- 1Center for Addictive Disorders, Department of Psychiatry, Psychotherapy, and Psychosomatics, Psychiatric Hospital, University of Zurich, Zurich, Switzerland
- 2Clinic for Adult Psychiatry, University Psychiatric Clinics, University of Basel, Basel, Switzerland
- 3Division of Adult Psychiatry, Department of Psychiatry, Geneva University Hospitals, Geneva, Switzerland
Background and aims: Alcohol Use Disorder (AUD) is characterized by a reduction in goal-directed behavior, with alcohol use taking precedence over other areas of life. These features in AUD resemble negative symptoms in schizophrenia, especially the reduction in motivation and pleasure (MAP). Given the clinical similarities of negative symptoms across diagnostic categories, it comes as a surprise that there are few investigations on negative symptoms in alcohol and other substance use disorders. To our knowledge, our study is the first to assess negative symptoms in AUD based on a two-factorial approach, and to investigate the interrelation of these dimensions with the severity of AUD, and alcohol craving.
Materials and methods: We examined a sample of 42 patients with AUD at the Psychiatric University Hospital in Zurich. Participants provided self-report and interview-based measures of the severity of AUD, negative symptoms, and alcohol craving. Finally, we used data from the electronic health records of the patients.
Results: Patients with AUD show negative symptoms to a similar extent as patients with schizophrenia or bipolar disorder. We found a positive correlation between the extent of impairment within the MAP factor and overall severity of AUD. Furthermore, MAP negative symptoms were correlated with alcohol craving. In a linear regression, negative symptoms predicted alcohol craving whereas depression did not.
Summary: Negative symptoms as conceptualized for schizophrenia are prevalent in patients with AUD and associated with the severity of AUD. More specifically, severity of AUD correlates with diminished motivation and pleasure, highlighting the importance of disturbances in motivational functions in AUD. This is further supported by the correlation between negative symptoms and craving, a hallmark of AUD. Taken together, our findings suggest that negative symptoms might be a highly relevant but hitherto often neglected therapeutic target in AUD.
Introduction
Alcohol is extensively used worldwide (1). Besides its desired acute effects, like euphoria and anxiolysis, excessive alcohol use has negative health consequences. Chronic alcohol use is among the leading causes for premature death and contributes to the global burden of neuropsychiatric and somatic diseases with enormous direct and indirect economic costs (2, 3). An estimated 4.3% of the Swiss population aged over 15 years show a chronic pattern of risky alcohol consumption (4). Lifetime prevalence of alcohol use disorder (AUD) is estimated to be 8.6% (1). Of those suffering from AUD, over 80% do not receive adequate treatment (5). After treatment, relapse is common (6). Monahan and Finney found abstinence rates of only 43% after treatment (7). Even after achieving long-term abstinence, there seems to be an annual relapse rate of 3% (8).
Among the features of AUD are substance craving, and a shift in goal-directed behavior toward the obtainment and use of alcohol (9). The upcoming ICD-11 considers this shift in behavior as one of the three main features that characterize alcohol dependence: “Substance use becomes an increasing priority in life such that its use takes precedence over other interests or enjoyments, daily activities, responsibilities, or health or personal care. Substance use takes an increasingly central role in the person’s life and relegates other areas of life to the periphery…” (10).
During the course of AUD and other substance use disorders (SUDs), substance use progresses from an initially voluntary to a more habitual and finally obsessive-compulsive stage (11). The brain’s reward system is profoundly dysregulated in addictive disorders and plays a key role in the development and maintenance of addiction (12–14). The adaptations affect different neurotransmitter systems including dopamine (15–17), glutamate (18–20), and GABA (21). In animal addiction models, different motivational states within the cycle of substance-seeking are paralleled by distinct oscillations in synaptic strength within the pathway between the prefrontal cortex and the nucleus accumbens (22), two important hubs for reward processing (23). Also in human imaging studies, functioning of those regions have been significantly altered in individuals with SUDs, indicated by increased activity in response to substance-related cues (24–26), which is linked to increased substance craving (27, 28). In contrast, the prefrontal cortex, and the nucleus accumbens show reduced responsiveness toward naturally rewarding cues such as social stimuli and monetary reinforcers (29–31).
In schizophrenia, the symptoms nowadays termed negative symptoms (32) have been considered a hallmark of the disease since Kraepelin and Bleuler (33, 34). As defined by the National Institute of Mental Health Measurement and Treatment Research to Improve Cognition in Schizophrenia (NIMH-MATRICS) Consensus Statement, negative symptoms include the following domains: blunted affect, alogia, asociality, anhedonia, and avolition (35). These domains can be summarized in two factors; the first, “motivation and pleasure” (MAP, sometimes referred to as “apathy”), consists of the domains asociality, anhedonia, and avolition. (36–38). The second factor, “diminished expression” (DIM), includes the domains blunted affect and alogia. The neurobiological basis for deficits in the motivation and pleasure domain is still debated; however, areas involved in reward prediction, like the ventral striatum, may be central (39). Negative symptoms are also present in patients with schizophrenia and comorbid SUD (40–42).
Anhedonia, which is defined as a reduced experience of pleasure is regarded as a core feature of schizophrenia and is also a key symptom of depression and common in various other psychiatric conditions (43). Recent research has shown that patients with schizophrenia, however, often report a normal or even elevated hedonic response to reward (44, 45). Their ability to anticipate pleasure in future reward, on the other hand, is diminished (46, 47). These patients show a social performance rather than a hedonic deficit (37). This has led to a distinction between anticipatory (“wanting”) and consummatory (“liking”) anhedonia (48). Interestingly, this distinction was first conceptualized in SUDs (17).
In SUDs, anhedonia has been regarded as part of the (prolonged) withdrawal symptomatology (49–54), a possible risk factor for relapse (55, 56) and as crucial for treatment outcome (57–59). Nguyen et al. found that anhedonia correlated with relapse rates in AUD (60). In studies in patients with cocaine use disorder, anhedonia had a negative impact on the effectiveness of contingency management treatment (57, 61, 62). A study by Huhn et al. in patients recovering from opioid use disorder showed a reduced activation of the prefrontal cortex for natural reward that in association with the extent of anhedonia (58). Janiri and colleagues found a significant correlation between anhedonia and substance craving (63). Furthermore, an anhedonic trait has been discussed as a risk factor in the development of addiction (64–66). For a systematic review of the literature on anhedonia in substance use disorders, see Garfield et al. (67).
The other two domains that comprise the motivation and pleasure factor of negative symptoms are asociality and avolition (35). Asociality can be defined as a lack of motivation to engage in social interaction. Avolition is a general reduction in the ability to initiate goal-directed behavior. In summary, the factor “motivation and pleasure” describes different aspects of an inability to anticipate and engage in behaviors usually regarded as pleasurable or otherwise rewarding. This factor shows a great degree of similarity with two of the diagnostic criteria of AUD in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (9):
– A great deal of time is spent in activities necessary to obtain alcohol, use alcohol, or recover from its effects.
– Important social, occupational, or recreational activities are given up or reduced because of alcohol use.
The second factor, “diminished expression,” signifies the reduced capability to experience and/or express emotions. The subdimension “blunted affect” refers to the subjective experience and non-verbal expression of emotions, whereas “alogia” means poverty of verbal expression (35).
Whereas anhedonia, in particular trait and consummatory anhedonia, has been studied in populations with SUDs, to our knowledge no study has yet applied a more extended model of negative symptoms to AUD. Considering the similarities between negative symptoms in schizophrenia and some of the clinical features in AUD, it seems plausible to examine whether the full spectrum of negative symptoms—not just consummatory anhedonia—can be found in patients with AUD (68).
