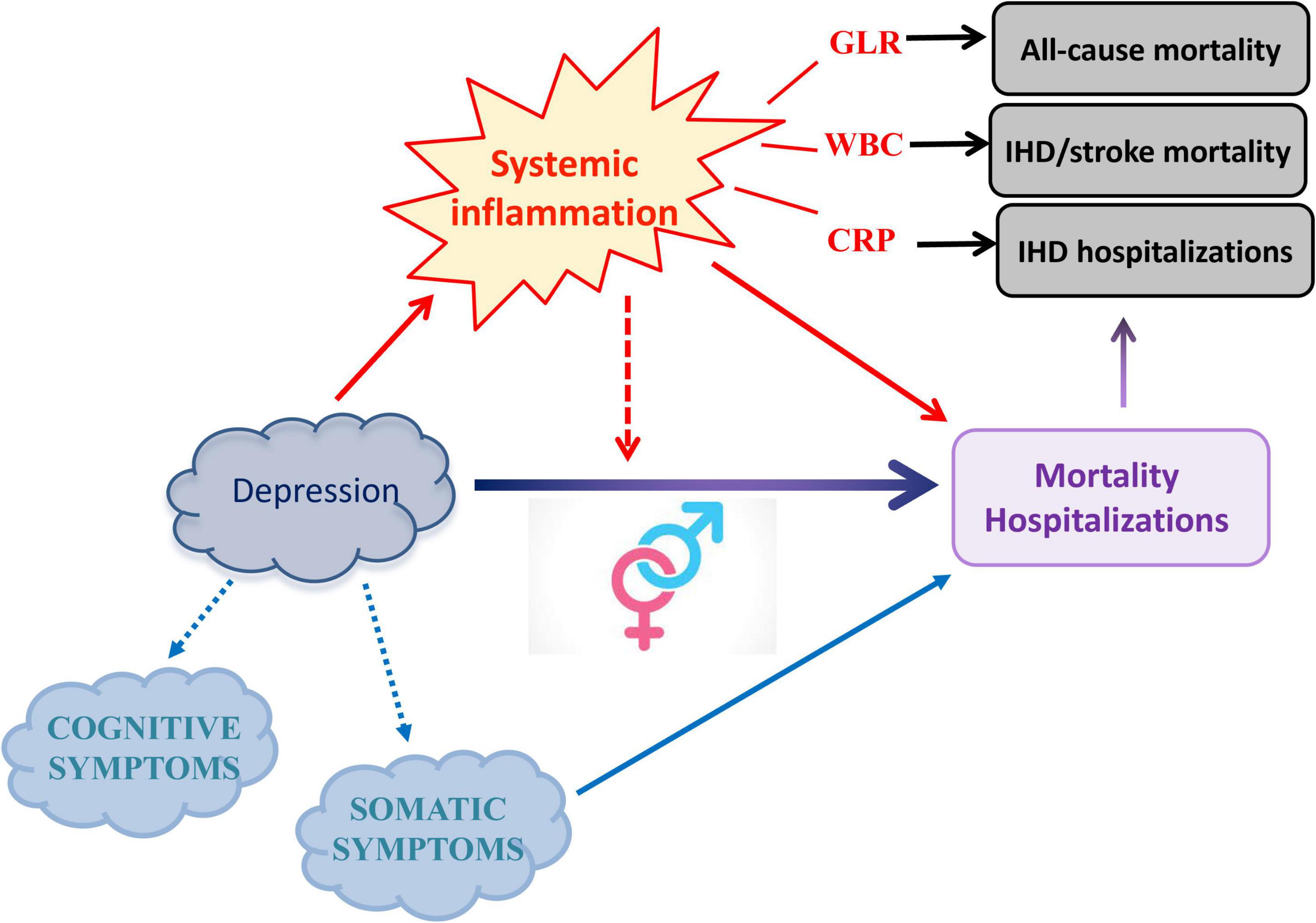
Figure 1. Main hypothesis investigated and evidence observed in the present study. Here, we untangled the role of specific circulating inflammatory markers in the influence of depression severity on mortality and hospitalization risk, further elucidating differential associations within gender strata and with depressive symptom domains. Figure adapted from 31.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://repository.neuromed.it/. Credentials are available upon request to the corresponding author (YWxlc3NhbmRyby5naWFsbHVpc2lAZ21haWwuY29t).