In this pilot study, we examined the two-factorial model of negative symptoms in a sample of patients with AUD. We further investigated whether MAP or DIM are specifically related to overall severity of AUD and craving.
Materials and methods
Study setting
This cross-sectional study was conducted at the Psychiatric University Hospital Zurich, Switzerland. Clinical interviews and assessments took place from July 2020 until January 2021.
The studies involving human participants were reviewed and approved by Cantonal Ethics Committee, Zurich, Switzerland. The participants provided their written informed consent to participant in this study.
Sample
Prior to the start of the study, all therapists at the Center for Addictive Disorders and the Center for Integrative Psychiatry at the Psychiatric University Hospital Zurich (Psychiatrische Universitätsklinik Zürich, PUK) were asked to check for eligible patients.
Inclusion criteria for study participation were as follows: diagnosis of AUD regardless of the stage of the disorder (e.g., abstinent, currently addicted, relapsed), age between 18 and 65 years, ability to provide written informed consent and to communicate in German. Exclusion criteria were a current or former diagnosis of schizophrenia or bipolar disorder, a diagnosis of severe neurological disorders or other somatic disorders which would impact the ability to participate. All other comorbidities were allowed.
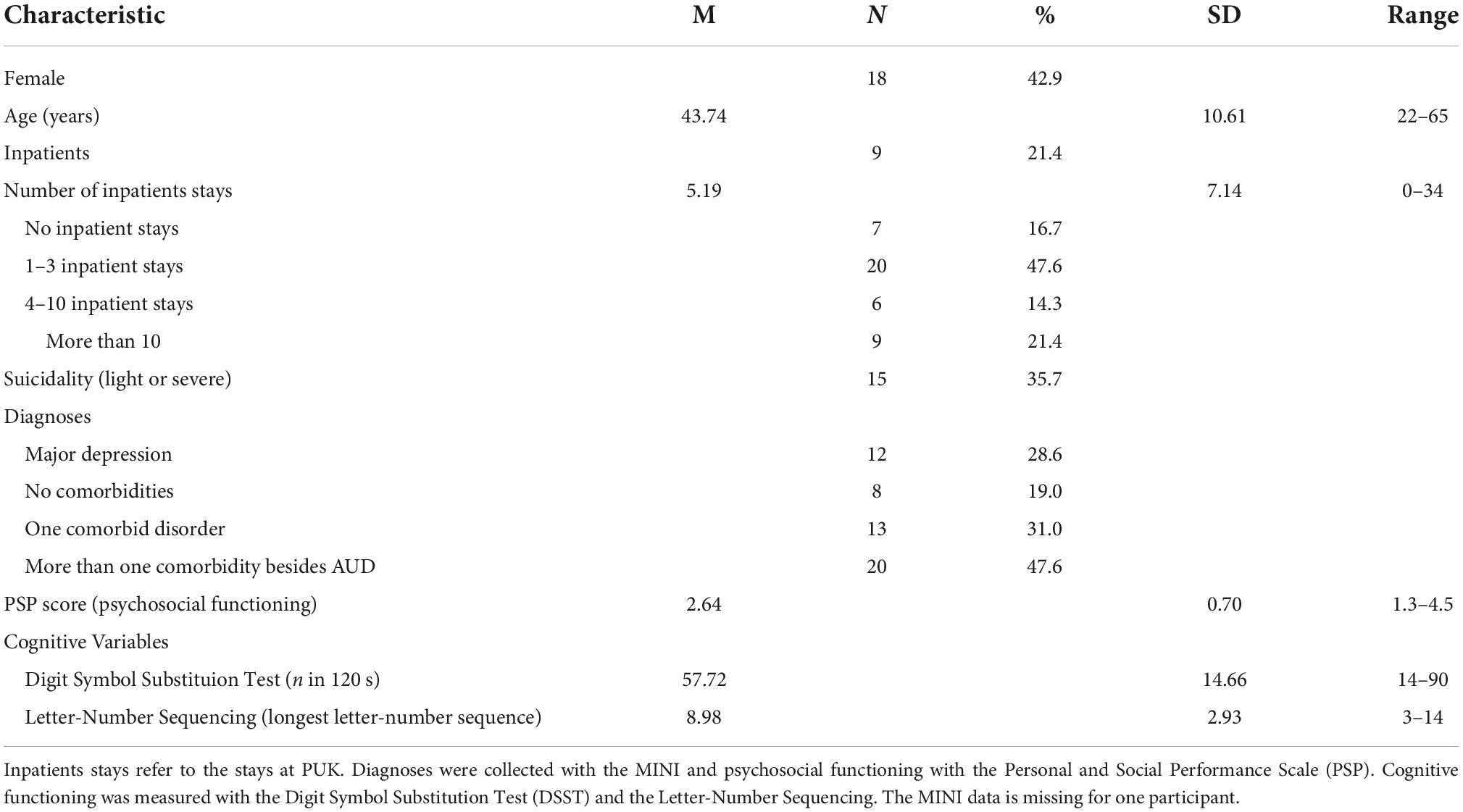
Sixty-three eligible patients were reported to us by their respective therapist, 14 of whom did not respond to our contact via telephone, six refused to participate, and one patient did not meet the inclusion criteria. Therefore, the final sample consisted of 42 patients in total, 33 of whom were outpatients and nine were inpatients. Ten participants have been abstinent from alcohol for a miminum of 30 days prior to their inclusion in the study. For details, see Table 1.
Measures
Clinical interviews and questionnaires
The Mini International Neuropsychiatric Interview (MINI) is a structured diagnostic interview consisting of up to 120 questions that allows for diagnosing axis-I disorders of the DSM-IV as well as suicidality (69). It is a structured, easy to conduct interview requiring only minimal training.
The Center for Epidemiological Studies Depression Scale (CES-D) in its German Version (Allgemeine Depressionsskala, ADS-L) was used to assess depressive symptoms (70, 71). The ADS-L is a 20-item self-report questionnaire, items are rated on a four-point Likert scale from 0 (rarely/not at all) to 4 (most of the time). A score of 23 or higher indicates clinically relevant depressive symptoms.
The Calgary Depression Scale for Schizophrenic Patients (CDSS) was developed to assess depressive symptoms in patients with schizophrenia in distinction from negative and extrapyramidal symptoms (72, 73). It has been validated in patients with major depressive disorder (74) as well as healthy subjects (75). It is a semi-structured interview consisting of nine items. The first eight items are open-ended questions; the interviewer rates participants’ answers on a four-point Likert scale ranging from severe to absent. For the last item, the interviewer rates the extent of depressive symptoms observed during the interview. A cut-off score of six allows for identification of depression in patients with schizophrenia.
Substance use was recorded using a Timeline Followback (TLFB) form. Any alcohol use was defined as drinking alcohol on a minimum of 3 days per week. For the last 7 days, the number of drinking days and the number of alcoholic beverages per drinking day were recorded. Currently abstinent participants were asked to name the number of alcoholic beverages usually consumed in 1 week. Harmful use of alcohol was defined as drinking more than one standard drink per day for women and two standard drinks for men, respectively.
The German Version of the Obsessive Compulsive Drinking Scale (OCDS-G) (76) is a self-assessment scale consisting of 18 questions. It captures cognitive aspects such as preoccupation with alcohol consumption, the amount of alcohol consumed typically, the subjective extent of substance craving, psychosocial impairments following alcohol consumption and the feeling of control over alcohol consumption. It also includes three visual analog scales on which participants rate the extent of craving.
The Brief Negative Symptoms Scale (BNSS) is a semi-structured 13-item interview on six domains, namely the five domains of negative symptoms defined by the NIMH MATRICS Consensus Definition Conference plus lack of normal distress as a sixth domain (77). These domains are assigned to two dimensions, diminished expression (DIM) and motivation and pleasure (MAP). The MAP dimension consists of the three domains, anhedonia, avolition and asociality, whereas the two domains, affective flattening and alogia (poverty of speech), form the DIM dimension. The interviewer asks open-ended questions regarding social and other activities as well as stressful events and rates the extent of impairment on a seven-point Likert scale.
The Self Evaluation of Negative Symptoms (SNS) is a 20-item questionnaire for the self-assessment of the five domains of negative symptoms blunted affect, alogia, social withdrawal, anhedonia, and avolition (78). Each item is rated on a 3-point Likert scale. The sum of all 20 items forms a total score, ranging from 0 to 40 corresponding to the severity of negative symptoms. A score below seven is considered non-pathological.
The Temporal Experience of Pleasure (TEPS) (79) measures anticipatory and consummatory hedonic capacity and consists of 18 items on a 6-point Likert scale. The average of the score of each item forms the total score with higher scores indicating higher hedonic capacity and lower scores indicating higher anhedonia, respectively. It has been tested in a sample with opioid-dependent participants (80).
To assess cognitive functioning, we used the Digit Symbol Substitution Test (DSST) and Letter-Number Sequencing, two subtests of the Wechsler Adult Intelligence Scale(81).
Therapist-rated questionnaires
The Rapid Addiction Profile (RAP) is rated by the therapist on a 4-point scale (82). It covers five dimensions: somatic level, psychiatric level, motivation level, crisis level and resource level. The total score ranges from 0 to 20 points, with higher scores indicating greater severity of AUD.
The Personal and Social Performance Scale (PSP) (83) is rated by the participants’ therapists to assess the level of impairment of social dysfunction during the last 30 days. The scale covers four areas of social functioning, namely socially useful activities such as occupation and study, personal and other social relationships, self-sufficiency, and aggressive or otherwise disturbing behavior. The level of dysfunction in each area is rated on a 6-point Likert scale.
Statistical analysis
According to the MINI interview, three participants did not fully meet diagnostic criteria for AUD. For each case, we contacted their therapist and re-evaluated the results of the MINI interview together with them. For all three patients we could ensure that diagnostic criteria for AUD were, in fact, fulfilled. One participant had been abstinent for approximately 13 months prior to the interview, formally being considered as remitted. Since the patient was still in outpatient treatment for AUD and, at the time of the first contact with the study personnel, still fulfilled the criteria for AUD, they were included in the study. One patient could only partially conduct the interview; his missing data was imputed by median scores.
First, the alcohol and substance use patterns and craving of abstinent versus and consuming participants were compared by either unpaired t-tests for continuous data or Mann–Whitney-U-tests or χ2 statistics for discrete data, where appropriate.
We used Kendall’s Tau b to assess correlations between negative symptoms scores and RAP and craving scores, respectively.
We compared our study sample in terms of negative symptomatology with two other subsamples consisting of patients with schizophrenia and bipolar disorder from a study by Kirschner et al. This sample is described in detail elsewhere (84). To test whether the study sample differed from patients with either schizophrenia or bipolar disorder on negative symptoms of interest, we performed oneway analysis of variance with disorder group as dependent variable and age, duration of disease, BNSS MAP, BNSS DIM and BNSS total scores as independent variables.
Finally, multiple linear regression was used to test whether depressive symptoms (CDSS total score), negative symptoms (BNSS MAP and DIM factor subscores), and alcohol drinking during the last 30 days were associated with the extent of craving (OCDS score). The conditions of linear independence, normal distribution of the dependent variable and residuals, homoscedasticity, and absence of multicollinearity (i.e., variance inflation factors all < 1.96) were met. As a goodness-of-fit measure for the model we used the adjusted R2 as it provides the percentage of variation explained by only the independent variables that actually affect the dependent variable.
All statistical analyses were conducted using SPSS Statistics Version 27. Given the exploratory nature of this pilot study we did not control for multiple comparisons and set the level of significance at p < 0.05 for all calculations.
Results
Demographics and sample characteristics
In total, 42 patients were included in the study and completed the clinical interviews. Detailed demographic characteristics of the sample are shown in Table 1. Over one third (n = 16) of the participants reached cut-off values for significant clinical depressive symptoms in the ADS-L and CDSS. Only eight participants had no psychiatric comorbidities, whereas almost half of all participants had more than one comorbid psychiatric disorder. Most patients (n = 35) had been formerly hospitalized more than once.
In detail, according to the MINI interview, the following comorbidities occurred within our sample: MDD: n = 12, dysthymia: n = 10, panic disorder: n = 10, agoraphobia: n = 9, social phobia = 7, generalized anxiety disorder: n = 10, obsessive-compulsive disorder (OCD): n = 1, posttraumatic stress disorder (PTSD): n = 9, bulimia nervosa: n = 3, antisocial personality disorder: n = 5. Consistent with the exclusion criteria, there were no patients with psychotic or bipolar disorder in our sample.
According to the MINI, more than one third (n = 15) of the patients fulfilled criteria for an additional substance use disorder, with cannabis use disorder being the most common (n = 8). sedative, hypontics and anxiolytic use disorder (n = 4), cocaine use disorder (n = 2), stimulant use disorder (n = 1), and opioid use disorder (n = 1) were also present.
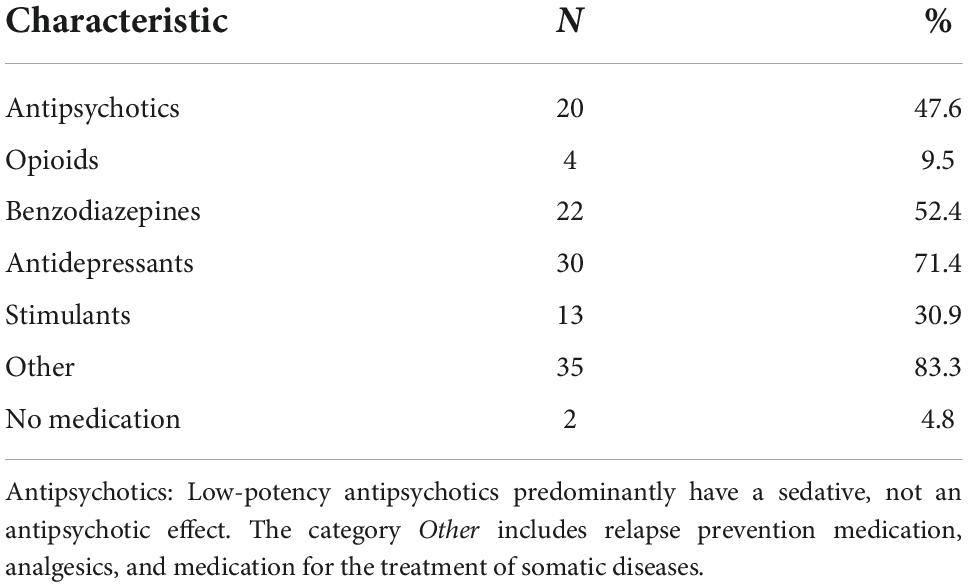
Table 2 displays the current psychopharmacological medication of the study participants. There were only two participants in the sample who did not report any intake of psychopharmacological medication. Almost half of the study population (n = 20) had been prescribed antipsychotics; antidepressants (n = 30) and benzodiazepines (n = 22) had been prescribed to more than half of the participants. Stimulants were also prevalent in the sample (n = 13).