The studies involving human participants were reviewed and approved by the Catholic University of Rome. The patients/participants provided their written informed consent to participate in this study.
AG and LI conceived the study. ADeC and SM carried out biological sample management and measurements. SC and ADi performed the statistical data elaboration and curation in the Moli-sani study. AG, FS, and SO analyzed the data. FB carried out psychometric assessment. AG and FB wrote the first draft of the manuscript, with contributions and critical review from all the co-authors. LI, MD, ADi, CC, and GG originally inspired the Moli-sani study.
The enrollment phase of the Moli-sani study was supported by research grants from the Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy) – Programma Triennale di Ricerca, Decreto no. 1588, and Instrumentation Laboratory, Milan, Italy. This study was partially supported by the Ricerca Corrente – Italian Ministry of Health. Funders had role neither in study design, collection, analysis, and interpretation of data nor in the writing and submission phase of the manuscript.
The Moli-sani research investigators thank Associazione Cuore Sano Onlus (Campobasso, Italy) for its cultural and financial support. We thank Prof. Marco Sarchiapone for inspiring and designing psychometric assessment in the Moli-sani study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.959171/full#supplementary-material
1. World Health Organization. Depression and other common mental disorders: global health estimates. Geneva: World Health Organization (2017).
2. Lux V, Aggen SH, Kendler KS. The DSM-IV definition of severity of major depression: inter-relationship and validity. Psychol Med. (2010) 40:1691–701. doi: 10.1017/S0033291709992066
3. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
4. Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. (2013) 10:e1001547. doi: 10.1371/journal.pmed.1001547
5. American Psychiatric Association. DSM-5 Manuale diagnostico e statistico dei disturbi mentali Cortina R editor. Virginia: American Psychiatric Association (2014). 1168 p.
6. Dhar AK, Barton DA. Depression and the Link with Cardiovascular Disease. Front Psychiatry. (2016) 7:33. doi: 10.3389/fpsyt.2016.00033
7. Benros ME, Waltoft BL, Nordentoft M, Østergaard SD, Eaton WW, Krogh J, et al. Autoimmune diseases and severe infections as risk factors for mood disorders. JAMA Psychiatry. (2013) 70:812. doi: 10.1001/jamapsychiatry.2013.1111
8. Darwish L, Beroncal E, Sison MV, Swardfager W. Depression in people with type 2 diabetes: current perspectives. Diabetes Metab Syndr Obes Targets Ther. (2018) 11:333–43. doi: 10.2147/DMSO.S106797
9. Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev. (2017) 52:58–70. doi: 10.1016/j.ctrv.2016.11.004
10. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. (2015) 172:1075–91. doi: 10.1176/appi.ajp.2015.15020152
11. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
12. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. (2011) 13:467–75. doi: 10.1007/s11920-011-0232-0
13. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. (2014) 140:774–815. doi: 10.1037/a0035302
14. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. (2019) 1437:57–67. doi: 10.1111/nyas.13712
15. Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. (2013) 11:200. doi: 10.1186/1741-7015-11-200
16. Gilman SE, Sucha E, Kingsbury M, Horton NJ, Murphy JM, Colman I. Depression and mortality in a longitudinal study: 1952–2011. Can Med Assoc J. (2017) 189:E1304–10. doi: 10.1503/cmaj.170125
17. Gialluisi A, Costanzo S, Castelnuovo AD, Bonaccio M, Bracone F, Magnacca S, et al. Combined influence of depression severity and low-grade inflammation on incident hospitalization and mortality risk in Italian adults. J Affect Disord. (2021) 279:173–82. doi: 10.1016/j.jad.2020.10.004
18. Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Skala JA, Dávila-Román VG. Depression and multiple rehospitalizations in patients with heart failure. Clin Cardiol. (2016) 39:257–62. doi: 10.1002/clc.22520
19. Hughes MF, Patterson CC, Appleton KM, Blankenberg S, Woodside JV, Donnelly M, et al. The predictive value of depressive symptoms for all-cause mortality: findings from the prime belfast study examining the role of inflammation and cardiovascular risk markers. Psychosom Med. (2016) 78:401–11. doi: 10.1097/PSY.0000000000000289
20. Kop WJ, Stein PK, Tracy RP, Barzilay JI, Schulz R, Gottdiener JS. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med. (2010) 72:626–35. doi: 10.1097/PSY.0b013e3181eadd2b
21. Hiles SA, Baker AL, de Malmanche T, McEvoy M, Boyle M, Attia J. The role of inflammatory markers in explaining the association between depression and cardiovascular hospitalisations. J Behav Med. (2015) 38:609–19. doi: 10.1007/s10865-015-9637-2
22. Lawes S, Demakakos P, Steptoe A, Lewis G, Carvalho LA. Combined influence of depressive symptoms and systemic inflammation on all-cause and cardiovascular mortality: evidence for differential effects by gender in the English Longitudinal Study of Ageing. Psychol Med. (2019) 49:1521–31. doi: 10.1017/S003329171800209X
23. Pounis G, Bonaccio M, Di Castelnuovo A, Costanzo S, De Curtis A, Persichillo M, et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thromb Haemost. (2016) 115:344–52. doi: 10.1160/th15-06-0487
24. Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Drevets WC, Cowen PJ, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. (2019) 214:11–9. doi: 10.1192/bjp.2018.66
25. Chu AL, Stochl J, Lewis G, Zammit S, Jones PB, Khandaker GM. Longitudinal association between inflammatory markers and specific symptoms of depression in a prospective birth cohort. Brain Behav Immun. (2018):74–81. doi: 10.1016/j.bbi.2018.11.007
26. Case SM, Stewart JC. Race/ethnicity moderates the relationship between depressive symptom severity and C-reactive protein: 2005-2010 NHANES data. Brain Behav Immun. (2014) 41:101–8. doi: 10.1016/j.bbi.2014.04.004
27. Duivis HE, Vogelzangs N, Kupper N, De Jonge P, Penninx BWJH. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: Findings from the netherlands study of depression and anxiety (NESDA). Psychoneuroendocrinology. (2013) 38:1573–85. doi: 10.1016/j.psyneuen.2013.01.002
28. Lamers F, Milaneschi Y, De Jonge P, Giltay EJ, Penninx BWJH. Metabolic and inflammatory markers: Associations with individual depressive symptoms. Psychol Med. (2018) 48:1102–10. doi: 10.1017/S0033291717002483
29. White J, Kivimäki M, Jokela M, Batty GD. Association of inflammation with specific symptoms of depression in a general population of older people: The English Longitudinal Study of Ageing. Brain Behav Immun. (2017) 61:27–30. doi: 10.1016/j.bbi.2016.08.012
30. Gialluisi A, Di Castelnuovo A, Bracone F, De Curtis A, Cerletti C, Donati MB, et al. Associations between systemic inflammation and somatic depressive symptoms: Findings from the Moli-sani study. Depress Anxiety. (2020) 37:935–43. doi: 10.1002/da.23070
31. Gialluisi A, Santonastaso F, Bonaccio M, Bracone F, Shivappa N, Hebert JR, et al. Circulating inflammation markers partly explain the link between the dietary inflammatory index and depressive symptoms. J Inflamm Res. (2021) 14:4955–68. doi: 10.2147/JIR.S312925
32. Iacoviello L, Bonanni A, Costanzo S, Curtis AD, Castelnuovo AD, Olivieri M, et al. The moli-sani project, a randomized, prospective cohort study in the molise region in italy; design, rationale and objectives. Ital J Public Health. (2007) 4:110–8.
33. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. (2002) 32:509–15. doi: 10.3928/0048-5713-20020901-06
34. Gialluisi A. Corrigendum to “Combined influence of depression severity and low-grade inflammation on incident hospitalization and mortality risk in Italian adults” (Journal of Affective Disorders, Volume 279, 15 January 2021, Pages 173-182). J Affect Disord. (2021) 292:2021. doi: 10.1016/j.jad.2021.06.031
35. Costanzo S, Mukamal KJ, Di Castelnuovo A, Bonaccio M, Olivieri M, Persichillo M, et al. Alcohol consumption and hospitalization burden in an adult Italian population: prospective results from the Moli-sani study. Addiction. (2019) 114:636–50. doi: 10.1111/add.14490
36. Bonaccio M, Di Castelnuovo A, Costanzo S, Ruggiero E, De Curtis A, Persichillo M, et al. Chili Pepper Consumption and Mortality in Italian Adults. J Am Coll Cardiol. (2019) 74:3139–49. doi: 10.1016/j.jacc.2019.09.068
37. Bonaccio M, Di Castelnuovo A, Costanzo S, Persichillo M, De Curtis A, Cerletti C, et al. Interaction between Mediterranean diet and statins on mortality risk in patients with cardiovascular disease: Findings from the Moli-sani Study. Int J Cardiol. (2019) 276:248–54. doi: 10.1016/j.ijcard.2018.11.117
38. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. (2004) 350:1387–97. doi: 10.1056/NEJMoa032804
39. Bonaccio M, Di Castelnuovo A, De Curtis A, Costanzo S, Persichillo M, Donati MB, et al. Adherence to the Mediterranean diet is associated with lower platelet and leukocyte counts: results from the Moli-sani study. Blood. (2014) 123:3037–44. doi: 10.1182/blood-2013-12-541672
40. Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. (2013) 88:218–30. doi: 10.1016/j.critrevonc.2013.03.010
41. Gialluisi A, Bonaccio M, Di Castelnuovo A, Costanzo S, De Curtis A, Sarchiapone M, et al. Lifestyle and biological factors influence the relationship between mental health and low-grade inflammation. Brain Behav Immun. (2020) 85:4–13. doi: 10.1016/j.bbi.2019.04.041
42. Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro. Boston, MA: Channing Laboratory (2012).
43. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a mediterranean diet and survival in a greek population. N Engl J Med. (2003) 348:2599–608. doi: 10.1056/NEJMoa025039
44. Wu Q, Liu J-H, Ma Q-H, Xu Y, Pan C-W. White blood cell count as a mediator of the relationship between depressive symptoms and all-cause mortality: A community-based cohort study. Arch Gerontol Geriatr. (2021) 94:104343. doi: 10.1016/j.archger.2021.104343
45. Köhler-Forsberg O, He W, Chang Y, Atlas SJ, Meigs JB, Nierenberg AA. White blood cell count at first depression diagnosis as predictor for risk of subsequent hospitalization with depression. Neurol Psychiatry Brain Res. (2017) 1:1–6. doi: 10.1016/j.npbr.2017.10.002
46. Köhler O, Sylvia LG, Bowden CL, Calabrese JR, Thase M, Shelton RC, et al. White blood cell count correlates with mood symptom severity and specific mood symptoms in bipolar disorder. Aust N Z J Psychiatry. (2017) 51:355–65. doi: 10.1177/0004867416644508
47. Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, et al. The relation between atherosclerosis and the neutrophil–lymphocyte ratio. Clin Appl Thromb. (2016) 22:405–11. doi: 10.1177/1076029615569568
48. Küçük E, Kocayiğit İ, Günel C, Düzenli H. Neutrophil-to-lymphocyte ratio in occlusive vascular diseases: the literature review of the past 10 years. World J Emerg Med. (2016) 7:165. doi: 10.5847/wjem.j.1920-8642.2016.03.001
49. Tan TP, Arekapudi A, Metha J, Prasad A, Venkatraghavan L. Neutrophil-lymphocyte ratio as predictor of mortality and morbidity in cardiovascular surgery: a systematic review. ANZ J Surg. (2015) 85:414–9. doi: 10.1111/ans.13036
50. Tokgoz S, Kayrak M, Akpinar Z, Seyithanoğlu A, Güney F, Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. (2013) 22:1169–74. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011
51. Demir S, Atli A, Bulut M, İbiloğlu AO, Güneş M, Kaya MC, et al. Neutrophil – lymphocyte ratio in patients with major depressive disorder undergoing no pharmacological therapy. Neuropsychiatr Dis Treat. (2015) 11:2253–8. doi: 10.2147/NDT.S89470
52. Demircan F, Gözel N, Kılınç F, Ulu R, Atmaca M. The impact of red blood cell distribution width and neutrophil/lymphocyte ratio on the diagnosis of major depressive disorder. Neurol Ther. (2016) 5:27–33. doi: 10.1007/s40120-015-0039-8
53. Aydin Sunbul E, Sunbul M, Yanartas O, Cengiz F, Bozbay M, Sari I, et al. Increased neutrophil/lymphocyte ratio in patients with depression is correlated with the severity of depression and cardiovascular risk factors. Psychiatry Investig. (2016) 13:121. doi: 10.4306/pi.2016.13.1.121
54. Ekinci O, Ekinci A. The connections among suicidal behavior, lipid profile and low-grade inflammation in patients with major depressive disorder: a specific relationship with the neutrophil-to-lymphocyte ratio. Nord J Psychiatry. (2017) 71:574–80. doi: 10.1080/08039488.2017.1363285
55. Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia. The National Heart, Lung, and Blood Institute-Sponsored WISE Study. J Am Coll Cardiol. (2007) 50:2044–50. doi: 10.1016/j.jacc.2007.07.069
56. Davidson KW, Schwartz JE, Kirkland SA, Mostofsky E, Fink D, Guernsey D, et al. Relation of Inflammation to Depression and Incident Coronary Heart Disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. (2009) 103:755–61. doi: 10.1016/j.amjcard.2008.11.035
57. Diniz BS, Reynolds CF, Butters MA, Dew MA, Firmo JOA, Lima-Costa MF, et al. THE effect of gender, age, and symptom severity in late-life depression on the risk of all-cause mortality: the bambuí cohort study of aging. Depress Anxiety. (2014) 31:787–95. doi: 10.1002/da.22226
58. Shi P, Yang A, Zhao Q, Chen Z, Ren X, Dai Q. A hypothesis of gender differences in self-reporting symptom of depression: implications to solve under-diagnosis and under-treatment of depression in males. Front Psychiatry. (2021) 12:589687. doi: 10.3389/fpsyt.2021.589687
59. De Jonge P, Ormel J, Van Den Brink RHS, Van Melle JP, Spijkerman TA, Kuijper A, et al. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry. (2006) 163:138–44. doi: 10.1176/appi.ajp.163.1.138
60. De Miranda Azevedo R, Roest AM, Hoen PW, De Jonge P. Cognitive/affective and somatic/affective symptoms of depression in patients with heart disease and their association with cardiovascular prognosis: A meta-analysis. Psychol Med. (2014) 44:2689–703. doi: 10.1017/S0033291714000063
61. Norton J, Pastore M, Ancelin M, Hotopf M, Tylee A, Mann A, et al. Time-dependent cognitive and somatic symptoms of depression as predictors of new cardiac-related events in at-risk patients?: the UPBEAT-UK cohort. Psychol Med. (2021) 30:1–8. doi: 10.1017/S0033291721000106
62. Piantella S, Dragano N, Marques M, Mcdonald SJ, Wright BJ. Prospective increases in depression symptoms and markers of inflammation increase coronary heart disease risk - The Whitehall II cohort study. J Psychosom Res. (2021) 151:110657. doi: 10.1016/j.jpsychores.2021.110657
63. Carney RM, Freedland KE. Does inflammation mediate the effects of depression on heart disease? That may depend on the symptoms. J Psychosom Res. (2022) 152:110683. doi: 10.1016/j.jpsychores.2021.110683
Keywords: depression, mortality, hospitalizations, inflammation, granulocytes, lymphocytes, cardiovascular disease, C-reactive protein
Citation: Gialluisi A, Bracone F, Costanzo S, Santonastaso F, Di Castelnuovo A, Orlandi S, Magnacca S, De Curtis A, Cerletti C, Donati MB, de Gaetano G and Iacoviello L (2022) Role of leukocytes, gender, and symptom domains in the influence of depression on hospitalization and mortality risk: Findings from the Moli-sani study. Front. Psychiatry 13:959171. doi: 10.3389/fpsyt.2022.959171
Received: 01 June 2022; Accepted: 13 September 2022;
Published: 13 October 2022.
Edited by:
Mario Andres Caro, Yale University, United StatesReviewed by:
Amir Moghadam Ahmadi, Rafsanjan University of Medical Sciences, IranCopyright © 2022 Gialluisi, Bracone, Costanzo, Santonastaso, Di Castelnuovo, Orlandi, Magnacca, De Curtis, Cerletti, Donati, de Gaetano and Iacoviello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Gialluisi, YWxlc3NhbmRyby5naWFsbHVpc2lAZ21haWwuY29t
†Present address: Federica Santonastaso, Human Technopole, Milan, Italy; European School of Molecular Medicine, University of Milan, Milan, Italy
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.