Alcohol use
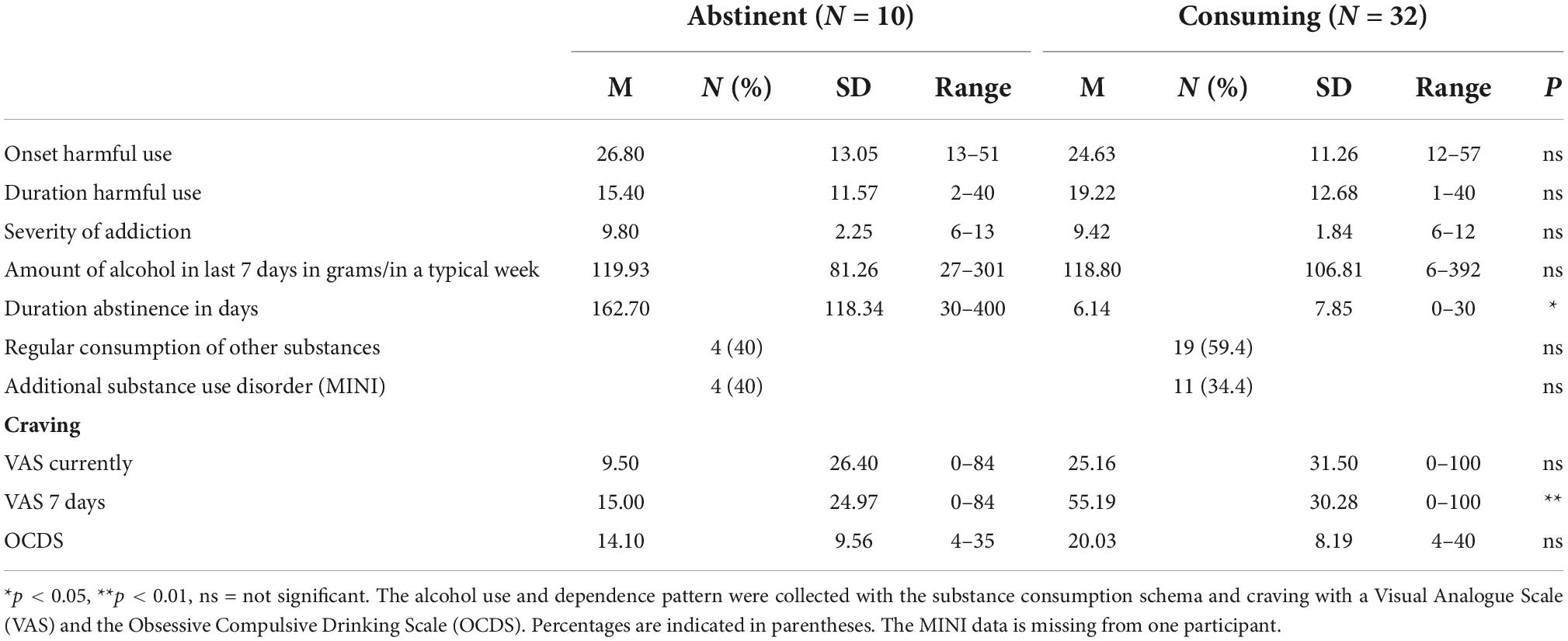
The pattern of alcohol use within the sample is shown in Table 3. Of 42 participants, 38 met diagnostic criteria for AUD within the past 12 months before inclusion in the study. Two participants had shown a harmful alcohol use within the past 30 days but did not meet the diagnostic criteria of current alcohol dependence. One had been abstinent for 13 months and was thus regarded as fully remitted (9). One participant did not answer questions concerning alcohol use.
Out of all participants, 10 had been abstinent from alcohol use for at least 30 days (30–400 days). Apart from the duration of abstinence, these participants did not differ significantly from the actively consuming group regarding their alcohol and substance use patterns and craving, respectively.
Group comparison with patients with schizophrenia and bipolar disorder
Using oneway ANOVA we compared our sample with two subsamples from another study population consisting of patients either with schizophrenia or with bipolar disorder. The three groups differed significantly in regards of age and duration of disease. With respect to the extent of negative symptoms, the ANOVA revealed no significant between-group differences for BNSS total scores as well as BNSS MAP and DIM scores (BNSS total: (F(2, 91) = 1.55, p = 0.219, BNSS MAP: F(2, 91) = 0.26, p = 0.773, BNSS DIM: F(2, 91) = 2.66, p = 0.075).
Negative symptoms and severity of alcohol use disorder
The BNSS total score was significantly correlated with the RAP score (τb = 0.228, p = 0.043, 95% CI [0.022, 0.416]). On the level of negative symptoms factors, we found a significant correlation between the BNSS MAP subscore and the RAP total score (τb = 0.223, p = 0.049, 95% CI [0.016, 0.411]). The DIM factor subscore of the BNSS, in contrast, did not significantly correlate with the RAP score (τb = 0.205, p = 0.076, 95% CI [-0.002, 0.395]). For details see Table 4.
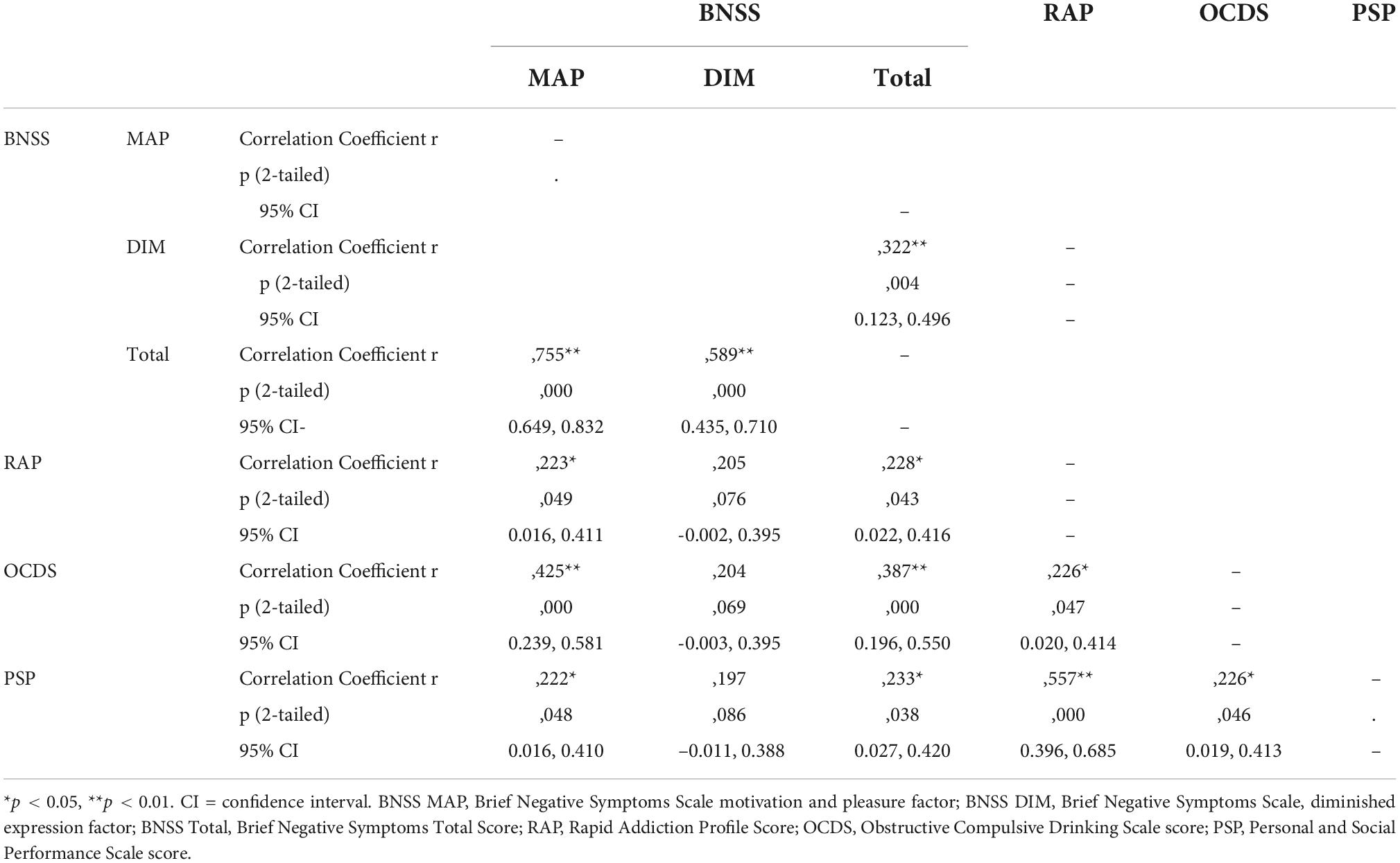
Table 4. Correlations between BNSS MAP, BNSS DIM, BNSS Total scores, and severity of AUD (RAP), craving (OCDS), and social functioning (PSP).
The SNS total score did not show a significant correlation with the RAP score (τb = 0.201, p = 0.076, 95% CI [−0.007, 0.392]).
TEPS scores (total score, as well as subscores for anticipatory and consummatory anhedonia) and the CDSS total score were also not correlated with the RAP score (data not shown).
Negative symptoms and craving
Non-parametric correlations
The total score of the BNSS scale was positively correlated with the OCDS total score as a measure of craving (τb = 0.387, p < 0.001, 95% CI [0.196, 0.550]). This was also the case for the MAP factor subscore of the BNSS (τb = 0.425, p < 0.001, [0.239, 0.581]). However, the DIM factor subscore did not show a significant correlation with the OCDS total score (τb = 0.204, p = 0.069, [−0.003, 0.395]). All data are provided in Table 4.
The SNS total score as a self-report measure for negative symptoms was significantly correlated with the OCDS total score (τb = 0.275, p = 0.013, 95% CI [0.072, 0.456]). TEPS scores (subscores for consummatory and anticipatory anhedonia as well as the total score), in contrast, did not show a significant correlation with the OCDS score (data not shown).
Depressive symptoms as measured with the CDSS total score were significantly correlated with the OCDS total score (τb = 0.387, p < 0.001, 95% CI [0.195, 0.550]).
Multiple regression analyses
The results obtained from the regression analysis are shown in Table 5. Multiple linear regression was used to test whether depressive symptoms. negative symptoms (MAP and DIM factor) and alcohol drinking during the last 30 days were associated with the extent of craving as measured by the OCDS. The overall regression model was significant [F(4, 37) = 9.003; < 0.001] and explained 44% of alcohol craving. with the BNSS MAP factor (β = 0.452; t = 2.78; p = 0.008) and number of drinking days in the last 30 days [(β = 0.233; t = 2.03; p = 0.049)] being significant predictors of craving. The CDSS score and the BNSS DIM factor subscore were not significantly associated with the OCDS score.
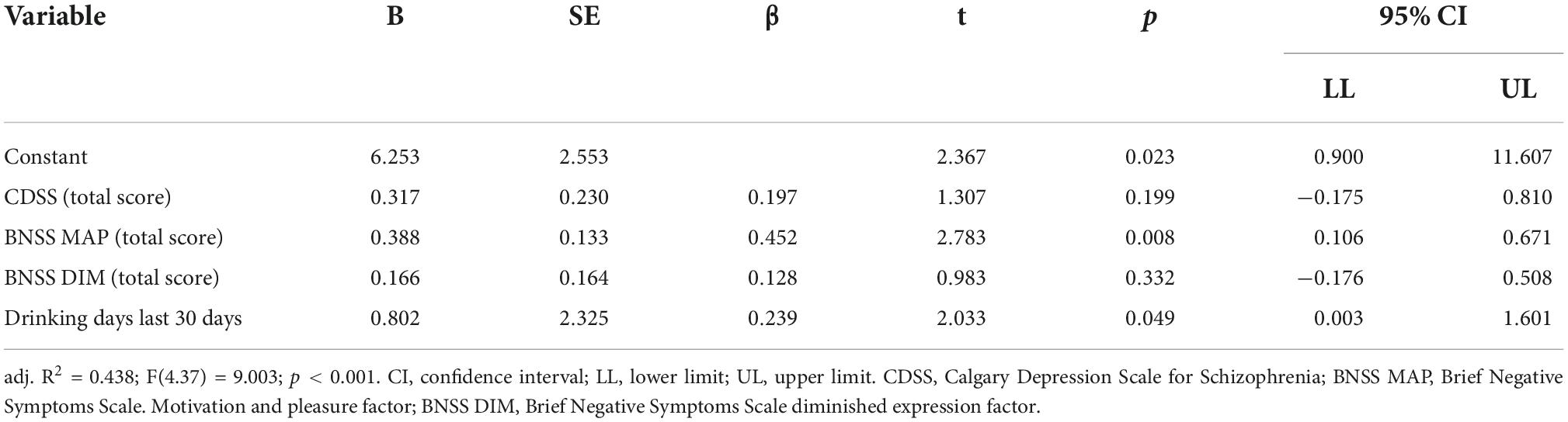
Table 5. Multiple linear regression with OCDS total score as the dependent variable and CDSS, BNSS MAP, BNSS DIM, and drinking days last 30 days as independent variables (N = 42).
Alcohol use pattern and negative symptoms
The duration of lifetime harmful alcohol consumption as assessed via TLFB did not correlate with the BNSS total score (τb = 0.040, p = 0.712, 95% CI [−0.168, 0.245]), the BNSS MAP score (τb = 0.162, p = 0.139, 95% CI [−0.047, 0.357]), or the BNSS DIM score (τb = −0.084, p = 0.450, 95% CI [−0.286, 0.125]). The amount of alcohol consumed during the last week was also not correlated with neither the BNSS total score (τb = 0.035, p = 0.745, 95% CI [−0.173, 0.241]), the BNSS MAP score (τb = 0.024, p = 0.828, 95% CI [the BNSS MAP score 0.184, 0.230]), nor the BNSS DIM score (τb = 0.080, p = 0.471, 95% CI [−0.129, 0.282]).
Social performance and negative symptoms
There was a significant correlation between the PSP total score and the BNSS total (τb = 0.233, p = 0.038, 95% CI [0.016, 0.410]), as well as the BNSS MAP score (τb = 0.226, p = 0.048, 95% CI [0.027, 0.420). The BNSS DIM score, in contrast, was not significantly correlated with the PSP total score (τb = 0.197, p = 0.086, 95% CI [−0.011, 0.388]). SNS, TEPS, and CDSS scores were not correlated with the PSP score (data not shown).
Discussion
To our knowledge, this pilot study is the first to apply the two-factor model of negative symptoms of schizophrenia to a sample of patients with AUD.
In comparison with two samples (84) of patients with either schizophrenia or bipolar disorder, our study sample of patients with AUD showed no significant difference in the extent of negative symptoms. This finding suggests that negative symptoms that have been established as a key element of psychotic disorders are also prominent in AUD. Furthermore, there was a positive correlation between the severity of AUD as measured with the therapist-rated RAP and both the total score and the MAP factor score of the BNSS. A possible explanation is that chronic elevated alcohol use leads to changes within neural circuits that are involved in motivation and reward similar to changes that occur in schizophrenia. Diminished expression, in contrast, was not correlated to the severity of AUD.
The BNSS total score as well as the MAP factor subscore showed a significant correlation with self-reported extent of alcohol craving. While other studies have already established a correlation between anhedonia and craving (51, 67), our findings suggest that a dysfunction in a somewhat broader motivational process may be a driving factor for craving. This finding was further supported by a multiple regression analysis comparing negative and depressive symptoms in their effect on the severity of AUD, which showed that 44% of the variation of the extent of craving within our population could be explained by the BNSS MAP score and the drinking days in the past 30 days. In contrast, the DIM factor subscore was not associated with the extent of craving.
These results support the hypothesis that during the course of AUD adaptations occur withing the neural pathways involved in motivation and reward. The fact that negative symptoms within the DIM domain were not correlated with craving is in line with this interpretation.
In our sample, there was no correlation between lifetime duration of harmful alcohol use as well as the total amount of alcohol consumed, and the extent of negative symptoms. This is probably due to the small sample size. However, factors other than substance use, e.g., comorbidity or psychosocial stressors, could theoretically be responsible for the development of negative symptoms in our participants.
The findings of this pilot study are exploratory in nature and have to be replicated in other samples. If reproduced, the association of negative symptoms with severity of AUD as well as the extent of alcohol craving within these patients may have therapeutic implications. In contrast to negative symptoms in schizophrenia which are often difficult to treat (85), there is growing evidence that motivational deficits in patients with AUD can be addressed therapeutically. In a study by Kirschner et al., for example, patients with cocaine use disorder successfully activated their reward system with mental imagery and real-time fMRI neurofeedback (86). In a pilot study, Pettoruso et al. successfully treated patients with cocaine use disorder with repetitive transcranial magnetic stimulation to improve anhedonia (87). Interestingly, the NMDA receptor antagonist ketamine which has shown remarkable preliminary results in the treatment of SUDs (88, 89), also seems to have significant anti-anhedonic effects (90, 91).
The present findings should be handled cautiously. Substantial study limitations include non-random sampling, a small sample size, the absence of a comparison group, and non-adjustments for multiple testing. Notably, the majority of patients within our sample had at least one psychiatric comorbidity limiting the internal validity of our study since it cannot be ruled out that these comorbid disorders contribute to the extent of negative symptoms. However, our naturalistic sample increases the external validity since psychiatric comorbidities are the rule rather than the exception in AUD and other SUDs (92, 93). We examined the correlation of MDD and severity of AUD and craving, respectively as MDD is among the most common comorbid disorders of AUD (94) and shares anhedonia as a common feature with negative symptoms. Anxiety disorders and antisocial personality disorder were also frequent in our sample. To our knowledge, there are no studies examining the occurrence of negative symptoms in these disorders but of course our study cannot rule out a possible impact of these comorbidities on negative symptoms.
Posttraumatic stress disorder was present in nine patients. Adverse childhood events have been linked to AUD (95, 96) as well as positive and negative symptoms in schizophrenia (97). Future research should investigate the nature of a possible interrelation between PTSD and negative symptoms in AUD. We further used a cross-sectional approach and did not assess important parameters, such as an objective measure of alcohol intake, a valid measure for the severity of AUD. Furthermore, the RAP that we used to assess overall severity of AUD, which is a reliable clinical tool, has not yet been validated in other studies.
Taken together, however, our findings provide first evidence that negative symptoms are prevalent in AUD to an extent that does not differ significantly from other major psychiatric disorders, are correlated with disease severity and craving, and therefore might constitute a novel and promising therapeutic target that should be addressed in future clinical trials to improvement treatment outcomes for patients with AUD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Cantonal Ethics Committee, Zurich, Switzerland. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MB, KD, and MH designed the study with assistance from MK and SK. GF and JH performed all the clinical interviews and collected all therapist-rated measurements. MK instructed GF and JH in the assessment of all negative symptom scales. GF, JH, KD, and MB analyzed the data. MB wrote the manuscript with input from all authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Glantz MD, Bharat C, Degenhardt L, Sampson NA, Scott KM, Lim CCW, et al. The epidemiology of alcohol use disorders cross-nationally: findings from the World Mental Health Surveys. Addict Behav. (2020) 102:106128. doi: 10.1016/J.ADDBEH.2019.106128
2. Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. (2009) 373:2223–33. doi: 10.1016/S0140-6736(09)60746-7
3. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. (2013) 382:1575–86. doi: 10.1016/S0140-6736(13)61611-6
4. Gmel G, Kuendig H, Notari L, Gmel C. Suchtmonitoring schweiz – konsum von alkohol, tabak und illegalen drogen in der schweiz im jahr 2016. Lausanne. (2017).
5. Mekonen T, Chan GCK, Connor J, Hall W, Hides L, Leung J. Treatment rates for alcohol use disorders: a systematic review and meta-analysis. Addiction. (2021) 116:2617–34. doi: 10.1111/add.15357
6. Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. (1971) 27:455–6. doi: 10.1002/1097-4679(197110)27:43.0.CO;2-R
7. Monahan SC, Finney JW. Explaining abstinence rates following treatment for alcohol abuse: a quantitative synthesis of patient, research design and treatment effects. Addiction. (1996) 91:787–805. doi: 10.1046/j.1360-0443.1996.9167876.x
8. Jin H, Rourke SB, Patterson TL, Taylor MJ, Grant I. Predictors of relapse in long-term abstinent alcoholics. J Stud Alcohol. (1998) 59:640–6. doi: 10.15288/JSA.1998.59.640
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
10. Saunders JB, Degenhardt L, Reed GM, Poznyak V. Alcohol use disorders in ICD-11: past, present, and future. Alcohol Clin Exp Res. (2019) 43:1617–31. doi: 10.1111/acer.14128
11. Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. (2009) 56:18–31. doi: 10.1016/J.NEUROPHARM.2008.07.043
12. Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. (2013) 4:72. doi: 10.3389/fpsyt.2013.00072
13. Luijten M, Schellekens AF, Kühn S, MacHielse MWJ, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. (2017) 74:387–98. doi: 10.1001/jamapsychiatry.2016.3084
14. Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev. (2019) 99:2115–40. doi: 10.1152/physrev.00014.2018
15. Martin-Soelch C, Chevalley AF, Künig G, Missimer J, Magyar S, Mino A, et al. Changes in reward-induced brain activation in opiate addicts. Eur J Neurosci. (2001) 14:1360–8. doi: 10.1046/J.0953-816X.2001.01753.X
16. Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. (2005) 63:101–54. doi: 10.1016/S0074-7742(05)63005-X
17. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. (1993) 18:247–91. doi: 10.1016/0165-0173(93)90013-P
18. Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. (2009) 10:561–72. doi: 10.1038/nrn2515
19. Engeli EJE, Zoelch N, Hock A, Nordt C, Hulka LM, Kirschner M, et al. Impaired glutamate homeostasis in the nucleus accumbens in human cocaine addiction. Mol Psychiatry. (2020) 26:5277–85. doi: 10.1038/s41380-020-0828-z
20. Steinegger CA, Zoelch N, Hock A, Henning A, Engeli EJE, Seifritz E, et al. Neurometabolic alterations in the nucleus accumbens of smokers assessed with 1H magnetic resonance spectroscopy: the role of glutamate and neuroinflammation. Addict Biol. (2021) 26:e13027. doi: 10.1111/ADB.13027
21. Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, et al. A molecular mechanism for choosing alcohol over an alternative reward. Science. (2018) 360:1321–6. doi: 10.1126/science.aao1157
22. Namba MD, Tomek SE, Olive MF, Beckmann JS, Gipson CD. The winding road to relapse: forging a new understanding of cue-induced reinstatement models and their associated neural mechanisms. Front Behav Neurosci. (2018) 12:17. doi: 10.3389/fnbeh.2018.00017
23. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. (2010) 35:4–26. doi: 10.1038/npp.2009.129
24. Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, Thomas Ross MJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. (2000) 157:1789–98. doi: 10.1176/appi.ajp.157.11.1789
25. Potenza MN, Hong KA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. (2012) 169:406–14. doi: 10.1176/appi.ajp.2011.11020289
26. Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN, et al. Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biol Psychiatry. (2013) 74:529–37. doi: 10.1016/j.biopsych.2013.04.029
27. Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. (2002) 159:1642–52. doi: 10.1176/appi.ajp.159.10.1642
28. Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. (2001) 58:334–41.
29. Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, et al. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend. (2007) 87:233–40. doi: 10.1016/j.drugalcdep.2006.08.022
30. Preller KH, Herdener M, Schilbach L, Stämpfli P, Hulka LM, Vonmoos M, et al. Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proc Natl Acad Sci U.S.A. (2014) 111:2842–7. doi: 10.1073/pnas.1317090111
31. Tobler PN, Preller KH, Campbell-Meiklejohn DK, Kirschner M, Kraehenmann R, Stämpfli P, et al. Shared neural basis of social and non-social reward deficits in chronic cocaine users. Soc Cogn Affect Neurosci. (2016) 11:1017–25. doi: 10.1093/scan/nsw030
32. Andreasen NC. Negative symptoms in schizophrenia. Arch Gen Psychiatry. (1982) 39:784. doi: 10.1001/archpsyc.1982.04290070020005
33. Kraepelin E. Dementia praecox. In: Cutting J, Shepherd M editors. The Clinical Roots of the Schizophrenia Concept: Translations of Seminal European Contributions on Schizophrenia (Reprinted from “Psychiatrie (5th ed.),” Leipzig: Barth, 426–41). Cambridge: Cambridge University Press (1987).
34. Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press (1950).
35. Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. (2006) 32:214–9. doi: 10.1093/SCHBUL/SBJ053
36. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. (2006) 32:238–45. doi: 10.1093/schbul/sbj013
37. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. (2010) 36:359–69. doi: 10.1093/schbul/sbn094
38. Kaiser S, Heekeren K, Simon JJ. The negative symptoms of schizophrenia: category or continuum? Psychopathology. (2011) 44:345–53. doi: 10.1159/000325912
39. Galderisi S, Merlotti E, Mucci A. Neurobiological background of negative symptoms. Eur Arch Psychiatry Clin Neurosci. (2015) 265:543–58. doi: 10.1007/s00406-015-0590-4
40. Batki SL, Leontieva L, Dimmock JA, Ploutz-Snyder R. Negative symptoms are associated with less alcohol use, craving, and “high” in alcohol dependent patients with schizophrenia. Schizophr Res. (2008) 105:201–7. doi: 10.1016/j.schres.2008.06.020
41. Tan JH, Shahwan S, Satghare P, Cetty L, Verma S, Sendren JR, et al. Binge drinking: prevalence, correlates, and expectancies of alcohol use among individuals with first-episode psychosis. Early Interv Psychiatry. (2019) 13:1136–45. doi: 10.1111/EIP.12744
42. Large M, Mullin K, Gupta P, Harris A, Nielssen O. Systematic meta-analysis of outcomes associated with psychosis and co-morbid substance use. Aust N Z J Psychiatry. (2014) 48:418–32. doi: 10.1177/0004867414525838
43. Leventhal AM, Unger JB, Audrain-McGovern J, Sussman S, Volk HE, Strong DR. Measuring anhedonia in adolescents: a psychometric analysis. J Pers Assess. (2015) 97:506–14. doi: 10.1080/00223891.2015.1029072
44. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. (2012) 169:364–73. doi: 10.1176/appi.ajp.2011.11030447
45. Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. (2006) 32:259–73. doi: 10.1093/schbul/sbj009
46. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. (2007) 93:253–60. doi: 10.1016/j.schres.2007.03.008
47. Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? J Abnorm Psychol. (2014) 123:771–82. doi: 10.1037/abn0000005
48. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. (2011) 35:537–55. doi: 10.1016/j.neubiorev.2010.06.006
49. Gawin FH. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Arch Gen Psychiatry. (1986) 43:107. doi: 10.1001/archpsyc.1986.01800020013003
50. Martinotti G, di Nicola M, Reina D, Andreoli S, Focà F, Cunniff A, et al. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst Use Misuse. (2009) 43:271–284. doi: 10.1080/10826080701202429
51. Hatzigiakoumis DS, Martinotti G, di Giannantonio M, Janiri L. Anhedonia and substance dependence: clinical correlates and: treatment options. Front Psychiatry. (2011) 2:10. doi: 10.3389/fpsyt.2011.00010
52. Pozzi G, Martinotti G, Reina D, Dario T, Frustaci A, Janiri L, et al. The assessment of post-detoxification anhedonia: influence of clinical and psychosocial variables. Subst Use Misuse. (2008) 43:722–32. doi: 10.1080/00952990701202954
53. Huhn AS, Meyer RE, Harris JD, Ayaz H, Deneke E, Stankoski DM, et al. Evidence of anhedonia and differential reward processing in prefrontal cortex among post-withdrawal patients with prescription opiate dependence. Brain Res Bull. (2016) 123:102–9. doi: 10.1016/J.BRAINRESBULL.2015.12.004
54. Carelli RM, West EA. When a good taste turns bad: neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology. (2014) 76:360–9. doi: 10.1016/J.NEUROPHARM.2013.04.025
55. Koob GF, le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. (2001) 24:97–129. doi: 10.1016/S0893-133X(00)00195-0
56. Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. (2002) 78:610–24. doi: 10.1006/NLME.2002.4099
57. Crits-Christoph P, Wadden S, Gaines A, Rieger A, Gallop R, McKay JR, et al. Symptoms of anhedonia, not depression, predict the outcome of treatment of cocaine dependence. J Subst Abuse Treat. (2018) 92:46–50. doi: 10.1016/J.JSAT.2018.06.010
58. Huhn AS, Brooner RK, Sweeney MM, Antoine D, Hammond AS, Ayaz H, et al. The association of prefrontal cortex response during a natural reward cue-reactivity paradigm, anhedonia, and demoralization in persons maintained on methadone. Addict Behav. (2021) 113:106673. doi: 10.1016/J.ADDBEH.2020.106673
59. Kiluk BD, Yip SW, DeVito EE, Carroll KM, Sofuoglu M. Anhedonia as a key clinical feature in the maintenance and treatment of opioid use disorder. Clin Psychol Sci. (2019) 7:1190–206. doi: 10.1177/2167702619855659
60. Nguyen LC, Durazzo TC, Dwyer CL, Rauch AA, Humphreys K, Williams LM, et al. Predicting relapse after alcohol use disorder treatment in a high-risk cohort: the roles of anhedonia and smoking. J Psychiatr Res. (2020) 126:1–7. doi: 10.1016/J.JPSYCHIRES.2020.04.003
61. McKay JR, Lynch KG, Coviello D, Morrison R, Cary MS, Skalina L, et al. Randomized trial of continuing care enhancements for cocaine-dependent patients following initial engagement. J Consult Clin Psychol. (2010) 78:111–20. doi: 10.1037/A0018139
62. Wardle MC, Vincent JN, Suchting R, Green CE, Lane SD, Schmitz JM. Anhedonia is associated with poorer outcomes in contingency management for cocaine use disorder. J Subst Abuse Treat. (2017) 72:32–9. doi: 10.1016/J.JSAT.2016.08.020
63. Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, et al. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. (2005) 52:37–44. doi: 10.1159/000086176
64. Sussman S, Leventhal A. Substance misuse prevention: addressing anhedonia. New Dir Youth Dev. (2014) 2014:45–56. doi: 10.1002/yd.20085
65. Leventhal AM, Cho J, Stone MD, Barrington-Trimis JL, Chou C-P, Sussman SY, et al. Associations between anhedonia and marijuana use escalation across mid-adolescence. Addiction. (2017) 112:2182–90. doi: 10.1111/add.13912
66. Martinotti G, Cloninger CR, Janiri L. Temperament and character inventory dimensions and anhedonia in detoxified substance-dependent subjects. Am J Drug Alcohol Abuse. (2008) 34:177–83. doi: 10.1080/00952990701877078
67. Garfield JBB, Lubman DI, Yücel M. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Austral N Z J Psychiatry. (2014) 48:36–51. doi: 10.1177/0004867413508455
68. Stull SW, Bertz JW, Panlilio LV, Kowalczyk WJ, Phillips KA, Moran LM, et al. I feel good? Anhedonia might not mean “without pleasure” for people treated for opioid use disorder. J Abnorm Psychol. (2021) 130:537–49. doi: 10.1037/ABN0000674
69. Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. (1997) 12:224–31. doi: 10.1016/S0924-9338(97)83296-8
71. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. (1977) 1:385–401. doi: 10.1177/014662167700100306
72. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. (1990) 3:247–51. doi: 10.1016/0920-9964(90)90005-R
73. Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. (1992) 6:201–8. doi: 10.1016/0920-9964(92)90003-N
74. Micoulaud-Franchi JA, Faugere M, Weibel S, Faget C, Lancon C, Richieri R, et al. Toward a transdiagnostic tool to evaluate depressive symptoms across mental disorders: validation of the calgary depression rating scale in patients with major depressive disorder. Psychiatry Res. (2018) 268:68–71. doi: 10.1016/J.PSYCHRES.2018.06.062
75. Müller MJ, Brening H, Gensch C, Klinga J, Kienzle B, Müller KM. The calgary depression rating scale for schizophrenia in a healthy control group: psychometric properties and reference values. J Affect Disord. (2005) 88:69–74. doi: 10.1016/J.JAD.2005.04.005
76. Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. (1995) 19:92–9. doi: 10.1111/J.1530-0277.1995.TB01475.X
77. Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. (2011) 37:300–5. doi: 10.1093/SCHBUL/SBQ059
78. Dollfus S, Mach C, Morello R. Self-evaluation of negative symptoms : a novel tool to assess negative symptoms. Schizophr Bull. (2016) 42:571–8. doi: 10.1093/SCHBUL/SBV161
79. Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers. (2006) 40:1086–102. doi: 10.1016/J.JRP.2005.11.001
80. Garfield JBB, Cotton SM, Lubman DI. Psychometric properties, validity, and reliability of the temporal experience of pleasure scale state version in an opioid-dependent sample. Drug Alcohol Depend. (2016) 161:238–46. doi: 10.1016/J.DRUGALCDEP.2016.02.011
81. Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS-IV) Administration and Scoring Manual. San Antonio, TX: Pearson (2008).
82. Besson J. Pour une approche globale de l’addiction à l’alcool. Forum Méd Suisse. (2002) 21:506–10. doi: 10.4414/fms.2002.04535
83. Nasrallah H, Morosini PL, Gagnon DD. Reliability, validity and ability to detect change of the personal and social performance scale in patients with stable schizophrenia. Psychiatry Res. (2008) 161:213–24. doi: 10.1016/J.PSYCHRES.2007.11.012
84. Kirschner M, Cathomas F, Manoliu A, Habermeyer B, Simon JJ, Seifritz E, et al. Shared and dissociable features of apathy and reward system dysfunction in bipolar i disorder and schizophrenia. Psychol Med. (2020) 50:936–47. doi: 10.1017/S0033291719000801
85. Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, et al. Treatment of negative symptoms: where do we stand, and where do we go? Schizophr Res. (2017) 186:55–62. doi: 10.1016/J.SCHRES.2016.05.015
86. Kirschner M, Sladky R, Haugg A, Stämpfli P, Jehli E, Hodel M, et al. Self-regulation of the dopaminergic reward circuit in cocaine users with mental imagery and neurofeedback. EBioMedicine. (2018) 37:489–98. doi: 10.1016/J.EBIOM.2018.10.052
87. Pettorruso M, Spagnolo PA, Leggio L, Janiri L, di Giannantonio M, Gallimberti L, et al. Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex may improve symptoms of anhedonia in individuals with cocaine use disorder: a pilot study. Brain Stimul. (2018) 11:1195–7. doi: 10.1016/J.BRS.2018.06.001
88. Dakwar E, Nunes EV, Hart CL, Foltin RW, Mathew SJ, Carpenter KM, et al. A Single ketamine infusion combined with mindfulness-based behavioral modification to treat cocaine dependence: a randomized clinical trial. Am J Psychiatry. (2019) 176:923–30. doi: 10.1176/appi.ajp.2019.18101123
89. Dakwar E, Levin F, Hart CL, Basaraba C, Choi J, Pavlicova M, et al. A single ketamine infusion combined with motivational enhancement therapy for alcohol use disorder: a randomized midazolam-controlled pilot trial. Am J Psychiatry. (2020) 177:125–33. doi: 10.1176/APPI.AJP.2019.19070684
90. Nogo D, Jasrai AK, Kim H, Nasri F, Ceban F, Lui LMW, et al. The effect of ketamine on anhedonia: improvements in dimensions of anticipatory, consummatory, and motivation-related reward deficits. Psychopharmacology. (2022) 1:1–29. doi: 10.1007/S00213-022-06105-9/TABLES/2
91. Pulcu E, Guinea C, Cowen PJ, Murphy SE, Harmer CJ. A translational perspective on the anti-anhedonic effect of ketamine and its neural underpinnings. Mol Psychiatry. (2021) 27:81–7. doi: 10.1038/s41380-021-01183-1
92. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the epidemiologic catchment area (ECA) study. JAMA. (1990) 264:2511–8. doi: 10.1001/JAMA.1990.03450190043026
93. Kessler RC. The epidemiology of dual diagnosis. Biol Psychiatry. (2004) 56:730–7. doi: 10.1016/J.BIOPSYCH.2004.06.034
94. Brière FN, Rohde P, Seeley JR, Klein D, Lewinsohn PM. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Compr Psychiatry. (2014) 55:526–33. doi: 10.1016/J.COMPPSYCH.2013.10.007
95. Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. (2010) 214:17–31. doi: 10.1007/S00213-010-1916-6
96. Dube SR, Anda RF, Felitti VJ, Edwards VJ, Croft JB. Adverse childhood experiences and personal alcohol abuse as an adult. Addict Behav. (2002) 27:713–25. doi: 10.1016/S0306-4603(01)00204-0
Keywords: alcohol use disorder, negative symptoms, anhedonia, craving, substance use disorder, addiction, motivation and pleasure
Citation: Buschner M, Dürsteler KM, Fischli G, Hess J, Kirschner M, Kaiser S and Herdener M (2022) Negative symptoms in alcohol use disorder: A pilot study applying the two-factor model of negative symptoms to patients with alcohol use disorder. Front. Psychiatry 13:957924. doi: 10.3389/fpsyt.2022.957924
Received: 31 May 2022; Accepted: 02 November 2022;
Published: 18 November 2022.
Edited by:
Liana Fattore, CNR Neuroscience Institute (IN), ItalyReviewed by:
Anna Franceschini, Center for Addiction Medicine, Public Health Unit, ItalyPhilippa Hüpen, RWTH Aachen University, Germany
Copyright © 2022 Buschner, Dürsteler, Fischli, Hess, Kirschner, Kaiser and Herdener. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maximilian Buschner, bWF4aW1pbGlhbi5idXNjaG5lckBibGkudXpoLmNo
 Maximilian Buschner
Maximilian Buschner Kenneth M. Dürsteler
Kenneth M. Dürsteler Gina Fischli
Gina Fischli Jelena Hess
Jelena Hess Matthias Kirschner
Matthias Kirschner Stefan Kaiser
Stefan Kaiser Marcus Herdener
Marcus Herdener